Abstract
Background
As life expectancy of HIV-infected persons increases, cancers have become an important cause of morbidity and mortality. Although cutaneous neoplasms are the most common malignancies in the general population, little data exist among HIV-positive persons especially regarding the impact of HIV-specific factors.
Methods
We evaluated the incidence rates and factors associated with the development of cutaneous malignancies among HIV-infected persons by examining prospectively collected data from a large HIV Natural History Study composed of 4,490 persons (1986 to 2006). Poisson regression and Cox proportional hazards models were performed.
Results
Six percent (n=254) of HIV-infected persons developed a cutaneous malignancy during 33,760 person-years of follow-up (mean 7.5 years). Since the advent of highly active antiretroviral therapy (HAART), incidence rates of cutaneous non-AIDS defining cancers (NADCs), in particular basal cell carcinoma, have exceeded rates of cutaneous AIDS-defining cancers such as Kaposi’s sarcoma. Factors associated with the development of cutaneous NADCs in the multivariate models included increasing age (hazard ratio (HR) 2.1; 95% CI 1.7–2.6) and race. Compared to white/non-Hispanic race, African Americans (HR 0.03, 95% CI 0.01–0.14) and other races (HR 0.14, 95% CI 0.03, 0.57) had a lower risk of cutaneous NADCs. There were no significant associations between cutaneous NADCs and time-updated CD4 counts, HIV RNA levels, or HAART receipt.
Conclusions
NADCs are now the most common cutaneous malignancies among HIV-infected persons. Cutaneous NADCs do not appear to be significantly associated with immune function or HAART, but rather are related to traditional factors, such as aging and skin color.
Keywords: Cutaneous malignancy, skin cancer, neoplasm, HIV, basal cell carcinoma, melanoma, epidemiology, HAART
Background
Cutaneous neoplasms are the most common malignancies in the U.S., with 70–80% diagnosed as basal cell carcinoma (BCC), followed by squamous cell carcinoma (SCC) and malignant melanoma (MM) [1, 2]. Risk factors include cumulative ultraviolet (UV) radiation exposure, race, advanced age, reduced DNA repair capability, and immunosuppression [3–6].
Multiple studies have demonstrated an increased risk of cutaneous malignancies in immunosuppressed populations. This risk is most pronounced in solid-organ transplant recipients, who have a 65–250 times increased risk as compared to the general population [4–6]. Patients with human immunodeficiency virus (HIV) infection also have an increased risk of some types of cancer [7–9]. Early in the HIV epidemic, Kaposi’s sarcoma (KS) was the most common malignancy in HIV-positive patients with a rate that was >1000-fold greater than the general population [7, 10]. Because of the increased propensity to develop KS, as well as non-Hodgkin’s lymphoma (NHL), these conditions were classified as AIDS-defining cancers (ADCs) [11]. Since the advent of highly active antiretroviral therapy (HAART), the rate of KS has significantly decreased [12].
The incidence and risk factors associated with cutaneous non-AIDS-defining cancers (NADCs) among HIV-infected persons are less defined. Although some studies have shown a higher incidence and/or a more aggressive course of cutaneous NADCs among HIV-infected patients, the number of studies is limited and the results have been mixed [9, 13–17]. Important risk factors for the development of skin cancer, regardless of HIV status, include fair skin, family history, and cumulative sun-exposure [18, 19]. However, it is currently unknown whether CD4 count, HIV RNA level, or receipt of HAART impacts the development of cutaneous NADCs among HIV-infected populations. We evaluated a large cohort of HIV-infected persons over the course of the HIV epidemic (1986–2006) to provide data on incidence rates and HIV-specific factors associated with cutaneous malignancies among HIV-infected persons.
Methods
We retrospectively examined data collected as part of the US Military HIV Natural History Study (NHS), a multicenter, prospective, observational study which enrolled over 4,500 persons at seven geographic locations in the US (1986 to 2006). Participants were military beneficiaries evaluated on a biannual basis utilizing standardized data collection procedures. All active duty US military personnel were HIV negative upon entry into the service and subsequently underwent HIV screening every one to five years. For this study, among those with a documented last known HIV negative date (55%), the mean seroconversion window was 20.7 (SD 17.0) months. Participants without a documented HIV positive date, those with a cancer diagnosis prior to 1986, and those with a diagnosis of cancer more than six months before the HIV diagnosis were excluded from the analyses, thereby yielding 4,490 participants for our study. Our study was approved by central and local Institutional Review Boards, and all participants provided informed consent.
Cutaneous malignancy cases were identified by database queries using the terms “malignancy”, “cancer”, “carcinoma”, and “tumor” [20] which had a location recorded as “skin”. Baseline was defined as the time of HIV seroconversion, conservatively estimated as 6 months prior to the first documented HIV-positive test. Cutaneous malignancies were only included if they occurred after this point. Cancers were defined as an ADC in cases of cutaneous KS or NHL, and NADC for all other primary skin cancers. The study included only cutaneous SCCs and excluded mucosal SCCs of the mouth, genital, or anal regions.
Data collected included demographics; cutaneous malignancy type, location, and date as well as subsequent cutaneous malignancies and their location/date when applicable; HIV last seronegative and first seropositive dates; CD4 counts and HIV RNA tests at baseline and sequentially over time; hepatitis B status at HIV diagnosis (defined by at least two of the following tests being concurrently positive: core antibody, surface antibody, or surface antigen tests); history of AIDS-defining conditions other than cancer; Walter Reed Stage at entry into the NHS, designated as 1–6 for ascending degrees of disease based on CD4 counts, opportunistic infections, lymphadenopathy, and delayed type hypersensitivity [21]; and use of antiretroviral therapy. HAART was defined as two or more nucleoside reverse transcriptase inhibitors (NRTIs) in combination with at least one protease inhibitor (PI) or one non-nucleoside reverse transcriptase inhibitors (NNRTI), or an abacavir or tenofovir containing regimen of three or more NRTIs.
