Abstract
We used an operant delayed spatial alternation task to examine the role of the dorsomedial prefrontal cortex (dmPFC) in spatial working memory. The task was designed to restrict movements during the delay period to minimize use of motor-mediating strategies. Inactivation of dmPFC (muscimol) resulted in increased errors and increased the temporal variability of responding. Animals did not show perseveration after errors (i.e., responding again at the erroneous location). Under control conditions, the time between spatial responses was greater and more variable prior to errors as compared to correct responses. These effects were eliminated when muscimol was infused into dorsomedial prefrontal cortex. Trial outcome also affected movement and delay times in the next trial. This effect was diminished with muscimol in dorsomedial prefrontal cortex. By contrast, when muscimol was infused in dorsal agranular insular cortex – a region that is strongly interconnected with dorsomedial prefrontal regions – there was no effect on delayed spatial alternation performance. These experiments confirm that dorsomedial prefrontal cortex is necessary for successful delayed spatial alternation and establish that there is a relationship between response time variability and trial outcome that depends on dorsomedial prefrontal function.
INTRODUCTION
Working memory is defined as the process of holding relevant data in mind when behavioral events are separated by temporal delays (Fuster, 2001), so that information from previous experiences can be used to plan future behaviors (Goldman-Rakic, 1995; Miller and Cohen, 2001). This ability also permits moment-to-moment control to optimize behavior (Funahashi, 2001; Tanji and Hoshi, 2008). The prefrontal cortex (PFC) has long been implicated in these processes. Lesions of lateral (e.g., Brozoski et al., 1979; Funahashi et al., 1993) or medial PFC (e.g., Bachevalier and Mishkin, 1986) in nonhuman primates and of dorsomedial PFC in rodents (e.g., Izaki et al., 2001; Dunnett et al., 2005; Wang and Cai, 2006; Yoon et al., 2008) result in erroneous responding during performance of working memory tasks.
Existing maze and operant versions of spatial working memory tasks have been criticized for the lack of control over the movements of rats during delays (Chudasama and Muir, 1997; Ennaceur and Aggleton, 1998; Euston and McNaughton, 2006; Cowen and McNaughton, 2007). Rats have been shown to use motor-mediating strategies that can circumvent the need for working memory processes and impact interpretation of the data. The experiments reported here address this issue by challenging spatial working memory capacity using a novel behavioral design that controls the orientation of rats during the delay period. Working memory impairment, measured as increased error responding, has also been reported in a number of human psychological conditions, including Attention-Deficit Hyperactivity Disorder (ADHD; e.g., Brocki et al., 2008) and normal aging (e.g., Salthouse and Babcock, 1991). In both ADHD and normal aging, there are reductions in the volume of PFC tissue (ADHD: Giedd et al., 2001; aging: Raz et al., 2000; Gunning-Dixon & Raz, 2000) and the complexity of PFC neurons (Grill and Riddle, 2002; Duan et al. 2003). It is becoming increasingly evident that an accompanying feature of impaired working memory performance is increased within-subject response time variability (e.g., Castellanos et al., 2005; Alderson et al., 2007; Geurts et al., 2008). This intra-individual variability in responding may be due to diminished executive control over behavior on a trial-by-trial basis, and may lead to increased erroneous responding.
Despite shared prefrontal dependence, few human studies have sought to examine the direct relationship between variable response times and working memory function. One recent study that examined this issue was by Buzy and colleagues (2009), who used an ex-Gaussian approach to demonstrate that subjects with ADHD committed more errors of omission, were more variable with regard to success, and showed skewed response time distributions that included more long responses than found in normal subjects. These performance differences correlated with differences in activity in medial regions of PFC (Fassbender et al., 2009).
There is a paucity of data from animal studies to support these results. The experiments reported here are some of the first to examine how inactivation of rat dorsomedial PFC (dmPFC) impact the relationships between delay durations and trial outcomes during the performance of an operant spatial working memory task. Under control conditions, rats had more variable response times prior to errors than to correct responses. However, when muscimol was infused to inactivate dmPFC, the intervals preceding correct responses became more variable and rats committed a greater number of errors. Dorsal agranular insular cortex (AId), which is densely interconnected with dmPFC (Gabbott et al., 2003), was inactivated as a control region. Infusions of muscimol into rat AId cortex did not result in impaired performance accuracy.
These experiments confirm previous reports of working memory impairment following lesion or inactivation of rat dmPFC under conditions in which the potential use of non-working memory strategies is minimized. The data also demonstrate that a lateral region of the PFC, AId, does not contribute to successful alternation performance, despite the extensive network of connections between these regions (Gabbott et al., 2003). Finally, these results show that response time variability may contribute to the increase in erroneous responding when dmPFC is inactivated, and that the performance of errors may influence response time variability.
MATERIALS AND METHODS
Subjects
Seven adult Long-Evans rats were trained using operant procedures to perform a self-paced delayed spatial alternation task. Naϊve rats were placed on regulated water for one week prior to training in the behavioral procedure and were handled ∼5 minutes per day. Once training commenced, rats received 7.1 ± 1.3 ml (n = 7 rats; mean ± standard error) of water during each behavioral session, which was supplemented by additional water in the home cage to maintain rats at ∼90% of their free access body weight. Food was available ad libitum. All procedures were approved by the Animal Care and Use Committee at the John B. Pierce Laboratory and conformed to the standards of the United States National Institutes of Health Guide for the Care and Use of Laboratory Animals. All efforts were taken to minimize the number of animal subjects in this study and to reduce pain and suffering.
Behavioral training apparatus
Training was carried out in standard operant arenas within sound attenuating chambers (MedAssociates, St. Albans, VT). Custom-built “left” and “right” nosepoke ports (John B. Pierce Machine Shop, New Haven, CT) were mounted on one wall of the chamber. Head entries into these response ports were captured by interruption of an infrared photobeam. A third nosepoke port was located in the center of the wall at the opposite end of the chamber and contained the reward spout, the tip of which was situated immediately behind the infrared photo beam to capture licking activity. Drinking tubes were mounted within the left and right response ports during early training and in the reward port for the duration of the experiment. Liquid reinforcements were delivered through standard spouts (Ancare, Bellmore, NY) connected via Tygon tubing to pumps (MedAssociates) outside the chamber. Illumination was provided during the session by a houselight (MedAssociates). An array of infrared light emitting diodes (B. G. Micro, Garland, TX) permitted video monitoring during periods when the houselight was extinguished. The “click” of a solid-state relay was played and a stimulus light above the spout (MedAssociates) was illuminated whenever water was available from the reward spout. See Figure 1A for a schematic layout of the operant chamber.
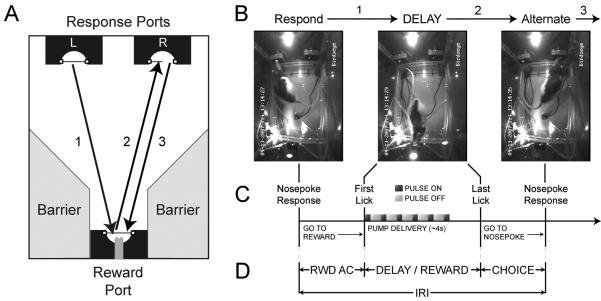
Figure 1. Delayed spatial alternation task.
A) Schematic of events in task. Rats were trained to alternate between responding in left and right response ports (marked “L” and “R”) for liquid rewards. Following a correct response – shown here in the left port – the rat crossed the chamber to collect reward (1). During this interval, the side-to-side movement is constrained by barriers to either side of the rat (marked “Barrier”). To complete another correct trial, the rat would then cross to the alternate (right) port (2) before returning to the reward port (3). B) Video captures of behavioral chamber depicting events in (A). Note that during the delay / reward collection period, the rat's back is to the response ports and side-to-side movement is restricted by two Plexiglas barriers. The rat was encouraged to maintain this posture by extending reward delivery in a pulsatile fashion (0.25 sec on, 0.50 sec off for ∼4.0 sec). C) Timeline of events in task. After a nosepoke response, the first lick captured at the reward port marked the beginning of the delay. The last lick captured designated the end of the delay / reward collection period. D) Time windows used in interval analyses (Figures 6 and 7). (RWD AC = reward acquisition; CHOICE = response port selection window; IRI = inter-response interval, time between consecutive responses.)
All experimental events were controlled and captured by computer software (MedPC). A fan in the wall of the external chamber provided masking noise at 60dBA. All behavioral sessions were monitored via four closed circuit video cameras (Swann, Melbourne, AU). Video recordings of all behavioral sessions were stored as digital video files and examined for evidence of potential behavioral strategies for mediating spatial information over the inter-trial interval (such as leaning to one side against the barriers or holding different patterns of the feet prior to left or right responses). No overt evidence for such behavioral strategies was apparent from the video recordings. Nevertheless, we must admit that it is possible that rats used a behavioral strategy that was subtle and not detectable by video analysis.
Behavioral testing apparatus
Once trained, all rats were transferred to a testing chamber, which was custom-built (John B. Pierce Machine Shop) and adapted to be suitable for neurophysiological recordings. The arena had the same basic layout as described for the training chamber, except that all metallic components were replaced with plastic equivalents. As shown in Figure 1B, behavior was monitored by a video system (Swann) and captured by a digital video recorder (Aventura Technologies).
