Abstract
A major limitation of current stroke therapies is the need to treat candidate patients within three hours of stroke onset. Human umbilical cord blood cell (HUCBC) and the sigma receptor agonist 1,3, di-o-tolylguanidine (DTG) administration both caused significant reductions in brain damage in the rat middle cerebral artery occlusion model of stroke when administered at delayed timepoints. In vivo, these treatments suppress the infiltration of peripheral lymphocytes into the brain in addition to decreasing neurodegeneration. An ex vivo organotypic slice culture (OTC) model was utilized to characterize the efficacy of these treatments in mitigating neurodegeneration in ischemic brain tissue in the absence of the peripheral immune system. Slice cultures subjected to oxygen glucose deprivation (OGD) had significantly elevated levels of degenerating neurons and microglial nitric oxide production when compared to their normoxic counterparts. In cultures subjected to OGD, HUCBC but not DTG treatment reduced the number of degenerating neurons and the production of microglial derived nitric oxide back to levels detected in normoxic controls. These data show that HUCBC treatment can mediate direct neuroprotection and suppress innate inflammation in ischemic brain tissue in the absence of the peripheral immune system, whereas DTG requires peripheral effects to mediate neuroprotection. These experiments yield insight into the mechanisms by which these neuroprotective treatments function at delayed timepoints following stroke.
Keywords: ischemia, inflammation, neuroprotection, sigma receptors, human umbilical cord blood
Introduction
Currently the only FDA approved treatment for ischemic stroke is recombinant tissue plasminogen activator (Alteplase) (Marler and Goldstein, 2003). This treatment works by dissolving the blood clot occluding the blood vessel, but its use is restricted to a short, 3–6 hour time window. Beyond this time, degradation of the basement membrane of the endothelium in addition to apoptotic and inflammatory processes greatly diminish the therapeutic benefits of current treatments. Furthermore, this treatment increases the risk for bleeding and only provides a modest (13%) benefit in mortality and morbidity to patients who are candidates for this treatment (Albers et al., 2004).
Two experimental treatments which have shown a great deal of promise in experimental rat models of focal ischemia are human umbilical cord blood cells (HUCBC) (Vendrame et al., 2004) and 1,3-di-o-tolylguanidine (DTG) (Ajmo Jr et al., 2006). The main advantage of these treatments over previous therapies lies in the fact that they can be given at clinically relevant timepoints (24 and 48 hours post stroke for DTG and HUCBC respectively). In the permanent middle cerebral artery occlusion (MCAO) model of stroke, injection of 106 HUCBC 48 hours after stroke (Newcomb et al., 2006) or administration of 15mg/kg DTG 24 hours after stroke (Ajmo Jr et al., 2006) reduces infarct volume by up to 80% when compared to vehicle treated controls. Originally, both treatments were thought to decrease infarct volumes by either reducing neuronal ion imbalances (DTG) (Katnik et al., 2006) or replacing neurons (HUCBC). Further studies have shown, however, that each of these treatments has profound effects on components of the immune system.
HUCBC migrate to injured brain and the spleen following ischemia (Vendrame et al., 2004). Subsequent studies showed that HUCBC infiltration into the brain was not required for neuroprotection (Borlongan et al., 2004). Sigma receptors are also found in the spleen at high densities and are expressed on lymphocytes and macrophages (Su, 1991). The spleen is important in ischemic pathology as splenectomy prior to stroke shows significant reductions in neurodegeneration (Ajmo et al., 2008). T-lymphocytes in particular are known to be present in the spleen in large numbers and to contribute to stroke induced neurodegeneration (Offner et al., 2006, Yilmaz et al., 2006). Peripheral immune cells such as these migrate to the infarct and interact with antigen presenting cells there to promote a pro-inflammatory immune response.
Despite encouraging data from in vivo studies, it has yet to be determined whether the efficacy of HUCBC and DTG are dependent solely on the peripheral immune system or also involve direct protective effects on ischemic brain tissue. This series of studies tested the hypothesis that DTG and HUCBC provide direct cellular protection in addition to modulating the peripheral immune system. To determine the efficacy of these therapies in the absence of the peripheral immune system, organotypic hippocampal slice cultures were subjected to 48 hrs oxygen glucose deprivation (OGD) in the presence or absence of either HUCBC or DTG. Neurodegeneration and nitric oxide production were evaluated to determine the protective effects of these compounds.
Experimental procedures
Animal Care
All animal procedures were conducted in accordance with the NIH Guide for the Care and Use of Laboratory Animals with a protocol approved by the Institutional Animal Care and Use Committee at the University of South Florida. Timed pregnant Sprague-Dawley dams were purchased from Harlan Labs (Indianapolis, IN), maintained on a 12 hr light/dark cycle (7 am – 7 pm) and given access to food and water ad libitum. Neonatal rats birthed from time-pregnant dams were used for all experiments.
Organotypic Slice Culture
Organotypic slice cultures (OTC) were prepared using a method previously described (Leonardo et al., 2009). Postnatal day 8–10 rat pups of mixed sex were sacrificed by decapitation. Brains were removed and intact hippocampi were isolated in cold isotonic buffer (136.89 mM NaCl, 5.37 mM KCl, 169 μM Na2HPO4, 220.4 μM KH2PO4, 5.5 mM glucose, 59.01 mM sucrose). 400 μm hippocampal slices were prepared using a McIlwain Tissue Chopper (Mickle Laboratory Engineering Co. Ltd. Gomshall, Surrey, England). Only slices that appeared thin and translucent were selected for culture. Slices were then incubated for 90 min at 4 °C. Cultures were maintained on Millicell CM (Millipore Corp., Billerica, MA) inserts and placed in 6-well plates containing Neurobasal media supplemented with B27 and 5 mM L-glutamine. Slices were cultured for 14 days in room air supplemented with 5%CO2 maintained at 37 °C, receiving partial media changes every 3–4 days prior to experimentation.
