Abstract
Aims
Pain depresses expression of many behaviors, and one goal of analgesic treatment is to restore pain-depressed behaviors. Assays that focus on pain-depressed behaviors may contribute to preclinical assessment of candidate analgesics.
Main Methods
This study compared effects of the mu opioid receptor agonist morphine (an acknowledged analgesic), the dopamine receptor antagonist haloperidol (a non-analgesic sedative), the adenosine receptor antagonist caffeine (a non-analgesic stimulant) and the neurokinin-1 receptor antagonist CJ 11,974-01 (a candidate analgesic) on acetic acid-induced writhing (a traditional pain-stimulated behavior) and acetic acid-induced suppression of locomotor activity (a pain-depressed behavior) in male ICR mice. Drug effects on non-depressed (baseline) locomotor activity were also examined.
Key findings
I.P. administration of acetic acid (0.18-1%) was equipotent in stimulating writhing and depressing locomotor activity. Morphine blocked both acid-induced stimulation of writhing and depression of locomotion, although it was 56-fold less potent in the assay of acid-depressed locomotion. Haloperidol and CJ 11,974-01 decreased acid-stimulated writhing, but failed to block acid-induced depression of locomotion. Caffeine had no effect on acid-stimulated writhing or acid-depressed locomotor activity, although it did increase non-depressed locomotion. Thus, morphine was the only drug to block both acid-stimulated writhing and acid-depressed locomotion.
Significance
Complementary assays of pain-stimulated and pain-depressed behaviors may improve the predictive validity of preclinical studies that assess candidate analgesic drugs. The low potency of morphine to block acid-induced depression of locomotion suggests that locomotor activity may be a relatively insensitive measure for studies of pain-depressed behavior.
Keywords: Pain model, Pain-depressed behavior, Pain-stimulated behavior, Mice, Acetic acid, Morphine, Locomotor activity
INTRODUCTION
In the absence of verbal reports, the existence of pain and analgesia in animals must be inferred from changes in other, non-verbal behaviors (Morton and Griffiths 1985; Flecknell 1994). Preclinical research on pain and analgesia has focused almost exclusively on pain-stimulated behaviors, which can be defined as behaviors that increase in frequency or intensity following presentation of a noxious stimulus (Dykstra 1985;Le Bars et al. 2001; Negus et al. 2006). Examples of pain-stimulated behaviors include withdrawal responses from noxious thermal or mechanical stimuli and writhing/flinching responses following injection of a noxious chemical. Although pain-stimulated behaviors have been a mainstay of preclinical research on pain and analgesia for many years, there are limitations to exclusive reliance on these behaviors to quantify preclinical pain states (Negus et al. 2006; Stevenson et al. 2006). For example, drugs may decrease these behaviors not only by blocking sensory/affective processing of the noxious stimulus (true analgesia) but also by producing motor effects that impair the ability of the subject to respond (a false positive analgesic effect).
A second limitation to this approach is its neglect of the fact that pain is commonly associated with a depression of many adaptive behaviors, and that one goal of analgesic treatment is a restoration of pain-depressed behavior (NRC 2003; ACPA 2007). The clinical value of these measures suggests that research efforts might also benefit from development of procedures that focus on pain-depressed behaviors, which can be operationally defined as behaviors that decrease in frequency or intensity following presentation of a noxious stimulus (Negus et al. 2006).
We have previously reported successful development of an assay of pain-depressed behavior in mice, using feeding as the primary dependent measure (Stevenson et al. 2006). The purpose of the present study was to evaluate an assay of noxious stimulus-depressed locomotor activity (LMA) in mice, to further evaluate the utility and sensitivity of preclinical methods that measure pain-depressed behavior. Assay development proceeded in three steps: 1) identification of conditions under which LMA occurred at a reliable and high rate, 2) characterization of a noxious stimulus (i.p. acetic acid) that reliably depressed LMA, and 3) assessment of drug effects on control and acid-depressed LMA. Locomotor activity was chosen as the behavioral endpoint because painful states in laboratory animals and humans are reliably associated with decreases in LMA and exercise (Lazarus and Neumann 2001; NRC 2003), suggesting that LMA is a reliable and clinically relevant endpoint in pain assessment.
Drug effects on control and acid-depressed LMA were compared to effects on acetic acid-induced stimulation of the writhing response, a common endpoint in studies of pain-stimulated behavior (Collier et al. 1968; Pearl et al. 1968). Four drugs were evaluated. Morphine (a mu opioid receptor agonist) was studied as a representative analgesic with acknowledged efficacy in human and veterinary medicine (NRC 2003; Meert and Vermeirsch 2005; ACPA 2007). Haloperidol (a dopamine D2 receptor antagonist and non-analgesic sedative) and caffeine (an adenosine receptor antagonist and non-analgesic stimulant) were studied as drugs that were expected to produce non-selective decreases and increases in behavior, respectively (Hitzemann et al. 1991; El Yacoubi et al. 2000). CJ 11,974-01 was studied as a representative neurokinin-1 receptor antagonist, a class of drugs that displayed promising effects in many conventional preclinical assays but that has failed to produce therapeutically useful analgesia in human clinical trials (Hill 2000; Urban and Fox 2000).
MATERIALS AND METHODS
Subjects
Adult male ICR mice (30-35g; Harlan, Indianapolis, IN) were used for all experiments. Mice were housed in groups of four to five in standard Plexiglas containers with food and water available ad libitum. Animals were maintained in a temperature and humidity controlled colony on a 12-h light/dark cycle (lights on at 7:00). All studies were conducted in accordance with the Guide for the Care and Use of Laboratory Animals as adopted by the National Institutes of Health. The University of New England Institutional Animal Care and Use Committee (IACUC) approved all protocols involving animals.
