SUMMARY
The activity and specificity of serine/threonine phosphatases is governed largely by their associated proteins. α4 is an evolutionarily conserved non-catalytic subunit for PP2A-like phosphatases. While α4 binds to only a minority of PP2A-related catalytic subunits, α4 deletion leads to progressive loss of all PP2A, PP4, and PP6 phosphatase complexes. In healthy cells, association with α4 renders catalytic (C) subunits enzymatically inactive while protecting them from proteasomal degradation until they are assembled into a functional phosphatase complex. During cellular stress, existing PP2A complexes can become unstable. Under such conditions, α4 sequesters released C subunits and is required for the adaptive increase in targeted PP2A activity that can dephosphorylate stress-induced phosphorylated substrates. Consistent with this, overexpression of α4 protects cells from a variety of stress stimuli, including DNA damage and nutrient limitation. These findings demonstrate that α4 plays a required role in regulating the assembly and maintenance of adaptive PP2A phosphatase complexes.
INTRODUCTION
Protein phosphatase 2A (PP2A) and the related phosphatases PP4 and PP6 account for the majority of cellular serine-threonine phosphatase activity (for review see (Janssens and Goris, 2001)). The catalytic subunit of PP2A is among the most conserved enzymes in eukaryotic cells and is able to dephosphorylate serine and threonine residues without a significant influence from the flanking residues in the substrate. The specificity of PP2A phosphatase activity is conferred by its assembly into a trimeric complex. Existing models propose that the C subunit first dimerizes with a 65 kDa scaffolding subunit A. This core dimer can then associate through the A subunit with any of over 16 regulatory B subunits. It is the regulatory B subunits that determine substrate specificity and subcellular localization (Arroyo and Hahn, 2005; Cohen et al., 1990; Millward et al., 1999; Virshup, 2000; Xu et al., 2006).
In addition to association with the conventional A and B subunits, the C subunit has been found to form a complex with a protein termed α4 (Tap42 in yeast). Biochemical studies estimate that only a small fraction of cellular C subunit is bound to α4 and these complexes do not contain B regulatory subunits (Di Como and Arndt, 1996; Murata et al., 1997; Zolnierowicz, 2000). Despite this, α4 is an essential gene in all cell types and organisms in which it has been studied, suggesting it targets PP2A to essential evolutionarily conserved substrates or plays a novel role in phosphatase biology (Di Como and Arndt, 1996; Kong et al., 2004; Murata et al., 1997).
One specific pathway in which PP2A activity has been shown to play a regulatory role is DNA double strand break repair. To determine whether α4 contributes to the dephosphorylation of DNA repair proteins, we first examined the phosphorylation/dephosphorylation of p53, ATM, and H2AX in cells upon deletion of α4. The dephosphorylation of these substrates is required to resolve DNA repair foci and this dephosphorylation has been shown to depend on PP2A activity (Chowdhury et al., 2005; Goodarzi et al., 2004; Shouse et al., 2008). Although others had implicated traditional heterotrimeric PP2A complexes in these events, we found that α4 was required for dephosphorylation of each of these proteins. Furthermore, additional studies revealed that deletion of α4 affected the dephosphorylation of not only DNA repair proteins but a wide variety of established PP2A substrates. Biochemically, α4 was found to be selectively associated with C subunits lacking a scaffolding A subunit and association with α4 rendered C enzymatically inactive. Despite this, deletion of α4 led to loss of cellular PP2A, PP4, and PP6, suggesting that trimeric PP2A complexes were not as stable as previously believed and depended on α4 for maintenance and/or assembly. Consistent with this, overexpression of α4 stabilized newly-synthesized free C subunit by preventing ubiquitination-mediated degradation. In addition, our data suggest that existing PP2A complexes can be destabilized by heat shock and the released C subunits quantitatively sequestered by α4. Finally we found that α4 binding to C subunits provides the cell with a reserve of catalytic subunits that can be rapidly assembled into adaptive phosphatase complexes that promoted cell survival under times of stress.
RESULTS
Deletion of α4 leads to increased phosphorylation of multiple signaling proteins
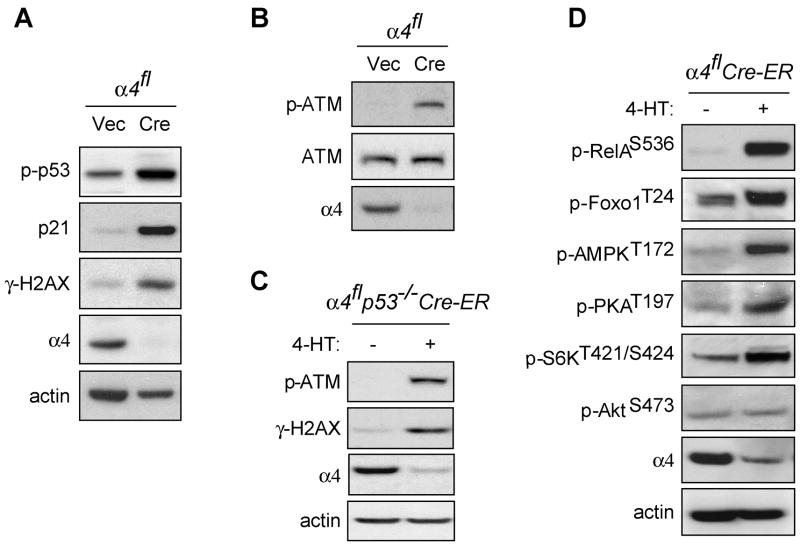
To investigate the essential role of α4 in PP2A function, male mouse embryonic fibroblasts (MEFs) carrying a floxed α4 allele on their single X chromosome were isolated. Retroviral Cre-mediated deletion of α4 in proliferating MEFs resulted in increased phosphorylation of a variety of DNA damage-response proteins, including p53, histone H2AX and ataxia-telangiectasia mutated (ATM) kinase (Figure 1A–B). This suggested that these proteins might be specific substrates of α4/C, particularly since the ability to resolve DNA damage-induced foci has recently been shown to depend on the combined activities of PP2A and PP4, the catalytic subunits of which share only α4 as a binding partner (Chowdhury et al., 2005; Chowdhury et al., 2008).
Figure 1. Deletion of α4 leads to increased phosphorylation of multiple signaling proteins.
A, B,α4fl MEFs were infected with MIGR1-GFP (Vec) or MIGR1-GFP-Cre (Cre) retrovirus. GFP-positive cells were sorted by flow cytometry after 24 h of infection and collected at 72 h. Immunoblotting was performed with antibodies as indicated in Experimental Procedures. The data presented are representative of at least four independent experiments. C, Tamoxifen inducible α4flp53−/− MEFs expressing Cre-ER fusion protein (α4flp53−/−Cre-ER) were treated with or without tamoxifen (4-HT) as described in Experimental Procedures. Immunoblotting was performed with antibodies as indicated. D, α4flCre-ER MEFs were treated with or without 4-HT for 48 h. Cell lysates were prepared and immunoblots probed with the indicated antibodies.
