Abstract
CD4+ T cell dysfunction in subjects with chronic HIV infection is in part due to an imbalance of costimulatory and coinhibitory receptors. We report that virus-specific CD4+ T cells expressing 4-1BB (CD137) or OX40 (CD134) produced more IL-2 than cells lacking these costimulatory receptors (P<0.05) and that 4-1BB was expressed at a lower level on HIV− than CMV-specific IFN-γ and IL-2 producing CD4+ T cells (P<0.0001 and P<0.01, respectively). Suppression of viral replication with antiretroviral therapy was associated with increased 4-1BB expression on HIV− and CMV-specific IL-2 producing CD4+ T cells (P<0.05 and P<0.01, respectively) and the percentage of IL-2 producing HIV-specific CD4+ T cells that expressed 4-1BB was inversely correlated with HIV plasma viral load (r=−0.75, P=0.007). These findings indicate that the loss of 4-1BB on HIV-specific CD4+ T cells is associated with viral replication and that it may contribute to reduced IL-2 production observed during chronic infection.
Keywords: 4-1BB expression, OX40 expression, HIV-specific CD4+ T cells, CMV-specific CD4+ T cells
INTRODUCTION
The inability of the immune system to control HIV replication has been associated with HIV-specific CD4+ T cell dysfunction [1–4] which is in part due to an imbalance of costimulatory and coinhibitory receptor expression [5–9]. We and others have previously shown that HIV-specific CD4+ T cells from subjects with untreated chronic infection are skewed towards poorly proliferating, IFN-γ producing effector memory cells [10, 11] that express high levels of the inhibitory receptors PD-1 and CTLA-4 [6, 8]. Blockade of the PD-1 pathway enhances HIV-specific CD8+ T cell function [7, 8, 12] and correlates with increased control of viral replication and decreased disease progression in the LCMV mouse model of chronic viral infection [13]. While coinhibitory receptors clearly contribute to the loss of virus-specific CD4+ T cell function [6–8], the role of costimulatory receptors in HIV disease progression remains largely unexplored.
CD28, which is constitutively expressed by naïve and central memory T cells, provides the costimulation (signal 2) required for initial activation following T cell receptor (TCR) recognition of the cognate antigen-MHC complex (signal 1). However, during chronic HIV infection expression of CD28 is decreased on virus-specific T cells [14–16]. Inducible costimulatory molecules, such as 4-1BB (CD137) and OX40 (CD134), can provide alternative signal 2 pathways when CD28 expression is decreased [17, 18]. 4-1BB and OX40, members of the tumour necrosis factor receptor (TNFR) super family which are expressed on activated CD4+ and CD8+ T cells, interact with their ligands, 4-1BBL (CD137L) and OX40L (CD134L), expressed on antigen presenting cells [19–21]. When engaged, they augment T cell proliferation, increase cytokine production, enhance cytolytic effector function, prevent activation-induced cell death, and thus, maintain protective T cell function during chronic anti-viral and anti-tumor responses[19, 22–25]. Vaccines targeting 4-1BB or OX40 enhance long-lasting antiviral cellular immune responses [26–29]. Moreover, 4-1BB and OX40 stimulation increases proliferation and cytokine production by HIV-specific CD8+ T cells [20, 30, 31]. However, the level of expression of these molecules on HIV-specific CD4+ T cells and their association with HIV disease progression has not been well described.
Recent studies have shown that HIV replication is associated with permutations of both positive and negative costimulatory molecules [5, 9] and that these changes contribute to HIV-specific CD4+ T cell dysfunction [6, 8]. In fact, an inverse correlation between costimulatory (CD28) and coinhibitory molecule (CTLA-4) expression has been identified in subjects with chronic HIV infection [32]. In this study we determined whether the expression of 4-1BB and OX40 is dysregulated and associated with the loss of antigen-specific CD4+ T cell function during chronic HIV infection. Using intracellular cytokine staining and multiparametric flow cytometry, we demonstrate that 4-1BB, but not OX40, expression is decreased on HIV-specific CD4+ T cells and that 4-1BB negative cells exhibit decreased IL-2 production. At the same time, we observed a negative correlation between 4-1BB expression and plasma viral load. Finally, we demonstrate that a greater percentage of virus-specific IL-2 producing CD4+ T cells from subjects receiving antiretroviral therapy (ART) express 4-1BB compared to untreated, chronically infected subjects. Thus, decreased expression of 4-1BB may contribute to the loss of HIV-specific CD4+ T cell function and disease progression in treatment naïve subjects with chronic HIV infection.
MATERIALS AND METHODS
Study population
Thirty-one HIV-1-infected subjects were enrolled into two clinical cohorts based on their treatment status: ART with virological suppression and untreated. Inclusion criteria for the suppressed cohort (n = 19) included receiving a combination of antiretroviral agents with suppression of plasma viral load to <20 copies of HIV-1 RNA per ml of plasma for ≥6 month (median CD4+ T cell count, 858 cells/µl; range, 252–1534 cells/µl). Untreated subjects (n = 12) were either treatment naive or off treatment for ≥6 months with a median viral load of 70856 copies HIV-1 RNA per ml of plasma (range, 11100–330,000 copies of HIV RNA per ml), and a median CD4+ T cell count of 450 cells/µl (range, 168–735 cells/µl). HIV-1-seronegative subjects (n = 10) were normal healthy adult volunteers. All study subjects participated voluntarily and gave informed consent. The study was approved by the University of Colorado Denver Institutional Review Board.
T cell stimulations for Ag-specific cytokine production
Blood was collected in Vacutainer tubes containing sodium heparin (BD Vacutainer). Within 4 hours of venipuncture, PBMC were isolated from whole blood by density gradient centrifugation on Ficoll (Amersham Biosciences). PBMCs (2.5–5 × 106) were resuspended in RPMI plus 10% human Ab serum and placed in 12- × 75-mm culture tubes. One µg/ml anti-CD28 and -CD49d mAbs (BD Biosciences) were added, and the cells were stimulated under the following conditions: pooled HIV-1 Gag 15mers (2.5 µg/ml final concentration of each peptide; clade B HXB2 strain HIV-1 (National Institutes of Health AIDS Research and Reference Reagent Program), CMV lysate (1/10 dilution, derived from a G-lung cell line infected with CMV strain AD169, virus titer 2 × 107 PFU/ml, provided by A. Weinberg, University of Colorado Denver), staphylococcal enterotoxin B (SEB, 1 µg/ml; Toxin Technologies), or medium alone. Cells were incubated at a 5-degree slant for a total of 24 hours at 37°C in a humidified 5% CO2 atmosphere with 1 µg/ml brefeldin A (BD Biosciences) added after 8 hours of stimulation. This length of stimulation was necessary to allow for the induction of 4-1BB expression on the CD4+ T cell surface (26).
Immunofluorescent staining of stimulated T cells
Stimulated PBMCs were washed and surfaced stained with anti-CD4 (PE-Cy5-5; BD Biosciences), anti-CD3 (PE Texas Red; Beckman Coulter) and anti-CD8 (Alexa Fluor 405; Caltag) for 30 min at 4°C. Cells were washed with PBS containing 1% BSA, fixed, permeabilized (Caltag), and stained with anti-IFN-γ (APC Alexa Flour 750; BD Biosciences), anti-IL-2 (APC; Caltag), anti-OX40 (CD134) (PE-Cy5; BD Biosciences), and anti-4-1BB (CD137) (PE; BD Biosciences) mAbs for 30 min at 4°C, washed, and fixed with 1% formaldehyde. Fluorescence−1 (FMO) controls were used in all staining.
Immunofluorescent staining of monocytes and dendritic cells
Six-parameter flow cytometry was employed to examine the expression of 4-1BB ligand (4-1BBL) and OX40 ligand (OX40L) on dendritic cells (DCs) and monocytes. DCs were identified using the following mAb panel: FITC-labeled anti-lineage mixture (CD3-CD14-CD16-CD19-CD20-CD56; BD Biosciences), APC-Cy7-labeled anti-HLA-DR (BD Biosciences), PECy5-labeled CD11c (BD Biosciences) and PerCP-Cy5-5-labeled CD123 (BD Biosciences). DC subsets were defined as follows: myeloid DCs (mDC) were lineage−HLA−DR+CD123−CD11c+ and plasmacytoid DCs (pDC) were lineage−HLA−DR+CD11c−CD123+. Pacific Blue CD14 (BD Biosciences) was used to identify CD14+ monocytes. Expression of 4-1BBL and OX40L was determined using PE-labeled 4-1BBL or OX40L (BD Biosciences). FMO controls were used in all staining.
