Recent studies have begun to elucidate how the endothelial lineage is specified from the nascent mesoderm [1, 2]; however, the molecular mechanisms which regulate this process remain largely unknown. We hypothesized that Notch signaling might play an important role in specifying endothelial progenitors from the mesoderm, since this pathway acts as a bipotential cell fate switch on equipotent progenitor populations in other settings [3, 4]. We found that zebrafish embryos with decreased levels of Notch signaling exhibited a significant increase in the number of endothelial cells, while embryos with increased levels of Notch signaling displayed a reduced number of endothelial cells. Interestingly, there was a concomitant gain of endothelial cells and loss of erythrocytes in embryos with decreased Notch activity, without a significant effect on cell proliferation or apoptosis. Lineage tracing analyses indicate that the ectopic endothelial cells in embryos with decreased Notch activity originate from mesodermal cells that normally produce erythrocytes. Taken together, these data suggest that Notch signaling negatively regulates the number of endothelial cells by limiting the number of endothelial progenitors within the mesoderm, possibly functioning as a cell fate switch between the endothelial and the hematopoietic lineages.
Results and Discussion
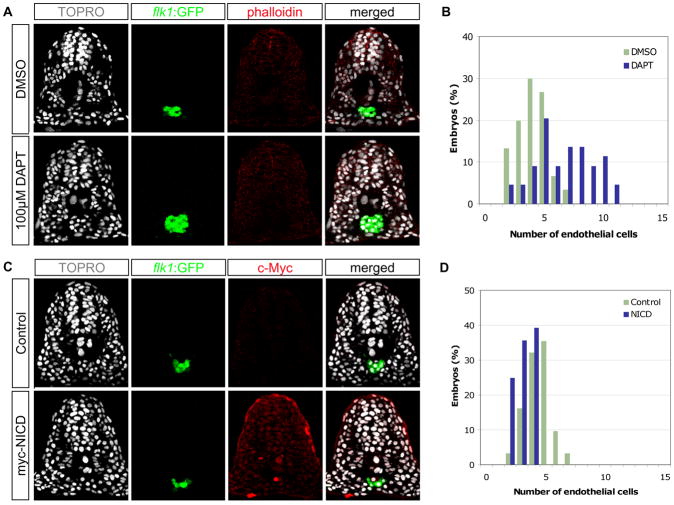
In order to determine the function of Notch signaling during the specification of the endothelial lineage, we first evaluated the effects of decreased Notch activity on endothelial cells at early stages of zebrafish development. Tg(kdrl:EGFP)s843 [5] embryos were treated with 100μM DAPT (in 5% DMSO), a known chemical antagonist of Notch signaling [6], or 5% DMSO (vehicle alone) from 6 to 18 hours post-fertilization (hpf), and the number of endothelial nuclei within a single transverse section of each embryo was quantified. At the 10th somite level, an average of 6.66 (s=2.42) endothelial nuclei was present in embryos treated with DAPT (n=44), compared to an average of 4.03 (s=1.27) endothelial nuclei in control embryos treated with DMSO (n=30), representing a 65% increase (ANOVA, p<10−6) (Fig. 1). Analysis at the 5th somite level yielded similar results (ANOVA, p<10−5; Suppl. Fig. 1). The observed increase in the number of GFP-positive endothelial cells in DAPT-treated embryos does not appear to be a transgene-dependent phenomenon since similar increases were observed in other transgenic lines, such as Tg(fli1a:nGFP)y7 [7] and Tg(kdrl:Ras-mCherry)s896 [8], that have labeled endothelial cells (data not shown). In addition, a significant increase in the number of endothelial cells was also observed in homozygous mindbomb (mibta56b) mutant embryos, which lack an E3 ubiquitin ligase required for the activation of Notch by Delta [9] (Suppl. Fig. 2).
Fig. 1. Notch signaling negatively regulates the number of endothelial cells during zebrafish development.
