Abstract
Hepatic complications of transplant are a common cause of mortality. While mild elevations of serum aminotransferase enzymes (AST, ALT) do not carry an adverse prognosis, this is not the case with severe hepatocellular injury. We reviewed 6,225 consecutive recipients to determine the incidence and outcomes of severe hepatocellular injury (AST >1500 U/L) before day 100, which occurred in 88 patients. Causes were Sinusoidal Obstruction Syndrome (SOS) (n=46), hypoxic hepatitis (n=33), Varicella Zoster Virus (VZV) hepatitis (n=4), drug-liver injury (N=2), and Unknown (n=3). The incidence declined from 1.9% in the 1990s to 1.1% recently (due to a 5-fold decline in SOS and disappearance of VZV hepatitis). In hypoxic hepatitis, peak serum AST was 3545 U/L (range 1380-25246) within days of shock or prolonged hypoxemia; case fatality rate was 88%. In SOS, AST increases occurred 2-6 weeks after diagnosis; peak AST was 2252 U/L (range 1437-8281); case fatality rate was 76%, with only serum bilirubin able to distinguish survivors (2.7 mg/dL vs. 11.3 mg/dL, p=0.0009). We conclude that circulatory insults (sinusoidal injury, hypotension, hypoxemia) and not infection are the most common cause of severe hepatocellular injury, whose frequency has declined because of a falling incidence of SOS and VZV hepatitis.
Keywords: hepatocellular injury, serum aspartate aminotransferase, sinusoidal obstruction syndrome, viral hepatitis, hematopoietic cell transplant, hypoxic hepatitis, outcomes
Introduction
Hepatic complications of hematopoietic cell transplantation (HCT) have been a common cause of morbidity and mortality since the inception of this procedure 40 years ago.1-4 Patients may come to transplant with underlying liver disease (for example, chronic hepatitis B or C, extramedullary hematopoiesis, tumor infiltration, fungal abscess), but are also at risk for a number of post-transplant liver disorders (for example, injury to hepatic sinusoids or Sinusoidal Obstruction Syndrome (SOS); graft-vs.-host disease; cholangitis lenta; viral hepatitis; drug-liver injury; biliary obstruction).1, 5, 6 Development of jaundice after transplant is an ominous prognostic sign, as both the level of jaundice and small increments in total serum bilirubin correlate with mortality.2
Elevation of serum aminotransferase enzymes in the months following HCT is a common occurrence, but most of the elevations are in the range of 2-10 times the normal upper limit, or ∼100-500 U/L for aspartate aminotransferase (AST) or alanine aminotransferase (ALT).4, 7-10 Extreme elevations of serum AST or ALT are uncommon. In the study reported here, we reviewed 6,225 consecutive patients undergoing transplant from 1992 to the current time, for the development of severe hepatocellular injury, defined as a serum AST level of 30-times the normal upper limit or greater (∼1500 U/L) during the first 100 days after HCT. The purpose of this study was to determine the incidence of acute hepatocellular injury over this time span, the causes of this injury, and the outcomes. Although the initial working diagnosis upon discovery of a rapidly rising serum AST is often an infectious process, particularly viral infection, the results of this 15 year experience show that decreased hepatic oxygenation – caused either by sinusoidal injury from myeloablative conditioning therapy, or by shock and hypoxemia – is a far more common etiology.
Methods
Patient selection
We retrospectively reviewed laboratory studies from 6,225 consecutive patients who received HCT at the Fred Hutchinson Cancer Research Center from July 1992 through June 2007. Cases were defined as having severe hepatocellular injury if there was an elevation of serum AST in excess of 30 times the upper limit of normal during the time from day 0 (the day of hematopoietic cell infusion) to day 100, along with evidence that serum AST elevation was of liver origin. Controls were defined as patients whose serum AST never exceeded this threshold during the same time period. Reviews of medical records were conducted under the aegis of a protocol approved by our Institutional Review Office.
Study end-points
Incidence
The number of cases during three-year periods of time (that is, 1992-1995 through 2004-2007) was divided by the number of transplants performed during these time periods to yield incidence figures during the 15-year period of observation.
Risk factors
The frequency of these baseline and demographic factors was compared among cases and controls: age, gender, diagnosis, type of transplant (allogeneic vs. autologous), conditioning regimen (myeloablative vs. reduced intensity), donor characteristics for allografts, (HLA-matched sibling vs. other donors), Cytomegalovirus serostatus, and ursodiol prophylaxis.
Causation
For all cases, we reviewed medical records, including laboratory chemistries, virology and microbiology results, medical imaging, clinical findings, and liver histology as determined by biopsy or autopsy. For each case, we then made a judgment about both the primary cause of severe hepatocellular injury and any secondary causes, when data suggested that there was more than one cause of liver injury in a given patient, using the following definitions:
Viral hepatitis11 was defined as the identification of a specific virus, by either culture, detection of viral antigen or nucleic acid (RNA for hepatitis C virus, DNA for hepatitis B virus and herpesviruses) in liver tissue or blood, in proximity to the development of hepatocellular injury.
