Abstract
Fifteen to 35% of the United States population experiences tinnitus, a subjective “ringing in the ears”. Up to 10 percent of those afflicted report severe and disabling symptoms. Tinnitus was induced in rats using unilateral, one-hour, 17 kHz-centered octave-band noise (116 dB SPL) and assessed using a gap-startle method. The dorsal cochlear nucleus (DCN) is thought to undergo plastic changes suggestive of altered inhibitory function during tinnitus development. Exposed rats showed near pre-exposure ABR thresholds for clicks and all tested frequencies 16 weeks post-exposure. Sound-exposed rats showed significantly worse gap detection at 24 and 32 kHz 16 weeks following sound exposure, suggesting the development of chronic, high frequency tinnitus.
Message and protein levels of α1–3, and β glycine receptor subunits (GlyRs), and the anchoring protein, gephyrin, were measured in DCN fusiform cells 4 months following sound exposure. Rats with evidence of tinnitus showed significant GlyR α1 protein decreases in the middle and high frequency regions of the DCN while α1 message levels were paradoxically increased. Gephyrin levels showed significant tinnitus-related increases in sound-exposed rats suggesting intracellular receptor trafficking changes following sound exposure. Consistent with decreased α1 subunit protein levels, strychnine binding studies showed significant tinnitus-related decreases in the number of GlyR binding sites, supporting tinnitus-related changes in the number and/or composition of GlyRs.
Collectively, these findings suggest the development of tinnitus is likely associated with functional GlyR changes in DCN fusiform cells consistent with previously described behavioral and neurophysiologic changes. Tinnitus related GlyR changes could provide a unique receptor target for tinnitus pharmacotherapy or blockade of tinnitus initiation.
Keywords: hearing loss, sound exposure, fusiform cell, auditory, glycine receptor, gephyrin
Tinnitus is a common audiologic complaint characterized by auditory perception without an external physical source. About 15 to 35% of the United States population experience tinnitus and 10 percent of tinnitus patients are subjected to chronic, persistent tinnitus that affects their ability to work, sleep, socialize and in some, resulting in severe clinical depression (Harrop-Griffiths et al., 1987; Sullivan et al., 1988; Jastreboff, 1990; Seidman and Jacobson, 1996; Zöger et al., 2001, 2006; Sanchez et al., 2002, 2005; Marciano et al., 2003; Dobie, 2003; Eggermont and Roberts, 2004; Ahmad and Seidman, 2004; Eggermont, 2007). The prevalence of sound-induced tinnitus is likely to increase, especially for young people exposed to loud sounds during leisure activities (Widen and Erlandsson, 2004; Chung et al., 2005). In spite of its prevalence, the pathophysiologic underpinnings of tinnitus remain poorly understood.
Recent evidence implicates hyperactivity in the dorsal cochlear nucleus (DCN) as a potential generator of tinnitus following acoustic exposure. This hyperactivity may result from plastic changes that develop over time and may depend, in part, on the magnitude of the insult to the periphery (Zhang and Kaltenbach, 1998; Kaltenbach et al., 2000a, b, 2004a, b, 2005; Brozoski et al., 2002). One hypothesis suggests that chronic tinnitus may develop from a net loss of glycinergic inhibition in the DCN and/or GABAergic inhibition at higher auditory structures (Brozoski et al., 2002, 2007; Bauer et al., 2008). Single unit data from tinnitus animal models found increased spontaneous activity and hyperactivity of DCN fusiform cell responses (Kaltenbach et al., 2000a, b, 2004; Brozoski et al., 2002) while Ma and Young (2006) found no evidence of increased spontaneous activity in DCN following a sound exposure that resulted in significant permanent threshold shift in a cat model of tinnitus. The present study examined putative tinnitus-related subunit and anchoring protein changes of the postsynaptic inhibitory glycine receptor (GlyR) in DCN fusiform cells.
Glycine receptors (GlyRs) are pentameric protein complexes constituting a ligand-gated ion channel permeable to chloride ions (Langosch et al., 1990; Betz et al., 1994; Takahashi and Momiyama, 1994; Lynch, 2004; Webb and Lynch, 2007). Heteromeric GlyRs are composed of three α and two β subunits (3α: 2β) or two α and three β subunits (2α: 3β) (Grudzinska et al., 2005). Homomeric α2 complexes are thought to predominate during development (Takahashi et al., 1992; Legendre; 2001; Lynch, 2004) with heteromeric α1 or α2 complexes abundant in adulthood in central auditory structures (Caspary et al., 1979, 1987; Moore and Caspary, 1983; Frostholm and Rotter, 1985; Finlayson and Caspary, 1989; Sato et al., 1992; Milbrandt and Caspary, 1995; Krenning et al., 1998; Lynch, 2004). Previous studies found that partial or total peripheral deafferentation alters neurochemical measures associated with GlyR function in the central auditory pathway (Milbrandt and Caspary, 1995; Willott et al., 1997; Suneja et al., 1998a, b; Holt et al., 2005). Suneja et al. (1998a, b) also found that in cases of profound cochlear damage, observed glycinergic changes could revert back toward pre-trauma control levels weeks or months following the initial peripheral insult.
Dorsal cochlear nucleus (DCN) studies (Milbrandt and Caspary, 1995; Wang et al., 2009) reported an age-related decrease in [3H] strychnine binding within the DCN of aged rats and similar results were observed in the bilateral DCN after unilateral cochlear ablation (UCA) (Suneja et al., 1998b). Gephyrin binds to β subunits and appears essential for proper GlyR anchoring at the postsynaptic membrane (Meier et al., 2000, 2001; Kirsch, 2006; Kneussel, 2006; Kneussel and Loebrich, 2007; Fritschy et al., 2008). Sound-induced loss of normal glycinergic input upon fusiform cells in the DCN could represent an activity-dependent plastic, synaptic change critical to the development of tinnitus (Brozoski et al., 2002).
The present study examined GlyR and gephyrin message and protein levels 16 weeks following a 1-hour sound-exposure in adult FBN rats behaviorally tested for psychophysical evidence of tinnitus (Bauer et al., 1999; Bauer and Brozoski, 2001; Brozoski et al., 2002, 2007; Turner et al., 2006).
EXPERIMENTAL PROCEDURES
Subjects
Adult male Fischer Brown Norway (FBN) rats (Harlan Sprague-Dawley Inc), 3–4 months old prior to sound exposure, were divided into control and sound-exposed groups. Twenty-nine FBN rats used in the quantitative immunocytochemistry and strychnine binding studies were behaviorally tested for the presence of tinnitus using the gap/startle detection method (Turner et al., 2006). For brevity, behavioral results from thirteen animals used for the in situ hybridization studies and tested using the conditioned-suppression method (Bauer et al., 1999, 2001; Brozoski et al., 2002; Brozoski and Bauer, 2005) will not be presented. All experimental protocols were approved by the Southern Illinois University School of Medicine LACUC committee.
Acoustic exposure
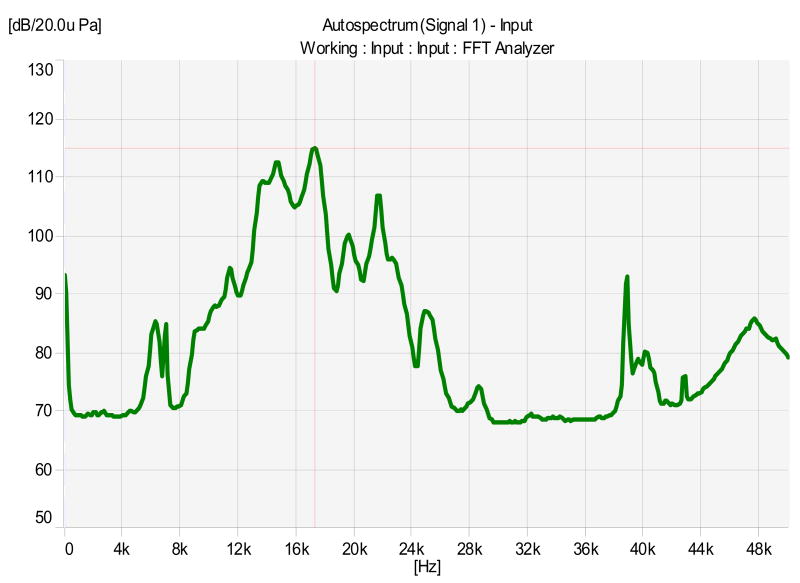
Control and sound-exposed rats were anesthetized with a ketamine HCl (50 mg/kg, Aveco, Fort Dodge, IA))/xylazine (9 mg/kg, Lloyd Laboratories, Shennandoah, IA) mixture and placed in a modified stereotaxic head frame. Choice of sound exposure was based on the model by Drs. Bauer and Brozoski (Bauer et al., 1999; Bauer and Brozoski, 2001) in an effort to develop a rat model with minimal threshold shift and with behavioral evidence of chronic tinnitus. Sound-exposed rats were exposed unilaterally using 116 dB SPL octave-band noise, centered at 17 kHz peak intensity for 1-hour (Fig. 1) (Bauer et al., 1999; Bauer and Brozoski, 2001; Brozoski and Bauer, 2005).
Figure 1.
Spectrum of the octave-band noise used for sound exposure in the present study. This octave band is centered at 17 kHz with a peak intensity of 116 dB. This exposure was sufficient to elevate ABR thresholds immediately post-exposure a maximum of approximately 30–40 dB SPL.
Auditory brainstem response
Threshold shift was measured by auditory brainstem response (ABR) for both ipsi- and contralateral ears from control and sound-exposed rats. Data were obtained prior to, immediately following and 16 weeks post sound-exposure. ABR testing was conducted in a double-walled sound chamber using subdermal electrodes inserted posterior to each pinna and at vertex, with a ground electrode in the animal’s hind leg.
ABR thresholds were obtained for clicks and 5 msec tone bursts presented at a rate of 50/sec. Tone bursts were gated using an exact Blackman envelope (2.5 msec rise/decay, 0 msec plateau). Evoked potentials were averaged over 1024 sweeps. Amplifier gain was set at 200 k and waveforms were filtered using a 100–3000 Hz bandpass filter. Data were collected using a modified Intelligent Hearing Systems (Miami, FL) high-frequency system.
