Abstract
Orexin-A, synthesized by neurons of the lateral hypothalamus, helps to maintain wakefulness through excitatory projections to nuclei involved in arousal. Obvious changes in eye movements, eyelid position and pupil reactions seen in the transition to sleep led to the investigation of orexin-A projections to visuomotor cell groups to determine whether direct pathways exist that may modify visuomotor behaviors during the sleep/wake cycle. Histological markers were used to define these specific visuomotor cell groups in monkey brainstem sections and combined with orexin-A immunostaining. The dense supply by orexin-A boutons around adjacent neurons in the dorsal raphe nucleus served as a control standard for a strong orexin-A input. The quantitative analysis assessing various functional cell groups of the oculomotor system revealed that almost no input from orexin-A terminals reached motoneurons supplying the singly-innervated muscle fibers of the extraocular muscles in the oculomotor nucleus, the omnipause neurons in the nucleus raphe interpositus and the premotor neurons in the rostral interstitial nucleus of the medial longitudinal fasciculus. In contrast, the motoneurons supplying the multiply-innervated muscle fibers of the extraocular muscles, the motoneurons of the levator palpebrae muscle in the central caudal nucleus, and especially the preganglionic neurons supplying the ciliary ganglion received a strong orexin input. We interpret these results as evidence that orexin-A does modulate pupil size, lid position, and possibly convergence and eye alignment via the motoneurons of multiply-innervated muscle fibres. However orexin-A does not directly modulate premotor pathways for saccades or the SIF motoneurons.
Keywords: oculomotor, eyelid, pupil, accommodation, saccade
The hypothalamus is involved in a wide variety of behavioural, autonomic visceral and endocrine functions. It is composed of numerous diffuse cell groups and a few magnocellular nuclei (for review: Saper, 2004; Niewenhuys et al., 2008). Le Gros Clark divided them rostrocaudally into four regions, the preoptic, supraoptic or anterior, tuberal and the mammillary region lying caudally (Le Cros Clark, 1938), while Crosby and Woodburne (1940) distinguished three zones arranged mediolaterally, the periventricular, medial and lateral zones. Orexins, also known as hypocretins, are neuropeptides produced by a rather restricted group of neurons in the lateral hypothalamus, that project throughout the central nervous system demonstrated in various species, e.g. rat (Peyron et al., 1998; Sakurai et al., 1998; de Lecea et al., 1998), hamster (Mintz et al., 2201), cat (Torterolo et al., 2006) and human (Thannickal et al., 2000). Aside from a role in the control of feeding, drug addiction, emotion, muscle tone and arousal (Yamada et al., 2000; Harris et al., 2005; Narita et al., 2006; Harris and Aston-Jones, 2006), orexin participates in the maintenance and stabilization of wakefulness in the sleep-wake cycle (Sakurai et al., 1998;Saper et al., 2005; Sakurai, 2007; Tsujino and Sakurai, 2009). The latter role was implied by the loss of orexin containing neurons in narcolepsy (Peyron et al., 2000; Nishino et al., 2000; Thannickal et al., 2000; Taheri et al., 2002). The control of sleep may be accomplished through strong excitatory projections of orexin neurons to the noradrenergic locus coeruleus (Hagan et al., 1999), serotoninergic cells of the dorsal raphe nucleus (DR) (Brown et al., 2001; Liu et al., 2002; Lee et al., 2005a) and cholinergic neurons of the laterodorsal tegmental and pedunculopontine tegmental nucleus (Peyron et al., 1998; Zhang et al., 2004). These regions are all part of the ascending arousal system that promotes wakefulness (for review: Sakurai, 2007). During sleep, orexin neurons are inhibited by the ventrolateral preoptic nucleus (for review: Saper et al., 2005)
Orexins appear in two forms, orexin-A and orexin-B, which correspond to hypocretin 1 and hypocretin 2, and show the same localization (Date et al., 1999). Both are derived from a common precursor by proteolytic cleavage, and show different binding affinities to two G-protein-coupled receptors. Whereas orexin-A has a high affinity to both orexin receptors, OxR1 and OxR2, OrxB has a much higher affinity to OxR2 than to OxR1 (Sakurai et al., 1998). Recording experiments showed that orexin neurons are most active during wakefulness, and they are virtually silent during non-REM and REM-sleep with only occasional bursts (Mileykovskiy et al., 2005; Lee et al., 2005b). With the onset of sleep there are rapid changes in eye movements, eyelid position and pupillary responses, e.g. loss of eye fixation with slow drifts, loss of conjugacy and drooping eyelids (Henn et al., 1984; Zhou and King 1997; Marquez-Ruiz and Escudero, 2008).