Hypoxic hepatitis12 was defined by the proximity of abrupt elevation of serum AST to a clinical event that was consistent with decreased delivery of oxygenated blood to the liver, for example, prolonged hypotension following either sepsis, blood loss, or cardiogenic shock, or respiratory failure that resulted in profound hypoxemia.
Sinusoidal Obstruction Syndrome13 was defined on the basis of either clinical criteria (painful hepatomegaly, jaundice, and gain in body weight due to retention of fluid, in the absence of another explanation for these findings) or histology demonstrating characteristic findings of sinusoidal injury.
Drug-induced Liver Injury (DILI)14 was defined by elevation of serum AST in proximity to exposure to a drug known to cause hepatocellular injury. Diagnosis of DILI also relied on demonstrating the absence of viral infection, or a clinical syndrome that would lead to inadequate liver perfusion, or SOS.
Cholestatic liver disease15 was defined as an elevation of total serum bilirubin in the presence of either extrahepatic biliary obstruction or intrahepatic cholestasis, evinced by either histologic evidence of cholestasis or consistent laboratory findings (concomitant elevation of serum alkaline phosphatase or gamma glutamyl transpeptidase enzymes).
Unknown was our designation for cases where none of the above definitions applied and no other cause of hepatocellular necrosis was apparent.
Clinical presentations
For cohorts of cases with hepatic injury with similar causation, we described the timing of the onset of elevation of serum AST, peak values, timing of peak values, and the total serum bilirubin levels.
Outcome
For each case, a judgment was made as to whether the patient recovered from the acute hepatocellular injury event, or died as a result of that event. Case fatality rates were determined for cohorts of patients with hepatic injury with similar causation, for example, viral hepatitis, hypoxic hepatitis, and SOS. Overall mortality by transplant day 200 was also determined for these same cohorts.
Statistical Methods
For categoric variables, Fisher's exact test was used to examine risk factors for severe hepatocellular injury and the differences in clinical characteristics among different causes. For continuous variables, the Wilcoxon rank sum test was used to compare the means of age and the levels of AST, albumin and total serum bilirubin in different causal groups.
Results
Incidence of severe hepatocellular injury
Eighty-eight cases with severe hepatic injury were identified from among 6,225 transplant recipients (1.4%). One potential case was excluded because elevated serum AST had been caused by rhabdomyolysis, not hepatocellular injury.16, 17 There were no significant differences between cases with severe hepatocellular injury and controls regarding baseline and demographic factors examined (Table 1).
Table 1. Baseline and demographic factors in cases with severe hepatocellular injury and concurrent controls.
| Cases (n=88) |
Controls (n=6137) |
p-value | |
|---|---|---|---|
| Age at transplant | 0.9764 | ||
| Mean ± SD | 40.6 ± 15.8 | 40.4 ± 17.1 | |
| Median (range) | 45.3 (2 - 68) | 43.3 (0 - 78) | |
| Male patients | 52 (59.0%) | 3433 (55.9%) | 0.5898 |
| Primary diagnosis | 0.4805 | ||
| Hematologic malignancies (non-lymphoma) | 48 (54.5%) | 3165 (51.6%) | |
| Lymphoma | 17 (19.3%) | 1027 (16.7%) | |
| Other malignancy | 17 (19.3%) | 1616 (26.3%) | |
| Other than malignancy | 6 (6.8%) | 328 (5.3%) | |
| Second or more HCT | 10 (11.4%) | 594 (9.7%) | 0.5848 |
| Allogeneic transplant | 62 (70.5%) | 4328 (70.5%) | 1.000 |
| Source of donor cells (allogeneic HCT only) | 0.3713 | ||
| Peripheral blood stem cells | 31 (50%) | 1940 (45%) | |
| Bone marrow | 31 (50%) | 2388 (55%) | |
| Other than HLA identical sibling donor (allogeneic HCT only) | 35 (56.5%) | 2487 (57.5%) | 0.8975 |
| Conditioning regimen | |||
| Myeloablative regimen1 | 81 (92.0%) | 5395 (87.9%) | 0.1835 |
| Reduced intensity regimen | 7 (8.0%) | 742 (12.1%) | |
| Positive CMV serostatus at transplant (donor or recipient) | 48 (54.6%) | 3940 (64.5%) | 0.0570 |
| Ursodiol prophylaxis | 19 (21.6%) | 1661 (27.1%) | 0.2779 |
Includes regimens received by 3 patients whose severe hepatocellular injury occurred later, following infusion of gemtuzumab ozogamicin for treatment of recurrent leukemia after transplant.18
The incidence of severe hepatocellular injury has declined remarkably from the 1990s to the current time: 1.9% during the three years after 1996 compared to 1.06% during the years from 2004-2007 (Table 2). This decline in incidence is due mostly to a falling incidence of SOS as a cause of hepatocellular injury (Table 2).
