Abstract
The supplementary motor area is thought to contribute to the generation of anticipatory postural adjustments (which act to stabilize supporting body segments prior to movement), but its precise role remains unclear. In addition, participants with Parkinson’s disease (PD) exhibit impaired function of the supplementary motor area as well as decreased amplitudes and altered timing of the anticipatory postural adjustment during step initiation, but the contribution of the supplementary motor area to these impairments also remains unclear. To determine how the supplementary motor area contributes to generating the anticipatory postural adjustment and to the impaired anticipatory postural adjustments of participants with PD, we examined the voluntary steps of 8 participants with PD and 8 participants without PD, before and after disrupting the supplementary motor area and dorsolateral premotor cortex, in separate sessions, with 1-Hz repetitive transcranial magnetic stimulation. Both groups exhibited decreased durations of their anticipatory postural adjustments after repetitive transcranial magnetic stimulation over the supplementary motor area but not over the dorsolateral premotor cortex. Peak amplitudes of the anticipatory postural adjustments were unaffected by repetitive transcranial magnetic stimulation to either site. The symptom severity of the participants with PD positively correlated with the extent that repetitive transcranial magnetic stimulation over the supplementary motor area affected the durations of their anticipatory postural adjustments. The results suggest that the supplementary motor area contributes to the timing of the anticipatory postural adjustment and that participants with PD exhibit impaired timing of their anticipatory postural adjustments, in part, due to progressive dysfunction of circuits associated with the supplementary motor area.
Keywords: gait, balance, posture, cerebral cortex, transcranial magnetic stimulation
Patients with Parkinson’s disease (PD) are at an increased risk for falls, and they fall most during dynamic transitions in their postural orientation (Bloem et al., 2001). Step initiation represents such a transition, during which patients with PD exhibit freezing, such that they are unable to step and, consequently, often fall (Bloem et al., 2004). Study participants with PD, even those without symptoms of freezing, exhibit diminished, prolonged, and more variable timing of their anticipatory postural adjustments (APAs) prior to lifting the foot during step initiation (Crenna et al., 1990; Gantchev et al., 1996; Burleigh-Jacobs et al., 1997; Rocchi et al., 2006). The APA represents an important stabilizing feature of step initiation, during which pressure increases under the swing limb to displace and stabilize the center of mass over the stance limb in preparation for the step (Elble et al., 1994). The neural substrates that underlie the impaired APAs of participants with PD, however, are not clear and need to be better understood in order to direct behavioral, pharmacological, and surgical therapies aimed to improve the step initiation of people with PD.
Relatively little is understood about how parkinsonian neuropathology contributes to step initiation, in part, because little detail is available regarding the neural control of step initiation in healthy participants, particularly at the level of the cerebral cortex. The supplementary motor area (SMA), however, represents a potential locus of control for generating the APA as well as a potential locus of neuropathology for the impaired APAs that are evident with PD during step initiation. In general, the SMA contributes to generating self-initiated, multi-segmental voluntary movements (Nachev et al., 2008). With specific regard to gait and step initiation, activation of the SMA is evident using single-photon or positron emission tomography during actual and imagined gait or step initiation (Hanakawa et al., 1999a,b; Malouin et al., 2003). In addition, gait apraxia with ignition failure is evident from individuals with SMA lesions (Della Sala et al., 2002; Nadeau, 2007), but these studies could not detail the specific contribution of the SMA to the generation of the step’s APA. Human lesion studies focused on APA function (Gurfinkel and Elner, 1988; Viallet et al., 1992) have shown that loss of the SMA leads to diminished APA amplitudes in preparation for upper limb movements, but these studies did not investigate step initiation, the lesions were often not localized to a single cortical region, and lesion studies are inherently subject to confounding long-term compensatory changes in cortical function (Ward, 2005). Therefore, it remains necessary to determine how the SMA specifically contributes to the generation of the APA during step initiation in order to better understand the neuropathology of impaired step initiation evident with disorders such as PD.
In addition to the studies implicating the SMA in the generation of the APA, people with PD exhibit altered SMA function when stepping. Specifically, people with PD exhibit hypoactivity of the SMA during gait (Hanakawa et al., 1999b) and diminished pre-movement electroencephalographic potentials during step initiation (Vidailhet et al., 1993), which are thought to represent SMA activation that contributes to the generation of APAs (Saitou et al., 1996). Therefore, impaired APAs appear coincidental with impaired SMA activity during stepping for people with PD, although changes in SMA activity have never been directly associated with changes in the characteristics of the APA during step initiation.
In order to provide a more detailed understanding of how the SMA contributes to the generation of the APA in people with and without PD, it would be useful to elicit a temporary disruption of the SMA and to record individuals with and without PD as they initiate steps during this period of disrupted SMA function. To do so, we selectively disrupted the SMA with subthreshold, 1-Hz repetitive transcranial magnetic stimulation (rTMS) and evaluated the effects of the stimulation on the APA during step initiation. We also applied rTMS over the dorsolateral premotor cortex (dlPMC) as a control site because it is not hypothesized to be involved in the control of APAs during self-initiated movement. To our knowledge, this is the first study to utilize rTMS over the SMA for the purpose of studying the APA during step initiation, as previous TMS studies evaluating the role of the cerebral cortex during dynamic postural tasks assessed the motor evoked potentials elicited by TMS to the primary motor cortex during step initiation (MacKinnon et al., 2007), an upper-limb unloading task (Kazennikov et al., 2008), steady-state gait (Schubert et al., 1997), or postural responses to an induced loss of balance (Taube et al., 2006).
Consistent with lesion studies (Gurfinkel and Elner, 1988; Viallet et al., 1992), we hypothesized that the SMA contributes to generating the amplitude and timing of the APA. Because decreased APA amplitudes and increased latencies appear to coincide with decreased SMA activity, we predicted that a temporary inhibition of the SMA by sub-threshold, 1-Hz rTMS (Touge et al., 2001) would decrease APA amplitudes and increase APA durations. We also hypothesized that participants with PD exhibit impaired step initiation due to dysfunction of the SMA. We thus predicted that rTMS over the SMA would alter the APAs of participants with PD such that the extent of these stimulation-induced changes would relate to the severity of their motor symptoms because increasing motor impairment would associate with escalating SMA dysfunction, thereby increasing susceptibility to rTMS.
EXPERIMENTAL PROCEDURES
Participants
Eight individuals with idiopathic PD (Hughes et al., 1992) and eight individuals without PD participated in the protocol after providing written informed consent in accordance with the Helsinki agreement. The local Institutional Review Board approved the protocol. Each group consisted of seven males and one female. Participants were chosen to ensure similar characteristics. Consequently, no significant differences were evident between the groups with and without PD, respectively, in mean (± SD) age (62 ± 11 versus 64 ± 10 yr), height (176 ± 6 versus 174 ± 11 cm), and weight (74 ± 10 versus 81 ± 9 kg) [respectively, T = 0.34, 0.37, and 1.58; P = 0.74, 0.72, and 0.14].
