Abstract
Context
There are no studies of the natural history of major depressive disorder that lack prevalence and clinic biases.
Objectives
To estimate risk factors for first lifetime onset and parameters of chronicity following the first episode, including duration, recovery, and recurrence, and to search for predictors of each parameter.
Design
Prospective population-based cohort study with 23 years of follow-up.
Setting
East Baltimore, Maryland, an urban setting.
Participants
Probability sample of 3481 adult household residents in 1981, including 92 with first lifetime onset of major depressive disorder during the course of the follow-up, and 1739 other participants followed up for at least 13 years.
Outcome Measures
Diagnostic Interview Schedule and Life Chart Interview.
Results
Female participants showed higher risk of onset of disorder, longer duration of episodes, and a nonsignificant tendency for higher risk of recurrence. Sex was not related to recovery. The median episode length was 12 weeks. About 15% of 92 individuals with first episodes did not have a year free of episodes, even after 23 years. About 50% of first episode participants recovered and had no future episodes. The evolution of the course was relatively stable from first to later episodes. Individuals with 1 or 2 short alleles of the serotonin transporter gene were at higher risk for an initial episode, but experienced episodes of shorter duration. There were few strong predictors of recovery or recurrence.
Conclusions
Major depressive disorder is unremitting in 15% of cases and recurrent in 35%. About half of those with a first-onset episode recover and have no further episodes.
Many individuals with depressive disorder do not seek treatment, and those who do presumably represent the most severe cases. For this reason, the natural history of depressive disorder is best studied using a population-based sample, in which individuals are selected from the general population without regard to treatment. This sample source avoids the Berkson bias.1 Many individuals with a depressive episode do not experience a recurrence, and those who do represent the more chronic and severe cases. For this reason, the natural history of depressive disorder is best studied through prospective follow-up of a sample of individuals experiencing first lifetime onsets—that is, from the first episode forward. This approach avoids the “clinician’s illusion,”2 sometimes called Neyman bias. These 2 biases are both likely to exaggerate the chronicity of depression.
The Baltimore Epidemiologic Catchment Area (ECA) Followup affords the opportunity, for the first time, to study the natural history of depressive disorder for more than 2 decades, using a population-based sample of first lifetime episode cases, and avoiding these 2 types of bias.
There have been about 24 long-term follow-up studies of depressive disorder in specialty psychiatric settings3 or family practice and community settings.4 Perhaps the best-known study is the National Institute of Mental Health Collaborative Study of the Psychobiology of Depression.5,6 This is a multisite study of a cohort of 955 persons who sought treatment for a so-called index episode (ie, the episode in which the individual sought treatment and entered the study), of whom 435 had major depressive disorder (MDD). The follow-up period was 15 years and there was intensive study of the clinical course. A report of 10 years of follow-up estimated the duration of depressive episodes to be 20 weeks, with median durations of 5 consecutive episodes of 22, 20, 21, 19, and 19 weeks, displaying a weak tendency for shorter episodes during the natural course.7 The probability of recurrence after 10 years was 67%8 and after 15 years was 85%.9
There are 4 population-based follow-up studies, including our prior work,10 the Lundby study from Sweden,11 the Zurich cohort,12 and the Netherlands Mental Health Survey and Incidence Study (NEMESIS) follow-up.13 Our prior 13- to 15-year follow-up of the Baltimore site of the ECA study10 was based on individuals identified through a household survey using the Diagnostic Interview Schedule (DIS).14 There were too few cases of first lifetime onset to make stable estimates of duration and recurrence, so the estimates we produced were based on all existing cases at the baseline. We reported a median duration of major depressive episodes of 3 months, with slightly shorter episodes after the first. About 80% of the individuals in depressive episodes at baseline recovered within 5 years, and 50% of those who recovered experienced a recurrence.10 The NEMESIS study15 is the closest to ours in its assessment method, using the Composite International Diagnostic Interview16 instead of the DIS,14 and the same Life Chart Interview ([LCI], recalibrated to 3-month periods instead of 1-year periods).17 The sample of 273 persons in a depressive episode included first lifetime onset cases and those who had been free of depression at baseline, but who had a recurrent episode during the follow-up period of 2 years. The estimated duration of depressive episodes was 3 months.15 As reported in the Baltimore ECA study,10 recurrent episodes were slightly shorter than initial episodes.13 The Lundby study11 was based on a geographically defined population followed from 1947 through 1997. The latest report from that study focused on 344 persons with first lifetime episodes, as we do here (the Lundby cohort excluded 92 persons with co-occurring disorders, unlike this ECA report). About 40% of the Lundby cohort participants had recurrent episodes.
Major depressive disorder is known to have a modest genetic basis, and there is a substantial body of work investigating this genetic underpinning of MDD, including association and linkage studies.18 The severity and age at onset of depression have been related to familial history.19 An insertion/deletion polymorphism in the promoter region of the serotonin transporter gene (5HTT) has gained attention as a plausible candidate for risk of MDD. Typically 2 alleles in this gene have been compared: a short allele (s) that is putatively related to lower gene expression,20 and a long allele (l). More recently, additional polymorphisms have been described that complicate this picture.21,22 There have been several studies reporting a relationship between the s allele and MDD; however, these have not been consistently replicated. There have been several meta-analytic studies that have also reported inconsistent findings regarding 5HTT, depression, and suicidal behavior.23 In addition to these case-control studies, there have been several studies reporting a relationship between 5HTT and depression in specific populations, such as the elderly,24 alcoholics,25–28 and patients with anxiety disorders, neuroticism,29–31 or obsessive-compulsive disorder.32,33 There has been 1 study that found a significant relationship between the polymorphism and MDD severity.34 Finally, there are reports of a relationship between this polymorphism and adverse life events culminating in MDD,35 although there have been conflicting findings.36 Less has been studied concerning the relationship between this polymorphism and the natural course of depression.