For those with cutaneous malignancy, the censoring date was the first cancer diagnosis date for the specific type of cancer considered. For those without cancer, the censoring date was the last study visit or the date of death. Follow-up for this report ended 31 December 2006. Participants were classified for stratification purposes as being documented as HIV positive in the pre-HAART era (prior to 1996) or the HAART era (1996 or later).
Statistical analyses included descriptive statistics. Means were given with standard deviations (SD). Chi-square tests were used to compare proportions; general linear models were used to compare continuous valued measurements. The number of events, person-years (PYs) at risk, and age-adjusted rates of events (per 100,000 PYs) were calculated for 5 year intervals, the pre-HAART era, and the HAART era. Rates for cutaneous ADCs were calculated for males only (due to the predominantly male study population and that most cases occurred among males), and age-adjusted with the US 2000 standard population for males, using the age at the mid-point of the interval. Rates for cutaneous NADC events were calculated for white/non-Hispanic males only (since almost all cases occurred among white males), and age-adjusted with the US 2000 standard population for white/non-Hispanic males. Rates were reported with 95% confidence intervals (CI). Poisson regression analyses were used to test the hypothesis that the cancer rates remained constant over time.
Univariate and multivariate Cox proportional hazard models were used to evaluate the association of specific factors with the type of initial skin cancer. Baseline was the time of HIV diagnosis or 01 January 1986, whichever occurred later. For CD4 count, HIV RNA, HAART use, and a history of a non-cancer AIDS defining event, we utilized both baseline and time-updated covariates, which included all available measurements from baseline through the event or censoring date. All models were stratified by HIV diagnosis era. Hazard ratios (HR) were reported with 95% CI. All analyses were conducted using SAS (version 9.1, Cary, NC).
Results
Baseline Characteristics of the Study Population
The study cohort consisted of 4,490 HIV-infected participants who were followed for a total of 33,760 PYs of follow-up (mean 7.5, SD 4.9 years). The mean age at HIV diagnosis was 30 (SD 7.9) years; 91% were male; self-reported race was 45% African-American, 44% white/non-Hispanic, and 11% other. Among participants with a documented Walter Reed Stage classification at HIV diagnosis (n=3,888), 65% were stage 1 or 2 (early stage). Among 2,435 participants with available serologic data, 34% were seropositive for hepatitis B. A baseline CD4 count was available for 3,483 participants with a mean value of 539 (SD 275) cells/mm3. Many participants were initially infected with HIV prior to the availability of HIV RNA testing. A baseline HIV RNA measurement was available for 1,317 participants with a mean of 4.2 (SD 0.9) log10 copies/ml.
Number and Incidence Rates of Cutaneous Malignancies
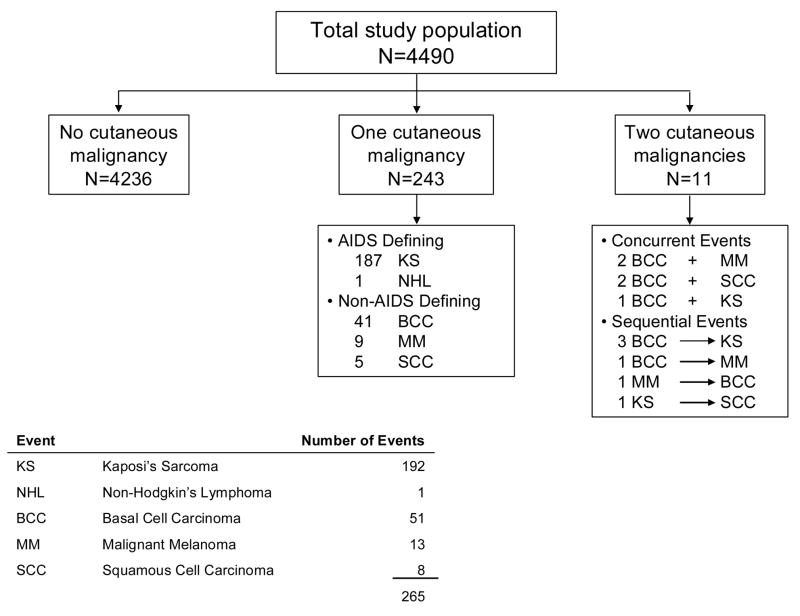
A total of 254 (5.7%) of the study cohort developed a cutaneous neoplasm during the study (Figure 1). Eleven participants developed two different cutaneous malignancies (five of which were concurrent and six developed during the follow-up time) for a total of 265 cutaneous cancer events. In total, there were 193 cutaneous ADCs (192 cases of KS and 1 NHL), and 72 cutaneous NADCs (51 BCC, 13 MM, and 8 SCC). All cancers occurred among males, except for one ADC which occurred in a female. No Merkel cell or other types of cutaneous malignancies were identified during the course of this study.
Figure 1.
Flow diagram of cutaneous malignancies among HIV-infected persons
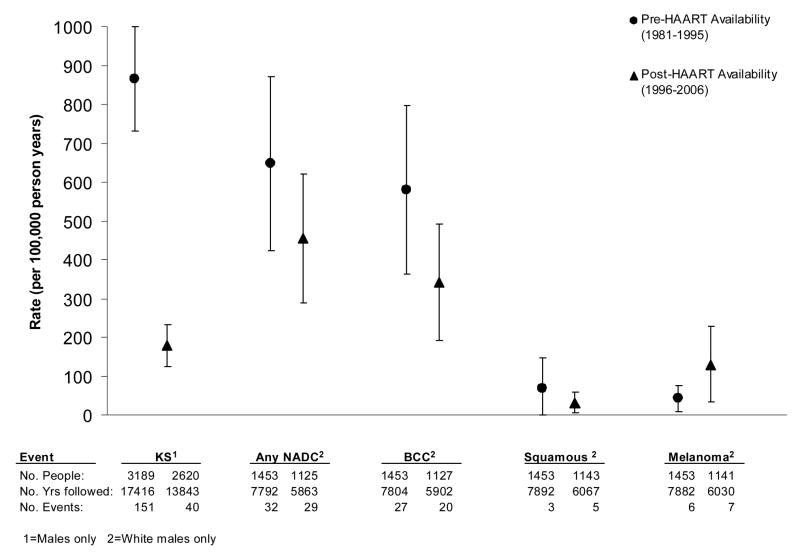
Age-adjusted rates of KS among males in the cohort varied over the course of the HIV epidemic, as shown in Table 1 and Figure 2. The highest rate was noted in 1991–1995, with an age-adjusted rate of 1,797 cases per 100,000 PYs. The rates of KS significantly declined during the HAART era (after 1995; p<0.001), with rates (per 100,000 PY) of 205 in 1996–2000 and 134 in 2001–2006. The age-adjusted rates of cutaneous NADCs did not significantly change over time, but since the advent of HAART, the incidence rates of cutaneous NADCs have exceeded cutaneous ADCs (Table 1, Figure 2). During the time period 2001–2006, the age-adjusted rate of BCCs among white/non-Hispanic males exceeded that of KS (397 cases per 100,000 PYs vs. 134 cases per 100,000 PYs, respectively). The rate of MM (114 per 100,000 PYs) was similar to KS, while SCC occurred at a lower rate (49 per 100,000 PYs).