Here, we investigated the orexin-A projections to specific motor and premotor cell groups of the oculomotor system and to preganglionic neurons of the pupillary and accommodation systems in monkey. We wished to find out whether direct orexin-A inputs are present that could account for changes seen in the transition to sleep or for rapid eye movements during sleep. Our study included the presumed twitch-motoneurons in the oculomotor nucleus (nIII), which supply the singly-innervated muscle fibers (SIF) of extraocular muscles and the presumed non-twitch motoneurons in the C- and S-groups, which supply the multiply-innervated muscle fibers (MIF) of the extraocular muscles (Büttner-Ennever et al., 2001; Büttner-Ennever, 2006). Furthermore, we examined the omnipause neurons in the nucleus raphe interpositus (RIP), which stabilize eye position and trigger the generation of saccades, the premotor neurons for vertical saccades in the rostral interstitial nucleus of the medial longitudinal fascicle (RIMLF), the motoneurons of the levator palpebrae superior muscle (LP) and the orbicularis oculi muscle (OO), and the preganglionic neurons supplying the ciliary ganglion found in Edinger-Westphal (EWpg) and anteromedian nuclei (AM) (for review: Büttner-Ennever et al., 1988; Porter et al., 1989; Horn, 2006; Horn et al., 2008; May et al., 2008a). In order to assess the strength of the orexin-A input, a quantitative analysis of orexin-A containing nerve endings associated with the visuomotor cell groups was performed. These data were compared with levels found in the nucleus raphe dorsalis (DR), which is known to receive strong projections from orexin-producing neurons (Peyron et al., 1998; Brown et al., 2001).
Previous studies in monkey have identified specific functional cell groups of the visuomotor system, close to the raphé nuclei of the brainstem, by their location and histochemical properties. The motoneurons of MIFs, SIFs, LP, OO, and the preganglionic neurons of the ciliary ganglion were identified by the cholinergic marker choline acetyltransferase (ChAT) (Eberhorn et al., 2005), and saccadic burst and omnipause neurons by immunostaining for parvalbumin or non-phosphorylated neurofilaments (Horn et al., 1994; Horn and Büttner-Ennever, 1998). The serotoninergic neurons of the dorsal raphe nucleus were identified with an antibody against tryptophan hydroxylase (Brownstein et al., 1975; Baker et al., 1991). Taken together these studies provide a unique opportunity to assess the significance of orexin inputs onto functionally identifiable cell groups in monkey.
Part of this work has been presented previously (Büttner-Ennever et al., 2007).
Experimental procedures
All animal tissue was obtained in accordance with state regulations and with approval of the appropriate state and university committees.
Antisera and Controls
Orexin-A
Orexin containing structures were detected with a polyclonal orexin-A antibody raised in rabbit (AB3098, Chemicon, Temecula, CA). The appearance of orexin-A-positive staining seen with this antibody in the present study resembles the data of previous reports (Sakurai et al., 1998; de Lecea et al., 1998).
Choline acetyltransferase (ChAT)
Cholinergic motoneurons were identified with a polyclonal ChAT antibody raised in goat (AB144P, Chemicon) against human placental ChAT. The appearance and distribution of ChAT-positive neurons seen with this antibody in the present study resembles the data of previous reports (Ichikawa and Shimizu, 1998).
Parvalbumin (PV)
The calcium-binding protein, parvalbumin, was detected in immunofluorescence and immunoperoxidase staining with a monoclonal parvalbumin antibody raised in mouse produced by hybridization of mouse myeloma cells with spleen cells from mice immunized with parvalbumin purified from carp muscle (235; Swant, Bellinzona, Switzerland; (Celio et al., 1988).
Tryptophan hydroxylase (TRH-PH8)
Serotoninergic neurons were detected with a monoclonal antibody raised in mouse (MAB5278; Chemicon, Temecula, CA) that binds an epitope of tryptophan hydroxylase (TRH), tyrosine hydroxylase and phenylalanine hydroxylase. TRH is the enzyme that converts 5-hydroxytryptophan to serotonin and therefore can be used as a marker for serotonin. The appearance of TRH-PH8-positive neurons seen with this antibody in the present study resembles the data of previous reports (Baker et al., 1991).
Non-phosphorylated neurofilaments (NP-NF)
NP-NFs were detected using a mouse monoclonal anti-nonphosphorylated ‘epitope in neurofilament H’ antibody (clone 02-135; SMI32, Sternberger Monoclonals Inc., Lutherville, MD). This antibody visualizes two bands (200kDa and 180 kDa) in conventional immunoblots (Tsang et al., 2000).
Controls
Controls for each reaction were carried out by the omission of primary antibodies, which in each case led to unstained sections.