Oxygen Glucose Deprivation
Organotypic slices were subjected to 48 hrs of normoxia or oxygen glucose deprivation (OGD). Slices were assigned to 1 of 2 exposures (normoxia or OGD) and 1 of 3 treatments (media, HUCBC, or DTG). Treatments were as follows: HUCBC=106 cells; DTG=30, 100, or 300 μM. This dose range of DTG has been used to reduce neuronal death (Katnik et al., 2006) and microglial activation in vitro (Hall et al., 2008), whereas the dosage for the HUCBC was that which afforded neuroprotection in vivo (Newcomb et al., 2006). Immediately prior to exposure, inserts were transferred into new 6-well plates containing media, HUCBC (Sigma Aldrich, St. Louis, MO) or DTG (Tocris, Ellisville, MO). Media consisted of Dulbecco’s Modified Eagles Medium (DMEM; Mediatech, Herndon, VA) for normoxia or DMEM without glucose (Invitrogen, Carlsbad, CA) for OGD. DTG was prepared in ethanol then diluted in media to achieve the proper concentration, ethanol levels were 0.3% final concentration in DTG samples as well as vehicle controls. HUCBC (AllCells, LLC, Inc.) were thawed and resuspended in DMEM containing DNase (1ug/ml) to prevent aggregation. HUCBC were washed twice and resuspended at a concentration of 108 cells/ml. Cultures were maintained in a standard tissue culture incubator during the normoxia exposure and a hypoxic chamber flushed with 1%O2, 5%CO2, and the balance N2 (CBS Scientific Co. Inc., Del Mar, CA) held at 37 °C during the OGD exposure.
Fluoro-Jade Staining
Organotypic slice cultures were stained with Fluoro-Jade (Histochem, Jefferson, AR), which labels degenerating neurons. This method was adapted from that originally developed by (Schmued et al., 1997) and has been described by (Duckworth et al., 2005). Tissue was thaw mounted and dried onto glass slides. Slices were rehydrated by exposure to absolute ethanol for 3 min followed by 70% ethanol and deionized water for 1 min each. Slices were oxidized using a 0.06% KMnO4 solution for 15 min followed by three rinses of double distilled water for 1 min each. Slices were stained in a 0.001% solution of Fluoro-Jade in 0.1% acetic acid for 30 min. Slides were again rinsed, allowed to dry at 45°C for 20 min, cleared with xylene and cover slipped with DPX mounting medium (Electron Microscopy Sciences, Ft. Washington, PA). Fluoro-jade staining was quantified using the NIH image J program
Nitric oxide imaging
Nitric oxide levels were measured using the NO sensitive dye 4-amino-5-methylamino-2′,7′-difluorofluorescein (DAF-FM). Organotypic slice cultures on Millicell inserts were incubated for 1 hour at 37°C in DMEM (Mediatech) containing 5 μM DAF-FM (Invitrogen) and 0.1 % DMSO. The slices were washed in physiological saline solution (PSS) consisting of: 140mM NaCl, 5.4 mM KCl, 1.3 mM CaCl2, 1.0 mM MgCl2, 20 mM glucose, and 25 mM 4-(2-hydroxyethyl)-1-piperazineethanesulfonic acid (HEPES) (pH adjusted to 7.4 with NaOH) prior to NO measurements. DAF-FM loaded slices were imaged using a DG-4 high speed wavelength switcher (Sutter Instruments Co., Novato, CA) which applied excitation light at 488 nm. Emitted fluorescent light was collected through the microscope objective (Zeiss; 2,5X Plan-Neofluar) and passed through an emission filter (510 nm) onto a Cooke SensiCam CCD camera (Cooke Corporation, Auburn Hills, MI). Images were recorded with Slidebook 3.0 software (Intelligent Imaging Innovations, Denver, CO). Nitric oxide levels were calculated using the Slidebook 3 software. Fluorescent intensity was expressed as arbitrary units.
Image analyses and quantification
Images were acquired using a Zeiss Axioscope 2 microscope controlled by Openlab (Improvision, Ltd., Lexington, MA, USA) software, and photomicrographs were captured with a Zeiss Axicam Color camera. All images subjected to direct comparisons were captured at the same exposure and digital gain settings. Fluorescence was quantified using NIH ImageJ software. Photomicrographs were imported into ImageJ and the threshold levels adjusted to be consistent with all slices analyzed. Fluorescence intensity values were calculated by dividing the total fluorescence by the total area of the slice.
Immunohistochemistry
Slice cultures were fixed with 4% paraformaldehyde in phosphate buffered saline (PBS) at 4°C overnight. Slice cultures were removed from the MilliCell inserts, thaw mounted and dried at 45°C for 1hr. The slides were rinsed with PBS (pH 7.2), and placed in permeabilization buffer containing 10% goat serum, 3% 1M lysine, and 0.3% Triton X-100 in PBS for 1 hr at room temperature. Slices were then incubated overnight at 4 °C in a PBS solution containing 2% goat serum, 0.3% Triton X-100 and primary antibody in a humidified chamber, protected from light when necessary. Slides were washed with PBS (3 × 5 min) and incubated with a secondary antibody solution containing PBS, 2% goat serum, and 0.3% Triton X-100 in a humidified chamber protected from light. The sections were incubated with OX-42 mouse anti-rat (1:3,000, Serotec) followed by Alexa-Fluor® 594 goat anti-mouse secondary (1:300, Molecular Probes). After final washing the slides were cover slipped with Vectashield hard set mounting media with DAPI (Vector Laboratories, Burlingame, CA).