Assay of acetic acid-induced writhing
Studies of acetic acid-induced writhing were conducted to permit a direct comparison of morphine, haloperidol, caffeine and CJ 11,974-01 effects on pain-depressed behavior (acid-depressed LMA) and pain-stimulated behavior (acid-stimulated writhing). Specifically, mice were removed from their home cages and injected i.p. with acetic acid (i.e. the same range of concentrations used in the LMA studies). Each mouse was then placed individually in a Plexiglas observation cylinder (14 cm diameter; 30 cm tall) and videotaped for 20 min. A writhe was operationally defined as a contraction of the abdomen followed by stretching of the hind limbs. First, the concentration (0-1%) and pretreatment time (0 – 100 min) of acetic acid were systematically manipulated, with the goal of identifying conditions under which acetic acid reliably produced writhing. On the basis of these results, an acetic acid concentration of 0.56% and a pretreatment time of 0 min were used for the remainder of the writhing experiments. Second, the effects of a range of doses of s.c. morphine (0.001-0.1 mg/kg), haloperidol (0.001-0.1mg/kg), caffeine (0.1-10 mg/kg) and CJ 11,974-01 (0.1-10mg/kg) were examined on acetic acid-stimulated writhing. Both morphine and haloperidol were tested using a 45 min pretreatment time (time of maximum effect), and both CJ 11,974-01 and caffeine were tested using a 15 min pretreatment time (time of maximum effect).
Assay of acetic acid-depressed locomotor activity
Locomotor activity (LMA) was measured using an activity monitoring system (Coulbourn Instruments, Allentown, PA) and Truscan software. Each of the eight chambers consisted of an arena (25.91 × 25.91 × 40.6 cm) surrounded by Plexiglas walls. The floor consisted of a removable plastic drop pan that was cleaned between sessions. A sensor ring surrounded the arena on the bottom outside edges of the four sides of the chamber and contained 16 infrared beams that transected both the length and the width of the chambers on all four sides (beam spacing = 1.52 cm, resolution of 32 × 32 squares). Measurement of the animals’ position was determined every 100 ms, and the software calculated a number of parameters related to aspects of locomotor activity. The total distance traveled (cm) by each mouse was used as the primary dependent measure. LMA sessions were conducted between 9am and 5pm. During baseline LMA sessions, mice were removed from their home cages, weighed and individually placed in LMA chambers. After 30 min, mice were returned to their home cages and the total distance traveled during the 30 min access period was recorded. Following baseline determination, testing was initiated and proceeded in two phases. First, the concentration (0-1%) and pretreatment time (0 – 300 min) of acetic acid were systematically manipulated, with the goal of identifying conditions under which acetic acid reliably depressed locomotor activity. On the basis of these results, an acetic acid concentration of 0.56% and a pretreatment time of 0 min were used for the remainder of the study. Second, the effects of a range of doses of subcutaneous (s.c.) morphine (1-10 mg/kg), haloperidol (.01-0.56mg/kg), caffeine (1-18 mg/kg) and CJ 11,974-01 (1-32mg/kg) were examined on acetic acid-depressed LMA and non-depressed (vehicle-treated condition) LMA. Both morphine and haloperidol were tested using a 45 min pretreatment time (time of maximum effect), and both CJ 11,974-01 and caffeine were tested using a 15 min pretreatment time (time of maximum effect). To limit the development of habituation to the locomotor chamber, separate groups of mice (n=8) were used for baseline and both phases of testing.
Drugs
Morphine sulfate (NIDA Drug Supply Program) was dissolved in sterile water. Haloperidol (Sigma Corp., St. Louis, MO) was dissolved in a vehicle composed of bacteriostatic water and 3% acetic acid. CJ 11,974-01 (Pfizer Corp., Groton, CT) was dissolved in sterile water. Caffeine (Sigma Corp., St. Louis, MO) was dissolved in a vehicle composed of bacteriostatic water and 5% acetic acid. All drugs were administered s.c. in a volume of 0.1ml/10g body weight. Acetic acid was diluted in sterile saline and administered i.p. in a volume of 0.1ml/10g body weight.
Data analysis
The primary dependent variable of the writhing studies was the number of writhes in a 20 min session. The primary dependent variable of the LMA studies was total distance traveled (cm) in a 30 min session. Statistical analysis was accomplished with one- or two-factor ANOVA as appropriate. A significant one-way ANOVA was followed by the Duncan post hoc test. Significance was set a priori at p ≤ 0.05.