Table 1.
Age-adjusted Rates for Specific Cutaneous Malignancies
| Type of Cutaneous Malignancy | Calendar Interval | No. of People Followed | No. of Years of Follow-up | No. of Events | Age- Adjusted Rate1 | 95% Confidence Interval | |
|---|---|---|---|---|---|---|---|
| Kaposi’s Sarcoma2 | 1986–1990 | 2349 | 7491 | 37 | 242 | 164 | 321 |
| 1991–1995 | 2867 | 9920 | 114 | 1797 | 1467 | 2126 | |
| 1996–2000 | 2298 | 7291 | 28 | 205 | 129 | 281 | |
| 2001–2006 | 1617 | 6548 | 12 | 134 | 58 | 211 | |
| P* < 0.001 | |||||||
| Any Non-AIDS Defining Skin Cancer3 | 1986–1990 | 1071 | 3342 | 10 | 426 | 162 | 690 |
| 1991–1995 | 1288 | 4465 | 22 | 698 | 406 | 990 | |
| 1996–2000 | 982 | 3146 | 11 | 516 | 211 | 820 | |
| 2001–2006 | 678 | 2715 | 18 | 453 | 244 | 663 | |
| P* = 0.10 | |||||||
| Basal Cell Carcinoma3 | 1986–1990 | 1071 | 3325 | 9 | 400 | 139 | 661 |
| 1991–1995 | 1288 | 4476 | 18 | 588 | 316 | 860 | |
| 1996–2000 | 984 | 3163 | 6 | 284 | 57 | 510 | |
| 2001–2006 | 684 | 2737 | 14 | 397 | 189 | 606 | |
| P* = 0.35 | |||||||
| Squamous Cell3,4 | 1986–1990 | 1071 | 3339 | 0 | 0.0 | ||
| 1991–1995 | 1295 | 4551 | 3 | 85 | 0.0 | 180 | |
| 1996–2000 | 1000 | 3239 | 2 | 24 | 0.0 | 58 | |
| 2001–2006 | 698 | 2827 | 3 | 49 | 0.0 | 104 | |
| P* = 0.12 | |||||||
| Melanoma3 | 1986–1990 | 1071 | 3337 | 1 | 26 | 0.0 | 77 |
| 1991–1995 | 1295 | 4542 | 5 | 89 | 11 | 168 | |
| 1996–2000 | 998 | 3220 | 3 | 165 | 0.0 | 351 | |
| 2001–2006 | 695 | 2808 | 4 | 114 | 2 | 226 | |
| P* = 0.21 | |||||||
Per 100,000 person years of follow-up.
For males only, age-adjusted to the US 2000 Standard Population for males
For white/non-Hispanic males only, age-adjusted to the US 2000 Standard Population for white/non-Hispanic males
Excludes oral, genital, and anal squamous cell cancers
P-values from Poisson regression testing the hypothesis that the event rates remained constant over the intervals
Figure 2.
Age-adjusted incidence rates (per 100,000 person-years) of cutaneous malignancies during the pre-HAART (1986–1995) era and HAART (1996–2006) era. Rates for Kaposi’s sarcoma are for males only; rates for non-AIDS defining cancers are for white/non-Hispanic males only. The rates are age-adjusted to the appropriate US 2000 standard population.
Clinical Characteristics of HIV-Infected Persons with Non-AIDS-Defining Cutaneous Malignancies
The most common cutaneous NADC diagnosed was BCC, which occurred in 51 participants; clinical characteristics are summarized in Table 2. Eight (16%) participants with BCC had more than one BCC lesion noted at initial presentation, all which occurred in the same body location. During a mean follow-up of 7.8 (SD 6.2) years after diagnosis of the first BCC, 12 (24%) participants developed new BCC lesions (range 1–5) at novel locations, for a total of 83 BCC events. In addition, four (8%) patients with an initial BCC subsequently developed a second type of cutaneous malignancy: three developed KS and one developed MM.
Table 2.