All participants with PD were tested while in the practical “off” medication state, at least 12 hours after their last dose of anti-Parkinson’s medication. Participants with other neurological, muscular, or psychiatric disorders (e.g., diabetes, peripheral neuropathies, uncorrected visual problems, hearing problems, joint pain, arthritis, fracture, stroke, seizure, migraine, or frequent severe headaches) were excluded. Participants with surgical implants, significant postural tremor, dyskinesia, or dementia were also excluded. Prior to each experiment, a neurologist trained in movement disorders evaluated the severity of the PD participants’ motor symptoms using the Unified Parkinson’s Disease Rating Scale (UPDRS) and Hoehn & Yahr scale (Hoehn and Yahr, 1967; Fahn and Elton, 1987). Total scores ranged from 9–28 on the motor exam of the UPDRS and from 2–3 on the Hoehn & Yahr scale. Based on these evaluations, all participants with PD exhibited mild to moderate PD with limb rigidity, impaired gait, and bradykinesia.
Stepping Protocol
The task was for the participants to stand with each foot on a force plate and then to take two self-initiated, forward voluntary steps with their eyes closed. The participants were asked to step without cues and with their eyes closed because participants with PD preferentially activate the dlPMC (Hanakawa et al., 1999a; Cunnington et al., 2001) as well as increase APA amplitude, step length, and step velocity toward healthy values when provided with sensory cues (Burleigh-Jacobs et al., 1997; Lewis et al., 2000; Morris et al., 1996, 2005; Suteerawattananon et al., 2004).
The participants stood in a stance width that equaled 11 % of their body height as measured from the center of one heel to the center of the other (McIlroy and Maki, 1997). The perimeters of the participants’ feet were marked with tape to ensure that stance width remained consistent throughout the experiment. We monitored the force distribution under the participants’ feet by an oscilloscope to ensure that the participants stood with an equal amount of weight under each foot prior to stepping. To prevent the participants from falling, they were harnessed to a ceiling-mounted track that did not provide any support during the task unless they began to fall.
The participants were instructed to close their eyes and, after a self-selected amount of time, to step forward with a pre-determined stepping foot, followed by a matching step with the initial stance limb to bring their feet back to parallel. The participants with PD were instructed to step with the leg most affected by the disease, as determined from the UPDRS motor exam, and those without PD stepped with the same leg as the participant with PD who was most closely matched for gender and age. Each participant performed nine steps before rTMS and nine steps after rTMS. The participants performed separate sessions in counter-balanced order for rTMS over the SMA and dlPMC. The sessions were separated by at least seven days, and the participants with PD always performed the experiment in the morning, after withholding their anti-Parkinson’s medications overnight.
As part of a larger protocol, the participants also performed visually cued voluntary steps, forced steps in response to platform translations, and quiet stance trials with their eyes closed. The tasks were ordered such that the participants first performed three trials of self-initiated steps, followed by three-trial blocks of the other tasks (Table 1). This sequence was repeated twice more to achieve a total of nine trials for each task. The first three self-initiated steps were, therefore, always ordered before the other tasks and, because the significant effects of rTMS were only evident for one trial after stimulation, the analyses for this study pertain only to the self-initiated steps with the eyes closed.
Table 1.
Presentation of the Self-Initiated Step Condition Within the Larger Protocol
| Trials | Condition |
|||
|---|---|---|---|---|
| Self-Initiated Stepping |
Cued Stepping |
Forced Stepping |
30 seconds Quiet Stance |
|
| 1–3 | X | |||
| 4–6 | X | |||
| 7–9 | X | |||
| 10 | X | |||
| 11–13 | X | |||
| 14–16 | X | |||
| 17–19 | X | |||
| 20 | X | |||
| 21–23 | X | |||
| 24–26 | X | |||
| 27–29 | X | |||
| 30 | X | |||
| 30 minutes rTMS |
||||
| 31–33 | X | |||
| 34–36 | X | |||
| 37–39 | X | |||
| 40 | X | |||
| 41–43 | X | |||
| 44–46 | X | |||
| 47–49 | X | |||
| 50 | X | |||
| 51–53 | X | |||
| 54–56 | X | |||
| 57–59 | X | |||
| 60 | X | |||
rTMS Protocol
After completing the stepping protocol, the participants sat upright in an adjustable dental chair mounted on locking wheels to prepare them for rTMS. For each participant, we first marked the scalp with a 1-cm grid of lines centered at the scalp’s vertex (according to the 10/20 system; Jasper, 1958) using a wax pencil. We defined the SMA and dlPMC locations of rTMS as a specified distance from the optimal positions to stimulate the tibialis anterior (TA, a distal leg muscle) and the first dorsal interosseous (FDI, a hand muscle) ipsilateral to each participant’s chosen stepping limb using single-pulse stimulations from a Magstim rapid rate device with a 70-mm, figure-eight, cooled-coil system (Magstim Company Ltd, Whitland, Dyfed, UK). We recorded muscle activity using pre-amplified differential electromyography from silver, silver-chloride electrodes placed over the muscles on the skin’s surface. To identify the optimal scalp locations for eliciting motor evoked potentials (MEPs) of maximal amplitude and shortest latency from the FDI and TA muscles (the hotspots), we applied stimulations at multiple locations separated by 1-cm increments, progressing to 0.5-cm increments. For the FDI muscle, the coil was positioned contralateral to the FDI muscle being stimulated and oriented so that its handle pointed approximately 45 degrees postero-lateral from the mid-sagittal line (Werhahn et al., 1994). For the TA muscle, the coil was oriented so that its handle pointed approximately perpendicular to the mid-sagittal line, ipsilateral to the stimulated TA muscle (Priori et al., 1993; Terao et al., 1994).
After locating the stimulation hotspots for the TA and FDI muscles, we determined the threshold for stimulating the FDI muscle at rest. The rest motor threshold was defined to be the stimulation intensity that elicited MEPs of at least 50 µV in five out of ten consecutive trials of single-pulse stimulations (Rossini et al., 1994). Although the participants performed a stepping task, we determined the rTMS intensities from the rest motor threshold of the FDI muscle because, in our experience, the FDI requires lower stimulation intensity than the TA to evoke muscle activation, and the FDI elicits more stable thresholds than the TA muscle when assessed on separate days. Therefore, using the FDI muscle’s threshold, we could produce more consistent stimulation intensities across the experimental sessions (which were separated by at least 7 days) and employ lower stimulation intensities that are less likely to induce adverse effects.
After determining the participants’ rest motor threshold, we prepared the participants for rTMS by reclining them in the adjustable chair and then fitting an elastic band around their head until the participants felt comfortable while maintaining their head in a stable position (Fig. 1A). For each participant, the intensity of stimulation during rTMS was set to 80% of the FDI’s rest motor threshold recorded during that day’s session. Repetitive TMS was delivered at one hertz for 30 minutes (1800 pulses) through the same stimulator and coil as when locating hotspots and determining motor thresholds. Sub-threshold, 1-Hz stimulations were chosen to maximize the safety of our protocol (Wassermann, 1998) and decrease spread of excitation to adjacent regions (Lang et al., 2006). Every 2.5 to 5 minutes during rTMS, we monitored the participants to ensure they remained awake and that their head position hadn’t shifted. When the 30 minutes of rTMS was complete, we rolled the participants in the chair to the force platform in order to minimize how much they actively moved before repeating the stepping protocol described above, because voluntary contraction can normalize cortical excitability after rTMS conditioning (Touge et al., 2001).
