Abstract
Biotribology is the science of biological surfaces in sliding contact encompassing the concepts of friction, wear, and lubrication of interacting surfaces. This bioscience field has emerged from the classical field of tribology and is of paramount importance to the normal function of numerous tissues, including articular cartilage, blood vessels, heart, tendons, ligaments, and skin. Surprisingly, relatively little attention has been given to the restoration of surface characteristics in the fields of tissue engineering and regenerative medicine—the science of design and manufacture of new tissues for the functional restoration of impaired or diseased organs that depend on inductive signals, responding stem cells, and extracellular matrix scaffolding. Analogous to ancient civilizations (c. 3000 B.C.) that introduced wheeled vehicles, sledges for transporting heavy blocks, and lubricants, modern biotribologists must aim to restore surface characteristics to regenerated tissues and develop novel biomaterials with optimal tribological properties. The objective of this article is to highlight the significance of functional biotribology in the physiology of body surfaces and provide a comprehensive overview of unresolved issues and controversies as it relates to regenerative medicine. Specific attention is placed on the molecular basis of lubrication, mechanical and biochemical regulation of lubricating molecules, and the need to study wear processes in articular cartilage, especially in light of degenerative diseases, such as osteoarthritis. Surface engineering of replacement tissues exhibiting low friction and high wear resistance is examined using articular cartilage as an illustrative model system.
Introduction
Friction, wear, and lubrication are central phenomena that are ubiquitous in diverse biological surfaces and systems. High friction is desirable between the foot and the floor for walking, whereas low friction is necessary for effortless flow of arterial blood cells. Wear facilitates tooth cleaning during brushing in oral hygiene and dentistry, but may result in excruciating pain during joint movement following cartilage degradation in osteoarthritis. The effectiveness of lubrication in reducing friction and wear is demonstrated in the blinking function of the eye and conjugal functions of human reproduction. Surface contact at cellular and tissue levels (Fig. 1) is dynamic and influences integrated functions, including sensing, communication, growth, morphogenesis, remodeling, and apoptosis.1–4 Surface contacts are likely to be unnoticed until they break down or become impaired following damage or disease. For routine activities, this may mean slipping on an icy sidewalk during walking. For cells and tissues, the result may be more profound and detrimental—arteries accumulate fatty detritus, endobronchial airways inflame and constrict, and joints become painful and immobile.
FIG. 1.
Surface interaction occurs at microscopic asperity contacts. Physical and/or chemical interactions between adhering asperities give rise to friction and wear. A molecular-scale boundary layer (not shown) prevents direct solid-to-solid contact at the asperity scale. Color images available online at www.liebertonline.com/ten.
Functional biotribology emphasizes surface characteristics and properties as a design endpoint for successful regeneration of tissues or biomaterials. The motivation for functional biotribology is practical and significant. It is expected that engineered tissues exhibiting suboptimal surface properties would result in poor function and decreased lifespan postimplantation in vivo. A fundamental design concept is to exploit lubricant substances that can reduce friction and wear of interacting surfaces. Thus, the focus of tissue engineering should not be simply on the bulk tissue but on the regeneration of specific biomolecules and cell types at surface tissue that can prolong the functional lifespan of the engineered construct.
The paradigm of regenerative medicine and tissue engineering is focused on the restoration of damaged tissues and organs through the use of morphogenetic signals, stem cells, and biomimetic materials.5 Knowledge of mechanical and biochemical signal transduction mechanisms is critical to optimizing biomolecular expression of key surface molecules. The main objective of this article is to relate concepts of basic mechanics, biochemistry, and molecular and cellular biology to biotribology of natural and tissue-engineered biomaterials, using the design of articular cartilage as a model system.
Living Bearings
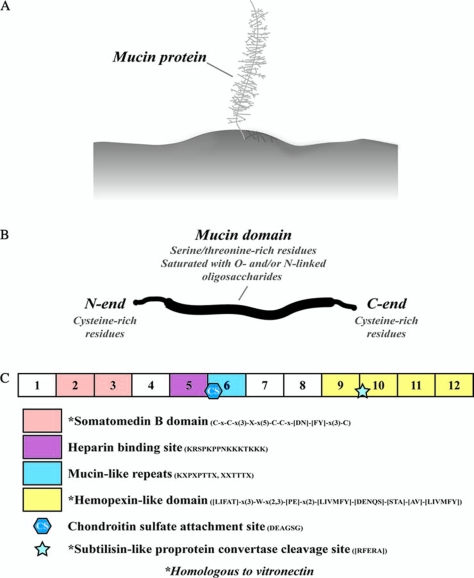
As living materials, bearings in the human body (e.g., articular cartilage and endothelium) facilitate constrained relative movement between two parts, and are far more complex than those found using traditional engineering materials, such as metals, plastics, and ceramics. Bearings of both engineering and living materials exploit the thickness and rheological behavior of the intervening viscous layer to produce low friction during relative surface movement. Lubrication of traditional engineering bearings can be achieved through the application of lubricious films consisting of natural (e.g., animal fats and mineral [petroleum] oils) or synthetic (e.g., hydrocarbons, esters, silicones, silanes, polyphenyl ethers, and perfluoropolyethers) substances6 that are often blended with special additives and can either physically adsorb at the surfaces (physisorption) or chemically react with the surfaces (chemisorption) to form and replenish low-friction, antiwear thin films.7 Living bearings contain cells and thus exhibit regenerative potential and capacity to repair damaged tissue through biosynthesis and replenishment of proteins and other biomolecules that can act as natural lubricants. Mucin proteins (Fig. 2), in particular, have received considerable attention in recent years due to their role as biological lubricants in diverse tissues, such as saliva, glycocalyx, respiratory tract, and synovial fluid.8–11 These proteins form gel-like (mono)layers adhering strongly to the underlying epithelium,12 hence imparting effective lubrication and, in turn, surface tissue protection against mechanical wear.
FIG. 2.
Mucin proteins as a molecular basis for boundary lubrication of cartilage surfaces. (A) Mucins bind to surface tissue to form a molecularly thin monolayer. (B) Mucin domains are heavily glycosylated with oxygen- and/or nitrogen-linked oligosaccharides. (C) The exons and amino acid sequence of the SZP found in synovial joint tissues demonstrate the functional significance of binding and lubricating surface regions at the molecular scale. Color images available online at www.liebertonline.com/ten.
A Dynamic Living Bearing: The Synovial Joint
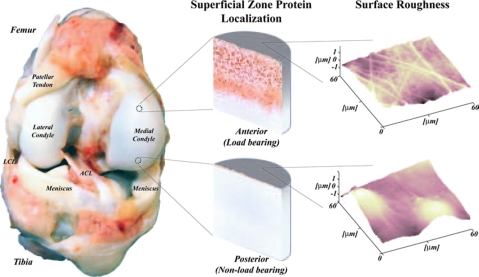
Why do human joints wear out during aging? Perhaps a more intriguing question is how do joints resist wear over a lifetime? Synovial (or freely movable) joints of the human body (e.g., knee, hip, and elbow) are complex living biological and mechanical systems consisting of articular cartilage, bone, menisci, ligaments, and synovium (Fig. 3) that allow for joint articulation and movement with minimal friction and wear. These joints are less constrained by ligamentous attachments compared to fibrous or cartilaginous joints, such as the skull and the spine.
FIG. 3.
The synovial joint is a living bearing system that can be studied at different hierarchical levels, from the whole joint scale (bone shape; contact pressure) through the cellular scale (lubricant protein distributions; cellular biosynthesis) to the atomic scale (surface roughness; protein binding).
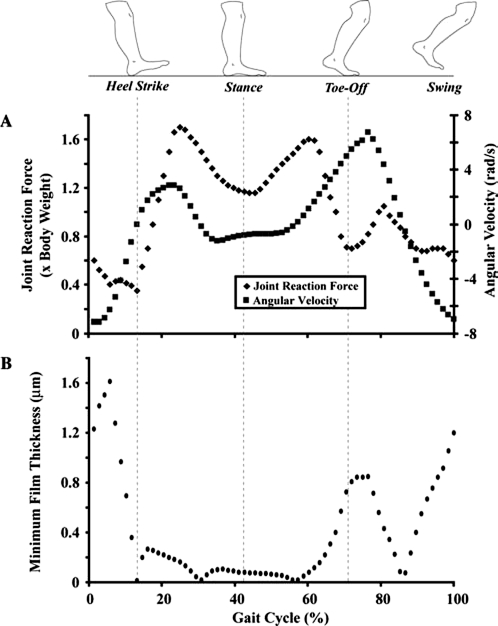
Articular cartilage is the primary bearing biomaterial lining the bones of the joint through which contact forces are transmitted. The cartilage in the average young (25–34 years) human male and female undergoes approximately 5400 and 4700 loading cycles, respectively, during normal daily activities13 and more than 108 loading cycles over an 80-year lifespan. Even during simple daily activities, such as walking, cartilage sustains mechanical forces several times higher than the body weight. Peak joint forces range from 1.2 to 7.2 times the body weight in the human knee14 (Fig. 4A) and from 2.5 to 5.8 times the body weight in the human hip joint.15 The macroscopic joint geometry and multiscale surface roughness produce nonuniform pressure distributions (determined from instrumented prostheses) with peak pressures approaching 18 MPa in the hip joint.15
FIG. 4.
