Abstract
The objective of this study was to determine the effects of temporal hydrostatic pressure (HP) on the properties of scaffoldless bovine articular cartilage constructs. The study was organized in three phases: First, a suitable control for HP application was identified. Second, 10 MPa static HP was applied at three different timepoints (6–10 days, 10–14 days, and 14–18 days) to identify a window in construct development when HP application would be most beneficial. Third, the temporal effects of 10–14-day static HP application, as determined in phase II, were assessed at 2, 4, and 8 weeks. Compressive and tensile mechanical properties, GAG and collagen content, histology for GAG and collagen, and immunohistochemistry for collagen types I and II were assessed. When a culture control identified in phase I was used in phase II, HP application from 10 to 14 days resulted in a significant 1.4-fold increase in aggregate modulus, accompanied by an increase in GAG content, while HP application at all timepoints enhanced tensile properties and collagen content. In phase III, HP had an immediate effect on GAG content, collagen content, and compressive stiffness, while there was a delayed increase in tensile stiffness. The enhanced tensile stiffness was still present at 8 weeks. For the first time, this study examined the immediate and long-term effects of HP on biomechanical properties, and demonstrated that HP has an optimal application time in construct development. These findings are exciting as HP stimulation allowed for the formation of robust tissue-engineered cartilage; for example, 10 MPa static HP resulted in an aggregate modulus of 273 ± 123 kPa, a Young's modulus of 1.6 ± 0.4 MPa, a GAG/wet weight of 6.1 ± 1.4%, and a collagen/wet weight of 10.6 ± 2.4% at 4 weeks.
Introduction
Cartilage is an avascular tissue and therefore has a limited intrinsic ability for repair after injury or osteoarthritis. Current treatments result in the formation of fibrocartilage, which is mechanically inferior to articular cartilage.1 Due to the limitations of current therapies, tissue engineering has emerged as a promising approach for treating cartilage degeneration, as a result of injury or osteoarthritis.
Scaffoldless approaches for tissue engineering articular cartilage2–5 bypass several of the problems associated with scaffold use, including stress shielding, biocompatibility, and biodegradation. In particular, the self-assembling process has allowed for significant increases in construct biochemical and biomechanical properties; however, these properties are still lacking in comparison to adult native tissue.6 Therefore, the use of mechanical stimulation modalities such as hydrostatic pressure (HP) appears to be a promising approach for enhancing the biomechanical and biochemical properties of engineered constructs.
Articular cartilage is exposed to HP in vivo, and efforts to stimulate chondrocytes with HP have focused on the physiological range of 3–18 MPa.7–16 It is believed that there are significant differences in the effects of static and intermittent HP. For example, several studies on human articular chondrocytes in monolayer have demonstrated that intermittent HP at 10 MPa, 1 Hz, results in increased aggrecan and collagen II mRNA,12,15,17 while static HP was shown to have no effects on mRNA levels.17 In contrast, in other work using explants or immature chondrocytes in three-dimensional culture, the beneficial effects of static HP have been demonstrated. Hall et al.18 demonstrated enhanced GAG production with static pressures in the physiological range, while both Hall et al.18 and Lammi et al.19 observed either no benefit or detrimental effects with pressures above the physiological range. In tissue-engineered constructs with immature bovine chondrocytes, Toyoda et al.20,21 and Mizuno et al.22 have also observed beneficial effects of static HP on GAG synthesis, aggrecan mRNA, and collagen type II mRNA. Finally, a recent study by our group compared the effects of 1, 5, and 10 MPa under static, 0.1 Hz, and 1 Hz conditions, and found that 10 MPa static HP significantly increased construct compressive and tensile properties, while 10 MPa, 1 Hz treatment only resulted in a significant increase in compressive properties.16 Based on these results, 10 MPa static HP was selected for this study. Additionally, our own prior work has suggested 10–14 days to be a potentially suitable timeframe to apply mechanical stimulation,6 while previous work by Ikenoue et al.12 demonstrated that 4 days of HP application had a greater effect on aggrecan and collagen II mRNA than 1 day of HP application had. Based on the results of these studies, a comparison among HP application times of 6–10, 10–14, and 14–18 days was made in this study.
Although several studies have been performed to assess the effects of HP on tissue-engineered constructs, no studies have determined the optimal timepoint in construct development for the application of HP. Additionally, studies assessing the immediate and delayed effects of HP on construct biomechanical properties are lacking. Therefore, the objective of this study was to determine when in construct development the biomechanical and biochemical properties were maximally sensitive to HP application. Further, this study sought to examine the effects of HP on construct biochemical and biomechanical properties immediately after HP application, as well as up to 6 weeks after HP application, to determine how long the beneficial effects of HP would last after removal of the stimulus. First, it was hypothesized that the bagging process used in applying HP would have no effect on construct properties. As prior studies involving mechanical stimulation demonstrated the benefit of application from 10 to 14 days, it was likewise hypothesized that HP would have an optimal application timepoint in construct development for the enhancement of construct biomechanical and biochemical properties. Finally, due to the slower turnover of collagen remodeling relative to GAG in the extracellular matrix (ECM), it was hypothesized that construct compressive properties would be increased immediately after HP application, while there would be a delayed increase in tensile properties. To test these hypotheses, three experiments were performed. First, 10 MPa static HP was applied to self-assembled constructs and compared to two different control groups. Second, 10 MPa static HP was applied to the constructs at three different times in construct development. Finally, the effects of HP application were assessed immediately at 2 weeks, as well as at later timepoints of 4 and 8 weeks.