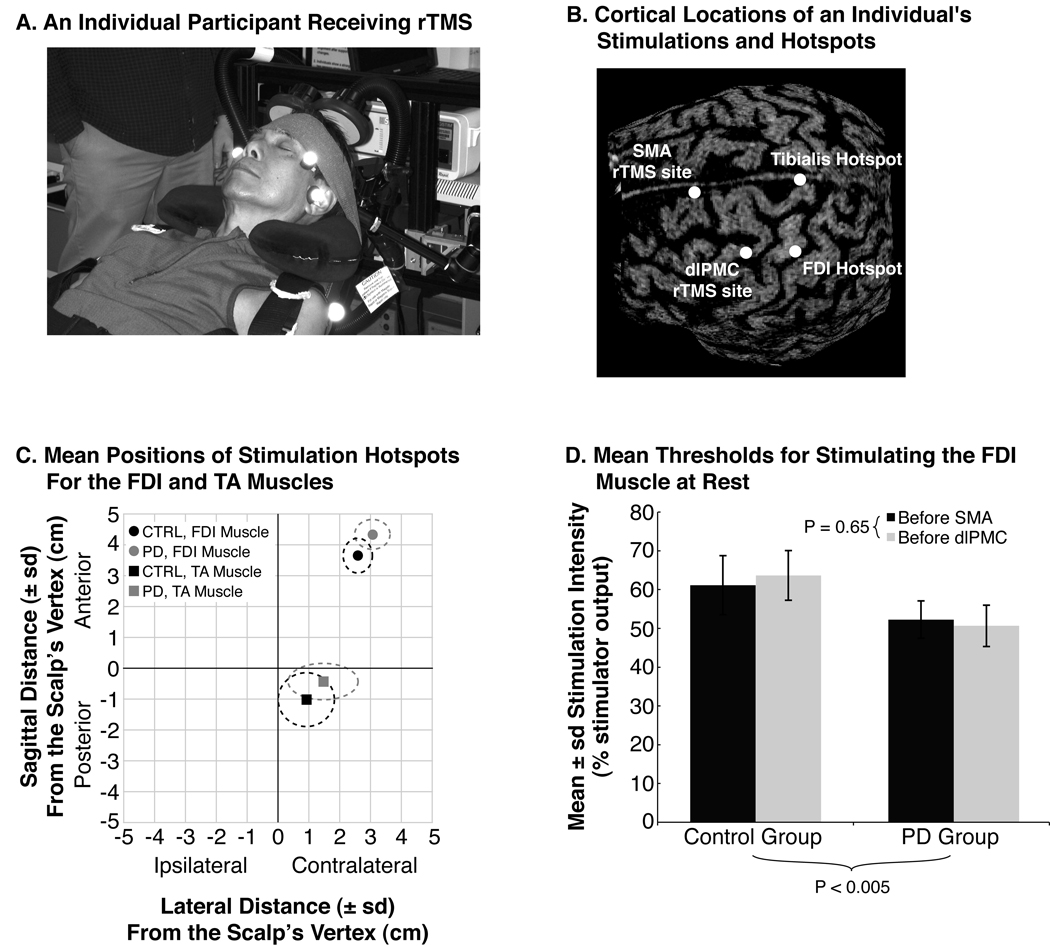
Fig. 1.
Characteristics of rTMS. (A) A participant receiving rTMS over the SMA. The participant sat reclined in an adjustable dental chair with a memory foam pillow supporting his head and neck. An elastic band was also wrapped around the forehead to prevent excessive movement. The air-cooled coil of the Magstim rapid device was held in place by an adjustable clamp. (B) Image-guided TMS, demonstrating the cortical locations of muscle hotspots and of rTMS. (C) The average (SD) hotspot locations for the participants with PD (gray symbols and dashed lines) and the participants without PD (black symbols and dashed lines), relative to the vertex of the skull. The squares represent the hotspots for stimulating the TA muscle, and circles represent those for stimulating the FDI muscle. (D) The average (SD) rest motor thresholds of the FDI muscle during the sessions for rTMS over the SMA (dark gray bars) and dlPMC (light gray bars). Repetitive TMS was applied at 80 % of each participant’s rest motor threshold for that day’s session. The p-value below the chart represents the main effect of group differences, and the p-value next to the inset legend represents the main effect of session differences.
When stimulating the SMA, the coil was positioned 5 cm anterior from the TA muscle’s hotspot along the mid-sagittal line. The coil was oriented with its handle pointing posterior along the mid-sagittal line (Cunnington et al., 1996; Obhi et al., 2002; Verwey et al., 2002). These coordinates are consistent with studies using image-guided TMS or functional imaging to localize the pre-SMA/SMA transition (Rushworth et al., 2002; Mayka et al., 2006) so that, when accounting for the caudal extent of the induced electric field from the coil’s hotspot (Pascual-Leone et al., 1999), this position likely elicited an induced electric field that spanned the SMA. When stimulating the dlPMC, the coil was positioned 2.5 cm anterior from the FDI muscle’s hotspot, with the handle oriented approximately 45 degrees postero-lateral from the mid-sagittal line (Gerschlager et al., 2001; Chen et al., 2003).
To confirm that our measured scalp locations placed the coil over the intended cortical regions, we obtained an anatomical magnetic resonance image (MRI) of the first healthy participant’s brain for use with image-guided TMS. The structural MRI was acquired with a 1.5 tesla magnet using multi-echo, multi-planar acquisition. Images were obtained in the coronal plane at 4-mm thickness. For image-guided TMS, the participant’s anatomical MRI was stereotactically co-registered with the participant’s head using a Polaris infrared tracking system (Northern Digital, Waterloo, Canada) interfaced with Brainsight software (Rogue Research, Montreal, Canada). The position of the TMS coil was then monitored with respect to the participant’s brain, and we acquired digital images of the coil’s locations when it was centered over the rTMS and hotspot locations outlined in the methods above.
Data Collection and Analysis
To capture the participants’ APAs, we recorded the lateral displacements of their center of pressure (CoP) from two force plates, one under each of the participants’ feet. Each force plate was equipped with four vertical and two horizontal strain gauge transducers. Force signals were amplified and sampled at 480 Hz. Total-body lateral CoP was calculated from the difference in loading of the right and left force plates as previously reported by Henry et al. (1998). Lateral CoP displacements were calculated after subtracting an initial CoP position, which was defined as the average CoP position over the first 500 ms of recording.
The onset of an APA was defined manually with an interactive plotting function programmed in Matlab software (Mathworks, Inc., Natick, MA, USA). Using this plotting function, we identified the moment when the CoP began to displace toward the swing limb prior to foot-lift. When identifying APA onsets, the CoP plots were unlabeled and randomly ordered to prevent biased identifications. The duration of an APA was calculated as the time when the lateral CoP displacement came back to its initial position just prior to when a participant lifted a foot off the force plate, minus the time when the APA began. Peak APA amplitudes were defined as the maximum lateral displacement of the CoP toward the swing limb just prior to foot-lift.