Flow cytometry
Cells were analyzed using a FACSAria flow cytometer (BD Immunocytometry Systems). Between 1 and 3 million events were collected. Electronic compensation was performed with Ab capture beads (BD Biosciences) stained separately with individual mAbs used in the test samples. The data files were analyzed using Diva software (BD). Lymphocytes were gated by their forward and side scatter profile. CD3+ cells were selected and expression of CD4 was analyzed in a bivariate dot plot with CD8 to exclude CD4/CD8 double positive T cells. Biexponential scaling was used in all dot plots. The expression of 4-1BB and OX40 was examined on cytokine-producing cells with frequencies ≥0.04% above the background (media control tube) to ensure an adequate number of events for analysis as previously validated by our laboratory [6, 10, 11]. The cutoff value was calculated by stimulating seronegative controls with HIV Gag to determine the background response of the assay and by calculating the frequency needed to obtain a minimum of 100 cytokine positive events when 2.5×106 cells were analyzed on the flow cytometer. FMO controls were used to set the gates for determining the percentage of 4-1BB and OX-40 positive T cells. To ensure the accuracy and precision of the measurements taken from day to day quality control was performed on the FACSAria daily using the Cytometer Setup & Tracking (CS&T) feature within BD FACSDiva software. The program uses standardized CS&T beads (BD Biosciences) to determine voltages, laser delays, and area scaling and to track these settings over time. A manual quality control (QC) using rainbow beads is also performed daily to verify the laser delay and area scaling determined by CS&T.
Statistical analysis
Statistical analysis was performed using GraphPad-Prism (Graphpad, San Diego, CA). The Kruskal-Wallis test with Dunns post test was used to examine the variation of 4-1BB and OX40 expression on antigen-specific cytokine-producing CD4+ T cells. The Mann-Whitney U test or Wilcoxon’s matched pairs test was utilized to determine significance of differences between groups. Correlations were calculated using the nonparametric Spearman test. P values of <0.05 were considered statistically significant.
RESULTS
Expression of 4-1BB is decreased on HIV-specific CD4+ T cells
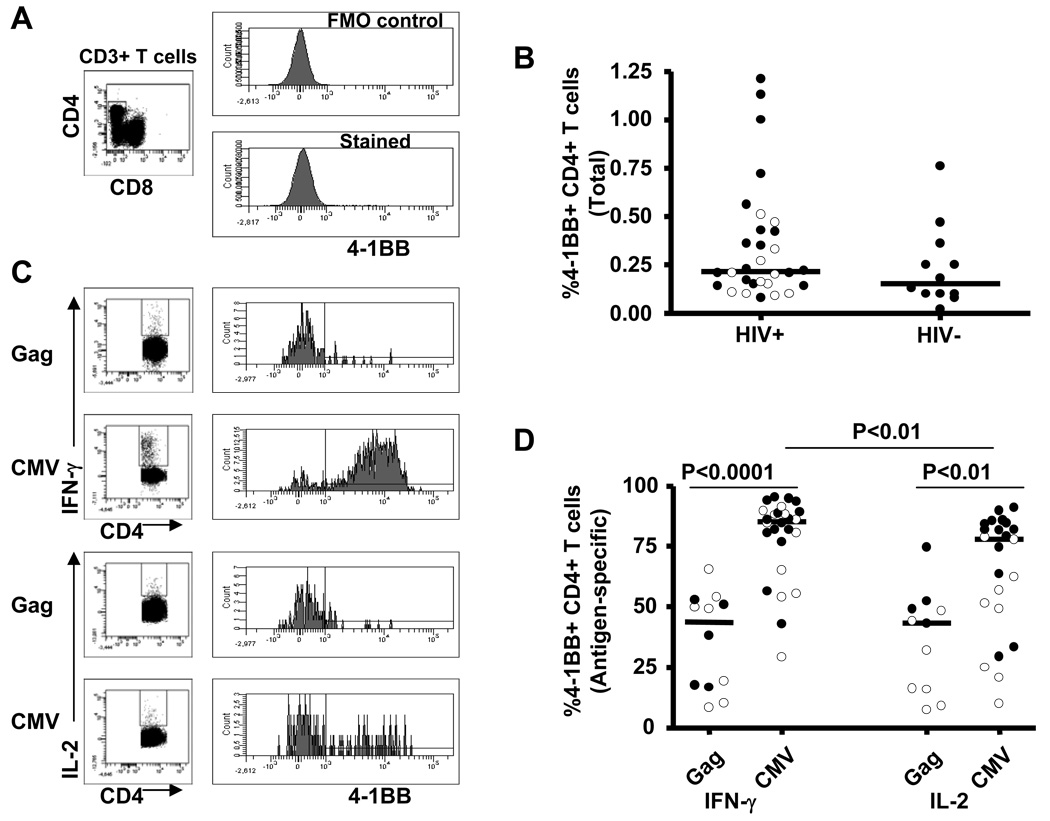
PBMCs obtained from 12 untreated HIV-infected viremic subjects, 19 ART-treated subjects, and 12 HIV seronegative subjects were examined for 4-1BB expression on CD4+ T cells using multiparametric flow cytomety. Figure 1A shows representative staining of 4-1BB on total CD4+ T cells. The percentage of 4-1BB expressing CD4+ T cells between HIV-infected (median; range, 0.22%; 0.08–1.21%) and HIV seronegative (0.16%; 0.02–0.76%) subjects was not significantly different (Figure 1B). In addition, expression of 4-1BB on total CD4+ T cells was not different between viremic and ART-suppressed HIV-infected subjects (Figure 1B, open circles and closed circles designate data from viremic and suppressed subjects, respectively). These findings follow the trend of previously published data [9].
Figure 1.
Expression of 4-1BB on total and antigen-specific CD4+ T cells. A, Representative plots illustrating staining of 4-1BB on total CD4+ T cells. B, 4-1BB expression on total CD4+ T cells from HIV-infected (n=31) and HIV seronegative controls (n=12). C, Representative plots illustrating staining of 4-1BB on antigen-specific IFN-γ and IL-2 producing CD4+ T cells. D, Expression of 4-1BB on Gag-specific and CMV-specific IFN-γ and IL-2 producing CD4+ T cells from HIV-infected subjects. Dots represent individual values and the solid lines indicate medians. Open and closed circles represent values from viremic and suppressed subjects, respectively. Statistical significance was determined using a Mann-Whitney U test.
Since 4-1BB is an inducible cell surface costimulatory molecule which is expressed maximally 24 hours after stimulation [9], we next stimulated PBMCs with either Gag or CMV for 24 hours and measured the proportion of IFN-γ or IL-2 producing CD4+ T cells expressing 4-1BB. Figure 1C illustrates representative plots of 4-1BB expression on antigen-specific cytokine producing CD4+ T cells. The percentage of 4-1BB expressing CD4+ T cells was significantly lower on both Gag-specific IFN-γ and IL-2 (43.74%; 8.42–65.49%, 43.18%; 7.26–74.55%, respectively) producing CD4+ T cells compared with CMV-specific IFN-γ and IL-2 (85.34%; 29.21–95.13%, P<0.0001, 77.75%; 9.92–91.02%, P<0.01, respectively) producing CD4+ T cells (Figure 1D). We also examined the effect of viral replication on 4-1BB expression by examining CD4+ T cells from subjects receiving successful ART. The percentage of 4-1BB expressing Gag-specific IL-2 producing CD4+ T cells was significantly lower in untreated viremic subjects (16.09%; 7.26–48.39%) compared to its level in subjects on ART with suppressed plasma viral load (50.68%; 43:18–74.55%, P<0.05). CMV-specific IL-2 producing CD4+ T cells from untreated subjects also expressed significantly lower levels of 4-1BB (51.39%; 9.92–78.64%) compared to the levels in ART treated subjects (81.88%; 29.56–91.02%, P<0.01) (Figure 1D). Taken together, the data in this cross-sectional study demonstrate that 4-1BB expression is lower on HIV-specific CD4+ T cells from untreated viremic subjects compared to those with ART-suppressed plasma viral load, suggesting there is an association between HIV replication and reduced expression of the 4-1BB receptor.