Statistical Analyses
All data were expressed as mean ± standard deviation. Group means were analyzed by ANOVA to determine statistical significance with a “p” value set at 0.05. Main effects for the Fluoro-Jade and DAF-FM time course were determined by one-way ANOVA and were subjected to a Dunnett’s post-hoc test using 0 hrs as the control group. Main effects from all other analyses were determined by two-way ANOVA and were subjected to Bonferroni post-hoc tests.
Confocal Microscopy
Confocal micrographs were captured on a Leica TCS SP5 Laser Scanning Confocal Microscope. Image analysis to determine pearson’s correlation coefficients was performed using the LAS AF version 1.8.2 software suite (Leica Microsystems). Number of cells positive for both NO production and CD11b immunoreactivity was evaluated by an investigator blind to the nature of the treatment. Means for each group were compared and significant differences were determined via two-way ANOVA with post-hoc Bonferroni tests.
Results
Oxygen glucose deprivation significantly increases neurodegeneration and nitric oxide production in OTC
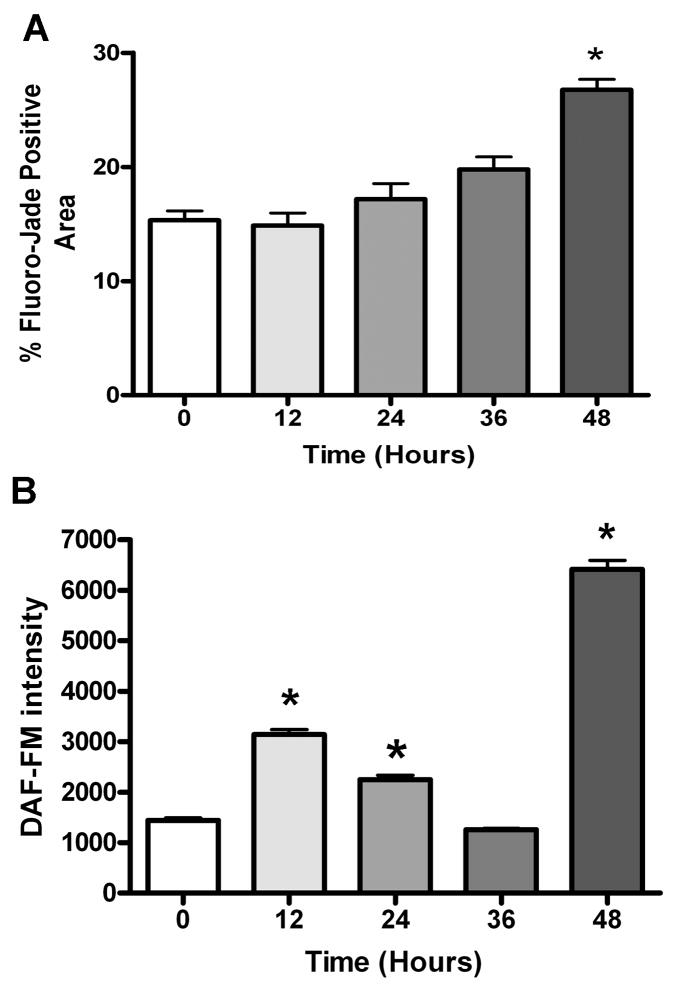
Slices were subjected to various durations of OGD (0, 12, 24, 36, and 48 hrs) to characterize the injury response in OTC (Figure 1). Following OGD exposure, neurodegeneration and nitric oxide production were assessed by staining with Fluoro-Jade and DAF-FM, respectively. OGD induced significant increases in Fluoro-Jade staining only at the 48 hour timepoint (p<0.05, n=4). Significant increases in DAF-FM staining were seen at the 12, 24, and 48 hour timepoints (p<0.01, n=6). Because significant increases in both Fluoro-Jade and DAF-FM staining were detected at 48 hrs, this timepoint was used for all subsequent experiments.
Figure 1. Oxygen glucose deprivation significantly increases neurodegeneration and nitric oxide production in organotypic slice cultures.
Organotypic slice cultures were subjected to various durations of oxygen glucose deprivation. Neurodegeneration was assessed using Fluoro-Jade staining while nitric oxide production was measured with DAF-FM staining. Data represent mean stained area values +\− SEM for Fluoro-Jade (A) and mean stained intensity values +\− SEM for DAF-FM staining (B). Asterisk denotes significance at p<0.05 and p<0.01 for (A) and (B), respectively.
