Abstract
GABAA receptors composed of the γ2 and δ subunits have distinct properties, functions and subcellular localization, and pathological conditions differentially modulate their surface expression. Recent studies demonstrate that acute seizure activity accelerated trafficking of the γ2 and β2/3 subunits but not that of the δ subunit. The trafficking of the γ2 and β2/3 subunits is relatively well understood but that of the δ subunit has not been studied. We compared intracellular accumulation of the δ and γ2 subunits in cultured hippocampal neurons using an antibody feeding technique. Intracellular accumulation of the δ subunit peaked between 3–6 hrs, whereas, maximum internalization of the γ2 subunit took 30 minutes. In the organotypic hippocampal slice cultures internalization of the δ subunit studied using a biotinylation assay revealed highest accumulation between 3–5 hr and that of the γ2 subunit between 15–45 min. The surface half-life of the δ subunit was 171 min in cultured hippocampal neurons and 102 min in the organotypic hippocampal slice cultures. In the subsequent studies, internalization of the δ subunit was found to be dependent on network activity but independent of ligand-binding. BDNF reduced buildup of the δ subunit in the cytoplasmic compartments and increased its surface expression, and this BDNF effect was independent of network activity. BDNF effect was mediated by activation of TrkB receptors, PLCγ and PKC. Increase in the basal PKC activity augmented cellsurface stability of the δ subunit. These results suggest that rate of intracellular accumulation of the δ subunit was distinct and modulated by BDNF.
Keywords: ligand-gated ion channels, internalization, hippocampus, PLCγ, PKC, biotinylation assay
GABAA receptors (GABARs) are ligand-gated chloride channels that mediate inhibitory neurotransmission in the adult brain and spinal cord. Based on sequence homology with the nicotinic acetylcholine receptors, and studies on stoichiometry and assembly in chimeric receptors, GABARs are thought to consist of five membrane spanning subunits (Schofield et al., 1987; Tretter et al., 1997). The subunits are encoded by 19 genes and can be divided into families α1-6, β1-3, γ1–3, δ, π, ε, ρ1-3 and θ based on sequence homology (Whiting, 1999). The majority of the GABARs present in the brain are thought to be made up of two α, two β, and one γ, δ, or ε subunits.
The δ subunit-containing GABARs show distinct properties compared to those containing the γ2 subunit. The δ and γ2 subunits are mutually exclusive in vivo (Araujo et al., 1998), and as the presence of the γ2 subunit is necessary for synaptic localization (Essrich et al., 1998; Schweizer et al., 2003) the δ subunit-containing receptors are restricted to peri- or extra-synaptic location (Nusser et al., 1998; Wei et al., 2003). Presence of the δ subunit also confers distinct channel and pharmacological properties such as high sensitivity to GABA, sensitivity to nanomolar concentrations of neurosteroids, anesthetics, and alcohol, to GABARs (Saxena and MacDonald, 1994; Haas and MacDonald, 1999; Wohlfarth et al., 2002; Wallner et al., 2003; Wei et al., 2004). The δ subunit-containing GABARs contribute to a persistent form of inhibition called tonic inhibition (Nusser and Mody, 2002; Glykys et al., 2008), which is a major regulator of neuronal excitability (Brickley et al., 1996; Semyanov et al., 2004). The plasticity of δ subunit-containing GABARs appears to play a role in conditions such as catamenial epilepsy, premenstrual syndrome, pregnancy and postpartum associated mood alterations, alcohol intoxication, depression, anxiety, and insomnia (see reviews (Harrison et al., 2007; Mody et al., 2007; Olsen et al., 2007; Santhakumar et al., 2007; Smith et al., 2007; Maguire and Mody 2009).
Several recent studies have suggested that the trafficking of the δ subunit-containing receptors is distinct from those containing a γ2 subunit. The effect of prolonged seizures (status epilepticus) in vivo on surface expression of γ2, β2/3 and δ subunits in hippocampal neurons was studied using biotinylation techniques (Goodkin et al., 2008; Terunuma et al., 2008). Seizures acutely diminished surface expression of γ2 subunit but the surface expression of the δ subunit was unchanged. Electrophysiological analysis demonstrated that these seizures diminished synaptic inhibition mediated by the γ2 and β2/3 subunit but tonic inhibition mediated by the δ subunit was intact (Goodkin et al., 2008). Another study demonstrated that ethanol intoxication increased surface expression of the γ2 subunit and decreased that of the δ subunit (Liang et al., 2007). These studies suggest that trafficking of the γ2 and δ subunit-containing receptors may be distinct. Trafficking to and from the membrane regulates the number of physiologically active receptors at the cell surface. To understand how trafficking alterations could contribute to the pathological conditions, it is crucial to identify mechanism of trafficking under physiological conditions. Although much is known about trafficking of the γ2 subunit-containing synaptic GABARs (Leidenheimer N, 2007), trafficking of δ subunit-containing receptors is poorly understood.
Synaptic GABARs are constitutively cycled between the membrane and intracellular compartments (Cinar and Barnes, Jr., 2001); studies have shown that trafficking of the β2/3 subunit-containing receptors is swift, occurs in a time scale of minutes (Kittler et al., 2000; Goodkin et al., 2005; Bogdanov et al., 2006). Proteins such as clathrin, adaptor protein 2 (AP2) complex, dynamin, GABAA receptor associated protein (GABARAP), huntingtin-associated protein 1 (HAP1), protein linking integrin-associated protein to cytoskeleton-1 (PLIC1), and gephyrin assist trafficking of GABARs (Kittler et al., 2000; Kittler et al., 2004; Herring et al., 2005; Kittler et al., 2005; Kanematsu et al., 2006; Yu et al., 2007; Michels and Moss, 2007). In the present study intracellular accumulation of the δ subunit of GABARs was compared with that of the γ2 subunit. The rate of accumulation of the δ subunit in intracellular space was slower than that of the γ2 subunit. We further studied effects of BDNF, which is known to modulate the trafficking of synaptic GABARs (Brunig et al., 2001; Cheng and Yeh, 2003; Jovanovic et al., 2004; Hewitt and Bains, 2006), on intracellular accumulation and surface expression of the δ subunit. BDNF inhibited intracellular accumulation of the δ subunit. These results demonstrate that in addition to distinct channel and pharmacological properties, trafficking of the δ subunit-containing receptors is also different compared to that of the γ2 subunit.
EXPERIMENTAL PROCEDURES
Materials
All the common chemicals, bicuculline, BDNF and U73343 were purchased from Sigma Aldrich Corporation (St. Louis, MO). Culture media components were obtained from Gibco (Invitrogen Corporation, Carlsbad, CA). Millicell culture inserts were obtained from Millipore (Millipore, Billerica, MA). PLCγ inhibitor U73122, PKC inhibitor calphostin c, and PKC activators phorbol 12, 13 dibutyrate (PDBu), and phorbol-12-myristate-13-acetate (PMA) as well as PI3K inhibitor LY294002 were procured from Calbiochem (EMD Chemicals Inc, San Diego, CA). Recombinant TrkB-Fc chimera was purchased from R&D Systems (Minneapolis, MN). Alpha-PDBu was procured from L C Laboratories (Woburn, MA).
Animals were handled according to guidelines set by the University of Virginia Health Sciences Center Animal Care and Use Committee and efforts were made to minimize animal stress and discomfort.
Hippocampal neuronal cultures
Hippocampal neurons were isolated and cultured as described previously (Mangan and Kapur, 2004). Briefly, the neurons were isolated from E18 rat fetuses, plated on poly-d-Lysine coated cover glass, and cultured on a glial layer. Low density cultures [10,000 neurons/cover slip (30 mm)] grown in vitro for 9–12 days were used for endocytosis studies.
Hippocampal slice cultures
Hippocampal slice cultures were prepared by the method of Gogolla et al. with few modifications (Gogolla et al., 2006). Briefly, hippocampi were isolated from 5–7 day old rat pups in Gey’s balanced salt solution [GBSS containing (in mM) NaCl 137, KCl 5, MgSO4 0.25, CaCl2 1.5, MgCl2 1.05, Na2HPO4 0.84, K2HPO4 0.22, NaHCO3 2.7, Dextrose 5.6, pH 7.2] supplemented with 6.5 mg/ml glucose, sliced on a McIlwain tissue chopper (section thickness 350 μm), and cultured on Millicell culture inserts (0.4 μm membrane thickness, 30 mm diameter) for 7 days in a humidified incubator at 37°C with 5% CO2. The culture medium consisted of 50% MEM, 25% heat-inactivated horse serum, 25% Hank’s balanced salt solution, 0.5% glutamax II, 10 mM HEPES, and 6.5 mg/ml glucose, pH 7.2. The cultures were fed every 2 days and used for experiments after 7 days in vitro. Cultures were treated with BDNF for various periods to study time course of BDNF effect. To study involvement of second messengers PLCγ and PKC, the cultures were treated with BDNF for 6 hr in the presence of their inhibitors. These inhibitors were added 30 min prior to BDNF.
Biotinylation and Western blotting
The surface expression of the δ subunit was studied by biotinylation (Goodkin et al., 2008). For these experiments, each sample comprised of proteins pooled from 12 slices, and the experiments were repeated 3–5 times. Hippocampal slice cultures were incubated in ice-cold PBS containing 1 mM CaCl2 and 0.5 mM MgCl2 (pH 7.4) and 1 mg/ml sulfo-NHS-LC-biotin (Pierce Biotechnology, Rockford, IL) for 30 min at 4°C with gentle shaking. Unbound biotin was removed by washing the slice cultures twice in TBS (25 mM Tris-Cl pH 7.4, 137 mM NaCl, 5 mM KCl, 2.3 mM CaCl2, and 0.5 mM MgCl2). The tissue was lysed in ice-cold RIPA lysis buffer containing 1 mM sodium orthovanadate and a protease inhibitor cocktail (Calbiochem, San Diego, CA) for 10 min on ice. The lysates were cleared by centrifugation at 14,000× g for 15 min at 4°C and the protein concentration was estimated using a DC protein assay kit (Bio-Rad Laboratories, Hercules, CA). The biotinylated proteins were purified by incubating lysates corresponding to 300 μg protein with 100 μl neurtravidin-agarose beads (Pierce Biotechnology, Rockford, IL) overnight at 4°C followed by extensive washing with the RIPA-lysis buffer. The pulled-down proteins were eluted in a non-reducing sample buffer and denatured at 95°C for 5 min. Total δ subunit expression was also studied using tissue lysates corresponding to 30 μg protein for normalization. The proteins were separated on 10% SDS-PAGE gels and transferred to hybond-P PVDF membranes (Amersham Biosciences, Piscataway, NJ). The membranes were blocked in 5% non-fat dry milk in TBST (25 mM Tris-Cl pH 7.6, 125 mM NaCl and 0.1% Tween 20) for 2 hr at room temperature (RT) and then incubated with either an anti-δ subunit antibody (Chemicon, Temecula, CA, 1:1000 in TBST containing 1% BSA) or anti-γ2 subunit antibody (Chemicon, Temecula, CA, 1:500 in TBST containing 1% BSA) overnight at 4°C with shaking. Membranes were subsequently incubated with horseradish peroxidase (HRP)-conjugated secondary antibody (1:4000 in TBST, Bio-Rad, Hercules, CA) for 1 hr at RT and developed using the Western lightning chemiluminescence reagent plus kit (Perkin Elmer, Boston, MA). The signal was detected using the Kodak gel Logic 2200 imaging system (Kodak, New Haven, CT). All blots were re-probed with an anti-β-actin antibody (1:5000, Sigma, St Louis, MO) to confirm absence of contaminating cytoplasmic proteins. The signal intensity was determined by densitometric scanning of the Western blots. Total expression of the δ subunit was normalized with β-actin expression, and a ratio of surface to normalized total proteins was calculated.