Expression of OX40 is higher on virus-specific IL-2 producing than IFN-γ producing CD4+ T cells
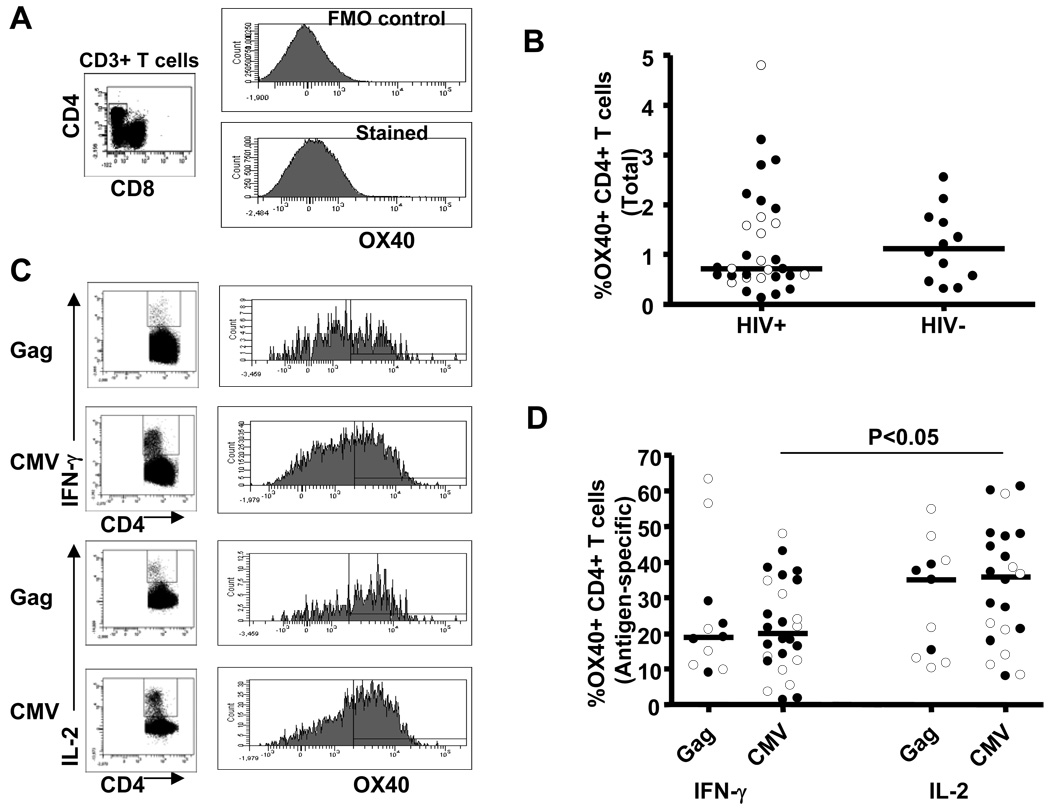
In a similar manner to 4-1BB, we also examined the expression of OX40 on unstimulated CD4+ T cells. Representative flow cytometric staining of OX40 on total CD4+ T cells is shown in Figure 2A. The percentage of OX40 expressing CD4+ T cells was not significantly different between HIV-infected (0.71%; 0.12–4.80%) and uninfected (1.12%; 0.30–2.55%) subjects (Figure 2B). Consistent with our observation for 4-1BB expression, OX40 expression on total CD4+ T cells was not different between viremic and suppressed HIV-infected subjects.
Figure 2.
Expression of OX40 on total and antigen-specific CD4+ T cells. A, Representative plots illustrating staining of OX40 on total CD4+ T cells. B, OX40 expression on total CD4+ T cells from HIV-infected (n=31) and HIV seronegative controls (n=12). C, Representative plots illustrating staining of OX40 on antigen-specific IFN-γ and IL-2 producing CD4+ T cells. D, Expression of OX40 on Gag-specific and CMV-specific IFN-γ and IL-2 producing CD4+ T cells from HIV-infected subjects. Dots represent individual values and the solid lines indicate medians. Open and closed circles represent values from viremic and suppressed subjects, respectively. Statistical significance was determined using a Mann-Whitney U test.
Given that OX40, like 4-1BB, is an inducible cell surface costimulatory molecule [9], we stimulated PBMCs with either Gag or CMV as described above and determined the proportion of antigen-specific IFN-γ and IL-2 producing CD4+ T cells that also express OX40. Figure 2C shows representative plots of OX40 on antigen-specific cytokine producing CD4+ T cells. The percentage of OX40 expressing CD4+ T cells was not significantly different between Gag- and CMV-specific IFN-γ producing or IL-2 producing CD4+ T cells (Figure 2D). However, when a subset of subjects which responded to both viruses were analyzed using a paired test, OX40 expression was significantly higher (P = 0.04) on Gag- than CMV-specific IL-2 producing CD4+ T cells (data not shown). Interestingly, the expression of OX40 was significantly higher on CMV-specific IL-2 producing CD4+ T cells (35.85%; 8.02–61.23%), compared with its level on CMV-specific IFN-γ producing CD4+ T cells (20.06%; 1.40–47.90%, P<0.05). Although the percentage of OX40 expressing Gag-specific IL-2 producing CD4+ T cells (35.0%; 10.28–54.84%) was higher than that in Gag-specific IFN-γ producing CD4+ T cells (18.99%; 9.14–63.33%), the difference did not achieve statistical significance (P=0.4) (Figure 2D). Increased expression of OX40 on virus-specific IL-2 producing CD4+ T cells suggests its role in T cell proliferation.
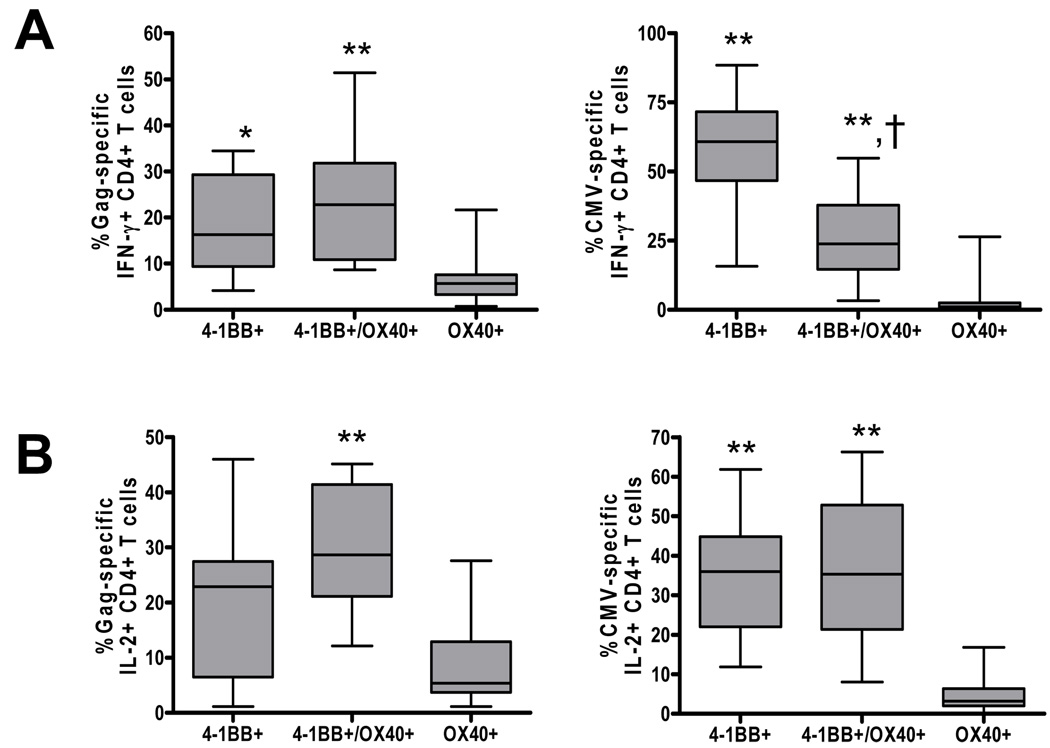
Expression of 4-1BB on antigen-specific CD4+ T cells is higher than OX40
In order to examine the relationship between the two costimulatory molecules, we determined the proportion of antigen-specific CD4+ T cells that express OX40 (OX40+/4-1BB−), 4-1BB (OX40−/4-1BB+) or both OX40 and 41BB (OX40+/4-1BB+). As shown in Figure 3A, the percentage of 4-1BB+ Gag- and CMV-specific IFN-γ producing CD4+ T cells was significantly higher than that of OX40+ Gag-specific (P<0.01) and CMV-specific (P<0.001) IFN-γ producing CD4+ T cells. Concomitantly, the percentage of double positive 4-1BB+/OX40+ Gag- and CMV-specific IFN-γ producing CD4+ T cells was significantly higher than that of single OX40+ Gag-specific (P<0.01) and CMV-specific (P<0.001) IFN-γ producing CD4+ T cells. Similar to IFN-γ, the proportion of OX40+ Gag- and CMV-specific IL-2 producing CD4+ T cells was also significantly lower than 41BB+/OX40+ Gag-specific (P<0.001) and CMV-specific (P<0.001) IL-2 producing CD4+ T cells (Figure 3B). There was no significant difference in the proportion of 4-1BB+ versus 4-1BB+/OX40+ expressing Gag- and CMV-specific IL-2 producing CD4+ T cells. When we analyzed the relationship between 4-1BB and OX40 on antigen-specific CD4+ T cells, we observed a strong significant positive correlation between the percentage of 4-1BB and OX40 expressing Gag-specific IFN-γ producing CD4+ T cells (r=0.74, P=0.009, data not shown). Taken together, the inducible expression of 4-1BB and OX40 on virus-specific CD4+ T cells, their dual expression on large proportion of Gag-specific CD4+ T cells, and their correlation to each others’ expression highlights the relatedness of these two costimulatory pathways.