HUCBC but not DTG significantly reduces Fluoro-Jade staining in OTC following OGD
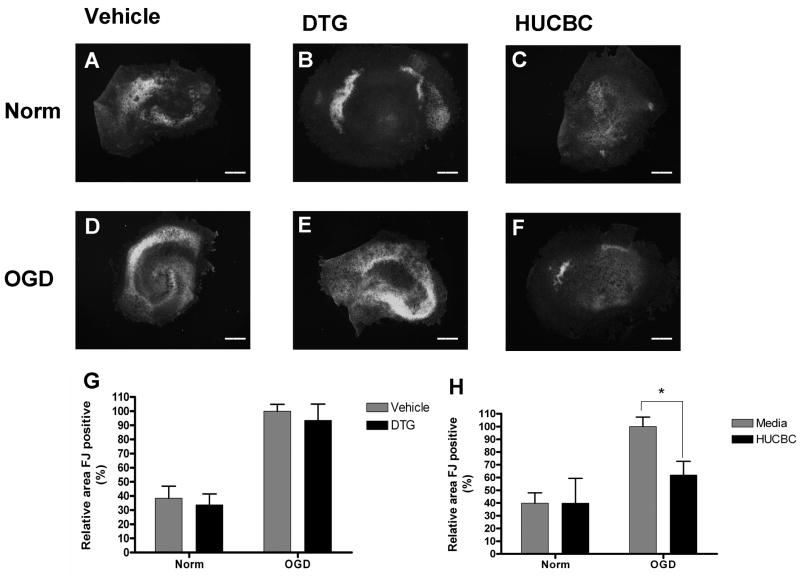
Fluoro-Jade staining was used to identify neuronal damage in each of the treatment groups. Extensive Fluoro-Jade staining was detected throughout the neuronal cell layers in slices exposed to OGD (Figure 2D) and was markedly increased when compared to normoxic slices (Figure 2A). Co-incubation with 100 μM DTG failed to reduce Fluoro-Jade staining in slices subjected to OGD (Figure 2E) and resembled OGD slices incubated with media alone (Figure 2D). In contrast, co-incubation with 106 HUCBC reduced Fluoro-Jade staining in slices exposed to OGD (Figure 2F). Normoxic samples treated with HUCBC (Figure 2C) and DTG (Figure 2B) showed similar levels of Fluoro-Jade staining relative to those incubated with media alone (Figure 2A). Quantification was performed to determine whether significant reductions in neuronal injury were achieved. Mean levels of Fluoro-Jade staining were significantly increased in OGD treated sections (p=<0.001, n=9) when compared to normoxic controls (Figure 2G, H). While DTG treatment (Figure 2G) did not significantly decrease Fluoro-Jade staining in slices subjected to OGD (p=0.5119, n=9), co-incubation with HUCBC (Figure 2H) significantly decreased Fluoro-Jade staining relative to media controls (p<0.05, n=6).
Figure 2. HUCBC but not DTG significantly reduces Fluoro-Jade staining in organotypic slice cultures following oxygen glucose deprivation.
Photomicrographs depict slices subjected to normoxia (A–C) or OGD (D–F) and treated with either vehicle (A,D), 100 μM DTG (B,E), or 106 HUCBC (C,F). Quantitative data (G–H) represent mean stained area values +\− SEM for Fluoro-Jade. Fluoro-Jade positive areas in DTG treated slices (G) showed no significant differences from vehicle controls in either normoxic or OGD conditions. Fluoro-Jade positive areas in HUCBC treated slices showed significant reductions in staining when compared to vehicle controls in OGD but not normoxic conditions (H). Asterisk denotes significance at p<0.05. Scale bars represent 500 μm.
DTG treatment is ineffective in reducing Fluoro-Jade staining at multiple concentrations
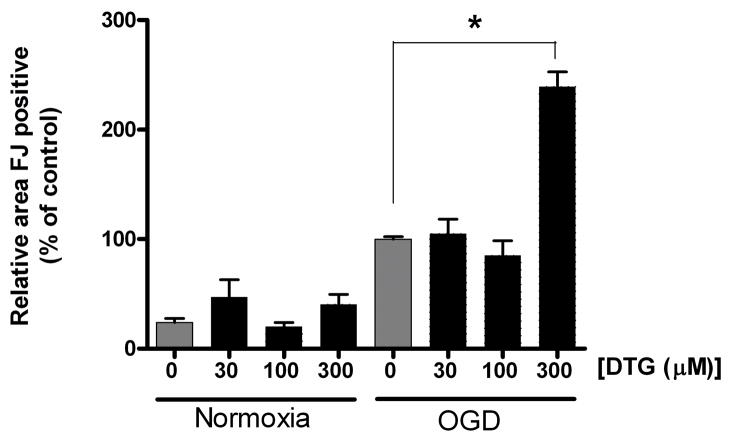
Because 100 μM DTG failed to show efficacy in reducing OGD induced injury, additional experiments were performed using 0, 30, 100 or 300 μM DTG to determine whether the protective effects of this compound are concentration dependent (Figure 3). This range has been used to reduce neuronal death (Katnik et al., 2006) and microglial activation in vitro (Hall et al., 2008). Here, none of the concentrations tested reduced Fluoro-Jade staining in organotypic slices following OGD. Furthermore, 300 μM DTG significantly increased Fluoro-Jade staining in slices subjected to OGD when compared to those incubated with media alone (139.22+/−14.34%, p<0.001, n=6). As only HUCBC showed efficacy in reducing Fluoro-Jade staining, only the effects of this treatment were explored with respect to NO production.
Figure 3. DTG treatment is ineffective in reducing Fluoro-Jade staining at multiple concentrations.
Levels of Fluoro-Jade stained area in slices co-incubated with 0, 30, 100, or 300 μM DTG and subjected to normoxia or OGD and normalized to OGD vehicle samples. While no concentrations tested decreased Fluoro-Jade staining, 300 μM DTG significantly increased Fluoro-Jade stained area in slices subjected to OGD compared to those incubated with media alone. Asterisk denotes significance at p<0.001.