To examine the possibility that α4 deletion led to rapid induction of apoptosis as a result of this persistent activation of p53, we generated α4flp53−/− MEFs that expressed Cre-ER fusion protein to create cell lines in which α4 could be deleted upon tamoxifen treatment. In the absence of p53, cells remained viable followingCre induction for prolonged periods of time (Supplemental Figure 1). In these cells, loss of α4 still led to increased phosphorylation of H2AX and ATM (Figure 1C). The ATM kinase has been proposed to be responsible for H2AX phosphorylation during DNA damage (Burma et al., 2001). To examine whether H2AX and p53 phosphorylation upon α4 deletion was dependent on ATM, we expressed shRNA against ATM in α4flCre-ER MEFs and selected stable cell lines. Even in the absence of ATM, α4 deletion still led to increased phosphorylation of p53 and H2AX, and cell death occurred at similar kinetics as in cells containing ATM (data not shown).
These data suggest the possibility that α4 deletion might lead to a more generalized defect in PP2A activity rather than having a selective effect on the dephosphorylation of either p53 or ATM. To test whether α4 might have an effect on other PP2A targets, we surveyed the phosphorylation status ofseveral other reported PP2A target proteins following α4 deletion (Eichhorn et al., 2008; Wu et al., 2007; Yan et al., 2008). The absence of α4 led to increased phosphorylation of RelA, Foxo1, AMPK, PKA, and S6K. Increased protein phosphorylation following α4 deletion was restricted to established PP2A targets. For example, no change occurred in Akt Ser473, a site dephosphorylated by the PH domain leucine-rich repeat protein phosphatase but not PP2A (Figure 1D) (Gao et al., 2005). Thus, α4 is required to maintain a basal dephosphorylated state of a wide variety of proposed PP2A targets.
α4 is essential to maintain PP2A activity and protein levels
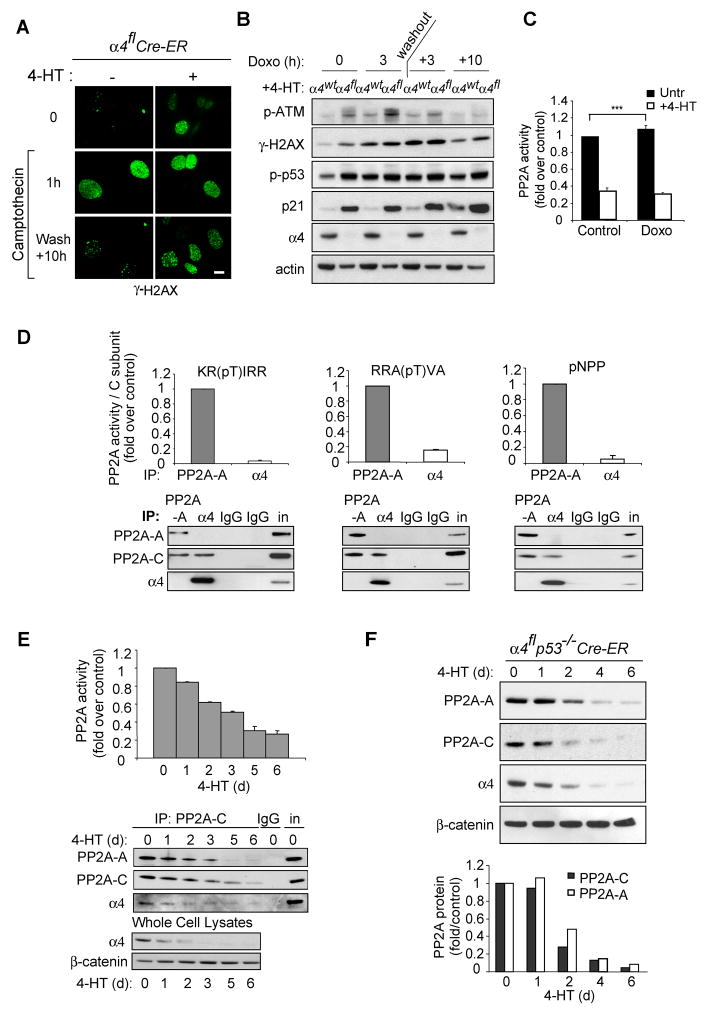
The ability to reverse stress-induced protein phosphorylation was also impaired in α4-deleted cells. In the absence of α4, induced levels of H2AX phosphorylation persisted following treatment with the DNA damaging agent, camptothecin (CPT) (Figure 2A). Biochemical analysis following treatment with another genotoxic reagent, doxorubicin, revealed that phosphorylation of ATM, H2AX and p53 was induced to a greater extent and took longer to resolve in α4-deficient cells (Figure 2B). In control cells, doxorubicin treatment was associated with a statistically significant increase in cellular PP2A activity as the phosphorylation of DNA repair proteins declined. In contrast, α4-depleted cells displayed an over 70% decline in total PP2A activity prior to treatment and total cellular PP2A activity decreased further following doxorubicin treatment (Figure 2C).
Figure 2. α4 is essential to maintain PP2A activity and protein levels.
A, α4flCre-ER MEFs were treated with or without 4-HT and then exposed to camptothecin (2 μM) for 1 h, and then allowed to recover in fresh medium. Immunofluorescence was performed with an antibody specific for γ-H2AX at the indicated timepoints. Scale bar = 10 μm. B, α4wtCre-ER MEFs (α4wt) or α4flCre-ER MEFs (α4fl) were treated with tamoxifen (4-HT) for 48 h and exposed to doxorubicin (Doxo) (2 μg/mL) for 3 h, and then allowed to recover for the indicated timepoints. Immunoblotting was performed with antibodies as indicated. The data are representative of three independent experiments. C, α4flp53&−/−Cre-ER MEFs were treated with or without 4-HT followed by doxorubicin (2 μg/mL) for 2 h. Phosphatase activity was determined as described in Experimental Procedures, and the data presented are mean ± S.D. of three independent experiments performed in duplicate (*** P<0.005 by Student’s t-test). D, 3T3 MEFs were lysed and immunoprecipitated (IP) with the indicated antibodies. Phosphatase activity was determined using the indicated substrates, and activity was normalized for the amount of C subunit as indicated in the immunoblots. Values are means ± S.D. E, α4flp53−/−Cre-ER MEFs were treated with 4-HT for the indicated periods of time, and phosphatase activity was assayed as described in Experimental Procedures. Immunoblots show the PP2A complex immunoprecipitated by the PP2A-C antibody or total protein levels in whole cell lysates. Values are means ± S.D. F, α4flp53−/−Cre-ER MEFs were treated with 4-HT for the indicated periods of time, and lysates were analyzed by immunoblotting. The bar graph represents quantitation of immunoblots using Image J software (NIH). The data are representative of at least three independent experiments.
Previous studies in yeast have estimated that only about 10% of cellular C subunit is bound to α4 (Di Como and Arndt, 1996). However, 60 hours after Cre-mediated deletion of the α4 gene in MEFs, we found a greater than 70% decrease in PP2A activity (Figure 2C), suggesting that α4-associated PP2A-C might be preferentially active against the substrate (KR(pT)IRR) used in the assay. To address this issue, we sought to determine the relative amount and specificity of the PP2A activity associated with α4. Phosphatase activity was assayed in immunoprecipitates from MEFs using antibodies against α4 or the A subunit, and normalized for the level of precipitated catalytic C subunit. Since different PP2A complexes can possess different substrate specificity, three different substrates were used for the PP2A activity assay, 2 phospho-peptides and p-Nitrophenyl Phosphate (pNPP) (Figure 2D). The catalytic subunit associated with α4 was inactive against all 3 substrates. In contrast, the catalytic subunit precipitated with the A subunit was able to dephosphorylate each substrate.