Characteristics at cancer diagnosis among HIV-infected participants diagnosed with a non-AIDS defining cutaneous malignancy
| Characteristic | Basal Cell | Malignant Melanoma | Squamous Cell |
|---|---|---|---|
| Number of people with diagnosis | 51 | 13 | 8 |
| Age, mean (SD) years | 44.1 (9.7) | 54.8 (16.4) | 43.5 (5.7) |
| Race/ethnicity, % | |||
| White/non-Hispanic | 92.2 | 100 | 100 |
| African American | 3.9 | 0 | 0 |
| Other | 3.9 | 0 | 0 |
| Male gender, % | 100 | 100 | 100 |
| Location of Malignancy, % | |||
| Head/neck | 49 | 0 | 50 |
| Trunk1 | 29 | 85 | 12 |
| Arm | 16 | 15 | 38 |
| Leg | 2 | 0 | 0 |
| Not reported | 4 | 0 | 0 |
| Hepatitis B positive, % | 36.7 | 18.2 | 75.0 |
| Duration of HIV infection, mean (SD) years | 5.8 (5.0) | 7.7 (5.5) | 8.3 (5.4) |
| CD4 cell count, mean (SD) cells/mm3 | 446 (215) | 408 (248) | 340 (192) |
| CD4 nadir prior to cancer diagnosis, mean (SD) cells/mm3 | 311 (163) | 236 (124) | 188 (116) |
| HIV RNA2, mean (SD) log10 copies/ml | 2.7 (1.1) | 3.0 (1.3) | 3.0 (0.9) |
| HIV RNA2 <400 copies/ml, % | 59.1 | 28.6 | 60 |
| Receipt of HAART, % | 24.0 | 30.8 | 50.0 |
| Died3 during follow-up, % | 35 | 23 | 38 |
Ten of the MM cases occurred on the back, and one on the anterior chest
Viral load available for 22 participants with BCC, 7 with MM, and 5 with SCC
None of the deaths were attributed to the cutaneous malignancy; two participants who died had two different cancer types
MM was diagnosed in 13 participants; three also had BCC (two concurrently and one subsequent to the diagnosis of MM). Table 2 summarizes the characteristics of participants with MM. During a mean follow-up of 4.9 years (SD 4.3) after diagnosis of the first MM, one (8%) participant developed an additional MM in a different location and one developed a BCC. SCC of the skin was identified in eight participants (Table 2), two also had BCC and one developed the SCC after having KS. During a mean follow-up of 2.1 years (SD 1.5) after diagnosis of the first SCC, no patient developed a subsequent cutaneous malignancy.
In summary, of the 65 participants followed for a mean of 5.1 (SD 4.3) years and diagnosed with a cutaneous NADC, 13 (20%) developed a second cutaneous malignancy of the same type and five (8%) developed a second cutaneous malignancy of a novel type. The mean time between the first and second cancers was 3.9 years for those with the same cancer type and 3.7 years for those with different cancer type. Twenty-two (34%) HIV-infected persons with a cutaneous NADC died during follow-up; however, none of the deaths was attributed to the cutaneous malignancy.
Comparisons of HIV-Infected Persons with an AIDS-Defining and Non-AIDS-Defining Cutaneous Malignancy
We compared the characteristics of participants whose first cancer event was a cutaneous NADC to those whose first cancer event was a cutaneous ADC (Table 3). The one participant who was diagnosed with concurrent cutaneous ADC and a NADC was included in the NADC group. Participants with a cutaneous NADC compared to a cutaneous ADC were older, more likely to be white/non-Hispanic, had a longer mean duration of HIV infection prior to cancer diagnosis, higher CD4 count and lower HIV RNA at the time of cancer diagnosis, and were more likely to be receiving HAART. Those with a cutaneous ADC were more likely hepatitis B seropositive compared to those with a cutaneous NADC.
Table 3.
Characteristics at time of HIV diagnosis and at time of initial cutaneous malignancy diagnosis1 by cancer type (non-AIDS defining and AIDS defining)
| Characteristic | Non-AIDS-Defining | AIDS-Defining | P-value |
|---|---|---|---|
| Number with diagnosis | 65 | 189 | |
| Age, mean (SD) years | |||
| At HIV diagnosis | 36.7 (9.2) | 30.6 (8.2) | <0.001 |
| At cancer diagnosis | 43.0 (9.7) | 35.8 (7.7) | <0.001 |
| Race/ethnicity, % | <0.001 | ||
| White/non-Hispanic | 93.9 | 56.1 | |
| African American | 3.1 | 35.5 | |
| Other | 3.1 | 8.5 | |
| Male gender, % | 100.0 | 99.5 | 0.56 |
| Hepatitis B positive at cancer diagnosis, % | 39.3 | 62.4 | 0.002 |
| Duration of HIV infection at cancer diagnosis, mean (SD) years | 6.3 (5.3) | 5.2 (3.2) | 0.04 |
| Walter Reed Stage2 at HIV diagnosis, % | 0.001 | ||
| 1 or 2 | 60.3 | 39.4 | |
| 3 or 4 | 27.6 | 23.9 | |
| 5 or 6 | 12.1 | 36.7 | |
| CD4 cell count3, mean (SD) cells/mm3 | |||
| At HIV diagnosis | 541 (250) | 451 (285) | 0.05 |
| At cancer diagnosis | 439 (207) | 128 (178) | <0.001 |
| Nadir prior to cancer diagnosis | 293 (155) | 106 (153) | <0.001 |
| HIV RNA4, mean (SD) log10 copies/ml | |||
| At HIV diagnosis | 4.7 (0.7) | 4.8 (0.6) | 0.75 |
| At cancer diagnosis | 2.8 (1.1) | 4.6 (1.0) | <0.001 |
| On HAART at cancer diagnosis, % | 28.1 | 7.0 | <0.001 |
Includes only the first type of cancer diagnosed among each participant
Of those with cancer, Walter Reed Stage is available for 94% at time of HIV diagnosis. Percent is of those with measurement available
Of those with cancer, CD4 cell count measurement is available for 76% and 98% at HIV diagnosis and during follow-up, respectively
Of those with cancer, HIV RNA measurement is available for 14% and 71% of those at HIV diagnosis and during follow-up, respectively
Factors Associated with Cutaneous Non-AIDS-Defining Cancers among HIV-Infected Persons
Using Cox proportional hazard models, we assessed factors associated with cutaneous NADC events (Table 4). Univariate factors associated with time to first cutaneous NADC included increasing age, white/non-Hispanic race, and prior non-cancer AIDS defining event. All cases occurred among males. In the univariate models, there were no significant associations between cutaneous NADC occurrence and Walter Reed stage at entry into the cohort, CD4 count, HIV RNA, or receipt of HAART. In the final multivariate model, factors associated with cutaneous NADCs included older age and white/non-Hispanic race.
Table 4.
