Abstract
Six morphine-dependent and two control macaques were infected in a SIV/SHIV non-human primate model of AIDS. Three animals in the morphine group rapidly developed clinical disease and died within the timeframe of this study. The sequence evolution of nef in plasma virus was assessed at 4, 12 and 20 weeks post infection. Cloned sequences were compared phylogenetically against each other as well as against the inoculum virus clones to determine the effect of morphine and rate of disease progression on diversity and divergence, respectively. Unlike our earlier studies of tat and env, nef evolution was not affected by morphine abuse or by rapid disease progression. The results suggest that although the evolution of other loci are inversely correlated to the onset and rate of clinical disease, differential evolution of nef is related neither to drug abuse nor rapid progression within the first twenty weeks of infection.
HIV-1 infection and the progression to AIDS can be altered by use of illegal drugs. Drug abuse itself portends a variety of risk behaviors that assist in the spread of infection, yet there are also cellular and biochemical changes caused by illicit drugs that may contribute to viral pathogenesis. Opioids, including heroin and morphine represent important drugs of abuse that have controversial effects on HIV or SIV infection and progression to AIDS. Some have reported enhanced pathogenesis and others have found a possible survival advantage of morphine use1-4. However, at the cellular level, opiates have been reported to increase viral replication rate, rate of infection of monocytes, upregulate chemokines and receptors, and upregulate viral gene expression1;2;5;6 A non-human primate model of HIV/AIDS in the setting of morphine addiction and AIDS has already been shown to be a useful alternative to more time consuming and variable studies in humans. Our macaque model of drug addiction and AIDS reinforces the studies that morphine abuse exacerbates both viral replication and disease progression2. Among morphine addicted monkeys, 50% progressed rapidly to AIDS and died within 20 weeks of infection. These animals showed significantly higher viral loads, lower CD4+ T cell counts and two thirds developed neuroAIDS2;7. The remaining half of the morphine addicted animals showed a normal rate of disease progression although they also had higher viral loads and lower CD4+ counts as compared to non-morphine addicted, normal progressor control macaques2.
In an effort to understand the relationship between morphine, disease progression and evolution of the virus, we have already studied the sequence evolution of tat and env. Rapid progression in the setting of morphine addiction was inversely correlated with viral evolution at these loci in plasma 8;9 and in cerebrospinal fluid10. Further, both morphine addiction and disease progression appeared to alter the nature of the mutations that occurred in tat clones isolated from the cerebrospinal fluid10. The lentiviral Nef protein is also widely reported to influence disease progression11;12. In addition, defects in Nef have been associated with reduced pathogenesis in both HIV and SIV13-15. However, the role of Nef in rapid disease progression in the setting of morphine abuse has not been studied. To fill this void and on the basis of the well known pathogenic role of Nef during progression to AIDS, we extended this analysis to the nef gene in our model.
Eight male Indian rhesus macaques were included in this study, six as part of a morphine-addicted cohort (three rapid progressors, Group A; three normal progressors, Group B) and two in a control, non-morphine cohort (Group C). The addiction protocol and virus infection were described previously2. Blood was drawn at 4, 12 and 20 weeks post infection for isolation of viral RNA. We amplified the nef gene using the One-Step RT-PCR kit (Qiagen, Valencia, CA) according to the manufacturer's instructions including Q solution with the following primers: Forward: 5′-CAGAGGATTCGAGAAGTCCTCAG,Reverse: 5′-ATCCCCTTGTGGAAAGTCCC. The forward primer was specific for SIV17E-Fr, position 9031-9053, while the reverse primer was common to all three virus in the inoculum (SIV17E-Fr, SHIV89.6P and SHIVKU-1B). Amplification of each of the three viral inoculum clones from lab stocks showed that only SIV17E-Fr produced a product using these semi-specific primers (data not shown). RT-PCR conditions were:50°C, 30 min; 95°C, 15 min; 35 cycles of 95°C, 10 sec; 55°C, 30 sec; 72°C, 1 min. Products were cloned and sequenced as reported previously8. Raw sequence data was processed and aligned using BioEdit16 and then subject to phylogenetic analysis, including tree construction, divergence and diversity calculations using Mega, v.3.017. Finally, sequences were translated to amino acids using BioEdit.