RESULTS
Acetic acid-induced stimulation of writhing and depression of locomotor activity
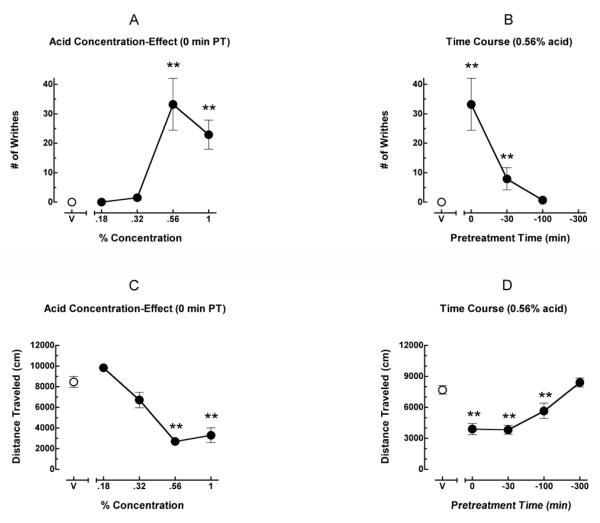
Figure 1A shows the mean number of writhes after treatment with a range of concentrations of acetic acid. Administration of acetic acid produced a biphasic concentration-effect curve. At 0 (vehicle) and 0.18% acid, mice did not writhe. At 0.32% acid mice writhed at very low levels. Writhing increased at an acid concentration of 0.56% (peak effect). A higher concentration of 1% acid was located on the descending limb of the acid concentration-effect curve. The number of writhes following 0.56 and 1% acid was significantly greater than the number of writhes following vehicle. Figure 1B shows writhing as a function of pretreatment interval. Pretreatment with 0.56% acetic acid at 0 and 30 min produced significant writhing relative to control levels (vehicle). However, the effects of acid were no longer apparent after 100 min. Figure 1C shows locomotor activity (LMA) across the same range of concentrations of acetic acid tested in the writhing procedure. Administration of acetic acid produced a concentration-dependent decrease in total distance traveled (cm). Locomotor activity following 0.56 and 1% acid was significantly decreased relative to vehicle. Figure 1D shows locomotor activity as a function of pretreatment interval. Pretreatment with 0.56% acetic acid at 0, 30 and 100 min produced significant decreases in total distance traveled relative to control levels. However, the effects of acid were no longer apparent after 300 min. On the basis of these results, subsequent experiments were conducted using a 0 min pretreatment with 0.56% acetic acid.
Figure 1.
Effects of concentration and pretreatment interval on acid-stimulated writhing and acid-depressed locomotor activity. All points show mean data of 8-10 mice, and error bars show S.E.M. Figure 1A shows the concentration-effect curve for acid-stimulated writhing. Abscissa: Percent concentration of acid (log scale). Data point above “V” represents number of writhes under control conditions (0% acid or vehicle). Ordinate: Number of writhes. **Significantly different from 0% acid (p < 0.01). Figure 1B shows the pretreatment time-effect curve for acid-stimulated writhing. Abscissa: pretreatment interval (min). Ordinate: Number of writhes. **Significantly different from 0% acid (p < 0.01). Figure 1C shows the concentration-effect curve for acid-depressed locomotor activity. Abscissa: Percent concentration of acid (log scale). Data point above “V” represents total distance traveled under control conditions (0% acid or vehicle). Ordinate: Distance traveled (cm) in the locomotor chamber. **Significantly different from 0% acid (p < 0.01). Figure 1D shows the pretreatment time-effect curve for acid-depressed locomotor activity. Abscissa: pretreatment interval (min). Ordinate: Distance traveled (cm) in the locomotor chamber. **Significantly different from 0% acid (p < 0.01).
Effects of morphine, haloperidol, caffeine and CJ 11,974-01 on acetic acid-stimulated writhing
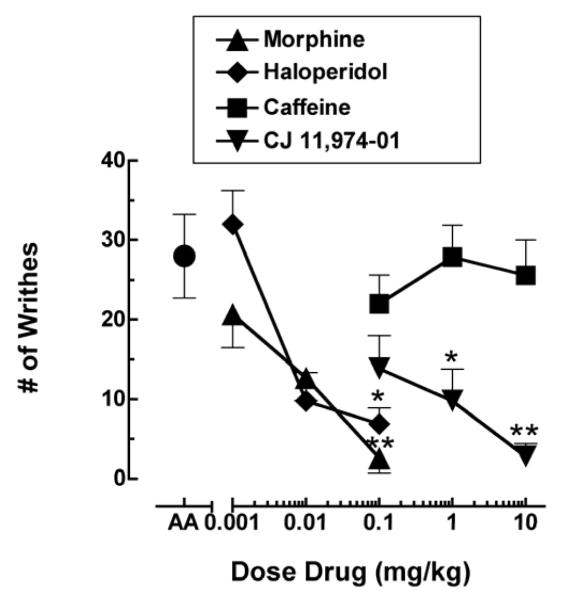
Figure 2 compares the effects of morphine (0.001-0.1 mg/kg), haloperidol (0.001-0.1 mg/kg), caffeine (0.1-10 mg/kg) and CJ 11,974-01 (0.1-10 mg/kg) on acetic acid-stimulated writhing. Acetic acid alone (closed circle) robustly induced a writhing response, producing a mean of approximately 29 writhes in 20 min. Morphine, haloperidol and CJ 11,974-01 produced dose-dependent and significant decreases in acid-induced writhing, whereas caffeine did not decrease writhing at the doses tested.
Figure 2.
Effects of morphine, haloperidol, caffeine and CJ-11,974-01 on acid-stimulated writhing. All points show mean data of 8-16 mice, and error bars show S.E.M. Abscissa: Dose of drug. Data point above “AA” represents number of writhes following acetic acid administration. Ordinate: number of writhes. *Significantly different from acid (AA) alone (p < 0.05). **Significantly different from acid (AA) alone (p < 0.01).