Gap detection method
Twenty-nine FBN rats (15 age-matched controls and 14 sound-exposed) were assessed for the presence of tinnitus using the gap detection method of Turner et al. (2006). Briefly, animals were tested to detect a silent gap embedded in acoustic background. Testing was conducted 20 days after sound exposure every 2 weeks up to 16 weeks using startle reflex hardware and software customized for this application by the manufacturer (Kinder Behavioral Testing Systems, Poway, CA). Briefly, animals were tested inside a sound-attenuating box with background noise presented through one speaker (Vifa XT25TG30-04) and the startle stimuli presented through a second speaker (Powerline CTS KSN-1005) mounted in the ceiling of the testing chamber 15 cm above the animal. A piezo transducer plate was attached to the animal holder and provided a measure of the startle force applied by the animal. Testing was performed using BBN, or bandpass filtered noise (1000 Hz bandpass: 48dB/octave roll off) centered at 4, 8, 10, 12, 16, 20, 24, and 32 kHz, each at an intensity of 60 dB SPL. The session began with a 2-min acclimation period followed by 2 startle-only trials (noise burst at 115 dB SPL, 20 msec in duration) to habituate the startle response to a more stable baseline. The remainder of the session consisted of additional startle-only trials mixed with gap trials in a counter-balanced fashion. Gap trials were identical to startle-only trials, except for the inserted silent gap. Gaps were 50 ms in length and shaped with a 0.1 ms rise/fall time. Gap trials consisted of startle stimuli preceded (100 ms) by a gap. The rationale for the gap testing is that tinnitus will fill in the silent gap in the background sound. If the background sound frequency is similar to the animal’s tinnitus, the result will be poorer gap detection and less inhibition of the startle response.
Frequency division of the DCN
Previous studies delineated a tonotopic organization along the mediolateral axis of the DCN. Low frequencies were found laterally with high frequencies represented medially. This organization was established using c-fos expression and electrophysiological methods in rats and cats (Rose et al., 1959; Yajima and Hayashi, 1989; Rouiller et al., 1992; Friauf, 1992; Saint Marie et al., 1999). Based on these data, the DCN was divided into three parts: the most medial third, the middle third and the most lateral third of DCN, a high, middle and low frequency area, respectively (Ryan et al., 1988; Yajima and Hayashi, 1989; Saint Marie et al., 1999; Wang et al., 2009). In the present study, frequency regions which GlyR neurochemistry changes occur not necessarily reflect animals’ behavioral tinnitus frequency.
In situ hybridization and autoradiography
Thirteen FBN rats (7 controls, 6 sound-exposed) were used for in situ hybridization experiments to assess mRNA/message levels of the GlyR subunits and gephyrin. All processing and analyses were carried out in parallel, with at least one control and one exposed animal processed simultaneously. Animals were sacrificed one week after the behavioral tests, their brains rapidly removed and immediately frozen on powdered dry ice and kept at −80°C until use. Serial coronal sections (16-μm thick) from bregma −10.52 to −11.6 were cut on a cryostat (Leica CM1850 Microsystems, Nussloch GmbH, Germany) at −20°C and thaw-mounted on Superfrost/Plus slides (Fisher Scientific, St. Louis, MO, USA). Tissue preparation and hybridization procedures were performed as described previously (Milbrandt et al., 1997; Krenning et al., 1998; Ling et al., 2005). In situ hybridization was performed with 3′ 35S-labelled oligonucleotide probes designed to detect GlyR subunits (α1–3, β) and gephyrin. Oligonucleotide probe sequences are depicted in Table 1.
Table 1.
GlyR subunits and gephyrin oligonucleotide probe sequences for in situ hybridization.
| Probes | Sequence 5′-3′ |
|---|---|
| GlyR α1 (Malosio et al., 1991) | 5′ GTT GGC ACC CTT GAC AGA GAT GCC ATC CTT GGC TTG CAG GCA GGC 3′ |
| GlyR α2 (Malosio et al., 1991) | 5′ CTT TTG GGG GTT GCG GAA GTG GGT TGG CAG GTG TAG CCT TGA CAG 3′ |
| GlyR α3 (Malosio et al., 1991) | 5′ GGC AGT GAA GCT GAG CCG ACT CTC CCT CAC CTC ATC ATC CGT GTC 3′ |
| GlyR β (Malosio et al., 1991) | 5′ GCA AGG TCC TCG GCC GAC TGT TGA GAT GGG CAC AAA TAC TGC TTC 3′ |
| Gephyrin (Kirsch et al., 1993) | 5′ CCT TCA ATA TCC AAA GTT GCA AAT GTT GTT GGC AAG CCT GGC TTC 3′ |
Finally, sections were dipped in Kodak NTB-2 photographic emulsion (VWR, West Chester, PA, USA) and stored at 4°C for 1 or 2 weeks (depending on the probe and labeling). Sections were developed in Kodak D-19 (Eastman-Kodak, Rochester, NY, USA) and counterstained with thionin. No labeling was observed in the two extra sections processed with control hybridization buffer using an unlabeled oligonucleotide probe.
Autoradiographs (corresponding to 12 or 14 sections per animal) were digitized using a CCD camera. All slides were blinded to the reviewing investigator to assure unbiased measurements. Hybridization signals (silver grains) were quantified with a computerized image analysis system (Cool Snap monochrome digital camera interfaced with a Nikon Microphot light microscope and coupled to an MCID imaging system running Elite 6.0 software). Approximately 15–30 fusiform cells were measured from each section. The numbers of grains over the fusiform cell soma were expressed as density of silver grains/100 μm2 and these values were independent of neuron size and/or neuronal shape change. Density values were corrected by subtracting background labeling obtained from five random areas located off the tissue sections on each slide (Milbrandt et al., 1997; Krenning et al., 1998).
Immunocytochemistry
The procedure used for quantitative immunocytochemistry was based on those published by Ling et al. (2005) and Abbott et al. (1999). Eight rats (4 control, 4 sound-exposed) were anesthetized followed by transcardial perfusion with 500ml of fixative containing 4% paraformaldehyde in 0.1M K-Na phosphate buffer pH 7.4 (PBS). The brains were removed, dehydrated in 20% sucrose and stored at −80°C until use.
Cryostat frozen sections (16 μm) were cut and blocked in 1.5% normal donkey serum in 0.1M PBS for 30 min, then transferred to purified primary antibody (for details see extra sheet for antibodies). Sections were incubated for 1.5 hrs and then at 4°C overnight with agitation. ABC kits (Vector Laboratories, Burlingame, CA, USA) were used, labeled with a secondary biotinylated antibody. Sections were visualized by exposure to diaminobenzidine (DAB) in PBS for 1–3 min. Images were captured using a CoolSnap camera (10×) interfaced with a Leitz Diaplan microscope coupled to an ImagePro Plus imaging system. Captured images at 10× included a calibration bar photograph and the slides were blinded to the reviewing investigator. Relative optical density (ROD) for each DCN fusiform cell was measured using NIH Scion Image. All values were corrected by subtracting background obtained from the measurements of negatively stained strial fibers deep into the DCN.
[3H]Strychnine binding
Four control and four sound-exposed rats were sacrificed, brains rapidly removed and stored at −80°C until sectioned. Strychnine binding sites were examined using a modified protocol from Milbrandt and Caspary (1995). Tissue sections (16 μm) were pre-washed twice in 50 mM Na-K phosphate buffer for 25 min and incubated with [3H] strychnine (0, 2, 4, 8, 12, 16, 32nM, 23–25 Ci/mmol, New England Nuclear, Boston, MA, USA) in 50 mM Na-K buffer for 20 min. Non-specific binding was determined by incubating companion slides with [3H] strychnine and 10 mM glycine. Incubation was terminated by a quick dip in 50 mM Na-K phosphate buffer followed by a 2 min wash in the same buffer twice and one quick dip in distilled water, then air dried. Sections were opposed to tritium sensitive phosphor screens (PerkinElmer Life and Analytical Sciences) with calibrated autoradiographic [3H]-Tritium standard (American Radiolabeled Chemicals Inc.) for 3 days. All slides were blinded to the investigator and images were scanned using a Cyclone storage phosphor system. Quantitative analysis of the images was obtained by using OptiQuant software (Version 4.0, Packard Instrument Co., USA). Specific binding was determined by subtracting nonspecific binding from total binding. For better resolution images, the slides were opposed to Tritium Hyperfilm (GE Healthcare, Piscataway, NJ) for 4–5 weeks at 4°C. Then the films were developed in Kodak D19 (Eastman-Kodak, Rochester, NY) for 4 minutes at room temperature, stopped in 1% acetic acid, fixed in Kodak rapid fixer, washed and air dried. Films were placed on a light box and digitized images were captured using a CCD video camera.
Statistical analysis
Data files generated during the experiments were imported to Excel spreadsheets (Excel, Microsoft Corp, Redmond, WA) and assessed for integrity and distributional characteristics. Descriptive statistics and graphical depictions were used to determine if transformations and or adjustments were necessary to meet the assumptions of the inferential statistical procedures employed. Statistical procedures were implemented within SPSS 14.0 (SPSS, Chicago, IL, USA). Statistical differences in ABR’s between control and sound exposed groups were determined by a mixed effects analysis of variance (ANOVA) with repeated measures. For individual animals, gap detection data yielded a single value expressed as a percentage (mean response in gap trials/mean response in startle-only trials). ANOVAs were used to compare differences in gap detection between groups. Differences between control and exposed groups for in situ and immunochemistry protein data were evaluated using factorial ANOVAs.
RESULTS
Acoustic Brainstem Response (ABR)
Data from 42 rats are presented in this study. ABR thresholds were obtained from 21 monaurally-exposed rats (one hour octave-band 17 kHz centered noise) and 21 unexposed control animals in response to clicks and six tone-burst frequencies (4, 10, 16, 20, 24 and 32 kHz). Thresholds were measured prior to and immediately following sound-exposure as well as 16 weeks following sound-exposure (end of the behavioral testing).