Specific pull-down of biotin-bound proteins by neutravidin agarose was confirmed in an experiment in which slice cultures (referred to as slices here onwards) were incubated with or without biotin and the tissue lysates were incubated with neutravidin agarose. Expression of the δ subunit was not observed in pulled-down proteins from slices which were not incubated with biotin (data not shown). In contrast, the δ subunit expression was observed in the samples incubated with biotin. This confirmed that neutravidin pulled-down only biotin tagged proteins.
Biotinylation assay to detect intracellular accumulation of the δ and g2 subunits in organotypic hippocampal slice cultures
Intracellular accumulation of the δ subunit was studied using the method of Martin and Henley (Martin and Henley, 2004). For these experiments also, each sample comprised of proteins pooled from 12 slices. Briefly, slice were incubated in a cleavable form of biotin, sulfo-NHS-SS-biotin, as described above. Unbound biotin was removed by TBS washes and the slice cultures were incubated at 37°C for various time points to facilitate intracellular accumulation of biotin-tagged surface receptors. At the end of the incubation period, biotin bound to the remaining surface receptors was cleaved off by incubating slices in the glutathione buffer (50 mM glutathione, 75 mM NaOH, 75 mM NaCl, 1 mM EDTA and 0.1% BSA pH 8.5), at 4°C twice for 20 min each. Glutathione is cell-impermeable and cleaves off biotin only from surface proteins. Biotin-bound proteins accumulated in the intracellular compartments during the incubation period are protected from the cleavage and can be isolated using neutravidin beads. The slices were lysed in RIPA lysis buffer and biotin-bound proteins purified using a neutravidin agarose pull-down. Surface and total expression was detected by Western blotting as described above. Western blot signal was quantified by densitometric scanning. Expression of the δ subunit in the biotin-bound fraction was normalized to the total δ subunit expression in the corresponding samples. The amount of receptors accumulated in the intracellular space was expressed as a percentage fraction of surface expressed δ subunit.
Antibody feeding assay to determine intracellular accumulation of GABARs subunits in the cultured dissociated hippocampal neurons
Intracellular accumulation of the β2/3 subunits was studied in the dissociated hippocampal neurons cultured 8 days in vitro using an antibody feeding technique and internalized receptors were detected as described before (Goodkin et al., 2005). Briefly, surface receptors were labeled by incubating cells with an anti-δ subunit, an anti-γ2 subunit antibodies (Chemicon, Tamacula, CA) directed against an epitope in the N-terminal extracellular domain (20 μg/ml) for 1 hr at 4°C. Anti-δ and anti-γ2 subunit antibodies were a kind gift from Dr. Sieghart (Medical University Vienna, Austria) and have been well characterized (Somogyi et al., 1996; Sperk et al., 1997; Nusser et al., 1998). The excess unbound antibody was removed by PBS washes and cells incubated at 37°C for 0–9 hr to facilitate intracellular receptor accumulation. To study the effect of BDNF on intracellular accumulation, neurons were incubated at 37°C for 6 hr in the presence of BDNF (50 ng/ml) following antibody feeding. The cultures were fixed in PBS containing 4% paraformaldehye and 4% sucrose for 15 min. To detect internalized δ and γ2 subunits, antibody-bound surface receptors were blocked by incubation with unlabeled secondary antibody (20 μg/ml). This concentration of secondary antibody was sufficient to block all the remaining surface receptors as determined by a second incubation with labeled secondary antibody. Minimal fluorescence was observed in these neurons, indicating that surface receptors were blocked by unlabeled secondary antibody (data not shown). Receptors accumulated in the intracellular compartments were visualized by permeabilization of the neurons in 0.1% Triton X100 in PBS for 10 min followed by blocking with 5% normal goat serum and 0.5% BSA in PBS for 30 min. The cells were subsequently incubated in Alexafluor-594-conjugated goat anti-rabbit secondary antibody (2 μg/ml PBS) for 1 hr at RT in the dark. The unbound secondary antibody was removed with PBS washes and coverslips were mounted on slides in Gel/Mount (Biomeda, Foster City, CA). The edge of each coverslip was sealed with clear nail polish and slides stored at -20°C. Fluorescent images of cells were captured with a Roper Scientific (Tucson, AZ) Photometrics CoolSNAPcf CCD camera mounted on a Nikon Eclipse TE200 fluorescent microscope equipped with a mercury lamp driven by the MetaMorph imaging software system (Universal Imaging Corporation, Downington, PA). Morphologically intact neurons were chosen for imaging and high-resolution digital images (16-bit) were acquired using a 60X 1.4 numerical aperture lens. Immunoreactivity was estimated in terms of the pixel area using the MetaMorph Imaging software in thresholded images and normalized to total cell area measured by differential interference contrast microscopy of the same cells. Adobe Photoshop 6 (Adobe, San Jose, CA) was used to increase overall brightness and contrast for final production.
Immunohistochemistry
Surface expression of the δ subunit was studied in the hippocampal slice cultures grown in vitro for 8 days using immunohistochemistry. The slices were fixed in PBS containing 4% paraformaldehyde and 1% gluteraldehyde for 1 hr at RT, washed extensively with PBS, and incubated in a blocking solution containing 5% normal goat serum and 0.5% BSA for 1 hr at RT. The slices were then incubated with the primary antibodies (anti-δ and anti-γ2 subunit antibodies directed against the extracellular epitope) for 36–48 hr at 4°C and antibody-bound receptors visualized by subsequent incubation with fluorescent secondary antibodies. The slices were also immunolabeled for neuronal marker protein NeuN by permeabilization of the tissue, overnight incubation with anti-NeuN antibody (Chemicon, Temecula, CA) at 4°C, followed by fluorescent secondary antibody. The images were captured using a Zeiss 510 confocal microscope with Ti Safire laser under 10X magnification.
Electrophysiological recordings in the hippocampal slice cultures
Tonic GABAergic inhibition was measured using the standard whole cell patch clamping technique as described previously (Mtchedlishvili and Kapur, 2006). The slices were perfused with aCSF containing (in mM) NaCl 127, KCl 2, CaCl21.5, MgSO4 1.5, NaHCO3 25.7, KH2PO4 1.1, glucose 10 (osmolarity, 300–305 mOsm) supplemented with 20 μM DNQX and 50 μM APV (blockers of ionotrophic glutamatergic receptors) in the presence/absence of bicuculline (100 μM). The change in baseline holding current and root mean square noise was analyzed following bicuculline application using the Mini analysis program as previously described (Goodkin et al., 2008).
RESULTS
Intracellular accumulation of the δ subunit of GABARs was distinct from that of the γ2 subunit
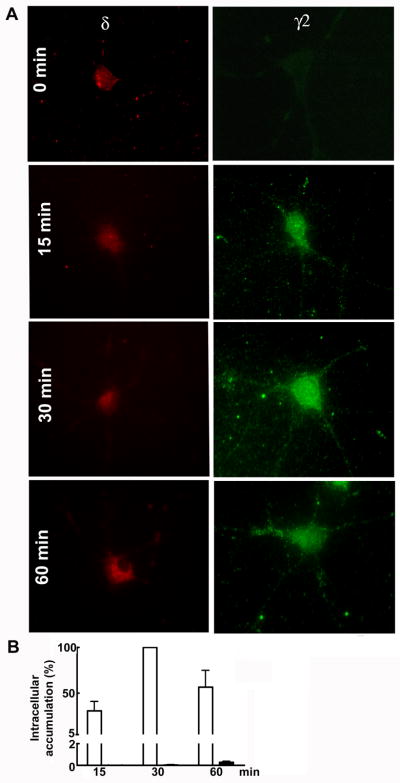
Previous studies have shown that the β2/3 subunits accumulated in the intracellular compartments within minutes (Cinar and Barnes, Jr., 2001; Goodkin et al., 2005). Since the β2/3 subunits assemble with either the δ and γ2 subunits (You and Dunn, 2007; Herd et al., 2008), we studied whether the time course of intracellular accumulation of the δ and γ2 subunits followed a similar pattern. An antibody feeding technique was used to study internalization in the cultured hippocampal neurons. Surface receptors were labeled using antibodies directed against an epitope on the N terminal extracellular domain of either the δ or γ2 subunits, and cell surface receptors that accumulated in the intracellular compartments within 60 min were detected as described in the materials and methods. In the neurons fixed immediately following antibody labeling, background immunoreactivity was observed (Fig. 1A, 0 min). This confirmed that unlabeled secondary antibody used to block the receptors remaining on the cell surface was effective, and that antibody-bound receptors remaining on the cell surface do not interfere with detection of receptors that had moved to the intracellular compartments.
Figure 1. Distinct pattern of intracellular accumulation of the δ and γ2 subunits.
A- Intracellular accumulation of the δ and γ2 subunits was studied in hippocampal neurons using an antibody feeding technique. Receptors accumulated in the intracellular space were detected. Minimum background fluorescence was observed in the neurons fixed immediately following antibody feeding (0 min). Intracellular immunoreactivity for the δ (red) remained unaltered till 60 min; in contrast, a gradual increase in the intracellular immunoreactivity for the γ2 subunit (green) was observed over the incubation time of 15–60 min and indicated fast intracellular accumulation of these receptors. Scale bar =10 μm. B- Intracellular accumulation of the δ (■) and γ2 (□) subunits was expressed as percentage of maximum internalization which was at 30 min for the γ2 subunit and 6 hr for the δ subunit (n=15–20 cells per time point).
In the cultures incubated with anti-δ subunit antibody, intracellular immunoreactivity remained unaltered in 30 min and slight increase in immunoreactivity was observed at 60 min (Fig. 1A). In contrast, in the neurons incubated with an antibody against the γ2 subunit there was increasing area of immunoreactivity over time (Fig. 1A).
To quantify the amount of receptors accumulated in the intracellular compartment, immunoreactive pixel area was normalized with the total cell area and background fluorescence, and expressed as percent of maximum fluorescence at 30 min for the γ2 subunit and 6 hr for the δ subunit. Significant accumulation of the γ2 subunits was observed as early as 15 min and increased further at 30 min (Fig 1B, n=20–30 cells in 4 experiments, p<0.05). In contrast only a little intracellular accumulation of the δ subunit was observed at 1 hr (n=15 cells, p<0.05). These results suggested that the γ2 subunit was trafficked to the intracellular compartments within 60 min, but most of the δ subunit remained on the cell surface.
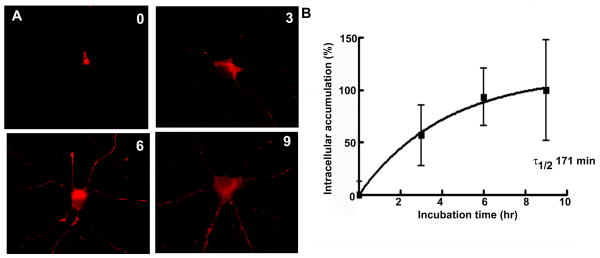
To determine peak intracellular accumulation of the δ subunit, internalization assay was performed over a longer period. Compared to 1 hr incubation, in the neurons incubated at 37°C for 3 hr and 6 hr, increasingly stronger immunoreactivity was observed (Fig. 2A). This observation suggested that intracellular accumulation of the δ subunit occurred in a time scale of hours. To determine the half life of internalization, the immunoreactivity was quantified as described above and expressed as a percentage of maxium fluorescence at 6 hr (Fig. 2B, n=21–40 cells per time point from 5 experiments). Data were fitted to an equation for single phase exponential association, Y= Ymax*(1-exp(−K*X)), where Ymax was the maximum number of receptors accumulated in the intracellular compartments and K the rate constant in minutes−1. The best fit of data to the equation revealed that the surface half-life for the δ subunit-containing receptors was 171 min.
Figure 2. Intracellular accumulation of the δ subunit peaked at 6 hr.