Table 2. Incidence of severe hepatocellular injury during a 15-year time span, by the causes of liver injury.
| Number of patients (%) | ||||||
|---|---|---|---|---|---|---|
| 1992-1995 N=1,315 |
1995-1998 N=1,213 |
1998-2001 N=1,139 |
2001-2004 N=1,233 |
2004-2007 N=1,325 |
1992-2007 N=6,225 |
|
| SOS | 16 (1.22%) | 13 (1.07%) | 9 (0.79%) | 5 (0.41%) | 3 (0.23%) | 46 (0.74%) |
| Hypoxic hepatitis | 6 (0.46%) | 7 (0.58%) | 5 (0.44%) | 6 (0.49%) | 9 (0.68%) | 33 (0.53%) |
| Viral infection | 1 (0.08%) | 3 (0.25%) | 0 | 0 | 0 | 4 (0.06%) |
| DILI | 0 | 0 | 1 (0.09%) | 0 | 1 (0.08%) | 2 (0.03%) |
| Unknown | 0 | 0 | 1 (0.09%) | 1 (0.08%) | 1 (0.08%) | 3 (0.05%) |
| All causes | 23 (1.75%) | 23 (1.90%) | 16 (1.40%) | 12 (0.97%) | 14 (1.06%) | 88 (1.41%) |
Causes of severe hepatocellular injury and their clinical and histologic features
The cause of severe hepatocellular injury was readily apparent in 78 cases but in 6 cases, there were multiple contributing causes (only the primary cause is listed in Table 2). In 3 cases, no cause could be identified; they are listed as cause unknown, and described more completely below. Liver injury in the remaining 85 cases was caused primarily by SOS (n=46), hypoxic hepatitis (n=33), viral infection (n=4) and DILI (n=2) (Table 2). Over 15 years, the frequency of severe hepatocellular injury caused by SOS decreased 5-fold from 1.22% during 1993-1995 to 0.23% during 2005-2007. In contrast, the incidence of hypoxic hepatitis has remained unchanged over this time period. Severe hepatocellular injury (as defined in Methods) caused by VZV infection has not been seen since 1999.
Sinusoidal Obstruction Syndrome
Sinusoidal liver injury in 46 cases was caused by either a myeloablative conditioning regimen (n=42) or a reduced intensity regimen (gemtuzumab ozogamicin – fludarabine – TBI 2 Gy) (n=1), or infusion of gemtuzumab ozogamicin for relapsed leukemia after transplant (n=3). Of 46 cases with severe hepatocellular injury, 27 were allogeneic graft recipients and 19 had received autologous cells. The majority of cases were male (33, or 72%).
For 42 patients whose SOS followed a myeloablative regimen, the onset of elevation of serum AST that defined severe injury did not occur near the time of diagnosis of SOS19 but several weeks later (the median day of the onset of serum AST rise was day 20 (range, day 6 to 46)). This delay in hepatocyte necrosis in patients with SOS was likely the result of deposition of matrix in hepatic sinusoids following initial injury to sinusoidal endothelial cells (Figure 1A). Peak AST levels occurred at a median of day 20 (range, day 6 to 47), that is, most patients had a very steep rise in serum AST to the threshold value of 30× normal upper limit. The median peak AST level was 2,252 U/L (range, 1,437 – 8,281). The myeloablative regimens received by these cases included busulfan - cyclophosphamide (n=13); busulfan – melphalan – thioTEPA (n=12); cyclophosphamide - total body irradiation (n=10); and several other regimens (n=7).
Only one case with SOS had received a reduced-intensity regimen consisting of gemtuzumab ozogamicin – fludarabine - TBI 2 Gy; the onset of his rise in serum AST was at day 10, with a peak AST of 2,616 U/L at day 12 (Figure 1A).
In 3 cases, the onset of SOS was not in proximity to their myeloablative conditioning regimens but rather followed infusion of gemtuzumab ozogamicin for recurrent leukemia after transplant on days 49, 67, and 70, respectively. Severe hepatocellular injury was then noted on days 63, 74, and 81, with peak serum AST values of 6,887 U/L, 2,801 U/L, and 3,091 U/L, respectively.
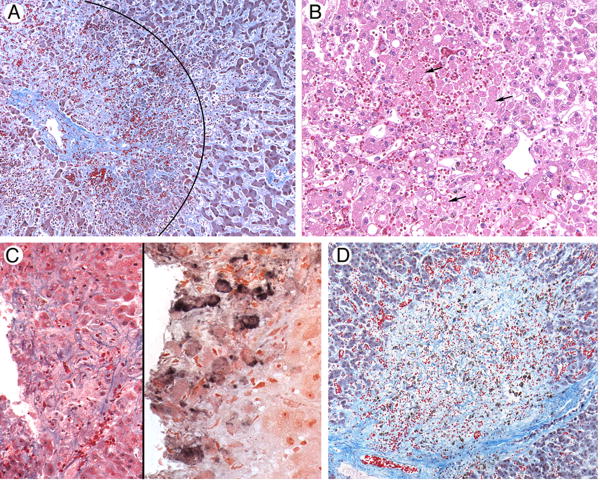
Figure 1.
Photomicrographs of liver tissue illustrating causes of severe hepatitis following hematopoietic cell transplant.