Figure 3.
Comparison of 4-1BB and OX40 expression on antigen-specific CD4+ T cells from HIV-infected subjects. Box plots showing expression of 4-1BB (4-1BB+OX40−), 4-1BB/OX40 (4-1BB+OX40+) and OX40 (4-1BB−OX40+) on Gag-specific and CMV-specific IFN-γ (A) and IL-2 (B) producing CD4+ T cells. Statistical significance was determined by ANOVA. *P<0.01 compared with OX40+, **P<0.001 compared with OX40+, †P<0.01 compared with 4-1BB+
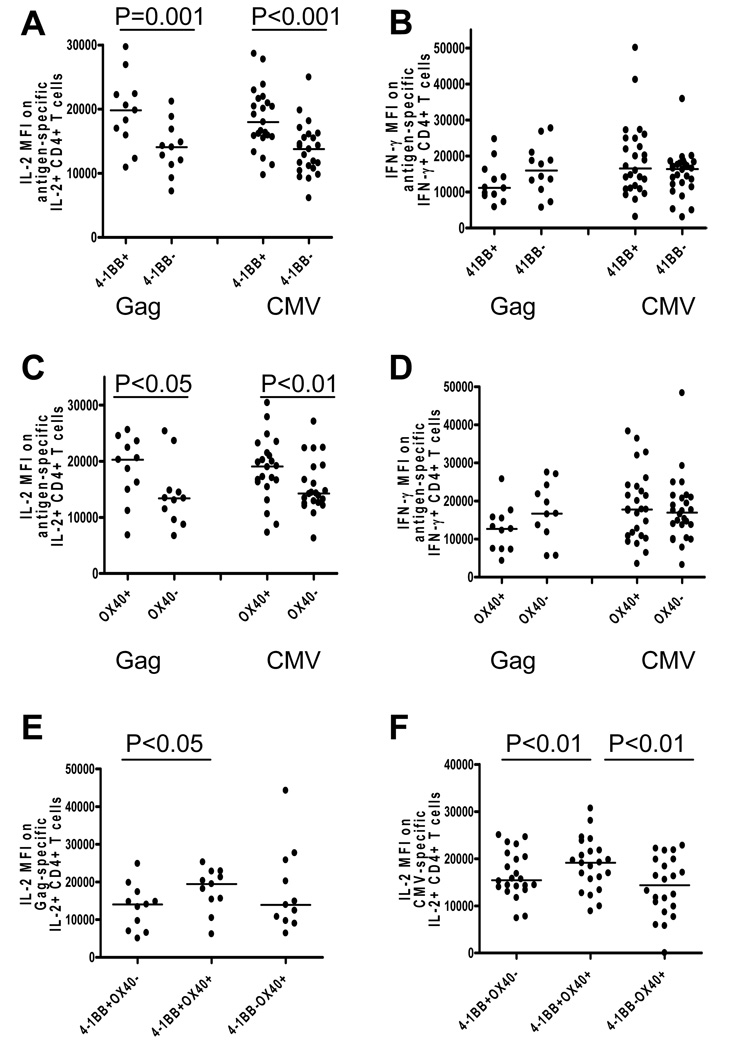
Expression of 4-1BB or OX40 is associated with increased amounts of IL-2
To determine if the expression of these costimulatory molecules correlated with CD4+ T cell function, we next examined the amount of IL-2 and IFN-γ, as determined by mean fluorescence intensity (MFI), on antigen-specific 4-1BB and OX40 expressing and nonexpressing CD4+ T cells. The MFI of each functional parameter within a given sample is related to the quantitative expression of that function on a per cell basis [33]. As a result, the relative amount of each cytokine per cell can be measured for each functional CD4+ T cell population. In addition, previous studies have shown that MFI measured by intracellular cytokine staining directly correlates with cytokine secretion as measured by ELISA [34]. As shown in Figure 4A, IL-2 production (MFI) in response to Gag stimulation was significantly higher in CD4+ T cells expressing 4-1BB (19811; 10905–29679) than 4-1BB negative CD4+ T cells (14060; 7181–21192, P=0.001). IL-2 production (MFI) in response to CMV antigens was also significantly higher in cells expressing 4-1BB (17976; 9742–28648) than 4-1BB negative CD4+ T cells (13768; 6126–24953, P<0.001). However, antigen-specific IFN-γ per cell was not significantly different between antigen-specific CD4+ T cells that expressed 4-1BB compared with those which did not express 4-1BB (Figure 4B). Similar to 4-1BB, IL-2 production (MFI) in response to Gag stimulation was significantly higher in cells expressing OX40 (20278; 6838–25602) compared to OX40 negative CD4+ T cells (13407; 6689–25355, P<0.05) (Figure 4C). In addition, IL-2 production (MFI) in response to CMV stimulation was significantly higher in cells expressing OX40 (19062; 7299–30403) than OX40 negative CD4+ T cells (14245; 6292–27084, P<0.01) (Figure 4C). As seen with 4-1BB, the amount of IFN-γ produced per cell was not significantly different when segregated by OX40 expression (Figure 4D). Given that expression of a single costimulatory molecule correlated with increased cytokine production, expression of multiple costimulatory molecules may be associated with even greater increase in cytokine production. To examine this possibility, we analyzed the amount of cytokine produced per cell in 4-1BB and OX40 single versus double positive antigen-specific CD4+ T cells. IL-2 production (MFI) in response to Gag- and CMV-specific stimulus was significantly higher in CD4+ T cells expressing both 4-1BB and OX40 than single positive 4-1BB cells (P<0.05, P<0.01, Figure 4E and 4F, respectively). Concomitantly, IL-2 production (MFI) in response to CMV stimulation was significantly higher in cells expressing both 4-1BB and OX40 than CD4+ T cells expressing only OX40 (P<0.01, Figure 4F). There was no significant difference in the amount of IFN-γ per cell (MFI) among 4-1BB and OX40 single and double positive Gag-specific CD4+ T cells (data not shown). CMV-specific IFN-γ producing CD4+ T cells that expressed only 4-1BB or both 4-1BB and OX40 produced significantly higher amount of IFN-γ per cell than those which expressed only OX40 (P<0.01 and P<0.001, respectively, data not shown). Taken together, these data indicate that both 4-1BB and OX40 pathways may augment antigen-specific IL-2 production.
Figure 4.
Association between expression of 4-1BB or OX40 on antigen-specific CD4+ T cells and the amount of cytokine produced per cell (determined by MFI). IL-2 (A) and IFN-γ (B) MFI on Gag-specific and CMV-specific IL-2 producing and IFN-γ producing CD4+ T cells in the context of 4-1BB expression. IL-2 (C) and IFN-γ (D) MFI on Gag-specific and CMV-specific IL-2 producing and IFN-γ producing CD4+ T cells in the context of OX40 expression. IL-2 MFI on 4-1BB+OX40−, 4-1BB+OX40+, and 4-1BB−OX40+ Gag-specific (E) and CMV-specific (F) CD4+ T cells. Dots represent individual values and the solid lines indicate medians. Statistical significance was determined by Wilcoxon’s matched pairs test.