A- Intracellular accumulation of the δ subunit was studied with a longer incubation period. Minimal background intracellular fluorescence for the δ subunit was observed at 0 hr. A gradual increase in the fluorescence intensity and immunoreactive area was observed over a period of 3–9 hr, indicating intracellular accumulation of the δ subunit.B- Intracellular accumulation of the δ subunit was plotted as a function of time (n=21–40 cells per time point from 5 experiments). The line was best fit to an equation for single phase exponential association.
Characterization of the rate of intracellular accumulation of the δ and γ2 subunits using a biotinylation assay in the organotypic hippocampal slice cultures
Internalization studies in the cultured neurons suggested that intracellular accumulation of the δ subunit was slower than that of the γ2 subunit. However, cultured neurons lack architecture and intrinsic circuitry of the hippocampus, which may influence subunit trafficking. Studies were therefore performed in the organotypic hippocampal slice cultures using a biotinylation assay. In preliminary studies, surface expression of the δ and γ2 subunits in the organotypic hippocampal slice cultures was confirmed by immunohistochemistry. In the slice cultures grown in vitro for 8 days, surface immunoreactivity for the δ subunit was observed in the dentate gyrus region and in the hilar interneurons (Supplementary figure 1A). Surface immunoreactivity of the γ2 subunit was observed in the dentate gyrus as well as CA regions of the hippocampus (Supplementary figure 1A). Expression of the β-actin a cytoplasmic protein was also studied to confirm that integrity of the slices was not lost during fixation. Surface immunoreactivity for β-actin was not observed (data not shown) confirming that immunoreactivity for the δ and γ2 was on the cell surface. These cells showed synaptic and tonic GABAergic inhibition detected using whole cell patch clamp technique (Supplementary figure 1B).
To study accumulation of the δ subunit in intracellular space, surface proteins were labeled with a cleavable form of biotin, and following removal of unbound biotin, the slices were incubated at 37°C to facilitate intracellular accumulation of biotin-bound surface proteins. At the end of the incubation period, biotin bound to proteins still remaining on the cell surface was cleaved off using a glutathione buffer. Biotin-bound surface proteins that accumulated in the intracellular compartment were purified using neutravidin agarose pull-down and the δ subunit expression was detected by Western blotting.
A representative Western blot shows surface expressed fraction of the δ subunit (lane TS, without glutathione cleavage) and intracellular accumulation of the δ subunit in 0–4 hr (lanes 0–4, after glutathione cleavage) in figure 3A. A small amount of the δ subunit accumulated intracellularly after 1 hr; this amount increased and reached a peak in 3 hours (Fig. 3A). Total δ subunit expression was similar in all the samples (Fig. 3A). The amount of δ subunit accumulated in the intracellular compartment was expressed as percent fraction of cell surface-expressed δ subunit at time 0 hr. In 5 experiments 7±2% of the surface receptors accumulated in the intracellular compartments within 1 hr, and this fraction increased to 29±5% at 3 hr and remained stable until 5 hr (Fig. 3B). The best fit of data to the equation for single-phase exponential association suggested that the surface half-life of the δ subunit-containing receptors was 102 min.
Figure 3. Intracellular accumulation of the δ subunit in organotypic hippocampal slice cultures.
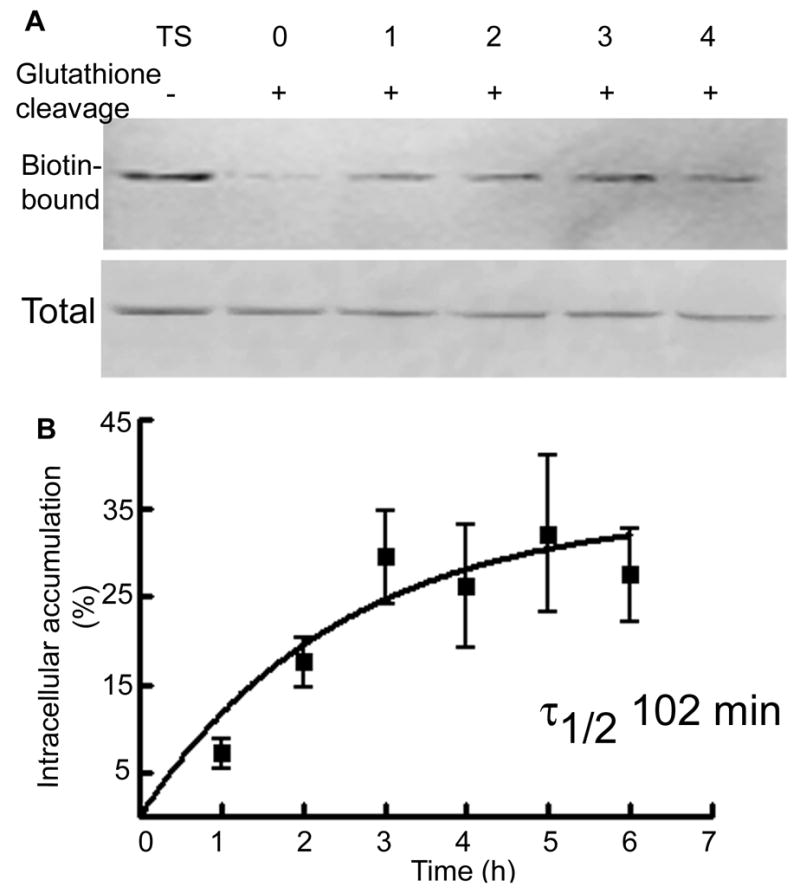
A- Intracellular accumulation of the δ subunit was studied using a biotinylation assay. A representative western blot shows biotin-bound proteins representing the surface expressed fraction of the δ subunit (Lane TS) and the amount of receptor accumulated in the intracellular compartment after incubation for 0, 1, 2, 3 and 4 hrs. Total expression of the δ subunit in tissue lysates was similar. B- A ratio of intracellular to surface δ subunit was plotted as a function of time (n=5).
Internalization of the γ2 subunit was faster than that of the δ subunit. Internalization of the γ2 subunit was observed at 15 min, which increased with longer incubation (Figure 4A and B). After 15 min of incubation 20±7% (n=4) of surface γ2 was internalized and the amount increased to 24±6% at 30 min (n=4) and then 35±9% (n=4) at 60 min. The equation for exponential function that best fit these data as above suggested that the surface half-life of the γ2 subunit was 14 min. These studies demonstrated that the intracellular accumulation of the δ subunit occurred in a time scale of hours compared to minutes for the γ2 subunit.
Figure 4. Intracellular accumulation of the γ2 subunit in organotypic hippocampal slice cultures.
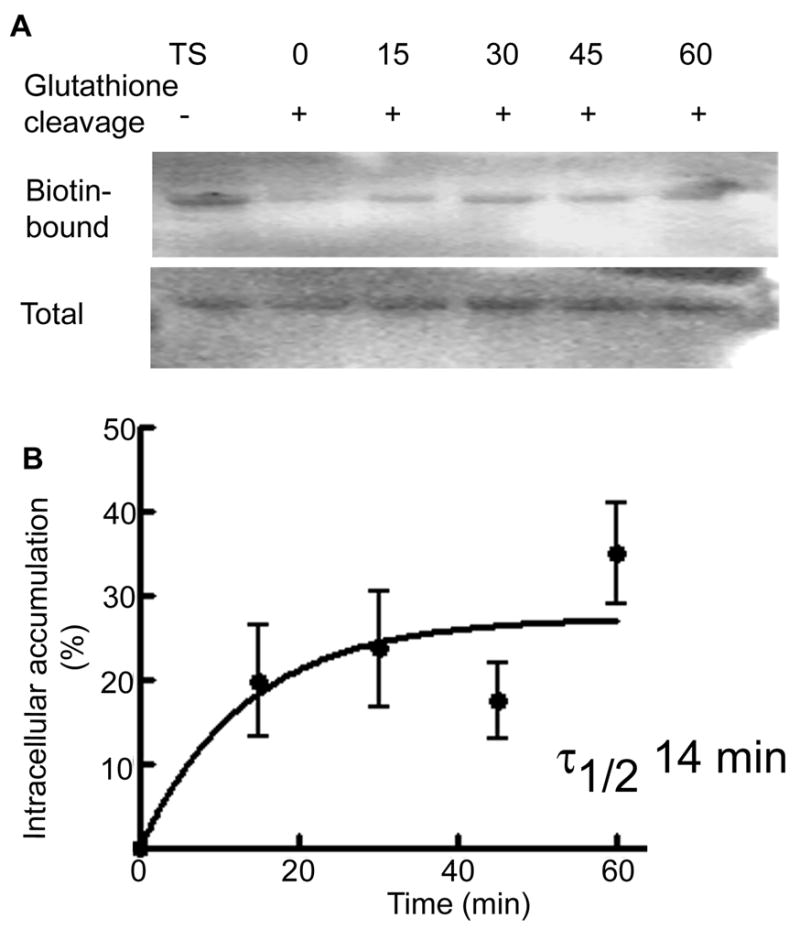
Intracellular accumulation of the γ2 subunit was studied using a biotinylation assay. A representative western blot shows biotin-bound proteins representing the surface expressed fraction of the γ2 subunit (Lane TS) and the amount of receptor accumulated in the intracellular compartment after incubation for 0, 15, 30, 45 and 60 min. Total expression of the γ2 subunit in tissue lysates was similar. B- A ratio of intracellular to surface γ2 subunit was plotted as a function of time (n=4).
Internalization of the δ subunit was dependent on network activity but not on the ligand binding
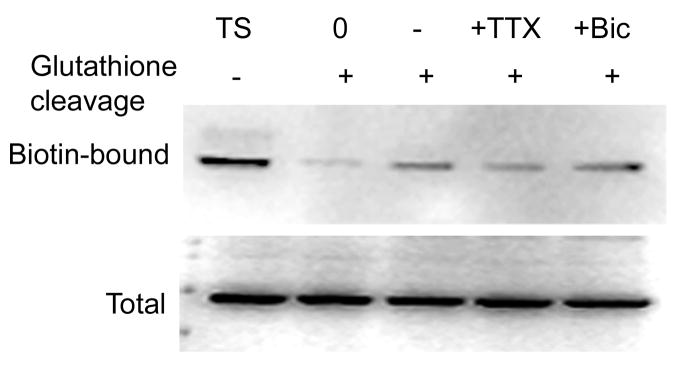
In addition to the constitutive internalization of GABARs, network activity as well as ligand binding may influence internalization. The δ and γ2 subunits confer different GABA affinity to the receptors (Saxena and MacDonald, 1994), which may result in differential internalization of these subunits. Hence we studied effect of TTX and bicuculline on internalization of the δ subunit. Reduced intracellular accumulation of the δ subunit was observed in the presence of 2 μM TTX (Figure 5). The amount of internalized δ subunit was only 45±12% after TTX treatment compared to that in untreated control cultures (100%) (p<0.05, n=4). Intracellular accumulation of the δ subunit was also studied in the presence of bicuculline (10 μM), a competitive GABAR antagonist. However, bicuculline did not alter the amount of internalized receptors (Figure 5), which was similar to control (116±15% p>0.05, n=4). These results suggest that internalization of the δ subunit was modulated by neuronal activity but not by ligand-binding. Our previous studies demonstrated internalization of the γ2 subunit was also independent of ligand-binding, but modulated by neuronal activity (Goodkin et al, 2008).
Figure 5. Internalization of the δ subunit was dependent of network activity and independent of ligand binding.
Intracellular accumulation of the δ subunit was studied in slice cultures using biotinylation assay. A representative western blot shows amount of the δ subunit accumulated in intracellular space during 4 hr of incubation with or without TTX (2 μM) or bicuculline (10 μM). A reduction in the amount of biotin-bound δ subunit after TTX treatment suggests reduced internalization, whereas amount of biotin-bound δ subunit was unaltered after bicuculline treatment. Total δ subunit expression was similar in all the samples.