Effects of morphine, haloperidol, caffeine and CJ 11,974-01 on locomotor activity in acetic acid-treated and saline-treated mice
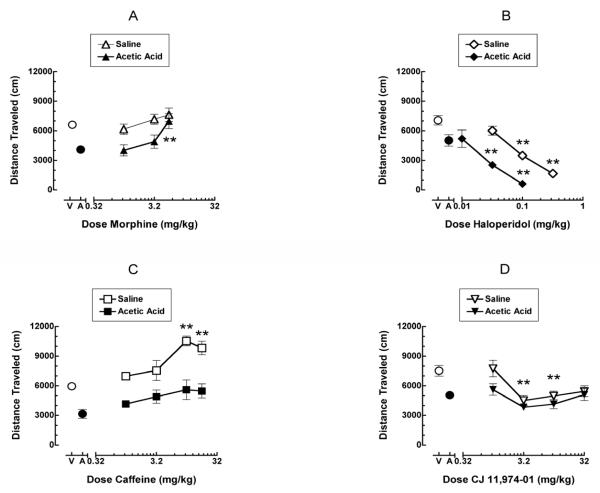
Figure 3 shows the effects of morphine, haloperidol, caffeine and CJ 11,974-01 pretreatment on acid-depressed LMA (assessed immediately after i.p. injection of 0.56% acetic acid, closed symbols) and control, non-depressed LMA (assessed immediately after i.p. saline treatment, open symbols). Figure 3A shows total distance traveled (cm) as a function of morphine dose (1-5.6 mg/kg). Morphine produced a dose-dependent blockade of acid-induced depression of LMA. Distance traveled following 5.6 mg/kg morphine was significantly greater than distance traveled following acid alone and similar to control distance traveled in mice treated with saline rather than with acetic acid. In contrast, these doses of morphine did not alter non-depressed LMA. Thus, morphine selectively increased acid-depressed LMA at doses that did not alter control, non-depressed LMA. Figure 3B shows total distance traveled (cm) as a function of haloperidol dose (0.01-0.32 mg/kg). Haloperidol produced a dose-dependent decrease in both acid-depressed and non-depressed LMA, and haloperidol was more potent in acid-treated mice. Thus, distance traveled following 0.032 and 0.1 mg/kg haloperidol was significantly less than distance traveled following acid alone, and distance traveled following 0.1 and 0.32 mg/kg haloperidol was significantly less than distance traveled following saline alone. Figure 3C shows total distance traveled (cm) as a function of caffeine dose (1-18 mg/kg). For non-depressed LMA, distance traveled following 10 and 18 mg/kg caffeine was significantly greater than distance traveled following saline vehicle alone. There was also a trend for caffeine to increase acid-depressed LMA, but this effect did not achieve statistical significance at any caffeine dose. Figure 3D shows total distance traveled (cm) as a function of CJ 11,974-01 dose (1-32 mg/kg). Doses of 3.2 and 10 mg/kg CJ 11,974-01 significantly decreased non-depressed LMA. There was also a trend for CJ 11,974-01 to further decrease acid-depressed LMA, but this effect did not achieve statistical significance at any dose.
Figure 3.
Effects of morphine, haloperidol, caffeine and CJ-11,074-01 on acid-depressed and non-depressed locomotor activity. All points show mean data of 8-16 mice, and error bars show S.E.M. Figure 3A shows the dose effect curve for morphine on acid-depressed (closed triangles) and non-depressed (open triangles) locomotor activity. Abscissa: Dose of morphine. Data point above “V” represents total distance traveled under control conditions (0% acid or vehicle). Data point above “A” represents total distance traveled following acetic acid administration. Ordinate: Distance traveled (cm) in the locomotor chamber. **Significantly different from acetic acid (A) alone (p <0.01). Figure 3B shows the dose effect curve for haloperidol on acid-depressed (closed diamonds) and non-depressed (open diamonds) locomotor activity. Abscissa: Dose of haloperidol. Data point above “V” represents total distance traveled under control conditions (0% acid or vehicle). Data point above “A” represents total distance traveled following acetic acid administration. Ordinate: Distance traveled (cm) in the locomotor chamber. **Significantly different from acetic acid (A) alone for closed diamonds or from vehicle (V) alone for open diamonds (p <0.01). Figure 3C shows the dose effect curve for caffeine on acid-depressed (closed squares) and non-depressed (open squares) locomotor activity. Abscissa: Dose of caffeine. Data point above “V” represents total distance traveled under control conditions (0% acid or vehicle). Data point above “A” represents total distance traveled following acetic acid administration. Ordinate: Distance traveled (cm) in the locomotor chamber. **Significantly different from vehicle (V) alone (p <0.01). Figure 3D shows the dose effect curve for CJ-11,974-01 on acid-depressed (closed inverted triangles) and non-depressed (open inverted triangles) locomotor activity. Abscissa: Dose of CJ-11,974-01. Data point above “V” represents total distance traveled under control conditions (0% acid or vehicle). Data point above “A” represents total distance traveled following acetic acid administration. Ordinate: Distance traveled (cm) in the locomotor chamber. **Significantly different from vehicle (V) alone (p <0.01).
Comparison of drug effects in assays of acid-stimulated writhing, acid-depressed LMA and non-depressed LMA
Table 1 shows the lowest dose of each drug to produce a significant change in acid-stimulated writhing, acid-depressed LMA and non-depressed LMA. The opioid analgesic morphine was the only drug to block both acid-induced stimulation of writhing and acid-induced depression of LMA at doses that did no alter non-depressed LMA. However, morphine was approximately 56-fold less potent in blocking acid-induced depression of LMA than acid-induced stimulation of writhing. The non-analgesic sedative haloperidol also blocked acid-stimulated writhing. However, doses of haloperidol that blocked writhing also decreased non-depressed LMA, and even lower haloperidol doses exacerbated acid-induced depression of LMA. In contrast, the non-analgesic stimulant caffeine had no significant effect on either acid-stimulated writhing or acid-depressed LMA, but increased non-depressed LMA. The candidate analgesic CJ 11,974-01 produced a profile of effects most similar to that produced by haloperidol. Thus, CJ 11,974-01 blocked acid-stimulated writhing at doses only slightly lower than those that also decreased non-depressed LMA and that tended to exacerbate acid-depressed LMA.