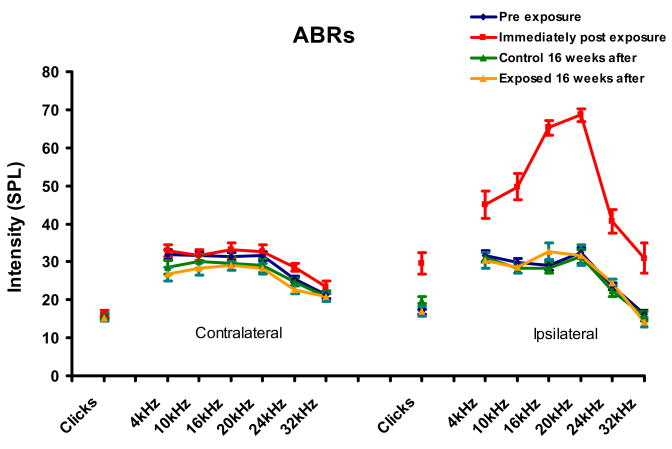
Immediately following sound-exposure, ipsilateral, sound-exposed ears showed significantly elevated thresholds for clicks and tones from 4 to 32 kHz (p < 0.02), while no significant contralateral threshold shifts were observed (Fig. 2). Sixteen weeks post-exposure, no significant threshold shifts were observed for any stimulus condition when compared to pre-exposure values (Fig. 2). Sixteen weeks post-exposure, control rats showed no threshold shifts at any frequency tested in either ear compared to their pre-exposure levels (Fig. 2). ABR threshold results from this sound exposure are suggestive of a temporary threshold shift in this set of adult FBN rats.
Figure 2.
Unilateral sound exposure effects on ABR thresholds for adult FBN rats. Mean ABR thresholds are shown for six frequencies and clicks. The sound-exposed ears (Ipsilateral) showed significant post-exposure threshold elevation but recovered at 16 weeks after. (ρ < 0.05, 21 control, 21 sound-exposed). Error bars represent SEMs.
Psychophysical evidence of tinnitus
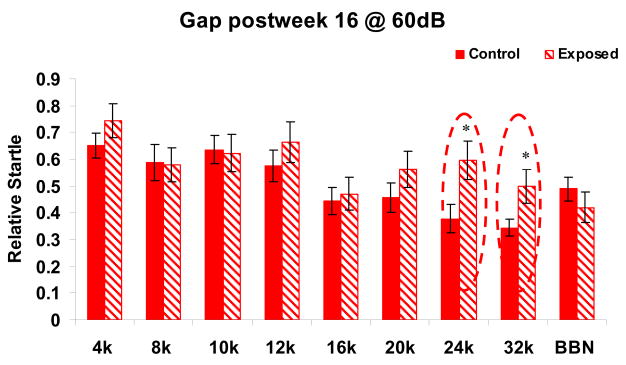
Behavioral evidence of tinnitus was assessed using a gap detection method. Based on previous studies, sound exposed Long-Evans rats did not exhibit significant tinnitus evidence until 4 months following sound exposure (Turner et al., 2006). In the present study, 15 exposed rats used to assess GlyR subunit protein levels and receptor binding displayed significantly worse gap detection (p < 0.05) when the gap was embedded in 24 or 32 kHz bandpass signals, suggesting that these rats developed a high frequency tinnitus 16 weeks following sound exposure (Fig. 3A). This frequency range for tinnitus was consistent with unpublished data showing evidence of 20 kHz tinnitus using conditioned-suppression method (Brozoski et al., 2002, 2007; Bauer et al., 2008) in a cohort (used for in situ hybridization in the present study) of FBN rats given the same sound exposure. Behavioral tinnitus evidence was frequency-specific since no significant startle differences between the control and sound-exposed groups were observed at any other frequencies or with BBN during the silent gap (Fig. 3). Together, these behavioral findings suggest that 75% of sound-exposed rats developed high frequency tinnitus following the sound exposure.
Figure 3.
Relative startle amplitude (response to gap+startle condition/response to startle only condition) as a function of background test frequency. A response of 1.0 would suggest no detection of the silent gap. Lower values correspond to better detection of the silent gap and more inhibition of the reflex. Exposed animals showed evidence of tinnitus 16 weeks following sound exposure in 24 and 32 kHz backgrounds, suggesting their tinnitus is between 24–32 kHz ranges. Significant changes are circled (ρ<.05, 15 control, 14 sound-exposed). (Kilohertz = kHz, BBN = broadband noise). Error bars represent SEMs.
GlyR subunits and gephyrin message level changes
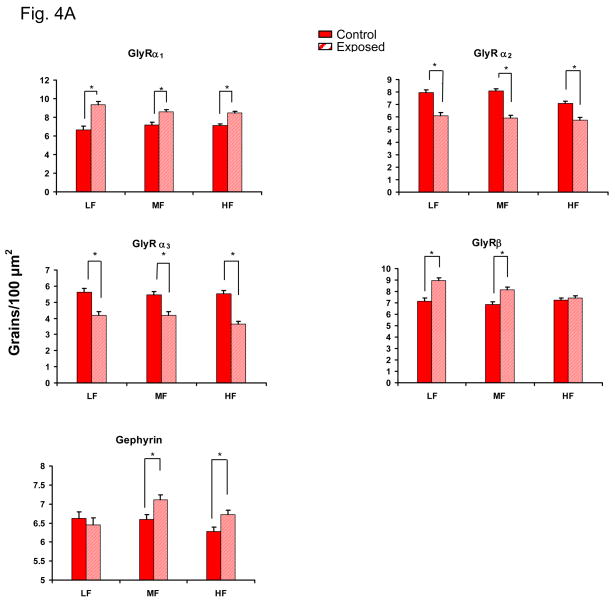
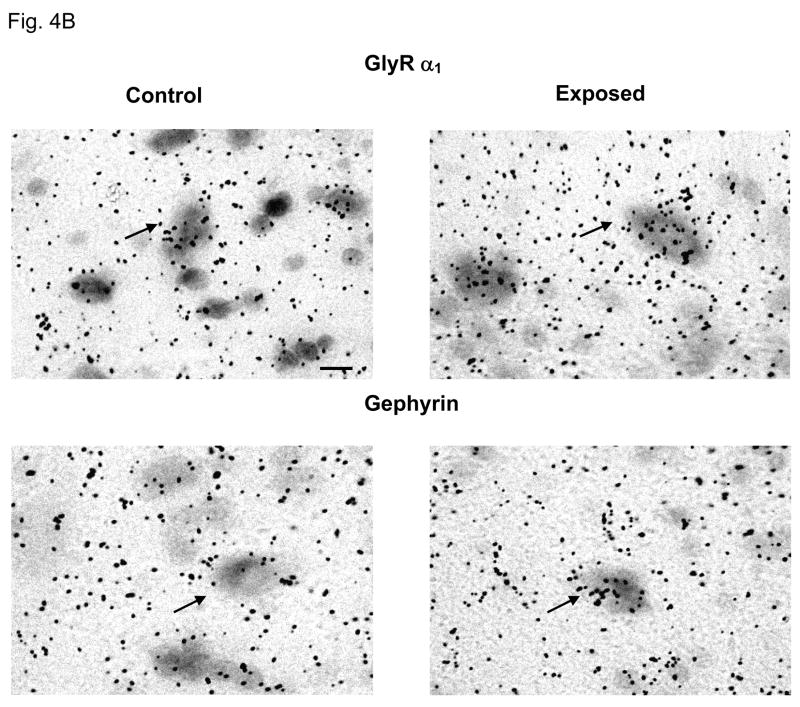
Distinct labeling indicating expression of GlyR α1–3 and β subunits mRNA was observed over DCN fusiform cells for all subjects (Fig. 4A, B). Sound-exposed rats showed significant exposure-related changes in GlyR subunit composition. Significant across frequency, increases in GlyR α1 message was observed in the presence of a significant decrease in α2 and α3 subunit message over fusiform cells across all frequency regions of DCN (p < 0.05 Fig. 4A). GlyR β subunit message levels were significantly increased in middle and low frequency regions over fusiform cells with no significant changes in the high frequency region of DCN (Fig. 4A). The scaffolding protein gephyrin, which is thought to anchor postsynaptic GlyR synaptic clusters by interacting with GlyR β subunits, showed significantly increased message over fusiform cells in high and middle frequency regions of DCN (Fig. 4A, B).
Figure 4.
Exposure-related changes in FBN rat mRNA levels for GlyRs α1–3, β subunits and anchoring protein gephyrin in DCN of adult FBN rats. Fig. 4A: Exposed subjects showed a significant message increase in GlyR α1 subunits and gephyrin. Message level of GlyR α2 and α3 significantly decreased across all frequency areas in DCN following exposure (*: p < 0.05, 7 control, 6 sound-exposed). Message level of GlyR β significant increase in low and middle frequency areas of DCN in exposed rats, which is similar to the changes of gephyrin. Error bars represent SEMs. Fig. 4B: In situ hybridization images are representative of the GlyRα1 and gephyrin changes plotted in Figure 4A. Arrows point to fusiform cell soma. LF = low frequency; MF = middle frequency; HF = high frequency. Scale bar = 5μm.