Given that PP2A activity lies predominantly in A/C complexes, we sought to more carefully evaluate the decrease in PP2A activity observed upon α4 deletion, and assessed PP2A activity upon α4 deletion in α4flp53−/−Cre-ER MEFs. The PP2A phosphatase activity in 500 μg of total cell extract diminished progressively following α4 deletion (Figure 2E). Western blot analysis of immunoprecipitates over this timecourse showed a comparable decrease in the levels of the A/C core enzyme. Since induction of PP2A activity has been reported to be required to resolve DNA damage foci, we also tested the effect of CPT treatment on PP2A holoenzyme expression in the presence or absence of α4. Strikingly, we found that cells lacking α4 had significantly decreased total levels of A and C protein in the presence and absence of CPT treatment (Supplemental Figure 2). Finally, when we monitored the levels of the individual proteins during a timecourse of α4 deletion, the protein level of the C subunit decreased in direct correlation with the extent of α4 loss (Figure 2F). The levels of the A subunit also displayed a significant decrease.
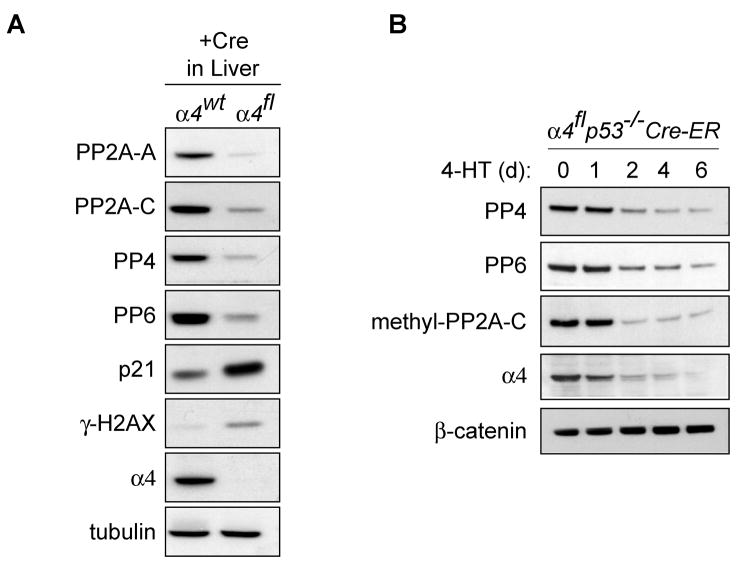
To test whether these findings in cultured cell lines also occur in vivo, we examined liver samples from α4wt or α4fl mice following infection with Adeno-Cre (Kong et al., 2004). Adult α4wt and α4fl mice were injected with an adenovirus encoding Cre through the tail vein, which selectively leads to infection of the liver parenchyma (Rohlmann et al., 1996). Strikingly, Western blot analysis of liver samples showed significant loss of A and C subunits upon α4 deletion. Accompanying this decline, there was increased phosphorylation of H2AX and induction of p21 and other stress response signals (Figure 3A) (Kong et al., 2004). In addition, two other α4-associated phosphatases, PP4 and PP6 (Chen et al., 1998), also substantially decreased upon α4 deletion in vivo (Figure 3A). To confirm this finding, the levels of PP4 and PP6 were analyzed following α4 deletion in α4flp53−/−Cre-ER MEFs. Both proteins declined progressively following α4 deletion (Figure 3B). Therefore, both in cell culture and in vivo, α4 was required to maintain active PP2A and PP2A-related catalytic subunits. Finally, a proportion of the mature PP2A-C that participates in trimeric PP2A holoenzyme complexes is reported to be methylated (Ikehara et al., 2007; Ogris et al., 1997; Tolstykh et al., 2000; Wei et al., 2001; Xing et al., 2008). α4 deletion results in depletion of this fraction of PP2A-C (Figure 3B). Thus, it appears that the stability of all PP2A-related catalytic subunits is disrupted following α4 deletion.
Figure 3. α4 deletion leads to loss of PP4, PP6 and methylated PP2A-C.
A, Liver samples from α4wt or α4fl mice at 6 days after injection with Adeno-Cre were analyzed by immunoblotting with antibodies as indicated. B, α4flp53−/−Cre-ER MEFs were treated with 4-HT for the indicated periods of time, and lysates were analyzed by immunoblotting.
α4 regulates PP2A A/C complex assembly and stability
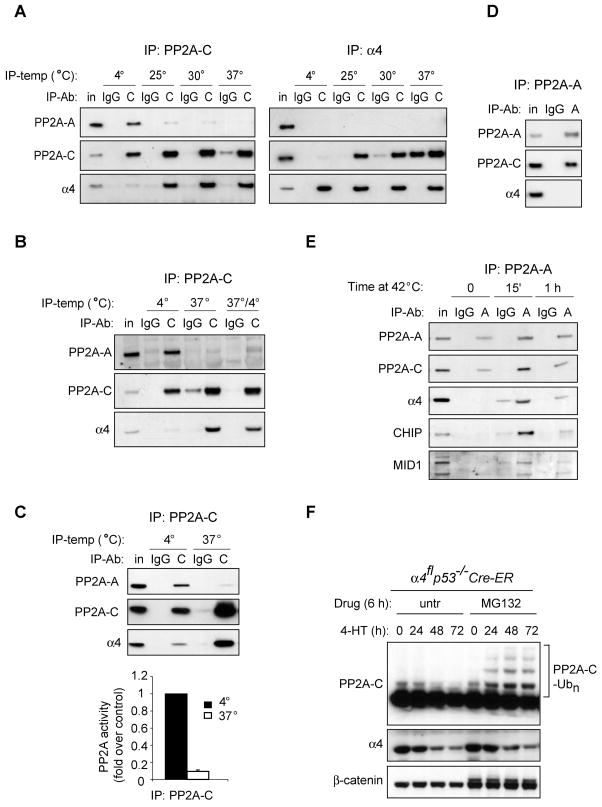
The above data suggest that active PP2A holoenzyme complexes turn over more rapidly and are potentially more unstable than previously believed. To test the stability of PP2A-C containing complexes, we performed immunoprecipitations at different temperatures. When cells were lysed on ice and immunoprecipitated with antibodies against α4 or C at 4°C, a relatively small fraction of C subunit associates with α4, as the C subunit preferentially pulled down the A subunit (Figure 4A). However, when the immunoprecipitations were carried out at higher temperatures, the association between C and A subunits was less and a corresponding increase in the association between α4 and C was observed. These changes occurred despite the fact that the total protein levels of all 3 proteins were unaffected by incubation at the different temperatures (Figure 4A and data not shown). If cells were lysed at 37°C, and then chilled to 4°C prior to immunoprecipitation, the C subunit was found to be preferentially associated with α4 (Figure 4B). Consistent with the above data that most C subunit associated with α4 is enzymatically inactive, immunoprecipitations of the C subunit performed at 37°C showed preferential association with α4 and very little PP2A phosphatase activity towards the phospho-peptide KR(pT)IRR (Figure 4C).
Figure 4. α4 can sequester and inhibit PP2A-C released from existing holoenzyme complexes.