Hydrodynamic lubrication is not the only operating regime of the joint during walking. Knee joint reaction force and angular velocity (A) and minimum film thickness (B) vary during a typical walking cycle. The film thickness between the cartilage surfaces was estimated using biomechanical contact data (adapted from Komistek et al.104 and Winter105) and the formula for loaded rigid contacts,106 h0/R = 4.9ηυ/W, where h0 is the minimum film thickness, R is the radius of the equivalent cylinder (approximated as 2.56 × 10−2 m for tibiofemoral contact), η is the viscosity (1 N·s/m2; Wright and Dowson34), υ is the sliding speed,105 and W is the normal load per unit length in the travel direction (i.e., joint reaction force104 times the body weight105 divided by the square root of the contact area107). Hydrodynamic theory fails to predict a film thickness larger than the surface roughness of cartilage (typical arithmetic average roughness Ra = 200 nm) for the duration of the walking cycle. Lubrication of the joint is largely due to elastohydrodynamic, boundary, and/or mixed lubrication mechanisms.
Articular cartilage is a highly organized structure. The tissue consists of cells, water, collagens, proteoglycans, and other matrix biomolecules. On a tissue and molecular scale, articular cartilage consists of surface, middle, and deep zones, each exhibiting unique cell architecture, biochemical composition, and mechanical properties16 (Fig. 3). The hydrated tissue is composed of biopolymers, such as type II collagen, aggrecan, chondroitin sulfate, keratan sulfate chains, and hyaluronan. The water and biopolymer contents vary with depth from the articular surface. Water accounts for more than 80% of the wet tissue weight at the surface and 65% in the deep zone. The collagen content (15–22% of the wet tissue weight overall) is highest in the surface zone, while the proteoglycan content (4–7% of the wet tissue weight overall) is lowest in the superficial zone and highest in the middle and deep zones. The viscoelastic properties of cartilage are attributed to the intrinsic properties of the macromolecules that form a solid porous matrix and the frictional drag of the interstitial fluid flow through this porous matrix.17–19 Material properties vary with tissue depth, as demonstrated in studies of strain patterns generated during simple uniaxial and physiologically relevant compression loading.20
Arthritis is a type of rheumatic disease involving joint inflammation. Osteoarthritis, commonly thought of as a degenerative joint disease or the “wear and tear” of human joints, is the most common form of arthritis, affecting 12.1% of adults in the United States (about 20.7 million people), and is a leading cause of disability in America.21 In the most extreme cases, the cartilage may be worn off completely, resulting in bone-on-bone surface rubbing. Although the etiologies of this disease are largely unknown, it is likely that they involve multiple factors, including a biochemical imbalance between catabolic cytokines and anabolic morphogens and growth factors, mechanical injury or trauma, and progressive surface deterioration due to mechanical wear. Moreover, cartilage is recalcitrant to repair, partly due to the avascularity of the tissue, the high concentration of protease inhibitors, and, presumably, the presence of growth inhibitors.5
Friction and Wear of Synovial Joints
When two bodies in contact slide over each other, surface interaction occurs through isolated microscopic contacts, referred to as asperity contacts (Fig. 1), resulting in the development of a friction force and the removal of material by different wear processes occurring at the asperity scale. The friction force represents the resistance encountered when a body slides against another body, and arises in the direction directly opposite to the direction of motion. A basic mechanism of sliding friction is described by the physical and/or chemical interactions occurring between adhesive asperity contacts, which must be sheared off for relative movement to occur. The first friction law, attributed to both Amontons and Leonardo da Vinci,
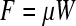 |
(1) |
relates the friction force F to the external normal load W through the coefficient of friction μ. In articular cartilage, the average coefficient of friction may vary from 0.005 (Charnley22) to 0.5 or even more (Pickard et al.23), and shows a strong dependence on the testing conditions and the operating lubrication regime. Equation (1) indicates a direct proportionality between friction force and external normal load—that is, constant coefficient of friction. While this relationship is followed at the macroscale, a nonlinear relationship is often encountered at the microscale due to the increased importance of surface adhesion forces, such as van der Waals, capillary, and molecular forces, implying a dependence of the coefficient of friction on the applied normal load. This is due to the fact that adhesion forces represent an additional normal force at the microscale that can result in significantly higher friction when the external load is on the same order of magnitude as the adhesion forces. A time-dependent coefficient of friction response is commonly observed with cartilage surfaces sliding against different solid surfaces that has often been attributed to interstitial fluid pressurization of the hydrated tissue.24 Variations in the coefficient of friction have also been observed over a wide range of length scales and have been attributed to differences in the operating conditions (e.g., magnitude of contact stresses, sample hardness, elastic modulus, apparent contact area, and total sliding distance) encountered at different scales.6,25 Therefore, a mechanistic understanding of the origins of the friction force in a particular test configuration requires careful consideration of multiple contributing factors.
Comparatively, fewer studies have been devoted to examine the origins and evolution of cartilage wear.26 This is surprising in light of the prevalence of osteoarthritis, a degenerative joint disease of multifactorial causalities characterized by progressive cartilage tissue loss, believed to be partly due to different wear mechanisms. Wear occurs by mechanical and/or chemical processes that could be enhanced by frictional heating produced from surface rubbing. Wear mechanisms include adhesion, abrasion, fatigue, impact, cavitation, erosion, and corrosion.27 Understanding of the different wear mechanisms of articular cartilage in the context of degenerative diseases, such as osteoarthritis, and basic knowledge of their contributions to cartilage wear require further study. Adhesion, the most common type of wear, is characterized by the shearing of asperity contacts formed at the sliding interface of two nominally flat solid bodies, resulting in the formation of wear particles. The classical relationship of adhesive wear,28
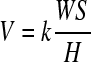 |
(2) |
is commonly used to relate the wear volume V to the wear coefficient k, normal load W, total sliding distance S, and hardness H of the worn surface. Wear rates (defined as the thickness of the worn layer from the cartilage surface per cycle) for total hip arthroplasties29 have been reported to be on the order of 10−6 mm/cycle; however, wear rate estimates for natural cartilage have yet to be determined. Descriptive wear patterns in articular cartilage are limited to load-bearing joint regions.30 In an ovine meniscectomy model of osteoarthritis, early osteoarthritis was associated with a loss in immunostaining and mRNA levels of cellular proteoglycan 4 (PRG4), considered to be a boundary lubricant in articular joints.31 In a living tissue, cells within the material produce molecules (e.g., structural proteins or boundary lubricants) that can replenish worn tissue. The specific conditions regulating tribological homeostasis (i.e., a balance between wear and replenishment mechanisms) to maintain tissue function over time remain unknown. In view of the empirical descriptions of wear and associated lubrication regimes, fundamental studies in cartilage biotribology are of paramount importance to regenerative medicine.