A. Sinusoidal Obstruction Syndrome. A liver acinus is shown here, with a central vein on the left; diffuse hepatocyte necrosis in zone 3, surrounding the vein (the area within the arc); and deposition of collagens within sinusoids in zone 2 of the acinus (outside the arc). Masson trichrome.
B. Hypoxic hepatitis following respiratory failure and hypotension. Zones 2 and 3 of a liver acinus, with the central vein in the lower right. Numerous hepatocytes that have lost nuclei as a result of ischemia are seen in zone 3; arrows point to some of these dying cells. Hematoxylin and eosin.
C. Varicella zoster virus infection. The left panel illustrates a punched-out area of confluent hepatocyte necrosis without distinguishing features (hematoxylin and eosin); the right panel shows reaction product from staining with a monoclonal antibody to VZV (immunohistochemistry).
D. Unknown. There is a large circular area of confluent hepatocyte necrosis surrounded by normal hepatocytes. There were no areas of viral cytopathic effect, and immunohistochemical stains were negative for viruses. Masson trichrome.
Hypoxic hepatitis
Hypoxic liver injury was the cause of extreme AST elevations in 33 cases, with median peak AST levels of 3,545 U/L (range, 1,380-25,246). The causes of liver injury were clinically obvious: septic shock in 14, severe respiratory failure in 10, cardiogenic shock in 7, and hemorrhagic shock in 2. Infection was the primary or a significant contributor to hypotension and hypoxemia in 23 cases. A major factor in development of infection was use of glucocorticoids, as 19 cases were receiving prednisone when hypotension developed (the indications were acute GVHD (n=14), idiopathic pneumonia syndrome (n=4), and graft rejection (n=1)). Most of the cases who developed hypoxic hepatitis had received myeloablative conditioning regimens (n=27) and allografts (n=27).
Viral hepatitis
All 4 cases were caused VZV infection, all before 1999, all following myeloablative conditioning and allogeneic transplantation, and all while acyclovir was not being given. Three patients were diagnosed with VZV infection during their lifetime, and the fourth only at autopsy. An 8-year old girl developed VZV hepatitis on day 71, after discontinuation of acyclovir, followed by elevation of serum AST to 1,892 U/L on day 74, followed by complete resolution after acyclovir therapy. A 43-year old man developed right upper quadrant pain, elevated serum AST, and disseminated zoster on day 58 while being treated for GVHD with prednisone and sirolimus. Despite prompt treatment with high-dose acyclovir and foscarnet, his serum AST reached 1,889 U/L on day 63, followed by jaundice, hemolytic-uremic syndrome, and multiorgan failure. Liver biopsy on day 68 showed both GVHD and VZV infection (Figure 1C). He died on day 76. A 50-year old woman with a history of zoster developed GVHD on day 21, treated successfully with prednisone. On day 29, she developed fever, diffuse skin erythema, and AST elevation to 2,240 U/L. Empiric treatment with acyclovir resulted in a rapid decline in serum AST. At day 60, typical cutaneous zoster developed, treated again with acyclovir. A 21 year-old woman with a history of zoster developed GVHD on day 11; worsening abdominal pain, diarrhea, and vomiting on day 31 led to increased prednisone dosing. Antithymocyte globulin was added as a treatment of severe gut GVHD on day 41. She subsequently developed severe hepatocellular injury with an AST level of 5,467 U/L on day 51, and died on day 52. Her autopsy revealed both disseminated VZV infection and GVHD involving liver and intestine.
Drug-induced liver injury
Two cases were attributed to drug toxicity on the basis of proximity to drugs known to cause hepatocyte necrosis and the lack of another tenable cause. A 41-year old woman developed respiratory failure on day 89. On day 91, intravenous liposomal amphotericin B (AmBisome®, 257mg/day) was started; on day 93, serum AST rose rapidly to 1,709 U/L, ALT to 2,060 U/L, total serum bilirubin to 3.2 mg/dL, and LDH to 3,784 U/L. She died on day 95. Autopsy showed fungal pneumonia, pericentral hepatocyte necrosis and bile duct damage with cholestasis typical of GVHD. We attributed her severe hepatocellular injury on day 93 to liposomal amphotericin B.20 A 2 year-old boy developed acute renal failure followed by respiratory failure on day 1 post-transplant. On day 2, his serum AST was 2,130 U/L and ALT level of 714 U/L. He did not meet criteria for a diagnosis of SOS and no viruses were identified. He developed myocardial ischemia and died on day 12. Autopsy was declined. We attributed his hepatocyte necrosis to his conditioning regimen, and considered hepatic hypoxia and sinusoidal liver injury to be contributing causes.
Unknown cause
Despite extensive evaluations, three cases proved enigmatic with regard to the cause of abrupt serum AST elevation.