Factors associated with initial non-AIDS defining cutaneous cancer
| Univariate Models1 |
Multivariate Model1 |
|||||
|---|---|---|---|---|---|---|
| Characteristic | HR | 95% CI | P-value | HR | 95% CI | P-value |
| Age at HIV Diagnosis, per 10 years | 2.4 | (1.9 – 2.9) | <0.001 | 2.1 | (1.7 – 2.6) | <0.001 |
| Race | ||||||
| White/non-Hispanic | 1.0 | 1.0 | ||||
| African American | 0.03 | (0.01 – 0.12) | <0.001 | 0.03 | (0.01 – 0.14) | <0.001 |
| Other | 0.13 | (0.03 – 0.52) | 0.004 | 0.14 | (0.03 – 0.57) | 0.01 |
| Hepatitis B Co-infection | 1.1 | (0.5 – 2.3) | 0.79 | |||
| Walter Reed Stage | ||||||
| Stage I/II | 1.0 | |||||
| Stage III/IV | 1.4 | (0.8 – 2.5) | 0.30 | |||
| Stage V/VI | 1.3 | (0.6 – 2.9) | 0.56 | |||
| CD4 Count, cells/mm3 | ||||||
| At HIV diagnosis, per 50 cells | 0.98 | (0.92 – 1.03) | 0.37 | |||
| Time-updated2, per 100 cells | 0.96 | (0.87 – 1.06) | 0.40 | |||
| HIV RNA, log10 copies/mL | ||||||
| At HIV diagnosis, per log | 2.20 | (1.01 – 4.64) | 0.05 | |||
| Time-updated2, per ½ log | 0.90 | (0.50 – 1.63) | 0.74 | |||
| HIV Diagnosed before 1996 | 1.1 | (0.5 – 2.3) | 0.89 | |||
| Non-cancer AIDS Event2 | 2.1 | (1.1 – 4.1) | 0.03 | |||
| HAART2 | 1.1 | (0.5 – 2.2) | 0.82 | |||
Hazard ratios (HR) are from proportional hazards regression models. All analyses are stratified by HIV diagnosis era (before 1996 versus 1996 or after)
Time-updated covariates use all available measurements from baseline through the event or censoring date
Discussion
NADCs are now the most frequent cause of cutaneous malignancies among HIV-infected persons. While KS was the most common cutaneous malignancy among HIV-infected persons before the introduction of HAART, BCC has now superseded it as the most frequent cutaneous malignancy. In our study, cutaneous NADCs were not associated with immune status or receipt of HAART, but most related to traditional risk factors such as aging and skin color.
Currently, cutaneous malignancies are the most frequently diagnosed cancers in the U.S. general population [1, 2]. Likewise, cutaneous malignancies likely account for the majority of cancers among HIV-infected persons [20]. However, most studies to date evaluating malignancies among HIV-infected persons have utilized registry data which often lacks information regarding cutaneous NADCs [22]. We evaluated 4,490 HIV-infected persons over a 20-year period and found that 6% of the cohort developed a cutaneous malignancy during a mean follow-up of 7.5 years.
In this study, the incidence of cutaneous ADCs significantly decreased after 1995, similar to other studies and coinciding with the introduction of HAART [12, 23, 24]. On the other hand, the age-adjusted incidence rates of cutaneous NADCs remained stable. As HIV-infected persons are experiencing increasing life expectancies and a reduction of ADCs [25], NADCs have become the most common cutaneous malignancies in this population.
The factors associated with the development of cutaneous NADCs in our study of HIV-infected persons included increased age and white/non-Hispanic race, similar to that seen in other HIV-positive cohorts and the general population [18, 19]. On the other hand, CD4 counts were not related to the development of cutaneous NADCs and were relatively high (mean 439 cells/mm3) at the time of cancer diagnosis. Other studies have shown that the depth of tumor spread and outcome are typically not associated with the CD4 count [17–19, 26, 27]. The lack of an association of cutaneous NADC with immune status in HIV-infected patients differs from immunosuppressed transplant patients whose risk of skin cancer correlates with lower CD4 counts [28].
Although a recent study demonstrated similarities in the types of cancers seen among HIV and transplant patients [29], differences among the risk factors and clinical characteristics of cutaneous malignancies exist between these populations. For example, 30–70% of solid organ transplant recipients develop a cutaneous malignancy, whereas only 6% of HIV-infected persons developed either a cutaneous ADC or NADC in our study [30, 31]. These differences may be attributed to the type or degree of immunosuppression in transplant recipients compared to HIV patients [30, 32]. Many transplant patients receive cyclosporine and/or azathioprine, which not only have immunosuppressive effects that reduce tumor surveillance and increase the risk of acquiring human papillomavirus (HPV) infection and perhaps other oncogenic viruses, they also display intrinsic carcinogenic and photosensitizing effects that may contribute to the development of cutaneous malignancies [6, 28].
The present study failed to show a protective benefit of HAART against the development of cutaneous NADCs. A recent analysis of the Strategies for Management of Antiretroviral Therapy (SMART) trial examined the impact of continuous HAART compared to drug conservation on cutaneous NADCs and found a similar rate for both arms (220 per 100,000 PYs, HR 1.1, p=0.98) [33]. Since interrupting HAART is associated with rapid CD4 declines and elevated inflammatory markers which may impact immune surveillance and cancer incidence, the fact that continuing HAART did not alter cutaneous NADC incidence rates lends further support to the finding that HAART may not reduce rates of cutaneous NADCs. The lack of benefit of HAART in our study may also reflect that any positive effects HAART may be offset by other factors such as increasing life expectancies and aging which might increase cancer risk. Although HAART has shown no protective effect in the development or prognosis of cutaneous NADCs to date, isolated case reports have shown that BCC and Merkel cell carcinoma may regress with antiretroviral therapy [34, 35].
It has been hypothesized that the chronic immunosuppressed state seen in HIV-infected persons, that may not be entirely reflected by the CD4 count or improved by HAART, may cause an overall immune dysregulation and lead to cutaneous neoplasms. Other postulated pathogenic mechanisms regarding the risk of cutaneous malignancies among HIV patients include the role of potentially oncogenic HIV proteins (e.g., tat and nef); the high incidence of HPV in this population and its potential role in the pathogenesis SCC; and/or elevated interleukin levels which result in keratinocyte and melanocyte proliferation [36–40].