Behavioral training
Rats were trained in the delayed spatial alternation task prior to implantation with cannulae. After 1-2 weeks of handling and regulated water access, rats were introduced to the operant chamber. Rats were first trained to collect water from the reward spout in response to periodic (every 5-20 seconds) illumination of the visual stimulus above the reward port. In subsequent sessions, an auditory click stimulus alerted rats to the onset of the visual stimulus, and rats were required to lick the spout to engage the pump (0.05 ml/sec for 2.0 seconds). During this phase of training, both left and right response ports were blocked to head entries. Pre-training sessions lasted 30 minutes or until the syringe was emptied, whichever came first.
Left and right response ports were uncovered on the first day of nosepoke training to encourage exploration. A response in either port resulted in presentation of the click stimulus and illumination of the light stimulus above the reward port, and rats were allowed 30 seconds to collect reward (1.0 sec water at 0.05 ml/sec). To facilitate alternation behavior, water (1.0 sec at 0.05 ml/sec) was delivered in the left or right response port when the rat alternated nosepoke responses, but not when rats continued to respond in the same location. Nosepoke training sessions were 90 minutes in length.
During the remainder of spatial alternation training and performance, rats had to alternate between left and right response locations on a trial-by-trial basis. During training, water rewards were shifted from the response location to the reward location and the amounts were reduced, until fully trained rats received fluid exclusively in the reward port (Figure 1A). In addition, the time allowed to collect reward was shortened incrementally over days (from 30 to 5 sec). Rats were not required to make a response in the reward port after selection of the correct response location, and if reward was not collected within the time allotted, the next trial would commence. All alternation sessions lasted 90 minutes.
Consecutive responses made in the same location were scored as errors. After an error all lights in the chamber were extinguished, and the trial had to be “reset” with a nosepoke entry in the reward port. Once the trial was reset, a correcting response could be made immediately for a reward.
To create a delay period, the reward period was extended by pulsing the pump on and off (6 “on” pulses of 0.25 sec each separated by 0.50 sec “off” pulses; see Figure 1C). During this period, rats' backs were to the response ports, preventing visual targeting of the next response location. Rats were allowed to continue licking the reward spout past the cessation of water delivery.
Existing maze and operant versions of spatial working memory tasks have been criticized for the lack of control over the movements of rats during delays (Chudasama and Muir, 1997; Ennaceur and Aggleton, 1998; Euston and McNaughton, 2006; Cowen and McNaughton, 2007). Rats have been shown to use motor-mediating strategies that can circumvent the need for working memory processes and impact interpretation of the data. The experiments reported here address this issue using several methods. Rats made spatial responses on one end of the behavioral arena (left and right photos in Figure 1B), and collected reward at the opposite end of the chamber, with their backs to the response locations (center photo in Figure 1B). They passed through a narrow alleyway that limited the angle of entry and exit and constrained side-to-side movements during the delay (noted as “Barrier” in Figure 1A). Fluid reward was delivered in a pulsatile manner to keep rats in this position for extended periods of time.
Surgery
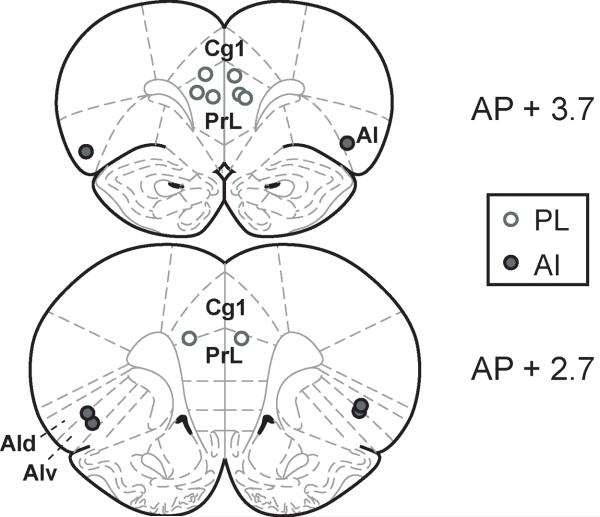
Anesthesia was initiated with ∼4% halothane and maintained with intraperitoneal injections of ketamine (75-100 mg/kg) and diazepam (5-10 mg/kg) or xylazine (10 mg/kg). Surgical levels of anesthesia were maintained over the course of surgery with supplements (∼25 mg/kg) of ketamine every 30-60 min. Under aseptic conditions, the scalp was retracted, and the skull was leveled between bregma and lambda. Craniotomies were made bilaterally above the target cannulae sites, and 26-gauge cannulae (Plastics One, Roanoke, VA) with stylettes cut flush to the cannulae tips were lowered slowly into place. Target locations were dorsal prelimbic cortex (n = 4 rats; AP +3.2, ML +/−1.4, DV −3.7 at an angle of 12° from the midline) or AId (n = 3 rats; AP +2.7, ML +/−4.5, DV −5.5). See Figure 2 for approximate locations of cannula tips.
Figure 2. Approximate location of cannula tips.
Cannulae were targeted at bilateral prelimbic (PL) cortex and the dorsal agranular insular (AId) cortex. (Images adapted from Paxinos and Watson (1998).)
After guide cannulae were placed, craniotomies were sealed with cyanoacrylate (SloZap, Pacer Technologies, Rancho Cucamonga, CA) accelerated by ZipKicker (Pacer Technologies). Dental cement (methyl methacrylate; AM Systems, Port Angeles, WA) was built up around the guide cannulae and skull screws to hold the implants in place. Rats were either given 0.05 mg/kg buprenorphine subcutaneously every 12 hours over the first 48 hours after surgery or carprofen (Rymadil®) at 25 mg in 500 ml drinking water for postoperative analgesia. Rats were maintained on oral antibiotic (3 ml of 200 mg/ml Baytril dissolved in 500 ml water; Bayer HealthCare LLC, Shawnee Mission, KS) and allowed to recover in their home cages for 7-10 days before behavioral testing.
Drug infusion
After at least one week of recovery from surgery, cannulated rats were placed on regulated water access and behavioral sessions were resumed. Infusion procedures were initiated once rats returned to pre-operative levels of performance in the delayed spatial alternation task. Muscimol, a potent GABA-A (gamma-aminobutyric acid) receptor agonist, was infused to reversibly inactivate the target regions (Narayanan et al., 2006; Martin and Ghez 1999).
Infusion procedures were carried out as described previously in Narayanan et al. (2006). Rats were lightly anesthetized with ∼4% halothane. Sterile saline (0.9%; Phoenix Scientific, Holliston, MA) or muscimol (Sigma-Aldrich, St. Louis, MO) dissolved in saline was delivered at a rate of 0.25 μl/min (Martin and Ghez, 1999) for two minutes (0.5 μl per hemisphere) via an injector assembly – a 33-gauge internal cannula (Plastics One) connected to a 10 μl Hamilton syringe (Hamilton, Reno, NV) by 0.38 mm diameter polyethylene tubing (Intramedic, New York, NY). The rate of infusion was controlled by a syringe infusion pump (KD Scientific, Holliston, MA). Injections were confirmed by monitoring movement of fluid in the tubing via a small air bubble. Each hemisphere was infused separately, and the injector was left in place for 2 min after infusion to allow diffusion of fluid. Behavioral testing took place 45 minutes after completion of infusion to allow full recovery from halothane and maximal inhibition of cortical activity by muscimol (Martin and Ghez 1999; Allen et al., 2008).
Rats with cannulae in dmPFC received infusions over a period of two weeks, each week beginning with sterile saline, followed by low-dose muscimol (0.01 μg/μL), either mid- (0.05 μg/μL) or high-dose (0.10 μg/μL) muscimol, and a recovery session in which no infusions were made. Rats with cannulae in AId cortex received infusions of saline, muscimol (0.05 μg/μL), and were run in a recovery session. The muscimol dose used for AId was selected based on its efficacy in impairing performance when infused into dmPFC. The AId rats received additional muscimol infusions at two higher doses in two consecutive sessions following the initial muscimol session (0.10 μg/μL and 1.0 μg/μL). These higher doses were used to examine potential differences in the effective dose for muscimol in mPFC and AId. However, as shown below, we observed no significant memory-related effects of any dose of AId in the delayed alternation task. For both areas, we used a progressive dosing schedule, testing lower doses before higher doses, in all animals. This was done to ensure that any potential carryover effects of prior inactivations would be the same direction over all animals. As prior studies involving testing with single doses of muscimol have failed to show such carryover effects, we believe that this issue did not influence any of the results reported in this manuscript.
Previous studies of rats performing a simple reaction time task used fluorescent muscimol infusions to confirm the approximate extent of cortical inactivation in medial prefrontal (Narayanan et al., 2006; Allen et al., 2008) and AId (N. K. Horst, N. S. Narayanan, and M. Laubach, unpublished observations). These experiments used a dose of fluorescent muscimol of the same molarity as a 1.0 μg/μL dose of plain muscimol. Bilateral infusions of 0.5 μL of this dose of fluorescent muscimol were delivered at a rate of 0.25 μL/min. In both cases, infusions were confined to the region of interest. The effective dose for the experiments described here was 0.05 μg/μL plain muscimol delivered at the same rate and volume. It is therefore expected that the effective spread would be equivalent or less than that observed at the higher dose.
Histology
Once experiments were complete, rats were anesthetized with 100 mg/kg sodium pentobarbital or 4-6 ml Euthasol (390 mg/ml pentobarbital sodium plus 50 mg/ml phenytoin sodium) and then transcardially perfused with either 10% formalin or 4% paraformaldehyde. Brains were sectioned coronally on a freezing microtome, mounted on subbed slides, and stained for Nissl with Thionin.