A 50-year old woman received busulfan-cyclophosphamide conditioning followed on day 9 by epigastric and right upper quadrant pain, fever, obtundation, pulmonary infiltrates, renal failure, and jaundice, with serum AST 1,859 U/L, alkaline phosphatase 1,300 U/L, and total serum bilirubin 22.3 mg/dL. Liver biopsy showed circular areas of confluent hepatic necrosis affecting multiple lobules (Figure 1D); viral studies were negative for herpesviruses (CMV, HSV, VZV, HHV-6), Adenovirus, and hepatitis viruses. Culture of blood, urine, bronchoalveolar lavage and cerebrospinal fluid were all negative as were CT, MRI and echocardiogram. At day 11, her AST had decreased to 218 U/L and completely normalized by day 32. Ursodiol was started on day 15 when her bilirubin level was 45.5 mg/dL, and her bilirubin then began to decline. She died from disseminated aspergillosis on day 70. We could not identify the cause of her acute liver injury at day 9, but speculate that the cause was an unidentified virus, based on the liver histology.
A 16 year-old boy had an uncomplicated course until day 87, when he suddenly developed severe abdominal pain, seizures, abnormal liver tests (AST 4,070 U/L, ALT 6,818 U/L), LDH 5,205 IU/L, serum lipase 6,991 U/L, D-dimer >20 μg/mL, serum haptoglobin <11 mg/dL, and lactic acidosis. Ultrasound demonstrated pancreatitis, a distended gallbladder without gallbladder wall thickening and no biliary obstruction. Blood cultures were all negative and he remained normotensive throughout. He was treated with antibiotics. His serum AST declined to 1,960 U/L on day 88 and was normal by day 100. Liver biopsy was not performed. We speculate that his severe hepatocellular injury, acute pancreatitis, and hemolysis were secondary to an infectious process that was never identified.
A 61-year old male was conditioned with cyclophosphamide 200 mg/kg, antithymocyte globulin, and TBI 2 Gy before his matched sibling allograft. He had history of hepatitis B and C and had undergone a previous transplant. He developed severe hepatocellular injury on day 9 post-transplant (serum AST 2,169 U/L, ALT 692 U/L), with total serum bilirubin 2.7 mg/dL and alkaline phosphatase 80 U/L. He did not have any gastrointestinal symptoms. His AST level normalized quickly by day 10. Abdominal ultrasound on day 12 showed no signs of SOS. He subsequently developed anasarca, pleural effusions, acute renal failure, and atrial fibrillation and died from multiorgan failure on day 21, when his total serum bilirubin was 6.6 mg/dL. Autopsy was declined. We speculate that his liver injury was caused by his high-dose cyclophosphamide conditioning therapy21, even though the cardinal features of SOS were absent.
Outcomes
The case fatality rate among patients who developed severe hepatocellular injury was 77%; these patients died both because of liver dysfunction and from the consequences of the underlying cause of the hepatic injury (Table 3). All-cause mortality by transplant day 200 was 88%. Most of the deaths occurred within days of the initial rise in serum AST, with some exceptions among patients whose liver injury was caused by SOS (Table 3).
Table 3. Outcome of severe hepatocellular injury, grouped by the causes of liver injury.
| Case fatality rate from hepatocellular injury and its underlying cause(s) (n, %) |
Time in days from onset of hepatocellular injury to death (median, range) |
All-cause mortality by day 200 (n, %) |
|
|---|---|---|---|
| SOS (n=46) | 35 (76%) | 8 (0-84) | 38 (83%) |
| Hypoxic hepatitis (n=33) | 29 (88%) | 1 (0-25) | 32 (97%) |
| VZV hepatitis (n=4) | 1 (25%) | 1 | 3 (75%) |
| Unknown (n=3) | 1 (33%) | 10 | 2 (67%) |
| DILI (n=2) | 2 (100%) | 2 and 10 | 2 (100%) |
| All cases (n=88) | 68 (77%) | 3 (0-84) | 77 (88%) |
Among 46 cases caused by SOS, there were 11 who recovered from the acute hepatocellular necrosis event and 35 who died without resolution of liver injury. We compared prognostic factors in this cohort of patients that were significantly different in those who recovered, compared to those who died. The only factor that predicted outcome was the total serum bilirubin at the time of peak serum AST (2.7±1.8 mg/dL vs. 11.3±12.5 mg/dL, p = 0.0009). There were no differences in age, underlying diagnoses, peak AST levels, or the day of onset of AST elevation in those who recovered compared to those who did not.
Among 33 cases caused by hypoxic hepatitis, there were only 4 patients who recovered from the hepatic necrosis event. They included 3 of 7 patients whose hypoxic hepatitis had been caused by cardiogenic shock and 1 of 2 whose hypoxic hepatitis was caused by hemorrhagic shock. No patient who developed hypoxic hepatitis following septic shock or respiratory failure survived the event. Patients who recovered had an earlier onset of elevated serum AST (median day 17 vs. 38), a lower peak serum AST (median 2721 vs. 3597 U/L), and lower total serum bilirubin levels (median 4.1 vs. 6.2 mg/dL).
Discussion
Although the frequency of elevations of serum aminotransferase enzymes (AST, ALT) following hematopoietic cell transplant is very high, most of the elevations are in the 100 – 500 U/L range and only rarely impact outcomes.7, 8 In contrast, the cases of severe hepatocellular injury reported here are far less common (<2%) but are associated with a greatly increased risk of death (the case fatality rate was 77% and mortality by day 200 was 88%). Fortunately, the incidence of severe hepatocellular injury has fallen steeply since 1999, due to the disappearance of VZV hepatitis and a marked reduction in hepatocellular necrosis caused by SOS.