(A) Transverse sections of 18 hpf embryos, DMSO- or DAPT-treated, visualized for TOPRO (white), kdrl:GFP (green), and phalloidin (red). (B) Quantification of endothelial nuclei per focal plane in DMSO-treated (n=30) or DAPT-treated (n=44) embryos. (C) Transverse sections of 18 hpf phenotypic wild-type siblings and Tg(hsp70l:Gal4)kca4;Tg(UAS:myc-NICD)kca3 embryos visualized for TOPRO (white), kdrl:GFP (green), and c-Myc as a surrogate measure for NICD expression (red). (D) Quantification of endothelial nuclei per focal plane in phenotypic wild-type (n=31) and NICD over-expressing (n=28) embryos. Embryos treated with DAPT contained 6.66 (s=2.42) endothelial nuclei per section, while those treated with DMSO had 4.03 (s=1.27) endothelial nuclei (ANOVA, p<10−6). Conversely, embryos over-expressing the NICD contained 3.14 (s=0.80) endothelial nuclei, compared to 4.42 (s=1.09) nuclei per section in phenotypic wild-type siblings (ANOVA, p<10−5), suggesting that Notch signaling negatively regulates endothelial cell number.
We utilized fluorescence activated cell sorting (FACS) to better quantify the effect of DAPT treatment on the entire endothelial population. To minimize interference from the GFP expression in the pharyngeal endoderm of the Tg(kdrl:EGFP)s843 embryos [5], casanovas4 (cass4) mutant embryos [10] were used. Previous studies have shown that the cass4 mutant embryos completely lack endoderm, with little effect on hematovascular development [10]. We found that DAPT-treated 18 hpf embryos contained 55% more GFP-positive endothelial cells compared to DMSO-treated embryos (Suppl. Fig. 3). Therefore, Notch signaling appears to negatively regulate the number of endothelial cells throughout the entire embryo. Consistent with our findings, all known notch genes and two delta genes are expressed in the zebrafish gastrula (Suppl. Fig. 4).
Previous studies have demonstrated that Notch signaling is required at multiple time points to regulate various aspects of vascular development [11–15]. To define the time frame in which Notch signaling modulates the number of endothelial cells, embryos were treated with DAPT during distinct stages of development. Embryos treated from 6 to 10 hpf and from 14 to 18 hpf did not exhibit obvious changes in the number of endothelial cells. In contrast, embryos incubated with DAPT from 10 to 14 hpf displayed a similar increase in the number of endothelial cells as those continuously treated from 6 to 18 hpf (Suppl. Fig. 5 and data not shown). Interestingly, decreased Notch activity at this stage did not inhibit the differentiation of arterial endothelial cells (as determined by EphrinB2a expression), suggesting that the function of Notch signaling in limiting the number of the endothelial cells can be separated from the previously reported function in promoting arterial endothelial cell fate.
In order to test whether increased Notch activity might cause the opposite effect on the number of endothelial cells, Tg(hsp70l:Gal4)kca4;Tg(UAS:myc-notch:intra)kca3 [16] embryos were heat-shocked at shield stage (6 hpf) to induce the expression of the intracellular domain of Notch (NICD). Embryos expressing the NICD (detected by presence of the myc epitope) exhibited a significant decrease in the number of endothelial cells compared to non-heat-shocked control siblings (Fig. 1C). An average of 4.42 (s=1.09) endothelial nuclei were present in the control embryos (n=31), while an average of 3.14 (s=0.80) endothelial nuclei were found in the embryos expressing NICD (n=28), representing a 29% loss in endothelial cell number (ANOVA, p<10−5; Fig. 1D). Therefore, increased Notch activity leads to a reduction in the number of endothelial cells. Taken together, our results show that Notch signaling negatively regulates the number of the endothelial cells during early zebrafish development.