Data analysis and statistics
All behavioral analyses were carried out using custom scripts for Matlab (The Mathworks, Natick, MA) and R (The R-Project for Statistical Computing, www.r-project.org). In the task described, rats were not required to collect reward after a correct response. Therefore, there are some trials for which a delay – defined as the time between the beginning and end of reward collection – did not occur. As behavior of the rats in these “incomplete” trials was uncontrolled, they were disregarded from analysis. The number of completed trials varied between subjects (129-299, 188.93 ± 26.4 (mean ± standard error) trials in saline sessions; 83-345, 174.3 ± 32.7 trials in muscimol sessions), but not between saline and muscimol conditions (p > 0.05, paired t-test; PFC: t = −0.10; AId: t = 1.59;. To standardize the number of trials used across subjects and conditions and to attempt to control for motivational effects, only the first 75 complete trials were used for further analyses.
Comparisons of overall performance accuracy between conditions (preoperative, saline weeks 1 and 2, 0.01 μg/μl muscimol weeks 1 and 2, 0.05 μg/μl muscimol, 0.1 μg/μl muscimol, and recovery weeks 1 and 2) were made using repeated measures analysis of variance (rmANOVA; aov in R) and paired t-tests (ttest.m in Matlab). Remaining analyses were carried out only on the effective dose (0.05 μg/μl muscimol) and its respective saline control session.
Comparisons of error patterns between conditions were carried out using paired t-tests in Matlab. Response bias was measured as the difference between the percentages of errors on the preferred versus the non-preferred side. The likelihood of win-stay (initiating) errors was calculated as the conditional probability that a trial was an error given that the previous trial was correct. The likelihood of repeated (perseverative) errors was calculated as the conditional probability that a trial was an error given that the previous trial was an error. Trials were not considered to be consecutive if an incomplete trial without reward collection occurred in between, and this was accounted for in these analyses. Geometric distributions were generated using the Rcmdr package in R. Comparison of geometric versus actual distributions and between distributions of error data in saline and muscimol sessions were conducted using the Wilcoxon rank-sum test (ranksum.m in Matlab) for non-normal samples.
Custom Matlab scripts were used to sort behavioral intervals based on previous or next trial outcome (correct vs. error) and by experimental session (saline vs. muscimol). First, the length of the inter-response interval (IRI, Figure 2D) was examined to determine if the time between consecutive nosepoke responses differed significantly between conditions. This interval was then subdivided into the phases of the task: Reward Acquisition, Delay, and Choice, as shown in Figure 2D. For each task phase, interval data were examined to determine whether their median and spread were affected by trial outcome, by session, or by an interaction between outcome and session. Significant differences in interval medians were determined using the Kruskal-Wallis test (kruskal.test in R) and differences in variability were assessed for significance using the Fligner-Killeen test for homogeneity of variance (fligner.test in R). These tests evaluate differences across all data from all subjects, so a jack-knifing procedure was used to confirm that single subjects did not drive significance levels. Data from each subject were iteratively removed from the interval data set, and data from the remaining three subjects were tested with the Kruskal-Wallis and Fligner-Killeen tests. Differences in behavior during each interval were considered significant if the p-value remained less than 0.05 after the removal of each subject's data.
RESULTS
Cannulae localization
Eight rats were trained to accurately perform the delayed spatial alternation task before being implanted bilaterally with guide cannulae. Cannulae were targeted to dmPFC (n = 4) or AId cortex (n = 3), and the locations of cannula tips were confirmed in 50 μm sections of thionin-stained tissue. Approximate locations of cannula tips are shown in Figure 2.
Effects of dmPFC inactivation on performance accuracy in delayed spatial alternation
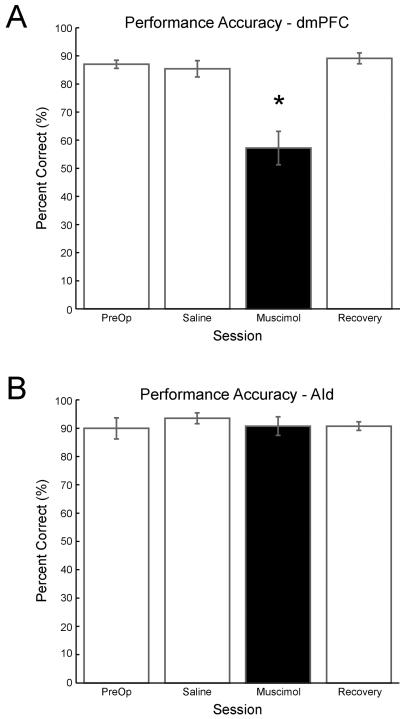
Muscimol infusions in rat dmPFC impaired performance accuracy in the delayed spatial alternation task (Figure 3). Repeated measures ANOVA on the percentage of correct trials across all test sessions revealed a main effect of drug (p << 0.001, F = 11.1). The percentage of correctly performed trials after infusions of 0.05 μg/μl muscimol (57.2 ± 5.9%) was significantly lower than in sessions with saline infusions (85.4 ± 2.9%; p < 0.05, t = 5.67).
Figure 3. Performance accuracy following muscimol inactivation of two regions in rat prefrontal cortex.
A) Rats (n = 4) with bilateral infusions of muscimol (0.5 μL of 0.05 μg/μL) in dorsomedial prefrontal cortex (dmPFC) show impaired performance accuracy compared to preoperative, saline, and recovery conditions. (* = p< 0.05 by rmANOVA; p < 0.02 by paired t-test with Bonferroni correction). B) Rats (n = 3) with muscimol (0.5 μL per hemisphere of 0.05 μg/μL) in dorsal agranular insular cortex (AId) were not impaired at this dose.
Performance accuracies within saline sessions (p > 0.05, t = −0.83), low-dose muscimol sessions (p > 0.05, t = 0.33), and recovery sessions (p > 0.05, t = −0.26) were not different by paired t-test. Cannulation, exposure to halothane, and infusion of fluid did not impact behavior, as there were no differences in the percentages of correct trials found between saline, recovery, and preoperative sessions (p > 0.05, paired t-test; week 1 saline session vs. week 1 recovery session: t = −0.79; week 2 saline vs. week 2 recovery: t = −0.19; week 1 saline vs. preoperative: t = 0.90; week 2 saline vs. preoperative t = −0.29; Figure 3).
At a five-fold lower dose (0.01 μg/μl) of muscimol, performance accuracy was not significantly impacted (p > 0.05, paired t-test; week 1 saline vs. week 1 low dose muscimol: t=2.01; week 2 saline vs. week 2 low dose muscimol: t = 1.37), although this may be due in part to the small number of subjects tested. At a two-fold higher dose (0.10 μg/μl muscimol), one of the rats did not complete any trials. Further analyses were carried out exclusively on the 0.05 μg/μl dose condition, for which all rats behaved and showed performance decrements.
AId cortex and dmPFC share substantial reciprocal connections (Gabbott et al., 2003). To determine whether this region contributes to accurate spatial alternation performance, muscimol was infused into AId cortex in three additional rats. There was no main effect of cannulation or drug by rmANOVA (p > 0.05, F = 0.32), even when muscimol was delivered at higher doses (0.1 μg/μl and 1.0 μg/μl; p > 0.05, F = 0.45). At the dose of muscimol that was effective in dmPFC, rats performed 90.7 ± 3.3% accurately as compared to 93.5 ± 1.9% under saline. Thus, the AId cortex does not appear to be critical for successful spatial alternation behavior in this task. The remaining analyses therefore focus on dmPFC and the aspects of behavior that are affected by muscimol in this region.
Patterns of errors after infusion of muscimol in dorsomedial prefrontal cortex
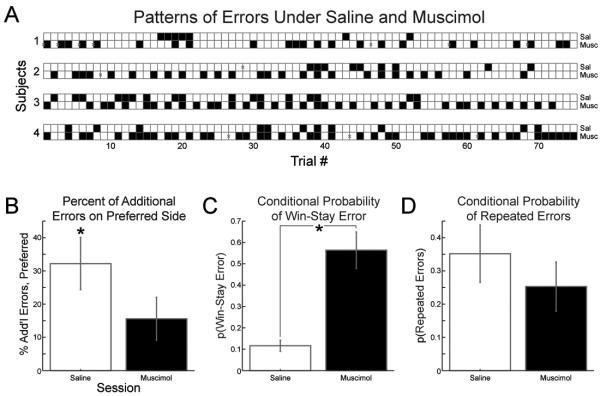
The above analyses describe an increase in the number of errors made after infusion of muscimol into rat dmPFC, but do not describe the type of errors being made. Figure 4A shows correct (white squares) and error (black squares) trials in each subject under saline and muscimol. The following analyses explore whether there were specific patterns of errors that emerged when dmPFC was inactivated by muscimol, which will aid in understanding the nature of the behavioral impairment following inactivation of dmPFC.
Figure 4. Patterns of errors following muscimol infusions in medial prefrontal cortex.
A) Trial outcomes for four rats implanted with cannulae in medial prefrontal cortex. Data are from sessions with 0.05 μg/μL muscimol and corresponding saline sessions, which occurred over adjacent days within a single week. Correct trials are represented by white boxes. Error trials are shown in black. Gray x's denote correct trials which were removed from analyses, because rat did not collect reward (see Methods). B) Percent of additional errors in preferred location. Rats showed response location bias under saline, but not when muscimol was infused in medial prefrontal cortex. (Asterisk denotes significantly more responses in preferred location under saline.) C) Conditional probability of an error, given the previous trial was correct. With muscimol in dmPFC, rats were more likely to return to the location at which they had just been rewarded. D) Conditional probability of an error given the previous response was an error. Rats made more repeated responses in the location for which they had responded erroneously (* = p < 0.05, paired t-test).