A, 3T3 MEFs were lysed and immunoprecipitated with antibodies against the PP2A C subunit, or α4, or control IgG at the indicated temperatures. In each panel, data are representative of experiments that have been replicated at least three times. B, 3T3 MEFs were lysed at 4°C and then immunoprecipitated at 4°C or 37°C. Alternatively cell lysates were incubated at 37°C for 3 h followed by immunoprecipitation at 4°C (37°/4°). C, 3T3 MEFs were lysed and immunoprecipitated with the C subunit antibody at either 4°C or 37°C. Immunoprecipitates were then used for the phosphatase assay as described in Experimental Procedures. Values are means ± S.D. D, 3T3 MEFs were lysed and immunoprecipitated with antibodies against the A subunit or control IgG at 4°C. E, 3T3 MEFs were subjected to heat shock (42°C) for the indicated amounts of time, then lysed and immunoprecipitated with antibody against the A subunit or IgG at 4°C. F, α4flp53−/−Cre-ER MEFs were treated with tamoxifen (4-HT) for the indicated periods of time, with or without proteasome inhibitor MG132 (10 μM) for the last 6 h before lysis. A–F, Immunoblots were probed with the specified antibodies and the bracket in F indicates polyubiquitination of the C subunit (PP2A-C-Ubn).
The above data suggest that PP2A A/C complexes can be unstable. To examine the ability of heat stress to affect the stability of A/C complexes, we subjected cells to transient heat shock. Despite having never observed binding between α4 and the A subunit under standard immunoprecipitation conditions (Figure 4D), immunoprecipitation using antibody to the A subunit consistently brought down α4 following heat shock (Figure 4E). Upon heat shock, the A subunit also immunoprecipitated Mid1 and CHIP, which are both E3 ubiquitin ligases reported to regulate the turnover of PP2A subunits (Luo et al., 2006; Trockenbacher et al., 2001). This suggests that recruitment of α4 to an established PP2A A/C complex protects the C subunit from degradation. As an alternative approach to determining if α4 contributed to protecting the C subunit from degradation, we repeated the α4 deletion experiments in the presence or absence of the proteasome inhibitor MG132. As α4 declined, there was an accumulation of ubiquitinated C when MG132 was present (Figure 4F).
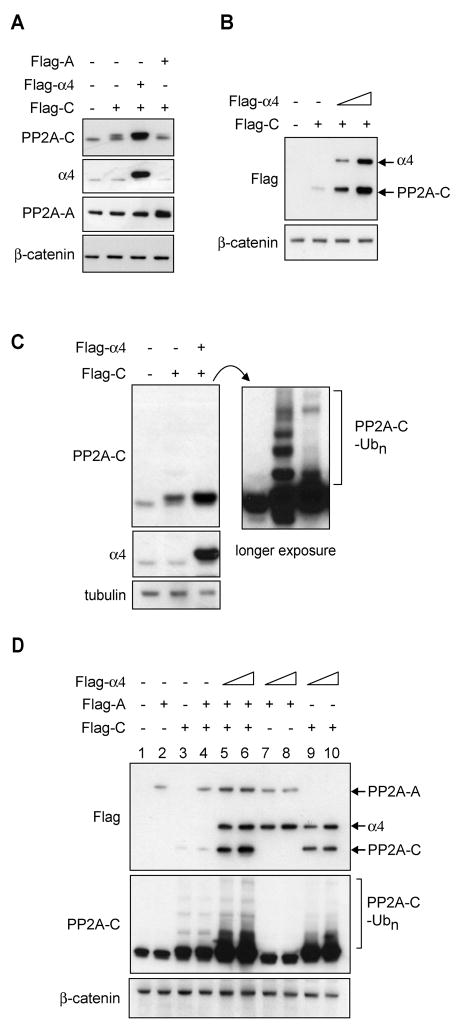
Enhanced expression of PP2A-C in cells has been difficult to achieve since cells tightly regulate PP2A protein levels (Baharians and Schonthal, 1998; Chung and Brautigan, 1999; Wadzinski et al., 1992). We next tested whether α4 plays a role in stabilizing newly-synthesized free C subunit. Consistent with observations from other groups, transfection of a C subunit expression plasmid led to only a small increase in PP2A-C expression (Figure 5A, lane 2). However, co-expression of α4 significantly increased protein levels of the C subunit (Figure 5A, lane 3) and the increase correlated with the amount of α4 co-expressed (Figure 5B). Overexpression of the C subunit alone resulted in a substantial increase in the amount of ubiquitinated C subunit that could be detected. Co-transfection of an α4-expression plasmid in contrast not only enhanced C expression but also resulted in a substantial reduction in the amount of ubiquitinated C subunit that could be detected (Figure 5C and Supplemental Figure 3). In contrast to α4 co-expression, co-expression of the A subunit failed to lead to enhanced expression of PP2A-C or prevent its ubiquitination (Figure 5A and D). When all three expression constructs were co-transfected an additional increase in both the A and C subunits was observed over the levels observed when only A and C were co-transfected (Figure 5D, lanes 5–6 versus lane 4). However, the increase in C associated with the transfection of all 3 constructs was accompanied by an increase in the level of ubiquitinated C that was observed (Figure 5D, lanes 5–6).
Figure 5. α4 promotes enhanced PP2A-C expression by preventing PP2A-C ubiquitination.
A, Cells (293T) were transiently transfected with constructs containing vector, Flag-PP2A-A, Flag-PP2A-C, or Flag-α4 (2.5 μg DNA each for a total of 5 μg DNA per sample) and protein was analyzed by Western blotting at 72 h post-transfection. B, Stabilization of Flag-PP2A-C is proportional to α4 expression levels. Cells were transfected with a total of 5 μg DNA containing vector alone, Flag-PP2A-C (2.5 μg), and/or increasing amounts of Flag-α4 (1.25 – 2.5 μg), and protein was analyzed at 72 h post-transfection. C, Cells expressing Flag-PP2A-C or equal amounts of Flag-PP2A-C and Flag-α4 as in panel A were harvested at 72 h after transfection. A shorter exposure is provided to assess relative levels of PP2A-C (left upper panel) and longer exposure (right panel) reveals ubiquitinated PP2A-C levels. D, Cells were transfected with 2.5 μg of constructs encoding Flag-PP2A-C, Flag-PP2A-A, and either 1.25 or 2.5 μg of Flag-α4 as indicated together with vector control for a total of 7.5 μg DNA. The levels of all three tagged proteins were determined through recognition of the Flag epitope by Western blotting (upper panel).