Materials and Methods
Chondrocyte isolation and seeding
Cartilage was obtained from the distal femur of 1-week-old male calves23–25 (Research 87, Boston, MA) less than 36 h after slaughter, and was digested with collagenase type II (Worthington, Lakewood, NJ) to yield chondrocytes. To reduce variability among animals, each leg was obtained from a different animal, and cells from all legs were combined together to create a mixture of chondrocytes; a mixture of cells from at least four legs was used in each study. Cell number was assessed on a hemocytometer, and viability remained >90%, as determined by a trypan blue exclusion test. Chondrocytes were frozen in culture medium supplemented with 20% fetal bovine serum (Biowhittaker, Walkersville, MD) and 10% dimethyl sulfoxide at −80°C for 1–2 weeks before use.
After thawing, viability remained greater than 85% in each study. A stainless steel mold consisting of 5-mm-dia. × 10-mm-long cylindrical prongs fit into 6 wells of a 48-well plate, and to construct each agarose well, sterile, molten 2% agarose was added to wells fitted with the stainless steel die. The agarose was allowed to gel at room temperature for 60 min, after which the mold was separated from the agarose. Culture medium was exchanged twice to completely saturate the agarose well with culture medium by the time of cell seeding. The medium was Dulbecco's modified Eagle's medium with 4.5 g/L-glucose and L-glutamine (Biowhittaker), 100 nM dexamethasone (Sigma, St. Louis, MO), 1% Fungizone/penicillin/streptomycin (Biowhittaker), 1% ITS+ (BD Scientific, Franklin Lakes, NJ), 50 μg/mL ascorbate-2-phosphate, 40 μg/mL L-proline, and 100 μg/mL sodium pyruvate (Fisher Scientific, Pittsburgh, PA). To each well, 5.5 × 106 cells were added in 125 μL of culture medium. The cells self-assembled within 24 h in the agarose wells and were maintained in the same well for a specified amount of time; t = 0 was defined as 24 h after seeding.
Preparation for specimen pressurization
Both bagged control (BC) and HP constructs were loaded into heat sealable bags (Kapak/Ampak Flexibles, Cincinnati, OH) previously sterilized by ethylene oxide. To each bag, 40 mL medium was added, and any air bubbles adhering to the bottom of the bag were released. The bags were then heat-sealed without any bubbles inside.
Specimen pressurization
BC specimens were placed into an opened pressure chamber maintained at 37°C, while pressure specimens were placed into a pressure chamber (Parr Instrument, Moline, IL), filled with water, and sealed underwater without any bubbles inside. The pressure chamber used has been described previously.10 Briefly, for 5 consecutive days, the specimens were pressurized to 10 MPa static HP for 1 h. After the execution of the desired regimen, the pressure chamber was disassembled, and the pouches were sterilized with 70% ethanol. In a sterile culture hood, the pouches were opened with autoclaved scissors, and the samples were returned to agarose-coated wells of six-well culture plates.
Phase I: selection of HP control
At 10 days, self-assembled constructs (n = 6/group) were removed from confinement in 5-mm-dia. agarose wells and exposed to 10 MPa static HP for 1 h/day for 5 days. The constructs were then placed in one well of a six-well culture plate coated with 2% agarose for the remainder of the study. A bagged control consisted of constructs removed from confinement in 5-mm-dia. agarose wells at 10 days, and placed in the HP chamber for 1 h/day for 5 days, but unpressurized. The constructs were then placed in one well of a six-well culture plate coated with 2% agarose for the remainder of the study. A culture control (CC) consisted of constructs removed from confinement in 5-mm-dia. agarose wells at 10 days, and cultured in one well of a six-well culture plate coated with 2% agarose for the remainder of the study. Five hundred microliters of medium per construct was changed daily, and all constructs were assessed at 4 weeks.
Phase II: temporal effects of HP application
At 6, 10, or 14 days, self-assembled constructs (n = 6/group) were removed from confinement in 5-mm-dia. agarose wells, and placed in one well of a six-well culture plate coated with 2% agarose. The constructs unconfined at 6 days were exposed to 10 MPa static HP, 1 h/day, from 6 to 10 days, and were cultured unconfined in the six-well plate for the remainder of the study. The 6-day culture control group (CC 6) remained unconfined in culture from 6 days until the conclusion of the study. The constructs unconfined at 10 days were exposed to 10 MPa static HP, 1 h/day, from 10 to 14 days, and were cultured unconfined in the six-well plate for the remainder of the study. The 10-day culture control group (CC 10) remained unconfined in culture from 10 days until the conclusion of the study. The constructs unconfined at 14 days were exposed to 10 MPa static HP, 1 h/day, from 14 to 18 days, and were cultured unconfined for the remainder of the study. The 14-day culture control group (CC 14) remained unconfined in culture from 14 days until the conclusion of the study. Five hundred microliters of medium per construct was changed daily, and all constructs were assessed at 4 weeks.
Phase III: short-term and long-term effects of HP application
At 10 days, constructs were removed from confinement, and 10 MPa static HP was applied for 1 h/day, from 10 to 14 days. A CC was treated as in phase I. Both HP and CC constructs (n = 6/group) were assessed at 2, 4, and 8 weeks.
Histology and immunohistochemistry
Samples were frozen and sectioned at 14 μm. GAG distribution was examined with a safranin-O/fast green stain.26,27 To examine collagen content, picrosirius red was used. Slides were also processed with immunohistochemistry (IHC) to test for the presence of collagen types I and II on a Biogenex (San Ramon, CA) i6000 autostainer. After fixation in chilled acetone, the slides were washed with IHC buffer (Biogenex), quenched of peroxidase activity with hydrogen peroxide/ methanol, and blocked with horse serum (Vectastain ABC kit; Vector Laboratories, Burlingame, CA). The slides were then incubated with either mouse anticollagen type I (Accurate Chemicals, Westbury, NY) or rabbit anticollagen type II (Cedarlane Labs, Burlington, NC) antibodies. Secondary antibody (anti-mouse or anti-rabbit IgG, Vectastain ABC kit) was applied, and color was developed using the Vectastain ABC reagent and DAB (Vectastain kit).