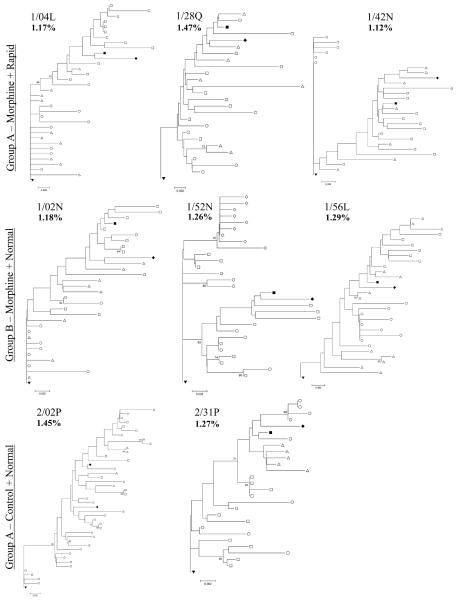
Phylogenetic analysis of nef indicates that neither morphine nor rapid progression correlate with different evolution within the span of 20 weeks, Figure 1. This pattern stands in distinct contrast to tat and env, where the complexity of the trees was notably less in Group A animals 8-10. The SIV17E-Fr (solid, inverted triangle, root of tree) and SHIV inoculum clones (SHIVKU-1B and SHIV89.6P, solid diamond and solid square, respectively) were included in the tree. Of note, among nef clones, very few are identical to the SIV17E-Fr inoculum and no clones are identical to either of the SHIV virus used for the infection, whereas with the Tat study 40-50% of clones from normal progressors and 60-70% of clones from the rapid progressors were identical to the starting SIV17E-Fr virus in both plasma and cerebrospinal fluid8;10. Thus the divergence of nef from the inoculum was not distinguished by morphine or rate of progression in 20 weeks, as we found earlier for both tat and env. Further, our PCR reaction was only partly biased in favor of SIV amplification, as the reverse primer was common to all nefs. A number of clones in each animal share similarity with the SHIVs and are likely a result of viral recombination which may occur readily in vivo.18 Any recombination between sequences near the 3′end of the SIV env and the 5′end of SHIV nef would render a template recognized in our assay. The presence of such apparently recombinant virus does not correlate with either morphine abuse (Groups A and B vs. Group C) or with rapid progression (Group A vs. Groups B and C).
Figure 1.
Each tree depicts the evolution of nef in a single macaque at four, twelve and twenty weeks (open circle, triangle and square, respectively) including the SIV17E-Fr (filled, inverted triangle) as a root and SHIVKU1B (filled diamond) and SHIV89.6P (filled square) as references. The three rapid progressor, morphine-dependent monkeys (1/04L, 1/28Q, and 1/42N) are included in Group A. These animals all died by the 20th week post infection. The three normal progressor, morphine-dependent macaques (1/02N, 1/52N, and 1/56L) are included in Group B. Finally, the normal progressor, non-morphine controls (2/02P and 2/31P) are included in Group C. Bootstrap values (150 repetitions) over 70 are provided at tree nodes. The average overall diversity is given as a percentage in bold under the monkey name in each tree. The scale bar in each tree represents 0.2% distance.
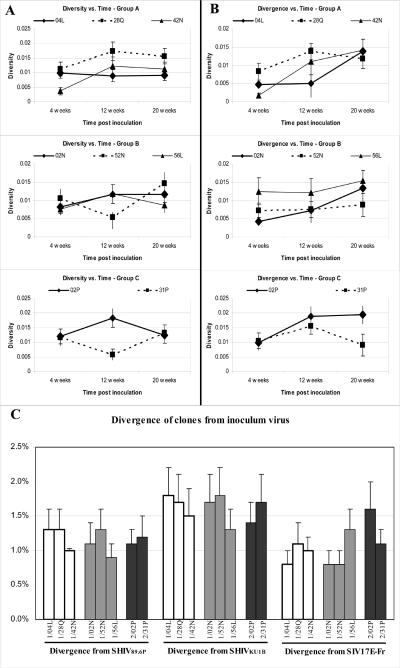
Previous examination of cloned tat sequences in plasma showed a general pattern of increasing diversity with time from 4 to 20 weeks in normal progressors and decreasing diversity with time during the same period in rapid progressors8. The nef clones all show a general trend of increasing diversity with time, regardless of morphine administration or rate of disease progression (Figure 2A). An examination of nef divergence from the SIV17E-Fr virus at 4, 12 and 20 weeks similarly fails to distinguish the three groups of macaques (Figure 2B). The analysis of total divergence (all time points together) from any of the three inoculum virus similarly failed to distinguish the groups (Figure 2C). In this case, however, it appears that SHUVKU1B virus experienced less recombination with the 3′end of the SIV env gene as the clones are less divergent from both the SIV17E-Fr and the SHIV89.6P virus.