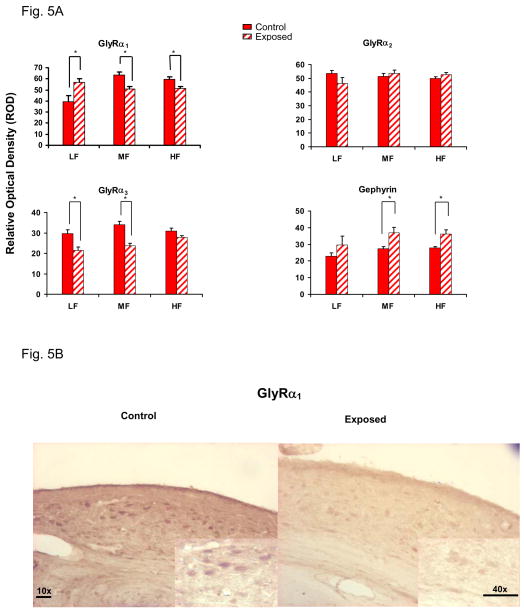
GlyR subunits and gephyrin protein level changes
Sound exposure-induced subunit protein changes were not necessarily coherent with observed message changes. GlyR α1 subunit positive cells were evenly distributed throughout the fusiform cell layer of DCN. In contrast to the observed exposure-related message increases, there were significant decreases in the relative optical density (ROD) of the α1 immunolabeling in the middle and high frequency areas of DCN (p <0.05, Fig. 5A, B). Sound-exposed animals showed significant GlyR α3 subunit protein level decreases in low and middle frequency regions of DCN (P < 0.05, Fig. 5A) while no significant α2 subunit protein changes were observed across frequency regions of DCN. Consistent with increased gephyrin message, a significant tinnitus-related increase in gephyrin protein levels was observed over fusiform cells in middle and high frequency regions, with a similar but not statistically significant trend in the low frequency regions of DCN of sound-exposed rats (Fig. 5A, B). We were not able to measure GlyR β subunit protein levels due to the lack of a specific antibody.
Figure 5.
5A: Unilateral sound exposure related GlyR subunits and gephyrin changes in DCN of adult control vs. exposed rats. GlyR α1 showed significant exposure–related decreases in high and middle frequency areas of DCN in adult exposed animals. A significant increase of gephyrin was seen in DCN middle and high frequency regions following sound exposure (ρ < 0.05, n = 4 control, 4 sound-exposed, ROD: relative optical density). Error bars represent SEMs. 5B: Transverse sections through the ipsilateral DCN immunolabeled with GlyR α1 probe. Inset micrograph shows stained fusiform cells from high frequency area in DCN. 10X: Scale bar = 10 μm; 40X: Scale bar = 5μm. All images were captured using exacting parallel settings and are representative of the quantitative data.
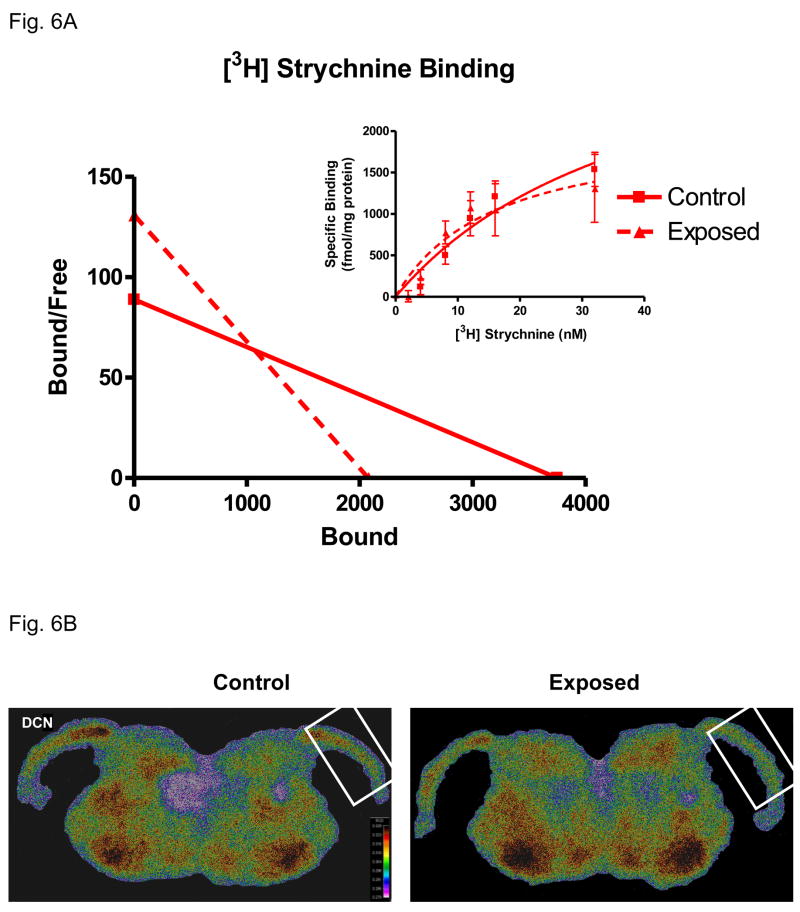
[3H] strychnine binding
The integrity and pharmacological properties of DCN GlyRs were assessed using the α1 subunit selective antagonist, strychnine, in a quantitative receptor binding study. [3H] strychnine binding levels were high throughout DCN and particularly prominent in the fusiform cell layer (Fig. 6B). Saturation analysis showed significantly altered specific strychnine binding across a series of concentrations in sound-exposed rats compared to control animals (Fig. 6A). Scatchard analysis revealed linear strychnine binding for sound-exposed and control groups, reflecting a single affinity binding site (Fig. 6A). There was a tinnitus-related decrease in the total number of binding sites (Bmax) based on equivalent protein concentrations (Fig. 6ABmax = 3746 fmol/mg for control; 2075 fmol/mg protein for sound-exposed). Exposure-related GlyR affinity changes (Kd) was calculated from saturation isotherms of control and exposed groups (Fig. 6A). Exposed animals showed a steeper slope which indicated a lower Kd although it failed to reach significance (6A).
Figure 6.
Exposure-related changes in strychnine binding between adult control and exposed FBN rats. Fig. 6A: Representative scatchard plots of [3H] strychnine binding in DCN from the control and exposed groups. A significant exposure-related loss of strychnine binding sites (X intercept = Bmax; Slope = Kd) is seen in exposed rats while a lower Kd suggests higher affinity in exposed rats (ρ < 0.05, 4 control, 4 sound-exposed). Fig. 6B: Color enhanced digitized images showing strychnine binding change in DCN between control and exposed rats. Ipsilateral DCNs were framed and the warmer colors indicate higher levels of binding.
DISCUSSION
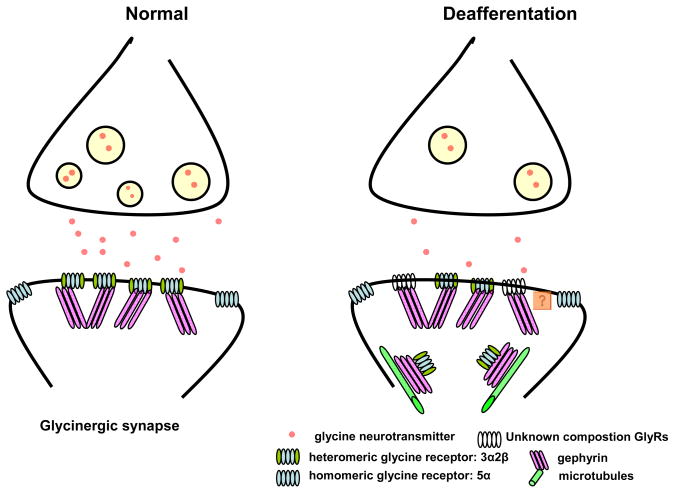
The present study found that adult FBN rats with statistically unchanged hearing 16 weeks following moderate sound exposure exhibited significant behavioral evidence of tinnitus. This modest acoustic insult decreased GlyR α1 subunit protein level and receptor binding over DCN fusiform cells. This sequence of changes may begin with decreased glycine release due to the resultant noise induced partial anatomical or functional deafferentation and resulting in compensatory receptor and trafficking changes summarized in Figure 7 and discussed below. The increased gephyrin protein level suggests a novel intracellular receptor-trafficking role for gephyrin. Altered GlyR composition and function in DCN fusiform cells is consistent with previously described tinnitus-related changes in the central auditory pathway (see below).
Figure 7.
Plastic changes of glycinergic synapses over fusiform cells in DCN following sound exposure. Glycine neurotransmitter releases from presynaptic terminals act on postsynaptic heteromeric and extrasynaptic homomeric GlyRs under normal conditions (left panel). Gephyrin represents an anchoring protein which directly interacts with the large intracellular loop of GlyR β subunits. Due to sound exposure induced peripheral auditory deafferentation, there may be less glycine release from presynaptic terminal to fusiform cells in DCN (right panel). As a compensate response, numbers of postsynaptic membrane GlyR decreased and/or receptor composition change due to unknown subunit switch. Not only as anchoring proteins, gephyrin may serve as the intracellular retrograde transport adaptor that links GlyRs with the microtubule dependent dynein motor complex to remove abnormal functional GlyRs from postsynaptic membrane.
ABR temporary threshold shift (TTS) and tinnitus
Acute exposure to loud sound under controlled experimental conditions can result in persistent tinnitus in humans (Atherley et al., 1968; Nottet et al., 2006) and rodents (Bauer et al., 1999; Bauer and Brozoski, 2001; Heffner and Koay, 2005). Depending on the magnitude of sound intensity and exposure duration, sound exposure can cause a temporary threshold shift (TTS), followed by threshold recovery, or some degree of permanent threshold shift (PTS) (Attias and Pratt, 1986; Clark, 1991; Quaranta et al., 1998). In the present study, a 1-h exposure to an octave band noise centered at 17 kHz (116 dB SPL) was considered a moderate exposure (McFadden et al., 1998; Fraenkel et al., 2003). Immediately following exposure, the sound-exposed group showed a maximum threshold shift between 16 and 20 kHz, which matched the peak exposure intensity suggesting a limited frequency extent of temporary cochlear distress. Sixteen weeks following exposure, rat ABR thresholds showed recovery to values not different from the pre-exposure control group suggesting a TTS (Fig. 2). These findings are similar to previous studies in chinchillas (McFadden et al., 1998; Brozoski et al., 2002), Long Evans rats and Wistar rats (Bauer and Brozoski, 2001; Fraenkel et al., 2003). Although numerous studies suggest that ABR tests reflect inner hair cell (IHC) activity (Nordmann et al., 2000; Harding et al., 2005), the present findings do not exclude the possibility that sound-exposed rats have significant spiral ganglion cell loss and/or some amount of outer hair cell damage (Bauer et al., 2007; Kujawa and Liberman, 2008). Recent findings by Bauer et al. (2007) and Kujawa and Liberman (2008) suggest significant spiral ganglion cell loss in similarly sound-exposed animals with non-significant threshold shifts and minimal hair cell loss.