To determine whether the observed gain of endothelial cells in embryos with decreased Notch activity was caused by changes in cell proliferation or cell death, we measured these events in DAPT- and DMSO-treated embryos at 12, 14, and 16 hpf. Neither BrdU incorporation nor staining with the mitotic marker phospho-HistoneH3 showed any discernable differences in cell proliferation within the endothelial cells between DAPT- and DMSO-treated embryos (Suppl. Fig. 6). In addition, Acridine Orange and anti-Caspase3/8 staining did not reveal any differences in cell death between the two groups (data not shown), suggesting that these processes are not likely to be the major cause for the increased number of endothelial cells in embryos with decreased Notch activity.
We then speculated that the excess endothelial cells might have resulted from the expansion of the endothelial progenitor population within the mesoderm, potentially at the expense of other related lineages. To test this hypothesis, we investigated the effect of modulated Notch signaling on the hematopoietic lineage, which develops in close spatiotemporal proximity to the endothelial lineage during embryogenesis [2, 17]. In DAPT-treated embryos, the expression level of hematopoietic markers, including gata1 [18], ikaros [19], and draculin [20], drastically decreased in comparison to DMSO-treated embryos (Fig. 2A and data not shown). The reduced expression of these in situ markers appears to be specific to the hematopoietic lineage, since the non-hematopoietic markers krox20 [21] and otx1 [22] (Fig. 2A) showed equivalent levels of expression.
Fig. 2. Notch signaling positively regulates the number of hematopoietic cells during zebrafish development.
(A) Whole mount RNA in situ hybridization showing the expression of gata1 in 18 hpf DMSO-treated or DAPT-treated embryos. Two additional markers, krox20 and otx1, which label the hindbrain and neuroectoderm, respectively, were used as positive controls. Notice the reduced expression of gata1 in DAPT-treated embryos (black arrow). (B) Transverse sections of 18 hpf DMSO- or DAPT-treated embryos visualized for TOPRO (white), gata1:GFP (green), and phalloidin (red). (C) Quantification of the hematopoietic nuclei per focal plane in DMSO-treated (n=27) or DAPT-treated (n=19) embryos. (D) Transverse sections of 18 hpf phenotypic wild-type siblings and Tg(hsp70l:Gal4)kca4;Tg(UAS:myc-NICD)kca3 embryos visualized for TOPRO (white), gata1:GFP (green), and c-Myc as a surrogate measure for NICD expression (red). (E) Quantification of the hematopoietic nuclei per focal plane in phenotypic wild-type siblings (n=28) and NICD over-expressing embryos (n=23). DAPT-treated embryos contained 4.89 (s=2.15) erythrocyte nuclei compared to 8.07 (s=2.38) in DMSO-treated embryos (ANOVA, p<10−4). Conversely, embryos over-expressing the NICD contained 9.89 (s=2.53) erythrocyte nuclei, compared to 6.72 (s=1.43) nuclei per section in phenotypic wild-type siblings (ANOVA, p<10−6), indicating that Notch signaling positively modulates the number of hematopoietic cells.
To confirm that the reduced expression of hematopoietic lineage markers detected by in situ hybridization was the result of a decreased number of hematopoietic cells, we used Tg(gata1:EGFP)la781 [23] embryos to count the number of GFP-positive erythrocytes in DAPT-and DMSO-treated embryos. Since DAPT-treated embryos exhibited a drastic increase in the number of endothelial cells in their posterior half where the majority of hematopoietic cells are erythrocytes, counting the number of erythrocytes serves as a simple surrogate measure to identify any discernable changes in the number of hematopoietic cells. Compared to an average of 8.07 (s=2.38) erythrocyte nuclei per section in DMSO-treated embryos (n=27), embryos treated with DAPT (n=19) had an average of 4.89 (s=2.15) erythrocyte nuclei (Fig. 2B), approximately a 39% decrease (ANOVA, p<10−4; Fig. 2C). Conversely, embryos over-expressing the NICD exhibited a significant increase in the number of erythrocytes compared to control siblings (ANOVA, p<10−6; Fig. 2D–E).