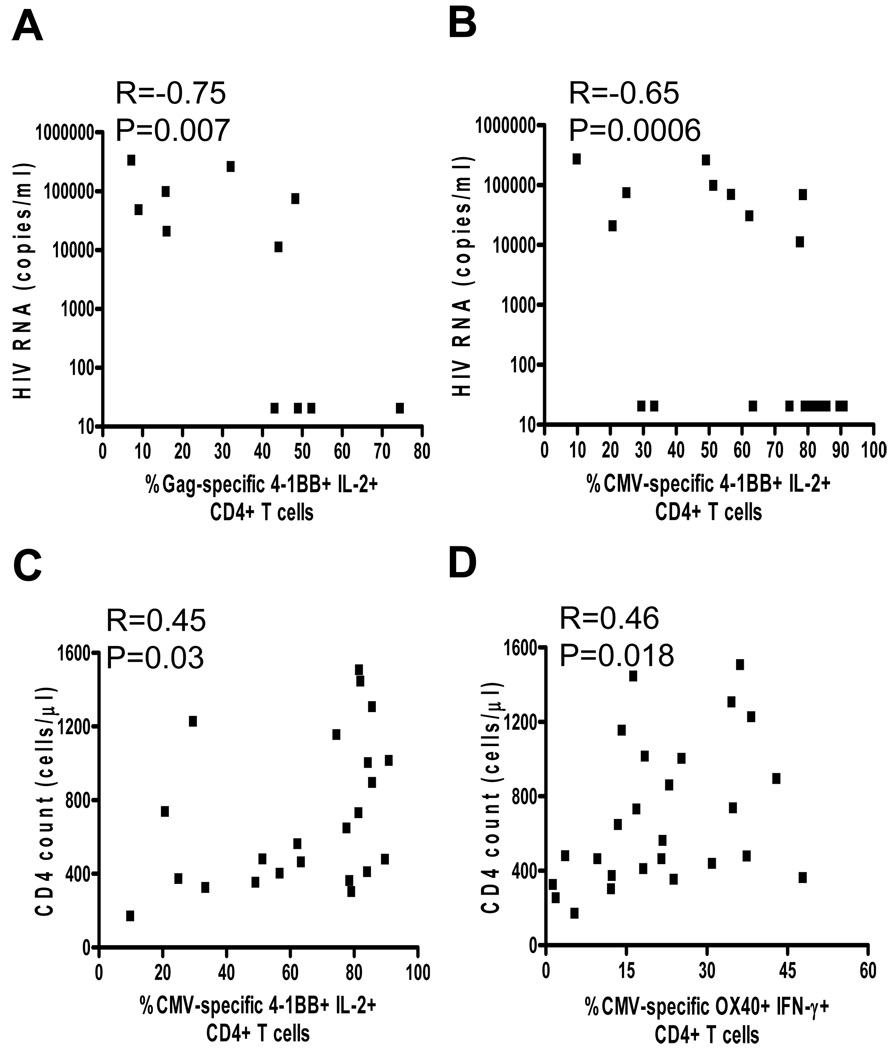
4-1BB and OX40 expression correlates negatively with plasma viral load and positively with CD4 count
To determine whether an association exists between 4-1BB or OX40 expression and markers of HIV disease progression, we evaluated the relationship between their expression on antigen-specific CD4+ T cells, viral load and CD4 count. All HIV infected subjects were included in these analyses to increase sample size. As shown in Figure 5, a strong significant inverse correlation was observed between HIV plasma viral load and 4-1BB expression on IL-2 producing Gag-specific (r=−0.75, P=0.007; Figure 5A) and CMV-specific CD4+ T cells (r=−0.65, P=0.0006; Figure 5B). When the ART-treated subjects were removed from the analysis to eliminate the variable of treatment (i.e. direct effect of ART on 4-1BB expression) the inverse correlations remain, although statistical significance was lost, presumably due to small sample size. Therefore, regardless of the nature of viral suppression (intrinsically by the immune system or extrinsically by ART), these analyses suggest that, 4-1BB expression is inversely associated with HIV viral load. Although we also observed a significant positive correlation between CD4 count and 4-1BB expression on CMV-specific CD4+ T cells (r=0.45, P=0.03) (Figure 5C), no correlation was observed for Gag-specific cells (data not shown). We also observed a significant correlation between CD4 count and OX40 expression on IFN-γ producing CMV-specific CD4+ T cells (r=0.46, P=0.018, Figure 5D). These observations suggest that HIV replication may play a role in down-regulating expression of these important costimulatory molecules on virus-specific CD4+ T cells and hence contribute to their dysfunction.
Figure 5.
Correlation of 4-1BB and OX40 expression with plasma viral load and CD4 count. A, A significant negative correlation between HIV RNA concentration and expression of 4-1BB on IL-2 producing HIV-specific CD4+ T cells. B, A significant negative correlation between HIV RNA concentration and expression of 4-1BB on IL-2 producing CMV-specific CD4+ T cells. C, A significant positive correlation between CD4 count and expression of 4-1BB on IL-2 producing CMV-specific CD4+ T cells. D, A significant positive correlation between CD4 count and expression of OX40 on IL-2 producing CMV-specific CD4+ T cells. Statistical significance was determined by Spearman’s correlation.
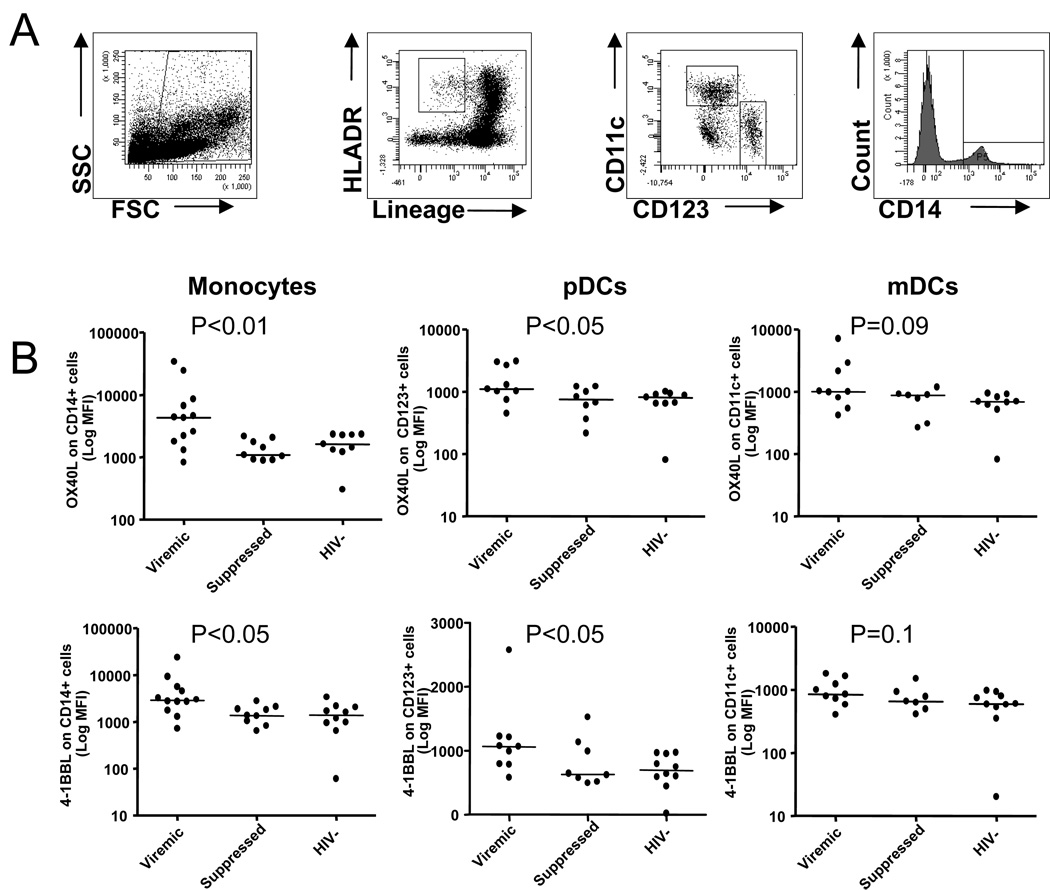
4-1BB and OX40 ligands are upregulated on antigen presenting cells from untreated HIV infected subjects
Since transduction of costimulatory signals through 4-1BB and OX40 receptors require binding of the molecules to their ligands expressed on activated APCs [9], we next examined the expression of 4-1BBL and OX40L on monocytes, mDCs, and pDCs in PBMCs from viremic (n=12) and suppressed (n=9) HIV-infected subjects and healthy controls (n=11) (Figure 6). Representative plots illustrating staining of mDCs, pDCs and monocytes are shown in Figure 6A. The expression (MFI) of 4-1BBL and OX40L on monocytes, pDCs, and mDCs was not significantly different between HIV-infected and uninfected subjects (data not shown). However, the expression of 4-1BBL and OX40L on CD14+ cells in viremic subjects was significantly higher than in suppressed subjects or in HIV seronegative controls (P<0.05) (Figure 6B). Although mDCs and pDCs exhibited higher 4-1BBL and OX40L expression levels in viremic subjects than in suppressed or healthy controls, the difference did not attain statistical significance (Figure 6B). This data suggest that 4-1BB and OX40 ligand expression on antigen presenting cells maybe intact since their expression is upregulated on monocytes and dendritic cells in viremic HIV-infected subjects.