Table 1.
Lowest doses of morphine, haloperidol, caffeine and CJ 11,974-01 to significantly alter acetic acid-stimulated writhing, acetic acid-depressed LMA, and non-depressed LMA. Arrows show the direction of effect (↑ = increase in rate of target behavior, decrease in rate of target behavior).
| Drug | Acid-Stimulated Writhing | Acid-Depressed LMA | Non-Depressed LMA |
|---|---|---|---|
| Morphine | 0.1 ↓ | 5.6 ↑ | >5.6 |
| Haloperidol | 0.1 ↓ | 0.032 ↓ | 0.1 ↓ |
| Caffeine | >10 | >18 | 10 ↑ |
| CJ 11,974-01 | 1.0 ↓ | >32 | 3.2 ↓ |
DISCUSSION
The purpose of the present paper was to continue development of procedures for evaluating drug effects on behavior depressed by a noxious stimulus. Our main findings were that high and stable levels of locomotor activity (LMA) were reliably depressed by i.p. administration of acetic acid, and that the opioid analgesic morphine was the only drug tested in this study to both restore acid-depressed LMA and block acid-stimulated writhing. These assays of acid-depressed LMA and acid-stimulated writhing were also used to generate profiles of effects produced by a representative non-analgesic sedative (haloperidol), a non-analgesic stimulant (caffeine) and a candidate analgesic from a drug class that has performed poorly in clinical trials (CJ 11,974-01). The effects produced by these drugs suggest that complementary use of procedures that measure pain-depressed and pain-stimulated behaviors may enhance predictive validity in translational research with candidate analgesics.
The first step in this study was to establish high and stable rates of LMA during relatively short experimental sessions. This was achieved by exposing mice to a locomotor chamber for 30 min. Baseline levels of LMA during this 30 min access period were approximately 8,500 cm. We then demonstrated that a noxious stimulus could reliably depress LMA. Specifically, LMA was decreased by i.p. injection of acetic acid, a chemical noxious stimulus that has been widely used for studies of pain-stimulated behaviors (Collier et al. 1968; Pearl et al. 1968). Our findings of acid-depressed LMA are consistent with reports showing that presumably painful events, such as abdominal surgery, are associated with decreases in locomotor activity (Flecknell 1994; Flecknell et al. 1999; Roughan and Flecknell 2000; Matson et al. 2007). The effects of acid were concentration- and time-dependent, with maximal depression of locomotor activity achieved at a concentration of 0.56% acid, and duration of depression at least 100 min. Acetic acid also produced concentration- and time-dependent stimulation of writhing. Acid was equipotent in producing depression of LMA and induction of writhing, as a concentration of 0.56% produced maximal suppression of LMA and maximal writhing. Acid-induced writhing was evident at 5 min and lasted less than 100 min. One should note that the acid-induced depression of LMA was not likely influenced by the competing behavior of writhing. Acetic acid induced an average of 1.5 writhes per min in this study (an average of 30 writhes in 20 min), and each writhe takes about 3-6 sec to complete, Thus, with writhing occurring at a high rate, mice spent only approximately 15% of their time actively writhing during a 30 min LMA session. Moreover, acetic acid depressed locomotion for a longer period of time than it stimulated writhing. This finding agrees with our previous finding that acetic acid was equipotent but longer lasting in depressing feeding than in stimulating writhing in mice (Stevenson et al. 2006).
Following demonstration of acid-depressed LMA, we assessed the degree to which an analgesic drug could selectively restore acid-depressed LMA. The opioid agonist morphine was chosen because it is widely used to treat moderate to severe pain in humans and has efficacy in a broad range of preclinical assays of nociception (NRC 2003; Meert and Vermeirsch 2005; ACPA 2007). Morphine was the only drug tested that both restored acid-depressed LMA and blocked acid-induced writhing. Once morphine was shown to be effective in restoring acid-depressed LMA, the next step was to determine whether morphine’s effects were selective for acid-suppressed LMA, or reflected a non-selective increase in LMA. This issue was important to consider because morphine has been shown to stimulate locomotion in mice at some doses (Raehal et al. 2005). However, one finding suggested that morphine-induced restoration of acid-depressed LMA represented a selective effect. Specifically, morphine produced increases in acid-depressed LMA at a dose that did not increase LMA in saline-treated mice. These results agree with our previous finding that morphine restored acid-depressed feeding in mice at a dose that did not stimulate baseline, non-depressed feeding.