Rats could have made more errors if a greater response bias for one location over the other emerged. Bias is operationally defined here as a greater number of errors committed on one side versus the other. Rats showed response biases under saline (66.1 ± 3.9% vs. 33.9 ± 3.9% (mean ± standard error); p < 0.05, paired t = 4.10), but not muscimol (57.8 ± 3.2% vs. 42.2 ± 3.2%; p > 0.05, t = 2.40). A bias index was calculated as the difference between the percentage of errors in the preferred compared to the non-preferred location. There was no increase in response bias with muscimol in dmPFC (p > 0.05, paired t = 1.32; Figure 4B).
Another possibility is that rats under muscimol may have returned to the response location for which they were previously rewarded, which would be reflected by the conditional probability of an error after a correct trial. The number of occurrences of correct → error (7.25 ± 1.7 vs. 22.5 ± 2.9 trials; p < 0.01, paired t = −7.7) increased when muscimol was infused in dmPFC. The conditional probability of an error after a correct trial increased significantly under muscimol inactivation (0.12 ± 0.03 vs. 0.56 ± 0.09; p<0.01, paired t = −6.5; Figure 4C).
Finally, rats could have made more perseverative errors after cortical inactivation. This potential result was examined by measuring the conditional probability of an error given the previous response was in error. Neither the number (saline = 3.75 ± 1.3, muscimol = 9.25 ± 4.3 errors; p > 0.05, paired t = −1.04) nor the likelihood (saline = 0.35 ± 0.09, muscimol = 0.25 ± 0.07; p > 0.05, paired t = 0.59) of repeated errors increased during muscimol inactivation of dmPFC (Fig 4D).
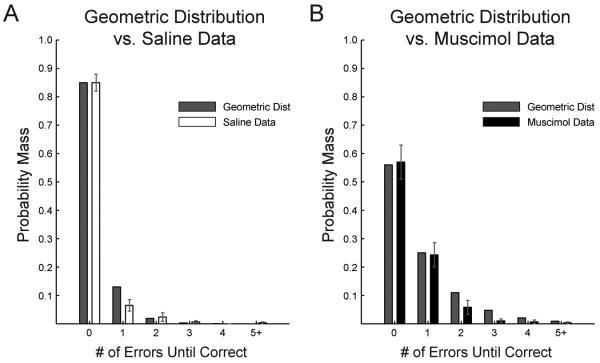
To further examine whether errors beyond the initiating “win-stay” error conformed to a nonrandom pattern, they were compared to a geometric distribution. The geometric distribution is the probability distribution of the number of failures before the first success in a series of Bernoulli trials. Thus, this distribution was an appropriate one to compare with the behavioral data. As shown in Figure 5, the patterns of non-initiating errors resembled the geometric distribution to which they were compared (p>0.05, Wilcoxon Rank-sum test; saline: U = 37.5; muscimol: U = 43.0), and therefore conformed to a random pattern. There was no difference by Wilcoxon Rank-sum between the distributions of data after saline or muscimol infusions (p > 0.05; U = 24).
Figure 5. Actual error distributions compared to expected error patterns from a geometric distribution.
Geometric distributions were based on the total percent correct under (A) saline (85.3 ± 2.9%) and (B) muscimol (55.7 ± 5.9%) conditions. A) Error patterns under saline were not significantly different from the geometric distribution (p = 0.86 by Wilcoxon rank-sum test). B) Error patterns were not significantly different from the geometric distribution following muscimol inactivation (p = 0.59 by Wilcoxon rank-sum test). The distributions of errors were not different between saline and muscimol (p = 0.52 by Wilcoxon rank-sum test).
Altogether, these data provide evidence that inactivation of rat dmPFC disrupts behavior by increasing the frequency of errors committed. Specifically, rats were more likely under muscimol to return to the previous response location. However, they were not more likely to perseverate at the port after the initial error.
Effects of movement times on trial outcome
Previous studies of delayed spatial alternation (e.g., Izaki et al., 2001) have shown that the percentage of correctly performed trials decreases as a function of increasing delay. As this version of the task was self-paced, standard delay lengths were not imposed between subjects, conditions, or trials. Accumulating evidence from studies of human subjects with ADHD suggests that within-subject variability in response times may be a defining feature of the condition (e.g., Castellanos et al., 2005; Alderson et al., 2007; Geurts et al., 2008; Buzy et al., 2009). Patients with ADHD have reduced PFC tissue volume (Giedd et al., 2001), and there is evidence for within-subject response time variability and altered function in medial regions of PFC during performance of a working memory task (Fassbender et al., 2009). The self-paced nature of the spatial alternation task described here permitted direct observation of the relationships between response time, response time variability, and trial outcomes under control conditions, and with dmPFC inactivated.
Inter-response interval (IRI) data were examined using non-parametric statistical methods. The Kruskal-Wallis ranksum (K-W) test was used to compare the central tendency (i.e., median) of IRIs under saline and muscimol and before or after correct or error trials. The Fligner-Killeen (F-K) test compared variability of IRIs between these same conditions. These tests were initially performed across pooled subject data. A jack-knifing procedure was used to confirm that significant results held true when each subject's data was removed from the full dataset.
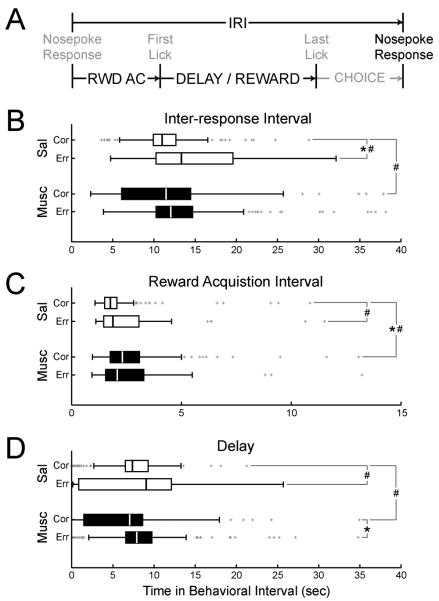
To determine if trial outcome was related to the time that had passed since the previous response, inter-response intervals were sorted by whether they were followed by a correct or an error response (Figure 6A). The median IRI was shorter prior to correct (11.1 ± 0.4 sec (mean ± standard error)) as compared to error (14.8 ± 0.9 sec) responses during saline sessions (p < 0.001, X2 = 12.7). The spread of data under saline was significantly less for correct responses (interquartile range: 2.6 ± 0.5 sec) as compared to errors (10.0 ± 2.1 sec; p << 0.001, X2 = 28.1). These differences were lost after muscimol inactivation (medians: 10.4 ± 2.5 sec for correct trials vs. 12.4 ± 1.3 sec for errors, p > 0.05 with jack-knife procedure, X2 = 2.77; spread: 6.1 ± 1.0 sec for correct trials vs. 5.3 ± 2.2 sec for errors, p > 0.05, X2 = 0.02 with jack-knife procedure). This appears to be due to an increase in the spread of IRIs preceding correct responses (6.1 ± 1.0 sec) when muscimol was infused (p << 0.001, X2 = 77.0). The median IRI was unchanged between saline and muscimol sessions for both correct and error responses (correct: 11.1 ± 0.38 sec for saline vs. 10.4 ± 2.5 sec for muscimol, p > 0.05, X2 = 0.34 across all subjects; error: 14.8 ± 0.9 sec for saline vs. 12.4 ± 1.3 sec for muscimol, p > 0.05, X2 = 1.97 across all subjects). The spread of data for error responses was not different between saline and muscimol sessions (10.0 ± 2.1sec for saline vs. 5.3 ± 2.2 sec for muscimol, p > 0.05, X2 = 0.38 across all subjects). Inter-response interval data are summarized in Figure 6B.
Figure 6. Distributions of interval lengths preceding correct or error responses.