Figure 6.
Expression of 4-1BB ligand (4-1BBL) and OX40 ligand (OX40L) on antigen presenting cells. A, Representative plots illustrating staining of myeloid dendritic cells (mDCs), plasmacytoid dendritic cells (pDCs) and monocytes. B, Scatter plots showing expression (MFI) of OX40 (upper panel) and 4-1BBL (lower panel) on monocytes, pDCs and mDCs, respectively, from viremic and suppressed HIV-infected subjects and HIV seronegative controls. Dots represent individual values and the solid lines indicate medians. Statistical significance was determined by ANOVA.
DISCUSSION
Although it is well described that HIV associated CD4+ T cell dysfunction is closely linked to viral replication and T cell exhaustion, the mechanisms responsible for decreased function remain largely unknown. We and others have previously demonstrated that CD4+ T cell dysfunction is characterized by altered cytokine patterns and increased expression of the coinhibitory receptors PD-1 and CTLA-4 [6, 8, 11]. We now report that HIV associated CD4+ T cell exhaustion is also associated with decreased expression of the costimulatory receptor 4-1BB. 4-1BB has been shown to be an important costimulatory molecule for induction of virus-specific memory T cell responses and its ligation enhances T cell function and promotes survival [9]. Although classic models emphasize the importance of 4-1BB for memory CD8+ T cell costimulation [35], recent data also suggests a role in CD4+ T cells [36–38]. We found that the percentage of 4-1BB, although slightly higher on virus-specific CD8+ T cells, was not significantly different from the levels found on virus-specific CD4+ T cells (data not shown). Furthermore, 4-1BB expression on antigen-specific CD4+ T cells was associated with IL-2 production and HIV replication. These findings suggest that reduced 4-1BB expression on HIV-specific CD4+ T cells from subjects with chronic HIV infection may contribute to the well described defects in proliferation which are correlated with HIV disease progression [4].
Consistent with previous reports, we observed that unstimulated peripheral blood CD4+ T cells from HIV infected and seronegative subjects expressed low levels of both 4-1BB and OX40 [9, 39, 40]. Due to the systemic immune activation inherent to HIV infection, 4-1BB expression is higher on CD4+ T cells following mitogenic stimulation in seropositive patients compared with seronegative controls [39]. However, there is no information regarding its expression on virus-specific CD4+ T cells. We were able to delineate the specific effects of HIV replication and chronic antigenic stimulation on the expression of 4-1BB and OX40 by comparing its expression on HIV− and CMV-specific CD4+ T cells. We observed that the induction of 4-1BB on IFN-γ and IL-2 producing CD4+ T cells producing was approximately 50% lower in response to HIV than CMV antigen stimulation, suggesting the expression of 4-1BB on HIV-specific, cytokine producing CD4+ T cells was dysregulated. Furthermore, we observed that 4-1BB expression on HIV-specific and CMV-specific IL-2 producing CD4+ T cells was significantly lower in untreated viremic subjects compared to those with ART-suppressed viral loads. Although longitudinal studies on pre and post ART-treated subjects would help to strengthen the association between 4-1BB expression and viral replication observed in this cross-sectional study, increased 4-1BB expression on virus-specific IL-2 producing CD4+ T cells from subjects on ART suggests that decreased expression of 4-1BB is associated with chronic viral replication and persistent stimulation of antigen-specific CD4+ T cells, which is the hallmark of HIV disease progression. However, it is worth noting that HIV preferentially infects HIV-specific CD4+ T [41] and that such preferential infection shortens the life span of HIV-specific CD4+ T cells [42, 43]. Thus, it is also possible that the decreased frequencies of Gag-specific CD4+ T cells expressing 4-1BB reflect a preferential loss of these cells.
Yu et al. previously reported that a higher percentage of CD4+ T cells from HIV-infected patients expressed OX40 than seronegative controls [40], despite overall low totals. We observed low expression levels of OX40 on circulating CD4+ T cells from both HIV infected and seronegative subjects. We also did not detect a difference in the expression of OX40 by HIV-specific vs. CMV-specific CD4+ T cells, unlike our observation for 4-1BB. However, in a subset of subjects which responded to both viruses, OX40 expression was significantly higher on Gag- compared to CMV-specific IL-2 producing CD4+ T cells suggesting that OX40 could be compensating for decreased 4-1BB expression. Interestingly, we did observe preferential expression of OX40 on IL-2 producing HIV− and CMV-specific CD4+ T cells. This is consistent with previous findings that demonstrate OX40 is expressed on less differentiated (CD27+/CD28+) CD4+ T cells [40]. Furthermore, it has been demonstrated that ligation of OX40 on CD4+ T cells enhances both HIV-specific CD4+ and CD8+ T cell proliferation [40]. While the current paradigm suggests OX40 preferentially costimulates CD4+ T cells and 4-1BB preferentially costimulates CD8+ T cells [23, 35], we observed higher expression of 4-1BB than OX40 on HIV-specific and CMV-specific CD4+ T cells. Previous murine studies have indicated that these costimulatory molecules are expressed at similar levels on activated CD4+ T cells [25]. Based on our observations with HIV− and CMV-specific cells, we suggest that in the context of chronic HIV infection, 4-1BB may play a dominant costimulatory role for human CD4+ T cells which lack the prototypical CD28 pathway [14–16].
To determine if an association between these costimulatory molecules and the function of antigen-specific CD4+ T cells existed, we examined the amount of IL-2 and IFN-γ per cell (determined by mean fluorescence intensity, MFI) on 4-1BB and OX40 expressing and non expressing cells after stimulation with HIV and CMV antigens. As previously mentioned, both costimulatory molecules are expressed on IFN-γ and IL-2 producing cells but we found that IL-2 producing virus-specific 4-1BB and OX40 expressing CD4+ T cells produced significantly more IL-2 per cell than their 4-1BB or OX40 non-expressing counterparts. These data suggest that not only are the CD4+ T cells skewed away from producing IL-2, as we and others have shown [6, 8, 11], but that a percentage of those IL-2 producing cells that remain and don’t express 4-1BB or OX40 produce less IL-2. It has been shown that stimulation via 4-1BBL and OX40L enhanced HIV-specific, influenza-specific and EBV-specific antiviral memory CD8+ T cell responses, as evidenced by greater expansion of tetramer+ T cells with enhanced cytokine production suggesting a role in IL-2 production by CD8+ T cells [30, 40, 44]. Furthermore, mice deficient in 4-1BBL showed defective memory responses to LCMV and influenza virus [45], indicating the crucial role of 4-1BB and OX40 pathways in antiviral immune response. Accordingly, we observed that virus-specific CD4+ T cells that expressed both 4-1BB and OX40 produced more IL-2 than single positive CD4+ T cells. CD8+ T cells that express both 4-1BB and OX40 have been dubbed ‘super effectors’ because after simultaneous ligation a preferential increase of common γc cytokines receptors CD25 and IL-7Rα on the dual costimulated CD8+ T cells versus the individually costimulated cells was observed [46]. Furthermore, we have previously demonstrated that differentiated IFN-γ producing HIV-specific CD4+ T cells from subjects with chronic untreated HIV infection express high levels of the co-inhibitory molecule PD-1, while less differentiated IL-2 producing CD4+ T cells express significantly lower levels of PD-1 [6]. Since blockade of the PD-1 pathway has been shown to increase HIV-specific proliferation [6, 7], presumably by enhancing the function of IFN-γ producing PD-1 expressing CD4+ T cells, it is likely that simultaneous blocking of the inhibitory pathways and stimulation through the 4-1BB and/or OX40 pathways may provide a potential target for enhancing the function of less differentiated IL-2 producing CD4+ T cells.
Taken together, these results demonstrate that the 4-1BB expression on HIV-specific CD4+ T cells from subjects with chronic HIV infection is significantly decreased. Importantly, decreased expression of 4-1BB on HIV− and CMV-specific IL-2 producing CD4+ T cells is ameliorated in subjects whose viral replication is suppressed by ART, suggesting that chronic viral replication and repeated stimulation induces decreased expression of this costimulatory receptor. Decreased expression of 4-1BB on HIV-specific CD4+ T cells and decreased IL-2 production by cells which do not express 4-1BB suggest that decreased expression of this costimulatory molecule may contribute to the loss of HIV-specific CD4+ T cell proliferation during chronic HIV infection.
