Abstract
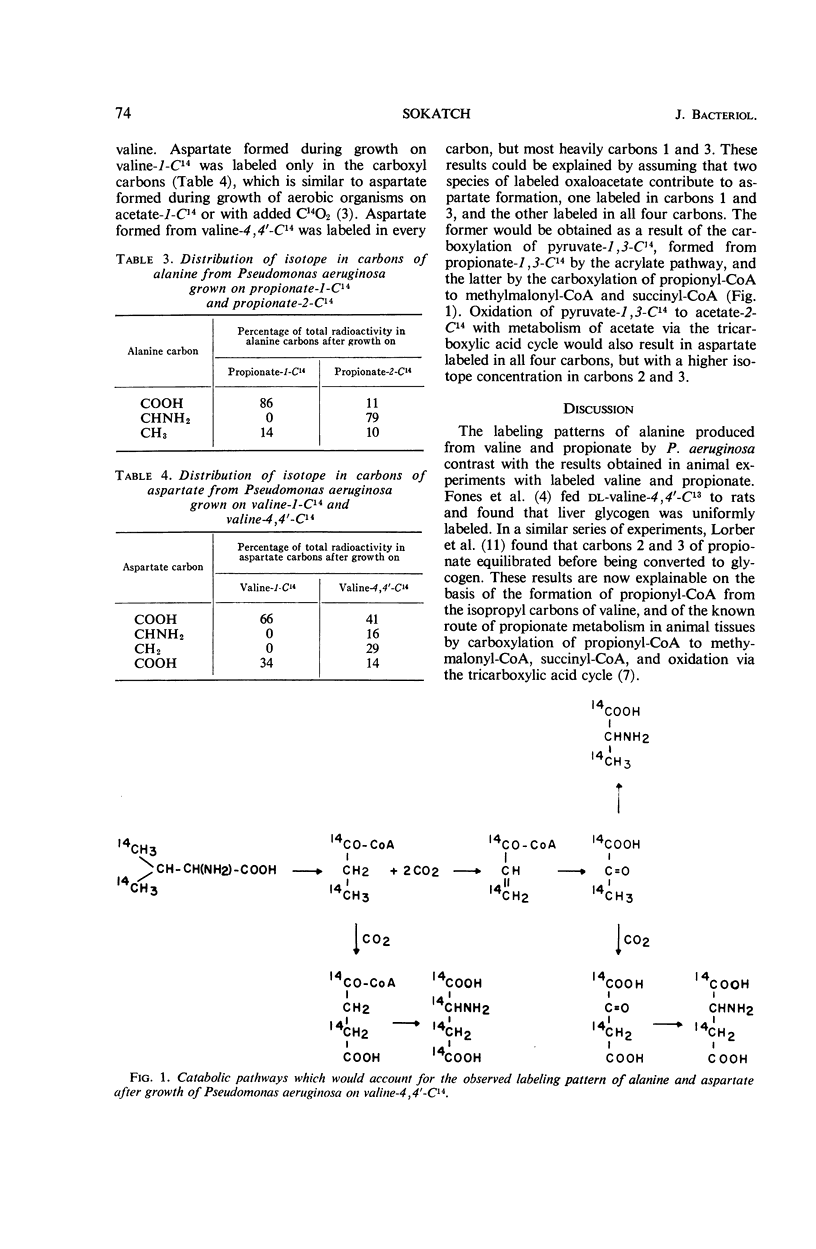
Sokatch, J. R. (University of Oklahoma School of Medicine, Oklahoma City). Alanine and aspartate formation during growth on valine-C14 by Pseudomonas aeruginosa. J. Bacteriol. 92:72–75. 1966.—Pseudomonas aeruginosa grown with dl-valine-4,4′-C14 synthesized alanine labeled mainly in carbons 1 and 3, indicating that the isopropyl carbons of valine were the precursors of pyruvate for alanine formation by a pathway which did not involve randomization of isotope. Alanine from cells grown on valine-1-C14 contained isotope only in the carboxyl carbon, suggesting another route to pyruvate from valine by carbon dioxide fixation. Oxidation of valine to propionyl-coenzyme A (CoA), as it occurs in animal tissues, followed by the oxidation of propionyl-CoA to acrylyl-CoA, lactyl-CoA, and pyruvate, would account for the isotope data. Cells grown on valine oxidized valine, isobutyrate, and propionate immediately, whereas cells grown on acetate did not oxidize valine or isobutyrate and required an induction period before propionate was oxidized. P. aeruginosa grown with propionate-1-C14 or propionate-2-C14 formed alanine-1-C14 and alanine-2-C14, respectively, which agrees with the contention that at least part of the propionate is oxidized via the acrylate pathway. Aspartate formed from valine-1-C14 was labeled only in the carboxyl carbons, whereas that formed from valine-4,4′-C14 was labeled in all four carbons, but most heavily in carbons 1 and 3. These data suggest that the main route for the formation of the carbon skeleton of aspartate was by a C3 plus C1 condensation, with the C3 unit derived from the isopropyl carbons of valine and the C1 unit probably from carbon dioxide.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- BALDWIN R. L., WOOD W. A., EMERY R. S. LACTATE METABOLISM BY PEPTOSTREPTOCOCCUS ELSDENII: EVIDENCE FOR LACTYL COENZYME A DEHYDRASE. Biochim Biophys Acta. 1965 Feb 15;97:202–213. doi: 10.1016/0304-4165(65)90084-x. [DOI] [PubMed] [Google Scholar]
- CRAWFORD L. V. Studies on the aspartic decarboxylase of Nocardia globerula. Biochem J. 1958 Feb;68(2):221–225. doi: 10.1042/bj0680221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- EHRENSVARD G. Metabolism of amino acids and proteins. Annu Rev Biochem. 1955;24:275–310. doi: 10.1146/annurev.bi.24.070155.001423. [DOI] [PubMed] [Google Scholar]