Quantitative biochemistry
Samples were frozen overnight and lyophilized for 72 h, followed by re-suspension in 0.8 mL of 0.05 M acetic acid with 0.5 M sodium chloride and 0.1 mL of a 10 mg/mL pepsin solution (Sigma) at 4°C for 72 h. Next, 0.1 mL of 10 × TBS was added along with 0.1 mL pancreatic elastase and mixed at 4°C overnight. From this digest, total DNA content was measured by Picogreen® Cell Proliferation Assay Kit (Molecular Probes, Eugene, OR). Total sulfated GAG was then quantified using the Blyscan Glycosaminoglycan Assay kit (Biocolor, Carrickfergus, United Kingdom), based on 1,9-dimethylmethylene blue binding.28,29 After being hydrolyzed by 2 N sodium hydroxide for 20 min at 110°C, samples were assayed for total collagen content by a chloramine-T hydroxyproline assay.30
Indentation testing
Samples were evaluated with an automated indentation apparatus.31 A step mass of 0.7 g (0.007 N) was applied with a 1 mm flat-ended, porous indenter tip, and specimens were allowed to creep until equilibrium, as described elsewhere.2 Preliminary estimations of the aggregate modulus of the samples were obtained using the analytical solution for the axisymmetric Boussinesq problem with Papkovich potential functions.32,33 The intrinsic mechanical properties of the samples, including aggregate modulus, Poisson's ratio, and permeability, were then determined using the linear biphasic theory.34
Tensile testing
Tensile tests were performed using a uniaxial materials testing system (Instron Model 5565, Canton, MA) with a 50 N load cell as described previously.35 Briefly, samples were cut into a dog-bone shape with a 1-mm-long gauge length. Samples were attached to paper tabs for gripping with cyanoacrylate glue outside of the gauge length. The 1-mm-long sections were pulled at a constant strain rate of 0.01 s−1. All samples broke within the gauge length. Stress–strain curves were created from the load–displacement curve and the cross-sectional area of each sample, and Young's modulus was calculated from each stress–strain curve.
Statistical analysis
All samples were assessed biochemically and biomechanically (n = 6). For phase I, a single-factor ANOVA was used to analyze the samples, and a Fisher LSD post hoc test was used when warranted. For phase II, a two-factor ANOVA was used to analyze the samples, and a Fisher LSD post hoc test was used when warranted. For phase III, a Student's t-test was used to compare the two groups at each timepoint. Significance was defined as p < 0.05.
Results
Gross appearance and histology
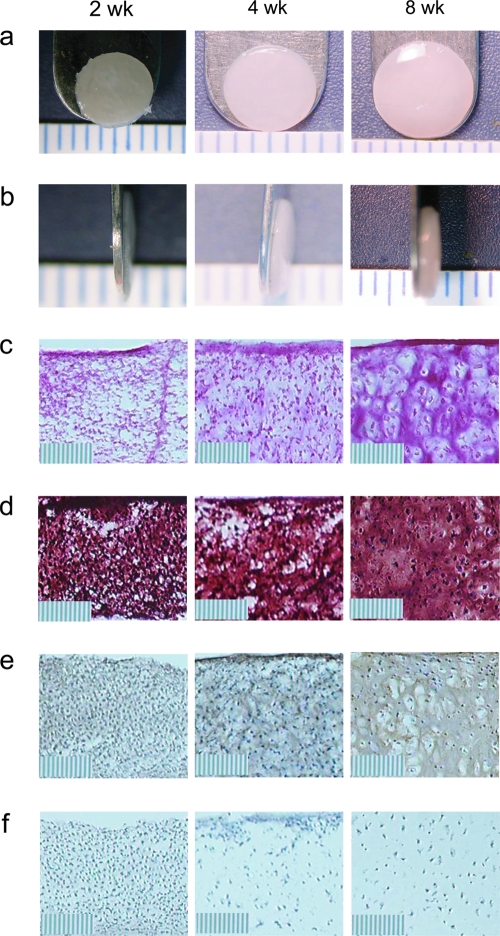
In all studies, the construct diameter slightly exceeded 6 mm at 4 weeks (Fig. 1a). In phase I, there were no differences in thickness among the HP, BC, and CC groups, with values of 0.46 ± 0.06, 0.41 ± 0.03, and 0.43 ± 0.06 mm, respectively (Fig. 1b). In phase II, there were no differences in thickness among the different groups, with values of 0.51 ± 0.03 and 0.50 ± 0.07 mm for the HP 6–10 and CC 6 groups, 0.52 ± 0.06 and 0.48 ± 0.07 mm for the HP 10–14 and CC 10 groups, and 0.54 ± 0.06 and 0.51 ± 0.09 mm for the HP 14–18 and CC 14 groups, respectively. In phase III, there were no differences in thickness among the groups, with values of 0.42 ± 0.03 and 0.41 ± 0.02 mm at 2 weeks, 0.70 ± 0.07 and 0.67 ± 0.05 mm at 4 weeks, and 0.93 ± 0.14 and 0.85 ± 0.16 mm at 8 weeks for the CC and HP groups, respectively.
FIG. 1.