METHODS
SAMPLE
In 1981, 175 211 adult residents of East Baltimore were sampled probabilistically for participation in the Baltimore site of the ECA Program.37–39 From 1993 through June 1996, 1920 of those interviewed in 1981 were interviewed again.10 Because most of the interviews occurred in 1993, this year is used as shorthand (ie, 1993) to designate this wave of the cohort study. In 2004 and the first half of 2005, 1071 of those interviewed in 1993 were interviewed again (likewise the shorthand 2004 is used for this wave). Attrition in the study was cumulative, in that the target sample for interviews in 2004 consisted only of those successfully interviewed in 1993.40 The relationship between depression during early waves and attrition during later waves was not strong or significant.
The focus of this analysis is the group of 92 participants who experienced an episode of depression (meeting criteria for DSM-IV) for the first time in their life during the follow-up. Seventy-one first lifetime episodes occurred between the baseline and 1993 follow-up, and 21 occurred between the 1993 and 2004 follow-ups. The comparison group for onset consists of the 1739 participants followed up through the 1993 wave who also had the opportunity for onset but for whom onset did not occur.
The research was approved by the Johns Hopkins Bloomberg School of Public Health institutional review board.
MEASURES
The DIS was used in all 3 waves in East Baltimore.14 Anticipating the possibility of analysis of trends, every effort was made to make the interview and survey procedures as similar as possible among waves.40 In the follow-up interviews in 1993 and 2004, the LCI17 was used to improve recall of the timing of psychopathology. At the beginning of the interview, 4 memory anchors were created by the respondent, who was asked to identify for each year since the prior interview their residence, marital status (and partner if they were married), job, and “the most important thing that happened to you that year,” which we call a landmark. Later in the interview, for those reporting a syndrome-level episode at any time since the baseline, the memory anchors were used to locate the year or years in which the episode or episodes occurred by inquiring individually about every single year since the prior wave, and providing information regarding the presence or absence of a depressive episode in each year of the follow-up from 1981 through 2004. The focus of the LCI was the syndrome of depression, defined by the respondent’s answer to the question, “During that year, was there ever a time when you were feeling sad, depressed, or blue [or respondent’s equivalent as determined earlier in the interview], and had some of these other problems, like [list of DSM-IV Criterion A symptoms reported by the respondent to have occurred at some time during their lifetime]?” All 92 respondents in this report had at some time in their lives met the full criteria for MDD, but the episodes reported here consist of all syndrome-level episodes, even if the particular episode did not reach full criteria for diagnosis.
Contradictions in the respondents’ reports occur within any wave of the survey interviews, and when comparing reports from different waves. Many of the contradictions concern the timing of episodes, and some, through a mistake in the interview process, are missing data where there should be confirmation. These contradictions were resolved one by one by listing each of the 92 respondents’ entire records regarding their courses of depression. Most of the contradictions are reports of earlier onset in later waves than in earlier waves among those with first lifetime onset. Our interpretation is that the respondent in this situation, having suffered recently through an episode, is more likely to attribute symptoms occurring earlier in life to depression than in prior waves, and may therefore have lowered the threshold for reporting such symptoms. Consistent with our earlier work,10 we consider the report at baseline, in which no or fewer symptoms are reported, to be more accurate, since the time of interview (baseline) is closer to the occurrence (or lack of occurrence) of the symptoms in question.
Respondents reported the duration of the first episode that occurred in any year in which there was an episode; we then converted the value to weeks. Consistent with the MacArthur group definitions41 and our prior work, time to recovery is defined as the number of years after an episode until a year occurs with no episode at all. Time to recurrence is the number of years between recovery and another episode.
Predictor variables were taken from the baseline, or, where available, the closest period of time prior to onset. The presence of a confidant, ordinal measures of the size of the family and friends’ network, and years of education were assessed at baseline. Other symptoms of psychopathology (panic attack, alcohol abuse, and drug abuse) were taken from the baseline wave. Marital status was taken from the wave of interviews prior to onset. Age was adjusted using the age at baseline. Family history of depression was taken from a follow-up wave which occurred 1 year after baseline (wave 2 was not used in this analysis since it was so close to baseline). Treatment for depression was defined as whether the respondent reported having talked to a doctor about 1 or more of the episodes. Impairment was defined as whether the respondent reported that the episode “interfered with their life or activities a lot.”
Information on the widely studied 44 base pair insertion/deletion polymorphism in the promoter region of 5HTT was available for a subsample of 839 participants interviewed in 2004 who agreed to provide blood or buccal sample for DNA analysis (61 incident cases and 778 comparison participants were included). There was not a strong relationship between depression and willingness to donate blood or buccal sample for DNA.42 The polymorphism was genotyped using polymerase chain reaction with products resolved on 2% NuSieve (Lonza Group Ltd, Basel, Switzerland), 1% agarose gel and visualized by ethidium bromide staining.43 Polymerase chain reaction primers and conditions are available upon request. The polymorphism, which has 2 common alleles, designated as the short (s) and long (l) alleles, was in Hardy-Weinberg equilibrium (P >.50) in the current sample population with genotype frequencies of 12% ss, 45% sl, and 43% ll.
ANALYSIS
The distribution of social and clinical characteristics in the 92 first-onset cases was compared with that of the 1739 participants with no prior episodes of depression at baseline and at at least 1 follow-up interview after 1982. Relative risks for incidence are estimated by Cox proportional hazards method, using baseline as the start point of time until the event of onset.44 Cases are censored at death, onset of depression, or their last follow-up interview. Kaplan-Meier plots display times to recovery and recurrence.44 Predictors of time to recovery, and time to recurrence for those who recovered, were estimated with the Cox model also. The predictors of the duration of episodes were estimated by linear regression, using the generalized estimating equation method to correct for the occurrence of multiple durations in individuals.45
RESULTS
The 92 participants with first lifetime onset of depressive disorder during follow-up were more likely to be women, in the youngest decade of age, separated or never married, had about 1 year more education, and were about twice as likely to have reported a parent with depression than the 1739 participants without onset (Table 1). Persons who reported incidents were more likely to have a history of panic attack or drug or alcohol abuse or dependence. The participants who reported incidents were less likely to have 2 long alleles of the 5HTT gene. Other differences between the 92 incident case participants and the 1739 participants with no history of depression were not strong. When age, sex, and years of education were adjusted in a Cox proportional hazards model of time to onset of depression in years since baseline (Table 2), the differences attributed to education and marital status were no longer significant, and the difference attributed to parental history of depression was also not statistically significant, using the traditional α value of .05. Women, younger persons, those with 1 or 2 short 5HTT alleles, and persons with a history of drug or alcohol disorders and panic attacks were at a higher risk. Other predictors, including social network variables, do not have a significant effect.