Molecular Basis Of Synovial Joint Lubrication
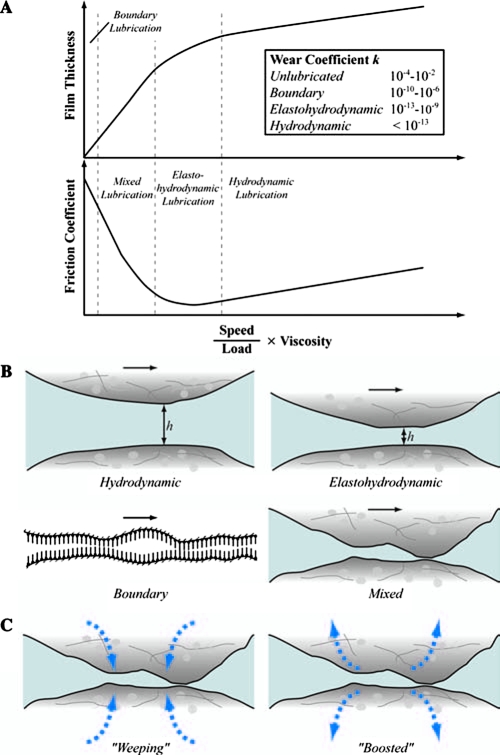
Lubricants generally reduce friction and wear of interacting surfaces. The main function of lubricants is to provide an easily sheared film between proximity surfaces in relative motion. Lubrication may be classified as fluid lubrication, when a thick film of fluid separates the surfaces, or boundary lubrication, when a molecularly thin (monolayer) film forms conformably on at least one of the sliding surfaces. Fluid lubrication may be further classified into three types: (i) hydrostatic lubrication, when a fluid film that separates the opposed surfaces is generated by external pressurization means (e.g., pump); (ii) hydrodynamic lubrication, when surface separation results from the formation of a thick fluid film due to the kinematics of the proximal surfaces, depending on the macroscopic bearing geometry (curvature effect), interfacial topography (roughness effect), normal load (pressure effect), relative speed (shear rate effect), and fluid film rheological properties (viscosity effect); and (iii) elastohydrodynamic lubrication, when the pressure in the self-generated hydrodynamic fluid film causes elastic deformation of the confining surfaces (i.e., the film thickness also depends on the elastic properties of the solid surfaces). The transition between lubrication regimes depends on the surface roughness and the film thickness, which is a function of the fluid viscosity, sliding speed, and applied normal load (or mean pressure)32 (Fig. 5A).
FIG. 5.
Lubrication influences friction and wear. (A) Variation of film thickness and friction coefficient with speed-to-load ratio multiplied by the viscosity of the interfacial viscous layer. (B) Schematics of different lubrication regimes. (C) Weeping and boosted mixed lubrication mechanisms. Although the lubrication mechanisms operating under physiologically relevant activities are not well understood, it is likely that elastohydrodynamic, boundary, and mixed lubrication mechanisms play synergistic roles at the joint interface. The prevalence of each of these lubrication mechanisms depends on the underlying tissue composition, structure, and contact parameters. The inset of (A) shows typical ranges of the wear coefficient k for different sliding conditions.39 Color images available online at www.liebertonline.com/ten.
Human joints are complex bearings that operate effectively under both fluid film and boundary lubrication conditions.33,34 During normal activities, such as walking, joints may also function under so-called mixed lubrication conditions, implying the coexistence of fluid and boundary lubrication conditions at the contact interface.10,35–37 Hydrodynamic theory fails to predict an adequate film thickness for complete surface separation throughout a typical walking cycle (Fig. 4B). Therefore, it is likely that hydrostatic,36 elastohydrodynamic,38 and/or mixed lubrication conditions can be encountered during the swing phase (high velocity-to-load ratio), when the film thickness is greater than the average surface roughness of cartilage, whereas boundary lubrication conditions dominate during the stance phase between heel strike and toe off (low velocity-to-load ratio), when the film thickness is significantly less than the average surface roughness of cartilage.6,39 The wear coefficient in hydrodynamic lubrication may be 7 orders of magnitude less than that in boundary lubrication and 11 orders of magnitude less than that obtained with unlubricated surfaces39 (Fig. 5A). While fluid film effects in cartilage lubrication are critical to providing normal function of the joint, it is likely that in the absence of a continuous and self-replenishing boundary lubricant, joint degeneration will occur rapidly.40
Hydrodynamic lubrication
Surface relative movement in the hydrodynamic lubrication regime is controlled by an interfacial fluid film of thickness much larger than the heights of the tallest asperities (Fig. 5B). Under these lubrication conditions, the normal load is transmitted through the pressurized fluid film, which exhibits a pressure-dependent shear resistance due to the exponential dependence of the fluid viscosity on pressure. Under isothermal conditions, the dependence of the fluid viscosity η on pressure p is given by a relationship of the form:
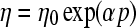 |
(3) |
where η0 is the ambient viscosity and α is the viscosity–pressure coefficient (expressed in units of m2/N), an intrinsic rheological property of the fluid.41 The classical hydrodynamic lubrication mechanism involves wedge or entraining flow generated when two surfaces slide past each other. The narrowing wedge-shaped gap produces a hydrodynamic pressure in the fluid that tends to push the two surfaces apart. A second classical mechanism, squeeze-film lubrication, occurs when the opposing surfaces approach each other at a relatively high speed, as in the case of dynamic contact loading, resulting in the pressurization of the fluid by the confining solid surfaces. The pressure distribution can be obtained by solving the Reynolds equation derived from the general Navier–Stokes equations of fluid flow. Hydrodynamic lubrication of human joints depends on the synovial fluid secreted from the synovium cells (synoviocytes). The synovium fluid is a dialysate of blood plasma that is devoid of clotting factors, erythrocytes, and hemoglobin, and contains proteoglycans, glycoproteins (e.g., hyaluronate and lubricin), and phospholipids.42–45 A dependence of the apparent viscosity of synovial fluid on shear rate and concentration of hyaluronan has been observed in previous studies.46–48
Elastohydrodynamic lubrication
In this type of lubrication, the bearing surfaces are separated by a highly pressurized fluid film that causes elastic deformation of the solid surfaces (Fig. 5B). Hence, the pressure and thickness of the hydrodynamic film depend on both the rheological properties of the fluid and the elastic deformation of the bearing surfaces. The high film pressure increases the fluid viscosity, producing a tendency for both the thickness and the shear resistance of the fluid film to increase due to the exponential dependence of viscosity on pressure (Eq. 3). The interdependence of local pressure, viscosity, surface deformation, and film thickness necessitates the implementation of a numerical iterative procedure to solve the Reynolds equation. It is likely that elastohydrodynamic lubrication is a significant mechanism in cartilage during normal activities, and is largely due to the multiphasic nature of the tissue.38,49
Boundary lubrication and mucins
Surface relative movement in the boundary lubrication regime does not favor the formation of a fluid film of thickness much larger than the roughness of the countersurfaces. Hence, the only barrier against direct solid-to-solid contact is an adsorbed molecular film that forms conformably with the surface topographies, preferably in a closed-pack arrangement that resembles a brush-like surface layer (Fig. 5B). In the absence of a strongly adsorbing, continuous, and self-replenishing boundary lubricant layer, intermittent asperity contact interactions promote rapid surface degradation by mechanical wear.