We calculated each participant’s average APA duration, the variability (i.e., the SD) of each participant’s APA durations, and the average peak APA amplitude prior to rTMS. Two-factor mixed-model ANOVA determined whether these measures were different between groups (with PD versus without PD) and stable between experimental sessions (SMA versus dlPMC). For each site of rTMS, a three-factor mixed-model ANOVA tested for differences in the dependent measures between groups (with PD versus without PD), trials (one through nine), and rTMS (before versus after). The factor for trial was included because it was unclear how long any effects due to rTMS would last. Pearson coefficients were analyzed to determine whether the effects of rTMS on the APAs correlated with the clinical severity of the PD participants’ lower-body motor symptoms. Because the results demonstrated that the effects of rTMS lasted for only one trial, the correlations were based on the difference between a measure’s value during the first trial after rTMS and its mean value from the trials before rTMS. The clinical severity of a PD participant’s lower-body motor symptoms was defined as the sum of the UPDRS items of leg tremor (sub-scores of item 20) and leg rigidity (sub-scores of item 22), as well as leg agility, arise from chair, posture, gait, postural stability, and body bradykinesia (items 26–31); with a possible range of scores from zero (no symptoms) to 44 (most severe symptoms), this subset of UPDRS items was chosen to render the score more directly relevant to the stepping task (Jacobs and Horak, 2006). Significance was defined as a P-value of less than or equal to 0.05.
RESULTS
Locations and Intensities of rTMS
The session of image-guided TMS confirmed that our measures located the FDI and TA muscles’ hotspots over the pre-central gyrus, and that the locations for rTMS over the SMA and dlPMC were consistent with previous reports localizing these regions (Fig. 1B; Gerschlager et al., 2001; Rushworth et al., 2002). Relative to the vertex of each participant’s scalp, the sagittal position of the FDI muscle’s hotspot was more anterior for the participants with PD than for the participants without PD [main effect of group: F = 7.54, P < 0.05] (Fig. 1C). The sagittal position of the TA muscle’s hotspot and the lateral positions of the TA and FDI muscles’ hotspots were not significantly different between the participants with and without PD [main effect of group: F = 1.13–2.94, P = 0.11–0.31]. Rest motor thresholds were significantly lower in the participants with PD compared to those without PD [main effect of group: F = 14.06, P < 0.005], but thresholds remained similar between experimental sessions [main effect of session: F = 0.22, P = 0.65] (Fig. 1D). Based on timestamps associated with the electronic files for each trial, the first two stepping trials were initiated within two minutes after rTMS for both participant groups.
APA Characteristics Before Stimulation
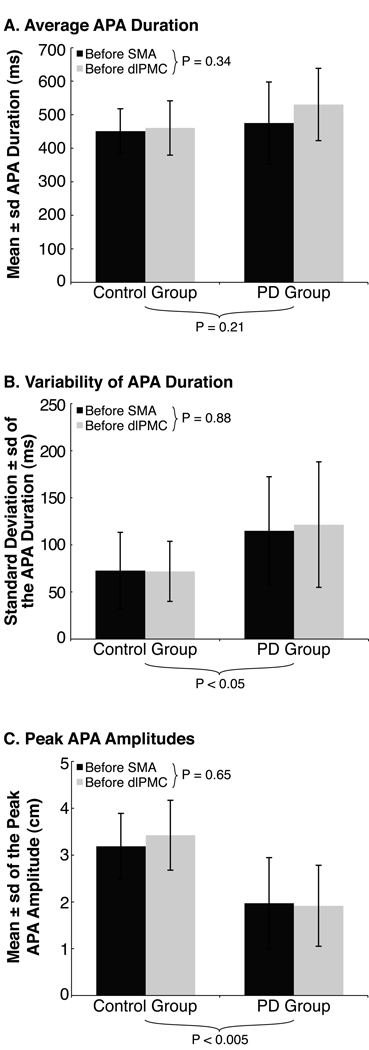
The participants with PD exhibited impaired APA control. Specifically, although APA durations were, on average, similar between participants with and without PD [main effect of group: F = 1.71, P = 0.21], APA durations were more variable for the participants with PD [main effect of group: F = 5.45, P < 0.05] (Fig. 2A and B). The participants with PD also exhibited smaller peak APA amplitudes than the participants without PD [main effect of group: F = 12.82, P < 0.005] (Fig. 2C). No significant differences were evident between the experimental sessions for any measure of the APA [main effects of session: F = 0.02–0.99, P = 0.34–0.88] (Fig. 2).
Fig. 2.
Characteristics of the APA prior to rTMS. The charts illustrate each group’s average (SD) (A) APA duration, (B) inter-trial variability of APA duration, and (C) peak APA amplitude prior to rTMS during the SMA (dark gray bars) and dlPMC (light gray bars) sessions. P-values below the charts represent main effects for group differences, those next to the inset legends represent main effects for session differences.
Effects of rTMS
APA durations significantly decreased for the first stepping trial after rTMS over the SMA [interaction effects of rTMS and trial: F = 2.25, P < 0.05], whereas APA durations remained similar after rTMS over the dlPMC [interaction effects of rTMS and trial: F = 1.26, P < 0.27] (Fig. 3A and B). No significant 2- or 3-way interactions were evident among the factor for group and the factors for trial and rTMS, either for rTMS over the SMA or the dlPMC [range of F = 0.09–1.46, range of P = 0.18–0.77]. The lack of significant interactions involving group differences appears due to high inter-individual variability within the group with PD: the mean (± SD) decrease in APA durations between the first trial after rTMS over the SMA and the mean of trials before rTMS was 130 ± 113 ms for the group with PD compared to 55 ± 18 ms for the group without PD.
Fig. 3.
Effects of rTMS on the APA. (A) An example of shortened APA duration for one trial after rTMS over the SMA from an individual with PD. The horizontal axis represents time relative to APA onset, and the vertical axis represents the lateral displacement of the CoP for individual trials before stimulation (the thin gray curves), the average of trials before stimulation (the thin black curve), and for the first trial after SMA stimulation (the thick gray curve). Negative displacements are directed toward the participant’s swing limb. (B) Average APA durations by trial for all participants, demonstrating how APA durations decreased for only one trial after SMA stimulation; no group effects were evident. The black line with squares represents the mean APA durations from the session of rTMS over the SMA; the gray line with circles, the session of rTMS over the dlPMC. The asterisk highlights the first trial after rTMS because this trial was significantly different from others. (C) Average peak APA amplitudes by trial for all participants, demonstrating how APA amplitudes were smallest for the sessions’ first trials compared to subsequent trials (asterisk); no significant changes following rTMS and no group effects were evident.