HUCBC treatment reduces DAF-FM staining in OTC following OGD
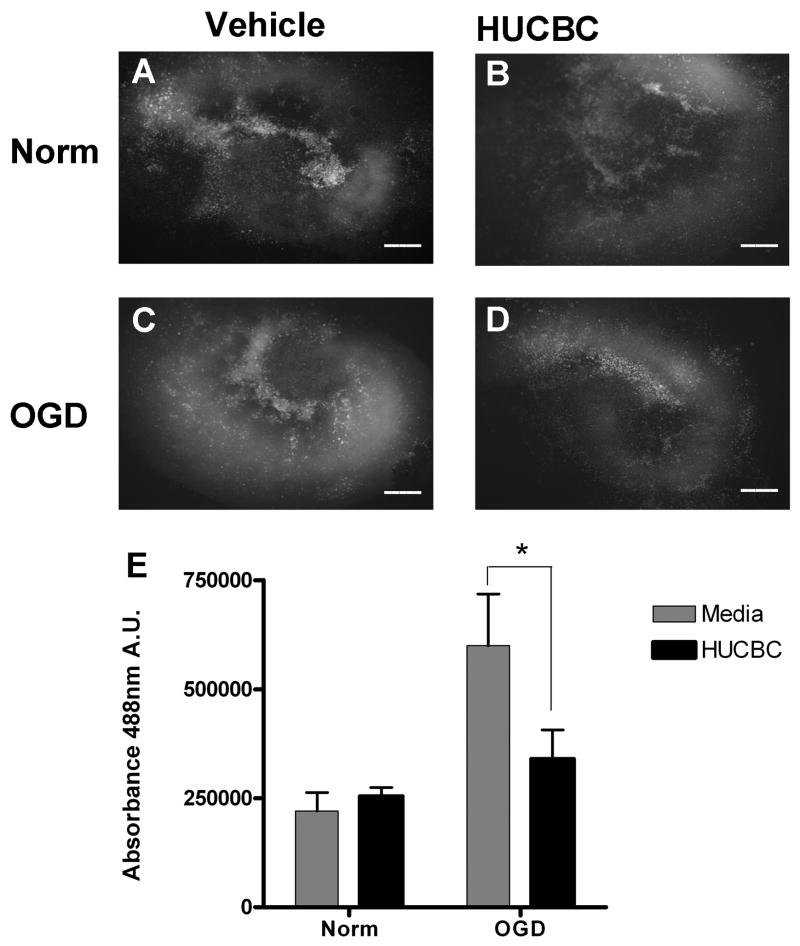
The intracellular dye DAF-FM was used to determine the extent of NO production in each of the treatment groups. Robust NO production was detected throughout the slices after exposure to OGD (Figure 4C) and was greatly increased relative to normoxic controls (Figure 4A). Co-incubation with 106 HUCBC reduced NO production in slices subjected to OGD (Figure 4D) relative to those incubated with media alone (Figure 4C). Normoxic samples co-incubated with HUCBC (Figure 4B) showed relatively low levels of NO production similar to normoxic controls (Figure 4A). Quantification was performed to determine whether DAF-FM staining was significantly reduced following HUCBC treatment (Figure 4E). Mean levels of DAF-FM staining were significantly increased in the OGD treated sections when compared to normoxic controls (172.01+/−75.21), and co-incubation with HUCBC significantly decreased DAF-FM staining in slices subjected to OGD relative to those incubated with media alone (43.3+/−15.86%%, p<0.05, n=3).
Figure 4. HUCBC treatment reduces DAF-FM staining in organotypic slice cultures following oxygen glucose deprivation.
Photomicrographs depict OTC subjected to normoxia (A,B) or OGD (C,D) and treated with either vehicle (A,C) or 106 HUCBC (B,D). DAF-FM intensities in HUCBC treated slices showed significant reductions in staining when compared to media controls subjected to OGD but not normoxia (E). Asterisk denotes significance at p<0.05. Scale bars represent 500 μm.
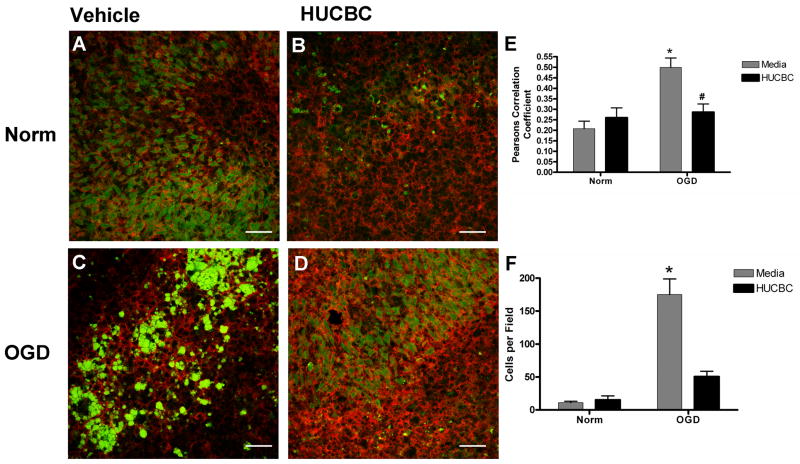
HUCBC reduces the number of nitric oxide producing microglia in ischemic OTC
To determine whether the observed NO production was due to activated microglia, immunohistochemistry was performed on DAF-FM stained slices probing for the CD11b cell surface antigen. In normoxic slices incubated with media alone, NO production was observed in cells which did not display CD11b immunoreactivity (Figure 5A). In contrast, NO production was much more prominent and overlapped with CD11b staining in slices incubated with media alone and subjected to OGD (Figure 5C). HUCBC treatment in both normoxic (Figure 5B) and OGD (Figure 5D) slices led to similar patterns of staining which were indistinguishable from untreated normoxic slices (Figure 5A). Pearson’s correlation coefficient values from multiple images were pooled and averaged to determine whether the signal overlap between the CD11b immunoreactivity and DAF-FM fluorescence was significantly different between treatment groups (Figure 5E). The mean correlation from slices subjected to OGD was significantly increased (140.21+/−46.99%) when compared to normoxic controls (p<0.0012, n=6), and HUCBC treatment significantly reduced the Pearson’s correlation in slices subjected to OGD (p<0.01, n=7) such that it was not different from normoxic controls (p=0.3819, n=5). The number of microglia producing NO was quantified in each image and this data was pooled and mean values were compared between groups (Figure 5F). OGD treatment resulted in a significant increase (1493.63+/−371.9%) in the number of NO producing microglia when compared to Normoxia controls (p,0.001, n=3) and HUCBC treatment significantly reduced the number of NO producing microglia subjected to OGD (p<0.001, n=3) such that it was not different from normoxic controls (p<0.05, n=3).