Mucins are a family of large and heavily glycosylated proteins (Fig. 2B). Glycosylation occurs as a posttranslational modification to the synthesized protein and provides hygroscopic characteristics. Mucin amino- and carboxyl-terminal regions are cysteine rich and likely involved in disulfide bonding, while the central region has multiple repeat residue sequences that are serine rich and threonine rich to allow glycosylation of primarily oxygen-linked oligosaccharides. Mucins can coat many surfaces in the human body, including teeth, respiratory and gastrointestinal tract, and reproductive organs, and are thought to act as boundary lubricants.12 Superficial zone protein (SZP) is a mucin domain–containing glycoprotein secreted from chondrocytes in the superficial layer of articular cartilage.50,51 This protein is homologous to lubricin45 and megakaryocyte stimulating factor (MSF) precursor52 and is encoded by the PRG4 gene.51 SZP is not retained in the matrix, but is mostly secreted into the synovial fluid50 or is bound to macromolecules in the lamina splendens and has also been localized in the lining joint cavities of the synovial membrane.53 In addition to its function as a boundary lubricant,45 SZP plays a role in the inhibition of integrative cartilage repair54 and synovial cell overgrowth.55 A mutation in the PRG4 gene has been linked to camptodactyly-arthropathy-coxa vara-pericarditis (CACP) syndrome, an autosomal recessive disease characterized by synovial hyperplasia without evidence of inflammation, where the lack of the mucin protein apparently results in premature joint wear.40 It has also been reported that the SZP gene is alternatively expressed in the synovium of rheumatoid arthritis and osteoarthritis, implying a possible role in the pathogenesis of these diseases.56 Knockout mice lacking the SZP gene have demonstrated abnormal protein deposits on the cartilage surface, disappearance of the underlying superficial zone, synovial hyperplasia, and precocious failure of joint function.55
The role of SZP in boundary lubrication is controversial.24,57,58 It has been suggested that surface-active phospholipid (SAPL) provides joint lubrication, as evidenced in part by the increased friction coefficient following phospholipase incubation.57 However, the role of phospholipids has been challenged, as it was later determined that commercial purified phospholipase contained trypsin-like activity.58 Digestion of bovine synovial fluid by phospholipase C in the presence of protease inhibitors did not affect negatively boundary lubricating efficacy compared to undigested control.58 Other authors reported that the removal of the superficial zone of bovine articular cartilage did not increase the friction coefficient of samples tested under reciprocating sliding motion to 2500 s.24 However, multiple factors were not controlled to specifically address the role of SZP in boundary lubrication, such as surface roughening due to microtoming that may influence the real area of contact and thus the friction coefficient.27 The boundary lubricant in synovial joints has been proposed as hyaluronan, SZP/lubricin/PRG4, SAPL, or a combination of these molecules.45,57,59–63 The synovial fluid constituents hyaluronan and PRG4 (either in physiologic or in pathophysiologic concentrations) contribute individually and concomitantly to boundary lubrication of articular cartilage.60
Mixed lubrication
Mixed lubrication is characterized by the coexistence of interfacial regions operating under elastohydrodynamic and boundary lubrication conditions (Fig. 5B). This implies that multiple lubrication mechanisms can occur simultaneously in this transition lubrication regime. For instance, there may be interfacial regions where surface separation is only a few molecular layers as opposed to other regions where the surfaces may be separated by a micrometer-thick hydrodynamic film. Two mixed lubrication mechanisms have been proposed for articular cartilage—namely, “weeping” and “boosted” lubrication62,64 (Fig. 5C). In addition to asperity contact, fluid pressurization arises in weeping lubrication through exudation of fluid from the cartilage during compression,36,64 and exhibits hydrostatic lubrication characteristics. In contrast, asperity and fluid pressurization in the boosted lubrication mechanism forces fluid into the cartilage, leaving behind trapped pools of concentrated lubricant.62 The nature of fluid flow (transport) through the tissue of cartilage under mixed lubrication conditions is a controversial subject.38,65,66 Another mechanism, termed interstitial fluid pressurization, is characterized by contact of the solid phase of cartilage (giving rise to friction at asperity contacts) and load support by the fluid phase (resulting in a small or perhaps negligible contribution to the friction force by viscous shear of the interstitial fluid and the synovial fluid).67–69 Surface asperity contact is inevitable in this mechanism, and thus the presence of surface lubricants (e.g., SZP) is important. The nonlinear nature of fluid depressurization under, for example, constant load causes a shift in the load support from the fluid to the solid phase over time. This time-dependent shift of the load can be observed in various normal physiologic activities, such as during a prolonged stance. In this case of increased load support by the solid phase, the presence of a boundary lubricant10,50 that influences surface contact of the solid phase becomes even more critical as time progresses. It is unclear to what extent interstitial fluid pressurization can be characterized as a mixed lubrication mechanism given the nature of solid contact and minimal fluid film thickness required for this mechanism to operate, and thus it may be more appropriately characterized as a mechanism operating in the boundary mode.
Mechanical Regulation Of Cartilage Lubrication
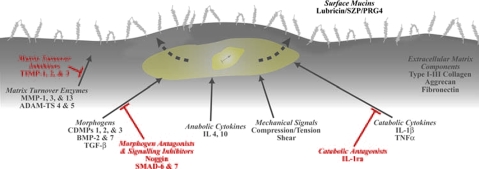
Cells in the cartilage (chondrocytes) respond to mechanical signals70,71 and through unknown mechanisms convert mechanical input ultimately into protein expression of extracellular matrix molecules. For example, proteoglycan synthesis is sensitive to the frequency of dynamic compressive loading and could be synthesized during dynamic loading at 0.001 Hz,71 although synthesis may be reduced by as much as 50% from that of controls subjected to 1 MPa dynamic pressure of 1 Hz frequency.70 The expression of SZP in chondrocytes is sensitive to mechanical signals (Fig. 6). Studies have shown that while compressive loading can decrease SZP expression level, shear loading increases SZP expression.72,73 Shear loading may mediate SZP expression level through transforming growth factor (TGF-β) signaling pathways.74
FIG. 6.
Mucins as a model boundary film for effective tissue lubrication. Regenerative medicine and tissue engineering strategies aimed at surface restoration of key design outcomes (e.g., surface mucin concentration) depend on several factors, including morphologic, mechanical, and other molecular signals. Color images available online at www.liebertonline.com/ten.
Mechanical regulation in boundary lubrication may involve a “sacrificial” layer mechanism characterized by the removal of the lubricant layer from the contacting surfaces to maintain a low friction coefficient, for example, through the formation of an easily sheared sacrificial layer, followed by the replenishment of the removed layer at the sliding interface. In this mechanism, the lubricating molecule has a strong affinity for surface attachment by physical adsorption. In the case of articular cartilage, SZP may bind to heparan sulfate or other binding partners only in the most superficial tissue layer (lamina splendens75) to form a sacrificial layer. Interfacial friction (shear) forces may promote the removal of SZP, resulting in the increase of the friction coefficient and, in turn, accelerate tissue degradation at the cartilage surface. Recent findings indicate that shear force–induced biosynthesis of superficial zone chondrocytes73,74 may be instrumental in SZP replenishment at the articular surface.
Biochemical Regulation of Cartilage Lubrication
Homeostasis of the major biomolecules (e.g., collagens and proteoglycans) for maintaining fluid film and boundary lubrication characteristics depends on various factors, including morphogens, growth factors, and cytokines (Fig. 6). Morphogenetic proteins and growth factors are molecules that specify cell identity during development.76 Bone morphogenetic proteins (BMPs) are a family of morphogens that promote new cartilage and bone growth.5,77 BMPs have chemotactic, mitogenic, and differentiation-inducing properties. The biological actions of BMPs are based on concentration-dependent thresholds. Articular cartilage contains endogenous morphogens, such as cartilage-derived morphogenetic protein (CDMP-1, a type of BMP). BMP-7, also called human osteogenic protein-1 (OP-1), plays an important role in human and bovine cartilage homeostasis and repair.78–80 Studies have shown that BMP-7 and other growth factors can synergistically promote increased survival and matrix synthesis by normal and osteoarthritic human articular chondrocytes.81–83 Other growth factors, such as TGF-β, basic fibroblast growth factor, insulin-like growth factor, and platelet-derived growth factor, have all been shown to be anabolic for cartilage and chondrocytes.83
Morphogenetic proteins and growth factors bind to the receptors of the cell surface membrane to initiate signaling cascades. In the case of BMP-4, BMP-7, and CDMP-1, binding to the cell membrane occurs at BMP receptors IA and IB,84 which are membrane-bound serine/threonine kinases. The BMP type II receptors phosphorylate the BMP type I receptors, which, in turn, phosphorylate signal-transducing Smad proteins.85 The transcription of BMP-response genes, which are likely homeobox genes, is initiated by Smad 1 and Smad 4 proteins. Additionally, the antagonism of cartilage and BMP actions may be mediated by other binding proteins, such as noggin.86,87
TGF-β is a potent regulator of SZP expression88,89 localized in the superficial zone of the tissue.89 SZP expression can either be up- or downregulated upon treatment with TGF-β1 and interleukin-1 (IL-1α), respectively.52,90 Such inhibition may be mediated by proinflammatory cytokines, such as tumor necrosis factor-α (TNF-α) and IL-1α, which promote cartilage matrix degradation in part by enhancing the expression of matrix metalloproteinases (MMPs).91 There is evidence that IL-1α and TNF-α colocalize with MMPs in the superficial layer of arthritic cartilage, illustrating the key role of this layer in the pathogenesis of arthritic diseases.92
Resurfacing and Regenerative Medicine
Functional biotribology refers to the restoration of the surface characteristics and properties. Through novel (simultaneous or sequential) combination of cells, acellular biomaterials, drugs, gene products, and genes, surfaces may be designed, specified, or fabricated as therapeutic agents.93 Particularly in cartilage, there has been limited success in the regeneration and repair of the tissue, with mixed reports of success94–96 and lack of characterization of the in vivo surface characteristics or properties.