Stimulation with rTMS over the SMA or dlPMC had no effect on APA amplitudes. Although significant differences in peak APA amplitudes were evident among trials [main effect of trial: F = 2.88, P < 0.01], with interactions among the trials before and after rTMS over the SMA [interaction effects of rTMS and trial: F = 2.67, P = 0.01], these effects were not related to the rTMS but were evident due to smaller peak APA amplitudes during the first trial of the session relative to subsequent trials (Fig. 3C). Although not statistically significant, similar trends were evident during the dlPMC session (Fig. 3C) [main effect of trial: F = 1.78, P = 0.09; interaction effects of rTMS and trial: F = 1.59, P = 0.16]. No significant 2- or 3-way interactions were evident among the factor for group and the factors for trial and rTMS, either for rTMS over the SMA or the dlPMC [range of F = 0.29–0.53, range of P = 0.60–0.83].
The effect of rTMS over the SMA on the APA durations of the participants with PD significantly correlated with the severity of their lower-body symptoms [Pearson r2 = 0.70, P < 0.01] (Fig. 4).
Fig. 4.
A scatter plot illustrating a significant correlation among the PD participants’ disease severity (measured by lower-body motor UPDRS scores) and the extent that rTMS over the SMA affected APA durations. The circles represent the values for individual participants with PD; the Xs, those of the participants without PD. Although the UPDRS was not assessed for those without PD, their values are depicted with an assumed UPDRS score of zero. The horizontal axis has been changed so that positive values represent a decrease in APA duration following rTMS in order to illustrate a positive correlation among disease severity and the effect of rTMS on APA duration.
DISCUSSION
The results demonstrated that rTMS over the SMA transiently shortened APA durations, whereas rTMS over the dlPMC did not, and no effects of rTMS were evident on APA amplitudes. In addition, the extent to which rTMS over the SMA affected the APA durations of the participants with PD positively correlated with the clinical severity of their lower-body motor symptoms. While rTMS over the SMA consistently decreased APA durations by less than 100 ms for the participants without PD, rTMS elicited far greater decreases in APA duration for the participants with PD who exhibited the greatest symptom severity. It has been suggested that the progression to the late stages of PD associates with a progressive degeneration of cortical structures such as the SMA (Braak et al., 2002). Therefore, we speculate that the increased efficacy of rTMS to alter the APA durations of participants with more severe parkinsonian symptoms likely reflects greater susceptibility of the SMA to rTMS due to its progressive degeneration. Any further speculation regarding the cellular basis of this effect is precluded, however, by a lack of understanding for whether sub-threshold, 1-Hz rTMS over the SMA elicits the same decrease in corticospinal excitability and the same dysfacilitation of cortico-cortical excitability as that which results from similar stimulations of the primary motor cortex (Romero et al., 2002). Further, it remains unclear whether the effects of rTMS over the SMA on APA durations result from alterations in corticospinal activity or in extra-pyramidal activity. Therefore, further study is necessary to identify the neurophysiologic basis by which rTMS over the SMA affects the APA during step initiation. Nevertheless, the specificity of the effects to only the SMA confirms that the effects were due to the stimulation of that cortical location and not due to trial order or the general procedures experienced during both rTMS sessions (such as lying reclined during stimulation). Taken together, then, the results suggest that the SMA contributes to coordinating the timing of the APA, and participants with PD exhibit impaired step initiation, in part, due to progressive dysfunction of circuits involving the SMA.
The direction and length of time with which rTMS effected APA durations, however, were contrary to our predictions: rTMS over the SMA shortened APA duration without altering APA amplitude, suggesting an increase in the velocity of the APA’s weight shift, and the effect of rTMS lasted for only one trial after stimulation. Regarding the duration of the effect, it has been reported that voluntary muscle activation can normalize rTMS-induced changes in cortical excitability (Touge et al. 2001). Thus, in our study, any rTMS-induced changes in the participants’ neuromotor state may have been normalized after the first trial due to feedback processing experienced during the first trial after stimulation. Regarding the direction of the rTMS effect on APA durations, our assumption of decreased SMA activity may be false: in a study investigating brain activation during sequential hand movements, participants with PD were reported to exhibit decreased activation of the rostral SMA but increased activation of the caudal SMA (Sabatini et al., 2000). Thus, the decrease in APA duration observed in this study may reflect either a more significant effect of rTMS to the caudal SMA than to the rostral SMA, or an inhibitory role of the SMA in defining the duration of the APA, such that activation of the SMA slows the APA.
The lack of effect of rTMS on APA amplitudes was also contrary to our predictions based on previous reports of diminished APA amplitudes with lesions to the SMA (Gurfinkel and Elner, 1988; Viallet et al., 1992). The decrease in APA amplitude with cortical lesions, however, may reflect a lack of regional specificity of the lesions or reflect effects of the lesions on other regions with input from the SMA that are also hypothesized to contribute to generating the APA, such as the primary motor cortex or the basal ganglia (Massion, 1992; MacKinnon et al., 2007). Thus, the SMA may contribute to the timing of the APA, whereas amplitude modulation may be relegated to the primary motor cortex or basal ganglia.
The effects of rTMS to a specific region of the cerebral cortex, however, may not represent a direct effect of that cortical region on the behavior. Studies have demonstrated that sub-threshold, 1-Hz rTMS over one site can elicit changes in the activity and excitability of other neural sites, presumably through communicating fibers (Gerschlager et al., 2001; Speer et al., 2003; Bestmann et al., 2005). Thus, in this study, changes in APA duration after rTMS over the SMA may represent an indirect influence of the stimulated site on other neural centers involved in regulating postural preparation during step initiation. We suggest, however, that the SMA likely exerts some direct influence because (1) no significant changes in APA duration were evident following rTMS over the dlPMC, which (like the SMA) represents an executive motor center with projections to the primary motor cortex and to the motor horn of the spinal cord (Dum and Strick, 2002), and (2) the effects of rTMS over the SMA on APA duration were evident in both the groups with and without PD, suggesting this effect was not indirectly related to the differential projections of the SMA and dlPMC to the basal ganglia (Leh et al., 2007).
Consistent with previous reports (Tremblay and Tremblay, 2002; Lou et al., 2003), the motor thresholds for stimulating the FDI muscle were lower for the participants with PD than for those without PD. Consequently, rTMS intensities were lower for the participants with PD and stimulating the groups with different absolute intensities may have diminished the effect of rTMS on the participants with PD. The intensities, however, were normalized to the corticospinal excitability of each participant, and our results never showed any group-by-stimulation interactions characterized by an effect of rTMS in the group without PD and no effect in the group with PD. In addition to decreased motor thresholds, hotspot locations were displaced for the participants with PD compared to those without PD, which is consistent with an altered somatotopic organization of the primary motor cortex in people with PD. Such a shift has been previously reported and postulated to be evident due to a shift in the synaptic excitability of inputs to the primary motor cortex consequent to the altered excitability of the striato-thalamo-cortical loops that occurs with PD (Thickbroom et al., 2006).