A) Intervals used in the analysis. B) Boxplot of inter-response intervals (IRIs) sorted by whether the next response was correct or erroneous. The median IRI was shorter prior to correct as compared to error responses during saline sessions. The spread of data under saline was significantly less for correct responses as compared to errors. These differences were lost following muscimol inactivation. This appears to be due to an increase in the spread of IRIs preceding correct responses when muscimol was infused. C) Time to collect reward was less variable prior to correct responses as compared to errors. With muscimol in medial prefrontal cortex, the median movement time to the reward port increased, as did the spread of movement times before correct responses. The alterations in movement times contributed to omission of the difference in spread between distributions before correct versus error responses. D) The length of the delay period tended to be more variable prior to correct responses than to errors under control conditions. This effect was omitted with muscimol in medial prefrontal cortex, in part due to an increase in the spread of delay lengths before correct responses. Under muscimol, the median delay length increased prior to error responses. (* = p < 0.05 by Kruskal-Wallis rank-sum test, confirmed using jack-knifing of subjects; # = p < 0.05 by Fligner-Killeen test for homogeneity of variance, confirmed using jack-knifing of subjects)
Changes in the inter-response interval could have been driven by specific phases of the task. After the previous nosepoke response, rats locomoted to the reward port during the phase of the task that is referred to as the “reward acquisition” period (Figure 6A). In saline sessions, time to collect reward was less variable prior to correct responses (interquartile range = 0.5 ± 0.04 sec) as compared to errors (1.6 ± 0.6 sec; p << 0.001, X2 = 25.5). However, the median movement time did not differ depending on trial outcome (correct: 1.7 ± 0.1 sec; error: 2.3 ± 0.2 sec; p > 0.05 X2 = 2.78). With muscimol in dmPFC, the median movement time to the reward port before a correct response increased compared to saline sessions (saline: 1.7 ± 0.1 sec; muscimol: 2.4 ± 0.4 sec; p << 0.001, X2 = 15.2), as did the spread of movement times before correct responses (interquartile range for saline: 0.5 ± 0.03 sec; muscimol: 0.8 ± 0.3 sec; p << 0.001, X2 = 21.1). The alterations in movement times contributed to omission of the difference in spread between distributions before correct versus error responses. There were no differences between the median (correct: 2.4 ± 0.4 sec; error: 2.4 ± 0.5 sec; p > 0.05, X2 = 2.5) or spread (correct: 0.8 ± 0.3 sec; error: 1.2 ± 0.4 sec; p > 0.05, X2 = 0.04) of movement times by trial outcome with muscimol in dmPFC. The median (saline: 2.3 ± 0.2 sec; muscimol: 2.3 ± 0.5; p > 0.05, X2 = 0.17) and spread (saline: 1.6 ± 0.6 sec; muscimol: 1.2 ± 0.4; p > 0.05, X2 = 0.09) of reward acquisition times did not vary for error trials between saline and muscimol. Reward acquisition period data are summarized in Figure 6C.
The “delay” phase of the task begins when the first lick is captured at the reward spout (Figure 6A). The last lick event captured designates the end of the delay. The median delay length was not different between correct (7.6 ± 0.6 sec) and error (9.4 ± 1.5 sec) trials in saline sessions. However, the length of the delay period was less variable prior to correct responses (interquartile range: 2.1 ± 0.8 sec) than to errors (10.5 ± 2.5 sec) under control conditions (p << 0.001, X2 = 43.4). This effect was omitted with muscimol in dmPFC, in part due to an increase in the spread of delay lengths before correct responses (saline: 2.1 ± 0.8 sec; muscimol: 5.2 ± 1.2 sec; p << 0.001, X2 = 25.8). Under muscimol, the median delay length was longer prior to errors (7.9 ± 0.5 sec), as compared to correct responses (5.9 ± 1.8 sec; p < 0.01, X2 = 9.9), but the spread of delays did not differ with jack-knifing of subject data (correct: 5.2 ± 1.2 sec; error: 3.9 ± 1.5 sec; p > 0.05, X2 = 0.01). Delay period data are summarized in Figure 6D.
The “choice” phase of the task occurred when rats left the reward port to make the next nosepoke response (Figure 6A). There were no significant differences in either median or spread of choice period lengths between trial outcomes or sessions.
Muscimol infusions in dmPFC led to increased behavioral variability prior to correct responses. Under control conditions, IRIs, reward acquisition intervals, and delays were more variable prior to error versus correct responses. When muscimol was infused in dmPFC, this difference was lost, due to an increase in variability in IRIs, reward acquisition intervals, and delays prior to correct responses. In addition, muscimol in dmPFC reduced the difference in median IRI prior to correct and error responses, increased the median reward acquisition time prior to correct responses, and increased the length of the delay before error responses.
Effects of trial outcome on movement times
Trial outcome has been shown to affect reaction times in a delayed response task (Narayanan and Laubach, 2008). Specifically, rats were slower to release a lever in response to a trigger stimulus when they had made an error on the previous trial. Muscimol infusions in dmPFC eliminated this slowing.
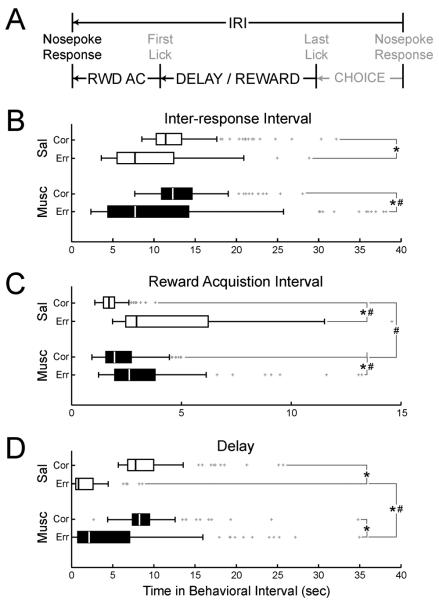
To determine whether delayed spatial alternation behavior would be similarly affected by trial outcome and muscimol, subsequent inter-response intervals were sorted by whether the previous trial was scored as correct or an error (Figure 7A). The median inter-response interval was shorter after errors (9.4 ± 1.8 sec) than correct responses (11.6 ± 0.5) under both saline (p < 0.05, X2 = 4.2) and muscimol (correct: 9.6 ± 3.1 sec; error: 12.5 ± 1.2 sec; p < 0.01, X2 = 8.1). In saline sessions, there was no difference in the spread of IRIs after correct (interquartile range: 2.8 ± 0.6 sec) or error responses (6.9 ± 2.2 sec) once data were assessed using jack-knifing of subjects (p > 0.05, X2 = 3.8). Muscimol increased the spread of IRIs after errors (interquartile range: 6.2 ± 3.3 sec) as compared to correct responses (2.8 ± 0.6 sec; p < 0.01, X2 = 8.7). There were no significant differences between the medians of reward acquisition times when compared between correct (saline: 11.6 ± 0.5 sec; muscimol: 12.5 ± 1.2 sec; p > 0.05, X2 = 3.1 after jack-knifing of subject data) or error (saline: 9.4 ± 1.8 sec; muscimol: 9.6 ± 3.1 sec; X2 = 0.02 across all subjects) responses across saline and muscimol sessions. Nor did the spread of reward acquisition data differ under these conditions (saline correct (2.8 ± 0.6 sec) vs. muscimol correct (2.8 ± 0.6 sec), p > 0.05, X2 = 1.2 after jack-knifing of subject data; saline error (6.8 ± 2.2 sec) vs. muscimol error (6.2 ± 3.3 sec), p > 0.05, X2 = 2.1 after jack-knifing of subject data). These data are summarized in Figure 7B.
Figure 7. Distributions of interval lengths following correct or error responses.
A) Intervals used in the analysis. B) Boxplot of inter-response intervals (IRIs) sorted by whether the prior response was correct or erroneous. The median inter-response interval was shorter after errors than correct responses under both saline and muscimol. Muscimol increased the spread of IRIs after error responses. C) Time to collect reward was longer and more variable following errors as compared to correct responses under both saline and muscimol. The spread of data following correct responses increased following muscimol inactivation. D) Median delay length was shorter following errors than correct responses under both saline and muscimol. This is due to the fact that rats would not collect fluid following error responses. The spread of delay lengths following error responses increased in muscimol sessions as compared to saline. (* = p < 0.05 by Kruskal-Wallis rank-sum test, confirmed using jack-knifing of subjects; # = p < 0.05 by Fligner-Killeen test for homogeneity of variance, confirmed using jack-knifing of subjects)
Time to collect reward (Figure 7A) was longer and more variable after errors as compared to correct responses under both saline (median: correct (1.6 ± 0.1 sec) vs. error (3.4 ± 0.4 sec), p << 0.001, X2 = 48.9; iqr: correct (0.3 ± 0.07 sec) vs. error (4.4 ± 1.2 sec), p << 0.001, X2 = 35.6) and muscimol (median: correct (2.1 ± 0.3 sec) vs. error (2.7 ± 0.6 sec), p < 0.01, X2 = 9.7; iqr: correct (0.8 ± 0.3 sec) vs. error (1.1 ± 0.3 sec), p < 0.01, X2 = 10.5). The spread of data after correct responses increased after muscimol inactivation (iqr: 0.8 ± 0.3 sec) as compared to saline sessions (0.3 ± 0.07 sec; p < 0.05, X2 = 6.0). This was not true for error trials (saline: 0.8 ± 0.3 sec; muscimol: 1.1 ± 0.3 sec; p > 0.05, X2 = 0.2). There was no difference in the median reward acquisition time between correct (saline: 1.6 ± 0.1 sec; muscimol: 2.1 ± 0.3 sec; p > 0.05, X2 = 2.7) or error (saline: 3.4 ± 0.4 sec; muscimol: 2.7 ± 0.6 sec; p > 0.05, X2 = 0.7) responses across saline and muscimol sessions.
Median delay length was shorter after errors than correct responses under both saline (correct: 8.3 ± 0.8 sec; error: 1.4 ± 0.6 sec; p << 0.001, X2 = 50.0) and muscimol (correct: 8.1 ± 0.4 sec; error: 3.4 ± 1.7 sec; p << 0.001, X2 = 36.4). This is due to the fact that rats would not collect fluid after error responses. There was no difference in the spread of delay lengths in saline sessions after correct (iqr: 2.2 ± 0.7 sec) or error responses (2.5 ± 0.8 sec; p > 0.05, X2 = 0.90 after jack-knifing of subject data). The median delay length after error responses increased in muscimol sessions (3.4 ± 1.7 sec) as compared to saline (1.4 ± 0.6 sec; p < 0.05, X2 = 5.8), and the spread of delay lengths after errors increased (saline: 2.5 ± 0.8 sec; muscimol: 5.1 ± 2.7 sec; p < 0.05, X2 = 4.3). No differences were observed in either the median (saline: 8.3 ± 0.8 sec; muscimol: 8.1 ± 0.4 sec; p > 0.05, X2 = 1.6) or spread (saline: 2.2 ± 0.7 sec; muscimol: 2.4 ± 0.5 sec; p > 0.05, X2 = 3.2) of delay lengths after correct trials across saline and muscimol sessions. There were no significant differences in either median or spread of choice period lengths between trial outcomes or sessions.