Histological and immunohistochemical images representative of all self-assembled constructs at 2, 4, and 8 weeks (10 × original magnification; scale bar marks are 10 μm). (a) Gross morphology. (b) Gross morphology profile. (c) Picrosirius red–stained sections. (d) Safranin-O/fast green–stained sections. (e) Collagen II IHC sections. (f) Collagen I IHC sections. Color images available online at www.liebertonline.com/ten.
In each phase, all constructs stained positive for collagen throughout their thickness (Fig. 1c). Based on Safranin-O staining, GAG production was observed throughout the constructs (Fig. 1d). Based on IHC, collagen II was expressed throughout each construct (Fig. 1e), while there was no collagen I production (Fig. 1f).
Quantitative biochemistry
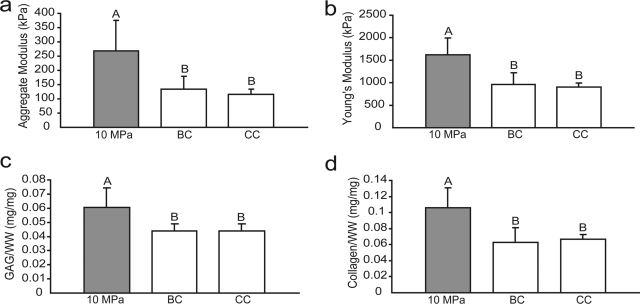
In phase I, there were no differences in wet weight (WW)/ construct or DNA/construct among the different treatment groups. The HP, BC, and CC groups had WW/construct values of 11.6 ± 1.9, 12.2 ± 0.6, and 12.0 ± 1.9 mg, and DNA/ construct values of 39.9 ± 8.9, 41.0 ± 11.0, and 36.9 ± 11.4 μg, respectively. The HP group had a significantly higher GAG/WW than either the BC or the CC groups, with values of 6.1 ± 1.4%, 4.4 ± 0.5%, and 4.1 ± 0.1%, respectively (Fig. 2c). The HP-treated group had a significantly higher collagen/WW than either the BC or the CC groups, with values of 10.6 ± 2.4%, 6.2 ± 1.9%, and 6.7 ± 0.6%, respectively (Fig. 2d).
FIG. 2.
Biomechanical and biochemical properties of self-assembled constructs in phase I. The HP-treated group exhibited a significantly higher (a) aggregate modulus, (b) Young's modulus, (c) GAG/WW, and (d) collagen/WW than BC or CC groups. Columns and error bars represent means and standard deviations. Groups denoted by different letters are significantly different (p < 0.05).
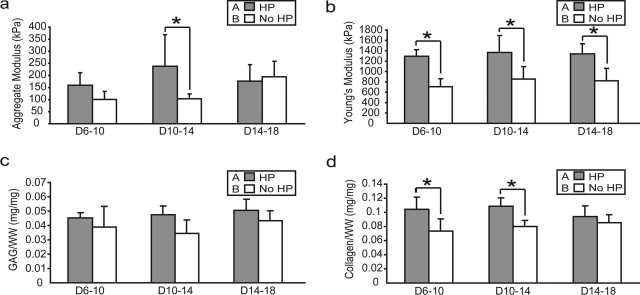
In phase II, there were no differences in WW/construct or DNA/construct among the different treatment groups. The WW/construct values were 10.7 ± 1.3 and 12.2 ± 1.5 mg for the HP 6–10 and CC 6 groups, 11.1 ± 1.2 and 11.0 ± 1.5 mg for the HP 10–14 and CC 10 groups, and 11.9 ± 1.7 and 11.0 ± 1.6 mg for the HP 14–18 and CC 14 groups, respectively. The DNA/construct values were 34.3 ± 7.5 and 32.3 ± 1.4 μg for the HP 6–10 and CC 6 groups, 43.6 ± 8.6 and 32.7 ± 7.6 μg for the HP 10–14 and CC 10 groups, and 44.6 ± 10.5 and 43.6 ± 14.2 μg for the HP 14–18 and CC 14 groups, respectively. HP was a significant factor for GAG/WW and collagen/WW. HP application from 6 to 10, 10 to 14, and 14 to 18 days increased GAG/WW from 3.9 ± 1.4% to 4.5 ± 0.4%, 3.5 ± 0.9% to 4.8 ± 0.6%, and 4.3 ± 0.7% to 5.1 ± 0.8%, respectively (Fig. 3c). HP application from 6 to 10, 10 to 14, and 14 to 18 days increased collagen/WW from 7.4 ± 1.7% to 10.4 ± 1.7%, 8.0 ± 0.9% to 10.8 ± 1.2%, and 8.5 ± 1.1% to 9.4 ± 1.5%, respectively (Fig. 3d).
FIG. 3.
Biomechanical and biochemical properties of self-assembled constructs in phase II. HP treatment was a significant factor for (a) aggregate modulus, (b) Young's modulus, (c) GAG/WW, and (d) collagen/WW. Columns and error bars represent means and standard deviations. Groups denoted by different letters are significantly different (p < 0.05) in the two-factor ANOVA (HP and application times). Asterisks (*) indicate significant difference from control (p < 0.05), based on the post hoc analysis comparing each individual group.