Unlike the hematopoietic lineage, no discernable changes in the number of somitic (detected with S58 and F59 antibodies [24]) or pronephric duct (Tg(pax2a:GFP)e1 [25]) cells were detected in embryos treated with DAPT, suggesting that the contribution of progenitors giving rise to other non-axial mesodermal lineages was negligible (Suppl. Fig. 7 and data not shown).
To further characterize the effects of perturbed Notch signaling on the endothelial and hematopoietic lineages, we treated Tg(kdrl:Ras-mCherry)s896;Tg(gata1: EGFP)la781 embryos with DAPT. Interestingly, the embryos exhibited a concomitant loss of erythrocytes and gain of endothelial cells (Fig. 3A), suggesting that Notch signaling might regulate the number of the endothelial and hematopoietic cells during the same developmental stages. To investigate the possibility of a transformation of hematopoietic progenitors into endothelial progenitors at early stages in embryos with compromised Notch signaling, homozygous bloodless (bds) mutant embryos were treated with DAPT. The bds mutation obliterates all primitive hematopoietic cells via lineage-specific apoptosis, without impacting the endothelial lineage [26]. Thus, if hematopoietic progenitors within the mesoderm are transformed to endothelial progenitors as a result of decreased Notch activity, there should be no discernable change in the number of endothelial cells in the bds mutant embryos.
Fig. 3. Reduced Notch activity causes an increase of endothelial cells at the expense of hematopoietic cells.
(A) Transverse sections of 18 hpf DMSO- or DAPT-treated embryos, visualized for TOPRO (white), kdrl:mCherry (red), and gata1:GFP (green). DAPT-treated embryos exhibited a concomitant gain of endothelial cells and loss of hematopoietic cells. (B) Transverse sections of 20 hpf DMSO- or DAPT-treated phenotypic bloodless (bds) mutant embryos, visualized for TOPRO (white), kdrl:GFP (green), and phalloidin (red). (C) Quantification of the number of endothelial nuclei per focal plane in DMSO-treated bds (n=22), DAPT-treated bds (n=20), and DAPT-treated wild-type sibling (n=24) embryos. DAPT treatment in embryos lacking primitive hematopoietic cells (bds + DAPT) did not result in a change in the number of endothelial cells, suggesting that the ectopic endothelial cells observed in DAPT-treated wild-type embryos might have originated from cells that normally produce hematopoietic cells.
In DAPT-treated bds mutant embryos (n=20), an average of 6.05 (s=1.32) endothelial nuclei were present per section, while DMSO-treated bds mutant embryos (n=22) and DAPT-treated phenotypic wild-type siblings (n=24) contained an average of 5.95 (s=1.13) and 8.04 (s=2.36) endothelial nuclei, respectively (Fig. 3B). Thus, we found no significant difference in the number of endothelial cells in bds mutant embryos treated with DAPT (ANOVA, p<0.80; Fig. 3C). These data suggest that decreased Notch activity causes a transformation of hematopoietic progenitors to endothelial progenitors, and that Notch signaling might function as a cell fate switch between the two lineages.
To further test the function of Notch signaling as a cell fate switch between the endothelial and hematopoietic lineages, lineage tracing experiments were performed [2]. Single-cell stage embryos were injected with DMNB-caged fluorescein conjugated dextran. At shield stage, approximately ten cells within the ventral margin of the zebrafish gastrula, which predominantly generate the primitive hematopoietic lineage, were uncaged with UV irradiation. Subsequently, embryos were treated with DAPT or DMSO (Fig. 4A). If there is a direct transformation of hematopoietic progenitors to endothelial progenitors as a result of decreased Notch activity, the increased contribution to the endothelial lineage from the ventral margin should be detected. As the cells from the ventral marginal zone rarely contribute to the endothelial lineage [2], an average of 0.16 (s=0.37) endothelial cells per focal plane contained uncaged fluorescein in DMSO-treated embryos (n=18) (Fig. 4B and C). In contrast, in DAPT-treated embryos (n=13), an average of 1.61 (s=1.01) endothelial cells per focal plane showed co-localization with uncaged fluorescein (Fig. 4B and C). These data support a model in which hematopoietic progenitors within the ventral marginal zone of the zebrafish gastrula are transformed to endothelial progenitors upon the inhibition of Notch signaling, which eventually leads to an increased number of endothelial cells (Fig. 4D).
