The prospect of applying differentiation therapies to the treatment of acute myeloid leukemias (AMLs) is highlighted by the use of all-trans retinoic acid (ATRA) in acute promyelocytic leukemia (APL). Treatment with ATRA in combination with chemotherapy or arsenic trioxide results in cure in the majority of patients with either newly diagnosed or recurrent APL.1 The effectiveness of ATRA is because of the induction of differentiation in the APL leukemic blasts and their conversion to a nonproliferative state. However, ATRA is largely ineffective at inducing differentiation in non-APL subtypes of AML, and differentiation therapies for these other subtypes have not been developed. Therefore, it is important to identify the cellular signaling pathways that are aberrantly activated and act to inhibit myeloid differentiation in AML cells.
Previous studies have shown that Src family kinases (SFKs) are overexpressed and/or hyperactivated in AML cell lines and primary cells. SFKs are non-receptor tyrosine kinases that have been implicated in a variety of physiological process, including proliferation, survival, differentiation and migration. In all nine different SFKs have been identified, although the expression in myeloid lineage cells appears more restricted to the SFKs Lyn, Fgr, Hck and c-Src.2 To determine the role of SFKs in regulating myeloid differentiation, we previously employed the pan-SFK inhibitors PP1 and PP2. Pharmacological inhibition of SFKs using PP1 or PP2 was found to markedly enhance ATRA-induced myeloid differentiation of AML cell lines, as well as primary cells from a patient with non-APL AML.3 These findings showed that SFKs act as negative regulators of ATRA-induced myeloid differentiation.
To understand the mechanism(s) whereby SFKs negatively regulate myeloid differentiation, in this report we sought to determine the target genes that are regulated by SFKs in myeloid lineage cells. To accomplish this, NB4 cells were treated for 10 h with vehicle alone, 0.01 µm ATRA alone, 10 µm PP2 alone or ATRA plus PP2, followed by microarray analysis using Affymetrix GeneChip Human Genome U133A 2.0 Array. Table 1 depicts the number of genes that were upregulated or downregulated by twofold or greater when comparing the following groups: (a) ATRA versus vehicle, (b) PP2 versus vehicle, (c) ATRA plus PP2 versus vehicle, (d) ATRA plus PP2 versus ATRA and (e) ATRA plus PP2 versus PP2. Supplementary Tables 1–10 list the top 25 upregulated and top 25 downregulated genes from each of these comparisons. Comparison of gene expression patterns in the ATRA versus vehicle groups revealed a cohort of ATRA regulated genes that corresponded closely with those previously reported by Yang et al.4 in ATRA-treated NB4 cells. This served to verify the accuracy of our microarray analysis. Moreover, we observed close correspondence between the ATRA-regulated genes identified when comparing the ATRA versus vehicle groups with those identified by comparing the ATRA plus PP2 versus PP2 groups (compare Supplementary Tables 1 and 9, and Supplementary Tables 2 and 10).
Table 1.
Number of upregulated and downregulated genes when comparing different treatment groups
| Sample Comparisons |
Genes upregulated (≥2-fold) |
Genes downregulated (≥2-fold) |
|---|---|---|
| ATRA vs Vehicle | 508 | 293 |
| PP2 vs Vehicle | 186 | 78 |
| ATRA+PP2 vs Vehicle |
676 | 414 |
| ATRA+PP2 vs ATRA |
206 | 109 |
| ATRA+PP2 vs PP2 |
508 | 278 |
Abbreviation: ATRA, all-trans retinoic acid.
NB4 cells were treated for 10 h with vehicle alone, 0.01 µM ATRA alone, 10 µM PP2 alone or ATRA plus PP2, followed by microarray analysis as described for Supplementary Table 1–10. The table depicts the number of genes that were up- or downregulated by twofold or greater when comparing treatment groups.
Of particular interest were those comparisons that allowed us to identify SFK gene targets. SFK gene targets were identified by comparing the PP2 versus vehicle groups, as well as the ATRA plus PP2 versus ATRA groups. Comparison of PP2 versus vehicle groups identified 186 genes that were upregulated (≥2-fold) in response to SFK inhibition and 78 that were downregulated (≥2-fold) (Table 1). Highly similar numbers of upregulated and downregulated genes were detected when comparing ATRA plus PP2 versus ATRA (206 and 109, respectively). The identities of SFK target genes identified in these two different comparisons were also similar (compare Supplementary Tables 3 and 7, and Supplementary Tables 4 and 8). Among the genes that were found to be dramatically upregulated following SFK inhibition (Supplementary Tables 3 and 7) were several encoding cytokines (IL-8, IL-1β and IL-16), chemokines (CXCL10, CCL4, CCL7 and CCL8) or proteins involved in cytokine or TNF signaling (IL-18 receptor accessory protein, IL-18 receptor 1, TNF alpha-induced protein 6 and IL-10 receptor alpha). SFK inhibition also led to marked upregulation of the genes encoding the adhesion proteins thrombospondin 1 and inter-cellular adhesion molecule-1 (ICAM-1). As these genes were upregulated following SFK inhibition, they represent genes that are negatively regulated by SFKs.