Combined immunoperoxidase labeling for orexin-A and different markers
Brainstem sections from macaque monkeys used in other anatomical projects were employed in this study. The animals were euthanized with an overdose of sodium-pentobarbital (80mg/kg body weight) and perfused transcardially with 0.9% saline followed by 4% paraformaldehyde in 0.1M phosphate buffer. The brains were removed from the skull and immersed in 10% sucrose in 0.1M phosphate buffer and transferred to 30% sucrose for frozen sectioning. The brainstems were cut at 40μm in the transverse stereotaxic plane. Selected sections through the hypothalamus, midbrain and pons combined immunolabelling were utilized either double-immunoperoxidase or double-immunofluorescence staining to simultaneously detect Orexin-A and one of the following: choline acetyltransferase (ChAT), parvalbumin (PV), non-phosphorylated filaments (NP-NF) or tryptophan hydroxylase (TRPH 8) (Table 1). For immunoperoxidase staining, free-floating sections were washed in 0.1M Tris-buffered saline (TBS, pH 7.4) and treated with 1% H2O2 in TBS for 30 min. After several washes, sections were preincubated with 2% normal horse serum containing 0.3% Triton-X 100 in TBS for 1 h at room temperature. The sections were then treated with rabbit anti-orexin-A (1:6000; Chemicon AB3098) in TBS with 2% horse serum and 0.3% Triton X-100 over two nights at room temperature. After washing in 0.1M TBS, the sections were incubated in biotinylated horse anti-rabbit IgG (1:200; Vector laboratories) in TBS containing 2% bovine serum albumin for 1h. For the detection of orexin-A, the sections were incubated in extravidin-peroxidase (1:1000; Sigma) for 1h. Two rinses were followed by one wash with 0.05M Tris-buffer (pH 7.6), and the reaction with 0.025% diaminobenzidine, 0.4% ammonium nickel sulfate and 0.015% H2O2 in 0.05M Tris-buffer (pH 7.6), for 10 min. This results in a black staining of orexin-A-positive structures. After a thorough washing and blocking of residual peroxidase activity with 1% H2O2 in 0.1M TBS, the sections were preincubated with 2% normal horse serum in 0.3% Triton-X 100 in TBS for 1 h at room temperature. Then the sections were treated with goat anti-ChAT (1:1000; Chemicon, AB144P), mouse anti-parvalbumin (1:2500; Swant, 235), mouse anti-non-phosphorylated neurofilaments (1:6000; Sternberger, SMI32) or mouse anti-tryptophan hydroxylase PH8-1 (1:2000; Chemicon, MAB5278) in TBS with 2% horse serum and 0.3% Triton X-100 overnight at room temperature. After washing in 0.1M TBS, the sections were incubated in biotinylated horse anti-goat IgG or horse anti-mouse IgG (1:200; Vector laboratories) in TBS containing 2% bovine serum albumin for 1h. The antigen binding sites were detected by incubating sections in extravidin peroxidase (1:1000; Sigma) for 1h and a subsequent reaction with 0.025% diaminobenzidine and 0.015% H2O2 in 0.05M Tris-buffer (pH 7.6) for 10 min to yield a brown stain. After washing, the sections were mounted, air-dried, dehydrated in alcohol and coverslipped with Depex.
Table 1.
List of antibodies and their sources with the applied methods used in this study.
| Antibody | Host | Antigen | Manufacturer | Cat. No. | Dilution | |
|---|---|---|---|---|---|---|
| Immunoperoxidase | Immunofluorescence | |||||
| Orexin A | rabbit | orexin A | Chemicon, Temecula, CA | AB3098 | 1:6000 | 1:2000 |
| ChAT | goat | choline acetyltransferase | Chemicon, Temecula, CA | AB144P | 1: 1000 | |
| PV | mouse | parvalbumin | Swant, Bellinzona, Switzerland | 235 | 1:2500 | 1:2000 |
| SMI32 | mouse | non-phosphorylated neurofilaments | Sternberger Monoclonals, Lutherville, MD | SMI32 | 1:6000 | 1:1000 |
| PH8-1 | mouse | tryptophan hydroxylase | Chemicon, Temecula, CA | MAB5278 | 1:2000 | |
Combined immunofluorescence labeling for orexin-A and other markers
Free floating sections of monkey brainstem were first treated with 2% normal donkey serum in 0.1M TBS (pH 7.4) containing 0.3% Triton-X 100 for 1 hour. Sections were then incubated in a cocktail containing rabbit anti-orexin-A (1:2000; Chemicon AB3098) and mouse anti-parvalbumin (1:2000; Swant, 235) in TBS with 2% normal donkey serum and 0.3% Triton X-100 for two nights at 4°C. Rinsed sections were then reacted with a cocktail of donkey carbocyanine 3 (Cy3)-tagged anti-rabbit (1:200; red) and donkey Alexa-488-tagged anti-mouse (1:200; green) in TBS containing 2% bovine serum albumin for 1h. After washing in 0.1M TBS, the sections were briefly rinsed with distilled water, and coverslipped with Gel/Mount.