The first diagnosis that comes to mind when a patient develops acute hepatitis after transplant is usually a viral infection.5 However, the only virus that led to severe hepatitis in this 15-year experience was VZV, and no cases have been seen here in the last 9 years. The routine use of acyclovir prophylaxis among patients seropositive for VZV before transplant is responsible for this decline.22 It is noteworthy that in each of our VZV hepatitis cases, acyclovir was not being taken at the onset of the infection. These data should not be interpreted as showing the absence of viral liver infection in the current era, as we have seen sporadic cases of hepatitis caused by adenovirus and hepatitis B and hepatitis C viruses during this time span. However, none of these infections have resulted in serum AST levels that were 30-times the normal upper limit (∼1,500 U/L), although both adenovirus and hepatitis B can cause extensive hepatocyte necrosis after hematopoietic cell transplant.6, 23-25 Hepatitis C virus seldom causes severe liver injury after transplant.7 Acute liver injury may also be seen after day 100, with a differential diagnosis that includes a hepatitic presentation of GVHD26, 27, a flare of hepatitis B or C following immune re-constitution or tapering of immune suppressive drugs7, 11, VZV hepatitis22, and drug-liver injury.28
The most common causes of severe liver injury in this series were caused by decreased oxygenation of hepatocytes, caused by SOS, decreased liver blood flow, and prolonged hypoxemia. The cases of hypoxic hepatitis were all typical of that diagnosis, that is, clinically obvious, protracted hypotension or hypoxemia followed by an abrupt rise in serum AST and ALT, usually within 1-3 days.12 One can make the argument that searching for a viral cause of acute hepatitis in a patient with this typical sequence of events is not necessary, given the high pre-test probability that hepatic hypoxia is the cause of increased AST and the expense of testing for viral DNA by PCR for herpesviruses, hepatitis B, and adenovirus. The cases of severe hepatocellular injury caused by SOS were far more subtle, as the typical clinical manifestations of SOS (painful hepatomegaly, weight gain, ascites, jaundice) occurred in the first 10 days after transplant19, but the initial rises in serum AST were days to weeks later. Half of the patients with hepatocyte necrosis caused by SOS had the onset of elevated AST after day 20, and as late as day 46. The pathogenesis of hepatocyte necrosis in patients with SOS is not the initial injury to sinusoidal endothelial cells (the histologic hallmark of SOS) but the later development of activation of hepatic stellate cells, deposition of extracellular matrix in the sinusoids, and falls in sinusoidal blood flow to zone 3 hepatocytes.13, 29, 30 An identical mechanism obtains in patients who received gemtuzumab ozogamicin either as part of conditioning therapy (one case in this series) or after transplant, as treatment for leukemic relapse.18, 31
There were three cases in this series that remain unexplained, one that looked viral on histology, one that followed a systemic illness that was probably infectious in nature, and one that followed high-dose cyclophophamide. The latter patient did not have signs and symptoms of SOS, but rather isolated elevation of AST and ALT, as has been reported previously with cyclophosphamide.21 Drug-liver injury was thought to be the cause of hepatitis in two cases, one related to liposomal amphotericin B20 and the other to the chemotherapy drugs carboplatin, etoposide, and melphalan.32 These data should not be interpreted as showing a lack of drug-liver injury, as several drugs in common use for supportive care of transplant patients are known liver toxins and the most likely explanation for AST/ALT elevations in the 100-500 U/L range (for example, trimethoprim-sulfamethoxazole, itraconazole, voriconazole, calcineurin inhibitors, imatinib).14, 32 None of these drugs, however, was a cause of severe liver injury during in the preceding 15 years.
We conclude that severe hepatocellular injury, as defined by serum ALT ≥1,500 U/L, is an uncommon event in the 100 days following transplant but one that is associated with a case fatality rate greater than 75%. The most common causes were not infections, but decreased hepatocyte oxygenation caused either by sinusoidal injury from conditioning regimens, shock, or hypoxemia. The frequency of severe hepatocellular injury has declined recently because SOS is now far less common than it was during the 1990s33 and because severe VZV hepatitis has not been seen in the last 9 years.
Acknowledgments
Our research in the field of gastrointestinal and hepatobiliary complications of hematopoietic cell transplant is supported by grants from the U.S. National Institutes of Health, National Cancer Institute (CA 18029, CA 15704). Dr. Sakai is supported by funding from the Bureau of Social Welfare and Public Health of the Tokyo Metropolitan Government and the Japan Clinical Research Support Unit.