Fig. 4. Notch signaling functions as a cell fate switch between the endothelial and the hematopoietic lineages in the nascent mesoderm.
(A) Experimental scheme of the lineage tracing experiment. Single-cell stage embryos were injected with DMNB-caged fluorescein conjugated dextran. At shield stage, ten cells within the ventral margin, which predominantly gives rise to hematopoietic cells, were uncaged with UV irradiation. Subsequently, embryos were treated with either DMSO or DAPT until 18 hpf. (B) Transverse sections of 18 hpf embryos treated with DMSO or DAPT after uncaging, visualized for TOPRO (white), kdrl:GFP (green), and uncaged fluorescein (red). White arrowheads point to endothelial cells that contain uncaged fluorescein in DAPT-treated embryos. (C) Quantification of the number of endothelial nuclei with uncaged fluorescein per focal plane in embryos treated with DMSO (n=18) or DAPT (n=13). Embryos treated with DAPT contained 1.61 (s=1.01) fluorescein-positive endothelial cells, compared to 0.16 (s=0.37) in DMSO-treated embryos. (D) Our proposed model: Notch signaling negatively regulates endothelial cell number by limiting the mesodermal cells that can differentiate into endothelial progenitors. Attenuated Notch activity causes mesodermal cells with hematopoietic potential to differentiate into endothelial progenitors, thus increasing the number of endothelial cells.
Considering the existence of common progenitors for the endothelial and hematopoietic lineages in zebrafish [2], it is tempting to speculate that Notch signaling might function as a bipotential cell fate switch for the two lineages by regulating the hemangioblasts, resulting in descendents preferentially adopting an endothelial fate. However, the observed magnitude of the increase in endothelial cells after inhibition of Notch signaling suggests that while such an event is possible, it probably does not account for the full effect.
Conclusion
Our experiments provide evidence that Notch signaling negatively regulates the number of endothelial cells by modulating the specification of endothelial progenitors. This novel function of Notch signaling can be separated from a previously known function in promoting the differentiation of arterial endothelial cells at later stages of development. In addition, our data indicate that the effect of Notch signaling on endothelial cell number is the result of the direct transformation of hematopoietic progenitors into endothelial progenitors. Taken together, our results suggest that Notch signaling functions as a cell fate switch between the endothelial and the hematopoietic lineages within the nascent mesoderm.
Experimental Procedures
Zebrafish husbandry, morpholino injection, chemical and heat-shock treatments
Zebrafish (Danio rerio) embryos were obtained from the UNC-CH Zebrafish Aquaculture Core Facility and raised as previously described [26]. The following mutant and transgenic lines were used: bdsa75 [26], cass4 [10], mibta56b [7], Tg(gata1:EGFP)la781 [23], Tg(hsp70l:Gal4)kca4 [16], Tg(fli1a:nGFP)y7 [8], Tg(kdrl:EGFP)s843 [5], Tg(kdrl:Ras-mCherry)s896 [9], Tg(pax2a:GFP)e1 [24], and Tg(UAS:myc-notch:intra)kca3 [16]. Homozygous mibta56a embryos were identified by PCR, and homozygous bdsa75 embryos were identified based on the lack of ikaros expression by RT-PCR.
Embryos were treated with 100μM DAPT (CalBiochem) as previously described[6]. Embryos were treated from 6 to 18 hours post-fertilization (hpf), unless noted otherwise, and subsequently fixed in 4% paraformaldehyde (PFA) overnight prior to processing. To induce ectopic Notch signaling, embryos from crosses between Tg(hsp70l:Gal4)kca4/+ and Tg(UAS:myc-notch:intra)kca3/+;Tg(kdrl:EGFP)s843 individuals were raised at 28°C until shield stage (6 hpf), and subjected to treatment at 40°C for 30 minutes. Bright-field microscopy and myoD [28] in situ hybridization were used to verify that the treated embryos exhibited no developmentally delays compared to the control embryos (Suppl. Fig. 8).