α4 expression protects cells from various stress stimuli
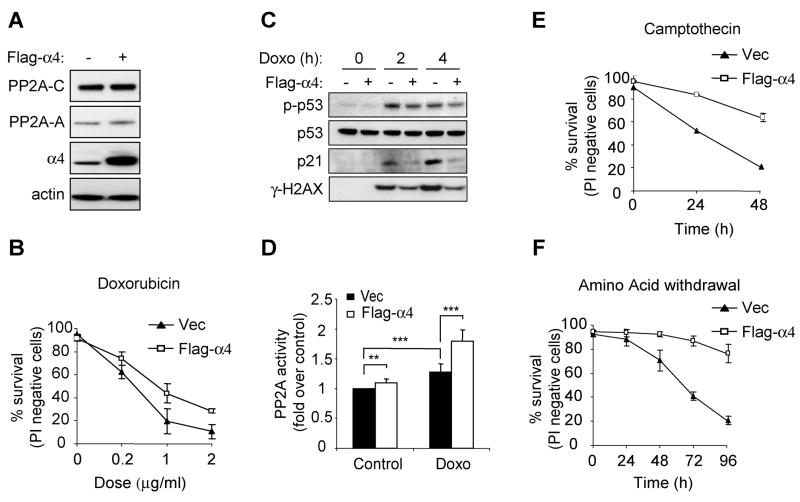
The above data suggest that α4 binding to the catalytic subunit stabilizes the free C subunit and inhibits its enzymatic activity, preventing promiscuous substrate dephosphorylation but leaving it available to be assembled into PP2A-A containing complexes that are enzymatically active but relatively unstable. Thus, α4 binding maintains a pool of the C subunit which could potentially be utilized to reassemble active PP2A complexes and promote cellular recovery from stresses that perturbed the kinase-phosphatase balance. To test this model, we examined whether overexpression of α4 plays a role in cellular recovery from stress. Overexpression of α4 alone had only a small (~10%) effect on the levels of steady state PP2A holoenzyme (Figure 6A). Despite this, cells expressing Flag-α4 showed significantly improved viability following exposure to the DNA damaging agent doxorubicin (Figure 6B). Upon doxorubicin treatment, cells expressing Flag-α4 more rapidly reversed the induced phosphorylation of H2AX and p53, and displayed reduced induction of p21 (Figure 6C). Cells expressing Flag-α4 displayed a significantly greater increase in PP2A activity during recovery from doxorubicin treatment than control cells (Figure 6D). Increased α4 expression also protected from other genotoxic reagents, such as camptothecin (Figure 6E). Finally, the ability of α4 to promote survival is not limited to DNA-damage responses, as α4 overexpression also provided cells with the potent ability to survive amino acid withdrawal (Figure 6F).
Figure 6. α4 expression protects cells from various stress stimuli.
A, 3T3 MEFs stably expressing vector or Flag-α4 were lysed and immunoblotting was performed using antibodies as indicated. In each panel, representative experiments are presented and are illustrative of at least three independent experiments. B, 3T3 MEFs expressing vector or Flag-α4 were treated with doxorubicin and viability was assessed by propidium iodide exclusion at 24 h. The data are presented as mean ± S.D. of triplicate samples in two independent experiments. C, 3T3 MEFs expressing vector (−) or Flag-α4 (+) were treated with doxorubicin (2 μg/mL). Cell lysates were prepared at 0, 2, or 4 h following treatment and immunoblots were probed with the indicated antibodies. D, Phosphatase activity was determined from immunoprecipitates of the C subunit in 3T3 MEFs expressing vector or Flag-α4 following 2 h treatment in the presence or absence of doxorubicin (Doxo, 2 μg/mL), as described in Experimental Procedures. The data presented are mean ± S.D. of three independent experiments. E–F, 3T3 MEFs expressing vector or Flag-α4 were treated with camptothecin (10 μM) or subjected to glutamine deprivation and viability was assessed by propidium iodide exclusion at the indicated timepoints. Data are representative of at least 2 independent clones for each cell type and are shown as the average of 3 experiments ± S.D. (**P<0.01, ***P<0.005 by Student’s t test).
DISCUSSION
Biochemical studies using purified enzymes demonstrate that catalytic subunits of serine-threonine phosphatases possess relatively promiscuous activity towards a range of phosphorylated substrates (Millward et al., 1999). However, accumulating evidence suggests that fully assembled PP2A phosphatase complexes exhibit a more restricted range of substrate specificity. How cells prevent the promiscuous activity of any uncomplexed catalytic subunit is largely unknown. Here we show that α4 serves as a binding partner of the C subunit that not only renders it catalytically inactive but also contributes to the ability of C to be assembled into functional PP2A phosphatase complexes. Without α4, not only PP2A but PP4 and PP6 catalytic subunit levels rapidly decline, and the cell is incapable of dephosphorylation of reported PP2A-family substrates. The ability to stabilize PP2A family catalytic subunits in an inactive conformation until they can be partnered with relevant A and B subunits explains why α4 is essential for eukaryotic cells. Protein phosphatases must be just as tightly regulated as their kinase counterparts. Disruption of α4 leads to such a profound deficiency in PP2A-related phosphatase activity that cells cannot appropriately modulate the phosphorylation of stress response proteins. Persistent stress signaling is linked to the initiation of apoptosis in mammals. However, even in yeast, which lack classical apoptosis, the inability to modulate stress signaling prevents the cells from proliferating following α4 (Tap42) deletion (Di Como and Arndt, 1996; Duvel et al., 2003; Santhanam et al., 2004).
It has been believed that α4 acts as a competitor to the A subunit since their association with the C subunit occurs in a mutually exclusive manner. The binding site of the A subunit on C partially overlaps with that of α4 on C (Jiang and Broach, 1999; Prickett and Brautigan, 2004). Prior studies have demonstrated that the binding of PP2A-C subunit to α4 increases in a temperature dependent fashion (Prickett and Brautigan, 2004). Furthermore, these authors found that PP2A-C was thermolabile in cell extracts, a finding consistent with reports that PP2A holoenzyme complexes can be unstable under physiologic conditions (Li et al., 2002; Silverstein et al., 2002; Strack et al., 2004). Correlated with these findings, our results show that when existing A/C complexes are destabilized by heat shock, α4 can bind and stabilize the released C subunit. However, rather than acting as a competitor to A/C binding, α4 binding to C is required for maintenance of PP2A holoenzyme activity. These data suggest that α4 binds and inhibits the enzymatic activity of either newly synthesized C or C released from active A/C complexes. Although α4-bound C is inactive, α4 binding is required to initiate its incorporation and/or reincorporation into active PP2A holoenzymes.
These data potentially fill a hole in our current understanding of the assembly of PP2A holoenzyme complexes. Previous studies have demonstrated that PP2A catalytic activity can also be regulated by reversible methylation and phosphorylation of the carboxyterminal tail of the C subunit. These modifications have been shown to influence the incorporation of specific regulatory B subunits into the final holoenzyme complex (Chung et al., 1999; Hombauer et al., 2007; Xing et al., 2008). In the absence of these modifications, the C subunit preferentially associates with α4 (Chung et al., 1999). Thus, the combined data suggests the primary regulation of C subunit inactivation results from its binding to α4. Based on our data, α4 binding inactivates and stabilizes the C subunit. Subsequent modification of the carboxyterminal tail may facilitate the recruitment of additional factors that direct the assembly of the C subunit into holoenzyme complexes. Recent structural analysis of the yeast α4-homologue, Tap42, revealed similarity to a tetratricopeptide repeat (TPR)-like fold, which is found in scaffold and chaperone-like proteins and is thought to mediate protein-protein interactions (Yang et al., 2007). Together with our data, these structural similarities suggest that α4 may act as a general scaffold/chaperone protein in maintaining proper PP2A function and assembly.
Our data support roles for α4 in maintaining PP2A function in both stressed and non-stressed conditions. In the absence of an imposed external stress, deletion of α4 resulted in the loss of PP2A as well as PP4 and PP6 catalytic subunits from the cell. α4 also appears to function in stress response pathways, as reduction of α4 led to a sustained phosphorylation of DNA damage responsive proteins and overexpression of α4 promoted recovery from DNA damage and survival from amino acid withdrawal. Recent studies have also shown that specific trimeric PP2A and PP4 complexes regulate the DNA damage response through dephosphorylation of γ-H2AX (Chowdhury et al., 2005; Chowdhury et al., 2008). The present data demonstrate that α4 is genetically upstream of these trimeric holoenzyme complexes in the regulation of the DNA damage response. α4 also contributes to the ability of cells to adaptively survive nutrient deprivation. The fact that PP2A activity rapidly declines and cannot be induced in response to a variety of stress stimuli upon α4 deletion demonstrates that a cell’s ability to adaptively regulatePP2A phosphatase activity depends on α4. The present data establish that α4 is a limiting component in the assembly and maintenance of PP2A phosphatase activity, as forced over-expression of α4 leads to a more rapid resolution of stress-induced serine/threonine phosphorylation and increased cell survival. Together, these data identify α4 as a key component in the ability of cells to maintain/modulate the activities of the PP2A-family of phosphatases.