Analysis of stained sections
The slides were examined with a Leica microscope DMRB (Bensheim, Germany) equipped with appropriate filters for red fluorescent Cy3 (N2.1) and green fluorescent Alexa- 488 (I3). Photographs of brightfield and fluorescence preparations were taken with a digital camera (Pixera Pro 600 ES; Klughammer, Markt Indersdorf, Germany) mounted on the microscope (Leica DMRB, Bensheim, Germany). The images were captured on a computer with Pixera Viewfinder software (Klughammer) and processed with Photoshop 7.0 software (Adobe). The sharpness, contrast and brightness were adjusted to reflect the appearance of the labeling seen through the microscope. The pictures were arranged and labeled with drawing software (Coreldraw 11.0; Corel Corporation). For a quantitative analysis, all orexin-A-positive endings associated with the soma and proximal dendrites of a given neuron were counted by focussing through the section, and the number documented in a spreadsheet table (Microsoft Excel, 2002). From there the average and mean terminal density was calculated, the latter was used for the statistical tests. The analysis of each chosen visuomotor cell group was performed on sections from two monkeys. At least 100 neurons taken from all levels of a given cell group were studied for orexin-A inputs. Statistical analysis was performed using a Wilcoxon two sample test.
Results
The application of the orexin-A antibody revealed two main clusters of strongly stained neuronal cell bodies in the tuberal and rostral mammillary region of the monkey hypothalamus (Fig. 1). One orexin-A positive cell group lies around the fornix (FO) in the perifornical region (PF) and spreads out in the lateral hypothalamic area (LHA) (Fig. B, C); the other orexin-A positive cell group occupies the area of the dorsomedial hypothalamic nuclei (DMH) with scattered neurons in the dorsal hypothalamic area (Fig. 1A, B). The orexin-A positive cells were medium-sized and typically give rise to two or three primary dendrites (Fig. 1D). Orexin-A immunostained fibres with numerous varicosities and terminal boutons were distributed throughout the whole brainstem with varying densities. As described before in rat and cat, a particularly strong supply of Orexin-A positive terminals was found in the locus coeruleus (not shown) and the raphe nuclei (Fig. 3G, I), whereas the cerebellar cortex was almost devoid of orexin-A fibres and terminals (Nambu et al., 1999; Zhang et al., 2004). The presence of immunostained structures throughout the whole section thickness indicated complete antibody penetration. This was an important prerequisite for the quantitative analysis that was performed.
Figure 1.
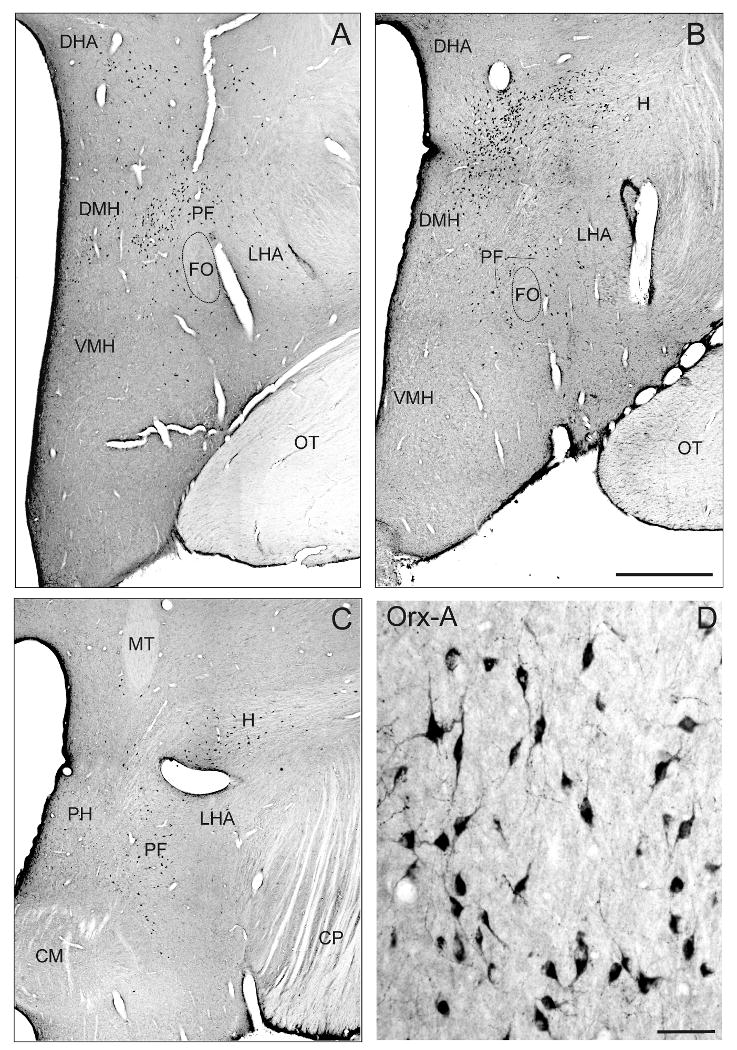
Frontal sections through 3 planes of the monkey hypothalamus (rostral to caudal) demonstrating the distribution of orexin-A positive neurons within the dorsomedial hypothalamicus (DMH) and in the perifornical region (PF) around the fornix (FO) spreading out in the lateral hypothalamic area (LHA) (A). The high-power photograph shows the morphology of orexin-A positive neurons in the DMH (B). Scale bar: A = 1mm; B = 50μm. CP = cerebral peduncle, DHA = dorsal hypothalamic area; H = H field of Forel; OT = optic tract.