Table 1.
Characteristics of 92 New Cases of Major Depressive Disorder and 1739 Noncases
| No. (%) |
||
|---|---|---|
| First Lifetime Onset Cases | Subjects Without Onset of Depression, Followed Through 1993 | |
| Sex | ||
| Male | 22 (23.9) | 663 (38.1) |
| Female | 70 (76.1) | 1076 (61.9) |
| Age at onset, ya | ||
| 10–19 | 14 (15.2) | 51 (2.9) |
| 20–29 | 21 (22.8) | 445 (25.6) |
| 30–39 | 28 (30.4) | 376 (21.6) |
| 40–49 | 19 (20.7) | 209 (12.0) |
| 50–59 | 6 (6.5) | 241 (13.9) |
| ≥60 | 4 (4.4) | 417 (24.0) |
| Marital statusb | ||
| Married | 45 (48.9) | 809 (46.5) |
| Widowed | 1 (1.1) | 204 (11.7) |
| Separated | 13 (14.1) | 142 (8.2) |
| Divorced | 8 (8.7) | 151 (8.7) |
| Never married | 25 (27.2) | 433 (24.9) |
| Confidantb | ||
| Yes | 87 (94.6) | 1538 (90.7) |
| No | 5 (5.4) | 157 (9.3) |
| No. of regular social contacts among friendsb | ||
| 0 or 1 | 7 (7.8) | 208 (12.1) |
| 2 | 22 (24.4) | 350 (20.3) |
| 3 | 22 (24.4) | 358 (20.8) |
| 4 | 39 (43.3) | 806 (46.8) |
| No. of regular social contacts among relativesb | ||
| 0 or 1 | 2 (2.2) | 73 (4.2) |
| 2 | 11 (12.2) | 249 (14.5) |
| 3 | 18 (20.0) | 334 (19.4) |
| 4 | 59 (65.6) | 1066 (61.9) |
| Mother or father depressedc | ||
| Yes | 22 (23.9) | 216 (12.4) |
| No | 70 (76.1) | 1523 (87.6) |
| 5HTTd | ||
| 1 or 2 short alleles | 42 (68.9) | 435 (55.9) |
| Both long alleles | 19 (31.1) | 343 (44.1) |
| Ever treated for depressione | ||
| Yes | 54 (58.7) | |
| Impaired from worst episode of depressione | ||
| Yes | 78 (84.8) | |
| Alcohol abuseb | ||
| Yes | 20 (21.7) | 192 (11.3) |
| Drug abuseb | ||
| Yes | 15 (16.5) | 82 (4.9) |
| Panic attackb | ||
| Yes | 14 (15.2) | 37 (2.2) |
| Mean (SD) education, y | 12.2 (2.3) | 11.0 (2.9) |
Abbreviation: 5HTT, serotonin transporter gene.
Age at baseline of those with no history of depressive disorder.
For both the first onset sample and those with no history of depressive disorder, measured at baseline.
Assessed at wave 2 in 1982.
Sample includes those donating blood or buccal swab for DNA in 2004.
Most severe value from wave following first onset.
Table 2.
Risk Factors for Incident Cases of Depressive Disorder Time to Onset From Wave 1 (92 Cases and 1739 Noncases)
| Hazard Ratio (95% CI) |
||||
|---|---|---|---|---|
| Characteristic | Person-Years | Events | Crude | Adjusteda |
| Age at baseline, y | 42 500 | 92 | 0.94 (0.92–0.96) | 0.94 (0.92–0.96) |
| Sex | ||||
| Female | 26 400 | 70 | 1.00 | 1.00 |
| Male | 16 100 | 22 | 0.53 (0.33–0.85) | 0.46 (0.28–0.74) |
| Marital status | ||||
| Married | 19 713 | 36 | 1.00 | 1.00 |
| Widowed | 4900 | 1 | 0.11 (0.02–0.84) | 0.35 (0.05–2.63) |
| Separated | 3485 | 13 | 1.97 (1.04–3.71) | 1.61 (0.85–3.04) |
| Divorced | 3692 | 7 | 1.04 (0.46–2.34) | 0.95 (0.42–2.13) |
| Never married | 10 686 | 35 | 1.76 (1.10–2.80) | 0.83 (0.50–1.38) |
| Education, y | 42 476 | 92 | 1.15 (1.07–1.24) | 1.06 (0.97–1.16) |
| Confidant present | 37 666 | 87 | 1.73 (0.70–4.27) | 1.27 (0.51–3.15) |
| Size of friends network | 42 055 | 90 | 1.03 (0.87–1.23) | 1.00 (0.83–1.21) |
| Size of relatives network | 42 055 | 90 | 1.13 (0.88–1.44) | 0.96 (0.74–1.23) |
| 5HTT | ||||
| Both long alleles | 8381 | 19 | 1.00 | 1.00 |
| 1 or 2 short alleles | 10 819 | 42 | 1.68 (0.98–2.88) | 1.73 (1.01–2.98) |
| Parent depressed | 5375 | 22 | 2.10 (1.30–3.40) | 1.60 (0.99–2.58) |
| Alcohol abuse | 4683 | 15 | 1.49 (0.86–2.59) | 2.43 (1.34–4.40) |
| Drug abuse | 2089 | 14 | 3.19 (1.81–5.64) | 1.94 (1.08–3.47) |
| Panic attack | 932 | 10 | 4.52 (2.34–8.71) | 3.46 (1.79–6.69) |
Abbreviations: CI, confidence interval; 5HTT, serotonin transporter gene.
Adjusted for age at baseline, sex, and years of education.