Reconstitution of fluid film lubrication at the tissue surface requires scaffolding structures with optimal collagen and proteoglycan content, organization, and spatial heterogeneity that can produce tissue of appropriate elastic modulus, porosity, and permeability.97 Localization of cells and other matrix-bound factors and molecules is critical for long-term maintenance of the surface properties. Engineering of cartilage has produced tissues with increased coefficients of friction (up to 0.6 for engineered constructs vs. less than 0.2 for the equilibrium friction coefficient of native tissue) under mixed lubrication conditions. Although the engineered tissue design promoted fluid exudation from the constructs that affected significantly the frictional properties, the tissue was not effective in producing low friction coefficients similar to those of native cartilage.98
Reconstitution of boundary lubrication at the tissue surface requires surface scaffolding with appropriate binding partners for biomolecules, such as proteoglycans. In addition, cellular localization is critical for maintaining the lubricant monolayer, especially in articular cartilage where a limited population of cells, particularly those in the tissue of load-bearing surface regions,74 are inductive to producing lubricating proteins. Initial efforts to restore the boundary lubricating ability of the superficial layer have involved stratified tissue constructs with specialized cell subpopulations specifically expressing SZP.99
It is expected that engineered tissues exhibiting suboptimal surface properties would result in poor function and decreased lifespan postimplantation in vivo. The strategy for restoration of the surface characteristics, such as lubricant molecule concentrations, may require the optimal combination of morphologic, mechanical, and other inductive signals (Fig. 6). It is believed that replenishment and regeneration of boundary lubricants (e.g., SZP) can be achieved through optimal use of morphogens, such as TGF-β and BMPs, in concert with mechanical signals. In addition to inductive signals, such as morphogens and biomechanical factors, successful engineering of cartilage will likely be the result of a complex array of independent variables, including cell type,100 cell seeding density,101 extracellular matrix scaffolding and bioreactor design,102 and controlled enzymatic matrix degradation.103
Outlook
Recent progress in biotribology has yielded valuable insight into the complex nature of friction, wear, and lubrication mechanisms encountered at interfaces of living systems. The lack of basic knowledge of the dominant wear processes in biological tissues presents a major obstacle in treating diseases, such as osteoarthritis. However, contemporary surface analyses techniques, such as surface force microscopy, provide powerful tools for characterizing normal, diseased, and regenerated tissues. Biotribology provides a context and design paradigm for the functional restoration and regeneration of articular cartilage and a host of other tissues with optimal surface characteristics and properties.
Acknowledgments
The authors gratefully acknowledge funding for this work provided by the National Institutes of Health (NIBIB), Grant No. 1F32 EB003371, the Lawrence J. Ellison Endowed Chair, and the National Science Foundation, Grant No. CMS–0528506.
References
- 1.Carter D.R. Beaupré G.S. Giori N.J. Helms J.A. Mechanobiology of skeletal regeneration. Clin Orthop Relat Res. 1998;355(Suppl):S41. doi: 10.1097/00003086-199810001-00006. [DOI] [PubMed] [Google Scholar]
- 2.D'Lima D.D. Hashimoto S. Chen P.C. Lotz M.K. Colwell C.W., Jr. Cartilage injury induces chondrocyte apoptosis. J Bone Joint Surg Am. 2001;83:S19. doi: 10.2106/00004623-200100021-00004. [DOI] [PubMed] [Google Scholar]
- 3.Lumpkin E.A. Caterina M.J. Mechanisms of sensory transduction in the skin. Nature. 2007;445:858. doi: 10.1038/nature05662. [DOI] [PubMed] [Google Scholar]
- 4.Rot-Nikcevic I. Reddy T. Downing K.J. Belliveau A.C. Hallgrimsson B. Hall B.K. Kablar B. Myf5-/-:MyoD-/- amyogenic fetuses reveal the importance of early contraction and static loading by striated muscle in mouse skeletogenesis. Dev Genes Evol. 2006;216:1. doi: 10.1007/s00427-005-0024-9. [DOI] [PubMed] [Google Scholar]
- 5.Reddi A.H. Role of morphogenetic proteins in skeletal tissue engineering and regeneration. Nat Biotechnol. 1998;16:247. doi: 10.1038/nbt0398-247. [DOI] [PubMed] [Google Scholar]
- 6.Bhushan B. Introduction to Tribology. New York: John Wiley & Sons, Inc.; 2002. [Google Scholar]
- 7.Komvopoulos K. Do V. Yamaguchi E.S. Ryason P.R. Effect of sulfur- and phosphorus-containing additives and metal deactivator on the tribological properties of boundary-lubricated steel surfaces. Tribol Trans. 2003;46:315. [Google Scholar]
- 8.Cárdenas M. Elofsson U. Lindh L. Salivary mucin MUC5B could be an important component of in vitro pellicles of human saliva: an in situ ellipsometry and atomic force microscopy study. Biomacromolecules. 2007;8:1149. doi: 10.1021/bm061055h. [DOI] [PubMed] [Google Scholar]
- 9.Carraway K.L. Perez A. Idris N. Jepson S. Arango M. Komatsu M. Haq B. Price-Schiavi S.A. Zhang J. Carraway C.A. Muc4/sialomucin complex, the intramembrane ErbB2 ligand, in cancer and epithelia: to protect and to survive. Prog Nucleic Acid Res Mol Biol. 2002;71:149. doi: 10.1016/s0079-6603(02)71043-x. [DOI] [PubMed] [Google Scholar]
- 10.Radin E.L. Swann D.A. Weisser P.A. Separation of a hyaluronate-free lubricating fraction from synovial fluid. Nature. 1970;228:377. doi: 10.1038/228377a0. [DOI] [PubMed] [Google Scholar]
- 11.Stonebraker J.R. Wagner D. Lefensty R.W. Burns K. Gendler S.J. Bergelson J.M. Boucher R.C. O'Neal W.K. Pickles R.J. Glycocalyx restricts adenoviral vector access to apical receptors expressed on respiratory epithelium in vitro and in vivo: role for tethered mucins as barriers to lumenal infection. J Virol. 2004;78:13755. doi: 10.1128/JVI.78.24.13755-13768.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Bansil R. Stanley E. LaMont J.T. Mucin biophysics. Annu Rev Physiol. 1995;57:635. doi: 10.1146/annurev.ph.57.030195.003223. [DOI] [PubMed] [Google Scholar]
- 13.Sequeira M.M. Rickenbach M. Wietlisbach V. Tullen B. Schutz Y. Physical activity assessment using a pedometer and its comparison with a questionnaire in a large population survey. Am J Epidemiol. 1995;142:989. doi: 10.1093/oxfordjournals.aje.a117748. [DOI] [PubMed] [Google Scholar]
- 14.Komistek R.D. Kane T.R. Mahfouz M. Ochoa J.A. Dennis D.A. Knee mechanics: a review of past and present techniques to determine in vivo loads. J Biomech. 2005;38:215. doi: 10.1016/j.jbiomech.2004.02.041. [DOI] [PubMed] [Google Scholar]
- 15.Hodge W.A. Fijan R.S. Carlson K.L. Burgess R.G. Harris W.H. Mann R.W. Contact pressures in the human hip joint measured in vivo. Proc Natl Acad Sci USA. 1986;83:2879. doi: 10.1073/pnas.83.9.2879. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Mow V.C. Ratcliffe A. Robin Poole A. Cartilage and diarthrodial joints as paradigms for hierarchical materials and structures. Biomaterials. 1992;13:67. doi: 10.1016/0142-9612(92)90001-5. [DOI] [PubMed] [Google Scholar]