BDNF is well known to modulate GABAR-mediated synaptic inhibition and alter trafficking of the β2/3 subunits of GABARs (Brunig et al., 2001; Cheng and Yeh, 2003). In the cultured hippocampal neurons, a shorter BDNF treatment potentiated synaptic GABAergic inhibition, however longer treatment resulted in depression of synaptic GABAergic inhibition (Jovanovich et al., 2004). We tested whether BDNF would modulate trafficking of GABARs containing the δ subunit.
BDNF reduced intracellular accumulation of the δ subunit of GABARs
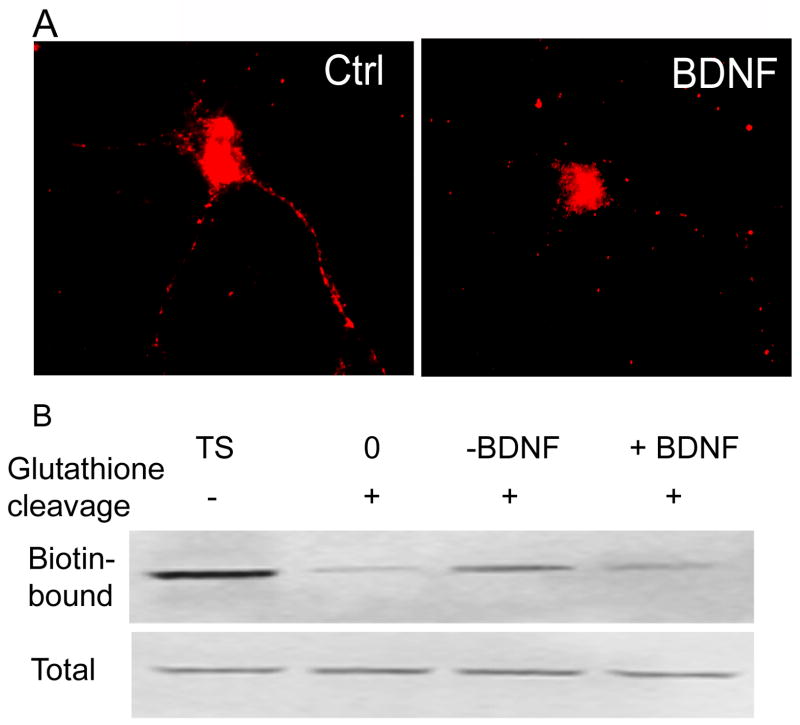
BDNF modulation of the δ subunit intracellular accumulation was studied. Since maximum intracellular accumulation of the δ subunit was observed after 6 hr in the dissociated neuronal cultures, the amount of receptors accumulated in the intracellular compartments was studied at the 6 hr time point. The surface receptors were labeled and the cultures were incubated at 37°C with or without BDNF (50 ng/ml) for 6 hr. Receptors accumulated in the intracellular compartments were detected as described above. A neuron treated with BDNF for 6 hr demonstrated less intracellular δ immunoreactivity than control, untreated neuron (Fig. 6A). This observation was confirmed in 4 separate cultures (28 neurons). Intracellular δ immunoreactivity was 48.32±27.2% of control (p<0.05, t-test).
Figure 6. BDNF reduced intracellular accumulation of the δ subunit.
A-Intracellular accumulation of the δ subunit was studied in cultured hippocampal neurons using an antibody feeding technique. Representative neuron show reduced immunoreactivity for the δ subunit after BDNF treatment, which suggested a reduction in the intracellular accumulation of the δ subunit. B- Reduced intracellular accumulation of the δ subunit in the presence of BDNF was confirmed in the organotypic hippocampal slice cultures using a biotinylation method. A representative blots shows reduction in the amount of biotin-bound δ subunit in BDNF treated slices indicated reduced intracellular accumulation. Total expression of the δ subunit was similar in all the samples.
Reduced intracellular accumulation of the δ subunit in the presence of BDNF was also confirmed in organotypic hippocampal slice cultures using the biotinylation assay. In these cultures, maximum intracellular accumulation was observed at 4 hr. Hence, the amount of accumulated δ subunit was compared between BDNF untreated and treated cultures at this time point. The surface proteins were biotinylated, slices were incubated with or without BDNF (50 ng/ml) for 4 hr, and the amount of intracellular δ subunit accumulation was determined by Western blotting. There was less biotin-bound δ subunit in the slices treated with BDNF as compared with untreated slices (Fig. 6B). In 3 experiments the amount of δ subunit accumulated in the intracellular compartments in BDNF-treated slices was only 50±29% of control (p<0.05, t-test).
BDNF increased the surface expression of the δ subunit in the organotypic hippocampal slice cultures
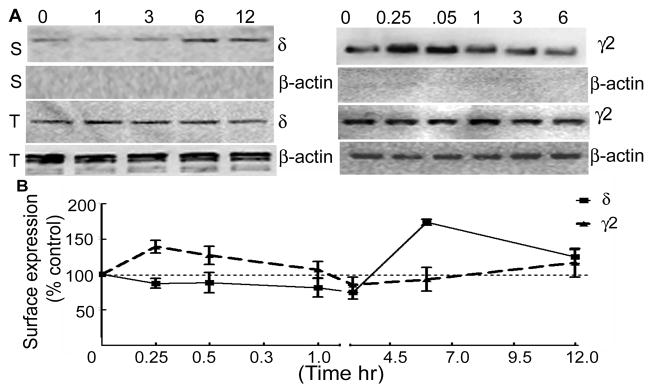
BDNF reduced the intracellular accumulation of the δ subunit, which predicted that more of the receptor remained on the cell surface, presumably due to constitutive exocytosis. The organotypic hippocampal slice cultures were treated with BDNF for 1, 3, 6, and 12 hr, and surface expression of the δ subunit was studied using biotinylation assay and Western blotting. Surface expression of the δ subunit increased in slices treated with BDNF for 6 hr and 12 hr (Fig. 7A, panel S), whereas total δ subunit expression was similar in all the samples (Fig. 7A, panel T). Blots were re-probed with β actin, a cytoplasmic protein, to confirm that the assay detected only surface proteins. The β-actin signal was not observed in the biotinylated proteins (Fig. 7A).
Figure 7. BDNF increased surface expression of the δ subunit of GABARs.
A- Effect of BDNF on surface expression of the δ and γ2 subunits was studied in hippocampal slice cultures. Surface (S) and total (T) expression of the δ and γ2 subunits was studied in slices treated for 1, 3, 6 and 12 hr and 0.25, 0.5, 1, 3 and 6 hr respectively. Surface expression of the δ subunit was higher in slices treated for 6 and 12 hr. On the other hand surface expression of the γ2 subunit was higher at 15 and 30 min. Total expression of the δ and γ2 subunits was similar in all the samples. Blots were re-probed with β-actin antibody and absence of β-actin signal in the surface proteins confirmed specificity of the biotinylation reaction. B- Surface expression of the δ or γ2 subunits in BDNF treated slices was plotted as a percent fraction of surface expressed δ or γ2 subunits in BDNF untreated slices (n=3–4).
In 3 experiments, BDNF treatment for 1 and 3 hr did not alter surface expression of the δ subunit (97±5% and 86±15% of control respectively), but longer 6 and 12 hr treatment increased it to 177±4% and 125±9% of control, respectively (Fig. 7B).
In the cultured neurons BDNF brings about an initial potentiation of GABAergic synaptic inhibition followed by depression (Jovanovic et al., 2004). We tested whether BDNF exerted similar effects in the hippocampal slice cultures. Similar to studies in cultured neurons, higher surface expression of the γ2 subunit was observed after 15 and 30 min of BDNF treatment (139±2% and 136±12% of control respectively, n=4), however the surface expression returned to the baseline expression after longer treatment (Fig 7A, panel γ2).
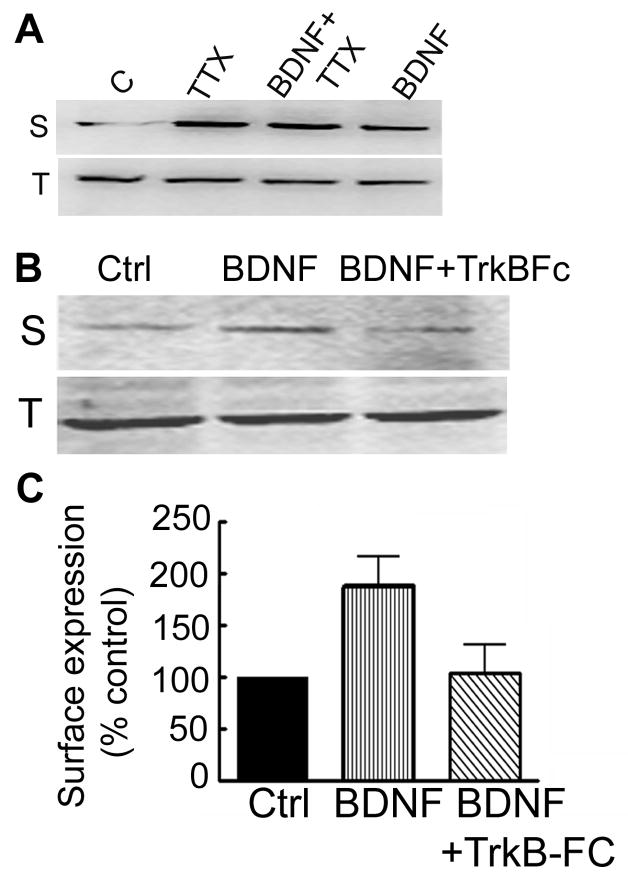
BDNF is known to induce epileptic activity (Rivera et al., 2002). This increase in the neuronal activity may contribute to the observed effects of BDNF on the δ subunit surface expression. To determine role of BDNF-induced network activity in surface expression of the δ subunit, the effect of BDNF was tested in the presence of TTX. Increased surface expression of the δ subunit was observed in the slice cultures treated with TTX (2 μM) alone and surface expression of the δ subunit remained elevated in the slice cultures treated with BDNF in the presence of TTX (Fig 8A), 354±84% of control (n=4, p<0.05). These results suggest that BDNF-induced increase in network activity, or seizures were not responsible for increased surface expression of the δ subunit, because this change occurred when activity was blocked.
Figure 8. BDNF effects were independent of network activity and mediated through activation of TrkB-Fc receptors.
A- Surface expression of the δ subunit was studied in hippocampal slice cultures treated with BDNF in the presence or absence of TTX (2 μM) for 6 hr. Surface (S) expression of the δ subunit was higher in TTX or BDNF treated slices. Total (T) expression of the δ subunit was similar in all the samples. B- Surface expression of the δ subunit was also studied in hippocampal slice cultures treated with BDNF in the presence of TrkB-Fc chimera (2 μg/ml). Surface expression (S) of the δ subunit was less in slices treated with BDNF and TrkB-Fc. Total expression (T) of the δ subunit was similar in all the samples. C- Surface expression of the δ subunit was expressed as a percent fraction of that in BDNF untreated control slices (n=3).
The effects of BDNF on the β2/3 and γ2 subunit-containing receptors are known to depend on activation of TrKB receptors, PLCγ, PI3K and PKC (Mizoguchi et al., 2003; Jovanovic et al., 2004; Hewitt and Bains, 2006; Kanematsu et al., 2006). We studied involvement of these second messengers in BDNF effects on surface expression of the δ subunit.
BDNF effects on the surface expression of the δ subunit were mediated through activation of TrkB receptors
TrkB receptors mediate the majority of the cellular effects of BDNF (Binder and Scharfman, 2004) and these receptors are expressed in the organotypic hippocampal slice cultures (Tyler and Pozzo-Miller, 2001). Therefore, BDNF effects on the δ subunit surface expression could be mediated through TrkB activation. Fusion proteins of the extracellular domain of Trk receptors with the Fc fragment of IgG are powerful neutralizing agents of the ligands (Shelton et al., 1995). We used a TrkB-Fc chimera (2 mg/ml) to test the involvement of TrkB receptors on the effects of BDNF on surface expression of the δ subunit. Maximum increase in the surface expression was observed after 6 hr of BDNF treatment, so subsequent studies were performed at this time point. Surface expression of the δ subunit was compared in slices treated with BDNF or BDNF and TrkB-Fc (Fig. 8B). In BDNF-treated slices, surface expression of the δ subunit was 188±28% (Figure 8C), whereas it was 107±27% in BDNF and TrkB-Fc treated slices. These results suggested that activation of TrkB receptors was important in the effects of BDNF on surface expression of the δ subunit.