Figure 3.
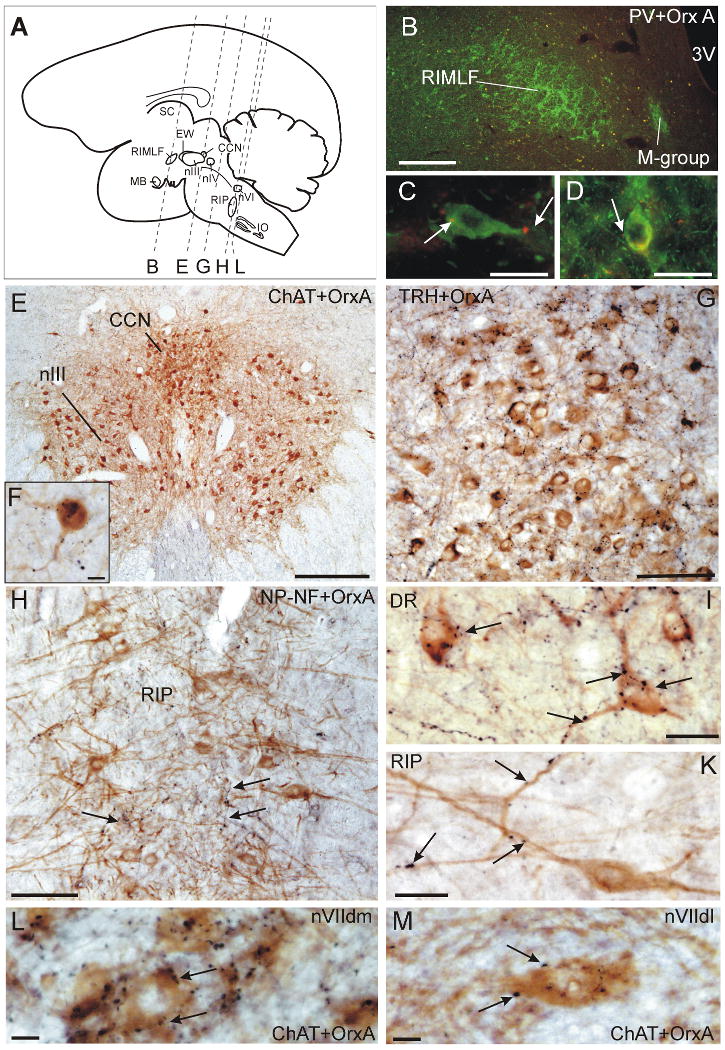
Schematic sagittal view of a monkey brain (A) which indicates the frontal cutting planes of B, E, G, H, L. Frontal section through the RIMLF immunostained for parvalbumin (PV; green) and orexin A (Orx-A; red) in the overview (B) and at high magnification (C,D). Note the putative PV-positive burst neurons in RIMLF are contacted by only few Orx-A boutons (arrows, C, D). Overview of a frontal section through the central caudal nucleus (CCN) immunostained for choline acetyltransferase (ChAT; brown) and Orx-A (black) (E). Highpower magnification of ChAT-positive motoneurons of the levator palpebrae in CCN associated with numerous Orx-A punctae on the soma and dendrites (F). Overview of a frontal section through the dorsal raphe nucleus (DRN) immunostained for tryptophan hydroxylase (TRH; brown) and Orx-A (black) (G). Highpower magnification of TRH-positive neurons in the DR covered by many Orx-A boutons (arrows) (I). Overview of a frontal section through the nucleus raphe interpositus (RIP) containing saccadic omnipause neurons immunostained for non-phosphorylated neurofilaments (NP-NF; brown) (H). Detailed view of a NP-NF-positive omnipause neuron associated with few Orx-A (black) boutons (arrows) (K). The ChAT-positive motoneurons in the dorsolateral subgroup in the facial nucleus (nVIIdl) innervating the orbicularis oculi muscle receive much less Orx-A boutons (M) than the motoneurons in the facial dorsomedial subgroup (nVIIdm) innervating the frontalis muscle (L). Scal bar: B, E = 500μm; C, D, I, K = 25μm; G, H = 100μm
Orexin-A input on motoneurons of the extraocular muscles
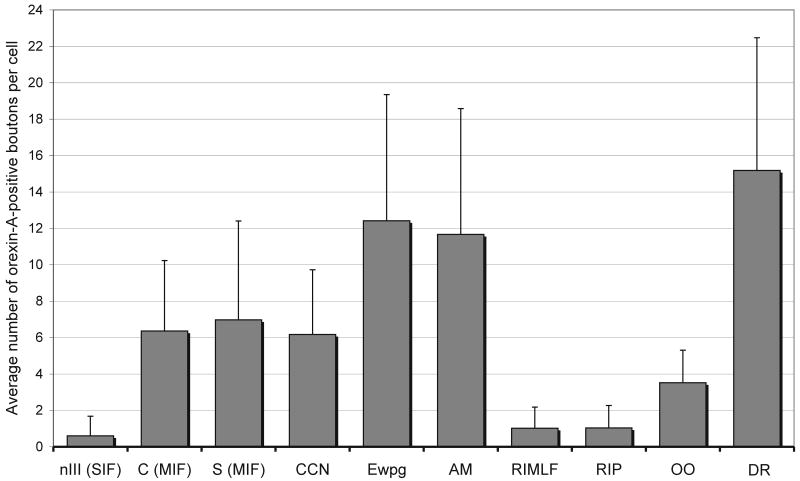