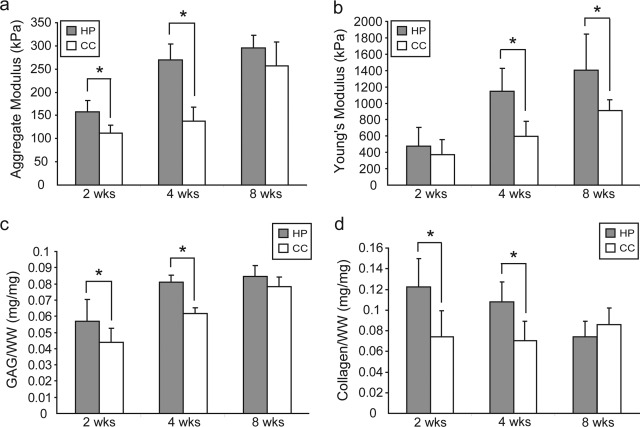
In phase III, the WW/construct values were 10.0 ± 1.2 and 7.7 ± 0.3 mg at 2 weeks, 17.1 ± 1.7 and 14.4 ± 2.2 mg at 4 weeks, and 28.5 ± 5.6 and 27.7 ± 3.9 mg at 8 weeks for the CC and HP groups, respectively. There were no differences in cellularity at each timepoint with DNA/construct values of 35.7 ± 4.7 and 34.0 ± 3.8 μg at 2 weeks, 37.1 ± 7.3 and 34.3 ± 2.0 μg at 4 weeks, and 33.5 ± 3.3 and 30.2 ± 3.0 μg at 8 weeks for the CC and HP groups, respectively. HP significantly increased GAG/WW from 4.4 ± 0.9% to 5.7 ± 1.3% at 2 weeks, and from 6.2 ± 0.3% to 8.1 ± 0.4% at 4 weeks. The GAG/WW was 7.8 ± 0.6% and 8.5 ± 0.7% for the CC and HP groups at 8 weeks (Fig. 4c). HP significantly increased collagen/WW from 7.4 ± 2.5% to 12.2 ± 0.3% at 2 weeks, and from 7.1 ± 1.8% to 10.8 ± 1.9% at 4 weeks. The collagen/WW was 8.6 ± 1.6% and 7.4 ± 1.5% for the CC and HP groups at 8 weeks (Fig. 4d).
FIG. 4.
Biomechanical and biochemical properties of self-assembled constructs in phase III. (a) Aggregate modulus was significantly increased by HP at 2 and 4 weeks. (b) Young's modulus was significantly increased by HP at 4 and 8 weeks. (c) GAG/WW and (d) collagen/WW were significantly increased at 2 and 4 weeks by HP application. Columns and error bars represent means and standard deviations. Asterisks (*) indicate significant difference from control (p < 0.05).
Mechanical evaluation
In phase I, the HP-treated group had a significantly higher aggregate modulus than the BC or CC groups, with values of 273 ± 123, 134 ± 45, and 116 ± 19 kPa, respectively (Fig. 2a). There were no differences among the groups in Poisson's ratio or permeability, with ranges of 0.14–0.19 and 3.94 ×10−14–9.78 × 10−14, respectively. Additionally, the HP-treated group had a significantly higher Young's modulus than the BC or CC groups, with values of 1.6 ± 0.4, 1.0 ± 0.3, and 0.9 ± 0.1 MPa, respectively (Fig. 2b).
In phase II, HP was a significant factor for aggregate modulus and Young's modulus. HP application from 10 to 14 days led to a significant increase in aggregate modulus from 101 ± 32 to 238 ± 131 kPa. HP application from 6 to 10 days increased aggregate modulus from 97 ± 24 to 159 ± 52 kPa, and HP application from 14 to 18 days decreased aggregate modulus slightly from 195 ± 64 to 177 ± 68 kPa (Fig. 3a). HP application did not significantly change Poisson's ratio from control for any group, with a range of 0.04–0.22. Additionally, there were no differences in permeability among the groups, with a range of 2.83 × 10−15–2.04 × 10−13. HP application from 6 to 10, 10 to 14, and 14 to 18 days increased Young's modulus from 0.9 ± 0.1 to 1.3 ± 0.1 MPa, 0.9 ± 0.2 to 1.4 ± 0.3 MPa, and 0.8 ± 0.2 to 1.3 ± 0.2 MPa, respectively (Fig. 3b).
In phase III, HP significantly increased aggregate modulus from 113 ± 16 to 158 ± 28 kPa at 2 weeks, and from 138 ± 30 to 270 ± 46 kPa at 4 weeks. There was no difference at 8 weeks, with values of 257 ± 51 and 296 ± 68 kPa for the CC and HP groups, respectively (Fig. 4a). There were no differences among the groups in Poisson's ratio or permeability, with ranges of 0.18–0.26 and 2.26 × 10−14–6.78 × 10−14. There was no difference in Young's modulus at 2 weeks, with values of 373 ± 182 and 476 ± 228 kPa for the CC and HP groups, respectively. HP significantly increased Young's modulus from 596 ± 185 to 1144 ± 281 kPa at 4 weeks, and from 912 ± 131 to 1404 ± 442 kPa at 8 weeks (Fig. 4b).
Discussion
This study utilized a three-phase approach to choose an appropriate control group for HP application, to determine the effects of temporal HP application, and to assess the temporal effects after HP application. To the best of our knowledge, this study is the first to assess the effects of HP application at different timepoints in construct development, and the first to examine short-term and long-term changes in construct properties after HP application.
In phase I, HP application significantly increased construct biomechanical and biochemical properties relative to both control groups, and the bagging process had no effect on construct properties. The application of 10 MPa static HP for 1 h/day, from days 10 to 14 led to a 120% increase in aggregate modulus and a 60% increase in Young's modulus, accompanied by significant increases in GAG and collagen content, respectively. Additionally, there were no differences in biomechanical, biochemical, histological, or gross morphological properties between the BC and CC groups. These results support our hypotheses, as HP application led to a significant increase in both compressive and tensile properties, and the bagging process inherent to HP application was shown to have no effect on construct biomechanical and biochemical properties. A comparison between the BC and CC groups was necessary to determine the effects of the bagging and handling process requisite for HP stimulation. In our setup, it is impossible to apply HP under sterile conditions without handling and bagging the constructs. As the handling and bagging is inherent to HP application, we consider HP application to include these inherent steps. Therefore, the CC group was selected for use in subsequent phases as it allows us to compare HP application in its entirety (including the handling and bagging process) to a control.