Figure 5. HUCBC reduces the number of nitric oxide producing microglia in ischemic organotypic slice cultures.
Confocal micrographs depict double-labeling with DAF-FM (NO, green) and CD11b (microglia, red) in slices subjected to normoxia (A,B) or OGD (C,D) and treated with either vehicle (A,C) or 106 HUCBC (B,D). Pearson’s correlation coefficients (E) demonstrated a significant increase in staining overlap in slices incubated with media alone and subjected to OGD (p<0.0012). The correlation in OGD slices co-incubated with HUCBC was significantly reduced relative to those incubated with media alone (p<0.01). Asterisk denotes significance from normoxia treated samples at p<0.05. Pound symbol represents significance from OGD media treated samples. Cell counts (F) confirmed a significant (p<0.001) increase in the number of NO producing microglia in OGD treated sections when compared to Normoxia, Normoxia+HUCBC and OGD+HUCBC. Scale bars represent 50 μm.
Discussion
HUCBC and DTG have shown great promise in reducing ischemic neurodegeneration in rats at delayed timepoints following MCAO. The mechanisms by which these treatments reduce infarct volume remain unclear. In vivo labeling studies have shown that both HUCBC and DTG interact with the brain and spleen (Su et al., 1988, Vendrame et al., 2004). There is both humoral and synaptic crosstalk between the brain and the spleen via the HPA axis and catecholaminergic signaling from the splenic nerve (Elenkov et al., 2000). Soluble catecholamines released by noradrenergic splenic nerve terminals or by the adrenal medulla act on splenic lymphocytes primarily via β-adrenergic receptors. Splenectomy prior to MCAO has recently been shown to combat ischemic injury (Ajmo et al., 2008), and while denervation of the spleen was not beneficial, administration of a pan selective adrenergic blocker also reduced cerebral infarction (Ajmo et al., 2009). To determine whether DTG and HUCBC exert direct effects on local microglial mediated inflammation and neurosurvival, an OTC model was employed to mimic ischemic conditions in the absence of the peripheral immune system. Because functional connections between different cell types are maintained (Noraberg et al., 2005), OTC is a superior model for the study of ischemic brain injury when compared to dissociated neuronal or microglial cell culture.
The characterization studies of the OGD model system revealed that there were Fluoro-Jade positive neurons present in normoxic slices. These are likely neurons injured during the preparation of the slice from the intact hippocampus. This observation is unsurprising as Fluoro-Jade positive neurons have been observed in injured brain tissue one month after insult. These degenerating neurons activate can activate microglia and are likely responsible for activated NO producing microglia observed in the normoxic slices. Interestingly nitric production increased in a biphasic manner over time with NO levels increasing at 12 and 24 hours of OGD but decreasing at 36hours of OGD before substantially increasing by the 48 hours of OGD. This observation was likely due to a transient increase in production of NO that was of non-microglial origin during the early phase of OGD which can be caused by calcium dysregulation and activation of neuronal nitric oxide synthase. These non microglial NO producing cells are less prevalent after 36 hours of OGD. By the 48hr timepoint the most prevalent cell type producing the NO are microglia.
While HUCBC treatment has been shown to reduce propridium iodide uptake in OTC, there have been no studies to our knowledge which directly measured neuronal damage, specifically, or the ensuing inflammatory response (Vendrame et al., 2005). Here we show that HUCBC reduced OGD induced neurodegeneration. HUCBC are composed of a mixture of cell types consisting predominantly of leukocytes and a small (<1%) fraction of stem cells (Pranke et al., 2001). The mechanisms by which HUCBC directly promote protection may include the secretion of neurotrophic growth factors such as glial cell–derived neurotrophic factor, epidermal growth factor, brain-derived neurotrophic factor, and fibroblast growth factor (Neuhoff et al., 2007). In the present study, direct cellular contact was prohibited by the selectively permeable membranes used to grow cultures. Thus, secreted factors such as the growth factors mentioned above may have contributed to the reduction in neurodegeneration observed after co-incubation with HUCBC.
HUCBC also decreased NO production in microglia. Consistent with the finding that HUCBC therapy in vivo dramatically reduced the number of microglia present in the infarct (Newcomb et al., 2006), these data demonstrate that HUCBC alter the microglial response to ischemic injury. Co-culture experiments in vitro demonstrated that HUCBC can decrease production of IL1β (Jiang L 2009). Similar experiments using a double transgenic PSAPP/Tg2576 mouse model of Alzheimer’s disease showed that HUCBC can decrease microglial activation by reducing CD40/CD40L interactions (Nikolic et al., 2008). As HUCBC decreased the incidence of NO producing microglia in the slice, decreasing microglial CD40 expression may be a mechanism by which HUCBC can cause this effect.
Sigma receptor activation is anti-inflammatory and can reduce neuronal death due to the release of inflammatory mediators (Vagnerova et al., 2006). Microglia are the only immune cell type present in the organotypic hippocampal slice, and sigma receptor activation was previously shown to reduce microglial activation in vitro. By blocking intracellular calcium signals, many aspects of microglial activation such as membrane ruffling, migration, and cytokine production were impaired (Hall et al., 2008). The 300 μM DTG concentration, which completely suppressed microglial activation in dissociated cultures, resulted in the highest levels of Fluoro-Jade staining in OTC. The complexity of the microenvironment associated with OTC in comparison to primary microglial cultures, however, requires that comparisons be made with caution. The lack of efficacy shown here in response to DTG treatment could reflect the beneficial aspects of microglial activation in a functional system. This is in agreement with previous work showing that ablation of microglia is neurodestructive (Lalancette-Hebert et al., 2007).