Under both saline and muscimol conditions, IRIs were longer after correct compared to error responses. After error responses, rats had to make a resetting response – which consisted of a single head entry – in the reward port. A correcting response could be made immediately following this resetting response. After correct responses, rats remained at the reward port to collect fluid reward, which was delivered over a 4 sec window. Given these differences between correct and error responses, it is not surprising that IRIs and delay periods were shorter after errors than after correct responses. With muscimol in dmPFC, IRIs were more variable after errors as compared to IRIs after errors following saline infusion. During the reward acquisition interval, the time it took to approach the reward port (for either reward or a resetting response) was longer and more variable after an error than after a correct response, in both saline and muscimol conditions. This result suggests that motivation to collect reward influenced how quickly rats moved to the reward port.
Potential behavioral strategies for mediating spatial information
Video analysis (from recordings made with four simultaneously active cameras) of behavior during the inter-trial interval failed to find evidence for potential behavioral strategies (e.g., leaning, foot shuffling) for mediating spatial information. There were no obvious differences in how animals stood between the barriers and no obvious differences in how rats placed their feet on trials with left and right responses. Nevertheless, it is possible that rats used a subtle behavioral strategy was used by the rats. Our inactivation results suggest that if such behavioral strategies were used, then they depend on mPFC, as rats performed poorly with mPFC inactivated.
DISCUSSION
Rats were trained to perform a self-paced version of the delayed spatial alternation task and implanted with cannulae bilaterally in dmPFC or AId. Muscimol was infused locally to reversibly inactivate each of these regions. Inactivation of dmPFC, but not AId resulted in a dramatic reduction of correctly performed trials. Impairments with muscimol infusion in the dmPFC were due to an increase in the number of errors initiated by rats after correct responses, but not to a greater number of perseverative errors or to an increase in biased responding. Behavioral success in this self-paced spatial alternation task depended upon the activity of rats during the inter-response interval. Under control conditions, inter-response intervals were shorter and less variable prior to correct responses as compared to errors. In particular, behavior before correct responses was more consistent across trials during the reward acquisition and delay periods. With muscimol in dmPFC, behavior during these epochs became more variable before correct responses, eliminating differences in the spread of movement times between correct and error trials and increasing overall within-subject variability in response times. We also observed that the outcome of the previous response influenced behavior in the next trial. In saline sessions, rats were slower to approach the reward port, and their movement times during this window were more variable after an error had been committed. The spread of reward acquisition times increased specifically for correct trials in muscimol sessions. Rats with saline in dmPFC spent significantly less time at the reward port during the delay period after an error response. When muscimol was infused in this region, the length of time and variability of delay lengths increased after error responses as compared to errors in saline sessions.
Dorsomedial prefrontal cortex is necessary for successful delayed spatial alternation
The data reported here support an important role for rat dmPFC in the execution of delayed spatial alternation behaviors. This is consistent with previously reported impairments in delayed spatial alternation following lesions or inactivations of this prefrontal region (e.g., Izaki et al., 2001; Dunnett et al., 2005; Wang and Cai, 2006; Yoon et al., 2008). Muscimol infusions were used in these experiments rather than permanent lesion methods to avoid the issue of functional recovery over time (e.g., Risterucci et al., 2003; Van Haaren et al., 1985).
All rats with cannula in dmPFC (n = 4) showed spatial response biases in control sessions, tending to make more errors on one side of the box than the other. When dmPFC was inactivated, response biases were not observed in these animals. In the earliest stages of training, rats were allowed to respond in either side for a reward, and may have developed preferences for a particular side. After alternation training, this preference may have served instead as a default response on trials in which the animal did not know where to respond, and muscimol may have impaired rats' ability to use this strategy.
Although rats were more likely to return to the location for which they were just rewarded, they did not perseverate in that location after the initial error. The return to a previously rewarded location suggests that spatial processing is less impaired than application of the alternation rule. Lesions of dmPFC have been shown to impair performance of a variety of non-spatial alternation tasks, including object working memory (Kesner et al., 1996), go/no-go alternation (Sakurai and Sugimoto, 1985; Delatour and Gisquet-Verrier, 1996), and olfactory alternation (Kinoshita et al., 2008). Alternatively, this behavior may be due to the lack of inhibitory control over neurons in the hippocampus (Johnson et al., 2007) or amygdala (Murray, 2007) that can also represent space and its association with reward. By contrast, rats did not return repeatedly to an error location, which could imply that circuits involved in error detection and correction were not impacted by these manipulations (Narayanan and Laubach, 2008).
As in spatial alternation tasks with delays that are assigned by the experimenter (e.g., Van Haaren et al., 1985), the time between trials was longer prior to errors than correct responses. The results reported here provide direct evidence for a relationship between within-subject response time variability and trial outcome. Interestingly, inactivation of rat mPFC resulted in greater overall response time variability, just as activity in human mPFC correlates with differences in response time variability within children with ADHD (Fassbender et al., 2009). This effect may be due in part to disruption of top-down control processes exerted on motor cortical regions by dmPFC, as has been shown previously in the simple reaction time task (Narayanan and Laubach, 2006).
We found that reward expectation and the subsequent delivery of reward after correct responses shortened the reward acquisition time and lengthened the delay (Figure 7C and D). Both of these windows contribute to the total time between trials (IRI, Figure 7B). These response times affect the next trial outcome and therefore the next reward acquisition interval, delay, and IRI – and the trial outcome after that. Under control conditions, the total IRI is longer following correct responses (Figure 7B) and correct responses are associated with shorter prior IRIs (Figure 6B). Conversely, the total IRI is shorter following an error (Figure 7B), while error responses are preceded by longer IRIs (Figure 6B).
Animals made few errors under control conditions, and the relationship we observed between movement times and trial outcomes is likely due to errors that occurred after long delays. When muscimol was infused in dmPFC, the relationship between IRIs after correct and error responses was qualitatively similar to control conditions. That is, IRIs were longer after correct responses and shorter after errors. It is possible that this contributed to the error pattern observed in Figure 4. This was not borne out by the relationship between previous IRI and response or by the previous reward acquisition interval. However, correct responses were preceded by shorter delays, suggesting that the delay period, and the reward collection – or lack thereof – which takes place during this period strongly influences the next response.
The AId cortex in lateral rat PFC shares extensive reciprocal connections with prelimbic cortex in the dmPFC (Gabbott et al., 2003). AId cortex has been implicated in the representation of reward value (Braun et al., 1982; Ragozzino and Kesner, 1999; Balleine and Dickinson, 2000; Kesner and Gilbert, 2007), and might be expected to play a significant role in appetitive behaviors such as delayed spatial alternation. However, muscimol infusions in AId did not affect performance accuracy, even at high doses. The spatial alternation task described here did not directly probe the question of reward values. At the highest dose (1.0 μg/μL) of muscimol, animals performed fewer trials over the session (p < 0.05, paired t = 6.79). This could support a role for this region in motivation. Further anecdotal evidence was observed when rats did not immediately consume water in their home cages following behavior, even though they had regulated water access and consumed little during the behavioral session.
Muscimol inactivation affects local, not distal, processing
Our use of the muscimol inactivation technique allowed for a within-subject experimental design that minimized the use of animal subjects. Local spread of muscimol within rat dmPFC (Narayanan et al., 2006; Allen et al., 2008) and AId (N. K. Horst, N. S. Narayanan, and M. Laubach, unpublished observations) has been demonstrated previously (see also Methods). Another concern that has been raised regarding the use of muscimol to inactivate brain regions is that infusion of this compound into an area may actually have significant impact on other anatomically connected areas. However, these experiments demonstrated differential behavioral results in two regions of cortex that are densely interconnected – namely the prelimbic cortex and AId cortex (Gabbott et al., 2003).
Importance of controlled behavior between spatial responses
Within the medial aspect of rat prefrontal cortex, several subregions have been implicated in the processing of spatial information. While some groups ascribe egocentric spatial processes to anterior cingulate cortex and allocentric processes to prelimbic cortex (Ragozzino and Kesner, 1999), others assert the opposite is true (Aggleton et al., 1995). These differential interpretations may arise from procedural variations between labs, such as the use of maze-based versus operant behaviors.
Rat dmPFC receives massive input from the CA1 (cornu ammonis) / subiculum region of the hippocampus (Thierry et al., 2000), home to location-specific neurons called “place-cells” (for a review, see Moser et al., 2008). Disconnection studies have demonstrated that contralateral inactivations of prelimbic cortex and ventral hippocampus are as deleterious to delayed spatial working memory as bilateral inactivations of either region (Floresco et al., 1997; Wang and Cai, 2006). It has also been shown that the apparent recovery of working memory function following dmPFC may be due to compensation by the hippocampus (Lee and Kesner, 2003), though the precise mechanisms of hippocampal function in the working memory task are not likely to be the same as those of medial prefrontal cortex (Yoon et al., 2008). Similarly, disconnection of dmPFC from the striatum can impair delayed spatial alternation performance (Dunnett et al., 2005).