Immunostaining for choline acetyltransferase (ChAT) revealed the complete population of eye muscle motoneurons within and around the oculomotor nucleus (nIII) in monkey midbrain sections. ChAT-immunoreactive neurons within nIII were recognized as the SIF-motoneurons of the inferior rectus (IR), medial rectus (MR), inferior oblique (IO) and superior rectus muscle (SR), which appear as multipolar, medium-sized neurons (Eberhorn et al., 2005) (Fig. 2A). They received the weakest orexin-A input of the examined cell groups with the least average terminal density per neuron (0.60 boutons/cell; SD: 1.08) (Fig. 2E; 4). ChAT-immunoreactive neurons within the well delineated C-group at the dorsomedial border of nIII represent the MIF-motoneurons of IR and MR, and those in the S-group, sandwiched between the nIII, the MIF-motoneurons of IO and SR (Büttner-Ennever, 2006) (Fig. 2A, B). In contrast to the SIF motoneurons within nIII, the MIF-motoneurons of the C-group and the S-group received a significant higher supply of orexin-A-positive boutons (C-MIF: 6.36 boutons/cell; SD: 3,87; S-MIF: 6.97 boutons/cell; SD: 5.43) (Fig. 2C, D; E; Fig. 4).
Figure 2.
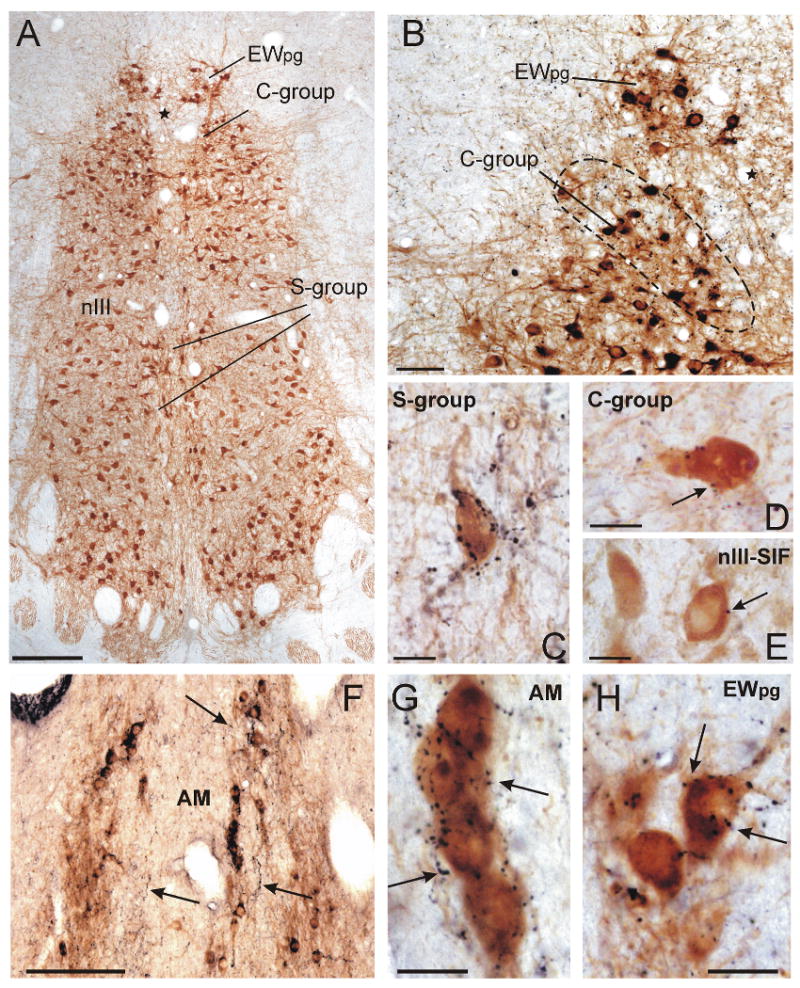
Frontal sections through the monkey oculomotor nucleus (nIII) stained for choline acetyltransferase (ChAT; brown) and orexin A (Orx-A; black) in the overview (A) and at high magnification (B). The star indicates the same blood vessel in A and B. The high-power photographs (C-H) show that the MIF-motoneurons in the C- and S-group are associated with far more orexin-A-positive terminals (C, D, arrows) than the SIF-motoneurons within nIII (E, arrow). Numerous orexin-A-positive fibres are distributed throughout the ChAT-positive preganglionic neurons of the ciliary ganglion in the Edinger-Westphal nucleus (EWpg) (B) and anteromedian nucleus (AM) (F; arrow). The high-power photographs demonstrate the dense supply of ChAT-positive preganglionic neurons by orexin-A-positive punctae in the AM and EWpg (G, H, arrows). Scale bar: A, F = 200μm; B = 50μm; C- E; G, H = 25μm
Figure 4.