HIV-infected persons have been suggested to have higher rates of some types of cutaneous malignancies, but comparing rates among HIV patients with the general population is challenging since cancer registries such as the National Cancer Institute Surveillance Epidemiology and End Results (SEER) Cancer Statistics Review do not provide data for BCC and SCC. Furthermore, age-adjusted rates are only comparable if the same standard population is utilized. For KS and MM, rates for the general population (age-adjusted to the US 2000 standard population) are available. KS rates from the general population before the HIV epidemic (1975–1979) was 4 cases per 100,000 PY [41] compared to our rates from 134 per 100,000 PY (2001–2006) to 1,797 (1991–1995), representing a 34–449 fold increased risk among HIV patients. For MM, the rate in the general population during 2001–2005 was 29 per 100,000 PY [41] compared to our rate of 114, suggesting a 3.9 fold higher rate. For rates of BCC and SCC, we recalculated age-adjusted rates using the 1970 US standard population for white males since there is data in the literature standardized to this population [42]. The rates for BCC were similar for HIV-infected persons (323, 537, 266 and 354 per 100,000 PY from earliest to most recent four time periods) compared to the general population (360 per 100,000 PY during 1977–1980) [42]. Comparison of rates for SCC showed the recalculated rates for SCC for our four time periods were 0, 68, 17 and 39, compared to 101 for the general population; however, these data are limited by the small number of SCCs in our cohort.
The current study demonstrated that HIV-infected persons presenting with a cutaneous NADC have a high likelihood to develop subsequent cutaneous malignancies at novel sites. In our study, 24% participants who initially presented with a BCC developed a subsequent BCC, and 8% developed a second type of cutaneous cancer. In addition, other studies have shown that HIV patients have high rates of recurrences [16, 18, 19, 43, 44]. These data suggests that HIV-infected persons with an initial cutaneous NADC should be carefully followed for both recurrent disease and the development of novel cutaneous malignancies.
Similar to other immunosuppressed populations, HIV patients develop cutaneous malignancies at a younger age than the general population [4, 6, 16, 26, 31]. In the our study, the mean age at the diagnosis of a cutaneous NADC was 43 years, as opposed to a mean age of 70 years in the general population [26].
The most frequent cutaneous NADC in study cohort was BCC, with a 6:1 ratio of BCC to SCC, which concurs with previous studies in HIV-infected populations and parallels the general population, but differs from transplant patients who disproportionally develop SCC [5, 14, 16, 19, 31]. In contrast to previous studies which found the trunk to be the most common location of BCC in HIV patients, the majority of BCC cases in our study occurred in the head and neck region, comparable to the general population [16, 19]. The current study differed from prior investigations as it included data during the HAART era. The most common locations of SCC (head/neck) and MM (trunk) were similar to that seen in the general population.
As in any study, the current study had limitations. First, 91% of the population consisted of men; hence, the current results may not be generalizable to HIV-infected women. The majority of our HIV-infected persons was diagnosed early during infection and had high initial CD4 counts; as such, our rates of cancer could be lower than rates for HIV-infected persons diagnosed at more advanced stages of disease, especially for ADCs. Our study does, however, provide important data given the recent emphasis on diagnosing HIV early. Data on an individual’s cumulative lifetime sun-exposure was not available, nor could the participants be segregated by geographic location since military personnel have frequent geographic relocations. These data are important because cumulative UV exposure plays an important role in skin cancer development by altering p53 tumor suppressor genes and reducing the number of Langerhans cells which are important for immune surveillance [1, 3]. Other limitations include incomplete data regarding the subtype and aggressiveness of the malignancies, and the method of treatment. Due to the observational nature of the study, the ascertainment of cutaneous malignancies may have varied over time in our cohort which may have affected incidence rates. Finally, no information was available regarding HPV co-infection [36–38].
In conclusion, since the advent of HAART, NADCs are now the most common cutaneous malignancy diagnosed among HIV-infected persons. The present study demonstrates that the development of cutaneous NADCs are associated with increased age and skin color, similar to the general population, but independent of CD4 count or receipt of HAART. These results suggest that regardless of the CD4 level, all HIV-infected persons should be counseled on UV avoidance and physicians should be aware of the risk of cutaneous malignancies in this population.
Acknowledgments
Support for this work was provided by the Infectious Disease Clinical Research Program (IDCRP), Uniformed Services University of the Health Sciences (USUHS), Bethesda, MD, of which the TriService AIDS Clinical Consortium (TACC) is a component. The IDCRP is a DoD Tri-service program executed through USUHS and the Henry M. Jackson Foundation for the Advancement of Military Medicine in collaboration with HHS/NIH/NIAID/DCR through Interagency Agreement HU0001-05-2-0011.
We wish to thank Ms. Waine MacAllister for editorial support and Dr. Edith Lederman for her critical review of the paper.
Footnotes
The opinions or assertions contained herein are the private views of the authors and are not to be construed as official or as reflecting the views of the Departments of the Army, Navy, or Air Force, or the Department of Defense. The authors have no commercial or other association that might pose a conflict of interest in this work.