Figure 2.
For each animal, all the sequenced clones for one time point (4, 12 or 20 weeks) were aligned (BioEdit) and a distance matrix was calculated (Mega, 3.0). The means of these distances for each time is plotted versus time for Group A − Morphine + Fast Progressors , Group B − Morphine + Normal Progressors, and Group C − Non-morphine + Normal Progressors in the top, middle and lower panels, respectively. A The overall diversity among all clones, not including the inoculum, is plotted against time post inoculation. B The average divergence of all clones from the SIV17E-Fr inoculum virus is graphed versus time. C The total divergence from each of the three infecting virus for all times points of the clones is plotted. Group A animals are graphed with solid white bars, Group B with gray bars and Group C with black bars. Individual animals and the inoculum virus used for divergence measurements are indicated below each bar.
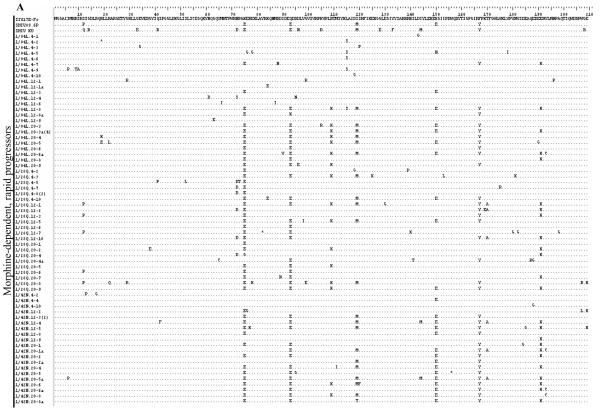
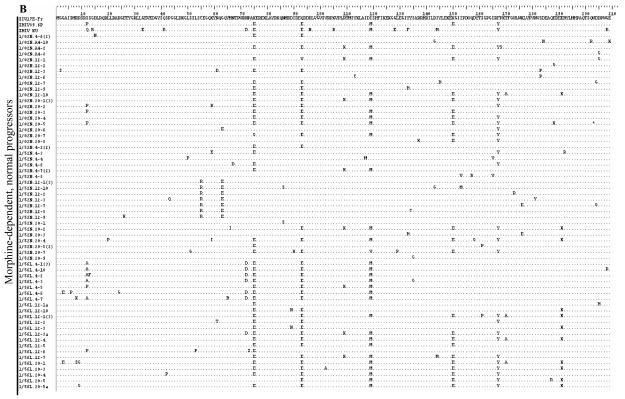
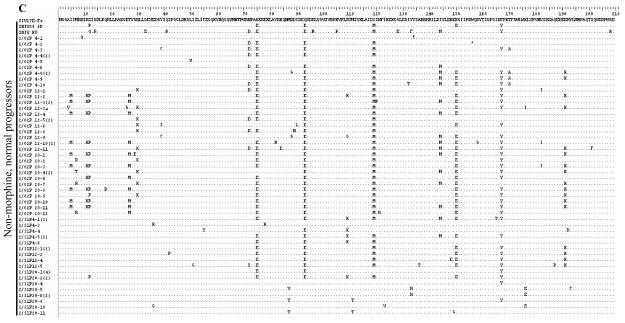
The deduced amino acid sequences for the first 209 amino acids of Nef are presented in Figure 3. In general, the most abundant changes from the reference sequence are common to monkeys in all three groups and appear to have resulted from recombination with SHIV. For example, positions E75 (glutamate at amino acid 75), E93, M120 and Y167 are common to many clones and both SHIVs. Glutamate at 150(E150) is also common to many clones and is present in the SHIV89.6P inoculum virus. The prevalence of other altered amino acids overall is roughly equal to all three groups. Interestingly, the rapid progressors appear to have a lower density of changes in the first 70 amino acids, followed by the morphine-dependent, normal progressors and then the control macaques. These changes do not appear to have resulted from recombination as neither of the SHIVs have the same changes. In addition, the control macaques, but not morphine-dependent animals, show a high frequency of methionine (as in SHIVKU-1B) in place of isoleucine at position 144, which is part of a reported MHC I-restricted CTL epitope19 and may indicate the presence of different selective environments based on the presence or absence of morphine.