EXPERIMENTAL PROCEDURES
Cell culture and cell death assay
MEFs and 293T cells were cultured in Dulbecco’s modified Eagle’s medium (DMEM) supplemented with 10% fetal bovine serum (FBS), 1% HEPES (N-2-hydroxyethylpiperazine-N′-2-ethanesulfonic acid), 1% L-glutamine, 100 units/mL of penicillin, and 100 μg/mL of streptomycin. Cell lines expressing Flag-α4 in 3T3 MEFs, or α4fl-Cre-ER MEFs have been described elsewhere (Kong et al., 2007). For doxorubicin (Sigma), and camptothecin (CPT, Sigma) treatment, drugs were added to the medium at the indicated doses. At the desired timepoints, cells were collected by trypsinization and incubated with propidium iodide (PI, 1 μg/mL; Molecular Probes). Cell death was determined using flow cytometry by PI exclusion. To examine recovery following exposure to drugs, cells were treated with camptothecin for 1 h or doxorubicin for 3 h, then washed and fed with fresh medium with no drugs, and cultured for indicated periods of time.
Generation of α4flp53−/− MEFs
α4flp53−/− MEFs were generated from breeding α4flp53−/− male mice with α4fl/wtp53−/− female mice. Mice were bred and maintained at the University of Pennsylvania in accordance with the Institutional Animal Care and Use Committee guidelines. α4flp53−/− MEFs were immortalized according to 3T3 protocols and were infected with lentiviral supernatant expressing Cre-estrogen receptor (ER) fusion protein provided by Dr. Eric Brown (University of Pennsylvania). Cre expression was induced in these cell lines by addition of 200 nM 4-hydroxytamoxifen (4-HT) to the medium for 48 hours if not indicated otherwise. Where indicated, proteasome inhibitor MG132 (10 μM, Sigma) was added to the medium for the last 6 h of incubation.
Immunoblotting and immunofluorescence
Cells were lysed in RIPA buffer (1% sodium deoxycholate, 0.1% SDS, 1% Triton X-100, 10 mM Tris at pH 8.0, 150 mM NaCl) with protease inhibitor complex (Roche). Equal amounts of protein (10–40 μg) were loaded on precast 4%–12% Bis-Tris NuPAGE gels (Invitrogen), followed by transfer onto nitrocellulose. Western blotting was performed with the following antibodies: FLAG (M2), PP6, tubulin and ATM (Sigma); PP4, p21 and actin (Santa Cruz); p-H2AX S139 (γ-H2AX), p-p53 S15, p-AMPK T172, p-PKA T197, p-S6K T421/S424, p-Foxo1 T24, p-RelA S536, p-Akt S473 and β-catenin (Cell Signaling); PP2A-C subunit (1D6, Upstate); p53 (Novocastra Laboratories); p-ATM S1981 (Rockland Immunochemical); CHIP/STUB1 and PP2A-C methyl L309 (Abcam), MID1 (gift from Timothy Cox, Seattle); α4 (rabbit (Kong et al., 2007), mouse Upstate); PP2A-A subunit (α and βisoforms) (6G3, Cell Signaling; or sheep, Abcam). For immunofluorescence, cells were fixed in 4% paraformaldehyde and permeabilized for 10 min in PBS containing 0.2% Triton X-100. Cells were washed with PBS containing 0.02% Triton X-100 and 1.5% FBS, followed by incubation with γ-H2AX antibody (Cell Signaling) for 1 h at room temperature. Cells were incubated with fluorescein isothiocyanate (FITC)-conjugated secondary antibody (Jackson ImmunoResearch Laboratories). Nuclei were visualized by staining with 1 μg/mL DAPI. Images were captured using a Nikon E800 fluorescence microscope equipped with a CCD camera at 100x objective and images were analyzed using Metamorph software package.
PP2A activity assay
Cellular protein phosphatase 2A (PP2A) activity was assayed using a PP2A immunoprecipitation phosphatase assay kit (Upstate). Cells were washed in TBS, then lysed on ice in phosphatase assay buffer (20mM imidazole-HCl, 2mM EDTA, 2mM EGTA, 0.1% NP-40, pH 7.0, with freshly added protease inhibitors). PP2A-C subunit was immunoprecipitated from total cell lysates (500 μg) using 3 μg of anti-PP2A-C antibody (clone 1D6, Upstate) and Protein G agarose for 2 h at 4°C (or at 37 °C where indicated). PP2A activity was assayed by incubating the immunoprecipitated protein with the synthetic phosphopeptide K-R-pT-I-R-R at 30°C for 10 min prior to detection with malachite green phosphate detection solution, according to the manufacturer’s instructions. Where indicated, phosphatase activity was normalized to the relative amount of immunoprecipitated C subunit according to Western blot quantitation using Image J software (NIH). Where indicated, the assay was modified by using alternative synthetic phosphopeptides RRA(pT)VA (Promega) or pNPP (Calbiochem).
Immunoprecipitation
Typically, one 15-cm plate containing cells grown to 80% confluence was used for each treatment condition and divided into aliquots for different immunoprecipitation conditions or antibodies. To harvest, MEFs were washed twice with PBS on the plate, then lysed on ice using a cell scraper with lysis buffer (150 mM KCl, 0.2% NP-40, 10% glycerol, 20 mM Tris pH 7.5, 0.5 mM DTT) containing freshly added protease inhibitor complex (Roche). Following 15 min incubation on ice, lysates were sonicated at a low setting (~7 watts) for 10 seconds, and then cleared at high speed centrifugation at 14000 rpm for 10 min before protein quantitation using the BCA assay (Pierce). Equal amounts of lysate (500 μg – 1 mg) were immunoprecipitated while rotating at 4°C with 3 μg of one of the following antibodies: PP2A-A subunit (6F9, Abcam), PP2A-C subunit (1D6, Upstate), α4 (Kong et al., 2007), or IgG controls: IgG-Rat (BD Biosciences), IgG-Mouse or IgG-Rabbit (Sigma). Protein G agarose beads (30 μl) were added to each immunoprecipitate and rotated for a total of 3 h. Beads were washed four times with lysis buffer, and then resuspended in SDS loading dye and boiled prior to Western blotting. Where described as IPs at different temperatures (IP-temp), cells were lysed and samples remained on ice until divided among the appropriate antibodies, and then rotated at the indicated temperature for 3 h. Washes were performed as above, except that wash buffer remained at room temperature or warmer as indicated. Where described as 37°C/4°C immunoprecipitation, cells were lysed on ice then rotated at 37°C for 3 h with no antibody, then returned to ice for 15 min. Antibodies were added at 3 μg/tube, and then immunoprecipitations were carried out at 4°C [37°C/4°C].