References
- 1.Strasser SI, McDonald GB. Gastrointestinal and hepatic complications. In: Appelbaum FR, Forman SJ, Negrin RS, Blume KG, editors. Thomas' Hematopoietic Cell Transplantation. Fourth. Oxford, UK: Wiley-Blackwell Publishing; 2009. pp. 1434–1455. [Google Scholar]
- 2.Gooley TA, Rajvanshi P, Schoch HG, McDonald GB. Serum bilirubin levels and mortality after myeloablative allogeneic hematopoietic cell transplantation. Hepatology. 2005;41:345–52. doi: 10.1002/hep.20529. [DOI] [PubMed] [Google Scholar]
- 3.El-Sayed MH, El-Haddad A, Fahmy OA, Salama II, Mahmoud HK. Liver disease is a major cause of mortality following allogeneic bone-marrow transplantation. European Journal of Gastroenterology & Hepatology. 2004;16:1347–54. doi: 10.1097/00042737-200412000-00019. [DOI] [PubMed] [Google Scholar]
- 4.Kim B, Chung K, Sun H, Suh J, Min W, Kang C, et al. Liver disease during the first posttransplant year in bone marrow transplantation recipients: retrospective study. Bone Marrow Transplantation. 2000;26:193–7. doi: 10.1038/sj.bmt.1702453. [DOI] [PubMed] [Google Scholar]
- 5.McDonald GB. Management of hepatic disease following hematopoietic cell tranplant. Alimentary Pharmacology & Therapeutics. 2006;24:441–52. doi: 10.1111/j.1365-2036.2006.03001.x. [DOI] [PubMed] [Google Scholar]
- 6.Shulman HM, McDonald GB. Hepatic complications of hematopoietic cell transplantation. In: Gershwin ME, Vierling JM, Manns M, editors. Liver Immunology: Principles and Practice. Totowa, NJ: Humana Press; 2007. pp. 409–21. [Google Scholar]
- 7.Strasser SI, Myerson D, Spurgeon CL, Sullivan KM, Storer B, Schoch HG, et al. Hepatitis C virus infection after bone marrow transplantation: A cohort study with 10 year follow-up. Hepatology. 1999;29:1893–9. doi: 10.1002/hep.510290609. [DOI] [PubMed] [Google Scholar]
- 8.Ruutu T, Eriksson B, Remes K, Juvonen E, Volin L, Remberger M, et al. Ursodeoxycholic acid for the prevention of hepatic complications in allogeneic stem cell transplantation. Blood. 2002;100:1977–83. doi: 10.1182/blood-2001-12-0159. [DOI] [PubMed] [Google Scholar]
- 9.Ho GT, Parker A, MacKenzie JF, Morris AJ, Stanley AJ. Abnormal liver function tests following bone marrow transplantation: aetiology and role of liver biopsy. European Journal of Gastroenterology & Hepatology. 2004;16:157–62. doi: 10.1097/00042737-200402000-00006. [DOI] [PubMed] [Google Scholar]
- 10.Sucak G, Yegin Z, Ozkurt Z, Aki S, Karakan T, Akyol G. The role of liver biopsy in the workup of liver dysfunction late after SCT: is the role of iron overload underestimated? Bone Marrow Transplantation. 2008 doi: 10.1038/bmt.2008.193. pre-published online. [DOI] [PubMed] [Google Scholar]
- 11.Lau GKK, Strasser SI, McDonald GB. Hepatitis virus infections in patients with cancer. In: Wingard JR, Bowden RA, editors. Management of Infection in Oncology Patients. London,UK: Martin Dunitz; 2003. pp. 321–42. [Google Scholar]
- 12.Henrion J, Schapira M, Luwaert R, Colin L, Delannoy A, Heller FR. Hypoxic hepatitis: clinical and hemodynamic study in 142 consecutive cases. Medicine. 2003;82:392–406. doi: 10.1097/01.md.0000101573.54295.bd. [DOI] [PubMed] [Google Scholar]
- 13.Deleve LD, Shulman HM, McDonald GB. Toxic injury to hepatic sinusoids: Sinusoidal obstruction syndrome (venocclusive disease) Seminars in Liver Disease. 2002;22:27–41. doi: 10.1055/s-2002-23204. [DOI] [PubMed] [Google Scholar]
- 14.Kaplowitz N, DeLeve LD, editors. Drug Induced Liver Disease. Second. New York: Informa Healthcare USA, Inc.; 2007. [Google Scholar]
- 15.Strasser SI, Shulman HM, McDonald GB. Cholestasis after hematopoietic cell transplantation. Clinics in Liver Disease. 1999;3:651–68. doi: 10.1016/s1089-3261(05)70089-1. [DOI] [PubMed] [Google Scholar]
- 16.Rossi MR, Longoni DV, Rovelli AM, Uderzo C. Severe rhabdomyolysis, hyperthermia and shock after amphotericin B colloidal dispersion in an allogeneic bone marrow transplant recipient. Pediatric Infectious Disease Journal. 2000;19:172–3. doi: 10.1097/00006454-200002000-00022. [DOI] [PubMed] [Google Scholar]