References
- 1.Diepgen TL, Mahler V. The epidemiology of skin cancer. Br J Dermatol. 2002;146(61):1–6. doi: 10.1046/j.1365-2133.146.s61.2.x. [DOI] [PubMed] [Google Scholar]
- 2.Christenson LJ, Borrowman TA, Vachon CM, Tollefson MM, Otley CC, Weaver AL, Roenigk RK. Incidence of basal cell and squamous cell carcinomas in a population younger than 40 years. JAMA. 2005;294:681–690. doi: 10.1001/jama.294.6.681. [DOI] [PubMed] [Google Scholar]
- 3.Saladi RN, Persaud AN. The causes of skin cancer: a comprehensive review. Drugs Today (Barc) 2005;41:37–53. doi: 10.1358/dot.2005.41.1.875777. [DOI] [PubMed] [Google Scholar]
- 4.Mehrany K, Weenig RH, Lee KK, Pittelkow MR, Otley CC. Increased metastasis and mortality from cutaneous squamous cell carcinoma in patients with chronic lymphocytic leukemia. J Am Acad Dermatol. 2005;53:1067–1071. doi: 10.1016/j.jaad.2005.08.055. [DOI] [PubMed] [Google Scholar]
- 5.Euvrard S, Kanitakis J, Claudy A. Skin cancers after organ transplantation. N Engl J Med. 2003;348:1681–1691. doi: 10.1056/NEJMra022137. [DOI] [PubMed] [Google Scholar]
- 6.Moloney FJ, Comber H, O’Lorcain P, O’Kelly P, Conlon PJ, Murphy GM. A population based study of skin cancer incidence and prevalence in renal transplant recipients. Br J Dermatol. 2006;154:498–504. doi: 10.1111/j.1365-2133.2005.07021.x. [DOI] [PubMed] [Google Scholar]
- 7.Engels EA, Biggar RJ, Hall HI, et al. Cancer risk in people infected with human immunodeficiency virus in the United States. Int J Cancer. 2008;123:187–194. doi: 10.1002/ijc.23487. [DOI] [PubMed] [Google Scholar]
- 8.Patel P, Hanson DL, Sullivan PS Adult and Adolescent Spectrum of Disease Project and HIV Outpatient Study Investigators. Incidence of types of cancer among HIV-infected persons compared with the general population in the United States, 1992–2003. Ann Intern Med. 2008;148:728–736. doi: 10.7326/0003-4819-148-10-200805200-00005. [DOI] [PubMed] [Google Scholar]
- 9.Goedert JJ, Cote TR, Virgo P, et al. Spectrum of AIDS-associated malignant disorders. Lancet. 1998;351:1833–1839. doi: 10.1016/s0140-6736(97)09028-4. [DOI] [PubMed] [Google Scholar]
- 10.Centers for Disease Control (CDC) Kaposi’s sarcoma and Pneumocystis pneumonia among homosexual men – New York City and California. MMWR Morb Mortal Wkly Rep. 1981;30:305–308. [PubMed] [Google Scholar]
- 11.Centers for Disease Control (CDC) 1993 revised classification system for HIV infection and expanded surveillance case definition for AIDS among adolescents and adults. MMWR Morb Mortal Wkly Rep. 1992;41:961–962. [PubMed] [Google Scholar]
- 12.Jacobson LP, Yamashita TE, Detels R, et al. Impact of potent antiretroviral therapy on the incidence of Kaposi’s sarcoma and non-Hodgkin’s lymphomas among HIV-1 infected individuals. Multicenter AIDS Cohort Study. J Acquire Immune Defic Syndr Hum Retroviral. 1999;21:S34–S41. [PubMed] [Google Scholar]
- 13.Franceschi S, Dal Maso L, Arniani S, et al. Risk of cancer other than Kaposi’s sarcoma and non-Hodgkin’s lymphoma in persons with AIDS in Italy. Cancer and AIDS Registry Linkage Study. Br J Cancer. 1998;78:966–970. doi: 10.1038/bjc.1998.610. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Wilkins K, Turner R, Dolev JC, LeBoit PE, Berger TG, Maurer TA. Cutaneous malignancy and human immunodeficiency virus disease. J Am Acad Dermatol. 2006;54:189–206. doi: 10.1016/j.jaad.2004.11.060. [DOI] [PubMed] [Google Scholar]
- 15.Wang CY, Brodland DG, Su WP. Skin cancers associated with acquired immunodeficiency syndrome. Mayo Clin Proc. 1995;70:766–772. doi: 10.4065/70.8.766. [DOI] [PubMed] [Google Scholar]
- 16.Smith KJ, Skelton HG, Yeager J, Angritt P, Wagner KF. Cutaneous neoplasms in a military population of HIV-1-positive patients. Military Medical Consortium for the Advancement of Retroviral Research. J Am Acad Dermatol. 1993;29:400–406. doi: 10.1016/0190-9622(93)70202-5. [DOI] [PubMed] [Google Scholar]
- 17.Rodrigues LK, Klencke BJ, Vin-Christian K, et al. Altered clinical course of malignant melanoma in HIV-positive patients. Arch Dermatol. 2002;138:765–770. doi: 10.1001/archderm.138.6.765. [DOI] [PubMed] [Google Scholar]
- 18.Maurer TA, Christian KV, Kerschmann RL, et al. Cutaneous squamous cell carcinoma in human immunodeficiency virus-infected patients. A study of epidemiologic risk factors, human papillomavirus, and p53 expression. Arch Dermatol. 1997;133:577–583. [PubMed] [Google Scholar]
- 19.Lobo DV, Chu P, Grekin RC, Berger TG. Nonmelanoma skin cancers and infection with the human immunodeficiency virus. Arch Dermatol. 1992;128:623–627. [PubMed] [Google Scholar]
- 20.Burgi A, Brodine S, Wegner S, et al. Incidence and risk factors for the occurrence of non-AIDS-defining cancers among human immunodeficiency virus-infected individuals. Cancer. 2005;104:1505–1511. doi: 10.1002/cncr.21334. [DOI] [PubMed] [Google Scholar]
- 21.Redfield RR, Wright DC, Tramont ED. The Walter Reed staging classification for HTLV-III/LAV infection. N Engl J Med. 1986;314:131–132. doi: 10.1056/NEJM198601093140232. [DOI] [PubMed] [Google Scholar]
- 22.Engels EA, Pfeiffer RM, Goedert JJ, et al. Trends in cancer risk among people with AIDS in the United States 1980–2002. AIDS. 2006;20:1645–1654. doi: 10.1097/01.aids.0000238411.75324.59. [DOI] [PubMed] [Google Scholar]