Although morphine blocked both acid-induced depression of LMA and acid-stimulated writhing in the present study, it was approximately 56-fold more potent in the writhing assay. The reasons for this discrepancy in potency are unknown. One possibility is that morphine’s effects in the writhing assay were mediated largely by peripheral opioid receptors, whereas its effects on acid-depressed LMA were centrally mediated. It is well-established that mu opioid agonists can decrease acid-stimulated writhing in mice by acting at peripheral opioid receptors (Takasuna et al. 1994), whereas opioid effects on baseline, non-depressed LMA appear to be centrally mediated (Teitelbaum et al. 1979; van Abeelen and van den Heuvel 1982). Moreover, the potency of morphine to block acid-stimulated writhing in the present study was similar to its potency to block writhing in previous studies (ED50 ranges of 0.1 – 1 mg/kg)(Elmer et al., 1998; Broadbear et al., 2000), whereas its potency to restore acid-depressed LMA was similar to its potency to produce antinociceptive effects (Ward and Takemori 1983; Wigdor and Wilcox 1987; Mogil and Wilson 1997; Elmer et al. 1998) and rewarding effects (Bohn et al. 2003; Elmer et al. 2005) that appear to require activation of central opioid receptors (ED50 ranges from approximately 1-10 mg/kg). However, the dose of morphine required to restore acid-depressed LMA in this study (5.6 mg/kg) was higher than the dose required to restore acid-depressed feeding in our previous study (1.0 mg/kg). A second possibility is that mice in the acid-depressed LMA experiments experienced LMA enhanced acid-induced nociception relative to mice in acid-stimulated writhing experiments (where test cylinders were small and restricted LMA for each mouse). In support of this, some reports suggest that general locomotor activity may exacerbate pain symptoms in veterinary and human populations (van Weering et al., 2007; Moseley et al., 2008), and therefore, higher doses of analgesics may be required to restore pain-depressed general locomotor activity levels than to inhibit more localized escape, limb-withdrawal or abdominal stretch responses. A third possibility is that morphine produced non-selective increases in locomotion. It is known that mu (morphine, DAGO), delta (DPDPE) and kappa (U50488H, bremazocine) opioid agonists produce dose-dependent increases in horizontal locomotor activity and opioid antagonists (naloxone and naltrexone) block mu- and kappa-stimulated LMA, indicating an opioid-mediated effect (Brase et al., 1977; Michael-Titus et al., 1989; Kuzmin et al., 2000). However, under the conditions examined in this study, morphine did not produce any change in control levels of LMA, suggesting that the morphine-induced restoration of acid-depressed LMA was a selective effect. Finally, pilot studies in our laboratory with the mu opioid agonist buprenorphine demonstrate a similar low-potency profile in restoring acid-depressed LMA (unpublished findings), and the potency of morphine was similar to block acid-stimulated writhing and restore acid-induced depression of intracranial self-stimulation in rats (Pereira Do Carmo et al. in press). This suggests that LMA may be a relatively insensitive behavioral endpoint for preclinical assays of pain-depressed behavior.
Haloperidol and caffeine were tested in the present study as non-analgesics that were expected to produce non-selective decreases and increases in behavior, respectively. Thus, like morphine, haloperidol produced a dose-dependent decrease in acid-stimulated writhing. However, doses of haloperidol that decreased writhing also decreased levels of control, non-depressed LMA, and even lower doses of haloperidol exacerbated acid-induced depression of LMA. Haloperidol also failed to prevent acid-induced depression of feeding in mice (Stevenson et al. 2006). Taken together, these findings are consistent with the conclusion that apparent antinociceptive effects of haloperidol in the writhing assay resulted not from a reduction in sensory detection of pain, but rather from a non-selective motor impairment that reduced a wide range of behaviors. The non-analgesic stimulant caffeine produced the expected increase in control, non-depressed LMA (El Yacoubi et al. 2000). However, caffeine did not significantly increase acid-depressed LMA, and in contrast to the effects of morphine, caffeine did not stimulate acid-depressed LMA to a greater degree than it stimulated non-depressed LMA. This finding provides further support for the proposition that caffeine effects on acid-depressed locomotion can be accounted for entirely by its ability to stimulate non-depressed LMA (Raehal et al. 2005). Also, unlike morphine, caffeine failed to block acid-stimulated writhing. Overall, these results with morphine, haloperidol and caffeine suggest that combined use of assays that measure pain-depressed and pain-stimulated behaviors can differentiate effects of true analgesics from effects produced by non-selective motor depressants or stimulants.
To further evaluate the utility of complementary assays of pain-depressed and pain-stimulated behaviors, the present study evaluated effects of the neurokinin-1 receptor antagonist CJ 11,974-01. NK-1 antagonists have been evaluated in preclinical assays that primarily involved measures of pain-stimulated behaviors, and results were considered sufficiently promising to promote some NK-1 antagonists to clinical trials. However, to date, this class of drugs has displayed poor analgesic efficacy in clinical pain trials (Hill 2000; Urban and Fox 2000). In agreement with previous studies assessing NK-1 antagonists, using assays of pain-stimulated behaviors (e.g., formalin-induced licking, acetic acid-induced writhing), CJ 11,974-01 decreased acid-stimulated writhing in the present study. However, CJ 11,974-01 also decreased control, non-depressed LMA and failed to restore acid-depressed LMA. Overall, this profile of activity is most similar to that of haloperidol in this study, and may suggest that CJ 11,074-01 produced a false positive antinociceptive effect in the writhing assay by inhibiting the ability of the animal to respond. These results also provide further evidence that combined use of assays of pain-depressed and pain-stimulated behavior may contribute to translational research by improving predictive validity of preclinical studies on candidate analgesics.
CONCLUSION
Taken together, these findings suggest that incorporating preclinical assays that measure transient pain-depressed behaviors into basic science research should proceed with careful, systematic evaluation of pain-depressed endpoints. An improved strategy for preclinical pain research and drug testing may consist of measuring both pain-depressed behaviors and more traditional pain-stimulated behaviors, though it may be necessary to choose different pain-depressed endpoints (e.g., feeding, exercise, affect).
Looking forward, assessment of pain-depressed LMA may be more predictive following chronic pain states. For example, it is widely known that chronic pain in veterinary and human populations is typically assessed with pain-depressed measures. In addition, the American Pain Society has reported that the primary goal for treating chronic pain patients is to increase functioning by restoring pain-depressed activities (ACPA 2007). Thus, systematic preclinical evaluation of chronic pain-depressed (as well as chronic pain-stimulated) behaviors may lead to more relevant quantification of clinical pain and analgesic efficacy.