- FONES W. S., WAALKES T. P., WHITE J. The conversion of L-valine to glucose and glycogen in the rat. Arch Biochem Biophys. 1951 Jun;32(1):89–95. doi: 10.1016/0003-9861(51)90241-x. [DOI] [PubMed] [Google Scholar]
- Hoare D. S., Gibson J. Photoassimilation of acetate and the biosynthesis of amino acids by Chlorobium thiosulphatophilum. Biochem J. 1964 Jun;91(3):546–559. doi: 10.1042/bj0910546. [DOI] [PMC free article] [PubMed] [Google Scholar]
- KAZIRO Y., OCHOA S. THE METABOLISM OF PROPIONIC ACID. Adv Enzymol Relat Areas Mol Biol. 1964;26:283–378. doi: 10.1002/9780470122716.ch7. [DOI] [PubMed] [Google Scholar]
- KNIGHT M. The photometabolism of propionate by Rhodospirillum rubrum. Biochem J. 1962 Jul;84:170–185. doi: 10.1042/bj0840170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- KREBS H. A., BELLAMY D. The interconversion of glutamic acid and aspartic acid in respiring tissues. Biochem J. 1960 Jun;75:523–529. doi: 10.1042/bj0750523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LEAVER F. W., WOOD H. G., STJERNHOLM R. The fermentation of three carbon substrates by Clostridium propionicum and Propionibacterium. J Bacteriol. 1955 Nov;70(5):521–530. doi: 10.1128/jb.70.5.521-530.1955. [DOI] [PMC free article] [PubMed] [Google Scholar]
- NORTON J. E., BULMER G. S., SOKATCH J. R. THE OXIDATION OF D-ALANINE BY CELL MEMBRANES OF PSEUDOMONAS AERUGINOSA. Biochim Biophys Acta. 1963 Oct 8;78:136–147. doi: 10.1016/0006-3002(63)91619-6. [DOI] [PubMed] [Google Scholar]
- SOKATCH J. T., GUNSALUS I. C. Aldonic acid metabolism. I. Pathway of carbon in an inducible gluconate fermentation by Streptococcus faecalis. J Bacteriol. 1957 Apr;73(4):452–460. doi: 10.1128/jb.73.4.452-460.1957. [DOI] [PMC free article] [PubMed] [Google Scholar]
- VAGELOS P. R., EARL J. M., STADTMAN E. R. Propionic acid metabolism. II. Enzymatic synthesis of lactyl pantethine. J Biol Chem. 1959 Apr;234(4):765–769. [PubMed] [Google Scholar]
- van der Linden A. C., Thijsse G. J. The mechanisms of microbial oxidations of petroleum hydrocarbons. Adv Enzymol Relat Areas Mol Biol. 1965;27:469–546. doi: 10.1002/9780470122723.ch10. [DOI] [PubMed] [Google Scholar]