- 17.Hayes W.C. Mockros L.F. Viscoelastic properties of human articular cartilage. J Appl Physiol. 1971;31:562. doi: 10.1152/jappl.1971.31.4.562. [DOI] [PubMed] [Google Scholar]
- 18.Mow V.C. Rosenwasser M. Articular cartilage: biomechanics. In: Woo S.L.-Y., editor; Buckwalter J.A., editor. Injury and Repair of the Musculoskeletal Soft Tissues. Park Ridge, IL: American Academy of Orthopaedic Surgeons; 1988. pp. 427–463. [Google Scholar]
- 19.Cohen N.P. Foster R.J. Mow V.C. Composition and dynamics of articular cartilage: structure, function, and maintaining healthy state. J Orthop Sports Phys Ther. 1998;28:203. doi: 10.2519/jospt.1998.28.4.203. [DOI] [PubMed] [Google Scholar]
- 20.Neu C.P. Hull M.L. Walton J.H. Heterogeneous three-dimensional strain fields during unconfined cyclic compression in bovine articular cartilage explants. J Orthop Res. 2005;23:1390. doi: 10.1016/j.orthres.2005.03.022.1100230622. [DOI] [PubMed] [Google Scholar]
- 21.Lawrence R.C. Helmick C.G. Arnett F.C. Deyo R.A. Felson D.T. Giannini E.H. Heyse S.P. Hirsch R. Hochberg M.C. Hunder G.G. Liang M.H. Pillemer S.R. Steen V.D. Wolfe F. Estimates of the prevalence of arthritis and selected musculoskeletal disorders in the United States. Arthritis Rheum. 1998;41:778. doi: 10.1002/1529-0131(199805)41:5<778::AID-ART4>3.0.CO;2-V. [DOI] [PubMed] [Google Scholar]
- 22.Charnley J. The lubrication of animal joints in relation to surgical reconstruction by arthroplasty. Ann Rheum Dis. 1960;19:10. doi: 10.1136/ard.19.1.10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Pickard J. Ingham E. Egan J. Fisher J. Investigation into the effect of proteoglycan molecules on the tribological properties of cartilage joint tissues. Proc Inst Mech Eng [H] 1998;212:177. doi: 10.1243/0954411981533953. [DOI] [PubMed] [Google Scholar]
- 24.Krishnan R. Caligaris M. Mauck R.L. Hung C.T. Costa K.D. Ateshian G.A. Removal of the superficial zone of bovine articular cartilage does not increase its frictional coefficient. Osteoarthritis Cartilage. 2004;12:947. doi: 10.1016/j.joca.2004.08.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Benz M. Chen N. Jay G. Israelachvili J. Static forces, structure and flow properties of complex fluids in highly confined geometries. Ann Biomed Eng. 2005;33:39. doi: 10.1007/s10439-005-8961-z. [DOI] [PubMed] [Google Scholar]
- 26.Lipshitz H. Glimcher M.J. In vitro studies of wear of articular cartilage. II. Characteristics of the wear of articular cartilage when worn against stainless steel plates having characterized surfaces. Wear. 1979;52:297. [Google Scholar]
- 27.Rabinowicz E. Friction and Wear of Materials. New York: John Wiley & Sons, Inc.; 1965. [Google Scholar]
- 28.Archard J.F. Contact and rubbing of flat surfaces. J Appl Phys. 1953;24:981. [Google Scholar]
- 29.Lundberg H.J. Stewart K.J. Callaghan J.J. Brown T.D. Kinetically critical sites of femoral head roughening for wear rate acceleration in total hip arthroplasty. Clin Orthop Relat Res. 2005;430:89. doi: 10.1097/01.blo.0000150450.42829.b8. [DOI] [PubMed] [Google Scholar]
- 30.Cook S.D. Thomas K.A. Kester M.A. Wear characteristics of the canine acetabulum against different femoral prostheses. J Bone Joint Surg Br. 1989;71:189. doi: 10.1302/0301-620X.71B2.2925733. [DOI] [PubMed] [Google Scholar]
- 31.Young A.A. McLennan S. Smith M.M. Smith S.M. Cake M.A. Read R.A. Melrose J. Sonnabend D.H. Flannery C.R. Little C.B. Proteoglycan 4 downregulation in a sheep meniscectomy model of early osteoarthritis. Arthritis Res Ther. 2006;8:R41. doi: 10.1186/ar1898. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Stribeck R. Die wesentlischen eigenschaften der gleit- und rollenlager. Z Ver Dt Ing. 1902;46:1341. [Google Scholar]
- 33.Mow V.C. Ratcliffe A. Structure and function of articular cartilage and meniscus. In: Mow V.C., editor; Hayes W.C., editor. Basic Orthopaedic Biomechanics. Philadelphia, PA: Lippincott-Raven Publishers; 1997. pp. 113–177. [Google Scholar]
- 34.Wright V. Dowson D. Lubrication and cartilage. J Anat. 1976;121:107. [PMC free article] [PubMed] [Google Scholar]
- 35.Mow V.C. Ateshian G.A. Lubrication and wear of diarthrodial joints. In: Mow V.C., editor; Hayes W.C., editor. Basic Orthopaedic Biomechanics. Philadelphia, PA: Lippincott-Raven Publishers; 1997. pp. 275–315. [Google Scholar]
- 36.Lewis P.R. McCutchen C.W. Mechanism of animal joints: experimental evidence for weeping lubrication in mammalian joints. Nature. 1959;184:1285. doi: 10.1038/1841285a0. [DOI] [PubMed] [Google Scholar]
- 37.Schwarz I.M. Hills B.A. Surface-active phospholipid as the lubricating component of lubricin. Br J Rheumatol. 1998;37:21. doi: 10.1093/rheumatology/37.1.21. [DOI] [PubMed] [Google Scholar]
- 38.Soltz M.A. Basalo I.M. Ateshian G.A. Hydrostatic pressurization and depletion of trapped lubricant pool during creep contact of a rippled indenter against a biphasic articular cartilage layer. J Biomech Eng. 2003;125:585. doi: 10.1115/1.1610020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Hutchings I.M. Tribology: Friction and Wear of Engineering Materials. Boca Raton, FL: CRC Press; 1992. [Google Scholar]
- 40.Marcelino J. Carpten J.D. Suwairi W.M. Gutierrez O.M. Schwartz S. Robbins C. Sood R. Makalowska I. Baxevanis A. Johnstone B. Laxer R.M. Zemel L. Kim C.A. Herd J.K. Ihle J. Williams C. Johnson M. Raman V. Alonso L.G. Brunoni D. Gerstein A. Papadopoulos N. Bahabri S.A. Trent J.M. Warman M.L. CACP, encoding a secreted proteoglycan, is mutated in camptodactyly-arthropathy-coxa vara-pericarditis syndrome. Nat Genet. 1999;23:319. doi: 10.1038/15496. [DOI] [PubMed] [Google Scholar]
- 41.Barus C. Isothermals, isopiestics, and isometrics relative to viscosity. Am J Sci. 1893;45:87. [Google Scholar]
- 42.Balazs E.A. Phillips G.O. Young M.D. Polyanions and their complexes. II. Light-induced paramagnetism in solid glycosaminoglycan-dye complexes. Biochim Biophys Acta. 1967;141:382. doi: 10.1016/0304-4165(67)90113-4. [DOI] [PubMed] [Google Scholar]
- 43.Davies D.V. Properties of synovial fluid. Proc Inst Mech Eng [H] 1967;181:25. [Google Scholar]
- 44.Hills B.A. Butler B.D. Surfactants identified in synovial fluid and their ability to act as boundary lubricants. Ann Rheum Dis. 1984;43:641. doi: 10.1136/ard.43.4.641. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Swann D.A. Slayter H.S. Silver F.H. The molecular structure of lubricating glycoprotein-I, the boundary lubricant for articular cartilage. J Biol Chem. 1981;256:5921. [PubMed] [Google Scholar]
- 46.King R.G. A rheological measurement of three synovial fluids. Rheol Acta. 1966;5:41. [Google Scholar]
- 47.Schurz J. Ribitsch V. Rheology of synovial fluid. Biorheology. 1987;24:385. doi: 10.3233/bir-1987-24404. [DOI] [PubMed] [Google Scholar]
- 48.Stitik T.P. Levy J.A. Viscosupplementation (biosupplementation) for osteoarthritis. Am J Phys Med Rehabil. 2006;85(Suppl):S32. doi: 10.1097/01.phm.0000245677.20294.c2. [DOI] [PubMed] [Google Scholar]
- 49.Tanner R.I. An alternative mechanism for lubrication of synovial joints. Phys Med Biol. 1966;11:119. doi: 10.1088/0031-9155/11/1/312. [DOI] [PubMed] [Google Scholar]