The different neurochemical subsets of sound-exposed rats were initially behaviorally tested two weeks following exposure using the gap detection method of Turner et al. (2006). The present study found that exposed rats showed poorer detection of a silent gap embedded within a 24 and 32 kHz background sound than did control animals, suggesting the presence of tinnitus near these frequencies (Fig. 3). Consistent with previous findings of chronic tinnitus in conditioned suppression-tested, similarly-exposed Long Evans rats (Bauer and Brozoski, 2001; Brozoski et al., 2002), gap detection deficits were present at 16 weeks following sound exposure. Collectively, these results support the notion that acute moderate sound exposure may induce chronic tinnitus in a subset of FBN rats.
Discordance between GlyR subunit message and protein changes following sound exposure
Significant exposure-related increase of GlyR α1 subunit message was evident over fusiform cells across all DCN frequency regions while GlyR α1 subunit protein levels were significantly decreased in middle and high frequency regions in sound-exposed animals. The decrease in α1 subunit protein was supported by the observed reduction in strychnine binding. The discordance between α1 subunit message and protein expression was similar to what has been reported in rat DCN aging studies, where α1 subunit message levels showed age-related increases while strychnine binding decreased (Wang et al., 2009; Caspary et al., 2002; Willott et al., 1997; Milbrandt et al., 1995). The mechanism for this dramatic mismatch between message and protein is unknown. A previous aging study by Banay-Schwartz et al (1989) implying an age-related reduction in the presynaptic glycine release could result in a compensatory upregulation of the transcription machinery. In turn, this would result in more α1 mRNA which cannot be translated into protein due to translational and or post-translational modifications. Decreased protein level not only correlate with synthesis but with posttranslational protein modifications and degradation (Rattan et al., 1992; Rattan, 1996; Ryazanov and Nefsky, 2002). There is significant evidence that activities associated with proteasome and lysosomal pathways of protein degradation are reduced within senescent cells (Cuervo and Dice, 2000; Friguet et al., 2000; Keller et al., 2000; Massey et al., 2006; Kiffin et al., 2007). A similar discordance has been observed following cochlear removal between Bcl-2 message and protein changes in nucleus magnocellularis (Bush and Hyson, 2008). Although cochlear removal is a more radical manipulation than sound exposure, observed changes suggest that reduction of peripheral auditory inputs could trigger altered regulation of transcriptional and/or translational processes in central brainstem.
Sound exposure induced GlyR composition and functional change in DCN
Partial peripheral auditory deafferentation appears to increase DCN fusiform cell spontaneous activity and this persistent neuronal hyperactivity has been suggested as an initiation mechanism for tinnitus (Zhang et al., 1998; Brozoski and Caspary, 2002). Previous studies demonstrated reductions in glycinergic inhibition in DCN following sound exposure, cochlear ablation or aging (Staatz-Benson et al., 1987; Milbrandt et al., 1995; Suneja et al., 1998b; Potashner et al., 2000; Caspary et al., 2001, 2006; Kaltenbach et al., 2004, 2005; Kaltenbach, 2006, 2007). The significant reduction of GlyR α1 and α3 subunit protein observed following sound exposure in the present study suggests a decreased number of heteromeric GlyRs or possible GlyR composition changes. These speculations were supported by the tinnitus-related significant loss of GlyR binding (decreased Bmax). Although Kd failed to reach significance, results were suggestive of an increase in receptor affinity over the fusiform cell layer of sound-exposed rats (Fig. 6A). Since receptor affinity is influenced by subunit composition, these data suggest that GlyR number and/or composition were altered in sound-exposed animals with behavioral evidence of tinnitus. Tinnitus-related binding results in the present study are similar to previous findings from rat and mouse DCN aging studies (Milbrandt and Caspary, 1995; Willott et al., 1995).
GlyR α2 protein levels were not significantly decreased following sound exposure, suggesting the presence of the remaining heteromeric membrane GlyRs containing relatively higher levels of α2 subunits (Fig. 5). The selective affinities of GlyR α1 and α2 for strychnine have not been examined. It is possible that α2 containing GlyRs could have a high affinity for strychnine, and since they would now comprise a relatively higher proportion or the total number of remaining constructs, that could possibly explain the relative low kd in DCN of sound exposed groups (Fig. 6). Immunocytochemical findings may reflect both assembled receptor subunits and non-assembled cytoplasmic subunits in the process of synthesis or degradation. High-affinity strychnine binding presumably labels fully assembled functional receptors (Betz and Laube, 2006). Specific changes in the properties of glycine transporters, postsynaptic membrane GlyRs, and their trafficking factors remain to be investigated.
Significance of intracellular receptor trafficking
Gephyrin binds both GlyR via the cytoplasmic loop of the β subunit and tubulin via a tubulin-binding domain to stabilize postsynaptic GlyRs on the membrane (Levi et al., 1998; Kneussel et al., 1999; Kneussel and Betz, 2000; Moss and Smart, 2001; Kneussel, 2005). The present study found a significant tinnitus-related increase in gephyrin message and protein levels following sound exposure in the high and middle frequency regions of DCN. Immunohistochemistry alone cannot accurately distinguish protein levels between/within synaptic and cytosolic compartments. Recent in vitro studies suggest gephyrin may not only anchor glycine membrane receptors but may be involved in intracellular transport of GlyRs (Maas et al., 2006; Kneussel and Loebrich, 2007; Fritschy et al., 2008). Gephyrin itself represents a transport adaptor that links vesicular GlyRs to anterograde or retrograde transport between synaptic sites and the intracellular domain. Moreover, the direction of movement may be activity dependent. External application of strychnine-induced intracellular retrograde movement of gephyrin suggests GlyRs may be predominately removed from membrane sites by blocking glycinergic inhibition (Maas et al., 2006). In the present study, it is tempting to speculate that sound exposure-induced reduction of glycinergic inhibition resulted in an upregulation of gephyrin retrograde intracellular receptor trafficking in exposed groups. This hypothesis will need further testing, including experiments which label endosomal and/or lysosomal markers.
Significance and relevance to tinnitus
Sound exposure is presumed to induce central plastic changes increasing spontaneous activity and reorganizing the neural activity of fusiform cells. Changes in the primary output neurons of the DCN may lead to altered spontaneous activity and bursting patterns at higher levels of the auditory neuraxis, including auditory cortex (Brozoski et al., 2002; Eggermont 2007). This is supported by studies in inferior colliculus (Bauer et al., 2008; Imig and Durham, 2005); auditory cortex (Komiya and Eggermont, 2000; Seki and Eggermont, 2003) and non-auditory structures including the locus coeruleus, lateral parabrachial nucleus, certain subregions of the hypothalamus, and amygdala (Wallhausser-Franke et al., 2003; Zhang et al., 2003; Mahlke and Wallhausser-Franke, 2004). Convergent neuronal activity in all these systems could collectively enhance development of tinnitus.
In conclusion, the present study indicated, that within weeks following a one-hour sound exposure, a subset of adult FBN rats developed high frequency chronic tinnitus without significant changes in their auditory thresholds. Subsequent neurochemical findings showed sound-exposure related postsynaptic GlyR subunit message and protein changes over fusiform cells, suggesting a net long-term reduction in inhibitory glycinergic synaptic transmission in the DCN. These findings are consistent with previous findings in chinchilla (Brozoski et al., 2002) and hamster (Kaltenbach et al., 1998, 2000a, b, 2004). These changes may reflect a compensatory net down-regulation of inhibitory function in the face of exposure-related partial peripheral auditory deafferentation. The present study establishes a direct connection between animals with psychophysical evidence of tinnitus and inhibitory glycinergic plastic changes in the DCN. Better understanding of plastic changes in the DCN of a tinnitus animal model could eventually lead to the development of selective pharmacotherapy for tinnitus treatment.
Table 2.
Exposure-related message and protein changes of GlyR subunits and gephyrin:
| mRNA | α1 | α2 | α3 | β | gephyrin | |
| HF | ↑ | ↓ | ↓ | NS | ↑ | |
| MF | ↑ | ↓ | ↓ | ↑ | ↑ | |
| LF | ↑ | ↓ | ↓ | ↑ | NS | |
| Protein | α1 | α2 | α3 | β | gephyrin | |
| HF | ↓ | NS | NS | X | ↑ | |
| MF | ↓ | NS | ↓ | X | ↑ | |
| LF | ↑ | NS | ↓ | X | NS |
Note: Arrow directions indicate significant up or down shift relative to control rats. HF: high frequency; MF: middle frequency; LF: low frequency. NS means non-significant change, X means no specific antibody.
Acknowledgments
The authors thank Patricia Jett, Jennifer Parrish and Judith Bryan for help in proofing/editing the manuscript. This research was supported by NIH grant DC008532 and American Tinnitus Association to DMC.