In phase II, it was determined that 10–14 days was the optimal time in construct development for HP application. HP application at all timepoints led to similar increases in tensile properties; however, HP application from 10 to 14 days had the greatest effect on aggregate modulus, a 140% increase. These results support our hypothesis, as HP application at a certain timepoint in construct development had the most beneficial effect on construct biomechanical properties. A 40% increase in GAG/WW accompanied the increased aggregate modulus of the 10–14 days HP group. On the other hand, HP application at all timepoints led to an approximately 0.5-fold increase in Young's modulus, accompanied by increases in collagen/WW. These results are interesting because they suggest that there may be different mechanisms for the effects of HP on compressive and tensile properties. Additionally, these results correlate with a prior study on self-assembled constructs that suggested that 10–14 days of construct development may be an important window for mechanical intervention.6
In phase III, HP application had immediate and delayed effects on construct properties. Application of static HP at 10 MPa for 1 h/day significantly increased compressive properties, GAG/WW, and collagen/WW immediately after the 5 days of HP application at 2 weeks, but the significant increase in tensile properties observed in the prior phases was delayed until 4 weeks. These results support our hypotheses, as the aggregate modulus was enhanced immediately after 10–14 days of HP application, while there was a delayed increase in tensile properties. This result was expected due to the slower turnover of collagen remodeling relative to GAG in the ECM. Since collagen content was quantified with a hydroxyproline assay, it is also possible that the measured collagen at 2 weeks was procollagen or immature collagen, which was not fully cross-linked or organized in the ECM until the next measurement at 4 weeks. Additionally, by 8 weeks, construct biomechanical and biochemical properties appear to level off, although a significant difference in tensile stiffness remains, likely as a result of the initial matrix formation present at the 4-week timepoint.
The results of these studies correlate with those of previous studies involving the use of static HP in physiologic magnitude ranges. For instance, Mizuno et al.22 found that the application of 2.8 MPa static HP to three-dimensional collagen sponges seeded with bovine articular chondrocytes led to a 3.1-fold increase in [(35)S]-sulfate incorporation in GAG. Additionally, Smith et al.17 observed a 32% increase in GAG synthesis with 10 MPa static HP application to high-density cultures of adult bovine articular chondrocytes. Likewise, Toyoda et al.21 found that 5 MPa static HP, applied to bovine articular chondrocytes cultured in agarose gels, significantly increased GAG synthesis and increased levels of aggrecan mRNA fourfold, while in a separate study, a 50% increase in the level of type II collagen mRNA was recorded with this same regimen.20 These results mirror the biochemical findings of the currently presented studies, as significant increases in both collagen content and GAG content were observed, that presumably led to significant increases in both compressive and tensile biomechanical properties.
Since HP application does not lead to cartilage deformation,36 it is difficult to envisage a mechanism to explain the beneficial effects of HP on construct biomechanical properties. However, as reviewed elsewhere,37 HP can deform the void spaces of cell transmembrane proteins, and at a certain pressure, the void space deformation leads to a change in protein conformation. This conformation change likely occurs in cell surface ion channels that act as “pressure sensors,” theoretically occurring over the pressures at which we see effects. For example, in chondrocytes, the Na/K pump and Na/K/2Cl transporter were shown to be sensitive to 10 MPa static HP application.38 Additionally, the Na/H pump39 and stretch-activated calcium channels40 in articular chondrocytes are affected by HP application. As ion concentration changes have been shown to alter protein synthesis,41 different ion channel responses to HP likely stimulate signal transduction cascades that eventually lead to upregulation of ECM-specific genes. The increased gene expression likely leads to increased ECM protein production, eventually resulting in enhanced biomechanical properties as observed in this study. Alternatively, although water has a high bulk modulus, it is not incompressible. Therefore, it is possible that 10 MPa HP results in stimulation of additional mechanotransduction pathways as a result of the compressibility of water, as this may result in small strains on the cells without a measurable construct deformation.
Although several studies have examined the effects of various HP regimens on construct gene expression and protein production, to our knowledge, this is the first study to assess the effects of temporal HP application, as well as the first to examine the immediate and long-term effects of HP on construct biomechanical and biochemical properties. Future studies should determine if combining HP with other mechanical stimulation, such as direct compression or shear, leads to additive or synergistic effects.
Acknowledgments
The authors would like to acknowledge funding from NIAMS R01 AR053286, as well as support from NIH grant No. 5T32 G-M008362.
Disclosure Statement
No competing financial interests exist.