BDNF-induced increase in the surface expression of the δ subunit was mediated through activation of PLCγ and PKC
Activation of TrkB receptors activates intracellular signaling cascades (Rose et al., 2004) including PLCγ and PI3K. Activation of PLCγ results in the formation of diacyl glycerol and IP3, release of calcium from intracellular stores, and activation of PKC (Huang and Reichardt, 2003). We tested involvement of PLCγ and PI3K in the effects of BDNF on the surface expression of the δ subunit in the slice cultures treated with BDNF and PLC or PI3K inhibitors.
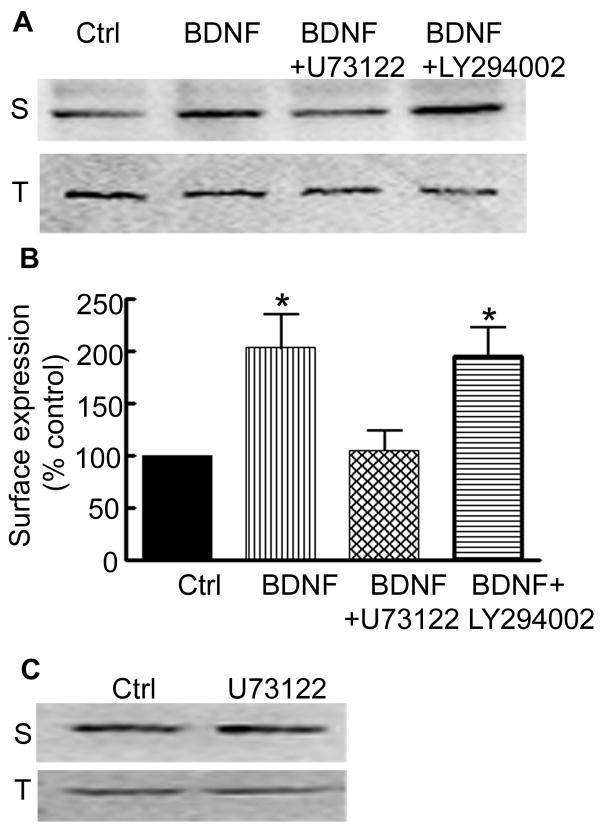
A broad spectrum inhibitor of PLC, U73122 with an IC50 1–2.1 μM (Bleasdale et al., 1990; Tanaka et al., 1997) and a specific cell-permeable PI3K inhibitor, LY294002 with an IC50 of 1.4 μM (Vlahos et al., 1994) were used. Surface expression of the δ subunit was compared between slice cultures treated for 6 hr with either BDNF alone, BDNF with U73122 (5 μM), or BDNF with LY294002 (5 μM) (Fig. 9A). U73122 blocked BDNF-induced increase in the δ subunit surface expression (117±11% vs 221±24%, n=5, p<0.05, ANOVA with posthoc Tukey’s multiple comparison test, Fig. 8B). LY294002 did not block BDNF effects. As a control, effect of U73343 an inactive analog of U73122 was also studied. U73343 (5 μM) failed to block BDNF effects and surface expression of the δ subunit in slice cultures treated with BDNF in the presence of U73343 was higher (supplementary figure 2A), 187±12% of control (n=4). These studies suggested involvement of PLCγ in BDNF effects on surface expression of the δ subunit.
Figure 9. BDNF effects on the surface expression of the δ subunit were mediated through activation of PLCγ.
A- Surface expression of the δ subunit was studied in the slices treated with BDNF for 6 hr in the presence of PLC inhibitor U73122 (5 μM) or PI3K inhibitor LY294002 (5 μM). Increased surface expression (S) of the δ subunit by BDNF was blocked by U73122 but not by LY294002. Total expression (T) of the δ subunit was similar in all the samples. B- Surface expression of the δ subunit after treatment with BDNF or BDNF and inhibitors (n=3–5, * p<0.05). C- A representative blot shows surface (S) and total (T) expression of the δ subunit was unaltered in slices treated with U73122.
To study whether inhibition of basal PLCγ activity also had an effect on the surface expression of the δ subunit, slice cultures were treated with U73122 alone for 12 hr. Surface expression of the δ subunit was unaltered in these slice cultures (Fig. 9C, 102±27% of control, n=4, p>0.05, t-test).
PKC phosphorylates the β1-3 and γ2 subunits of GABARs (McDonald et al., 1998; Moss et al., 1992a) and enhances or reduces their surface expression (Connolly et al., 1999; Terunuma et al., 2008). PKC modulation of the basal surface expression of the δ subunit was studied. Calphostin c is a cell-permeable, highly specific competitive inhibitor of PKC which interacts with its regulatory domain by competing at the diacylglycerol and phorbol esters binding site. It inhibits PKA and myosin light chain only at higher concentrations (Kobayashi et al., 1989).
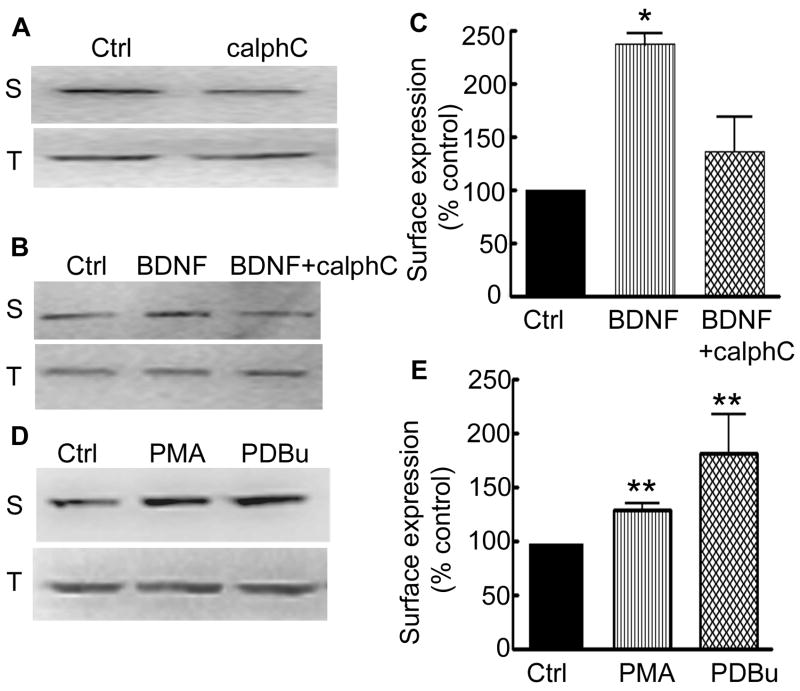
In the slices treated with calphostin c (5 μM) for 12 hr, surface expression of the δ subunit was less than that in the untreated control slices (Fig. 10A). It was 45±30% (n=3, p<0.05, t-test). The effect of PKC inhibition on BDNF-induced increase in the surface expression of the δ subunit was studied (Fig. 10B). In slices treated with BDNF alone surface expression of the δ subunit was 237±25%, whereas it was 136±32% in slices treated with BDNF and calphostin c (Fig. 10C, n=3, p<0.05, ANOVA). These results suggested that PKC in part mediated BDNF effects on the surface expression of the δ subunit.
Figure 10. Basal and BDNF-induced increase in the surface expression of the δ subunit was mediated through PKC activation.
A- Surface (S) and total (T) expression of the δ subunit was studied in slices treated with PKC inhibitor calphostin c (5 μM) for 12 hr. A reduction in the surface expression of the δ subunit suggested that basal PKC activity was necessary for surface expression of the δ subunit. B- Calphostin c also blocked BDNF-induced increase in the surface expression of the δ subunit. C- Inhibitory effect of calphostin c on BDNF effects (n=3, * p<0.05). D- Surface (S) and total (T) expression of the δ subunit was studied in slice cultures treated with PKC activators PDBu (5 μM) for 6 hr and PMA (1 μM) for 12 hr. Both activators of PKC increased surface expression of the δ subunit. E- Surface expression of the δ subunit (n=4, ** p<0.01).
To further confirm PKC regulation of the surface expression of the δ subunit, potent PKC activators PDBu (5 μM) and PMA (1 μM) were used. In the cultured neurons, nanomolar quantities of PKC activators are sufficient to induce activation of PKC (Connolly et al., 1999). However, concentrations in micromolar scale have been used in the slices (Huang and Hsu, 1999; Stocca and Lovinger, 2003; Sung and Blakely, 2007; Hu et al., 2008). Slices were treated with PMA or PDBu, and both increased surface expression of the δ subunit (Fig. 10D, E, 129±6% and 181±35% respectively, n=4, p<0.05, ANOVA). As a control, effect of α-PDBu, an inactive isoform of PDBu was tested. Treatment of slice cultures with α-PDBu resulted in only a slight increase in the surface expression of the δ subunit (Supplementary figure 2B), 110±8% of control (n=4), suggesting a specific action of PDBu on surface expression of the δ subunit. These studies suggested that basal and BDNF-induced increase in the surface expression of the δ subunit required PKC activity.
PKC activators reduced intracellular accumulation of the δ subunit
Since PKC activators increased surface expression of the δ subunit, we tested their effect on the intracellular accumulation of the δ subunit. The surface receptors were biotinylated, slice cultures were incubated with PMA or PDBu for 4 hr, and the amount of intracellular accumulation was determined by Western blotting. In the PDBu and PMA treated slices intracellular accumulation was only 42±29% and 51±19% of control (n=3, p<0.05, ANOVA). These results indicated that PKC activation inhibits accumulation of the δ subunit in the intracellular compartment.
DISCUSSION
The key findings of this study are 1) Intracellular accumulation of the δ subunit was distinctly slower than that of the γ2 subunit, 2) Internalization of the δ subunit was modulated by network activity but independent of ligand-binding, similar to that of the γ2 subunit, 3) BDNF reduced intracellular accumulation of the δ subunit, and 4) BDNF increased surface expression of the δ subunit through activation of TrkB receptors, PLCγ and PKC.
Internalization of the δ subunit has not been studied in the past. Here we report that the rate of intracellular accumulation of the δ subunit was distinct from that of the β2/3 and γ2 subunits. The δ subunit accumulated in the intracellular compartment slowly and reached a peak between 3–6 hr. In contrast, peak intracellular accumulation of the γ2 subunit occurred within 30–60 min. Recent findings have demonstrated differential surface expression of GABAR subunits under pathological conditions. Prolonged seizures of status epilepticus reduced surface expression of the β3 and γ2 subunits, whereas surface expression of the δ subunit either increased or remained unaltered (Goodkin et al., 2008; Terunuma et al., 2008). In contrast, alcohol intoxication reduced surface expression of the δ subunit and increased surface expression of the γ2 subunit (Liang et al., 2007). These studies suggested that trafficking of the δ and γ2 subunit-containing receptors is differentially modulated in pathological conditions. The data presented here show that the trafficking of these subunits is distinct even under the physiological condition.
Internalization of the δ subunit was modulated by neuronal activity; treatment of slice cultures with TTX reduced internalization and increased surface expression, suggesting a negative correlation between activity and surface expression of the δ subunit. In contrast, in the animals in status epilepticus, surface expression of the δ subunit increased or remained unaltered, suggesting a correlation between activity and surface expression of the δ subunit (Goodkin et al., 2008; Terunuma et al., 2008). However, in these studies surface expression was determined only after an hour of increased neuronal activity, whereas we have used 6 hr TTX treatment. Bicuculline did not alter internalization of the δ subunit, suggesting that binding to the GABA had minimal effect on internalization of these receptors. Our previous studies suggest a similar ligand independent internalization of the γ2 subunit as well (Goodkin et al., 2008). Hence the difference in the kinetics of internalization of the δ and γ2 subunit is not likely to be due to difference in GABA affinity for receptors composed of these subunits.