ACKNOWLEDGMENTS
The authors thank the NIDA Drug Supply Program for morphine sulfate and Pfizer Corporation for CJ 11,974-01. Research supported by NIAMS, NIH grant 1 R15 AR054975-01 to G.W.S. and R01-DA11460 to S.S.N. from NIDA.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
REFERENCES
- American Chronic Pain Association . ACPA Medications & Chronic Pain. Rocklin, CA: 2007. [Google Scholar]
- Bohn LM, Gainetdinov RR, Sotnikova TD, Medvedev IO, Lefkowitz RJ, Dykstra LA, Caron MG. Enhanced rewarding properties of morphine, but not cocaine, in beta(arrestin)-2 knock-out mice. The Journal of Neuroscience. 2003;23:10265–10273. doi: 10.1523/JNEUROSCI.23-32-10265.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brase DA, Loh HH, Way EL. Comparison of the effects of morphine on locomotor activity, analgesia and primary and protracted physical dependence in six mouse strains. Journal of Pharmacology and Experimental Therapeutics. 1977;201:368–374. [PubMed] [Google Scholar]
- Broadbear JH, Sumpter TL, Burke TF, Husbands SM, Lewis JW, Woods JH, Traynor JR. Methocinnamox is a potent, long-lasting, and selective antagonist of morphine-mediated antinociception in the mouse: comparison with clocinnamox, beta-funaltrexamine, and beta-chlornaltrexamine. Journal of Pharmacology and Experimental Therapeutics. 2000;294:933–940. [PubMed] [Google Scholar]
- Collier HO, Dinneen LC, Johnson CA, Schneider C. The abdominal constriction response and its suppression by analgesic drugs in the mouse. British Journal of Pharmacology and Chemotherapy. 1968;32:295–310. doi: 10.1111/j.1476-5381.1968.tb00973.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dykstra LA. Effects of buprenorphine on shock titration in squirrel monkeys. Journal of Pharmacology and Experimental Therapeutics. 1985;235:20–25. [PubMed] [Google Scholar]
- El Yacoubi M, Ledent C, Menard JF, Parmentier M, Costentin J, Vaugeois JM. The stimulant effects of caffeine on locomotor behaviour in mice are mediated through its blockade of adenosine A(2A) receptors. British Journal of Pharmacology. 2000;129:1465–1473. doi: 10.1038/sj.bjp.0703170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Elmer GI, Pieper JO, Levy J, Rubinstein M, Low MJ, Grandy DK, Wise RA. Brain stimulation and morphine reward deficits in dopamine D2 receptor-deficient mice. Psychopharmacology (Berlin) 2005;182:33–44. doi: 10.1007/s00213-005-0051-2. [DOI] [PubMed] [Google Scholar]
- Elmer GI, Pieper JO, Negus SS, Woods JH. Genetic variance in nociception and its relationship to the potency of morphine-induced analgesia in thermal and chemical tests. Pain. 1998;75:129–140. doi: 10.1016/S0304-3959(97)00215-7. [DOI] [PubMed] [Google Scholar]
- Flecknell PA. Refinement of animal use--assessment and alleviation of pain and distress. Lab Animal. 1994;28:222–231. doi: 10.1258/002367794780681660. [DOI] [PubMed] [Google Scholar]
- Flecknell PA, Roughan JV, Stewart R. Use of oral buprenorphine (‘buprenorphine jello’) for postoperative analgesia in rats--a clinical trial. Lab Animal. 1999;33:169–174. doi: 10.1258/002367799780578381. [DOI] [PubMed] [Google Scholar]
- Hill R. NK1 (substance P) receptor antagonist s--why are they not analgesic in humans? Trends in Pharmacological Sciences. 2000;21:244–246. doi: 10.1016/s0165-6147(00)01502-9. [DOI] [PubMed] [Google Scholar]
- Hitzemann R, Dains K, Bier-Langing CM, Zahniser NR. On the selection of mice for haloperidol response and non-response. Psychopharmacology (Berlin) 1991;103:244–250. doi: 10.1007/BF02244211. [DOI] [PubMed] [Google Scholar]
- Kuzmin A, Sandin J, Terenius L, Ogren SO. Dose- and time-dependent bimodal effects of kappa-opioid agonists on locomotor activity in mice. Journal of Pharmacology and Experimental Therapeutics. 2000;295:1031–1042. [PubMed] [Google Scholar]
- Lazarus H, Neumann C. Assessing undertreatment of pain: the patients’ perspectives. Journal of Pharmaceutical Care in Pain and Symptom Control. 2001;9:5–34. [Google Scholar]
- Le Bars D, Gozariu M, Cadden SW. Animal models of nociception. Pharmacological Reviews. 2001;53:597–652. [PubMed] [Google Scholar]
- Matson DJ, Broom DC, Carson SR, Baldassari J, Kehne J, Cortright DN. Inflammation-induced reduction of spontaneous activity by adjuvant: A novel model to study the effect of analgesics in rats. Journal of Pharmacology and Experimental Therapeutics. 2007;320:194–201. doi: 10.1124/jpet.106.109736. [DOI] [PubMed] [Google Scholar]
- Meert TF, Vermeirsch HA. A preclinical comparison between different opioids: antinociceptive versus adverse effects. Pharmacology, Biochemistry and Behavior. 2005;80:309–326. doi: 10.1016/j.pbb.2004.12.002. [DOI] [PubMed] [Google Scholar]