There were 345 total episodes during the follow-up of the 92 first-onset cases. A linear regression was estimated with weeks of the duration of the episode as the dependent variable and the predictors presented in Table 3, using the generalized estimating equation method. Each variable in the model was adjusted for onset age, sex, and education (second column). Men had episodes that were greater than 20 weeks shorter on average than those of women. Each year of education reduced the length of episodes by an average of 5 weeks. Although widowed, separated, and never-married individuals had shorter episodes than married persons (not statistically significant), divorced individuals had episodes that were much longer on average. Persons with larger networks of relatives had longer episodes. Persons with a history of panic attack had longer episodes as well. Individuals with 1 or 2 short alleles of the 5HTT gene had episodes that were about 20 weeks shorter.
Table 3.
Predictors of Durations of Episodes; Generalized Estimating Equation Model of 345 Episodes in 92 New Casesa
| Regression Coefficient (95% CI) |
||
|---|---|---|
| Characteristic | Crude | Adjustedb |
| Age at onset, y | 0.76 (0.07 to 1.45) | 0.54 (−0.19 to 1.27) |
| Sex | ||
| Female | 1 [Reference] | |
| Male | −19.01 (−40.63 to 2.60) | −22.05 (−42.28 to 1.82) |
| Marital status | ||
| Married | 1 [Reference] | |
| Widowed | −22.51 (−83.75 to 38.73) | −23.29 (−83.08 to 36.51) |
| Separated | −17.48 (−41.28 to 6.32) | −21.41 (−45.29 to 2.47) |
| Divorced | 79.21 (42.49 to 115.94) | 67.26 (30.09 to 104.43) |
| Never married | −8.47 (−27.70 to 10.77) | −11.02 (−30.72 to 8.68) |
| Education, y | −6.03 (−10.04 to −2.02) | −5.29 (−9.08 to −1.51) |
| Confidant present | 2.60 (−32.32 to 37.53) | 10.50 (−22.38 to 43.39) |
| Size of friends network | −8.42 (−17.43 to 0.60) | −5.01 (−14.15 to 4.13) |
| Size of relatives network | 9.55 (0.59 to 18.51) | 9.63 (0.85 to 18.42) |
| 1 or 2 short 5HTT alleles | −20.56 (−38.82 to −2.31) | −21.26 (−38.54 to −3.99) |
| Mother or father depressed | −14.31 (−34.96 to 6.35) | −17.55 (−36.75 to 1.65) |
| Treated in worst episode | 14.97 (−2.85 to 32.79) | 9.73 (−7.44 to 26.91) |
| Impaired in worst episode | 5.35 (−19.85 to 30.54) | 7.41 (−15.93 to 30.76) |
| Alcohol abuse or dependence | −4.99 (−27.24 to 17.26) | −2.67 (−24.26 to 18.92) |
| Drug abuse or dependence | −8.69 (−33.19 to 15.81) | 4.73 (−18.85 to 28.31) |
| Panic attack | 17.91 (−6.66 to 42.47) | 25.47 (2.76 to 48.18) |
| Total No. of episodesc | −3.27 (−4.85 to −1.69) | −3.40 (−5.67 to −1.14) |
| Each additional episodec | −2.34 (−4.39 to −0.28) | 0.14 (−2.38 to 2.66) |
Abbreviations: CI, confidence interval; 5HTT, serotonin transporter gene.
Exchangeable correlation structure.
Adjusted by age at onset, sex, and education.
Adjusted by age at onset, sex, education, total number of episodes, and the temporal order rank of each episode.
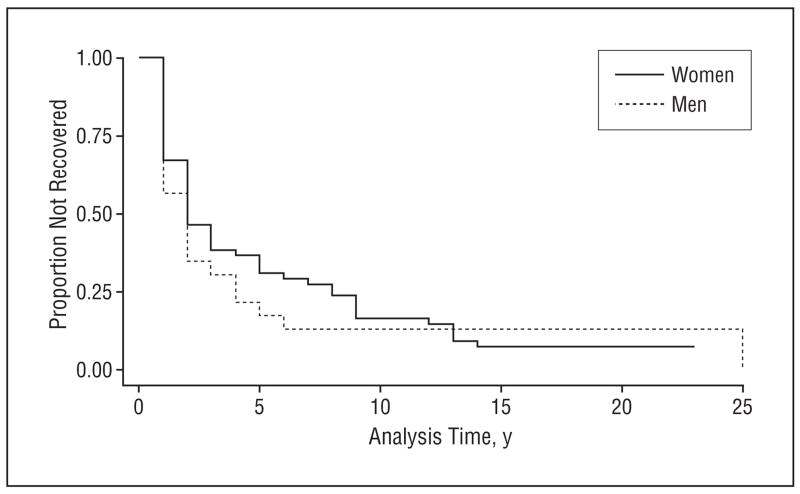
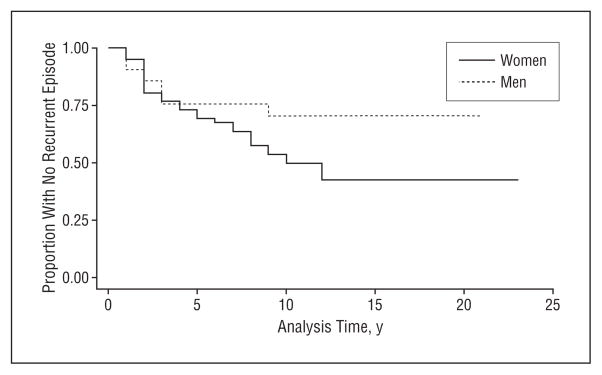
Median time to recovery was 2 or 3 years for both men and women (Figure 1). After 10 years, about 15% had not had at least 1 year free from an episode of depression—that is, 85% recovered. An analysis of time to recovery from the first episode was performed using all the predictors in Table 1. There were no significant predictors of time to recovery (data not shown). For those who recovered, women were more likely to have a recurrent episode than men (Figure 2), but even after 15 years, only about half of the first-onset group have had even 1 recurrent episode. In a Cox proportional hazards model (not shown) similar to that in Table 2, only age at onset was a significant predictor of time to recurrence, with each additional year of age at onset lowering the risk for recurrence by 0.96 (95% confidence interval [CI], 0.93–0.99).
Figure 1.
Time to recovery by gender.