The δ and γ2 subunits are mutually exclusive in vivo (Araujo et al., 1998) and may have different intracellular accumulation rates. The β2 and δ subunits are known to co-assemble and form functional receptors (You and Dunn, 2007; Herd et al., 2008). If these subunits are part of the same receptors, their internalization ought to be similar. However, we found that the rate of intracellular accumulation of the δ and β2/3 subunits differed. The reasons for this difference are presently unclear, though, several possibilities may account for the difference. One possibility is that the majority of the δ subunit may assemble with the β1 subunit. Immunochemical studies in cultured hippocampal neurons support this possibility. In these neurons immunoreactivity of the β1 subunit and the δ subunit appeared diffuse and was mostly concentrated in the cell soma, whereas immunoreactivity of the β2/3 subunits appeared distinct and spread in the cell soma as well as processes (Mangan et al., 2005).
Another possibility is that there may be two pools of the β2/3 subunits: a fast-trafficked pool assembled with the γ2 subunits, and a slow-trafficked pool assembled with the δ subunit. Homomeric β3 receptors have been observed in recombinant systems (Wooltorton et al., 1997; Taylor et al., 1999) though whether such receptors are also present in vivo is not known. In the hippocampus, αβ3 receptors are also expressed (Mortensen and Smart, 2006). Hence internalized β2/3 subunits can originate from three pools of membrane expressed receptors: αxβ2/3δ, αxβ2/3γ2 and αxβ3. A slow-trafficked fraction of the β2/3 subunits assembled with the δ subunit may not be detected against the large population of the fast-trafficked fraction of the β2/3 subunits. It is unclear whether the β2 and β3 subunits have distinct rate of intracellular accumulation in neurons, though studies using recombinant receptors suggest that both the subunits are trafficked quickly (Connolly et al., 1999; Kittler et al., 2000; Cinar and Barnes, Jr., 2001). Determining the percentage of the δ subunit assembled with the β1, β2 and β3 subunits under basal conditions may help to explain distinct rates of intracellular accumulation observed in our studies.
Rules of internalization may also change depending on the partnering subunits. Such differential dominance of subunits is known for AMPA receptors (Lee et al., 2004). In these receptors, internalization of GluR1 and GluR2 subunits is distinct; however, under heteromeric condition GluR1/GluR2 receptors internalize similarly to those consisting of only GluR2 subunits. Homomeric δ subunit-containing GABARs have not been reported under in vivo conditions however, the δ subunit may dominate other subunits in the pentamer.
Intracellular accumulation depends not only on the rate of internalization, but also the rate of reinsertion and rate of degradation. Fast recycling or degradation of the internalized δ subunit may result in a delay in peak intracellular accumulation. Studies addressing the rate of reinsertion and degradation of the internalized δ subunit may help to explain slow intracellular accumulation.
The β2/3 and γ2 subunits undergo clathrin-dependent endocytosis though non-clathrin mediated endocytosis has also been observed in recombinant receptors (Cinar and Barnes, Jr., 2001). Clathrin, dynamin, AP2 complex, and amphiphysin regulate internalization of the β2/3 and γ2 subunits [(Kittler et al., 2000) see reviews (Michels and Moss, 2007; Jacob et al., 2008)]. Proteins which mediate the internalization of the δ subunit are not known. So far, only the μ2 subunit of the AP2 complex has been shown to interact with the δ subunit (Kittler et al., 2005) and the significance of this interaction remains to be examined. A weaker interaction between the δ subunit and the μ2 subunit of the AP2 complex may result in slower removal of the δ subunit from the membrane, resulting in delayed peak intracellular accumulation.
BDNF reduced intracellular accumulation of the δ subunit and increased its surface expression and these effects were independent of activation of TrkB receptors. Although activation of TrkB receptors is known to induce epileptic activity (Rivera et al., 2002), the effect of BDNF on surface expression of the δ subunit appeared to be independent of this action, because TTX failed to block BDNF effects. In fact, both BDNF and TTX increased surface expression of the δ subunit. Enhanced neuronal excitation is prevalent action of BDNF (Koyama and Ikegaya, 2005), however it also increases expression of glutamic acid decarboxylase (GAD), a GABA synthesizing enzyme (Yamada et al., 2002), and the density of inhibitory synapses in the hippocampal slice cultures (Marty et al., 2000), suggestive of a potentiation of inhibitory neurotransmission. Our results suggest that neuronal activity and BDNF potentially act on the δ subunit via independent mechanisms. BDNF treatment for 6 hr is also known to increase total expression of the α4 subunit in the hippocampal neurons (Roberts et al., 2006), which is a preferred partner of the δ subunit (Sur et al., 1999; Peng et al., 2002). Although BDNF did not alter total expression of the δ subunit, increased expression of the α4 subunit may result in augmentation of surface receptors. Further studies are necessary to understand whether this BDNF effect was through direct modulation of the δ subunit or whether any other GABAR subunit was involved. In the hippocampus, GABARs composed of the δ subunit are major mediators of the tonic inhibition. BDNF-induced increased surface expression of the δ subunit is likely to increase tonic inhibition.
Increased surface expression of the δ subunit in BDNF-treated slice cultures was dependent on activation of PLCγ but not PI3K. This is unlike that of the β2/3 subunits, which involve both PLCγ and PI3K in mediating BDNF effects in the hippocampal neurons (Jovanovic et al., 2004). PI3K mainly regulates recycling of endosomes (Martys et al., 1996; Kurashima et al., 1998) and insertion of GABARs at the membrane (Wang et al., 2003). The results of the present study suggest that PI3K may not play a significant role in mediating BDNF effects on the δ subunit surface expression. PKC inhibition reduced, and its activation increased, the surface expression of the δ subunit. PKC phosphorylates S409 in β1, 408/409 in β2/3, and S327/343 in γ2 GABARs subunits (Moss et al., 1992b; McDonald and Moss, 1997) and a positive correlation between phosphorylation and surface stability has been observed (Kittler et al., 2005). Similar PKC-mediated phosphorylation of the δ subunit may regulate its stability at the cell surface; however, direct phosphorylation of the δ subunit has not yet been demonstrated. An indirect effect of PKC may include a yet unidentified protein that can regulate membrane stability of the δ subunit. Approximately 30% of the surface-expressed δ subunit had moved to the intracellular space within 4 hr, and BDNF treatment reduced this by half. However, treatment with BDNF resulted in 60–80% increase in the surface expression of the δ subunit. This difference suggests that in addition to internalization, BDNF may also affect the rate of forward trafficking.
In conclusion, our results demonstrate slower intracellular accumulation of the δ subunit compared with that of the β2/3 and γ2 subunits. BDNF effects on surface expression of the δ and γ2 subunits were distinct.
Supplementary Material
A-Hippocampal slice cultures grown in vitro for 8 days were immunolabeled for the surface-expressed δ or γ2 subunits. Immunoreactivity of the δ subunit was primarily observed in the dentate granule layer, whereas that of the γ2 subunit was observed in the dentate granule layer as well as CA regions. Immunoreactivity of NeuN, a neuronal marker protein studied in permeabilized slices, was observed in the dentate granule cells, CA neurons and interneurons. B- GABAergic currents were recorded from the dentate gyrus granule neurons in the hippocampal slice cultures grown in vitro for 8 days in the presence of 20 μM DNQX and 50 μM APV, blockers of ionotrophic glutamatergic receptors, by whole cell patch clamp recordings. Tonic GABAergic inhibition was measured in terms of a characteristic shift in the baseline holding current after application of 100 μM bicuculline, a competitive GABARs antagonist. The baseline-holding current shifted from -71.1 ± 15.6 pA to -36.0 ± 16.0 pA (n=3, p<0.01, paired t-test).
A- Hippocampal slice cultures were treated with U73343 (5 μM), an inactive analog of U73122, a PLC inhibitor, in the presence of BDNF for 6 hr. U73343 did not inhibit BDNF-induced increase in the surface expression (S) of the δ subunit. Total expression (T) of the δ subunit was similar in all the samples. B- Hippocampal slice cultures were treated with α-PDBu (5 μM), an inactive isoform of PKC activator PDBu for 6 hr. Surface expression (S) of the δ subunit was not altered in the presence of α-PDBu. Total expression (T) of the δ subunit was similar in all the samples.
Acknowledgments
We thank Charisse Winston and Hui Wei Chen for the preparation of the hippocampal neuronal and organotypic slice cultures, Amogh Sivarapatna for technical assistance and Drs. Howard Goodkin, Karthik Rajasekaran, and Chengsan Sun for constructive comments on the article. We also thank Dr. Werner Sieghart for the gift of anti-GABAA receptor δ antibody. This work was supported by National Institutes of Health Grants RO1 NS 040337, RO1 NS 044370 and UO1NS58204 to JK.