Figure 3.
The nef coding sequences were translated to amino acids using BioEdit. Only sequences with nucleotide changes causing an amino acid substitution (non-synonymous change) are included in each group. The SIV17E-Fr sequence serves as the reference and the SHIV sequences are included to show differences from the SIV. Each sequence is represented by animal week-clone in the left column and all changes from 17E are indicated in the right column. Positions of identity are indicated by a dot (.) and stop codons by an asterisk (*). A Group A, B Group C, C Group C.
This is the first report examining the role of the evolution of SIV Nef in disease progression and morphine abuse in a macaque model of AIDS. The results stand in stark contrast to the pattern evident with tat in the blood or cerebrospinal fluid, or with env in the blood, where a significant inverse correlation was found between disease progression and sequence evolution. This lack of a discernable role for nef evolution is surprising in light of the widely reported involvement of Nef during disease progression and pathogenesis; however, it is conceivable that Nef is not a meaningful component of rapid disease progression associated with abuse of morphine. It will be interesting to follow the evolution of nef over a longer duration as it remains possible that a greater pathogenic role exists during prolonged disease.
ACKNOWLEDGEMENTS
The authors would like to Ms. Viani Ramirez for technical support during the early phase of this project. This work was supported by National Institute on Drug Abuse (DA015013) and National Center for Research Resources (G12RR003050). Clones used in this study can be found in Genebank under the accession numbers: AY033146, AY006474, AF038398, and DQ345966-DQ346194.
Reference List
- 1.Chuang RY, Suzuki S, Chuang TK, Miyagi T, Chuang LF, Doi RH. Opioids and the progression of simian AIDS. Front Biosci. 2005;10:1666–77. doi: 10.2741/1651. 1666-1677. [DOI] [PubMed] [Google Scholar]
- 2.Kumar R, Torres C, Yamamura Y, Rodriguez I, Martinez M, Staprans S, et al. Modulation by morphine of viral set point in rhesus macaques infected with simian immunodeficiency virus and simian-human immunodeficiency virus. J Virol. 2004;78(20):11425–11428. doi: 10.1128/JVI.78.20.11425-11428.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Donahoe RM. Multiple ways that drug abuse might influence AIDS progression: clues from a monkey model. J Neuroimmunol. 2004;147(12):28–32. doi: 10.1016/j.jneuroim.2003.10.011. [DOI] [PubMed] [Google Scholar]
- 4.Kapadia F, Vlahov D, Donahoe RM, Friedland G. The role of substance abuse in HIV disease progression: reconciling differences from laboratory and epidemiologic investigations. Clin Infect Dis. 2005;41(7):1027–1034. doi: 10.1086/433175. [DOI] [PubMed] [Google Scholar]
- 5.Li Y, Merrill JD, Mooney K, Song L, Wang X, Guo CJ, et al. Morphine enhances HIV infection of neonatal macrophages. Pediatr Res. 2003;54(2):282–288. doi: 10.1203/01.PDR.0000074973.83826.4C. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Steele AD, Henderson EE, Rogers TJ. Mu-opioid modulation of HIV-1 coreceptor expression and HIV-1 replication. Virology. 2003;309(1):99–107. doi: 10.1016/s0042-6822(03)00015-1. [DOI] [PubMed] [Google Scholar]
- 7.Kumar R, Tirado G, Rodriguez N, Staprans S, Yamamura Y, Kumar A. Morphine Addiction Causes Pronounced Virus Replication in Cerebral Compartment and Accelerated Onset of AIDS in SIV/SHIV-infected Indian Rhesus Macaques. 2006 doi: 10.1016/j.virol.2006.06.020. submitted. [DOI] [PubMed] [Google Scholar]