Immunohistochemistry, in situ hybridization, and BrdU Incorporation
Immunohistochemistry and whole mount in situ hybridization were performed as previously described [5]. The antibodies and riboprobes used can be found in Supplemental Data. Polymerized actin was visualized by phalloidin (Molecular Probes) at 1:300 and nuclei were visualized with TOPRO (Molecular Probes) at 1:10,000. For immunohistochemistry, processed samples were mounted in Vectashield (Vector Laboratories) and the images were acquired using a Zeiss 510 Meta confocal microscope (Michael Hooker Microscopy Facility, UNC-CH) or a Zeiss LSM5 Pascal confocal microscope. For whole mount in situ hybridization, embryos were mounted in benzylbenzoate:benzyl alcohol and documented with Zeiss Axiocam.
For BrdU incorporation, homozygous Tg(kdrl:EGFP)s843 embryos were treated with DAPT or DMSO from 10 to 18 hpf and also incubated in 10mM BrdU in 5% DMSO from 14 hpf to 16 hpf. At 18 hpf, embryos were fixed in 4% PFA overnight and then incubated in rat monoclonal anti-BrdU followed by anti-rat Alexa Fluor 568.
Lineage tracing
Lineage tracing was performed as previously described [2]. Briefly, Tg(kdrl:EGFP)s843 embryos were injected with 4.6nl of 0.2% DNMB-caged, biotinylated, lysine fixable fluorescein dextran (Molecular probes) in 0.2M KCl and allowed to develop until shield stage (6 hpf). The cells located in the first three vegetal tiers of the ventral margin were activated using pulses from a 360 nm laser focused through a 20× objective. Embryos were then treated with DAPT or DMSO and fixed at 18 hpf. To detect activated fluorescein and distinguish it from GFP, embryos were incubated in biotinylated rabbit IgG anti-FITC antibody followed by Alexa Fluor 568 Streptavidin (Molecular Probes).
Analysis of Variance (ANOVA)
The data were analyzed with one-way ANOVA for the effect of different treatments on the number of cells of interest. A p-value smaller than 0.01 was regarded as significant.
Supplementary Material
Acknowledgments
The authors would like to thank the members of the Jin lab for helpful discussions and Drs. Cheol-Hee Kim and Hae-Chul Park for in situ probes. We also thank Drs. Victoria Bautch, Frank Conlon, Wiebke Herzog, Mark Majesky, Cam Patterson, and John Rawls for discussion and critical reading of the manuscript. This study was supported by an NSF Pre-doctoral Fellowship to K. M. V, Agency for Science, Technology and Research, Singapore (A*STAR) to Y.-J. J., the NHLBI (HL54737) and Packard foundation to D.Y.R.S, and a Basil O’Connor Scholar Award from the March of Dimes Foundation, the NHLBI (HL090960), and the University of North Carolina at Chapel Hill Research Fund to S.-W. J.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Ueno H, Weissman IL. Clonal analysis of mouse development reveals a polyclonal origin for yolk sac blood islands. Dev Cell. 2006;11:519–533. doi: 10.1016/j.devcel.2006.08.001. [DOI] [PubMed] [Google Scholar]
- 2.Vogeli KM, Jin SW, Martin GR, Stainier DY. A common progenitor for haematopoietic and endothelial lineages in the zebrafish gastrula. Nature. 2006;443:337–339. doi: 10.1038/nature05045. [DOI] [PubMed] [Google Scholar]