- 50.Schumacher B.L. Block J.A. Schmid T.M. Aydelotte M.B. Kuettner K.E. A novel proteoglycan synthesized and secreted by chondrocytes of the superficial zone of articular cartilage. Arch Biochem Biophys. 1994;311:144. doi: 10.1006/abbi.1994.1219. [DOI] [PubMed] [Google Scholar]
- 51.Ikegawa S. Sano M. Koshizuka Y. Nakamura Y. Isolation, characterization and mapping of the mouse and human PRG4 (proteoglycan 4) genes. Cytogenet Cell Genet. 2000;90:291. doi: 10.1159/000056791. [DOI] [PubMed] [Google Scholar]
- 52.Flannery C.R. Hughes C.E. Schumacher B.L. Tudor D. Aydelotte M.B. Kuettner K.E. Caterson B. Articular cartilage superficial zone protein (SZP) is homologous to megakaryocyte stimulating factor precursor and is a multifunctional proteoglycan with potential growth-promoting, cytoprotective, and lubricating properties in cartilage metabolism. Biochem Biophys Res Commun. 1999;254:535. doi: 10.1006/bbrc.1998.0104. [DOI] [PubMed] [Google Scholar]
- 53.Schumacher B.L. Hughes C.E. Kuettner K.E. Caterson B. Aydelotte M.B. Immunodetection and partial cDNA sequence of the proteoglycan, superficial zone protein, synthesized by cells lining synovial joints. J Orthop Res. 1999;17:110. doi: 10.1002/jor.1100170117. [DOI] [PubMed] [Google Scholar]
- 54.Englert C. McGowan K.B. Klein T.J. Giurea A. Schumacher B.L. Sah R.L. Inhibition of integrative cartilage repair by proteoglycan 4 in synovial fluid. Arthritis Rheum. 2005;52:1091. doi: 10.1002/art.20986. [DOI] [PubMed] [Google Scholar]
- 55.Rhee D.K. Marcelino J. Baker M. Gong Y. Smits P. Lefebvre V. Jay G.D. Stewart M. Wang H. Warman M.L. Carpten J.D. The secreted glycoprotein lubricin protects cartilage surfaces and inhibits synovial cell overgrowth. J Clin Invest. 2005;115:622. doi: 10.1172/JCI200522263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Jüsten H.P. Grünewald E. Totzke G. Gouni-Berthold I. Sachinidis A. Wessinghage D. Vetter H. Schulze-Osthoff K. Ko Y. Differential gene expression in synovium of rheumatoid arthritis and osteoarthritis. Mol Cell Biol Res Commun. 2000;3:165. doi: 10.1006/mcbr.2000.0211. [DOI] [PubMed] [Google Scholar]
- 57.Hills B.A. Monds M.K. Enzymatic identification of the load-bearing boundary lubricant in the joint. Br J Rheumatol. 1998;37:137. doi: 10.1093/oxfordjournals.rheumatology.a031463. [DOI] [PubMed] [Google Scholar]
- 58.Jay G.D. Cha C.J. The effect of phospholipase digestion upon the boundary lubricating ability of synovial fluid. J Rheumatol. 1999;26:2454. [PubMed] [Google Scholar]
- 59.Benz M. Chen N. Israelachvili J. Lubrication and wear properties of grafted polyelectrolytes, hyaluronan and hylan, measured in the surface forces apparatus. J Biomed Mater Res A. 2004;71:6. doi: 10.1002/jbm.a.30123. [DOI] [PubMed] [Google Scholar]
- 60.Schmidt T.A. Gastelum N.S. Nguyen Q.T. Schumacher B.L. Sah R.L. Boundary lubrication of articular cartilage: role of synovial fluid constituents. Arthritis Rheum. 2007;56:882. doi: 10.1002/art.22446. [DOI] [PubMed] [Google Scholar]
- 61.Tadmor R. Chen N. Israelachvili J.N. Thin film rheology and lubricity of hyaluronic acid solutions at a normal physiological concentration. J Biomed Mater Res. 2002;61:514. doi: 10.1002/jbm.10215. [DOI] [PubMed] [Google Scholar]
- 62.Walker P.S. Dowson D. Longfield M.D. Wright V. “Boosted lubrication” in synovial joints by fluid entrapment and enrichment. Ann Rheum Dis. 1968;27:512. doi: 10.1136/ard.27.6.512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Wilkins J. Proteolytic destruction of synovial boundary lubrication. Nature. 1968;219:1050. doi: 10.1038/2191050a0. [DOI] [PubMed] [Google Scholar]
- 64.McCutchen C.W. The frictional properties of animal joints. Wear. 1962;5:1. [Google Scholar]
- 65.Mann R.W. Letter to the editor commenting on “hydrostatic pressurization and depletion of trapped lubricant pool during creep and sliding contact of a rippled indenter against a biphasic articular cartilage layer.”. J Biomech Eng. 2004;126(538) author reply 539. [PubMed] [Google Scholar]
- 66.McCutchen C.W. Comment on “Hydrostatic pressurization and depletion of trapped lubricant pool during creep contact of a rippled indenter against a biphasic articular cartilage layer.”. J Biomech Eng. 2004;126 536; author reply 537. [PubMed] [Google Scholar]
- 67.Ateshian G.A. A theoretical formulation for boundary friction in articular cartilage. J Biomech Eng. 1997;119:81. doi: 10.1115/1.2796069. [DOI] [PubMed] [Google Scholar]
- 68.Ateshian G.A. Mow V.C. Friction, lubrication, and wear of articular cartilage and diarthrodial joints. In: Mow V.C., editor; Huiskes R., editor. Basic Orthopaedic Biomechanics and Mechano-Biology. Philadelphia, PA: Lippincott Williams & Wilkins; 2005. pp. 447–494. [Google Scholar]
- 69.Ateshian G.A. Wang H. Lai W.M. The role of interstitial fluid pressurization and surface porosities on the boundary friction of articular cartilage. J Tribol. 1998;120:241. [Google Scholar]
- 70.Torzilli P.A. Grigiene R. Continuous cyclic load reduces proteoglycan release from articular cartilage. Osteoarthritis Cartilage. 1998;6:260. doi: 10.1053/joca.1998.0119. [DOI] [PubMed] [Google Scholar]
- 71.Kim Y.J. Sah R.L. Grodzinsky A.J. Plaas A.H. Sandy J.D. Mechanical regulation of cartilage biosynthetic behavior: physical stimuli. Arch Biochem Biophys. 1994;311:1. doi: 10.1006/abbi.1994.1201. [DOI] [PubMed] [Google Scholar]
- 72.Grad S. Lee C.R. Gorna K. Gogolewski S. Wimmer M.A. Alini M. Surface motion upregulates superficial zone protein and hyaluronan production in chondrocyte-seeded three-dimensional scaffolds. Tissue Eng. 2005;11:249. doi: 10.1089/ten.2005.11.249. [DOI] [PubMed] [Google Scholar]
- 73.Nugent G.E. Aneloski N.M. Schmidt T.A. Schumacher B.L. Voegtline M.S. Sah R.L. Dynamic shear stimulation of bovine cartilage biosynthesis of proteoglycan 4. Arthritis Rheum. 2006;54:1888. doi: 10.1002/art.21831. [DOI] [PubMed] [Google Scholar]
- 74.Neu C.P. Khalafi A. Komvopoulos K. Schmid T. Reddi A.H. Mechanotransduction of bovine articular cartilage superficial zone protein by transforming growth factor β signaling. Arthritis Rheum. 2007;56:3706. doi: 10.1002/art.23024. [DOI] [PubMed] [Google Scholar]
- 75.Schmid T. Homandberg G. Madsen L. Su J. Kuettner K. Superficial zone protein (SZP) binds to macromolecules in the lamina splendens of articular cartilage. 51st Annual Meeting of the Orthopaedic Research Society; Dallas, TX. 2002. p. 81. [Google Scholar]
- 76.Lodish H. Berk A. Zipursky S.L. Matsudaira P. Baltimore D. Darnell J.E. Molecular Cell Biology. New York: W.H. Freeman and Company; 2000. [Google Scholar]
- 77.Reddi A.H. Introduction. In: Hascall V.C., editor; Kuettner K.E., editor. The Many Faces of Osteoarthritis. Basel, Switzerland: Birkhauser Verlag; 2002. pp. 63–66. [Google Scholar]
- 78.Flechtenmacher J. Huch K. Thonar E.J.-M.A. Mollenhauer J.A. Davies S.R. Schmid T.M. Puhl W. Sampath T.K. Aydelotte M.B. Kuettner K.E. Recombinant human osteogenic protein 1 is a potent stimulator of the synthesis of cartilage proteoglycans and collagens by human articular chondrocytes. Arthritis Rheum. 1996;39:1896. doi: 10.1002/art.1780391117. [DOI] [PubMed] [Google Scholar]
- 79.Hidaka C. Goodrich L.R. Chen C.T. Warren R.F. Crystal R.G. Nixon A.J. Acceleration of cartilage repair by genetically modified chondrocytes over expressing bone morphogenetic protein-7. J Orthop Res. 2003;21:573. doi: 10.1016/S0736-0266(02)00264-4. [DOI] [PubMed] [Google Scholar]