- 8.Noel RJ, Kumar A. Virus Replication and Disease Progression Inversely Correlate with SIVtat Evolution in Morphine-Dependent and SIV/SHIV-infected Indian Rhesus Macaques. Virology. 2006 doi: 10.1016/j.virol.2005.10.026. in press. [DOI] [PubMed] [Google Scholar]
- 9.Tirado G, Kumar A. Evolution of SIV Envelope in Morphine-dependent Rhesus Macaques with Rapid Disease Progression. AIDS Research and Human Retroviruses. 2006 doi: 10.1089/aid.2006.22.114. in press. [DOI] [PubMed] [Google Scholar]
- 10.Noel RJ, Marrero-Otero Z, Kumar R, Chompre GB, Yamamura Y, Kumar A. Correlation between SIV Tat Evolution and AIDS progression in Cerebrispinal Fluid of Morphine-dependent and Control Macaques Infected with SIV and SHIV. 2006 doi: 10.1016/j.virol.2006.03.032. submitted. [DOI] [PubMed] [Google Scholar]
- 11.Overholser ED, Coleman GD, Bennett JL, Casaday RJ, Zink MC, Barber SA, et al. Expression of simian immunodeficiency virus (SIV) nef in astrocytes during acute and terminal infection and requirement of nef for optimal replication of neurovirulent SIV in vitro. J Virol. 2003;77(12):6855–6866. doi: 10.1128/JVI.77.12.6855-6866.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Geyer M, Fackler OT, Peterlin BM. Structure--function relationships in HIV-1 Nef. EMBO Rep. 2001;2(7):580–585. doi: 10.1093/embo-reports/kve141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Kirchhoff F, Greenough TC, Brettler DB, Sullivan JL, Desrosiers RC. Brief report: absence of intact nef sequences in a long-term survivor with nonprogressive HIV-1 infection. N Engl J Med. 1995;332(4):228–232. doi: 10.1056/NEJM199501263320405. [DOI] [PubMed] [Google Scholar]
- 14.Iafrate AJ, Carl S, Bronson S, Stahl-Hennig C, Swigut T, Skowronski J, et al. Disrupting surfaces of nef required for downregulation of CD4 and for enhancement of virion infectivity attenuates simian immunodeficiency virus replication in vivo. J Virol. 2000;74(21):9836–9844. doi: 10.1128/jvi.74.21.9836-9844.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Deacon NJ, Tsykin A, Solomon A, Smith K, Ludford-Menting M, Hooker DJ, et al. Genomic structure of an attenuated quasi species of HIV-1 from a blood transfusion donor and recipients. Science. 1995;270(5238):988–991. doi: 10.1126/science.270.5238.988. [DOI] [PubMed] [Google Scholar]
- 16.Hall TA. BioEdit: a user-friendly biological sequence alignment editor and analysis program for Windows95/98/NT. Nucleic Acids Symp. 1999;41:95–98. [Google Scholar]
- 17.Kumar S, Tamura K, Nei M. MEGA3: Integrated software for Molecular Evolutionary Genetics Analysis and sequence alignment. Brief Bioinform. 2004;5(2):150–163. doi: 10.1093/bib/5.2.150. [DOI] [PubMed] [Google Scholar]
- 18.Bocharov G, Ford NJ, Edwards J, Breinig T, Wain-Hobson S, Meyerhans A. A genetic-algorithm approach to simulating human immunodeficiency virus evolution reveals the strong impact of multiply infected cells and recombination. J Gen Virol. 2005;86(Pt 11):3109–3118. doi: 10.1099/vir.0.81138-0. [DOI] [PubMed] [Google Scholar]
- 19.Evans DT, O'Connor DH, Jing P, Dzuris JL, Sidney J, da Silva J, et al. Virus-specific cytotoxic T-lymphocyte responses select for amino-acid variation in simian immunodeficiency virus Env and Nef. Nat Med. 1999;5(11):1270–1276. doi: 10.1038/15224. [DOI] [PubMed] [Google Scholar]