- 17.Tong J, Laport G, Lowsky R. Rhabdomyolysis after concomitant use of cyclosporine and simvastatin in a patient transplanted for multiple myeloma. Bone Marrow Transplantation. 2005;36:739–40. doi: 10.1038/sj.bmt.1705128. [DOI] [PubMed] [Google Scholar]
- 18.Rajvanshi P, Shulman HM, Sievers EL, McDonald GB. Hepatic sinusoidal obstruction following Gemtuzumab ozogamicin (Mylotarg) therapy. Blood. 2002;99:4245–6. doi: 10.1182/blood.v99.7.2310. [DOI] [PubMed] [Google Scholar]
- 19.McDonald GB, Hinds MS, Fisher LB, Schoch HG, Wolford JL, Banaji M, et al. Venocclusive disease of the liver and multiorgan failure after bone marrow transplantation: A cohort study of 355 patients. Ann Intern Med. 1993;118:255–67. doi: 10.7326/0003-4819-118-4-199302150-00003. [DOI] [PubMed] [Google Scholar]
- 20.Fischer MA, Winkelmayer WC, Rubin RH, Avorn J. The hepatotoxicity of antifungal medications in bone marrow transplant recipients. Clinical Infectious Diseases. 2005;41:301–7. doi: 10.1086/431586. [DOI] [PubMed] [Google Scholar]
- 21.Honjo I, Suou T, Hirayama C. Hepatotoxicity of cyclophosphamide in man: Pharmacokinetic analysis. Res Commun Chem Pathol Pharmacol. 1988;61:149–65. [PubMed] [Google Scholar]
- 22.Gnann J. Herpes simplex and varicella zoster virus infection after hemopoietic stem cell or solid organ transplantation. In: Bowden RA, Ljungman P, Paya CV, editors. Transplant Infections. Second. Philadelphia: Lippincott Williams and Wilkins; 2003. pp. 350–66. [Google Scholar]
- 23.Shields AF, Hackman RC, Fife KH, Corey L, Meyer JD. Adenovirus infections in patients undergoing bone marrow transplantation. N Engl J Med. 1985;312:529–33. doi: 10.1056/NEJM198502283120901. [DOI] [PubMed] [Google Scholar]
- 24.Neofytos D, Ojha A, Mookerjee B, Wagner J, Filicko J, Ferber A, et al. Treatment of adenovirus disease in stem cell transplant recipients with cidofovir. Biology of Blood & Marrow Transplantation. 2007;13:74–81. doi: 10.1016/j.bbmt.2006.08.040. [DOI] [PubMed] [Google Scholar]
- 25.Wang WH, Wang HL. Fulminant adenovirus hepatitis following bone marrow transplantation. A case report and brief review of the literature. Archives of Pathology & Laboratory Medicine. 2003;127:e246–8. doi: 10.5858/2003-127-e246-FAHFBM. [DOI] [PubMed] [Google Scholar]
- 26.Strasser SI, Shulman HM, Flowers ME, Reddy R, Margolis DA, Prumbaum M, et al. Chronic graft-vs-host disease of the liver: presentation as an acute hepatitis. Hepatology. 2000;32:1265–71. doi: 10.1053/jhep.2000.20067. [DOI] [PubMed] [Google Scholar]
- 27.Akpek G, Boitnott JK, Lee LA, Hallick JP, Torbenson M, Jacobsohn DA, et al. Hepatitic variant of graft-versus-host disease after donor lymphocyte infusion. Blood. 2002;100:3903–7. doi: 10.1182/blood-2002-03-0857. [DOI] [PubMed] [Google Scholar]
- 28.Sakai M, McDonald GB. Gastrointestinal and hepatic manifestations of chronic GVHD. In: Vogelsang G, Pavletic S, editors. Chronic graft-versus-host disease Principles and practice of interdisciplinary management. New York, New York: Cambridge University Press; in press. [Google Scholar]
- 29.Shulman HM, Fisher LB, Schoch HG, Henne KW, McDonald GB. Venocclusive disease of the liver after marrow transplantation: Histologic correlates of clinical signs and symptoms. Hepatology. 1994;19:1171–80. [PubMed] [Google Scholar]
- 30.Traber PG, Chianale J, Gumucio JJ. Physiologic significance and regulation of hepatocellular heterogeneity. Gastroenterology. 1988;95:1130–43. doi: 10.1016/0016-5085(88)90194-1. [DOI] [PubMed] [Google Scholar]
- 31.McKoy JM, Angelotta C, Bennett CL, Tallman MS, Wadleigh M, Evens AM, et al. Gemtuzumab ozogamicin-associated sinusoidal obstructive syndrome (SOS): an overview from the research on adverse drug events and reports (RADAR) project. Leukemia Research. 2007;31:599–604. doi: 10.1016/j.leukres.2006.07.005. [DOI] [PubMed] [Google Scholar]
- 32.McDonald GB, Frieze D. A problem-oriented approach to liver disease in oncology patients. Gut. 2008;57:987–1003. doi: 10.1136/gut.2007.131136. [DOI] [PubMed] [Google Scholar]
- 33.McDonald GB, McDonald SH, Gooley T. Liver complications of allogeneic hematopoietic cell transplant: Declining incidence related to changes in practice (Abstract) Hepatology Hepatology. 2008;48(Supplement):318A–319A. [Google Scholar]