- 80.Chubinskaya S. Merrihew C. Cs-Szabo G. Mollenhauer J. McCartney J. Rueger D.C. Kuettner K.E. Human articular chondrocytes express osteogenic protein-1. J Histochem Cytochem. 2000;48:239. doi: 10.1177/002215540004800209. [DOI] [PubMed] [Google Scholar]
- 81.Yaeger P.C. Masi T.L. Buck de Ortiz J.L. Binette F. Tubo R. McPherson J.M. Synergistic action of transforming growth factor-β and insulin-like growth factor-I induces expression of type II collagen and aggrecan genes in adult human articular chondrocytes. Exp Cell Res. 1997;237:318. doi: 10.1006/excr.1997.3781. [DOI] [PubMed] [Google Scholar]
- 82.Loeser R.F. Pacione C.A. Chubinskaya S. The combination of insulin-like growth factor 1 and osteogenic protein 1 promotes increased survival of and matrix synthesis by normal and osteoarthritic human articular chondrocytes. Arthritis Rheum. 2003;48:2188. doi: 10.1002/art.11209. [DOI] [PubMed] [Google Scholar]
- 83.O'Connor W.J. Botti T. Khan S.N. Lane J.M. The use of growth factors in cartilage repair. Orthop Clin North Am. 2000;31:399. doi: 10.1016/s0030-5898(05)70159-0. [DOI] [PubMed] [Google Scholar]
- 84.ten Dijke P. Yamashita H. Sampath T.K. Reddi A.H. Estevez M. Riddle D.L. Ichijo H. Heldin C.H. Miyazono K. Identification of type I receptors for osteogenic protein-1 and bone morphogenetic protein-4. J Biol Chem. 1994;269:16985. [PubMed] [Google Scholar]
- 85.Reddi A.H. Cartilage-derived morphogenetic proteins and cartilage morphogenesis. Microsc Res Tech. 1998;43:131. doi: 10.1002/(SICI)1097-0029(19981015)43:2<131::AID-JEMT6>3.0.CO;2-C. [DOI] [PubMed] [Google Scholar]
- 86.Re'em-Kalma Y. Lamb T. Frank D. Competition between noggin and bone morphogenetic protein 4 activities may regulate dorsalization during Xenopus development. Proc Natl Acad Sci USA. 1995;92:12141. doi: 10.1073/pnas.92.26.12141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Wijgerde M. Karp S. McMahon J. McMahon A.P. Noggin antagonism of BMP4 signaling controls development of the axial skeleton in the mouse. Dev Biol. 2005;286:149. doi: 10.1016/j.ydbio.2005.07.016. [DOI] [PubMed] [Google Scholar]
- 88.Khalafi A. Schmid T.M. Neu C. Reddi A.H. Increased accumulation of superficial zone protein (SZP) in articular cartilage in response to bone morphogenetic protein-7 and growth factors. J Orthop Res. 2007;25:293. doi: 10.1002/jor.20329. [DOI] [PubMed] [Google Scholar]
- 89.Niikura T. Reddi A.H. Differential regulation of lubricin/superficial zone protein by transforming growth factor beta/bone morphogenetic protein superfamily members in articular chondrocytes and synoviocytes. Arthritis Rheum. 2007;56:2312. doi: 10.1002/art.22659. [DOI] [PubMed] [Google Scholar]
- 90.Schmidt T.A. Schumacher B.L. Han E.H. Klein T.J. Voegtline M.S. Sah R.L. Synthesis and secretion of lubricin/superficial zone protein by chondrocytes in cartilage explants: modulation by TGF-β1 and Il-1α. 50th Annual Meeting of the Orthopaedic Research Society; San Francisco, CA. 2004. p. 900. [Google Scholar]
- 91.Moos V. Fickert S. Müller B. Weber U. Sieper J. Immunohistological analysis of cytokine expression in human osteoarthritic and healthy cartilage. J Rheumatol. 1999;26:870. [PubMed] [Google Scholar]
- 92.Tetlow L.C. Adlam D.J. Woolley D.E. Matrix metalloproteinase and proinflammatory cytokine production by chondrocytes of human osteoarthritic cartilage: associations with degenerative changes. Arthritis Rheum. 2001;44:585. doi: 10.1002/1529-0131(200103)44:3<585::AID-ANR107>3.0.CO;2-C. [DOI] [PubMed] [Google Scholar]
- 93.Boyce S.T. Regulatory issues and standardization. In: Atala A., editor; Lanza R.P., editor. Methods of Tissue Engineering. San Diego, CA: Academic Press; 2002. pp. 3–17. [Google Scholar]
- 94.Brittberg M. Lindahl A. Nilsson A. Ohlsson C. Isaksson O. Peterson L. Treatment of deep cartilage defects in the knee with autologous chondrocyte transplantation. N Engl J Med. 1994;331:889. doi: 10.1056/NEJM199410063311401. [DOI] [PubMed] [Google Scholar]
- 95.Breinan H.A. Minas T. Hsu H.P. Nehrer S. Sledge C.B. Spector M. Effect of cultured autologous chondrocytes on repair of chondral defects in a canine model. J Bone Joint Surg Am. 1997;79:1439. doi: 10.2106/00004623-199710000-00001. [DOI] [PubMed] [Google Scholar]
- 96.Peterson L. Minas T. Brittberg M. Nilsson A. Sjögren-Jansson E. Lindahl A. Two- to 9-year outcome after autologous chondrocyte transplantation of the knee. Clin Orthop Relat Res. 2000;374:212. doi: 10.1097/00003086-200005000-00020. [DOI] [PubMed] [Google Scholar]
- 97.Hollister S.J. Porous scaffold design for tissue engineering. Nat Mater. 2005;4:518. doi: 10.1038/nmat1421. [DOI] [PubMed] [Google Scholar]
- 98.Morita Y. Tomita N. Aoki H. Sonobe M. Wakitani S. Tamada Y. Suguro T. Ikeuchi K. Frictional properties of regenerated cartilage in vitro. J Biomech. 2006;39:103. doi: 10.1016/j.jbiomech.2004.10.031. [DOI] [PubMed] [Google Scholar]
- 99.Klein T.J. Schumacher B.L. Schmidt T.A. Li K.W. Voegtline M.S. Masuda K. Thonar E.J.-M.A. Sah R.L. Tissue engineering of stratified articular cartilage from chondrocyte subpopulations. Osteoarthritis Cartilage. 2003;11:595. doi: 10.1016/s1063-4584(03)00090-6. [DOI] [PubMed] [Google Scholar]
- 100.Kinner B. Spector M. Cell-based therapies for the treatment of articular cartilage injury. In: Atala A., editor; Lanza R.P., editor. Methods of Tissue Engineering. San Diego, CA: Academic Press; 2002. pp. 1059–1073. [Google Scholar]
- 101.Mauck R.L. Seyhan S.L. Ateshian G.A. Hung C.T. Influence of seeding density and dynamic deformational loading on the developing structure/function relationships of chondrocyte-seeded agarose hydrogels. Ann Biomed Eng. 2002;30:1046. doi: 10.1114/1.1512676. [DOI] [PubMed] [Google Scholar]
- 102.Sittinger M. Bujia J. Rotter N. Reitzel D. Minuth W.W. Burmester G.R. Tissue engineering and autologous transplant formation: practical approaches with resorbable biomaterials and new cell culture techniques. Biomaterials. 1996;17:237. doi: 10.1016/0142-9612(96)85561-x. [DOI] [PubMed] [Google Scholar]
- 103.Quinn T.M. Hunziker E.B. Controlled enzymatic matrix degradation for integrative cartilage repair: effects on viable cell density and proteoglycan deposition. Tissue Eng. 2002;8:799. doi: 10.1089/10763270260424150. [DOI] [PubMed] [Google Scholar]
- 104.Komistek R.D. Stiehl J.B. Dennis D.A. Paxson R.D. Soutas-Little R.W. Mathematical model of the lower extremity joint reaction forces using Kane's method of dynamics. J Biomech. 1997;31:185. doi: 10.1016/s0021-9290(97)00128-0. [DOI] [PubMed] [Google Scholar]
- 105.Winter D.A. Biomechanics and Motor Control of Human Movement. New York: John Wiley & Sons, Inc.; 1990. [Google Scholar]
- 106.Dowson D. Higginson G.R. Elastohydrodynamic Lubrication. New York: Pergamon Press, Inc.; 1977. [Google Scholar]
- 107.Lee S.J. Aadalen K.J. Malaviya P. Lorenz E.P. Hayden J.K. Farr J. Kang R.W. Cole B.J. Tibiofemoral contact mechanics after serial medial meniscectomies in the human cadaveric knee. Am J Sports Med. 2006;34:1334. doi: 10.1177/0363546506286786. [DOI] [PubMed] [Google Scholar]