In addition to the genes mentioned above, another gene that was found to be negatively regulated by SFKs was the gene encoding early growth response-1 (Egr-1). An 8.5-fold upregulation of Egr-1 mRNA was observed when comparing the PP2 versus vehicle groups (Supplementary Table 3), and a 13.1-fold upregulation was seen when comparing the ATRA plus PP2 versus ATRA groups (Supplementary Table 7). Egr-1 is a zinc finger transcription factor that has previously been implicated as playing a key role in myeloid differentiation. Expression of Egr-1 is induced during monocytic differentiation, and ectopic expression of Egr-1 promotes monocytic differentiation of HL-60 and M1 cells in the absence of a differentiation stimulus.5–7 Further, ectopic expression of Egr-1 in M1 cells overcomes the blockade to differentiation imposed by c-Myc and E2F-1.8 Our observation that SFKs negatively regulate the Egr-1 gene suggests a mechanism whereby hyperactivated or overexpressed SFKs may act to inhibit the differentiation of AML cells.
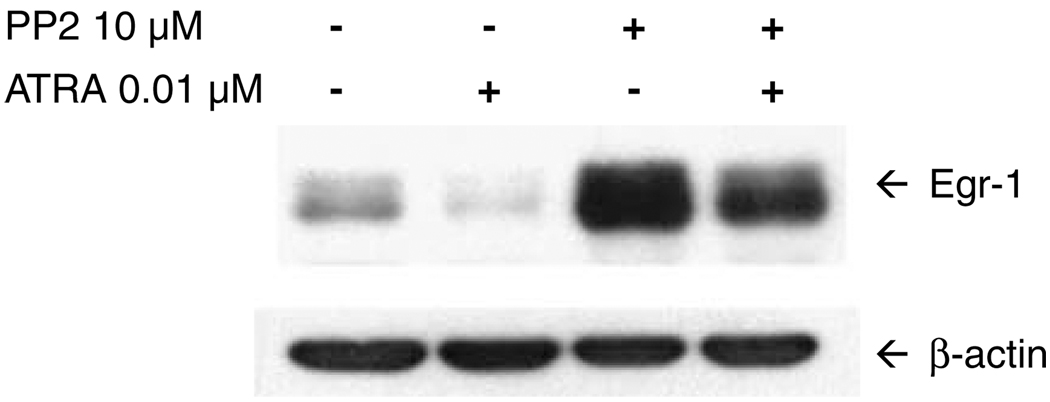
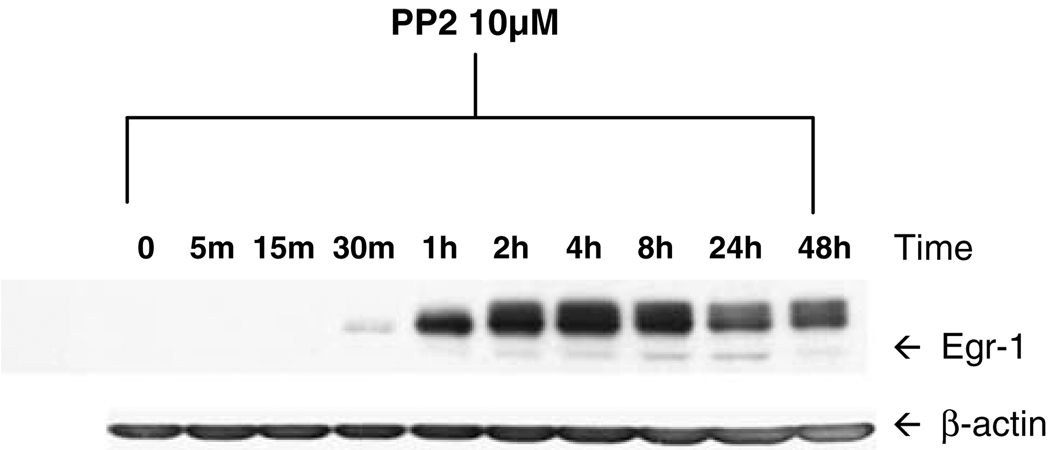
To verify that SFKs negatively regulate Egr-1 expression, we performed immunoblotting experiments following inhibition of the SFK enzymes (Figure 1). NB4 cells were left untreated, or were treated for 2 h with ATRA alone, PP2 alone or ATRA plus PP2. In the absence of treatment, low levels of Egr-1 protein were detected. Interestingly, treatment with ATRA alone resulted in reduction in Egr-1 levels, consistent with a previous report that Egr-1 is associated with monocytic, but not granulocytic differentiation.6,7 By contrast, treatment with PP2 alone resulted in dramatic upregulation of Egr-1, supporting our contention that the Egr-1 gene is a negatively regulated target of SFKs. In Figure 2 we performed an immunoblotting time course of cells treated with PP2 alone. Induction of Egr-1 protein was detected as soon as 30–60 min after the addition of SFK inhibitor. Expression of Egr-1 peaked at around 4 h, but was maintained at above-basal levels at 48 h and beyond.