- 3.Iso T, Hamamori Y, Kedes L. Notch signaling in vascular development. Arterioscler Thromb Vasc Biol. 2003;23:543–553. doi: 10.1161/01.ATV.0000060892.81529.8F. [DOI] [PubMed] [Google Scholar]
- 4.Lai EC. Notch signaling: control of cell communication and cell fate. Development. 2004;131:965–973. doi: 10.1242/dev.01074. [DOI] [PubMed] [Google Scholar]
- 5.Jin SW, Beis D, Mitchell T, Chen JN, Stainier DY. Cellular and molecular analyses of vascular tube and lumen formation in zebrafish. Development. 2005;132:5199–5209. doi: 10.1242/dev.02087. [DOI] [PubMed] [Google Scholar]
- 6.Geling A, Steiner H, Willem M, Bally-Cuif L, Haass C. A gamma-secretase inhibitor blocks Notch signaling in vivo and causes a severe neurogenic phenotype in zebrafish. EMBO Rep. 2002;3:688–694. doi: 10.1093/embo-reports/kvf124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Roman BL, Pham VN, Lawson ND, Kulik M, Childs S, Lekven AC, Garrity DM, Moon RT, Fishman MC, Lechleider RJ, Weinstein BM. Disruption of acvrl1 increases endothelial cell number in zebrafish cranial vessels. Development. 2002;129:3009–3019. doi: 10.1242/dev.129.12.3009. [DOI] [PubMed] [Google Scholar]
- 8.Chi NC, Shaw RM, Jungblut B, Huisken J, Ferrer T, Arnaout R, Scott I, Beis D, Xiao T, Baier H, Jan LY, Tristani-Firouzi M, Stainier DY. Genetic and physiologic dissection of the vertebrate cardiac conduction system. PLoS Biol. 2008;6:e109. doi: 10.1371/journal.pbio.0060109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Itoh M, Kim CH, Palardy G, Oda T, Jiang YJ, Maust D, Yeo SY, Lorick K, Wright GJ, Ariza-McNaughton L, Weissman AM, Lewis J, Chandrasekharappa SC, Chitnis AB. Mind bomb is a ubiquitin ligase that is essential for efficient activation of Notch signaling by Delta. Dev Cell. 2003;4:67–82. doi: 10.1016/s1534-5807(02)00409-4. [DOI] [PubMed] [Google Scholar]
- 10.Alexander J, Rothenberg M, Henry GL, Stainier DY. casanova plays an early and essential role in endoderm formation in zebrafish. Dev Biol. 1999;215:343–357. doi: 10.1006/dbio.1999.9441. [DOI] [PubMed] [Google Scholar]
- 11.Hellstrom M, Phng LK, Hofmann JJ, Wallgard E, Coultas L, Lindblom P, Alva J, Nilsson AK, Karlsson L, Gaiano N, Yoon K, Rossant J, Iruela-Arispe ML, Kalen M, Gerhardt H, Betsholtz C. Dll4 signalling through Notch1 regulates formation of tip cells during angiogenesis. Nature. 2007;445:776–780. doi: 10.1038/nature05571. [DOI] [PubMed] [Google Scholar]
- 12.Lawson ND, Vogel AM, Weinstein BM. sonic hedgehog and vascular endothelial growth factor act upstream of the Notch pathway during arterial endothelial differentiation. Dev Cell. 2002;3:127–136. doi: 10.1016/s1534-5807(02)00198-3. [DOI] [PubMed] [Google Scholar]
- 13.Mandal L, Banerjee U, Hartenstein V. Evidence for a fruit fly hemangioblast and similarities between lymph-gland hematopoiesis in fruit fly and mammal aorta-gonadal-mesonephros mesoderm. Nat Genet. 2004;36:1019–1023. doi: 10.1038/ng1404. [DOI] [PubMed] [Google Scholar]
- 14.Siekmann AF, Lawson ND. Notch signalling limits angiogenic cell behaviour in developing zebrafish arteries. Nature. 2007;445:781–784. doi: 10.1038/nature05577. [DOI] [PubMed] [Google Scholar]