Figure 2.
Time to recurrence by gender.
The data suggest little or no acceleration or amplification of the course through time. For this analysis of acceleration we report distinct episodes without regard to whether they occur before or after the year required by the “recovery” criterion cited above, so that there are slightly more episodes included. In the follow-up period, which varied from 1 to 23 years depending on the year of onset, the first episode had a median duration of 20 weeks; 60 of the 92 incident patients had 2 or more episodes, with a median duration of 12 weeks; 42 of the 60 patients with 2 episodes had 3 or more, with a median duration of 20 weeks; 31 of the 42 had 4 or more, with a median duration of 24 weeks; 22 of the 31 had 5 or more, with a median duration of 12 weeks; and so forth, up to 10 of the 11 with 9 or more episodes having a tenth episode, with a median duration of 6 weeks. To further analyze the possibility of time trend in the duration of episodes requires adjusting by the total number of episodes, which must almost by necessity be related to shorter durations (3.40 weeks shorter), as seen in Table 3. Once the total number of episodes is adjusted, there is not a strong or significant effect for episodes earlier in the course to be shorter or longer than episodes occurring later in the course (Table 3).
In prior work,10 and in this paper, the focus has been on the diagnosis of MDD using the DIS as the assessment device, with no requirement that the interviewers have any clinical background or experience. In a separate analysis,46 we have compared diagnoses from the DIS with those obtained via the Schedules for Clinical Assessment in Neurobiology (SCAN), with psychiatrists, blinded to DIS results, conducting the interviews. There was moderate but not complete agreement, with discrepancies consisting mainly of patients diagnosed as having MDD by the SCAN procedure who were negative on the DIS. For the current analysis, data was available for 46 individuals who were positive on the SCAN diagnosis but negative on the DIS diagnosis. Incorporating those 46 individuals into the analyses in Tables 1 and 2 had no marked effect on the results (not shown). In particular, results for 5HTT were nearly identical between the 92 DIS cases and the 46 SCAN cases. It was not possible to conduct identical analyses for duration, recurrence, and recovery, because the SCAN did not obtain sufficiently parallel data. However, there was an estimate of the duration of the initial episode of MDD for 27 of the 46 false positive cases, and these had a median duration of 12 weeks, slightly lower than the DIS/LCI–based estimate of 20 weeks for the first episode, but similar to the duration of later episodes.
COMMENT
The results showed that female sex, younger age, 1 or 2 short alleles of the 5HTT polymorphism, and prior alcohol abuse, drug abuse, or panic attack raised the risk of first lifetime onset of depressive disorder. The effects of social networks, marital status, and educational attainment were not significant in adjusted models. The course is not progressive for up to 10 episodes occurring as far apart as 23 years. Women, divorced persons, those with large family networks, and persons with prior panic attacks had longer episodes. About 50% recover and have no future episodes. There were few predictors of recovery or recurrence.
These findings are limited in the same way that many long-term follow-up studies are: in the attrition in the cohort, and in the difficulties of recall for long periods of time.
There are more similarities across the wide range of long-term follow-up studies than might have been imagined, given the differences in the origins of the sample, the varying measures used, and the definitions of such crucial indicators of chronicity as recovery and recurrence. The 2 population-based studies (this one and NEMESIS15) estimate episodes of depression to last 3 months, somewhat longer (20 weeks) in the clinic-based Collaborative Study of Depression.7 Close comparisons of recovery and recurrence are difficult to make because the definitions differ.47 However, we may attempt to estimate the proportion of individuals with a first lifetime onset of depressive disorder who will ever in their lifetime either not recover, or suffer at least 1 further episode. This can be considered a crude indicator of the chronicity of depression. In the Baltimore ECA Followup, about 15% have not recovered, even after 20 years of follow-up (Figure 1). Of the 78 subjects who recovered, 35, or about 45%, have had a recurrent episode—that is, about 38% of the entire sample after 10 years (0.85×0.45=0.38) have had a recurrent episode. By this estimate, 53% of those with a lifetime episode of depressive disorder either do not recover at all or have at least 1 recurrence. This estimate is higher than the 40% estimated in the Lundby study,11 which included a wider range of less severe forms of depression. It is similar to 4 clinic-based studies reviewed elsewhere, in which long-term recurrence proportions ranged from 42% to 60%,3 and to an additional study with a 5-year recurrence rate estimated at 41%.48 It is somewhat higher than the family-practice studies included in another review that had a shorter minimum duration of 5 years.4 These ECA figures are calibrated, for the most part, at the level of syndrome or disorder, so that there may be sadness or other isolated aspects of depression occurring in periods designated as recovered, or episodes shorter than 2 weeks occurring as well.49,50 But the evidence is consistent across a range of studies that about 50% of those with an occurrence of major depressive episode will recover and not have another episode.
The natural history of depressive disorder does not appear to evolve during its course, except for the possibility of a slight tendency for the first episode to be longer. Risk of recurrence increases after each subsequent episode, as reported by Solomon et al,8 and is also apparent in these ECA data, where the proportion of participants with a subsequent episode rises as time passes, as discussed above. But this increase is deceptive, and does not necessarily signal a change in the structure of the course; it may suggest preexisting heterogeneity as to the propensity for chronicity.51
Another consistent finding is that of the difficulty of locating any variables at all which predict recurrence. This study, and most others, were unable to find social variables, or clinical variables not drawn from earlier aspects of the course, that predicted recurrence. This lack of predictive ability pertains even to sex,11,12,52 which is reliably related to prevalence and incidence of depressive disorder. The assessment of treatment is limited in this epidemiologic study, but here, and in other studies with more intensive assessment of treatment, there was no obvious long-term effect of treatment for depressive disorder.