In summary, the results support a neural control model for voluntary step initiation in which the SMA coordinates the timing of the APA, independent of control on APA amplitude. In addition, patients with PD likely exhibit abnormal APA timing due to dysfunction of the SMA, whereas diminished APA amplitudes may be a result of pathology to other affected regions such as the primary motor cortex or the basal ganglia. The results suggest that the impaired balance and mobility of individuals with PD may be associated with dysfunction of the SMA and that treatments targeted to improve this dysfunction may be useful to ameliorate the disabilities associated with impaired step initiation.
ACKNOWLEDGEMENT
We thank our research participants for their participation, Andrew Owings and Ryan Eaton for hardware assistance, as well as Dr. John Nutt and the staff of the Parkinson’s Center of Oregon for their help with participant recruitment. We also thank Triana Nagel-Nelson and the other members of the Balance Disorders Laboratory for assisting the participants during data collection.
This research was supported by the National Institutes of Health grants F31NS048800 (Jacobs) from the National Institute of Neurological Disorders and Stroke, and AG-06457 (Horak) from the National Institute of Aging.
LIST OF ABBREVIATIONS
- ANOVA
analysis of variance
- APA
anticipatory postural adjustment
- CoP
center of pressure
- dlPMC
dorsolateral premotor cortex
- FDI
first dorsal interosseous
- MRI
magnetic resonance image
- PD
Parkinson’s disease
- rTMS
repetitive transcranial magnetic stimulation
- SD
standard deviation
- SMA
supplementary motor area
- TA
tibialis anterior
- UPDRS
Unified Parkinson’s Disease Rating Scale
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
REFERENCES
- Bestmann S, Baudewig J, Siebner HR, Rothwell JC, Frahm J. BOLD MRI responses to repetitive TMS over human dorsal premotor cortex. Neuroimage. 2005;28:22–29. doi: 10.1016/j.neuroimage.2005.05.027. [DOI] [PubMed] [Google Scholar]
- Bloem BR, Grimbergen YA, Cramer M, Willemsen M, Zwinderman AH. Prospective assessment of falls in Parkinson’s disease. J Neurol. 2001;248:950–958. doi: 10.1007/s004150170047. [DOI] [PubMed] [Google Scholar]
- Bloem BR, Hausdorff JM, Visser JE, Giladi N. Falls and freezing of gait in Parkinson’s disease: a review of two interconnected, episodic phenomena. Mov Disord. 2004;19:871–884. doi: 10.1002/mds.20115. [DOI] [PubMed] [Google Scholar]
- Boylan LS, Pullman SL, Lisanby SH, Spicknall KE, Sackeim HA. Repetitive transcranial magnetic stimulation to SMA worsens complex movements in Parkinson’s disease. Clin Neurophysiol. 2001;112:259–264. doi: 10.1016/s1388-2457(00)00519-8. [DOI] [PubMed] [Google Scholar]
- Braak H, Del Tredici K, Bratzke H, Hamm-Clement J, Sandmann-Keil D, Rüb U. Staging of the intracerebral inclusion body pathology associated with idiopathic Parkinson’s disease (preclinical and clinical stages) J Neurol. 2002;249 Suppl 3:1–5. doi: 10.1007/s00415-002-1301-4. [DOI] [PubMed] [Google Scholar]
- Buhmann C, Gorsler A, Baumer T, Hidding U, Demiralay C, Hinkelmann K, Weiller C, Siebner HR, Munchau A. Abnormal excitability of premotor-motor connections in de novo Parkinson’s disease. Brain. 2004;127:2732–2746. doi: 10.1093/brain/awh321. [DOI] [PubMed] [Google Scholar]
- Burleigh-Jacobs A, Horak FB, Nutt JG, Obeso JA. Step initiation in Parkinson’s disease: influence of levodopa and external sensory triggers. Mov Disord. 1997;12:206–215. doi: 10.1002/mds.870120211. [DOI] [PubMed] [Google Scholar]
- Chen WH, Mima T, Siebner HR, Oga T, Hara H, Satow T, Begum T, Nagamine T, Shibasaki H. Low-frequency rTMS over lateral premotor cortex induces lasting changes in regional activation and functional coupling of cortical motor areas. Clin Neurophysiol. 2003;114:1628–1637. doi: 10.1016/s1388-2457(03)00063-4. [DOI] [PubMed] [Google Scholar]
- Crenna P, Frigo C, Giovannini P, Piccolo I. The initiation of gait in Parkinson’s disease. In: Berardelli A, Benecke M, Manfredi M, Marsden CD, editors. Motor Disturbances II. London: Academic Press; 1990. pp. 161–173. [Google Scholar]
- Cunnington R, Lalouschek W, Dirnberger G, Walla P, Lindinger G, Asenbaum S, Brucke T, Lang W. A medial to lateral shift in pre-movement cortical activity in hemi Parkinson’s disease. Clin Neurophysiol. 2001;112:608–618. doi: 10.1016/s1388-2457(01)00467-9. [DOI] [PubMed] [Google Scholar]
- Cunnington R, Iansek R, Thickbroom GW, Laing BA, Mastaglia FL, Bradshaw JL, Phillips JG. Effects of magnetic stimulation over supplementary motor area on movement in Parkinson’s disease. Brain. 1996;119:815–822. doi: 10.1093/brain/119.3.815. [DOI] [PubMed] [Google Scholar]
- DellaSala S, Francescani A, Spinnler H. Gait apraxia after bilateral supplementary motor area lesion. J Neurol Neurosurg Psychiatry. 2002;72:77–85. doi: 10.1136/jnnp.72.1.77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dum RP, Strick PL. Motor areas in the frontal lobe of the primate. Physiol Behav. 2002;77:677–682. doi: 10.1016/s0031-9384(02)00929-0. [DOI] [PubMed] [Google Scholar]
- Elble RJ, Moody C, Leffler K, Sinha R. The initiation of normal walking. Mov Disord. 1994;9:139–146. doi: 10.1002/mds.870090203. [DOI] [PubMed] [Google Scholar]
- Fahn S, Elton R. Recent Developments in Parkinson Diseases. In: Fahn S, Marsden D, Calne D, editors. Unified Parkinson’s disease rating scale. London: Macmillan; 1987. pp. 153–163. [Google Scholar]
- Fitzgerald PB, Brown TL, Daskalakis ZJ, Chen R, Kulkarni J. Intensity-dependent effects of 1 Hz rTMS on human corticospinal excitability. Clin Neurophysiol. 2002;113:1136–1141. doi: 10.1016/s1388-2457(02)00145-1. [DOI] [PubMed] [Google Scholar]
- Gantchev N, Viallet F, Aurenty R, Massion J. Impairment of posturo-kinetic co-ordination during initiation of forward oriented stepping movements in parkinsonian patients. Electroencephalogr Clin Neurophysiol. 1996;101:110–120. doi: 10.1016/0924-980x(95)00253-h. [DOI] [PubMed] [Google Scholar]