ACKNOWLEDGEMENTS
This study was supported by the Colorado Center for AIDS Research (CFAR) Pilot Project Grant and NIH grant AI076161 to BP.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
REFERENCES
- 1.Day CL, Walker BD. Progress in defining CD4 helper cell responses in chronic viral infections. J. Exp. Med. 2003;198:1773–1777. doi: 10.1084/jem.20031947. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.McMichael AJ, Rowland-Jones SL. Cellular immune responses to HIV. Nature. 2001;410:980–987. doi: 10.1038/35073658. [DOI] [PubMed] [Google Scholar]
- 3.Pantaleo G, Koup RA. Correlates of immune protection in HIV-1 infection: what we know, what we don't know, what we should know. Nat. Med. 2004;10:806–810. doi: 10.1038/nm0804-806. [DOI] [PubMed] [Google Scholar]
- 4.Rosenberg ES, Billingsley JM, Caliendo AM, Boswell SL, Sax PE, Kalams SA, Walker BD. Vigorous HIV-1-specific CD4+ T cell responses associated with control of viremia. Science. 1997;278:1447–1450. doi: 10.1126/science.278.5342.1447. [DOI] [PubMed] [Google Scholar]
- 5.Chen L. Co-inhibitory molecules of the B7-CD28 family in the control of T-cell immunity. Nat. Rev. Immunol. 2004;4:336–347. doi: 10.1038/nri1349. [DOI] [PubMed] [Google Scholar]
- 6.D'Souza M, Fontenot AP, Mack DG, Lozupone C, Dillon S, Meditz A, Wilson CC, Connick E, Palmer BE. Programmed death 1 expression on HIV-specific CD4+ T cells is driven by viral replication and associated with T cell dysfunction. J. Immunol. 2007;179:1979–1987. doi: 10.4049/jimmunol.179.3.1979. [DOI] [PubMed] [Google Scholar]
- 7.Day CL, Kaufmann DE, Kiepiela P, Brown JA, Moodley ES, Reddy S, Mackey EW, Miller JD, Leslie AJ, DePierres C, Mncube Z, Duraiswamy J, Zhu B, Eichbaum Q, Altfeld M, Wherry EJ, Coovadia HM, Goulder PJ, Klenerman P, Ahmed R, Freeman GJ, Walker BD. PD-1 expression on HIV-specific T cells is associated with T-cell exhaustion and disease progression. Nature. 2006;443:350–354. doi: 10.1038/nature05115. [DOI] [PubMed] [Google Scholar]
- 8.Kaufmann DE, Kavanagh DG, Pereyra F, Zaunders JJ, Mackey EW, Miura T, Palmer S, Brockman M, Rathod A, Piechocka-Trocha A, Baker B, Zhu B, Le Gall S, Waring MT, Ahern R, Moss K, Kelleher AD, Coffin JM, Freeman GJ, Rosenberg ES, Walker BD. Upregulation of CTLA-4 by HIV-specific CD4+ T cells correlates with disease progression and defines a reversible immune dysfunction. Nat. Immunol. 2007;8:1246–1254. doi: 10.1038/ni1515. [DOI] [PubMed] [Google Scholar]
- 9.Watts TH. TNF/TNFR family members in costimulation of T cell responses. Annu. Rev. Immunol. 2005;23:23–68. doi: 10.1146/annurev.immunol.23.021704.115839. [DOI] [PubMed] [Google Scholar]
- 10.Palmer BE, Blyveis N, Fontenot AP, Wilson CC. Functional and phenotypic characterization of CD57+CD4+ T cells and their association with HIV-1-induced T cell dysfunction. J. Immunol. 2005;175:8415–8423. doi: 10.4049/jimmunol.175.12.8415. [DOI] [PubMed] [Google Scholar]
- 11.Palmer BE, Boritz E, Wilson CC. Effects of sustained HIV-1 plasma viremia on HIV-1 Gag-specific CD4+ T cell maturation and function. J. Immunol. 2004;172:3337–3347. doi: 10.4049/jimmunol.172.5.3337. [DOI] [PubMed] [Google Scholar]
- 12.Trautmann L, Janbazian L, Chomont N, Said EA, Gimmig S, Bessette B, Boulassel MR, Delwart E, Sepulveda H, Balderas RS, Routy JP, Haddad EK, Sekaly RP. Upregulation of PD-1 expression on HIV-specific CD8+ T cells leads to reversible immune dysfunction. Nat. Med. 2006;12:1198–1202. doi: 10.1038/nm1482. [DOI] [PubMed] [Google Scholar]
- 13.Barber DL, Wherry EJ, Masopust D, Zhu B, Allison JP, Sharpe AH, Freeman GJ, Ahmed R. Restoring function in exhausted CD8 T cells during chronic viral infection. Nature. 2006;439:682–687. doi: 10.1038/nature04444. [DOI] [PubMed] [Google Scholar]
- 14.Choremi-Papadopoulou H, Viglis V, Gargalianos P, Kordossis T, Iniotaki-Theodoraki A, Kosmidis J. Downregulation of CD28 surface antigen on CD4+ and CD8+ T lymphocytes during HIV-1 infection. J. Acquir. Immune. Defic. Syndr. 1994;7:245–253. [PubMed] [Google Scholar]
- 15.Gamberg J, Pardoe I, Bowmer MI, Howley C, Grant M. Lack of CD28 expression on HIV-specific cytotoxic T lymphocytes is associated with disease progression. Immunol. Cell. Biol. 2004;82:38–46. doi: 10.1111/j.1440-1711.2004.01204.x. [DOI] [PubMed] [Google Scholar]
- 16.Trimble LA, Shankar P, Patterson M, Daily JP, Lieberman J. Human immunodeficiency virus-specific circulating CD8 T lymphocytes have down-modulated CD3zeta and CD28, key signaling molecules for T- cell activation. J. Virol. 2000;74:7320–7330. doi: 10.1128/jvi.74.16.7320-7330.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Bukczynski J, Wen T, Watts TH. Costimulation of human CD28− T cells by 4-1BB ligand. Eur. J. Immunol. 2003;33:446–454. doi: 10.1002/immu.200310020. [DOI] [PubMed] [Google Scholar]
- 18.DeBenedette MA, Shahinian A, Mak TW, Watts TH. Costimulation of CD28− T lymphocytes by 4-1BB ligand. J. Immunol. 1997;158:551–559. [PubMed] [Google Scholar]
- 19.Bertram EM, Dawicki W, Sedgmen B, Bramson JL, Lynch DH, Watts TH. A switch in costimulation from CD28 to 4-1BB during primary versus secondary CD8 T cell response to influenza in vivo. J. Immunol. 2004;172:981–988. doi: 10.4049/jimmunol.172.2.981. [DOI] [PubMed] [Google Scholar]
- 20.Bukczynski J, Wen T, Wang C, Christie N, Routy JP, Boulassel MR, Kovacs C, Macdonald KS, Ostrowski M, Sekaly RP, Bernard NF, Watts TH. Enhancement of HIV-specific CD8 T cell responses by dual costimulation with CD80 and CD137L. J. Immunol. 2005;175:6378–6389. doi: 10.4049/jimmunol.175.10.6378. [DOI] [PubMed] [Google Scholar]
- 21.Croft M. Co-stimulatory members of the TNFR family: keys to effective T-cell immunity? Nat. Rev. Immunol. 2003;3:609–620. doi: 10.1038/nri1148. [DOI] [PubMed] [Google Scholar]
- 22.Cannons JL, Lau P, Ghumman B, DeBenedette MA, Yagita H, Okumura K, Watts TH. 4-1BB ligand induces cell division, sustains survival, and enhances effector function of CD4 and CD8 T cells with similar efficacy. J. Immunol. 2001;167:1313–1324. doi: 10.4049/jimmunol.167.3.1313. [DOI] [PubMed] [Google Scholar]
- 23.Gramaglia I, Weinberg AD, Lemon M, Croft M. Ox-40 ligand: a potent costimulatory molecule for sustaining primary CD4 T cell responses. J. Immunol. 1998;161:6510–6517. [PubMed] [Google Scholar]
- 24.Hurtado JC, Kim YJ, Kwon BS. Signals through 4-1BB are costimulatory to previously activated splenic T cells and inhibit activation-induced cell death. J. Immunol. 1997;158:2600–2609. [PubMed] [Google Scholar]