Abbreviations
- ABR
auditory brainstem response
- ANOVA
analysis of variance
- AVCN
anteroventral cochlear nucleus
- BBN
broadband noise
- CF
characteristic frequency
- CN
cochlear nucleus
- DAB
diaminobenzidine
- DAS
dorsal acoustic striae
- DCN
dorsal cochlear nucleus
- FBN
Fischer Brown Norway
- GlyR
glycine receptor
- GlyRs
glycine receptors
- HF
high frequency
- IAS
intermediate acoustic striae
- IC
inferior colliculus
- LF
low frequency
- MF
middle frequency
- NIDCD
National Institute on Deafness and Other Communication Disorders
- PBS
phosphate buffered saline
- PKC
protein kinase C
- RLF
rate-level function
- ROD
relative optical density
- SGNs
spiral ganglion neurons
- SIU
Southern Illinois University
- VCN
ventral cochlear nucleus
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Abbott SD, Hughes LF, Bauer CA, Salvi R, Caspary DM. Detection of glutamate decarboxylase isoforms in rat inferior colliculus following acoustic exposure. Neurosci. 1999;93(4):1375–1381. doi: 10.1016/s0306-4522(99)00300-0. [DOI] [PubMed] [Google Scholar]
- Ahmad N, Seidman M. Tinnitus in the older adult: epidemiology, pathophysiology and treatment options. Drugs Aging . 2004;21(5):297–305. doi: 10.2165/00002512-200421050-00002. Review. [DOI] [PubMed] [Google Scholar]
- Atherley GR, Hempstock TI, Noble WG. Study of tinnitus induced temporarily by noise. J Acoust Soc Am. 1968;44(6):1503–1506. doi: 10.1121/1.1911288. [DOI] [PubMed] [Google Scholar]
- Attias J, Pratt H. Follow-up of auditory-evoked potentials and temporary threshold shift in subjects developing noise-induced permanent hearing loss. Audiology. 1986;25(2):116–123. doi: 10.3109/00206098609078377. [DOI] [PubMed] [Google Scholar]
- Banay-Schwartz M, Lajtha A, Palkovits M. Changes with aging in the levels of amino acids in rat CNS structural elements. II. Taurine and small neutral amino acids. Neurochem Res Jun. 1989;14(6):563–70. doi: 10.1007/BF00964919. [DOI] [PubMed] [Google Scholar]
- Bauer CA, Brozoski TJ, Rojas R, Boley J, Wyder M. Behavioral model of chronic tinnitus in rats. Otolaryngol Head Neck Surg. 1999;121(4):457–462. doi: 10.1016/S0194-5998(99)70237-8. [DOI] [PubMed] [Google Scholar]
- Bauer CA, Brozoski TJ. Assessing tinnitus and prospective tinnitus therapeutics using a psychophysical animal model. J Assoc Res Otolaryngol. 2001;2(1):54–64. doi: 10.1007/s101620010030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bauer CA, Brozoski TJ, Myers K. Primary afferent dendrite degeneration as a cause of tinnitus. J Neurosci Res. 2007;85(7):1489–98. doi: 10.1002/jnr.21259. [DOI] [PubMed] [Google Scholar]
- Bauer CA, Turner JG, Caspary DM, Myers KS, Brozoski TJ. Tinnitus and inferior colliculus activity in chinchillas related to three distinct patterns of cochlear trauma. J Neurosci Res. 2008;86(11):2564–2578. doi: 10.1002/jnr.21699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Betz H, Kuhse J, Fischer M, Schmieden V, Laube B, Kuryatov A, Langosch D, Meyer G, Bormann J, Rundström N. Structure, diversity and synaptic localization of inhibitory glycine receptors. J Physiol Paris. 1994;88(4):243–248. doi: 10.1016/0928-4257(94)90087-6. Review. [DOI] [PubMed] [Google Scholar]
- Betz H, Laube B. Glycine receptors: recent insights into their structural organization and functional diversity. J Neurochem. 2006;97(6):1600–10. doi: 10.1111/j.1471-4159.2006.03908.x. Review. [DOI] [PubMed] [Google Scholar]
- Brozoski TJ, Bauer CA, Caspary DM. Elevated fusiform cell activity in the dorsal cochlear nucleus of chinchillas with psychophysical evidence of tinnitus. J Neurosci. 2002;22(6):2383–2390. doi: 10.1523/JNEUROSCI.22-06-02383.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brozoski TJ, Bauer CA. The effect of dorsal cochlear nucleus ablation on tinnitus in rats. Hear Res. 2005;206(1–2):227–236. doi: 10.1016/j.heares.2004.12.013. [DOI] [PubMed] [Google Scholar]
- Brozoski TJ, Ciobanu L, Bauer CA. Central neural activity in rats with tinnitus evaluated with manganese-enhanced magnetic resonance imaging (MEMRI) Hear Res. 2007;228(1–2):168–179. doi: 10.1016/j.heares.2007.02.003. [DOI] [PubMed] [Google Scholar]
- Bush AL, Hyson RL. Effects of lithium and deafferentation on expression of glycogen synthase kinase-3beta, NFkappaB, beta-catenin and pCreb in the chick cochlear nucleus. Brain Res. 2008;1203:18–25. doi: 10.1016/j.brainres.2008.01.076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Caspary DM, Havey DC, Faingold CL. Effects of microiontophoretically applied glycine and GABA on neuronal response patterns in the cochlear nuclei. Brain Res. 1979;172(1):179–185. doi: 10.1016/0006-8993(79)90909-0. [DOI] [PubMed] [Google Scholar]
- Caspary DM, Pazara KE, Kössl M, Faingold CL. Strychnine alters the fusiform cell output from the dorsal cochlear nucleus. Brain Res. 1987;417(2):273–282. doi: 10.1016/0006-8993(87)90452-5. [DOI] [PubMed] [Google Scholar]
- Caspary DM, Salvi RJ, Helfert RH, Brozoski TJ, Bauer CA. Neuropharmacology of noise induced hearing loss in brainstem auditory structures. In: Henderson D, Prasher D, Kopke R, Salvi RJ, Hamernik R, editors. Noise induced hearing loss: mechanisms of damage and means of prevention. London: NRN; 2002. pp. 169–186. [Google Scholar]
- Caspary DM, Hughes LF, Schatteman TA, Turner JG. Age-related changes in the response properties of cartwheel cells in rat dorsal cochlear nucleus. Hear Res. 2006 Jun;216–217:207–215. doi: 10.1016/j.heares.2006.03.005. [DOI] [PubMed] [Google Scholar]
- Chung JH, Des Roches CM, Meunier J, Eavey RD. Evaluation of noise-induced hearing loss in young people using a web-based survey technique. Pediatrics. 2005;115(4):861–867. doi: 10.1542/peds.2004-0173. [DOI] [PubMed] [Google Scholar]
- Clark WW. Recent studies of temporary threshold shift (TTS) and permanent threshold shift (PTS) in animals. J Acoust Soc Am. 1991;90(1):155–163. doi: 10.1121/1.401309. Review. [DOI] [PubMed] [Google Scholar]
- Cuervo AM, Dice JF. When lysosomes get old. Exp Gerontol. 2000 Mar;35(2):119–31. doi: 10.1016/s0531-5565(00)00075-9. Review. [DOI] [PubMed] [Google Scholar]
- Dobie RA. Depression and tinnitus. Otolaryngol Clin North Am. 2003;36(2):383–388. doi: 10.1016/s0030-6665(02)00168-8. Review. [DOI] [PubMed] [Google Scholar]
- Eggermont JJ. Pathophysiology of tinnitus. Prog Brain Res. 2007;166:19–35. doi: 10.1016/S0079-6123(07)66002-6. Review. [DOI] [PubMed] [Google Scholar]
- Eggermont JJ, Roberts LE. The neuroscience of tinnitus. Trends Neurosci . 2004;27(11):676–682. doi: 10.1016/j.tins.2004.08.010. Review. [DOI] [PubMed] [Google Scholar]
- Finlayson PG, Caspary DM. Synaptic potentials of chinchilla lateral superior olivary neurons. Hear Res. 1989;38(3):221–228. doi: 10.1016/0378-5955(89)90067-1. [DOI] [PubMed] [Google Scholar]
- Fraenkel R, Freeman S, Sohmer H. Susceptibility of young adult and old rats to noise-induced hearing loss. Audiol Neurootol. 2003;8(3):129–139. doi: 10.1159/000069476. [DOI] [PubMed] [Google Scholar]
- Friauf E. Tonotopic Order in the Adult and Developing Auditory System of the Rat as Shown by c-fos Immunocytochemistry. Eur J Neurosci. 1992;4(9):798–812. doi: 10.1111/j.1460-9568.1992.tb00190.x. [DOI] [PubMed] [Google Scholar]
- Friguet B, Bulteau AL, Chondrogianni N, Conconi M, Petropoulos I. Protein degradation by the proteasome and its implications in aging. Ann N Y Acad Sci. 2000 Jun;908:143–54. doi: 10.1111/j.1749-6632.2000.tb06643.x. Review. [DOI] [PubMed] [Google Scholar]
- Fritschy JM, Harvey RJ, Schwarz G. Gephyrin: where do we stand, where do we go? Trends Neurosci. 2008;31(5):257–264. doi: 10.1016/j.tins.2008.02.006. [DOI] [PubMed] [Google Scholar]
- Frostholm A, Rotter A. Glycine receptor distribution in mouse CNS: autoradiographic localization of [3H]strychnine binding sites. Brain Res Bull. 1985;15(5):473–486. doi: 10.1016/0361-9230(85)90038-3. [DOI] [PubMed] [Google Scholar]
- Grudzinska J, Schemm R, Haeger S, Nicke A, Schmalzing G, Betz H, Laube B. The beta subunit determines the ligand binding properties of synaptic glycine receptors. Neuron. 2005;45(5):727–739. doi: 10.1016/j.neuron.2005.01.028. [DOI] [PubMed] [Google Scholar]
- Harding GW, Bohne BA, Vos JD. The effect of an age-related hearing loss gene (Ahl) on noise-induced hearing loss and cochlear damage from low-frequency noise. Hear Res. 2005;204(1–2):90–100. doi: 10.1016/j.heares.2005.01.004. [DOI] [PubMed] [Google Scholar]