Heat Shock
Medium was changed to medium prewarmed to 42°C and cells were placed in an incubator maintained at 42°C. At the indicated timepoints, cells were gently washed with PBS (prewarmed to 42°C) and lysed on ice with lysis buffer (150 mM KCl, 0.2% NP-40, 10% glycerol, 20 mM Tris pH 7.5, 0.5 mM DTT) containing protease inhibitors. Immunoprecipitations were carried out as above at 4°C for 3 h.
Expression studies in 293T cells
The expression construct for murine Flag-PP2A-C (α isoform) was generated by reverse-transcription PCR from RNA isolated from MEFs. FLAG-tagged PP2A-C was created using the forward primer 5′-AGC TGC GGA TCC GCC ACC ATG GAT TAC AAG GAT GAC GAC GAT AAG GAC GAG AAG TTG TTC ACC AAG - 3′ and reverse primer 5′-AGC TGC GAA TTC TTA CAG GAA GTA GTC TGG GGT ACG ACG AGT GAC ATG TGG CTC GCC - 3′. A construct for the coding region of PP2A-A (α isoform) was obtained from Open Biosystems (accession # BC052678, cat# MMM1013-9201907) and then amplified using Flag-tagged forward primer 5′-AGC TGC GGA TCC GCC ACC ATG GAC TAC AAG GAC GAC GAT GAC AAG ACC ATG GCA GCT GCC GAC - 3′ and reverse primer 5′-AGC TGC GAA TTC TCA GGC AAG AGA GAG AAC AGT CAG AGC - 3′. Both PP2A-A and PP2A-C constructs were subcloned into the LPC retroviral vector using BamH1 and EcoR1 restriction enzymes. 293T cells were transfected with 2.5 μg of LPC vector alone, Flag-PP2A-A/LPC, Flag-PP2A-C/LPC, or Flag-α4/LPC using Lipofectamine2000 (Invitrogen) according to the manufacturer’s guidelines. At 72 h post-transfection cells were harvested on ice in lysis buffer and protein was analyzed by Western blotting.
Supplementary Material
Acknowledgments
We thank Dr. Eric Brown (University of Pennsylvania) for providing Cre-ER viral supernatant; Dr. Timothy Cox (University of Washington) for antibody against Mid-1; and members of the Thompson laboratory for helpful comments on the manuscript. Supported by grants from the National Cancer Institute. M. K. is a Leukemia & Lymphoma Society Special Fellow.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Arroyo JD, Hahn WC. Involvement of PP2A in viral and cellular transformation. Oncogene. 2005;24:7746–7755. doi: 10.1038/sj.onc.1209038. [DOI] [PubMed] [Google Scholar]
- Baharians Z, Schonthal AH. Autoregulation of protein phosphatase type 2A expression. J Biol Chem. 1998;273:19019–19024. doi: 10.1074/jbc.273.30.19019. [DOI] [PubMed] [Google Scholar]
- Burma S, Chen BP, Murphy M, Kurimasa A, Chen DJ. ATM phosphorylates histone H2AX in response to DNA double-strand breaks. J Biol Chem. 2001;276:42462–42467. doi: 10.1074/jbc.C100466200. [DOI] [PubMed] [Google Scholar]
- Chen J, Peterson RT, Schreiber SL. Alpha 4 associates with protein phosphatases 2A, 4, and 6. Biochem Biophys Res Commun. 1998;247:827–832. doi: 10.1006/bbrc.1998.8792. [DOI] [PubMed] [Google Scholar]
- Chowdhury D, Keogh MC, Ishii H, Peterson CL, Buratowski S, Lieberman J. gamma-H2AX dephosphorylation by protein phosphatase 2A facilitates DNA double-strand break repair. Mol Cell. 2005;20:801–809. doi: 10.1016/j.molcel.2005.10.003. [DOI] [PubMed] [Google Scholar]
- Chowdhury D, Xu X, Zhong X, Ahmed F, Zhong J, Liao J, Dykxhoorn DM, Weinstock DM, Pfeifer GP, Lieberman J. A PP4-phosphatase complex dephosphorylates gamma-H2AX generated during DNA replication. Mol Cell. 2008;31:33–46. doi: 10.1016/j.molcel.2008.05.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chung H, Brautigan DL. Protein phosphatase 2A suppresses MAP kinase signalling and ectopic protein expression. Cell Signal. 1999;11:575–580. doi: 10.1016/s0898-6568(99)00033-9. [DOI] [PubMed] [Google Scholar]
- Chung H, Nairn AC, Murata K, Brautigan DL. Mutation of Tyr307 and Leu309 in the protein phosphatase 2A catalytic subunit favors association with the alpha 4 subunit which promotes dephosphorylation of elongation factor-2. Biochemistry. 1999;38:10371–10376. doi: 10.1021/bi990902g. [DOI] [PubMed] [Google Scholar]
- Cohen PT, Brewis ND, Hughes V, Mann DJ. Protein serine/threonine phosphatases; an expanding family. FEBS Lett. 1990;268:355–359. doi: 10.1016/0014-5793(90)81285-v. [DOI] [PubMed] [Google Scholar]
- Di Como CJ, Arndt KT. Nutrients, via the Tor proteins, stimulate the association of Tap42 with type 2A phosphatases. Genes Dev. 1996;10:1904–1916. doi: 10.1101/gad.10.15.1904. [DOI] [PubMed] [Google Scholar]
- Duvel K, Santhanam A, Garrett S, Schneper L, Broach JR. Multiple roles of Tap42 in mediating rapamycin-induced transcriptional changes in yeast. Mol Cell. 2003;11:1467–1478. doi: 10.1016/s1097-2765(03)00228-4. [DOI] [PubMed] [Google Scholar]
- Eichhorn PJ, Creyghton MP, Bernards R. Protein phosphatase 2A regulatory subunits and cancer. Biochim Biophys Acta. 2008 doi: 10.1016/j.bbcan.2008.05.005. [DOI] [PubMed] [Google Scholar]
- Gao T, Furnari F, Newton AC. PHLPP: a phosphatase that directly dephosphorylates Akt, promotes apoptosis, and suppresses tumor growth. Mol Cell. 2005;18:13–24. doi: 10.1016/j.molcel.2005.03.008. [DOI] [PubMed] [Google Scholar]
- Goodarzi AA, Jonnalagadda JC, Douglas P, Young D, Ye R, Moorhead GB, Lees-Miller SP, Khanna KK. Autophosphorylation of ataxia-telangiectasia mutated is regulated by protein phosphatase 2A. Embo J. 2004;23:4451–4461. doi: 10.1038/sj.emboj.7600455. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hombauer H, Weismann D, Mudrak I, Stanzel C, Fellner T, Lackner DH, Ogris E. Generation of active protein phosphatase 2A is coupled to holoenzyme assembly. PLoS Biol. 2007;5:e155. doi: 10.1371/journal.pbio.0050155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ikehara T, Ikehara S, Imamura S, Shinjo F, Yasumoto T. Methylation of the C-terminal leucine residue of the PP2A catalytic subunit is unnecessary for the catalytic activity and the binding of regulatory subunit (PR55/B) Biochem Biophys Res Commun. 2007;354:1052–1057. doi: 10.1016/j.bbrc.2007.01.085. [DOI] [PubMed] [Google Scholar]