- 25.Taraban VY, Rowley TF, O'Brien L, Chan HT, Haswell LE, Green MH, Tutt AL, Glennie MJ, Al-Shamkhani A. Expression and costimulatory effects of the TNF receptor superfamily members CD134 (OX40) and CD137 (4-1BB), and their role in the generation of anti-tumor immune responses. Eur. J. Immunol. 2002;32:3617–3627. doi: 10.1002/1521-4141(200212)32:12<3617::AID-IMMU3617>3.0.CO;2-M. [DOI] [PubMed] [Google Scholar]
- 26.Calarota SA, Hokey DA, Dai A, Jure-Kunkel MN, Balimane P, Weiner DB. Augmentation of SIV DNA vaccine-induced cellular immunity by targeting the 4-1BB costimulatory molecule. Vaccine. 2008;26:3121–3134. doi: 10.1016/j.vaccine.2008.02.017. [DOI] [PubMed] [Google Scholar]
- 27.Du X, Zheng G, Jin H, Kang Y, Wang J, Xiao C, Zhang S, Zhao L, Chen A, Wang B. The adjuvant effects of co-stimulatory molecules on cellular and memory responses to HBsAg DNA vaccination. J. Gene. Med. 2007;9:136–146. doi: 10.1002/jgm.1004. [DOI] [PubMed] [Google Scholar]
- 28.Munks MW, Mourich DV, Mittler RS, Weinberg AD, Hill AB. 4-1BB and OX40 stimulation enhance CD8 and CD4 T-cell responses to a DNA prime, poxvirus boost vaccine. Immunol. 2004;112:559–566. doi: 10.1111/j.1365-2567.2004.01917.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Harrison JM, Bertram EM, Boyle DB, Coupar BE, Ranasinghe C, Ramshaw IA. 4-1BBL coexpression enhances HIV-specific CD8 T cell memory in a poxvirus prime-boost vaccine. Vaccine. 2006;24:6867–6874. doi: 10.1016/j.vaccine.2006.06.007. [DOI] [PubMed] [Google Scholar]
- 30.Serghides L, Bukczynski J, Wen T, Wang C, Routy JP, Boulassel MR, Sekaly R, Ostrowski M, Bernard NF, Watts TH. Evaluation of OX40 ligand as a costimulator of human antiviral memory CD8 T cell responses: comparison with B7.1 and 4-1BBL. J. Immunol. 2005;175:6368–6377. doi: 10.4049/jimmunol.175.10.6368. [DOI] [PubMed] [Google Scholar]
- 31.Wang C, Wen T, Routy JP, Bernard NF, Sekaly RP, Watts TH. 4-1BBL induces TNF receptor-associated factor 1-dependent Bim modulation in human T cells and is a critical component in the costimulation-dependent rescue of functionally impaired HIV-specific CD8 T cells. J. Immunol. 2007;179:8252–8263. doi: 10.4049/jimmunol.179.12.8252. [DOI] [PubMed] [Google Scholar]
- 32.Stone SF, Price P, French MA. Dysregulation of CD28 and CTLA-4 expression by CD4 T cells from previously immunodeficient HIV-infected patients with sustained virological responses to highly active antiretroviral therapy. HIV Med. 2005;6:278–283. doi: 10.1111/j.1468-1293.2005.00307.x. [DOI] [PubMed] [Google Scholar]
- 33.Precopio ML, Betts MR, Parrino J, Price DA, Gostick E, Ambrozak DR, Asher TE, Douek DC, Harari A, Pantaleo G, Bailer R, Graham BS, Roederer M, Koup RA. Immunization with vaccinia virus induces polyfunctional and phenotypically distinctive CD8(+) T cell responses. J. Exp. Med. 2007;204:1405–1416. doi: 10.1084/jem.20062363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Darrah PA, Patel DT, De Luca PM, Lindsay RW, Davey DF, Flynn BJ, Hoff ST, Andersen P, Reed SG, Morris SL, Roederer M, Seder RA. Multifunctional TH1 cells define a correlate of vaccine-mediated protection against Leishmania major. Nat. Med. 2007;13:843–850. doi: 10.1038/nm1592. [DOI] [PubMed] [Google Scholar]
- 35.Shuford WW, Klussman K, Tritchler DD, Loo DT, Chalupny J, Siadak AW, Brown TJ, Emswiler J, Raecho H, Larsen CP, Pearson TC, Ledbetter JA, Aruffo A, Mittler RS. 4-1BB costimulatory signals preferentially induce CD8+ T cell proliferation and lead to the amplification in vivo of cytotoxic T cell responses. J. Exp. Med. 1997;186:47–55. doi: 10.1084/jem.186.1.47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Dawicki W, Bertram EM, Sharpe AH, Watts TH. 4-1BB and OX40 act independently to facilitate robust CD8 and CD4 recall responses. J. Immunol. 2004;173:5944–5951. doi: 10.4049/jimmunol.173.10.5944. [DOI] [PubMed] [Google Scholar]
- 37.Dawicki W, Watts TH. Expression and function of 4-1BB during CD4 versus CD8 T cell responses in vivo. Eur. J. Immunol. 2004;34:743–751. doi: 10.1002/eji.200324278. [DOI] [PubMed] [Google Scholar]
- 38.Mack DG, Lanham AK, Palmer BE, Maier LA, Watts TH, Fontenot AP. 4-1BB enhances proliferation of beryllium-specific T cells in the lung of subjects with chronic beryllium disease. J. Immunol. 2008;181:4381–4388. doi: 10.4049/jimmunol.181.6.4381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Wang S, Kim YJ, Bick C, Kim SH, Kwon BS. The potential roles of 4-1BB costimulation in HIV type 1 infection. AIDS Res. Hum. Retroviruses. 1998;14:223–231. doi: 10.1089/aid.1998.14.223. [DOI] [PubMed] [Google Scholar]
- 40.Yu Q, Yue FY, Gu XX, Schwartz H, Kovacs CM, Ostrowski MA. OX40 ligation of CD4+ T cells enhances virus-specific CD8+ T cell memory responses independently of IL-2 and CD4+ T regulatory cell inhibition. J. Immunol. 2006;176:2486–2495. doi: 10.4049/jimmunol.176.4.2486. [DOI] [PubMed] [Google Scholar]
- 41.Douek DC, Brenchley JM, Betts MR, Ambrozak DR, Hill BJ, Okamoto Y, Casazza JP, Kuruppu J, Kunstman K, Wolinsky S, Grossman Z, Dybul M, Oxenius A, Price DA, Connors M, Koup RA. HIV preferentially infects HIV-specific CD4+ T cells. Nature. 2002;417:95–98. doi: 10.1038/417095a. [DOI] [PubMed] [Google Scholar]
- 42.Yue FY, Kovacs CM, Dimayuga RC, Gu XX, Parks P, Kaul R, Ostrowski MA. Preferential apoptosis of HIV-1-specific CD4+ T cells. J. Immunol. 2005;174:2196–2204. doi: 10.4049/jimmunol.174.4.2196. [DOI] [PubMed] [Google Scholar]
- 43.Brenchley JM, Ruff LE, Casazza JP, Koup RA, Price DA, Douek DC. Preferential infection shortens the life span of human immunodeficiency virus-specific CD4+ T cells in vivo. J. Virol. 2006;80:6801–6809. doi: 10.1128/JVI.00070-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Bukczynski J, Wen T, Ellefsen K, Gauldie J, Watts TH. Costimulatory ligand 4-1BBL (CD137L) as an efficient adjuvant for human antiviral cytotoxic T cell responses. Proc. Natl. Acad. Sci. USA. 2004;101:1291–1296. doi: 10.1073/pnas.0306567101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.DeBenedette MA, Wen T, Bachmann MF, Ohashi PS, Barber BH, Stocking KL, Peschon JJ, Watts TH. Analysis of 4-1BB ligand (4-1BBL)-deficient mice and of mice lacking both 4-1BBL and CD28 reveals a role for 4-1BBL in skin allograft rejection and in the cytotoxic T cell response to influenza virus. J. Immunol. 1999;163:4833–4841. [PubMed] [Google Scholar]
- 46.Lee SJ, Rossi RJ, Lee SK, Croft M, Kwon BS, Mittler RS, Vella AT. CD134 costimulation couples the CD137 pathway to induce production of supereffector CD8 T cells that become IL-7 dependent. J. Immunol. 2007;179:2203–2214. doi: 10.4049/jimmunol.179.4.2203. [DOI] [PubMed] [Google Scholar]