Abbreviations used are
- GABA
γ amino butyric acid
- BDNF
brain derived neurotrophic factor
- PKC
protein kinase C
- PLCγ
phospholipase C gamma isoform
- PI3K
phosphoinositide 3 kinase
- IP3
inositol triphosphate
- PDBu
phorbol 12, 13 dibutyrate
- PMA
phorbol-12-myristate-13-acetate
- Trk B
tyrosine receptor kinase B
- GAD
glutamic acid decarboxylase
- TTX
tetrodotoxin
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Reference List
- Araujo F, Ruano D, Vitorica J. Absence of association between δ and γ2 subunits in native GABAA receptors from rat brain. Eur J Pharmacol. 1998;347:347–353. doi: 10.1016/s0014-2999(98)00122-8. [DOI] [PubMed] [Google Scholar]
- Binder DK, Scharfman HE. Brain-derived neurotrophic factor. Growth Factors. 2004;22:123–131. doi: 10.1080/08977190410001723308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bleasdale JE, Thakur NR, Gremban RS, Bundy GL, Fitzpatrick FA, Smith RJ, Bunting S. Selective inhibition of receptor-coupled phospholipase C-dependent processes in human platelets and polymorphonuclear neutrophils. J Pharmacol Exp Ther. 1990;255:756–768. [PubMed] [Google Scholar]
- Bogdanov Y, Michels G, Armstrong-Gold C, Haydon PG, Lindstrom J, Pangalos M, Moss SJ. Synaptic GABAA receptors are directly recruited from their extrasynaptic counterparts. EMBO J. 2006;25:4381–4389. doi: 10.1038/sj.emboj.7601309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brickley SG, Cull-Candy SG, Farrant M. Development of a tonic form of synaptic inhibition in rat cerebellar granule cells resulting from persistent activation of GABAA receptors. J Physiol. 1996;497(4):753–759. doi: 10.1113/jphysiol.1996.sp021806. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brunig I, Penschuck S, Berninger B, Benson J, Fritschy JM. BDNF reduces miniature inhibitory postsynaptic currents by rapid down regulation of GABAA receptor surface expression. Eur J Neurosci. 2001;13:1320–1328. doi: 10.1046/j.0953-816x.2001.01506.x. [DOI] [PubMed] [Google Scholar]
- Cheng Q, Yeh HH. Brain-derived neurotrophic factor attenuates mouse cerebellar granule cell GABAA receptor-mediated responses via postsynaptic mechanisms. J Physiol. 2003;548:711–721. doi: 10.1113/jphysiol.2002.037846. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cinar H, Barnes EM., Jr Clathrin-independent endocytosis of GABAA receptors in HEK 293 cells. Biochemistry. 2001;40:14030–14036. doi: 10.1021/bi011025t. [DOI] [PubMed] [Google Scholar]
- Connolly CN, Kittler JT, Thomas P, Uren JM, Brandon NJ, Smart TG, Moss SJ. Cell surface stability of gamma-aminobutyric acid type A receptors. Dependence on protein kinase C activity and subunit composition. J Biol Chem. 1999;274:36565–36572. doi: 10.1074/jbc.274.51.36565. [DOI] [PubMed] [Google Scholar]
- Essrich C, Lorez M, Benson J, Fritschy JM, Luscher B. Postsynaptic clustering of major GABAA receptor subtypes requires the γ2 subunit and gephyrin. Nat Neurosci. 1998;1:563–571. doi: 10.1038/2798. [DOI] [PubMed] [Google Scholar]
- Glykys J, Mann EO, Mody I. Which GABAA receptor subunits are necessary for tonic inhibition in the hippocampus? J Neurosci. 2008;28(6):1421–1426. doi: 10.1523/JNEUROSCI.4751-07.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gogolla N, Galimberti I, DePaola V, Caroni P. Preparation of organotypic hippocampal slice cultures for long-term live imaging. Nat Protoc. 2006;1:1165–1171. doi: 10.1038/nprot.2006.168. [DOI] [PubMed] [Google Scholar]
- Goodkin HP, Joshi S, Mtchedlishvili Z, Brar J, Kapur J. Subunit-specific trafficking of GABAA receptors during status epilepticus. J Neurosci. 2008;28:2527–2538. doi: 10.1523/JNEUROSCI.3426-07.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goodkin HP, Yeh JL, Kapur J. Status epilepticus increases the intracellular accumulation of GABAA receptors. J Neurosci. 2005;25:5511–5520. doi: 10.1523/JNEUROSCI.0900-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haas KF, MacDonald RL. GABAA receptor subunit γ2 and δ subtypes confer unique kinetic properties on recombinant GABAA receptor currents in mouse fibroblasts. J Physiol. 1999;514 (Pt 1):27–45. doi: 10.1111/j.1469-7793.1999.027af.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harrison NL. Mechanisms of sleep induction by GABAA receptor agonists. J Clin Psychiatry. 2007;68 (Suppl 5):6–12. [PubMed] [Google Scholar]
- Herd MB, Haythornthwaite AR, Rosahl TW, Wafford KA, Homanics GE, Lambert JJ, Belelli D. The expression of GABAA β subunit isoforms in synaptic and extrasynaptic receptor populations of mouse dentate gyrus granule cells. J Physiol. 2008;586:989–1004. doi: 10.1113/jphysiol.2007.146746. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Herring D, Huang R, Singh M, Dillon GH, Leidenheimer NJ. PKC modulation of GABAA receptor endocytosis and function is inhibited by mutation of a dileucine motif within the receptor β2 subunit. Neuropharmacology. 2005;48:181–194. doi: 10.1016/j.neuropharm.2004.09.015. [DOI] [PubMed] [Google Scholar]
- Hewitt SA, Bains JS. Brain-derived neurotrophic factor silences GABA synapses onto hypothalamic neuroendocrine cells through a postsynaptic dynamin-mediated mechanism. J Neurophysiol. 2006;95:2193–2198. doi: 10.1152/jn.01135.2005. [DOI] [PubMed] [Google Scholar]
- Hu CL, Zeng XM, Zhou MH, Shi YT, Cao H, Mei YA. Kv 1.1 is associated with neuronal apoptosis and modulated by protein kinase C in the rat cerebellar granule cell. J Neurochem. 2008;106:1125–1137. doi: 10.1111/j.1471-4159.2008.05449.x. [DOI] [PubMed] [Google Scholar]
- Huang CC, Hsu KS. Protein tyrosine kinase is required for the induction of long-term potentiation in the rat hippocampus. J Physiol. 1999;520:783–796. doi: 10.1111/j.1469-7793.1999.00783.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang EJ, Reichardt LF. Trk receptors: roles in neuronal signal transduction. Annu Rev Biochem. 2003;72:609–642. doi: 10.1146/annurev.biochem.72.121801.161629. [DOI] [PubMed] [Google Scholar]
- Jacob TC, Moss SJ, Jurd R. GABAA receptor trafficking and its role in the dynamic modulation of neuronal inhibition. Nat Rev Neurosci. 2008;9:331–343. doi: 10.1038/nrn2370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jovanovic JN, Thomas P, Kittler JT, Smart TG, Moss SJ. Brain-derived neurotrophic factor modulates fast synaptic inhibition by regulating GABAA receptor phosphorylation, activity, and cell-surface stability. J Neurosci. 2004;24:522–530. doi: 10.1523/JNEUROSCI.3606-03.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kanematsu T, Yasunaga A, Mizoguchi Y, Kuratani A, Kittler JT, Jovanovic JN, Takenaka K, Nakayama KI, Fukami K, Takenawa T, Moss SJ, Nabekura J, Hirata M. Modulation of GABAA receptor phosphorylation and membrane trafficking by phospholipase C-related inactive protein/protein phosphatase 1 and 2A signaling complex underlying brain-derived neurotrophic factor-dependent regulation of GABAergic inhibition. J Biol chem. 2006;281:22180–22189. doi: 10.1074/jbc.M603118200. [DOI] [PubMed] [Google Scholar]
- Kittler JT, Delmas P, Jovanovic JN, Brown DA, Smart TG, Moss SJ. Constitutive endocytosis of GABAA receptors by an association with the adaptin AP2 complex modulates inhibitory synaptic currents in hippocampal neurons. J Neurosci. 2000;20:7972–7977. doi: 10.1523/JNEUROSCI.20-21-07972.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kittler JT, Thomas P, Tretter V, Bogdanov YD, Haucke V, Smart TG, Moss SJ. Huntingtin-associated protein 1 regulates inhibitory synaptic transmission by modulating GABAA receptor membrane trafficking. Proc Natl Acad Sci USA. 2004;101:12736–12741. doi: 10.1073/pnas.0401860101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kittler JT, Chen G, Honing S, Bogdanov Y, McAinsh K, rancibia-Carcamo IL, Jovanovic JN, Pangalos MN, Haucke V, Yan Z, Moss SJ. Phospho-dependent binding of the clathrin AP2 adaptor complex to GABAA receptors regulates the efficacy of inhibitory synaptic transmission. Proc Natl Acad Sci USA. 2005;102:14871–14876. doi: 10.1073/pnas.0506653102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kobayashi E, Nakano H, Morimoto M, Tamaoki T. Calphostin C (UCN-1028C), a novel microbial compound, is a highly potent and specific inhibitor of protein kinase C. Biochem Biophys Res Commun. 1989;159:548–553. doi: 10.1016/0006-291x(89)90028-4. [DOI] [PubMed] [Google Scholar]
- Koyama R, Ikegaya Y. To BDNF or Not to BDNF: That Is the Epileptic Hippocampus. Neuroscientist. 2005;11:282–287. doi: 10.1177/1073858405278266. [DOI] [PubMed] [Google Scholar]
- Kurashima K, Szabo EZ, Lukacs G, Orlowski J, Grinstein S. Endosomal recycling of the Na+/H+ exchanger NHE3 isoform is regulated by the phosphatidylinositol 3-kinase pathway. J Biol Chem. 1998;273:20828–20836. doi: 10.1074/jbc.273.33.20828. [DOI] [PubMed] [Google Scholar]
- Lee SH, Simonetta A, Sheng M. Subunit rules governing the sorting of internalized AMPA receptors in hippocampal neurons. Neuron. 2004;43:221–236. doi: 10.1016/j.neuron.2004.06.015. [DOI] [PubMed] [Google Scholar]
- Leidenheimer N. Regulation of excitation by GABAA receptor internalization. Results Probl Cell Differ. 2007;44:1–28. doi: 10.1007/400_2007_039. [DOI] [PubMed] [Google Scholar]
- Liang J, Suryanarayanan A, Abriam A, Snyder B, Olsen RW, Spigelman I. Mechanisms of reversible GABAA receptor plasticity after ethanol intoxication. J Neurosci. 2007;27:12367–12377. doi: 10.1523/JNEUROSCI.2786-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maguire J, Mody I. Steroid hormone fluctuations and GABAA receptor plasticity. Psychoendocrinology. 2009 doi: 10.1016/j.psyneuen.2009.06.019. Article in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mangan PS, Kapur J. Factors underlying bursting behavior in a network of cultured hippocampal neurons exposed to zero magnesium. J Neurophysiol. 2004;91:946–957. doi: 10.1152/jn.00547.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mangan PS, Sun C, Carpenter M, Goodkin HP, Sieghart W, Kapur J. Cultured hippocampal pyramidal neurons express two kinds of GABAA receptors. Mol Pharmacol. 2005;67:775–788. doi: 10.1124/mol.104.007385. [DOI] [PubMed] [Google Scholar]
- Martin S, Henley JM. Activity-dependent endocytic sorting of kainate receptors to recycling or degradation pathways. EMBO J. 2004;23:4749–4759. doi: 10.1038/sj.emboj.7600483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marty S, Wehrlé R, Sotelo C. Neuronal activity and brain-derived neurotrophic factor regulate the density of inhibitory synapses in organotypic slice cultures of postnatal hippocampus. J Neurosci. 2000;20 (21):8087–8095. doi: 10.1523/JNEUROSCI.20-21-08087.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martys JL, Wjasow C, Gangi DM, Kielian MC, McGraw TE, Backer JM. Wortmannin-sensitive trafficking pathways in chinese hamster ovary cells. J Biol Chem. 1996;271:10953–10962. doi: 10.1074/jbc.271.18.10953. [DOI] [PubMed] [Google Scholar]
- McDonald BJ, Moss SJ. Conserved phosphorylation of the intracellular domains of GABAA receptor β2 and β3 subunits by cAMP-dependent protein kinase, cGMP-dependent protein kinase protein kinase C and Ca2+/calmodulin type II-dependent protein kinase. Neuropharmacology. 1997;36:1377–1385. doi: 10.1016/s0028-3908(97)00111-1. [DOI] [PubMed] [Google Scholar]
- McDonald BJ, Amato A, Connolly CN, Benke D, Moss SJ, Smart TG. Adjacent phosphorylation sites on GABAA receptor βγρ subunits determine regulation by cAMP-dependent protein kinase. Nat Neurosci. 1998;1:23–28. doi: 10.1038/223. [DOI] [PubMed] [Google Scholar]
- Michels G, Moss SJ. GABA A receptors: properties and trafficking. Crit Rev Biochem Mol Biol. 2007;42:3–14. doi: 10.1080/10409230601146219. [DOI] [PubMed] [Google Scholar]
- Mizoguchi Y, Kanematsu T, Hirata M, Nabekura J. A rapid increase in the total number of cell surface functional GABAA receptors induced by brain-derived neurotrophic factor in rat visual cortex. J Biol Chem. 2003;278:44097–44102. doi: 10.1074/jbc.M305872200. [DOI] [PubMed] [Google Scholar]