- Harrop-Griffiths J, Katon W, Dobie R. Chronic tinnitus: association with psychiatric diagnosis. J Psychosom Res. 1987;31:613–621. doi: 10.1016/0022-3999(87)90040-7. [DOI] [PubMed] [Google Scholar]
- Heffner HE, Koay G. Tinnitus and hearing loss in hamsters (Mesocricetus auratus) exposed to loud sound. Behav Neurosci. 2005;119(3):734–742. doi: 10.1037/0735-7044.119.3.734. [DOI] [PubMed] [Google Scholar]
- Holt AG, Asako M, Lomax CA, MacDonald JW, Tong L, Lomax MI, Altschuler RA. Deafness-related plasticity in the inferior colliculus: gene expression profiling following removal of peripheral activity. J Neurochem. 2005;93(5):1069–1086. doi: 10.1111/j.1471-4159.2005.03090.x. [DOI] [PubMed] [Google Scholar]
- Imig TJ, Durham D. Effect of unilateral noise exposure on the tonotopic distribution of spontaneous activity in the cochlear nucleus and inferior colliculus in the cortically intact and decorticate rat. J Comp Neurol. 2005;490(4):391–413. doi: 10.1002/cne.20674. [DOI] [PubMed] [Google Scholar]
- Jastreboff PJ. Phantom auditory perception (tinnitus): mechanisms of generation and perception. Neurosci Res. 1990;8:221–254. doi: 10.1016/0168-0102(90)90031-9. [DOI] [PubMed] [Google Scholar]
- Kaltenbach JA, Afman CE. Hyperactivity in the dorsal cochlear nucleus after intense sound exposure and its resemblance to tone-evoked activity: a physiological model for tinnitus. Hear Res. 2000a;140(1–2):165–172. doi: 10.1016/s0378-5955(99)00197-5. [DOI] [PubMed] [Google Scholar]
- Kaltenbach JA, Zhang J, Afman CE. Plasticity of spontaneous neural activity in the dorsal cochlear nucleus after intense sound exposure. Hear Res. 2000b;147(1–2):282–292. doi: 10.1016/s0378-5955(00)00138-6. [DOI] [PubMed] [Google Scholar]
- Kaltenbach JA, Zacharek MA, Zhang J, Frederick S. Activity in the dorsal cochlear nucleus of hamsters previously tested for tinnitus following intense tone exposure. Neurosci Lett. 2004;355(1–2):121–125. doi: 10.1016/j.neulet.2003.10.038. [DOI] [PubMed] [Google Scholar]
- Kaltenbach JA, Zhang J, Finlayson P. Tinnitus as a plastic phenomenon and its possible neural underpinnings in the dorsal cochlear nucleus. Hear Res. 2005;206(1–2):200–226. doi: 10.1016/j.heares.2005.02.013. Review. [DOI] [PubMed] [Google Scholar]
- Kaltenbach JA. The dorsal cochlear nucleus as a participant in the auditory, attentional and emotional components of tinnitus. Hear Res. 2006;216–217:224–234. doi: 10.1016/j.heares.2006.01.002. [DOI] [PubMed] [Google Scholar]
- Kaltenbach JA. The dorsal cochlear nucleus as a contributor to tinnitus: mechanisms underlying the induction of hyperactivity. Prog Brain Res. 2007;166:89–106. doi: 10.1016/S0079-6123(07)66009-9. Review. [DOI] [PubMed] [Google Scholar]
- Keller JN, Huang FF, Markesbery WR. Decreased levels of proteasome activity and proteasome expression in aging spinal cord. Neuroscience. 2000;98(1):149–56. doi: 10.1016/s0306-4522(00)00067-1. [DOI] [PubMed] [Google Scholar]
- Kiffin R, Kaushik S, Zeng M, Bandyopadhyay U, Zhang C, Massey AC, Martinez-Vicente M, Cuervo AM. Altered dynamics of the lysosomal receptor for chaperone-mediated autophagy with age. J Cell Sci. 2007 Mar 1;120(Pt 5):782–91. doi: 10.1242/jcs.001073. Epub Feb 6. [DOI] [PubMed] [Google Scholar]
- Kirsch J. Glycinergic transmission. Cell Tissue Res. 2006;326(2):535–40. doi: 10.1007/s00441-006-0261-x. Review. [DOI] [PubMed] [Google Scholar]
- Kneussel M, Hermann A, Kirsch J, Betz H. Hydrophobic interactions mediate binding of the glycine receptor beta-subunit to gephyrin. J Neurochem. 1999;72(3):1323–1326. doi: 10.1046/j.1471-4159.1999.0721323.x. [DOI] [PubMed] [Google Scholar]
- Kneussel M. Postsynaptic scaffold proteins at non-synaptic sites. The role of postsynaptic scaffold proteins in motor-protein-receptor complexes. EMBO Rep. 2005;6(1):22–27. doi: 10.1038/sj.embor.7400319. Review. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kneussel M, Loebrich S. Trafficking and synaptic anchoring of ionotropic inhibitory neurotransmitter receptors. Biol Cell. 2007;99(6):297–309. doi: 10.1042/BC20060120. Review. [DOI] [PubMed] [Google Scholar]
- Kneussel M. Dynamic stabilization: Structural plasticity at inhibitory postsynaptic sites. Traffic. 2006;7(12):1604–1606. doi: 10.1111/j.1600-0854.2006.00501.x. [DOI] [PubMed] [Google Scholar]
- Kneussel M, Betz H. Clustering of inhibitory neurotransmitter receptors at developing postsynaptic sites: the membrane activation model. Trends Neurosci. 2000;23(9):429–435. doi: 10.1016/s0166-2236(00)01627-1. Review. [DOI] [PubMed] [Google Scholar]
- Komiya H, Eggermont JJ. Spontaneous firing activity of cortical neurons in adult cats with reorganized tonotopic map following pure-tone trauma. Acta Otolaryngol. 2000;120(6):750–756. doi: 10.1080/000164800750000298. [DOI] [PubMed] [Google Scholar]
- Krenning J, Hughes LF, Caspary DM, Helfert RH. Age-related glycine receptor subunit changes in the cochlear nucleus of Fischer-344 rats. Laryngoscope. 1998;108(1 Pt 1):26–31. doi: 10.1097/00005537-199801000-00005. [DOI] [PubMed] [Google Scholar]
- Kujawa SG, Liberman MC. Noise-Induced Primary Neuronal Degeneration in Ears with Complete Threshold Recovery. Assoc Res Otolaryngol. 2008;31:251. [Google Scholar]
- Langosch D, Becker CM, Betz H. The inhibitory glycine receptor: a ligand-gated chloride channel of the central nervous system. Eur J Biochem. 1990;194(1):1–8. doi: 10.1111/j.1432-1033.1990.tb19419.x. Review. [DOI] [PubMed] [Google Scholar]
- Legendre P. The glycinergic inhibitory synapse. Cell Mol Life Sci. 2001;58(5–6):760–93. doi: 10.1007/PL00000899. Review. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lévi S, Vannier C, Triller A. Strychnine-sensitive stabilization of postsynaptic glycine receptor clusters. J Cell Sci. 1998;111(Pt 3):335–345. doi: 10.1242/jcs.111.3.335. [DOI] [PubMed] [Google Scholar]
- Ling LL, Hughes LF, Caspary DM. Age-related loss of the GABA synthetic enzyme glutamic acid decarboxylase in rat primary auditory cortex. Neurosci. 2005;132(4):1103–1113. doi: 10.1016/j.neuroscience.2004.12.043. [DOI] [PubMed] [Google Scholar]
- Lynch JW. Molecular structure and function of the glycine receptor chloride channel. Physiol Rev. 2004;84(4):1051–1095. doi: 10.1152/physrev.00042.2003. Review. [DOI] [PubMed] [Google Scholar]
- Maas C, Tagnaouti N, Loebrich S, Behrend B, Lappe-Siefke C, Kneussel M. Neuronal cotransport of glycine receptor and the scaffold protein gephyrin. J Cell Biol. 2006;172(3):441–451. doi: 10.1083/jcb.200506066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mahlke C, Wallhäusser-Franke E. Evidence for tinnitus-related plasticity in the auditory and limbic system, demonstrated by arg3.1 and c-fos immunocytochemistry. Hear Res. 2004;195(1–2):17–34. doi: 10.1016/j.heares.2004.03.005. [DOI] [PubMed] [Google Scholar]
- Marciano E, Carrabba L, Giannini P. Psychiatric comorbidity in a population of outpatients affected by tinnitus. Int J Audiol. 2003;42:4–9. doi: 10.3109/14992020309056079. [DOI] [PubMed] [Google Scholar]
- Mascia MP, Mihic SJ, Valenzuela CF, Schofield PR, Harris RA. A single amino acid determines differences in ethanol actions on strychnine-sensitive glycine receptors. Mol Pharmacol. 1996;50:402–406. [PubMed] [Google Scholar]
- Massey AC, Kiffin R, Cuervo AM. Autophagic defects in aging: looking for an “emergency exit”? Cell Cycle. 2006 Jun;5(12):1292–6. doi: 10.4161/cc.5.12.2865. Epub Jun 15. [DOI] [PubMed] [Google Scholar]
- McFadden SL, Campo P, Ding D, Quaranta N. Effects of noise on inferior colliculus evoked potentials and cochlear anatomy in young and aged chinchillas. Hear Res. 1998;117(1–2):81–96. doi: 10.1016/s0378-5955(98)00013-6. [DOI] [PubMed] [Google Scholar]
- Meier J, Meunier-Durmort C, Forest C, Triller A, Vannier C. Formation of glycine receptor clusters and their accumulation at synapses. J Cell Sci. 2000;113(Pt 15):2783–2795. doi: 10.1242/jcs.113.15.2783. [DOI] [PubMed] [Google Scholar]
- Meier J, Vannier C, Sergé A, Triller A, Choquet D. Fast and reversible trapping of surface glycine receptors by gephyrin. Nat Neurosci. 2001;4(3):253–260. doi: 10.1038/85099. [DOI] [PubMed] [Google Scholar]
- Milbrandt JC, Caspary DM. Age-related reduction of [3H]strychnine binding sites in the cochlear nucleus of the Fischer 344 rat. Neurosci. 1995;67(3):713–719. doi: 10.1016/0306-4522(95)00082-t. [DOI] [PubMed] [Google Scholar]