Sigma receptor activation also suppresses T-cell mediated immunity by upregulating production of the anti-inflammatory cytokine IL10 (Zhu et al., 2003). As microglia can interact with T-cells as antigen presenting cells (Morioka et al., 1991), it is possible that modulation of this interaction by sigma receptor agonists such as DTG could result in the robust neuroprotection seen in vivo and therefore show no protective effect ex vivo where T-cells are absent. Results here suggest that the neuroprotective effects of DTG at delayed timepoints in vivo are mediated by effects on systems outside of the CNS, such as the peripheral immune system. This notion is also supported by a previous study performed in vivo using dehydroepiandrosterone. Data from these experiments showed that sigma receptor activation prior to or during the onset of ischemia exacerbated neuronal death, while sigma receptor activation 24 hours after ischemia was neuroprotective (Li et al., 2009). Another likely therapeutic target of DTG in vivo is the lymphocyte population that infiltrates the infarct (Gee et al., 2007). This interaction of peripheral immune cells and injured brain tissue could also mediate the protective effects of HUCBC seen in this study, as they are composed primarily of lymphocytes and monocytes (Pranke et al., 2001).
While HUCBC and DTG are both potent suppressors of ischemia induced neurodegeneration at delayed timepoints in vivo (Vendrame et al., 2004, Ajmo Jr et al., 2006), only HUCBC exerted direct neuroprotective effects in the OTC model of ischemia. Understanding the processes by which these treatments confer neuroprotection should shed light on mechanisms to delay or reverse the pathophysiological effects of ischemic injury. As mounting evidence indicates that the response to ischemia consists of complex, concerted actions of the CNS and the peripheral immune system, further research into these interactions will likely lead to breakthroughs in the treatment of stroke.
Acknowledgments
This work was supported by the National Institute of Neurological Disorders and Stroke (RO 1-NS052839-03 to K.R.P. and A.E.W.; 5R21NS060907 to K.R.P. and A.E.W.) and the American Heart Association (0715096B to A.A.H.). We would also like to acknowledge Karen Lai M.S. for her contributions with image analysis.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Ajmo CT, Jr, Collier LA, Leonardo CC, Hall AA, Green SM, Womble TA, Cuevas J, Willing AE, Pennypacker KR. Blockade of adrenoreceptors inhibits the splenic response to stroke. Exp Neurol. 2009 doi: 10.1016/j.expneurol.2009.03.044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ajmo CT, Jr, Vernon DO, Collier L, Hall AA, Garbuzova-Davis S, Willing A, Pennypacker KR. The spleen contributes to stroke-induced neurodegeneration. J Neurosci Res. 2008 doi: 10.1002/jnr.21661. in press, Epub ahead of print. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ajmo C, Jr, Vernon D, Collier L, Pennypacker K, Cuevas J. Sigma receptor activation reduces infarct size at 24 hours after permanent middle cerebral artery occlusion in rats. Cur Neurovascular Res. 2006;3:89–98. doi: 10.2174/156720206776875849. [DOI] [PubMed] [Google Scholar]
- Albers GW, Amarenco P, Easton JD, Sacco RL, Teal P. Antithrombotic and thrombolytic therapy for ischemic stroke: the Seventh ACCP Conference on Antithrombotic and Thrombolytic Therapy. Chest. 2004;126:483S–512S. doi: 10.1378/chest.126.3_suppl.483S. [DOI] [PubMed] [Google Scholar]
- Borlongan CV, Hadman M, Sanberg CD, Sanberg PR. Central nervous system entry of peripherally injected umbilical cord blood cells is not required for neuroprotection in stroke. Stroke. 2004;35:2385–2389. doi: 10.1161/01.STR.0000141680.49960.d7. [DOI] [PubMed] [Google Scholar]
- Duckworth EA, Butler TL, De Mesquita D, Collier SN, Collier L, Pennypacker KR. Temporary focal ischemia in the mouse: technical aspects and patterns of Fluoro-Jade evident neurodegeneration. Brain Res. 2005;1042:29–36. doi: 10.1016/j.brainres.2005.02.021. [DOI] [PubMed] [Google Scholar]
- Elenkov IJ, Wilder RL, Chrousos GP, Vizi ES. The sympathetic nerve--an integrative interface between two supersystems: the brain and the immune system. Pharmacol Rev. 2000;52:595–638. [PubMed] [Google Scholar]
- Gee JM, Kalil A, Shea C, Becker KJ. Lymphocytes: potential mediators of postischemic injury and neuroprotection. Stroke. 2007;38:783–788. doi: 10.1161/01.STR.0000248425.59176.7b. [DOI] [PubMed] [Google Scholar]
- Hall AA, Herrera Y, Ajmo CT, Jr, Cuevas J, Pennypacker KR. Sigma receptors suppress multiple aspects of microglial activation. Glia. 2008 doi: 10.1002/glia.20802. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jiang LWT, Saporta S, Chen N, Sanberg CD, Sanberg PR, Willing AE. Human umbilical cord blood cells decrease microglial survival in vitro. Stem Cells and Development. 2009 doi: 10.1089/scd.2009.0170. In press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Katnik C, Guerrero WR, Pennypacker KR, Herrera Y, Cuevas J. Sigma-1 receptor activation prevents intracellular calcium dysregulation in cortical neurons during in vitro ischemia. J Pharmacol Exp Ther. 2006;319:1355–1365. doi: 10.1124/jpet.106.107557. [DOI] [PubMed] [Google Scholar]