- 15.Kim YH, Hu H, Guevara-Gallardo S, Lam MT, Fong SY, Wang RA. Artery and vein size is balanced by Notch and ephrin B2/EphB4 during angiogenesis. Development. 2008;135:3755–3764. doi: 10.1242/dev.022475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Scheer N, Riedl I, Warren JT, Kuwada JY, Campos-Ortega JA. A quantitative analysis of the kinetics of Gal4 activator and effector gene expression in the zebrafish. Mech Dev. 2002;112:9–14. doi: 10.1016/s0925-4773(01)00621-9. [DOI] [PubMed] [Google Scholar]
- 17.Warga RM, Kane DA, Ho RK. Fate mapping embryonic blood in zebrafish: multi- and unipotential lineages are segregated at gastrulation. Dev Cell. 2009;16:744–755. doi: 10.1016/j.devcel.2009.04.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Detrich HW, 3rd, Kieran MW, Chan FY, Barone LM, Yee K, Rundstadler JA, Pratt S, Ransom D, Zon LI. Intraembryonic hematopoietic cell migration during vertebrate development. Proc Natl Acad Sci U S A. 1995;92:10713–10717. doi: 10.1073/pnas.92.23.10713. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Willett CE, Kawasaki H, Amemiya CT, Lin S, Steiner LA. Ikaros expression as a marker for lymphoid progenitors during zebrafish development. Dev Dyn. 2001;222:694–698. doi: 10.1002/dvdy.1223. [DOI] [PubMed] [Google Scholar]
- 20.Herbomel P, Thisse B, Thisse C. Ontogeny and behaviour of early macrophages in the zebrafish embryo. Development. 1999;126:3735–3745. doi: 10.1242/dev.126.17.3735. [DOI] [PubMed] [Google Scholar]
- 21.Woo K, Fraser SE. Specification of the hindbrain fate in the zebrafish. Dev Biol. 1998;197:283–296. doi: 10.1006/dbio.1998.8870. [DOI] [PubMed] [Google Scholar]
- 22.Li Y, Allende ML, Finkelstein R, Weinberg ES. Expression of two zebrafish orthodenticle-related genes in the embryonic brain. Mech Dev. 1994;48:229–244. doi: 10.1016/0925-4773(94)90062-0. [DOI] [PubMed] [Google Scholar]
- 23.Long Q, Meng A, Wang H, Jessen JR, Farrell MJ, Lin S. GATA-1 expression pattern can be recapitulated in living transgenic zebrafish using GFP reporter gene. Development. 1997;124:4105–4111. doi: 10.1242/dev.124.20.4105. [DOI] [PubMed] [Google Scholar]
- 24.Barresi MJ, D’Angelo JA, Hernández LP, Devoto SH. Distinct mechanisms regulate slow-muscle development. Curr Biol. 2001;11:1432–8. doi: 10.1016/s0960-9822(01)00428-6. [DOI] [PubMed] [Google Scholar]
- 25.Picker A, Scholpp S, Bohli H, Takeda H, Brand M. A novel positive transcriptional feedback loop in midbrain-hindbrain boundary development is revealed through analysis of the zebrafish pax2.1 promoter in transgenic lines. Development. 2002;129:3227–3239. doi: 10.1242/dev.129.13.3227. [DOI] [PubMed] [Google Scholar]
- 26.Liao EC, Trede NS, Ransom D, Zapata A, Kieran M, Zon LI. Non-cell autonomous requirement for the bloodless gene in primitive hematopoiesis of zebrafish. Development. 2002;129:649–659. doi: 10.1242/dev.129.3.649. [DOI] [PubMed] [Google Scholar]
- 27.Westerfield M. The Zebrafish Book. Univ. Oregon Press; Eguene, OR: 1993. [Google Scholar]
- 28.Halpern ME, Thisse C, Ho RK, Thisse B, Riggleman B, Trevarrow B, Weinberg ES, Postlethwait JH, Kimmel CB. Cell-autonomous shift from axial to paraxial mesodermal development in zebrafish floating head mutants. Development. 1995;121:4257–4264. doi: 10.1242/dev.121.12.4257. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.