- 23.Grulich AE, Li Y, McDonald AM, Correll PK, Law MG, Kaldor JM. Decreasing rates of Kaposi’s sarcoma and non-Hodgkin’s lymphoma in the era of potent combination anti-retroviral therapy. AIDS. 2001;15:629–633. doi: 10.1097/00002030-200103300-00013. [DOI] [PubMed] [Google Scholar]
- 24.International Collaboration on HIV and Cancer. Highly active antiretroviral therapy and incidence of cancer in human immunodeficiency virus-infected adults. J Natl Cancer Inst. 2000;92:1823–1830. doi: 10.1093/jnci/92.22.1823. [DOI] [PubMed] [Google Scholar]
- 25.Lohse N, Hansen AB, Pedersen G, et al. Survival of persons with and without HIV infection in Denmark, 1995–2005. Ann Intern Med. 2007;146:87–95. doi: 10.7326/0003-4819-146-2-200701160-00003. [DOI] [PubMed] [Google Scholar]
- 26.Nguyen P, Vin-Christian K, Ming ME, Berger T. Aggressive squamous cell carcinomas in persons infected with the human immunodeficiency virus. Arch Dermatol. 2002;138:758–763. doi: 10.1001/archderm.138.6.758. [DOI] [PubMed] [Google Scholar]
- 27.Tindall B, Finlayson R, Mutimer K, Billson FA, Munro VF, Cooper DA. Malignant melanoma associated with human immunodeficiency virus infection in three homosexual men. J Am Acad Dermatol. 1989;20:587–591. doi: 10.1016/s0190-9622(89)70068-2. [DOI] [PubMed] [Google Scholar]
- 28.Ducloux D, Carron PL, Racadot E, Rebibou JM, Bresson-Vautrin C, Saint-Hillier Y, Chalopin JM. CD4 lymphocytopenia in long-term renal transplant recipients. Transplant Proc. 1998;30:2859–2860. doi: 10.1016/s0041-1345(98)00843-4. [DOI] [PubMed] [Google Scholar]
- 29.Grulich AE, van Leeuwen MT, Falster MO, Vajdic CM. The incidence of cancers in people with HIV/AIDS compared with immunosuppressed transplant recipients: a meta-analysis. Lancet. 2007;370:59–67. doi: 10.1016/S0140-6736(07)61050-2. [DOI] [PubMed] [Google Scholar]
- 30.Hampton T. Skin cancer’s ranks rise: immunosuppression to blame. JAMA. 2005;294:1476–1480. doi: 10.1001/jama.294.12.1476. [DOI] [PubMed] [Google Scholar]
- 31.Berg D, Otley CC. Skin cancer in organ transplant recipients: Epidemiology, pathogenesis, and management. J Am Acad Dermatol. 2002;47:1–17. doi: 10.1067/mjd.2002.125579. [DOI] [PubMed] [Google Scholar]
- 32.Randle HW. The historical link between solid-organ transplantation, immunosuppression, and skin cancer. Dermatol Surg. 2004;30:595–597. doi: 10.1111/j.1524-4725.2004.30142.x. [DOI] [PubMed] [Google Scholar]
- 33.Silverberg MJ, Neuhaus J, Bower M, et al. Risk of cancers during interrupted antiretroviral therapy in the SMART study. AIDS. 2007;21:1957–1963. doi: 10.1097/QAD.0b013e3282ed6338. [DOI] [PubMed] [Google Scholar]
- 34.Chan SY, Madan V, Lear JT, Helbert M. Highly active antiretroviral therapy-induced regression of basal cell carcinomas in a patient with acquired immunodeficiency and Gorlin syndrome. Br J Dermatol. 2006;155:1079–1080. doi: 10.1111/j.1365-2133.2006.07479.x. [DOI] [PubMed] [Google Scholar]
- 35.Burack J, Altschuler EL. Sustained remission of metastatic Merkel cell carcinoma with treatment of HIV infection. JR Soc Med. 2003;96:238–239. doi: 10.1258/jrsm.96.5.238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Forslund O, Iftner T, Andersson K, et al. Viraskin Study Group. Cutaneous human papillomaviruses found in sun-exposed skin: Beta-papillomavirus species 2 predominates in squamous cell carcinoma. J Infect Dis. 2007;196:876–883. doi: 10.1086/521031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Harwood CA, McGregor JM, Proby CM, Breuer J. Human papillomavirus and the development of non-melanoma skin cancer. J Clin Pathol. 1999;52:249–253. doi: 10.1136/jcp.52.4.249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Karagas MR, Nelson HH, Sehr P, et al. Human papillomavirus infection and incidence of squamous cell and basal cell carcinomas of the skin. J Natl Cancer Inst. 2006;98:389–395. doi: 10.1093/jnci/djj092. [DOI] [PubMed] [Google Scholar]
- 39.Smith KJ, Skelton HG, Heimer W, Baxter D, Angritt P, Frisman D, Wagner KF. Melanocytic activation in HIV-1 disease: HMB-45 staining in common acquired nevi. Military Medical Consortium for the Advancement of Retroviral Research. J Am Acad Dermatol. 1993;29:539–544. doi: 10.1016/0190-9622(93)70218-i. [DOI] [PubMed] [Google Scholar]
- 40.Barillari G, Ensoli B. Angiogenic effects of extracellular human immunodeficiency virus type 1 Tat protein and its role in the pathogenesis of AIDS-associated Kaposi’s sarcoma. Clin Microbiol Rev. 2002;15:310–326. doi: 10.1128/CMR.15.2.310-326.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Ries LAB, Melbert D, Krapcho M, Stinchcomb DG, Howlader N, Horner MJ, et al., editors. SEER Cancer Statistics Review. National Cancer Institute; Bethesda, MD: 1975–2005. [Accessed November 20, 2008]. http://seer.cancer.gov/csr/1975_2005/ based on November 2007 SEER data submission, posted to the SEER web site, 2008. [Google Scholar]
- 42.Karagas MR, Wainstock MA, Nelson HH. Keratinocyte Carcinomas (Basal and squamous cell carcinomas of the skin) In: Schottenfeld D, Fraumeni JF Jr, editors. Cancer Epidemiology and Prevention. 3. 2006. pp. 1230–1250. [Google Scholar]
- 43.Honda KS. HIV and skin cancer. Dermatol Clin. 2006;24:521–30. doi: 10.1016/j.det.2006.06.011. [DOI] [PubMed] [Google Scholar]
- 44.Cooksley CD, Hwang LY, Waller DK, Ford CE. HIV-related malignancies: community-based study using linkage of cancer registry and HIV registry data. Int J STDs AIDS. 1999;10:795–802. doi: 10.1258/0956462991913574. [DOI] [PubMed] [Google Scholar]