- Mody I, Glykys J, Wei W. A new meaning for "Gin & Tonic": tonic inhibition as the target for ethanol action in the brain. Alcohol. 2007;41(3):145–53. doi: 10.1016/j.alcohol.2007.03.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mortensen M, Smart TG. Extrasynaptic αβ subunit GABAA receptors on rat hippocampal pyramidal neurons. J Physiol. 2006;577:841–856. doi: 10.1113/jphysiol.2006.117952. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moss SJ, Doherty CA, Huganir RL. Identification of the cAMP-dependent protein kinase and protein kinase C phosphorylation sites within the major intracellular domains of the β1, γ2S, and γ2L subunits of the GABAA receptor. J Biol Chem. 1992a;267:14470–14476. [PubMed] [Google Scholar]
- Moss SJ, Smart TG, Blackstone CD, Huganir RL. Functional modulation of GABAA receptors by cAMP-dependent protein phosphorylation. Science. 1992b;257:661–665. doi: 10.1126/science.1323140. [DOI] [PubMed] [Google Scholar]
- Mtchedlishvili Z, Kapur J. High-affinity, slowly desensitizing GABAA receptors mediate tonic inhibition in hippocampal dentate granule cells. Mol Pharmacol. 2006;69:564–575. doi: 10.1124/mol.105.016683. [DOI] [PubMed] [Google Scholar]
- Nusser Z, Sieghart W, Somogyi P. Segregation of different GABAA receptors to synaptic and extrasynaptic membranes of cerebellar granule cells. J Neurosci. 1998;18:1693–1703. doi: 10.1523/JNEUROSCI.18-05-01693.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nusser Z, Mody I. Selective modulation of tonic and phasic inhibitions in dentate gyrus granule cells. J Neurophysiol. 2002;87(5):2624–2628. doi: 10.1152/jn.2002.87.5.2624. [DOI] [PubMed] [Google Scholar]
- Olsen RW, Hanchar HJ, Meera P, Wallner M. GABAA receptor subtypes: the "one glass of wine" receptors. Alcohol. 2007;41(3):201–9. doi: 10.1016/j.alcohol.2007.04.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peng Z, Hauer B, Mihalek RM, Homanics GE, Sieghart W, Olsen RW, Houser CR. GABA A receptor changes in δ subunit-deficient mice: altered expression of α4 and γ2 subunits in the forebrain. J Comp Neurol. 2002;446:179–197. doi: 10.1002/cne.10210. [DOI] [PubMed] [Google Scholar]
- Rivera C, Li H, Thomas-Crusells J, Lahtinen H, Viitanen T, Nanobashvili A, Kokaia Z, Airaksinen MS, Voipio J, Kaila K, Saarma M. BDNF-induced TrkB activation down-regulates the K+-Cl-cotransporter KCC2 and impairs neuronal Cl− extrusion. J Cell Biol. 2002;159:747–752. doi: 10.1083/jcb.200209011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roberts DS, Hu Y, Lund IV, Brooks-Kayal AR, Russek SJ. Brain-derived neurotrophic factor (BDNF)-induced synthesis of early growth response factor 3 (Egr3) controls the levels of GABAA receptor α4 subunits in hippocampal neurons. J Biol Chem. 2006;281:29431–29435. doi: 10.1074/jbc.C600167200. [DOI] [PubMed] [Google Scholar]
- Rose CR, Blum R, Kafitz KW, Kovalchuk Y, Konnerth A. From modulator to mediator: rapid effects of BDNF on ion channels. Bioessays. 2004;26:1185–1194. doi: 10.1002/bies.20118. [DOI] [PubMed] [Google Scholar]
- Santhakumar V, Wallner M, Otis TS. Ethanol acts directly on extrasynaptic subtypes of GABAA receptors to increase tonic inhibition. Alcohol. 2007;41(3):211–21. doi: 10.1016/j.alcohol.2007.04.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saxena NC, MacDonald RL. Assembly of GABAA receptor subunits: role of the δ subunit. J Neurosci. 1994;14:7077–7086. doi: 10.1523/JNEUROSCI.14-11-07077.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schofield PR, Darlison MG, Fujita N, Burt DR, Stephenson FA, Rodriguez H, Rhee LM, Ramachandran J, Reale V, Glencorse TA, Seeburg PH, Barnard EA. Sequence and functional expression of the GABAA receptor shows a ligand-gated receptor super-family. Nature. 1987;328:221–227. doi: 10.1038/328221a0. [DOI] [PubMed] [Google Scholar]
- Schweizer C, Balsiger S, Bluethmann H, Mansuy IM, Fritschy JM, Mohler H, Lüscher B. The γ2 subunit of GABAA receptors is required for maintenance of receptors at mature synapses. Mol Cell Neurosci. 2003;24(2):442–450. doi: 10.1016/s1044-7431(03)00202-1. [DOI] [PubMed] [Google Scholar]
- Semyanov A, Walker MC, Kullmann DM, Silver RA. Tonically active GABAA receptors: modulating the gain and maintaining the tone. Trends Neurosci. 2004;27(5):262–269. doi: 10.1016/j.tins.2004.03.005. [DOI] [PubMed] [Google Scholar]
- Shelton DL, Sutherland J, Gripp J, Camerato T, Armanini MP, Phillips HS, Carroll K, Spencer SD, Levinson AD. Human trks: molecular cloning, tissue distribution, and expression of extracellular domain immunoadhesins. J Neurosci. 1995;15:477–491. doi: 10.1523/JNEUROSCI.15-01-00477.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith SS, Shen H, Gong QH, Zhou X. Neurosteroid regulation of GABAA receptors: Focus on the α4 and δ subunits. Pharmacol Ther. 2007;116(1):58–76. doi: 10.1016/j.pharmthera.2007.03.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Somogyi P, Fritschy JM, Benke D, Roberts JDB, Sieghart W. The γ2 subunit of the GABAA receptor is concentrated in synaptic junctions containing the α1 and β subunits in hippocampus, cerebellum and globus pallidus. Neuropharmacology. 1996;35:1425–1444. doi: 10.1016/s0028-3908(96)00086-x. [DOI] [PubMed] [Google Scholar]
- Stocca G, Lovinger DM. Phorbol ester uncouples adenosine inhibition of presynaptic Ca2+ transients at hippocampal synapses. Hippocampus. 2003;13:355–360. doi: 10.1002/hipo.10088. [DOI] [PubMed] [Google Scholar]
- Sung U, Blakely RD. Calcium-dependent interactions of the human norepinephrine transporter with syntaxin 1A. Mol Cell Neurosci. 2007;34:251–260. doi: 10.1016/j.mcn.2006.11.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sur C, Farrar SJ, Kerby J, Whiting PJ, Atack JR, McKernan RM. Preferential coassembly of α4 and δ subunits of the GABAA receptor in rat thalamus. Mol Pharmacol. 1999;56:110–115. doi: 10.1124/mol.56.1.110. [DOI] [PubMed] [Google Scholar]
- Tanaka T, Saito H, Matsuki N. Inhibition of GABAA synaptic responses by brain-derived neurotrophic factor (BDNF) in rat hippocampus. J Neurosci. 1997;17:2959–2966. doi: 10.1523/JNEUROSCI.17-09-02959.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taylor PM, Thomas P, Gorrie GH, Connolly CN, Smart TG, Moss SJ. Identification of amino acid residues within GABAA receptor β subunits that mediate both homomeric and heteromeric receptor expression. J Neurosci. 1999;19:6360–6371. doi: 10.1523/JNEUROSCI.19-15-06360.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Terunuma M, Xu J, Vithlani M, Sieghart W, Kittler J, Pangalos M, Haydon PG, Coulter DA, Moss SJ. Deficits in phosphorylation of GABAA receptors by intimately associated protein kinase C activity underlie compromised synaptic inhibition during Status Epilepticus. J Neurosci. 2008;28:376–384. doi: 10.1523/JNEUROSCI.4346-07.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tretter V, Ehya N, Fuchs K, Sieghart W. Stoichiometry and assembly of a recombinant GABAA receptor subtype. J Neurosci. 1997;17:2728–2737. doi: 10.1523/JNEUROSCI.17-08-02728.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tyler WJ, Pozzo-Miller LD. BDNF enhances quantal neurotransmitter release and increases the number of docked vesicles at the active zones of hippocampal excitatory synapses. J Neurosci. 2001;21:4249–4258. doi: 10.1523/JNEUROSCI.21-12-04249.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vlahos CJ, Matter WF, Hui KY, Brown RF. A specific inhibitor of phosphatidylinositol 3-kinase, 2-(4- morpholinyl)-8-phenyl-4H-1-benzopyran-4-one (LY294002) J Biol Chem. 1994;269:5241–5248. [PubMed] [Google Scholar]
- Wallner M, Hanchar HJ, Olsen RW. Ethanol enhances α4β3δ and α6β3δ GABAA receptors at low concentrations known to affect humans. Proc Natl Acad Sci USA. 2003;100:15218–15223. doi: 10.1073/pnas.2435171100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang Q, Liu L, Pei L, Ju W, Ahmadian G, Lu J, Wang Y, Liu F, Wang YT. Control of synaptic strength, a novel function of Akt. Neuron. 2003;38:915–928. doi: 10.1016/s0896-6273(03)00356-8. [DOI] [PubMed] [Google Scholar]
- Wei W, Faria LC, Mody I. Low ethanol concentrations selectively augment the tonic inhibition mediated by δ subunit-containing GABAA receptors in hippocampal neurons. J Neurosci. 2004;24:8379–8382. doi: 10.1523/JNEUROSCI.2040-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wei W, Zhang N, Peng Z, Houser CR, Mody I. Perisynaptic localization of δ subunit-containing GABAA receptors and their activation by GABA spillover in the mouse dentate gyrus. J Neurosci. 2003;23:10650–10661. doi: 10.1523/JNEUROSCI.23-33-10650.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Whiting PJ. The GABAA receptor gene family: new targets for therapeutic intervention. Neurochem Internatl. 1999;34:387–390. doi: 10.1016/s0197-0186(99)00048-0. [DOI] [PubMed] [Google Scholar]
- Wohlfarth KM, Bianchi MT, Macdonald RL. Enhanced neurosteroid potentiation of ternary GABAA receptors containing the δ Subunit. J Neurosci. 2002;22:1541–1549. doi: 10.1523/JNEUROSCI.22-05-01541.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wooltorton JR, Moss SJ, Smart TG. Pharmacological and physiological characterization of murine homomeric β3 GABAA receptors. Eur J Neurosci. 1997;9:2225–2235. doi: 10.1111/j.1460-9568.1997.tb01641.x. [DOI] [PubMed] [Google Scholar]
- Yamada MK, Nakanishi K, Ohba S, Nakamura T, Ikegaya Y, Nishiyama N, Matsuki N. Brain-derived neurotrophic factor promotes the maturation of GABAergic mechanisms in cultured hippocampal neurons. J Neurosci. 2002;22 (17):7580–7585. doi: 10.1523/JNEUROSCI.22-17-07580.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- You H, Dunn SM. Identification of a domain in the δ subunit (S238-V264) of the α4β3δ GABAA receptor that confers high agonist sensitivity. J Neurochem. 2007;103:1092–1101. doi: 10.1111/j.1471-4159.2007.04817.x. [DOI] [PubMed] [Google Scholar]
- Yu W, Jiang M, Miralles CP, Li Rw, Chen G, de Blas AL. Gephyrin clustering is required for the stability of GABAergic synapses. Mol Cell Neurosci. 2007;36:484–500. doi: 10.1016/j.mcn.2007.08.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
A-Hippocampal slice cultures grown in vitro for 8 days were immunolabeled for the surface-expressed δ or γ2 subunits. Immunoreactivity of the δ subunit was primarily observed in the dentate granule layer, whereas that of the γ2 subunit was observed in the dentate granule layer as well as CA regions. Immunoreactivity of NeuN, a neuronal marker protein studied in permeabilized slices, was observed in the dentate granule cells, CA neurons and interneurons. B- GABAergic currents were recorded from the dentate gyrus granule neurons in the hippocampal slice cultures grown in vitro for 8 days in the presence of 20 μM DNQX and 50 μM APV, blockers of ionotrophic glutamatergic receptors, by whole cell patch clamp recordings. Tonic GABAergic inhibition was measured in terms of a characteristic shift in the baseline holding current after application of 100 μM bicuculline, a competitive GABARs antagonist. The baseline-holding current shifted from -71.1 ± 15.6 pA to -36.0 ± 16.0 pA (n=3, p<0.01, paired t-test).
A- Hippocampal slice cultures were treated with U73343 (5 μM), an inactive analog of U73122, a PLC inhibitor, in the presence of BDNF for 6 hr. U73343 did not inhibit BDNF-induced increase in the surface expression (S) of the δ subunit. Total expression (T) of the δ subunit was similar in all the samples. B- Hippocampal slice cultures were treated with α-PDBu (5 μM), an inactive isoform of PKC activator PDBu for 6 hr. Surface expression (S) of the δ subunit was not altered in the presence of α-PDBu. Total expression (T) of the δ subunit was similar in all the samples.