An intriguing result of this analysis is the paradoxical effects of the 5HTT on the natural history of MDD. Individuals with 2 long alleles are protected from the occurrence of first lifetime onset of depressive disorder (Table 2), consistent with other research. However, the same configuration is related to longer, not shorter, episodes of depression, something not studied in the literature prior to this analysis. Our speculative explanation is based on the notion that depressive disorder has multiple causes which endure in varying degrees throughout the course of life. Individuals with the protective genetic configuration sometimes are exposed to other causes whose force is sufficient to break through this protective effect, and presumably these other causes are stronger than in individuals with first episodes and a less protective genetic constellation. After the occurrence of the first episode, these causal forces remain in place, producing longer episodes and more difficult recovery. This pattern may or may not pertain to the other areas of psychopathology which have been associated with this polymorphism (reviewed above), and may have more general implications for genetic studies that rely on prevalent cases.
Is MDD “chronic”? This is the first population-based study to provide an answer without well-known clinic and prevalence sample biases. The results are not very different from the results of patient samples from clinics and studies whose samples include cases late in the course. About 15% of first lifetime onsets have unremitting course, and 35% recover but have 1 or more future episodes. About 50% of first lifetime onsets recover and have no future episodes. What remains is to isolate factors predicting the chronicity.
Acknowledgments
Funding/Support: This study was supported by National Institutes of Mental Health grant MH47447.
Financial Disclosure: None reported.
References
- 1.Berkson J. Limitations of the application of fourfold table analysis to hospital data. Biometrics Bull. 1946;2(3):47–53. [PubMed] [Google Scholar]
- 2.Cohen P, Cohen J. The clinician’s illusion. Arch Gen Psychiatry. 1984;41(12):1178–1182. doi: 10.1001/archpsyc.1984.01790230064010. [DOI] [PubMed] [Google Scholar]
- 3.Lee AS. Better outcomes for depressive disorders? Psychol Med. 2003;33(5):769–774. doi: 10.1017/s003329170300802x. [DOI] [PubMed] [Google Scholar]
- 4.van Weel-Baumgarten EM, Schers HJ, van den Bosch WJ, van den Hoogen HJ, Zitman FG. Long-term follow-up of depression among patients in the community and in family practice settings: a systematic review. J Fam Pract. 2000;49(12):1113–1120. [PubMed] [Google Scholar]
- 5.Keller MB, Lavori PW, Mueller TI, Endicott J, Coryell W, Hirschfeld RM, Shea T. Time to recovery, chronicity, and levels of psychopathology in major depression: a 5-year prospective follow-up of 431 subjects. Arch Gen Psychiatry. 1992;49(10):809–816. doi: 10.1001/archpsyc.1992.01820100053010. [DOI] [PubMed] [Google Scholar]
- 6.Keller MB, Shapiro RW. Major depressive disorder: initial results from a one-year prospective naturalistic follow-up study. J Nerv Ment Dis. 1981;169(12):761–768. [PubMed] [Google Scholar]
- 7.Solomon DA, Keller MB, Leon AC, Mueller TI, Shea MT, Warshaw M, Maser JD, Coryell W, Endicott J. Recovery from major depression: a 10-year prospective follow-up across multiple episodes. Arch Gen Psychiatry. 1997;54(11):1001–1006. doi: 10.1001/archpsyc.1997.01830230033005. [DOI] [PubMed] [Google Scholar]
- 8.Solomon DA, Keller MB, Leon AC, Mueller TI, Lavori PW, Shea MT, Coryell W, Warshaw M, Turvey C, Maser JD, Endicott J. Multiple recurrences of major depressive disorder. Am J Psychiatry. 2000;157(2):229–233. doi: 10.1176/appi.ajp.157.2.229. [DOI] [PubMed] [Google Scholar]
- 9.Mueller TI, Leon AC, Keller MB, Solomon DA, Endicott J, Coryell W, Warshaw M, Maser JD. Recurrence after recovery from major depressive disorder during 15 years of observational follow-up. Am J Psychiatry. 1999;156(7):1000–1006. doi: 10.1176/ajp.156.7.1000. [DOI] [PubMed] [Google Scholar]
- 10.Eaton WW, Anthony JC, Gallo J, Cai G, Tien A, Romanoski A, Lyketsos C, Chen LS. Natural history of Diagnostic Interview Schedule/DSM-IV major depression: the Baltimore Epidemiologic Catchment Area follow-up. Arch Gen Psychiatry. 1997;54(11):993–999. doi: 10.1001/archpsyc.1997.01830230023003. [DOI] [PubMed] [Google Scholar]
- 11.Mattisson C, Bogren M, Horstmann V, Munk-Jorgensen P, Nettelbladt P. The long-term course of depressive disorders in the Lundby Study. Psychol Med. 2007;37(6):883–891. doi: 10.1017/S0033291707000074. [DOI] [PubMed] [Google Scholar]
- 12.Angst J, Gamma A, Sellaro R, Lavori PW, Zhang H. Recurrence of bipolar disorders and major depression: a life-long perspective. Eur Arch Psychiatry Clin Neurosci. 2003;253(5):236–240. doi: 10.1007/s00406-003-0437-2. [DOI] [PubMed] [Google Scholar]
- 13.Spijker J, de Graaf R, Bijl RV, Beekman AT, Ormel J, Nolen WA. Determinants of persistence of major depressive episodes in the general population: results from the Netherlands Mental Health Survey and Incidence Study (NEMESIS) J Affect Disord. 2004;81(3):231–240. doi: 10.1016/j.jad.2003.08.005. [DOI] [PubMed] [Google Scholar]