- Milbrandt JC, Hunter C, Caspary DM. Alterations of GABAA receptor subunit mRNA levels in the aging Fischer 344 rat inferior colliculus. J Comp Neurol. 1997;379(3):455–465. doi: 10.1002/(sici)1096-9861(19970317)379:3<455::aid-cne10>3.0.co;2-f. [DOI] [PubMed] [Google Scholar]
- Moore MJ, Caspary DM. Strychnine blocks binaural inhibition in lateral superior olivary neurons. J Neurosci. 1983;3(1):237–242. doi: 10.1523/JNEUROSCI.03-01-00237.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moss SJ, Smart TG. Constructing inhibitory synapses. Nat Rev Neurosci. 2001;2(4):240–250. doi: 10.1038/35067500. Review. [DOI] [PubMed] [Google Scholar]
- Nordmann AS, Bohne BA, Harding GW. Histopathological differences between temporary and permanent threshold shift. Hear Res. 2000;139(1–2):13–30. doi: 10.1016/s0378-5955(99)00163-x. [DOI] [PubMed] [Google Scholar]
- Nottet JB, Moulin A, Brossard N, Suc B, Job A. Otoacoustic emissions and persistent tinnitus after acute acoustic trauma. Laryngoscope. 2006;116(6):970–975. doi: 10.1097/01.MLG.0000216823.77995.13. [DOI] [PubMed] [Google Scholar]
- Potashner SJ, Suneja SK, Benson CG. Altered glycinergic synaptic activities in guinea pig brain stem auditory nuclei after unilateral cochlear ablation. Hear Res. 2000;147(1–2):125–136. doi: 10.1016/s0378-5955(00)00126-x. [DOI] [PubMed] [Google Scholar]
- Quaranta A, Portalatini P, Henderson D. Temporary and permanent threshold shift: an overview. Scand Audiol Suppl. 1998;48:75–86. Review. [PubMed] [Google Scholar]
- Rattan SI, Derventzi A, Clark BF. Protein synthesis, posttranslational modifications, and aging. Ann N Y Acad Sci. 1992 Nov 21;663:48–62. doi: 10.1111/j.1749-6632.1992.tb38648.x. Review. [DOI] [PubMed] [Google Scholar]
- Rattan SI. Synthesis, modifications, and turnover of proteins during aging. Exp Gerontol. 1996 Jan-Apr;31(1–2):33–47. doi: 10.1016/0531-5565(95)02022-5. Review. [DOI] [PubMed] [Google Scholar]
- Rouiller EM, Wan XS, Moret V, Liang F. Mapping of c-fos expression elicited by pure tones stimulation in the auditory pathways of the rat, with emphasis on the cochlear nucleus. Neurosci Lett. 1992;144(1–2):19–24. doi: 10.1016/0304-3940(92)90706-d. [DOI] [PubMed] [Google Scholar]
- Ryan AF, Furlow Z, Woolf NK, Keithley EM. The spatial representation of frequency in the rat dorsal cochlear nucleus and inferior colliculus. Hear Res. 1988;36(2–3):181–189. doi: 10.1016/0378-5955(88)90060-3. [DOI] [PubMed] [Google Scholar]
- Ryazanov AG, Nefsky BS. Protein turnover plays a key role in aging. Mech Ageing Dev. 2002 Jan;123(2–3):207–13. doi: 10.1016/s0047-6374(01)00337-2. Review. [DOI] [PubMed] [Google Scholar]
- Saint Marie RL, Benson CG, Ostapoff EM, Morest DK. Glycine immunoreactive projections from the dorsal to the anteroventral cochlear nucleus. Hear Res. 1991;51(1):11–28. doi: 10.1016/0378-5955(91)90003-r. [DOI] [PubMed] [Google Scholar]
- Saint Marie RL, Luo L, Ryan AF. Spatial representation of frequency in the rat dorsal nucleus of the lateral lemniscus as revealed by acoustically induced c-fos mRNA expression. Hear Res. 1999;128(1–2):70–74. doi: 10.1016/s0378-5955(98)00188-9. [DOI] [PubMed] [Google Scholar]
- Sanchez TG. Reabilitação do paciente com zumbido. In: Tratado de Otorrinolaringologia. Campos CA, Costa HO, editors. Vol. 2. São Paulo: Roca; 2002. pp. 311–324. [Google Scholar]
- Sanchez TG, Medeiros IR, Levy CP, Ramalho Jda R, Bento RF. Tinnitus in normally hearing patients: clinical aspects and repercussions. Rev Bras Otorrinolaringol (Engl Ed) 2005;71(4):427–431. doi: 10.1016/S1808-8694(15)31194-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sato K, Kiyama H, Tohyama M. Regional distribution of cells expressing glycine receptor alpha 2 subunit mRNA in the rat brain. Brain Res. 1992;590(1–2):95–108. doi: 10.1016/0006-8993(92)91085-s. [DOI] [PubMed] [Google Scholar]
- Seidman MD, Jacobson GP. Update on tinnitus. Otolaryngol Clin North Am. 1996;29:455–465. [PubMed] [Google Scholar]
- Seki S, Eggermont JJ. Changes in spontaneous firing rate and neural synchrony in cat primary auditory cortex after localized tone-induced hearing loss. Hear Res. 2003;180(1–2):28–38. doi: 10.1016/s0378-5955(03)00074-1. [DOI] [PubMed] [Google Scholar]
- Smith PA, Moran TD, Abdulla F, Tumber KK, Taylor BK. Spinal mechanisms of NPY analgesia. Peptides. 2007;28(2):464–474. doi: 10.1016/j.peptides.2006.09.029. Review. [DOI] [PubMed] [Google Scholar]
- Staatz-Benson C, Potashner SJ. Uptake and release of glycine in the guinea pig cochlear nucleus. J Neurochem. 1987;49(1):128–137. doi: 10.1111/j.1471-4159.1987.tb03404.x. [DOI] [PubMed] [Google Scholar]
- Sullivan MD, Katon W, Dobie R. Disabling tinnitus. Association with affective disorder. Gen Hosp Psychiatry. 1988;10:285–291. doi: 10.1016/0163-8343(88)90037-0. [DOI] [PubMed] [Google Scholar]
- Suneja SK, Potashner SJ, Benson CG. Plastic changes in glycine and GABA release and uptake in adult brain stem auditory nuclei after unilateral middle ear ossicle removal and cochlear ablation. Exp Neurol. 1998a;151(2):273–288. doi: 10.1006/exnr.1998.6812. [DOI] [PubMed] [Google Scholar]
- Suneja SK, Benson CG, Potashner SJ. Glycine receptors in adult guinea pig brain stem auditory nuclei: regulation after unilateral cochlear ablation. Exp Neurol. 1998b;154(2):473–88. doi: 10.1006/exnr.1998.6946. [DOI] [PubMed] [Google Scholar]
- Takahashi T, Momiyama A, Hirai K, Hishinuma F, Akagi H. Functional correlation of fetal and adult forms of glycine receptors with developmental changes in inhibitory synaptic receptor channels. Neuron. 1992;9(6):1155–1156. doi: 10.1016/0896-6273(92)90073-m. [DOI] [PubMed] [Google Scholar]
- Takahashi T, Momiyama A. Glycine-gated Cl- channels underlying synaptic currents. Jpn J Physiol. 1994;44(Suppl 2):S97–99. [PubMed] [Google Scholar]
- Turner JG, Brozoski TJ, Bauer CA, Parrish JL, Myers K, Hughes LF, Caspary DM. Gap detection deficits in rats with tinnitus: a potential novel screening tool. Behav Neurosci. 2006;120(1):188–195. doi: 10.1037/0735-7044.120.1.188. [DOI] [PubMed] [Google Scholar]
- Wallhäusser-Franke E, Mahlke C, Oliva R, Braun S, Wenz G, Langner G. Expression of c-fos in auditory and non-auditory brain regions of the gerbil after manipulations that induce tinnitus. Exp Brain Res. 2003;153(4):649–654. doi: 10.1007/s00221-003-1614-2. [DOI] [PubMed] [Google Scholar]
- Wang H, Turner JG, Ling L, Parrish JL, Hughes LF, Caspary DM. Age-related changes in glycine receptor subunit composition and binding in dorsal cochlear nucleus. Neurosci. 2009;160:227–239. doi: 10.1016/j.neuroscience.2009.01.079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Webb TI, Lynch JW. Molecular pharmacology of the glycine receptor chloride channel. Curr Pharm Des. 2007;13(23):2350–2367. doi: 10.2174/138161207781368693. Review. [DOI] [PubMed] [Google Scholar]
- Widén SE, Erlandsson SI. Self-reported tinnitus and noise sensitivity among adolescents in Sweden. Noise Health. 2004;7(25):29–40. [PubMed] [Google Scholar]
- Willott JF, Milbrandt JC, Bross LS, Caspary DM. Glycine immunoreactivity and receptor binding in the cochlear nucleus of C57BL/6J and CBA/CaJ mice: effects of cochlear impairment and aging. J Comp Neurol. 1997;385(3):405–414. [PubMed] [Google Scholar]
- Yajima Y, Hayashi Y. Response properties and tonotopical organization in the dorsal cochlear nucleus in rats. Exp Brain Res. 1989;75(2):381–389. doi: 10.1007/BF00247945. [DOI] [PubMed] [Google Scholar]
- Zhang JS, Kaltenbach JA. Increases in spontaneous activity in the dorsal cochlear nucleus of the rat following exposure to high-intensity sound. Neurosci Lett J. 1998;250(3):197–200. doi: 10.1016/s0304-3940(98)00482-0. Erratum in: Neurosci Lett. 252(2):668. [DOI] [PubMed] [Google Scholar]
- Zhang JS, Kaltenbach JA, Wang J, Kim SA. Fos-like immunoreactivity in auditory and nonauditory brain structures of hamsters previously exposed to intense sound. Exp Brain Res. 2003;153(4):655–660. doi: 10.1007/s00221-003-1612-4. [DOI] [PubMed] [Google Scholar]
- Zöger S, Svedlund J, Holgers KM. Psychiatric disorders in tinnitus patients without severe hearing impairment: 24 months follow-up of patients at an audiological clinic. Audiology. 2001;40:133–140. doi: 10.3109/00206090109073108. [DOI] [PubMed] [Google Scholar]
- Zöger S, Svedlund J, Holgers KM. Relationship between tinnitus severity and psychiatric disorders. Psychosomatics. 2006;47(4):282–288. doi: 10.1176/appi.psy.47.4.282. [DOI] [PubMed] [Google Scholar]