- Gerschlager W, Siebner HR, Rothwell JC. Decreased corticospinal excitability after subthreshold 1 Hz rTMS over lateral premotor cortex. Neurology. 2001;57:449–455. doi: 10.1212/wnl.57.3.449. [DOI] [PubMed] [Google Scholar]
- Gurfinkel VS, Elner AM. Participation of the secondary motor area of the frontal lobe of the brain in organizing postural components of human voluntary movement. Neirofiziologiia. 1988;20:7–15. [PubMed] [Google Scholar]
- Hanakawa T, Fukuyama H, Katsumi Y, Honda M, Shibasaki H. Enhanced lateral premotor activity during paradoxical gait in Parkinson’s disease. Ann Neurol. 1999a;45:329–336. doi: 10.1002/1531-8249(199903)45:3<329::aid-ana8>3.0.co;2-s. [DOI] [PubMed] [Google Scholar]
- Hanakawa T, Katsumi Y, Fukuyama H, Honda M, Hayashi T, Kimura J, Shibasaki H. Mechanisms underlying gait disturbance in Parkinson’s disease: a single photon emission computed tomography study. Brain. 1999b;122:1271–1282. doi: 10.1093/brain/122.7.1271. [DOI] [PubMed] [Google Scholar]
- Henry SM, Fung J, Horak FB. Control of stance during lateral and anterior/posterior surface translations. IEEE Trans Rehabil Eng. 1998;6:32–42. doi: 10.1109/86.662618. [DOI] [PubMed] [Google Scholar]
- Hoehn MM, Yahr MD. Parkinsonism: onset, progression and mortality. Neurology. 1967;17:427–442. doi: 10.1212/wnl.17.5.427. [DOI] [PubMed] [Google Scholar]
- Hughes AJ, Ben-Shlomo Y, Daniel SE, Lees AJ. What features improve the accuracy of clinical diagnosis in Parkinson’s disease: a clinicopathologic study. Neurology. 1992;42:1142–1146. doi: 10.1212/wnl.42.6.1142. [DOI] [PubMed] [Google Scholar]
- Jacobs JV, Horak FB. Abnormal proprioceptive-motor integration contributes to hypometric postural responses of participants with Parkinson’s disease. Neuroscience. 2006;141:999–1009. doi: 10.1016/j.neuroscience.2006.04.014. [DOI] [PubMed] [Google Scholar]
- Jasper HH. The Ten Twenty Electrode System of the International Federation. Clin Neurophysiol. 1958;10:371–375. [PubMed] [Google Scholar]
- Kazennikov O, Solopova I, Talis V, Ioffe M. Anticipatory postural adjustment: the role of motor cortex in the natural and learned bimanual unloading. Exp Brain Res. 2008;186:215–223. doi: 10.1007/s00221-007-1224-5. [DOI] [PubMed] [Google Scholar]
- Lang N, Harms J, Weyh T, Lemon RN, Paulus W, Rothwell JC, Siebner HR. Stimulus intensity and coil characteristics influence the efficacy of rTMS to suppress cortical excitability. Clin Neurophysiol. 2006;117:2292–2301. doi: 10.1016/j.clinph.2006.05.030. [DOI] [PubMed] [Google Scholar]
- Leh SE, Ptito A, Chakravarty MM, Strafella AP. Fronto-striatal connections in the human brain: a probabilistic diffusion tractography study. Neurosci Lett. 2007;419:113–118. doi: 10.1016/j.neulet.2007.04.049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lewis GN, Byblow WD, Walt SE. Stride length regulation in Parkinson’s disease: the use of extrinsic, visual cues. Brain. 2000;123:2077–2090. doi: 10.1093/brain/123.10.2077. [DOI] [PubMed] [Google Scholar]
- Lou JS, Benice T, Kearns G, Sexton G, Nutt J. Levodopa normalizes exercise related cortico-motoneuron excitability abnormalities in Parkinson’s disease. Clin Neurophysiol. 2003;114:930–937. doi: 10.1016/s1388-2457(03)00040-3. [DOI] [PubMed] [Google Scholar]
- MacKinnon CD, Bissig D, Chiusano J, Miller E, Rudnick L, Jager C, Zhang Y, Mille ML, Rogers MW. Preparation of anticipatory postural adjustments prior to stepping. J Neurophysiol. 2007;97:4368–4379. doi: 10.1152/jn.01136.2006. [DOI] [PubMed] [Google Scholar]
- Malouin F, Richards CL, Jackson PL, Dumas F, Doyon J. Brain activations during motor imagery of locomotor-related tasks: a PET study. Hum Brain Mapp. 2003;19:47–62. doi: 10.1002/hbm.10103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Massion J. Movement, posture and equilibrium: interaction and coordination. Prog Neurobiol. 1992;38:35–56. doi: 10.1016/0301-0082(92)90034-c. [DOI] [PubMed] [Google Scholar]
- Mayka MA, Corcos DM, Leurgans SE, Vaillancourt DE. Three-dimensional locations and boundaries of motor and premotor cortices as defined by functional brain imaging: a meta-analysis. Neuroimage. 2006;31:1453–1474. doi: 10.1016/j.neuroimage.2006.02.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McIlroy WE, Maki BE. Preferred placement of the feet during quiet stance: development of a standardized foot placement for balance testing. Clin Biomech. 1997;12:66–70. doi: 10.1016/s0268-0033(96)00040-x. [DOI] [PubMed] [Google Scholar]
- Morris ME, Iansek R, Matyas TA, Summers JJ. Stride length regulation in Parkinson's disease. Normalization strategies and underlying mechanisms. Brain. 1996;119:551–568. doi: 10.1093/brain/119.2.551. [DOI] [PubMed] [Google Scholar]
- Morris M, Iansek R, McGinley J, Matyas T, Huxham F. Three-dimensional gait biomechanics in Parkinson's disease: evidence for a centrally mediated amplitude regulation disorder. Mov Disord. 2005;20:40–50. doi: 10.1002/mds.20278. [DOI] [PubMed] [Google Scholar]
- Nachev P, Kennard C, Husain M. Functional role of the supplementary and pre supplementary motor areas. Nat Rev Neurosci. 2008;9:856–869. doi: 10.1038/nrn2478. [DOI] [PubMed] [Google Scholar]
- Nadeau SE. Gait apraxia: further clues to localization. Eur Neurol. 2007;58:142–145. doi: 10.1159/000104714. [DOI] [PubMed] [Google Scholar]
- Obhi SS, Haggard P, Taylor J, Pascual-Leone A. rTMS to the supplementary motor area disrupts bimanual coordination. Motor Control. 2002;6:319–332. doi: 10.1123/mcj.6.4.319. [DOI] [PubMed] [Google Scholar]
- Pascual-Leone A, Bartres-Faz D, Keenan JP. Transcranial magnetic stimulation: studying the brain-behaviour relationship by induction of ‘virtual lesions’. Philos Trans R Soc Lond B Biol Sci. 1999;354:1229–1238. doi: 10.1098/rstb.1999.0476. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Priori A, Bertolasi L, Dressler D, Rothwell JC, Day BL, Thompson PD, Marsden CD. Transcranial electric and magnetic stimulation of the leg area of the human motor cortex: single motor unit and surface EMG responses in the tibialis anterior muscle. Electroencephalogr Clin Neurophysiol. 1993;89:131–137. doi: 10.1016/0168-5597(93)90095-7. [DOI] [PubMed] [Google Scholar]