- Lalancette-Hebert M, Gowing G, Simard A, Weng YC, Kriz J. Selective ablation of proliferating microglial cells exacerbates ischemic injury in the brain. J Neurosci. 2007;27:2596–2605. doi: 10.1523/JNEUROSCI.5360-06.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leonardo CC, Hall AA, Collier LA, Gottschall PE, Pennypacker KR. Inhibition of gelatinase activity reduces neural injury in an ex vivo model of hypoxia-ischemia. Neuroscience. 2009 doi: 10.1016/j.neuroscience.2009.02.080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li Z, Cui S, Zhang Z, Zhou R, Ge Y, Sokabe M, Chen L. DHEA-neuroprotection and -neurotoxicity after transient cerebral ischemia in rats. J Cereb Blood Flow Metab. 2009;29:287–296. doi: 10.1038/jcbfm.2008.118. [DOI] [PubMed] [Google Scholar]
- Marler JR, Goldstein LB. Medicine. Stroke--tPA and the clinic. Science. 2003;301:1677. doi: 10.1126/science.1090270. [DOI] [PubMed] [Google Scholar]
- Morioka T, Kalehua AN, Streit WJ. The microglial reaction in the rat dorsal hippocampus following transient forebrain ischemia. J Cereb Blood Flow Metab. 1991;11:966–973. doi: 10.1038/jcbfm.1991.162. [DOI] [PubMed] [Google Scholar]
- Neuhoff S, Moers J, Rieks M, Grunwald T, Jensen A, Dermietzel R, Meier C. Proliferation, differentiation, and cytokine secretion of human umbilical cord blood-derived mononuclear cells in vitro. Exp Hematol. 2007;35:1119–1131. doi: 10.1016/j.exphem.2007.03.019. [DOI] [PubMed] [Google Scholar]
- Newcomb JD, Ajmo CT, Sanberg CD, Sanberg PR, Pennypacker KR, Willing AE. Timing of cord blood treatment after experimental stroke determine therapeutic efficacy. Cell Transplant. 2006;15:213–223. doi: 10.3727/000000006783982043. [DOI] [PubMed] [Google Scholar]
- Nikolic WV, Hou H, Town T, Zhu Y, Giunta B, Sanberg CD, Zeng J, Luo D, Ehrhart J, Mori T, Sanberg PR, Tan J. Peripherally administered human umbilical cord blood cells reduce parenchymal and vascular beta-amyloid deposits in Alzheimer mice. Stem Cells Dev. 2008;17:423–439. doi: 10.1089/scd.2008.0018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Noraberg J, Poulsen FR, Blaabjerg M, Kristensen BW, Bonde C, Montero M, Meyer M, Gramsbergen JB, Zimmer J. Organotypic hippocampal slice cultures for studies of brain damage, neuroprotection and neurorepair. Curr Drug Targets CNS Neurol Disord. 2005;4:435–452. doi: 10.2174/1568007054546108. [DOI] [PubMed] [Google Scholar]
- Offner H, Subramanian S, Parker SM, Afentoulis ME, Vandenbark AA, Hurn PD. Experimental stroke induces massive, rapid activation of the peripheral immune system. J Cereb Blood Flow Metab. 2006;26:654–665. doi: 10.1038/sj.jcbfm.9600217. [DOI] [PubMed] [Google Scholar]
- Pranke P, Failace RR, Allebrandt WF, Steibel G, Schmidt F, Nardi NB. Hematologic and immunophenotypic characterization of human umbilical cord blood. Acta Haematol. 2001;105:71–76. doi: 10.1159/000046537. [DOI] [PubMed] [Google Scholar]
- Schmued L, Albertson C, Slikker W. Fluoro-Jade: a novel fluorochrome for the sensitive and reliable histochemical localization of neuronal degeneration. Brain Res. 1997;751:37–46. doi: 10.1016/s0006-8993(96)01387-x. [DOI] [PubMed] [Google Scholar]
- Su TP. Sigma receptors. Putative links between nervous, endocrine and immune systems. Eur J Biochem. 1991;200:633–642. doi: 10.1111/j.1432-1033.1991.tb16226.x. [DOI] [PubMed] [Google Scholar]
- Su TP, London ED, Jaffe JH. Steroid binding at sigma receptors suggests a link between endocrine, nervous, and immune systems. Science. 1988;240:219–221. doi: 10.1126/science.2832949. [DOI] [PubMed] [Google Scholar]
- Vagnerova K, Hurn PD, Bhardwaj A, Kirsch JR. Sigma 1 receptor agonists act as neuroprotective drugs through inhibition of inducible nitric oxide synthase. Anesth Analg. 2006;103:430–434. doi: 10.1213/01.ane.0000226133.85114.91. table of contents. [DOI] [PubMed] [Google Scholar]
- Vendrame M, Cassady CJ, Newcomb J, Butler T, Pennypacker KR, Zigova T, Davis Sanberg C, Sanberg PR, AE W. Infusion of human umbilical cord blood cells in a rat model of stroke dose-dependently rescues behavioral deficits and reduces infarct volume. Stroke. 2004;35:2390–2395. doi: 10.1161/01.STR.0000141681.06735.9b. [DOI] [PubMed] [Google Scholar]
- Vendrame M, Gemma C, De Mesquita D, Collier L, Bickford PC, Sanberg CD, Sanberg PR, Pennypacker KR, Willing AE. Anti-inflammatory effects of human cord blood cells in a rat model of stroke. Stem Cells Dev. 2005;14:595–604. doi: 10.1089/scd.2005.14.595. [DOI] [PubMed] [Google Scholar]
- Yilmaz G, Arumugam TV, Stokes KY, Granger DN. Role of T lymphocytes and interferon-gamma in ischemic stroke. Circulation. 2006;113:2105–2112. doi: 10.1161/CIRCULATIONAHA.105.593046. [DOI] [PubMed] [Google Scholar]
- Zhu LX, Sharma S, Gardner B, Escuadro B, Atianzar K, Tashkin DP, Dubinett SM. IL-10 mediates sigma 1 receptor-dependent suppression of antitumor immunity. J Immunol. 2003;170:3585–3591. doi: 10.4049/jimmunol.170.7.3585. [DOI] [PubMed] [Google Scholar]