Quantitative comparison of the average number of orexin-A positive boutons associated with single neurons of the visuomotor brain regions. The exact average of density values for orexin-A positive inputs and the standard deviations are written in the text. Note that the preganglionic neurons of the ciliary ganglion receive the strongest supply of orexin-A afferents compared to SIF-motoneurons and premotor neurons of saccades.
Orexin-A input on motoneurons of the eyelid
In ChAT-immunostained sections the motoneurons of the levator palpebrae muscle (LP) can be easily identified as the central caudal nucleus (CCN) dorsomedial to the caudal part of nIII (Porter et al., 1989; Schmidtke and Büttner-Ennever, 1992) (Fig. 3A, E). The quantitative analysis revealed that the LP motoneurons received a substantial input (6.17 boutons/cell; SD: 3.56) of orexin-A-positive terminals (Fig. 3F; Fig. 4). Similarily the motoneurons of the eyelid closing orbicularis oculi muscle (OO) were identified by ChAT- immunoreactivity in the dorsolateral group of the facial nucleus (Porter et al., 1989). A slightly weaker orexin-A input was noted to the motoneurons of the lid closing orbicularis oculi muscle (3.52 boutons/cell; SD: 1.78) compared to those of the levator palpebrae muscle (Fig. 3M; Fig. 4). Interestingly the adjacent dorsomedial subgroup of the facial nucleus containing motoneurons of the frontalis muscle (Welt and Abbs, 1990) exhibit a very dense supply with orexin-A positive boutons comparable to that in the dorsal raphe nucleus (Fig. 3L), although a systematic quantitative assessment hast not been performed for this group.
Orexin-A input on preganglionic neurons of the ciliary ganglion
In monkey, the preganglionic neurons of the ciliary ganglion form a compact group of ChAT-positive, medium-sized, multipolar cells with well developed dendrites in the Edinger-Westphal nucleus (EWpg) dorsal to nIII (May et al., 2008a; Horn et al., 2008) (Fig. 2A, B). The rostrally adjoining anteromedian nucleus (AM) contains medium-sized ChAT-positive preganglionic neurons as well. These are are located around the midline, with their long axis oriented vertically (Fig. 2F). The ChAT-positive populations of presumed preganglionic neurons in these nuclei received the strongest supply of orexin-A-positive terminals of all the cell groups studied (EWpg: 12.42 boutons/cell; SD: 6.93; AM:11,67 boutons/cell; SD: 6.91). This was approximately twice as many as contact motoneurons of MIFs and the LP (Fig. 2G, H; Fig. 4).
Orexin-A input to premotor cell groups of the saccadic system
In primates, the saccadic omnipause neurons (OPN) lie in the pontine reticular formation around the midline and form the nucleus raphe interpositus (RIP; Fig. 3A) (Büttner-Ennever et al., 1988). The OPNs were identified by their expression of parvalbumin (PV) or non-phosphorylated neurofilaments (NP-NF) (Fig. 3H, K). They appear as two vertical cell columns their long, horizontally-oriented dendrites reaching across the midline, and are found at the same level that rootlets of the abducens nerve (VI) traverse the pons (Horn et al., 1994). At first sight, a considerable number of orexin-A fibres and boutons appeared to be distributed within the OPN area (Fig. 3H, arrows) compared to the adjacent reticular formation. But with close inspection, it became obvious that only few orexin-A positive boutons are directly associated with individual OPNs, and these were mostly associated with dendrites (Fig. 3K). Systematic analysis of OPNs revealed a rather weak orexin-A input (1.04 boutons/cell; 1.23) (Fig. 4).
According to previous studies, the premotor burst neurons for vertical saccades may be identified in the mesencephalic reticular formation within the rostral interstitial nucleus of the medial longitudinal fasciculus (RIMLF) by their immunoreactivity for parvalbumin (PV) (Horn and Büttner-Ennever, 1998). Thereby, the RIMLF appears as a wing-shaped nucleus located ventrolateral to the third ventricle (Fig. 3A, B). In single staining for orexin-A, we found that the RIMLF contains only a few orexin-A-positive traversing fibres and boutons. Accordingly, quantitative analysis revealed only a weak supply of orexin-A positive punctae (1.02 boutons/cell; SD: 1.16) around PV-immunoreactive presumed vertical saccadic burst neurons in the RIMLF (Fig. 3C, D; Fig. 4).