- Rocchi L, Chiari L, Mancini M, Carlson-Kuhta P, Gross A, Horak FB. Step initiation in Parkinson’s disease: Influence of initial stance conditions. Neurosci Lett. 2006;406:128–132. doi: 10.1016/j.neulet.2006.07.027. [DOI] [PubMed] [Google Scholar]
- Romero JR, Anschel D, Sparing R, Gangitano M, Pascual-Leone A. Subthreshold low frequency repetitive transcranial magnetic stimulation selectively decreases facilitation in the motor cortex. Clin Neurophysiol. 2002;113:101–107. doi: 10.1016/s1388-2457(01)00693-9. [DOI] [PubMed] [Google Scholar]
- Rossini PM, Barker AT, Berardelli A, Caramia MD, Caruso G, Cracco RQ, Dimitrijevic MR, Hallett M, Katayama Y, Lucking CH, Maertens de Noordhout AL, Marsden CD, Murray NMF, Rothwell JC, Swash M, Tomberg C. Non-invasive electrical and magnetic stimulation of the brain, spinal cord and roots: basic principles and procedures for routine clinical application. Report of an IFCN committee. Electroencephalogr Clin Neurophysiol. 1994;91:79–92. doi: 10.1016/0013-4694(94)90029-9. [DOI] [PubMed] [Google Scholar]
- Rushworth MF, Hadland KA, Paus T, Sipila PK. Role of the human medial frontal cortex in task switching: a combined fMRI and TMS study. J Neurophysiol. 2002;87:2577–2592. doi: 10.1152/jn.2002.87.5.2577. [DOI] [PubMed] [Google Scholar]
- Saitou K, Washimi Y, Koike Y, Takahashi A, Kaneoke Y. Slow negative cortical potential preceding the onset of postural adjustment. Electroencephalogr Clin Neurophysiol. 1996;98:449–455. doi: 10.1016/0013-4694(96)95004-x. [DOI] [PubMed] [Google Scholar]
- Sabatini U, Boulanouar K, Fabre N, Martin F, Carel C, Colonnese C, Bozzao L, Berry I, Montastruc JL, Chollet F, Rascol O. Cortical motor reorganization in akinetic patients with Parkinson’s disease: a functional MRI study. Brain. 2000;123:394–403. doi: 10.1093/brain/123.2.394. [DOI] [PubMed] [Google Scholar]
- Schubert M, Curt A, Jensen L, Dietz V. Corticospinal input in human gait: modulation of magnetically evoked motor responses. Exp Brain Res. 1997;115:234–246. doi: 10.1007/pl00005693. [DOI] [PubMed] [Google Scholar]
- Speer AM, Willis MW, Herscovitch P, Daube-Witherspoon M, Shelton JR, Benson BE, Post RM, Wassermann EM. Intensity-dependent regional cerebral blood flow during 1-Hz repetitive transcranial magnetic stimulation (rTMS) in healthy volunteers studied with H215O positron emission tomography: I. Effects of primary motor cortex rTMS. Biol Psychiatry. 2003;54:818–825. doi: 10.1016/s0006-3223(03)00002-7. [DOI] [PubMed] [Google Scholar]
- Suteerawattananon M, Morris GS, Etnyre BR, Jankovic J, Protas EJ. Effects of visual and auditory cues on gait in individuals with Parkinson’s disease. J Neurol Sci. 2004;219:63–69. doi: 10.1016/j.jns.2003.12.007. [DOI] [PubMed] [Google Scholar]
- Taube W, Schubert M, Gruber M, Beck S, Faist M, Gollhofer A. Direct corticospinal pathways contribute to neuromuscular control of perturbed stance. J Appl Physiol. 2006;101:420–429. doi: 10.1152/japplphysiol.01447.2005. [DOI] [PubMed] [Google Scholar]
- Terao Y, Ugawa Y, Sakai K, Uesaka Y, Kohara N, Kanazawa I. Transcranial stimulation of the leg area of the motor cortex in humans. Acta Neurol Scand. 1994;89:378–383. doi: 10.1111/j.1600-0404.1994.tb02650.x. [DOI] [PubMed] [Google Scholar]
- Thickbroom GW, Byrnes ML, Walters S, Stell R, Mastaglia FL. Motor cortex reorganisation in Parkinson’s disease. J Clin Neurosci. 2006;13:639–642. doi: 10.1016/j.jocn.2005.06.013. [DOI] [PubMed] [Google Scholar]
- Touge T, Gerschlager W, Brown P, Rothwell JC. Are the after-effects of low-frequency rTMS on motor cortex excitability due to changes in the efficacy of cortical synapses? Clin Neurophysiol. 2001;112:2138–2145. doi: 10.1016/s1388-2457(01)00651-4. [DOI] [PubMed] [Google Scholar]
- Tremblay F, Tremblay LE. Cortico-motor excitability of the lower limb motor representation: a comparative study in Parkinson’s disease and healthy controls. Clin Neurophysiol. 2002;113:2006–2012. doi: 10.1016/s1388-2457(02)00301-2. [DOI] [PubMed] [Google Scholar]
- Verwey WB, Lammens R, van Honk J. On the role of the SMA in the discrete sequence production task: a TMS study. Transcranial Magnetic Stimulation. Neuropsychologia. 2002;40:1268–1276. doi: 10.1016/s0028-3932(01)00221-4. [DOI] [PubMed] [Google Scholar]
- Viallet F, Massion J, Massarino R, Khalil R. Coordination between posture and movement in a bimanual load lifting task: putative role of a medial frontal region including the supplementary motor area. Exp Brain Res. 1992;88:674–684. doi: 10.1007/BF00228197. [DOI] [PubMed] [Google Scholar]
- Vidailhet M, Stocchi F, Rothwell JC, Thompson PD, Day BL, Brooks DJ, Marsden CD. The Bereitschaftspotential preceding simple foot movement and initiation of gait in Parkinson’s disease. Neurology. 1993;43:1784–1788. doi: 10.1212/wnl.43.9.1784. [DOI] [PubMed] [Google Scholar]
- Ward NS. Neural plasticity and recovery of function. Prog Brain Res. 2005;150:527–535. doi: 10.1016/S0079-6123(05)50036-0. [DOI] [PubMed] [Google Scholar]
- Wassermann EM. Risk and safety of repetitive transcranial magnetic stimulation: report and suggested guidelines from the International Workshop on the Safety of Repetitive Transcranial Magnetic Stimulation, June 5–7, 1996. Electroencephalogr Clin Neurophysiol. 1998;108:1–16. doi: 10.1016/s0168-5597(97)00096-8. [DOI] [PubMed] [Google Scholar]
- Werhahn KJ, Fong JK, Meyer BU, Priori A, Rothwell JC, Day BL, Thompson PD. The effect of magnetic coil orientation on the latency of surface EMG and single motor unit responses in the first dorsal interosseous muscle. Electroencephalogr Clin Neurophysiol. 1994;93:138–146. doi: 10.1016/0168-5597(94)90077-9. [DOI] [PubMed] [Google Scholar]