- 14.Robins LN, Helzer JE, Croughan J, Ratcliff KS. National Institute of Mental Health Diagnostic Interview Schedule: its history, characteristics, and validity. Arch Gen Psychiatry. 1981;38(4):381–389. doi: 10.1001/archpsyc.1981.01780290015001. [DOI] [PubMed] [Google Scholar]
- 15.Spijker J, de Graaf R, Bijl RV, Beekman AT, Ormel J, Nolen WA. Duration of major depressive episodes in the general population: results from The Netherlands Mental Health Survey and Incidence Study (NEMESIS) Br J Psychiatry. 2002;181:208–213. doi: 10.1192/bjp.181.3.208. [DOI] [PubMed] [Google Scholar]
- 16.Essau CA, Wittchen H-U. An overview of the composite international diagnostic interview (CIDI) Int J Methods Psychiatr Res. 1993;3:79–85. [Google Scholar]
- 17.Lyketsos CG, Nestadt G, Cwi J, Heithoff K, Eaton WW. The Life Chart Interview: a standardized method to describe the course of psychopathology. Int J Methods Psychiatr Res. 1994;4:143–155. [Google Scholar]
- 18.Levinson DF. The genetics of depression: a review. Biol Psychiatry. 2006;60(2):84–92. doi: 10.1016/j.biopsych.2005.08.024. [DOI] [PubMed] [Google Scholar]
- 19.Mondimore FM, Zandi PP, MacKinnon DF, McInnis MG, Miller EB, Schweizer B, Crowe RP, Scheftner WA, Weissman MM, Levinson DF, DePaulo JR, Jr, Potash JB. A comparison of the familiality of chronic depression in recurrent early-onset depression pedigrees using different definitions of chronicity. J Affect Disord. 2007;100(1–3):171–177. doi: 10.1016/j.jad.2006.10.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Heils A, Teufel A, Petri S, Stober G, Bengel D, Lesch KP. Allelic variation of human serotonin transporter gene expression. J Neurochem. 1996;66(6):2621–2624. doi: 10.1046/j.1471-4159.1996.66062621.x. [DOI] [PubMed] [Google Scholar]
- 21.Hu X, Oroszi G, Chun J, Smith TL, Goldman D, Schuckit MA. An expanded evaluation of the relationship of four alleles to the level of response to alcohol and the alcoholism risk. Alcohol Clin Exp Res. 2005;29(1):8–16. doi: 10.1097/01.alc.0000150008.68473.62. [DOI] [PubMed] [Google Scholar]
- 22.Hu XZ, Lipsky RH, Zhu G, Akhtar LA, Taubman J, Greenberg BD, Xu K, Arnold PD, Richter MA, Kennedy JL, Murphy DL, Goldman D. Serotonin transporter promoter gain-of-function genotypes are linked to obsessive-compulsive disorder. Am J Hum Genet. 2006;78(5):815–826. doi: 10.1086/503850. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Li D, He L. Meta-analysis supports association between serotonin transporter (5-HTT) and suicidal behavior. Mol Psychiatry. 2007;12(1):47–54. doi: 10.1038/sj.mp.4001890. [DOI] [PubMed] [Google Scholar]
- 24.Grünblatt E, Löffler C, Zehetmayer S, Jungwirth S, Tragl KH, Riederer P, Fischer P. Association study of the 5-HTTLPR polymorphism and depression in 75-year-old nondemented subjects from the Vienna Transdanube Aging (VITA) study. J Clin Psychiatry. 2006;67(9):1373–1378. doi: 10.4088/jcp.v67n0907. [DOI] [PubMed] [Google Scholar]
- 25.Feinn R, Nellissery M, Kranzler HR. Meta-analysis of the association of a functional serotonin transporter promoter polymorphism with alcohol dependence. Am J Med Genet B Neuropsychiatr Genet. 2005;133(1):79–84. doi: 10.1002/ajmg.b.30132. [DOI] [PubMed] [Google Scholar]
- 26.Nellissery M, Feinn RS, Covault J, Gelernter J, Anton RF, Pettinati H, Moak D, Mueller T, Kranzler HR. Alleles of a functional serotonin transporter promoter polymorphism are associated with major depression in alcoholics. Alcohol Clin Exp Res. 2003;27(9):1402–1408. doi: 10.1097/01.ALC.0000085588.11073.BB. [DOI] [PubMed] [Google Scholar]
- 27.Munafò MR, Lingford-Hughes AR, Johnstone EC, Walton RT. Association between the serotonin transporter gene and alcohol consumption in social drinkers. Am J Med Genet B Neuropsychiatr Genet. 2005;135(1):10–14. doi: 10.1002/ajmg.b.30162. [DOI] [PubMed] [Google Scholar]
- 28.Samochowiec J, Kucharska-Mazur J, Grzywacz A, Jabloński M, Rommelspacher H, Samochowiec A, Sznabowicz M, Horodnicki J, Sagan L, Pelka-Wysiecka J. Family-based and case-control study of DRD2, DAT, 5HTT, COMT genes polymorphisms in alcohol dependence. Neurosci Lett. 2006;410(1):1–5. doi: 10.1016/j.neulet.2006.05.005. [DOI] [PubMed] [Google Scholar]
- 29.Lesch KP, Bengel D, Heils A, Sabol SZ, Greenberg BD, Petri S, Benjamin J, Müller CR, Hamer DH, Murphy DL. Association of anxiety-related traits with a polymorphism in the serotonin transporter gene regulatory region. Science. 1996;274 (5292):1527–1531. doi: 10.1126/science.274.5292.1527. [DOI] [PubMed] [Google Scholar]
- 30.Schinka JA, Busch RM, Robichaux-Keene N. A meta-analysis of the association between the serotonin transporter gene polymorphism (5-HTTLPR) and trait anxiety. Mol Psychiatry. 2004;9(2):197–202. doi: 10.1038/sj.mp.4001405. [DOI] [PubMed] [Google Scholar]
- 31.Munafò MR, Clark T, Flint J. Does measurement instrument moderate the association between the serotonin transporter gene and anxiety-related personality traits? a meta-analysis. Mol Psychiatry. 2005;10(4):415–419. doi: 10.1038/sj.mp.4001627. [DOI] [PubMed] [Google Scholar]
- 32.Dickel DE, Veenstra-VanderWeele J, Bivens NC, Wu X, Fischer DJ, Van Etten-Lee M, Himle JA, Leventhal BL, Cook EH, Jr, Hanna GL. Association studies of serotonin system candidate genes in early-onset obsessive-compulsive disorder. Biol Psychiatry. 2007;61(3):322–329. doi: 10.1016/j.biopsych.2006.09.030. [DOI] [PubMed] [Google Scholar]