Figure 1.
Inhibition of Src family kinases (SFKs) results in Egr-1 induction. NB4 cells were treated for 2 h with vehicle alone, 0.01 µm all-trans retinoic acid (ATRA) alone, 10 µm PP2 alone, or ATRA plus PP2. Following treatment, whole-cell lysates were prepared and proteins (20 µg per lane) were electrophoresed on a 10% SDS-PAGE gel, transferred to nitrocellulose, and probed with anti-Egr-1 monoclonal antibody (Cell Signaling Technology). The filter was stripped and reprobed with anti-β-actin to demonstrate equal protein loading. The experiment was performed three times, with similar results each time.
Figure 2.
Time course of Egr-1 induction following Src family kinase (SFK) inhibition. NB4 cells were left untreated for 48 h, or were treated for varying lengths of time with 10 µm PP2 to inhibit SFKs. Following treatment, whole-cell lysates were subjected to immunoblotting for Egr-1 or β-actin as described for Figure 1. The experiment was performed twice, with similar results.
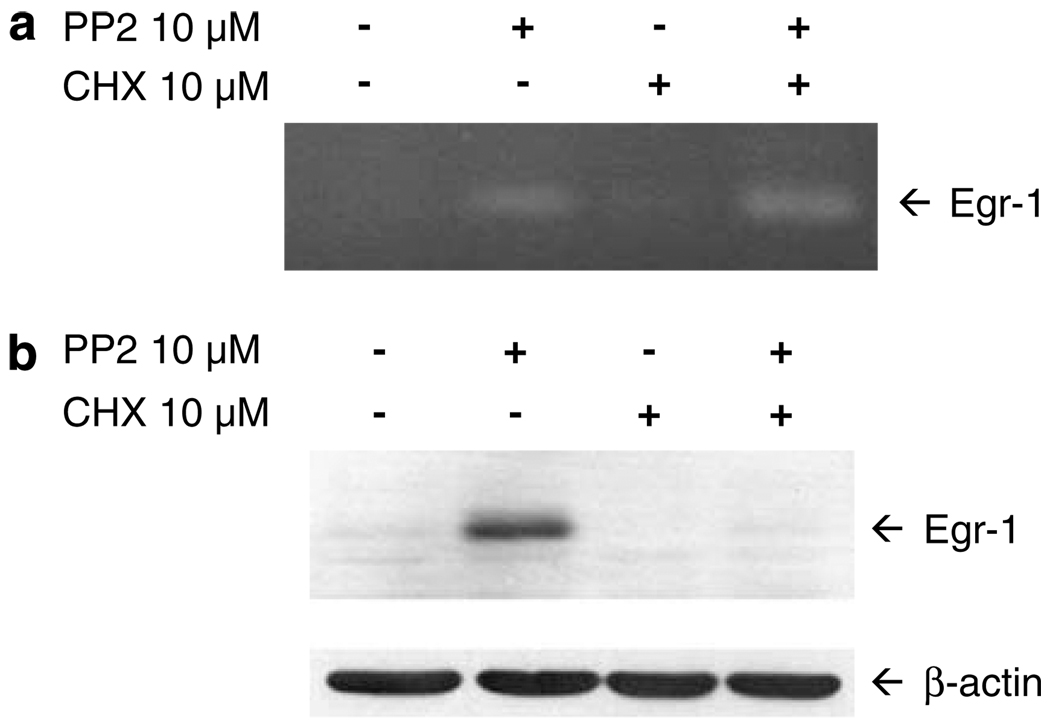
The rapid induction of Egr-1 protein following SFK inhibition suggested that SFKs may act directly to repress the Egr-1 gene. To determine whether SFKs directly repress the Egr-1 gene, we performed reverse transcription-polymerase chain reaction (RT-PCR) analysis of Egr-1 mRNA induction in the absence or presence of the protein synthesis inhibitor cycloheximide. As shown in Figure 3a, treatment of NB4 cells with PP2 alone resulted in increased expression of Egr-1 mRNA, relative to vehicle-treated cells. Cycloheximide, while blocking PP2 induction of Egr-1 protein (Figure 3b), failed to block the induction of Egr-1 mRNA (Figure 3a). This indicates that the induction of Egr-1 mRNA following SFK inhibition does not depend on the synthesis of new proteins. These results support the contention that SFKs directly regulate the expression of the Egr-1 gene through post-translational mechanisms.