- Janssens V, Goris J. Protein phosphatase 2A: a highly regulated family of serine/threonine phosphatases implicated in cell growth and signalling. Biochem J. 2001;353:417–439. doi: 10.1042/0264-6021:3530417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jiang Y, Broach JR. Tor proteins and protein phosphatase 2A reciprocally regulate Tap42 in controlling cell growth in yeast. Embo J. 1999;18:2782–2792. doi: 10.1093/emboj/18.10.2782. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kong M, Bui TV, Ditsworth D, Gruber JJ, Goncharov D, Krymskaya VP, Lindsten T, Thompson CB. The PP2A-associated protein alpha4 plays a critical role in the regulation of cell spreading and migration. J Biol Chem. 2007;282:29712–29720. doi: 10.1074/jbc.M703159200. [DOI] [PubMed] [Google Scholar]
- Kong M, Fox CJ, Mu J, Solt L, Xu A, Cinalli RM, Birnbaum MJ, Lindsten T, Thompson CB. The PP2A-associated protein alpha4 is an essential inhibitor of apoptosis. Science. 2004;306:695–698. doi: 10.1126/science.1100537. [DOI] [PubMed] [Google Scholar]
- Li X, Scuderi A, Letsou A, Virshup DM. B56-associated protein phosphatase 2A is required for survival and protects from apoptosis in Drosophila melanogaster. Mol Cell Biol. 2002;22:3674–3684. doi: 10.1128/MCB.22.11.3674-3684.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luo J, Shen G, Yan J, He C, Zhang H. AtCHIP functions as an E3 ubiquitin ligase of protein phosphatase 2A subunits and alters plant response to abscisic acid treatment. Plant J. 2006;46:649–657. doi: 10.1111/j.1365-313X.2006.02730.x. [DOI] [PubMed] [Google Scholar]
- Millward TA, Zolnierowicz S, Hemmings BA. Regulation of protein kinase cascades by protein phosphatase 2A. Trends Biochem Sci. 1999;24:186–191. doi: 10.1016/s0968-0004(99)01375-4. [DOI] [PubMed] [Google Scholar]
- Murata K, Wu J, Brautigan DL. B cell receptor-associated protein alpha4 displays rapamycin-sensitive binding directly to the catalytic subunit of protein phosphatase 2A. Proc Natl Acad Sci U S A. 1997;94:10624–10629. doi: 10.1073/pnas.94.20.10624. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ogris E, Gibson DM, Pallas DC. Protein phosphatase 2A subunit assembly: the catalytic subunit carboxy terminus is important for binding cellular B subunit but not polyomavirus middle tumor antigen. Oncogene. 1997;15:911–917. doi: 10.1038/sj.onc.1201259. [DOI] [PubMed] [Google Scholar]
- Prickett TD, Brautigan DL. Overlapping binding sites in protein phosphatase 2A for association with regulatory A and alpha-4 (mTap42) subunits. J Biol Chem. 2004;279:38912–38920. doi: 10.1074/jbc.M401444200. [DOI] [PubMed] [Google Scholar]
- Rohlmann A, Gotthardt M, Willnow TE, Hammer RE, Herz J. Sustained somatic gene inactivation by viral transfer of Cre recombinase. Nat Biotechnol. 1996;14:1562–1565. doi: 10.1038/nbt1196-1562. [DOI] [PubMed] [Google Scholar]
- Santhanam A, Hartley A, Duvel K, Broach JR, Garrett S. PP2A phosphatase activity is required for stress and Tor kinase regulation of yeast stress response factor Msn2p. Eukaryot Cell. 2004;3:1261–1271. doi: 10.1128/EC.3.5.1261-1271.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shouse GP, Cai X, Liu X. Serine 15 phosphorylation of p53 directs its interaction with B56gamma and the tumor suppressor activity of B56gamma-specific protein phosphatase 2A. Mol Cell Biol. 2008;28:448–456. doi: 10.1128/MCB.00983-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Silverstein AM, Barrow CA, Davis AJ, Mumby MC. Actions of PP2A on the MAP kinase pathway and apoptosis are mediated by distinct regulatory subunits. Proc Natl Acad Sci U S A. 2002;99:4221–4226. doi: 10.1073/pnas.072071699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Strack S, Cribbs JT, Gomez L. Critical role for protein phosphatase 2A heterotrimers in mammalian cell survival. J Biol Chem. 2004;279:47732–47739. doi: 10.1074/jbc.M408015200. [DOI] [PubMed] [Google Scholar]
- Tolstykh T, Lee J, Vafai S, Stock JB. Carboxyl methylation regulates phosphoprotein phosphatase 2A by controlling the association of regulatory B subunits. Embo J. 2000;19:5682–5691. doi: 10.1093/emboj/19.21.5682. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Trockenbacher A, Suckow V, Foerster J, Winter J, Krauss S, Ropers HH, Schneider R, Schweiger S. MID1, mutated in Opitz syndrome, encodes an ubiquitin ligase that targets phosphatase 2A for degradation. Nat Genet. 2001;29:287–294. doi: 10.1038/ng762. [DOI] [PubMed] [Google Scholar]
- Virshup DM. Protein phosphatase 2A: a panoply of enzymes. Curr Opin Cell Biol. 2000;12:180–185. doi: 10.1016/s0955-0674(99)00074-5. [DOI] [PubMed] [Google Scholar]
- Wadzinski BE, Eisfelder BJ, Peruski LF, Jr, Mumby MC, Johnson GL. NH2-terminal modification of the phosphatase 2A catalytic subunit allows functional expression in mammalian cells. J Biol Chem. 1992;267:16883–16888. [PubMed] [Google Scholar]
- Wei H, Ashby DG, Moreno CS, Ogris E, Yeong FM, Corbett AH, Pallas DC. Carboxymethylation of the PP2A catalytic subunit in Saccharomyces cerevisiae is required for efficient interaction with the B-type subunits Cdc55p and Rts1p. J Biol Chem. 2001;276:1570–1577. doi: 10.1074/jbc.M008694200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu Y, Song P, Xu J, Zhang M, Zou MH. Activation of protein phosphatase 2A by palmitate inhibits AMP-activated protein kinase. J Biol Chem. 2007;282:9777–9788. doi: 10.1074/jbc.M608310200. [DOI] [PubMed] [Google Scholar]
- Xing Y, Li Z, Chen Y, Stock JB, Jeffrey PD, Shi Y. Structural mechanism of demethylation and inactivation of protein phosphatase 2A. Cell. 2008;133:154–163. doi: 10.1016/j.cell.2008.02.041. [DOI] [PubMed] [Google Scholar]
- Xu Y, Xing Y, Chen Y, Chao Y, Lin Z, Fan E, Yu JW, Strack S, Jeffrey PD, Shi Y. Structure of the protein phosphatase 2A holoenzyme. Cell. 2006;127:1239–1251. doi: 10.1016/j.cell.2006.11.033. [DOI] [PubMed] [Google Scholar]
- Yan L, Lavin VA, Moser LR, Cui Q, Kanies C, Yang E. PP2A regulates the pro-apoptotic activity of FOXO1. J Biol Chem. 2008;283:7411–7420. doi: 10.1074/jbc.M708083200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang J, Roe SM, Prickett TD, Brautigan DL, Barford D. The structure of Tap42/alpha4 reveals a tetratricopeptide repeat-like fold and provides insights into PP2A regulation. Biochemistry. 2007;46:8807–8815. doi: 10.1021/bi7007118. [DOI] [PubMed] [Google Scholar]
- Zolnierowicz S. Type 2A protein phosphatase, the complex regulator of numerous signaling pathways. Biochem Pharmacol. 2000;60:1225–1235. doi: 10.1016/s0006-2952(00)00424-x. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.