- Michael-Titus A, Dourmap N, Costentin J. Mu and delta opioid receptors control differently the horizontal and vertical components of locomotor activity in mice. Neuropeptides. 1989;13:235–242. doi: 10.1016/0143-4179(89)90076-0. [DOI] [PubMed] [Google Scholar]
- Mogil JS, Wilson SG. Nociceptive and morphine antinociceptive sensitivity of 129 and C57BL/6 inbred mouse strains: implications for transgenic knock-out studies. European Journal of Pain. 1997;1:293–297. doi: 10.1016/s1090-3801(97)90038-0. [DOI] [PubMed] [Google Scholar]
- Morton DB, Griffiths PH. Guidelines on the recognition of pain, distress and discomfort in experimental animals and an hypothesis for assessment. The Veterinary Record. 1985;116:431–436. doi: 10.1136/vr.116.16.431. [DOI] [PubMed] [Google Scholar]
- Moseley GL, Zalucki N, Birklein F, Marinus J, van Hilten JJ, Luomajoki H. Thinking about movement hurts: the effect of motor imagery on pain and swelling in people with chronic arm pain. Arthritis and Rheumatism. 2008;59:623–631. doi: 10.1002/art.23580. [DOI] [PubMed] [Google Scholar]
- Negus SS, Vanderah TW, Brandt MR, Bilsky EJ, Becerra L, Borsook D. Preclinical assessment of candidate analgesic drugs: recent advances and future challenges. Journal of Pharmacology and Experimental Therapeutics. 2006;319:507–514. doi: 10.1124/jpet.106.106377. [DOI] [PubMed] [Google Scholar]
- National Research Council . Guide for the Care and Use of Mammals in Neuroscience and Behavioral Research. National Academy Press; Washington, DC.: 2003. [Google Scholar]
- Pearl J, Aceto MD, Harris LS. Prevention of writhing and other effects of narcotics and narcotic antagonists in mice. Journal of Pharmacology and Experimental Therapeutics. 1968;160:217–230. [PubMed] [Google Scholar]
- Do Carmo G Pereira, Stevenson GW, Carlezon WA, Negus SS. Effects of pain- and analgesia-related manipulations on intracranial self-stimulation in rats: Further studies on pain-depressed behavior. Pain. 2009 doi: 10.1016/j.pain.2009.04.010. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raehal KM, Lowery JJ, Bhamidipati CM, Paolino RM, Blair JR, Wang D, Sadee W, Bilsky EJ. In vivo characterization of 6beta-naltrexol, an opioid ligand with less inverse agonist activity compared with naltrexone and naloxone in opioid-dependent mice. Journal of Pharmacology and Experimental Therapeutics. 2005;313:1150–1162. doi: 10.1124/jpet.104.082966. [DOI] [PubMed] [Google Scholar]
- Roughan JV, Flecknell PA. Effects of surgery and analgesic administration on spontaneous behaviour in singly housed rats. Research in Veterinary Science. 2000;69:283–288. doi: 10.1053/rvsc.2000.0430. [DOI] [PubMed] [Google Scholar]
- Stevenson GW, Bilsky EJ, Negus SS. Targeting pain-suppressed behaviors in preclinical assays of pain and analgesia: effects of morphine on acetic acid-suppressed feeding in C57BL/6J mice. Journal of Pain. 2006;7:408–416. doi: 10.1016/j.jpain.2006.01.447. [DOI] [PubMed] [Google Scholar]
- Takasuna M, Negus SS, DeCosta BR, Woods JH. Opioid pharmacology of the antinociceptive effects of loperamide in mice. Behavioral Pharmacology. 1994;5:189–195. doi: 10.1097/00008877-199404000-00010. [DOI] [PubMed] [Google Scholar]
- Teitelbaum H, Giammatteo P, Mickley GA. Differential effects of localized lesions of n. accumbens on morphine- and amphetamine-induced locomotor hyperactivity in the C57BL/6J mouse. Journal of Comparative Physiology and Psychology. 1979;93:745–751. doi: 10.1037/h0077605. [DOI] [PubMed] [Google Scholar]
- Urban LA, Fox AJ. NK1 receptor antagonists--are they really without effect in the pain clinic? Trends in Pharmacological Sciences. 2000;21:462–464. doi: 10.1016/s0165-6147(00)01578-9. author reply 465. [DOI] [PubMed] [Google Scholar]
- van Abeelen JH, van den Heuvel CM. Behavioural responses to novelty in two inbred mouse strains after intrahippocampal naloxone and morphine. Behavioural Brain Research. 1982;5:199–207. doi: 10.1016/0166-4328(82)90053-5. [DOI] [PubMed] [Google Scholar]
- van Weering M, Vollenbroek-Hutten MM, Kotte EM, Hermens HJ. Daily physical activities of patients with chronic pain or fatigue versus asymptomatic controls. A systematic review. Clinical Rehabilitation. 2007;21:1007–1023. doi: 10.1177/0269215507078331. [DOI] [PubMed] [Google Scholar]
- Ward SJ, Takemori AE. Relative involvement of mu, kappa and delta receptor mechanisms in opiate-mediated antinociception in mice. Journal of Pharmacology and Experimental Therapeutics. 1983;224:525–530. [PubMed] [Google Scholar]
- Wigdor S, Wilcox GL. Central and systemic morphine-induced antinociception in mice: contribution of descending serotonergic and noradrenergic pathways. Journal of Pharmacology and Experimental Therapeutics. 1987;242:90–95. [PubMed] [Google Scholar]