- 33.Lin PY. Meta-analysis of the association of serotonin transporter gene polymorphism with obsessive-compulsive disorder. Prog Neuropsychopharmacol Biol Psychiatry. 2007;31(3):683–689. doi: 10.1016/j.pnpbp.2006.12.024. [DOI] [PubMed] [Google Scholar]
- 34.Zalsman G, Huang YY, Oquendo MA, Burke AK, Hu XZ, Brent DA, Ellis SP, Goldman D, Mann JJ. Association of a triallelic serotonin transporter gene promoter region (5-HTTLPR) polymorphism with stressful life events and severity of depression. Am J Psychiatry. 2006;163(9):1588–1593. doi: 10.1176/ajp.2006.163.9.1588. [DOI] [PubMed] [Google Scholar]
- 35.Caspi A, Sugden K, Moffitt TE, Taylor A, Craig IW, Harrington H, McClay J, Mill J, Martin J, Braithwaite A, Poulton R. Influence of life stress on depression: moderation by a polymorphism in the 5-HTT gene. Science. 2003;301(5631):386–389. doi: 10.1126/science.1083968. [DOI] [PubMed] [Google Scholar]
- 36.Chipman P, Jorm AF, Prior M, Sanson A, Smart D, Tan X, Easteal S. No interaction between the serotonin transporter polymorphism (5-HTTLPR) and childhood adversity or recent stressful life events on symptoms of depression: results from two community surveys. Am J Med Genet B Neuropsychiatr Genet. 2007;144(4):561–565. doi: 10.1002/ajmg.b.30480. [DOI] [PubMed] [Google Scholar]
- 37.Regier DA, Myers JK, Kramer M, Robins LN, Blazer DG, Hough RL, Eaton WW, Locke BZ. The NIMH Epidemiologic Catchment Area (ECA) Program: historical context, major objectives, and study population characteristics. Arch Gen Psychiatry. 1984;41(10):934–941. doi: 10.1001/archpsyc.1984.01790210016003. [DOI] [PubMed] [Google Scholar]
- 38.Eaton WW, Kessler LG. Epidemiologic Field Methods in Psychiatry: The NIMH Epidemiologic Catchment Area Program. Orlando, Florida: Academic Press, Inc; 1985. [Google Scholar]
- 39.Eaton WW, Holzer CE, III, Von Korff M, Anthony JC, Helzer JE, George L, Burnam A, Boyd JH, Kessler LG, Locke BZ. The design of the Epidemiologic Catchment Area surveys: the control and measurement of error. Arch Gen Psychiatry. 1984;41(10):942–948. doi: 10.1001/archpsyc.1984.01790210024004. [DOI] [PubMed] [Google Scholar]
- 40.Eaton WW, Kalaydjian A, Scharfstein DO, Mezuk BM, Ding Y. Prevalence and incidence of depressive disorder: the Baltimore ECA followup, 1981–2004. Acta Psychiatr Scand. 2007;116(3):182–188. doi: 10.1111/j.1600-0447.2007.01017.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Frank E, Prien RF, Jarrett RB, Keller MB, Kupfer DJ, Lavori PW, Rush AJ, Weissman MM. Conceptualization and rationale for consensus definition of terms in major depressive disorder. Arch Gen Psychiatry. 1991;48(9):851–855. doi: 10.1001/archpsyc.1991.01810330075011. [DOI] [PubMed] [Google Scholar]
- 42.Mezuk BM, Zandi P, Eaton WW. Participant characteristics that influence consent for genetic research in a population-based survey: the Baltimore Epidemiologic Catchment Area Followup. Community Genet. doi: 10.1159/000113880. In press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Furlong RA, Ho L, Walsh C, Rubinsztein JS, Jain S, Paykel ES, Easton DF, Rubinsztein DC. Analysis and meta-analysis of two serotonin transporter gene polymorphisms in bipolar and unipolar affective disorders. Am J Med Genet. 1998;81(1):58–63. [PubMed] [Google Scholar]
- 44.Lawless JF. Statistical Models and Methods for Lifetime Data. New York: John Wiley & Sons; 1982. [Google Scholar]
- 45.Liang K-Y, Zeger SL. Longitudinal data analysis using generalized linear models. Biometrika. 1986;73(1):13–22. [Google Scholar]
- 46.Eaton WW, Neufeld K, Chen L, Cai G. A comparison of self-report and clinical diagnostic interviews for depression: DIS and SCAN in the Baltimore ECA Followup. Arch Gen Psychiatry. 2000;57(3):217–222. doi: 10.1001/archpsyc.57.3.217. [DOI] [PubMed] [Google Scholar]
- 47.Prien RF, Carpenter LL, Kupfer DJ. The definition and operational criteria for treatment outcome of major depressive disorder: a review of the current research literature. Arch Gen Psychiatry. 1991;48(9):796–800. doi: 10.1001/archpsyc.1991.01810330020003. [DOI] [PubMed] [Google Scholar]
- 48.Van Londen L, Molenaar RP, Goekoop JG, Zwinderman AH, Rooijmans HG. Three- to 5-year prospective follow-up of outcome in major depression. Psychol Med. 1998;28(3):731–735. doi: 10.1017/s0033291797006466. [DOI] [PubMed] [Google Scholar]
- 49.Judd LL, Akiskal HS, Maser JD, Zeller PJ, Endicott J, Coryell W, Paulus MP, Kunovac JL, Leon AC, Mueller TI, Rice JA, Keller MB. A prospective 12-year study of subsyndromal and syndromal depressive symptoms in unipolar major depressive disorders. Arch Gen Psychiatry. 1998;55(8):694–700. doi: 10.1001/archpsyc.55.8.694. [DOI] [PubMed] [Google Scholar]
- 50.Angst J, Merikangas K. The depressive spectrum: diagnostic classification and course. J Affect Disord. 1997;45(1–2):31–40. doi: 10.1016/s0165-0327(97)00057-8. [DOI] [PubMed] [Google Scholar]
- 51.Eaton WW. Studying the natural history of psychopathology. In: Tsuang MT, Tohen M, editors. Textbook in Psychiatric Epidemiology. 2. New York: Wiley-Liss; 2002. pp. 215–238. [Google Scholar]
- 52.Simpson HB, Nee JC, Endicott J. First-episode major depression: few sex differences in course. Arch Gen Psychiatry. 1997;54(7):633–639. doi: 10.1001/archpsyc.1997.01830190059006. [DOI] [PubMed] [Google Scholar]