Previous attempts at spatial working memory procedures have been criticized for lack of control over movements during the delay period (Chudasama and Muir, 1997; Ennaceur and Aggleton, 1998; Euston and McNaughton, 2006; Cowen and McNaughton, 2007). To maximally engage prefrontal circuitry, and minimize the contributions of non-dmPFC regions such as hippocampus and striatum, rats were stationary during the delay period between spatial responses. Information about location or posture during the delay should not have informed subjects of the previous or next response, because these varied minimally between trials.
Conclusions
Our results support previous findings on increased error responding following lesion or inactivation of dmPFC in rats performing delayed spatial alternation tasks. By controlling the movement of rats during the delay period, we can be fairly confident that we are observing processes that do not depend on motor-mediation. We also demonstrate, for the first time, that there is a relationship between response time variability and trial outcome that depends on activity in dmPFC. As the medial PFC is conserved across mammalian species (Preuss, 1995), dysfunction of this regions likely underlies the intra-individual variations in response times and increased error responding observed in clinical disorders such as ADHD (e.g., Brocki et al., 2008) and in normal aging (e.g., Myerson et al., 1989; Cerella and Hale, 1994; Gooch et al., 2009; cf. Robertson et al., 2006 and Myerson et al., 2007). Inactivation of dmPFC may have led to an inability to integrate actions together over time (Fuster, 2008) and in a context-dependent manner (Braver and Barch, 2002). Moreover, considerable effort should be required to process spatial information in the absence of normal dmPFC function, and effort-based processing is known to depend on dmPFC (Walton et al., 2002, 2003). Based on our finding that dmPFC inactivation led to changes in multiple measures of the delayed alternation task (accuracy, timing, post-error processing), we suggest that dmPFC is critical to processing mnemonic, temporal and control related information during delayed spatial working memory tasks.
Acknowledgments
We thank the Instruments Shop at the John B. Pierce Laboratory for their technical support, Ivan de Araujo, Amy Arnsten, and Xiao-Jing Wang for critical feedback on this research, and Matthew Krause for assisting with the initial design and training procedures for the operant task.
Funding. This research was supported by NIH Grant 2-T32-NS007224 (NKH), the Kavli Foundation (ML), and NIH Grant P01-AG030004-01A1 (ML).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Competing interests. The authors declare that no competing interests exist.
REFERENCES
- Aggleton J, Neave N, Nagle S, Sahgal A. A comparison of the effects of medial prefrontal, cingulate cortex, and cingulum bundle lesions on tests of spatial memory: evidence of a double dissociation between frontal and cingulum bundle contributions. J Neurosci. 1995;15:7270–7281. doi: 10.1523/JNEUROSCI.15-11-07270.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alderson RM, Rapport MD, Kofler MJ. Attention-deficit/hyperactivity disorder and behavioral inhibition: a meta-analytic review of the stop-signal paradigm. J Abnorm Child Psychol. 2007;35:745–758. doi: 10.1007/s10802-007-9131-6. [DOI] [PubMed] [Google Scholar]
- Allen T, Narayanan N, Kholodar-Smith D, Zhao Y, Laubach M, Brown T. Imaging the spread of reversible brain inactivations using fluorescent muscimol. J Neurosci Methods. 2008;171:30–38. doi: 10.1016/j.jneumeth.2008.01.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Balleine B, Dickinson A. The effect of lesions of the insular cortex on instrumental conditioning: evidence for a role in incentive memory. J Neurosci. 2000;20:8954–8964. doi: 10.1523/JNEUROSCI.20-23-08954.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bachevalier J, Mishkin M. Visual recognition impairment follows ventromedial but not dorsolateral prefrontal lesions in monkeys. Behav Brain Res. 1986;20:249–261. doi: 10.1016/0166-4328(86)90225-1. [DOI] [PubMed] [Google Scholar]
- Braun JJ, Lasiter PS, Kiefer SW. The gustatory neocortex of the rat. Physiol Psychol. 1982;10:13–45. [Google Scholar]
- Braver TS, Barch DM. A theory of cognitive control, aging cognition, and neuromodulation. Neurosci Biobehav Rev. 2002;26:809–17. doi: 10.1016/s0149-7634(02)00067-2. [DOI] [PubMed] [Google Scholar]
- Brocki KC, Randall KD, Bohlin G, Kerns KA. Working memory in school-aged children with attention-deficit/hyperactivity disorder combined type: Are deficits modality specific and are they independent of impaired inhibitory control? J Clin Exp Neuropsychol. 2008:1–11. doi: 10.1080/13803390701754720. [DOI] [PubMed] [Google Scholar]
- Brozoski T, Brown R, Rosvold H, Goldman P. Cognitive deficit caused by regional depletion of dopamine in prefrontal cortex of rhesus monkey. Science. 1979;205:929–932. doi: 10.1126/science.112679. [DOI] [PubMed] [Google Scholar]
- Buzy WM, Medoff DR, Schweitzer JB. Intra-Individual Variability Among Children with ADHD on a Working Memory Task: An Ex-Gaussian Approach. Child Neuropsychol. 2009:1–19. doi: 10.1080/09297040802646991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Castellanos FX, Sonuga-Barke EJ, Scheres A, Di Martino A, Hyde C, Walters JR. Varieties of attention-deficit/hyperactivity disorder-related intra-individual variability. Biol Psychiatry. 2005;57:1416–1423. doi: 10.1016/j.biopsych.2004.12.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cerella J, Hale S. The rise and fall in information-processing rates over the life span. Acta Psychologica. 1994;86:109–197. doi: 10.1016/0001-6918(94)90002-7. [DOI] [PubMed] [Google Scholar]
- Chudasama Y, Muir J. A behavioural analysis of the delayed non-matching to position task: the effects of scopolamine, lesions of the fornix and of the prelimbic region on mediating behaviours by rats. Psychopharmacology (Berl) 1997;134:73–82. doi: 10.1007/s002130050427. [DOI] [PubMed] [Google Scholar]
- Cowen S, McNaughton B. Selective delay activity in the medial prefrontal cortex of the rat: contribution of sensorimotor information and contingency. J Neurophysiol. 2007;98:303–316. doi: 10.1152/jn.00150.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Delatour B, Gisquet-Verrier P. Prelimbic cortex specific lesions disrupt delayed-variable response tasks in the rat. Behav Neurosci. 1996;110:1282–1298. doi: 10.1037//0735-7044.110.6.1282. [DOI] [PubMed] [Google Scholar]
- Duan H, Wearne SL, Rocher AB, Macedo A, Morrison JH, Hof PR. Age-related dendritic and spine changes in corticocortically projecting neurons in macaque monkeys. Cereb Cortex. 2003;13:950–61. doi: 10.1093/cercor/13.9.950. [DOI] [PubMed] [Google Scholar]
- Dunnett S, Meldrum A, Muir J. Frontal-striatal disconnection disrupts cognitive performance of the frontal-type in the rat. Neuroscience. 2005;135:1055–1065. doi: 10.1016/j.neuroscience.2005.07.033. [DOI] [PubMed] [Google Scholar]
- Ennaceur A, Aggleton JP. An attempt to overcome the problem of motor mediation by rats in the delayed non matching-to-position task. Neuroscience Research Communications. 1998;22:153–162. [Google Scholar]
- Euston D, McNaughton B. Apparent encoding of sequential context in rat medial prefrontal cortex is accounted for by behavioral variability. J Neurosci. 2006;26:13143–13155. doi: 10.1523/JNEUROSCI.3803-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fassbender C, Zhang H, Buzy WM, Cortes CR, Mizuiri D, Beckett L, Schweitzer JB. A lack of default network suppression is linked to increased distractibility in ADHD. Brain Res. EPub. 2009 doi: 10.1016/j.brainres.2009.02.070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Floresco S, Seamans J, Phillips A. Selective roles for hippocampal, prefrontal cortical, and ventral striatal circuits in radial-arm maze tasks with or without a delay. J Neurosci. 1997;17:1880–1890. doi: 10.1523/JNEUROSCI.17-05-01880.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Funahashi S, Bruce C, Goldman-Rakic P. Dorsolateral prefrontal lesions and oculomotor delayed-response performance: evidence for mnemonic “scotomas”. J Neurosci. 1993;13:1479–1497. doi: 10.1523/JNEUROSCI.13-04-01479.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Funahashi S. Neuronal mechanisms of executive control by the prefrontal cortex. Neurosci Res. 2001;39:147–165. doi: 10.1016/s0168-0102(00)00224-8. [DOI] [PubMed] [Google Scholar]
- Fuster J. The prefrontal cortex--an update: time is of the essence. Neuron. 2001;30:319–333. doi: 10.1016/s0896-6273(01)00285-9. [DOI] [PubMed] [Google Scholar]