Figure 3.
The gene encoding Egr-1 is a direct target of Src family kinases (SFKs). (a) NB4 cells were treated for 1 h with vehicle alone, 10 µm PP2, 10 µm cycloheximide (CHX) or cycloheximide plus PP2. After the preparation of total RNA, reverse transcription-polymerase chain reaction (RT-PCR) was carried out using primers specific for Egr-1 mRNA (forward primer: 5′-AGCCCTACGAGCACCTGAC-3′; reverse primer: 5′-TGGGTTGGTCATGCTCACTA-3′). The RT-PCR products were electrophoresed on a 2% agarose gel and stained with ethidium bromide. β-Actin was used as an internal control. (b) NB4 cells were treated as in (a), followed by immunoblotting for Egr-1 or β-actin. The experiment was performed three times with similar results.
In conclusion, in an effort to understand how SFK enzymes act to negatively regulate myeloid differentiation, we have used microarray studies to identify SFK gene targets. We have discovered that the gene encoding Egr-1, a key regulator of myeloid differentiation, is directly and negatively regulated by SFKs. Pharmacological inhibition of SFKs led to rapid induction of Egr-1 mRNA and protein. Collectively, these findings suggest that SFKs, which are frequently overexpressed and/or hyper-activated in AML, act, in part, to inhibit the differentiation of leukemic cells by repressing the expression of Egr-1.
Supplementary Material
Footnotes
Conflict of interest
The authors declare no conflict of interest.
Supplementary Information accompanies the paper on the Leukemia website (http://www.nature.com/leu)
References
- 1.Tallman MS, Nabhan C, Feusner JH, Rowe JM. Acute promyelocytic leukemia: evolving therapeutic strategies. Blood. 2002;99:759–767. doi: 10.1182/blood.v99.3.759. [DOI] [PubMed] [Google Scholar]
- 2.Miranda MB, Johnson DE. Signal transduction pathways that contribute to myeloid differentiation. Leukemia. 2007;21:1363–1377. doi: 10.1038/sj.leu.2404690. [DOI] [PubMed] [Google Scholar]
- 3.Miranda MB, Redner RL, Johnson DE. Inhibition of Src family kinases enhances retinoic acid-induced gene expression and myeloid differentiation. Mol Cancer Ther. 2007;6:3081–3090. doi: 10.1158/1535-7163.MCT-07-0514. [DOI] [PubMed] [Google Scholar]
- 4.Yang L, Zhao H, Li S, Ahrens K, Collins C, Eckenrode S, et al. Gene expression profiling during all-trans retinoic acid-induced cell differentiation of acute promyelocytic leukemia cells. J Mol Diagn. 2003;5:212–221. doi: 10.1016/S1525-1578(10)60476-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Kharbanda S, Nakamura T, Stone R, Hass R, Bernstein S, Datta R, et al. Expression of the early growth response 1 and 2 zinc finger genes during induction of monocytic differentiation. J Clin Invest. 1991;88:571–577. doi: 10.1172/JCI115341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Nguyen HQ, Hoffman-Liebermann B, Liebermann DA. The zinc finger transcription factor Egr-1 is essential for and restricts differentiation along the macrophage lineage. Cell. 1993;72:197–209. doi: 10.1016/0092-8674(93)90660-i. [DOI] [PubMed] [Google Scholar]
- 7.Krishnaraju K, Hoffman B, Liebermann DA. The zinc finger transcription factor Egr-1 activates macrophage differentiation in M1 myeloblastic leukemia cells. Blood. 1998;92:1957–1966. [PubMed] [Google Scholar]
- 8.Gibbs JD, Liebermann DA, Hoffman B. Leukemia suppressor function of Egr-1 is dependent on transforming oncogene. Leukemia. 2008;22:1909–1916. doi: 10.1038/leu.2008.189. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.