References
- 1.Buckwalter JA. Articular cartilage: injuries and potential for healing. J Orthop Sports Phys Ther. 1998;28(4):192–202. doi: 10.2519/jospt.1998.28.4.192. [DOI] [PubMed] [Google Scholar]
- 2.Hu JC. Athanasiou KA. A self-assembling process in articular cartilage tissue engineering. Tissue Eng. 2006;12(4):969–979. doi: 10.1089/ten.2006.12.969. [DOI] [PubMed] [Google Scholar]
- 3.Furukawa KS. Suenaga H. Toita K. Numata A. Tanaka J. Ushida T et al. Rapid and large-scale formation of chondrocyte aggregates by rotational culture. Cell Transplant. 2003;12(5):475–479. doi: 10.3727/000000003108747037. [DOI] [PubMed] [Google Scholar]
- 4.Stewart MC. Saunders KM. Burton-Wurster N. Macleod JN. Phenotypic stability of articular chondrocytes in vitro: the effects of culture models, bone morphogenetic protein 2, and serum supplementation. J Bone Miner Res. 2000;15(1):166–174. doi: 10.1359/jbmr.2000.15.1.166. [DOI] [PubMed] [Google Scholar]
- 5.Waldman SD. Couto DC. Grynpas MD. Pilliar RM. Kandel RA. A single application of cyclic loading can accelerate matrix deposition and enhance the properties of tissue-engineered cartilage. Osteoarthritis Cartilage. 2006;14(4):323–330. doi: 10.1016/j.joca.2005.10.007. [DOI] [PubMed] [Google Scholar]
- 6.Elder BD. Athanasiou KA. Effects of confinement on the mechanical properties of self-assembled articular cartilage constructs in the direction orthogonal to the confinement surface. J Orthop Res. 2008;26(2):238–246. doi: 10.1002/jor.20480. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Afoke NY. Byers PD. Hutton WC. Contact pressures in the human hip joint. J Bone Joint Surg Br. 1987;69(4):536–541. doi: 10.1302/0301-620X.69B4.3611154. [DOI] [PubMed] [Google Scholar]
- 8.Hodge WA. Carlson KL. Fijan RS. Burgess RG. Riley PO. Harris WH et al. Contact pressures from an instrumented hip endoprosthesis. J Bone Joint Surg Am. 1989;71(9):1378–1386. [PubMed] [Google Scholar]
- 9.Huberti HH. Hayes WC. Patellofemoral contact pressures. The influence of q-angle and tendofemoral contact. J Bone Joint Surg Am. 1984;66(5):715–724. [PubMed] [Google Scholar]
- 10.Hu JC. Athanasiou KA. The effects of intermittent hydrostatic pressure on self-assembled articular cartilage constructs. Tissue Eng. 2006;12(5):1337–1344. doi: 10.1089/ten.2006.12.1337. [DOI] [PubMed] [Google Scholar]
- 11.Carver SE. Heath CA. Increasing extracellular matrix production in regenerating cartilage with intermittent physiological pressure. Biotechnol Bioeng. 1999;62(2):166–174. [PubMed] [Google Scholar]
- 12.Ikenoue T. Trindade MC. Lee MS. Lin EY. Schurman DJ. Goodman SB et al. Mechanoregulation of human articular chondrocyte aggrecan and type II collagen expression by intermittent hydrostatic pressure in vitro. J Orthop Res. 2003;21(1):110–116. doi: 10.1016/S0736-0266(02)00091-8. [DOI] [PubMed] [Google Scholar]
- 13.Parkkinen JJ. Ikonen J. Lammi MJ. Laakkonen J. Tammi M. Helminen HJ. Effects of cyclic hydrostatic pressure on proteoglycan synthesis in cultured chondrocytes and articular cartilage explants. Arch Biochem Biophys. 1993;300(1):458–465. doi: 10.1006/abbi.1993.1062. [DOI] [PubMed] [Google Scholar]
- 14.Smith RL. Carter DR. Schurman DJ. Pressure and shear differentially alter human articular chondrocyte metabolism: a review. Clin Orthop Relat Res. 2004;427 Suppl:S89–S95. [PubMed] [Google Scholar]
- 15.Smith RL. Lin J. Trindade MC. Shida J. Kajiyama G. Vu T et al. Time-dependent effects of intermittent hydrostatic pressure on articular chondrocyte type II collagen and aggrecan mRNA expression. J Rehabil Res Dev. 2000;37(2):153–161. [PubMed] [Google Scholar]
- 16.Elder BD. Athanasiou KA. Synergistic and additive effects of hydrostatic pressure and growth factors on tissue formation. PLoS ONE. 2008;3(6):e2341. doi: 10.1371/journal.pone.0002341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Smith RL. Rusk SF. Ellison BE. Wessells P. Tsuchiya K. Carter DR et al. In vitro stimulation of articular chondrocyte mRNA and extracellular matrix synthesis by hydrostatic pressure. J Orthop Res. 1996;14(1):53–60. doi: 10.1002/jor.1100140110. [DOI] [PubMed] [Google Scholar]
- 18.Hall AC. Urban JP. Gehl KA. The effects of hydrostatic pressure on matrix synthesis in articular cartilage. J Orthop Res. 1991;9(1):1–10. doi: 10.1002/jor.1100090102. [DOI] [PubMed] [Google Scholar]
- 19.Lammi MJ. Inkinen R. Parkkinen JJ. Hakkinen T. Jortikka M. Nelimarkka LO et al. Expression of reduced amounts of structurally altered aggrecan in articular cartilage chondrocytes exposed to high hydrostatic pressure. Biochem J. 1994;304 (Pt 3):723–730. doi: 10.1042/bj3040723. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Toyoda T. Seedhom BB. Kirkham J. Bonass WA. Upregulation of aggrecan and type II collagen mRNA expression in bovine chondrocytes by the application of hydrostatic pressure. Biorheology. 2003;40(1–3):79–85. [PubMed] [Google Scholar]