- Gabbott P, Warner T, Jays P, Bacon S. Areal and synaptic interconnectivity of prelimbic (area 32), infralimbic (area 25) and insular cortices in the rat. Brain Res. 2003;993:59–71. doi: 10.1016/j.brainres.2003.08.056. [DOI] [PubMed] [Google Scholar]
- Geurts HM, Grasman RP, Verte S, Oosterlaan J, Roeyers H, van Kammen SM, Sergeant JA. Intra-individual variability in ADHD, autism spectrum disorders and Tourette's syndrome. Neuropsychologia. 2008;46:3030–3041. doi: 10.1016/j.neuropsychologia.2008.06.013. [DOI] [PubMed] [Google Scholar]
- Giedd JN, Blumenthal J, Molloy E, Castellanos FX. Brain imaging of attention deficit/hyperactivity disorder. Ann N Y Acad Sci. 2001;931:33–49. doi: 10.1111/j.1749-6632.2001.tb05772.x. [DOI] [PubMed] [Google Scholar]
- Goldman-Rakic P. Cellular basis of working memory. Neuron. 1995;14:477–485. doi: 10.1016/0896-6273(95)90304-6. [DOI] [PubMed] [Google Scholar]
- Gooch CM, Stern Y, Rakitin BC. Evidence for age-related changes to temporal attention and memory from the choice time production task. Neuropsychol Dev Cogn B Aging Neuropsychol Cogn. 2009 May;16(3):285–310. doi: 10.1080/13825580802592771. 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grill JD, Riddle DR. Age-related and laminar-specific dendritic changes in the medial frontal cortex of the rat. Brain Res. 2002;937:8–21. doi: 10.1016/s0006-8993(02)02457-5. [DOI] [PubMed] [Google Scholar]
- Gunning-Dixon F, Raz N. The cognitive correlates of white matter abnormal aging: a quantitative review. Neuropsychology. 2000;14:224–232. doi: 10.1037//0894-4105.14.2.224. [DOI] [PubMed] [Google Scholar]
- Izaki Y, Maruki K, Hori K, Nomura M. Effects of rat medial prefrontal cortex temporal inactivation on a delayed alternation task. Neurosci Lett. 2001;315:129–132. doi: 10.1016/s0304-3940(01)02366-7. [DOI] [PubMed] [Google Scholar]
- Johnson A, van der Meer M, Redish A. Integrating hippocampus and striatum in decision-making. Curr Opin Neurobiol. 2007;17:692–697. doi: 10.1016/j.conb.2008.01.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Karatekin C. A test of the integrity of the components of Baddeley's model of working memory in attention-deficit/hyperactivity disorder (ADHD) J Child Psychol Psychiatry. 2004;45:912–926. doi: 10.1111/j.1469-7610.2004.t01-1-00285.x. [DOI] [PubMed] [Google Scholar]
- Kesner R, Gilbert P. The role of the agranular insular cortex in anticipation of reward contrast. Neurobiol Learn Mem. 2007;88:82–86. doi: 10.1016/j.nlm.2007.02.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kesner R, Hunt M, Williams J, Long J. Prefrontal cortex and working memory for spatial response, spatial location, and visual object information in the rat. Cereb Cortex. 1996;6:311–318. doi: 10.1093/cercor/6.2.311. [DOI] [PubMed] [Google Scholar]
- Kinoshita S, Yokoyama C, Masaki D, Yamashita T, Tsuchida H, Nakatomi Y, Fukui K. Effects of rat medial prefrontal cortex lesions on olfactory serial reversal and delayed alternation tasks. Neurosci Res. 2008;60:213–218. doi: 10.1016/j.neures.2007.10.012. [DOI] [PubMed] [Google Scholar]
- Klein C, Wendling K, Huettner P, Ruder H, Peper M. Intra-subject variability in attention-deficit hyperactivity disorder. Biol Psychiatry. 2006;60:1088–1097. doi: 10.1016/j.biopsych.2006.04.003. [DOI] [PubMed] [Google Scholar]
- Lee I, Kesner R. Time-dependent relationship between the dorsal hippocampus and the prefrontal cortex in spatial memory. J Neurosci. 2003;23:1517–1523. doi: 10.1523/JNEUROSCI.23-04-01517.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martin J, Ghez C. Pharmacological inactivation in the analysis of the central control of movement. J Neurosci Methods. 1999;86:145–159. doi: 10.1016/s0165-0270(98)00163-0. [DOI] [PubMed] [Google Scholar]
- Miller E, Cohen J. An integrative theory of prefrontal cortex function. Annu Rev Neurosci. 2001;24:167–202. doi: 10.1146/annurev.neuro.24.1.167. [DOI] [PubMed] [Google Scholar]
- Moser E, Kropff E, Moser M. Place cells, grid cells, and the brain's spatial representation system. Annu Rev Neurosci. 2008;31:69–89. doi: 10.1146/annurev.neuro.31.061307.090723. [DOI] [PubMed] [Google Scholar]
- Murray E. The amygdala, reward and emotion. Trends Cogn Sci. 2007;11:489–497. doi: 10.1016/j.tics.2007.08.013. [DOI] [PubMed] [Google Scholar]
- Myerson J, Hale S, Hirschman R, Hansen C, Christiansen B. Global increase in response latencies by early middle age: Complexity effects in individual performances. Journal of the Experimental Analysis of Behavior. 1989;52:353–362. doi: 10.1901/jeab.1989.52-353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Myerson J, Robertson S, Hale S. Aging and intraindividual variability in performance: analyses of response time distributions. J Exp Anal Behav. 2007;88:319–37. doi: 10.1901/jeab.2007.88-319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Narayanan N, Horst N, Laubach M. Reversible inactivations of rat medial prefrontal cortex impair the ability to wait for a stimulus. Neuroscience. 2006;139:865–876. doi: 10.1016/j.neuroscience.2005.11.072. [DOI] [PubMed] [Google Scholar]
- Narayanan N, Laubach M. Top-down control of motor cortex ensembles by dorsomedial prefrontal cortex. Neuron. 2006;52:921–931. doi: 10.1016/j.neuron.2006.10.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Narayanan N, Laubach M. Neuronal correlates of post-error slowing in the rat dorsomedial prefrontal cortex. J Neurophysiol. 2008;100:520–525. doi: 10.1152/jn.00035.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ragozzino M, Kesner R. The role of the agranular insular cortex in working memory for food reward value and allocentric space in rats. Behav Brain Res. 1999;98:103–112. doi: 10.1016/s0166-4328(98)00058-8. [DOI] [PubMed] [Google Scholar]
- Raz N, Williamson A, Gunning-Dixon F, Head D, Acker J. Neuroanatomical and cognitive correlates of adult age differences in acquisition of a perceptual-motor skill. Microsc Res Tech. 2000;51:85–93. doi: 10.1002/1097-0029(20001001)51:1<85::AID-JEMT9>3.0.CO;2-0. [DOI] [PubMed] [Google Scholar]
- Risterucci C, Terramorsi D, Nieoullon A, Amalric M. Excitotoxic lesions of the prelimbic-infralimbic areas of the rodent prefrontal cortex disrupt motor preparatory processes. Eur J Neurosci. 2003;17:1498–1508. doi: 10.1046/j.1460-9568.2003.02541.x. [DOI] [PubMed] [Google Scholar]
- Robertson S, Myerson J, Hale S. Are there age differences in intraindividual variability in working memory performance? J Gerontol B Psychol Sci Soc Sci. 2006;61:18–24. doi: 10.1093/geronb/61.1.p18. [DOI] [PubMed] [Google Scholar]
- Sakurai Y, Sugimoto S. Effects of lesions of prefrontal cortex and dorsomedial thalamus on delayed go/no-go alternation in rats. Behav Brain Res. 1985;17:213–219. doi: 10.1016/0166-4328(85)90045-2. [DOI] [PubMed] [Google Scholar]
- Salthouse TA, Babcock RL. Decomposing adult age differences in working memory. Dev Psych. 1991;27:763–776. [Google Scholar]
- Tanji J, Hoshi E. Role of the lateral prefrontal cortex in executive behavioral control. Physiol Rev. 2008;88:37–57. doi: 10.1152/physrev.00014.2007. [DOI] [PubMed] [Google Scholar]
- Thierry A, Gioanni Y, Dégénétais E, Glowinski J. Hippocampo-prefrontal cortex pathway: anatomical and electrophysiological characteristics. Hippocampus. 2000;10:411–419. doi: 10.1002/1098-1063(2000)10:4<411::AID-HIPO7>3.0.CO;2-A. [DOI] [PubMed] [Google Scholar]
- van Haaren F, De Bruin J, Heinsbroek R, Van de Poll N. Delayed spatial response alternation: effects of delay-interval duration and lesions of the medial prefrontal cortex on response accuracy of male and female Wistar rats. Behav Brain Res. 1985;18:41–49. doi: 10.1016/0166-4328(85)90167-6. [DOI] [PubMed] [Google Scholar]
- Walton ME, Bannerman DM, Rushworth MF. The role of rat medial frontal cortex in effort-based decision making. J Neurosci. 2002;22:10996–1003. doi: 10.1523/JNEUROSCI.22-24-10996.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walton ME, Bannerman DM, Alterescu K, Rushworth MF. Functional specialization within medial frontal cortex of the anterior cingulate for evaluating effort-related decisions. J Neurosci. 2003;23:6475–9. doi: 10.1523/JNEUROSCI.23-16-06475.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang G-W, Cai J-X. Disconnection of the hippocampal-prefrontal cortical circuits impairs spatial working memory performance in rats. Behavioural Brain Research. 2006;175:329–336. doi: 10.1016/j.bbr.2006.09.002. [DOI] [PubMed] [Google Scholar]
- Yoon T, Okada J, Jung M, Kim J. Prefrontal cortex and hippocampus subserve different components of working memory in rats. Learn Mem. 2008;15:97–105. doi: 10.1101/lm.850808. [DOI] [PMC free article] [PubMed] [Google Scholar]