- 21.Toyoda T. Seedhom BB. Yao JQ. Kirkham J. Brookes S. Bonass WA. Hydrostatic pressure modulates proteoglycan metabolism in chondrocytes seeded in agarose. Arthritis Rheum. 2003;48(10):2865–2872. doi: 10.1002/art.11250. [DOI] [PubMed] [Google Scholar]
- 22.Mizuno S. Tateishi T. Ushida T. Glowacki J. Hydrostatic fluid pressure enhances matrix synthesis and accumulation by bovine chondrocytes in three-dimensional culture. J Cell Physiol. 2002;193(3):319–327. doi: 10.1002/jcp.10180. [DOI] [PubMed] [Google Scholar]
- 23.Khalafi A. Schmid TM. Neu C. Reddi AH. Increased accumulation of superficial zone protein (SZP) in articular cartilage in response to bone morphogenetic protein-7 and growth factors. J Orthop Res. 2007;25(3):293–303. doi: 10.1002/jor.20329. [DOI] [PubMed] [Google Scholar]
- 24.Mauck RL. Nicoll SB. Seyhan SL. Ateshian GA. Hung CT. Synergistic action of growth factors and dynamic loading for articular cartilage tissue engineering. Tissue Eng. 2003;9(4):597–611. doi: 10.1089/107632703768247304. [DOI] [PubMed] [Google Scholar]
- 25.Saini S. Wick TM. Effect of low oxygen tension on tissue-engineered cartilage construct development in the concentric cylinder bioreactor. Tissue Eng. 2004;10(5–6):825–832. doi: 10.1089/1076327041348545. [DOI] [PubMed] [Google Scholar]
- 26.Shimizu M. Minakuchi K. Kaji S. Koga J. Chondrocyte migration to fibronectin, type I collagen, and type II collagen. Cell Struct Funct. 1997;22(3):309–315. doi: 10.1247/csf.22.309. [DOI] [PubMed] [Google Scholar]
- 27.Rosenberg L. Chemical basis for the histological use of safranin O in the study of articular cartilage. J Bone Joint Surg Am. 1971;53(1):69–82. [PubMed] [Google Scholar]
- 28.Brown AN. Kim BS. Alsberg E. Mooney DJ. Combining chondrocytes and smooth muscle cells to engineer hybrid soft tissue constructs. Tissue Eng. 2000;6(4):297–305. doi: 10.1089/107632700418029. [DOI] [PubMed] [Google Scholar]
- 29.Pietila K. Kantomaa T. Pirttiniemi P. Poikela A. Comparison of amounts and properties of collagen and proteoglycans in condylar, costal and nasal cartilages. Cells Tissues Organs. 1999;164(1):30–36. doi: 10.1159/000016640. [DOI] [PubMed] [Google Scholar]
- 30.Woessner JF., Jr. The determination of hydroxyproline in tissue and protein samples containing small proportions of this imino acid. Arch Biochem Biophys. 1961;93:440–447. doi: 10.1016/0003-9861(61)90291-0. [DOI] [PubMed] [Google Scholar]
- 31.Athanasiou KA. Agarwal A. Dzida FJ. Comparative study of the intrinsic mechanical properties of the human acetabular and femoral head cartilage. J Orthop Res. 1994;12(3):340–349. doi: 10.1002/jor.1100120306. [DOI] [PubMed] [Google Scholar]
- 32.Sneddon I. The relaxation between load and penetration in the axisymmetric Boussinesq problem for a punch of arbitrary profile. Int J Eng Sci. 1965;3(1):47–57. [Google Scholar]
- 33.Hayes WC. Keer LM. Herrmann G. Mockros LF. A mathematical analysis for indentation tests of articular cartilage. J Biomech. 1972;5(5):541–551. doi: 10.1016/0021-9290(72)90010-3. [DOI] [PubMed] [Google Scholar]
- 34.Mow VC. Kuei SC. Lai WM. Armstrong CG. Biphasic creep and stress relaxation of articular cartilage in compression: theory and experiments. J Biomech Eng. 1980;102(1):73–84. doi: 10.1115/1.3138202. [DOI] [PubMed] [Google Scholar]
- 35.Aufderheide AC. Athanasiou KA. Assessment of a bovine co-culture, scaffold-free method for growing meniscus-shaped constructs. Tissue Eng. 2007;13(9):2195–2205. doi: 10.1089/ten.2006.0291. [DOI] [PubMed] [Google Scholar]
- 36.Bachrach NM. Mow VC. Guilak F. Incompressibility of the solid matrix of articular cartilage under high hydrostatic pressures. J Biomech. 1998;31(5):445–451. doi: 10.1016/s0021-9290(98)00035-9. [DOI] [PubMed] [Google Scholar]
- 37.Kornblatt JA. Kornblatt MJ. The effects of osmotic and hydrostatic pressures on macromolecular systems. Biochim Biophys Acta. 2002;1595(1–2):30–47. doi: 10.1016/s0167-4838(01)00333-8. [DOI] [PubMed] [Google Scholar]
- 38.Hall AC. Differential effects of hydrostatic pressure on cation transport pathways of isolated articular chondrocytes. J Cell Physiol. 1999;178(2):197–204. doi: 10.1002/(SICI)1097-4652(199902)178:2<197::AID-JCP9>3.0.CO;2-3. [DOI] [PubMed] [Google Scholar]
- 39.Browning JA. Walker RE. Hall AC. Wilkins RJ. Modulation of Na+ x H+ exchange by hydrostatic pressure in isolated bovine articular chondrocytes. Acta Physiol Scand. 1999;166(1):39–45. doi: 10.1046/j.1365-201x.1999.00534.x. [DOI] [PubMed] [Google Scholar]
- 40.Mizuno S. A novel method for assessing effects of hydrostatic fluid pressure on intracellular calcium: a study with bovine articular chondrocytes. Am J Physiol Cell Physiol. 2005;288(2):C329–C337. doi: 10.1152/ajpcell.00131.2004. [DOI] [PubMed] [Google Scholar]
- 41.Horowitz SB. Lau YT. A function that relates protein synthetic rates to potassium activity in vivo. J Cell Physiol. 1988;135(3):425–434. doi: 10.1002/jcp.1041350309. [DOI] [PubMed] [Google Scholar]