Orexin-A input on serotoninergic neurons of the dorsal raphe nucleus
Staining for tryptophan hydroxylase (TRH) was used to identify the serotoninergic neurons of the dorsal raphe nucleus (DR), which lies immediately caudal to the trochlear nucleus (nIV) and extends dorsally into the ventral periaqueductal gray (Brownstein et al., 1975; Charara and Parent, 1998). Combined immunostaining revealed that orexin-A positive fibres and boutons were densely distributed among the serotoninergic neurons of the dorsal raphe nucleus (Fig. 3G; for location see Fig. 3A). The somata and proximal dendrites of most TRH-positive were densely contacted by orexin-A-positive boutons (15.18 boutons/cell; SD: 7.23). Accordingly this terminal pattern was considered as a very strong orexin-A input. (Fig. 3 G, I; Fig. 4).
Discussion
Using double-immunostaining for orexin-A and histological markers defining functional cell groups of the visuomotor system, we found three orexin-A termination patterns. 1. Weak orexin-A input to cell groups involved in the saccadic system, e.g. SIF-motoneurons of the extraocular muscles, the omnipause neurons in RIP and the premotor burst neurons in the RIMLF. 2. Medium density orexin-A inputs to MIF-motoneurons of the extraocular muscles and the LP and OO motoneurons in the central caudal nucleus and facial nucleus, respectively. 3. Dense orexin-A input to the preganglionic neurons of the ciliary ganglion in the EWpg and AM.
The localization of the orexin neurons appears to be conserved across species when comparing the rat, hamster, cat and human (Nambu et al., 1999; Mintz et al., 2001; Zhang et al., 2001, Thannickal et al., 2004; Saper, 2004). As in these species the orexin neurons of monkey lie in the diffuse cell groups of the tuberal region with one cluster in the perifornical region (PF) scattering in the lateral hypothalamic area (LHA), and another cluster in the adjacent dorsomedial hypothalamus (DMH). Efferents from this hypothalamic region have been carefully studied previously using autoradiographic techniques (Holstege, 1987). They form two different descending fiber bundles, a lateral tract arising mainly from the paraventricular hypothalamic nucleus; and a medial tract, probably originating in part from the orexin neurons, that targets the raphé magnus and raphé pallidus nuclei, lamina X of the spinal cord and the sympathetic preganglionic neurons in the upper thoracic intermediolateral nuclei.
Furthermore, the observed dense distribution of orexin-A fibres and boutons around serotoninergic neurons of the dorsal raphe nucleus in monkey confirms previous pharmacological and anatomical work in cat and rat (Zhang et al., 2004; Lee et al., 2005a) that shows this nucleus to be strongly influenced by orexin-A.
Orexin-A input to motoneurons of the extraocular muscles
As described in rat and cat (Peyron et al., 1998; Nambu et al., 1999; Zhang et al., 2004), only very few orexin-A terminals were present within the motonuclei of extraocular muscles in monkey, which contain the SIF-motoneurons (Büttner-Ennever, 2006). In contrast, the MIF-motoneurons of the C- and S-group received a considerable supply of orexin-A-positive boutons. This implies that orexin-A plays a role in their function. Their differential orexin-A input supports a current hypothesis that SIF- and MIF- motoneurons subserve different functions reflected in the differences in histochemical properties of their target muscle fibers (Spencer and Porter, 2006), as well as differences in afferent input (Büttner-Ennever et al., 1996a; Wasicky et al., 2004; Eberhorn et al., 2005; Ugolini et al., 2006). Based on combined anatomical and physiological work, SIF-motoneurons are considered to be twitch motoneurons, which primarily drive the eye movements including saccades. In contrast, MIF-motoneurons represent non-twitch motoneurons, which may subserve a tonic action in gaze holding or eye alignment (Goldberg et al., 1981; Nelson et al., 1986; Shall and Goldberg, 1992; Büttner-Ennever et al., 2002; Büttner-Ennever, 2006). Transneuronal tract-tracing methods have revealed that in contrast to SIF-motoneurons, the MIF-motoneurons do not receive premotor afferents from areas involved in eye movement generation, e.g. the paramedian pontine reticular formation or the magnocellular region of the medial vestibular nuclei, but do receive input from centers involved in gaze stabilization, e.g. the prepositus hypoglossus nucleus and marginal zone (Büttner-Ennever and Gerrits, 2004; Ugolini et al., 2006; McCrea and Horn, 2006). During the transition from the awake state, which is stabilized by orexin, to sleep, in which the orexin-neurons are silent, there is a loss of precise fixation, and slow “drifting”, “rolling” or “pendular eye movements occur (Henn et al., 1984; Mileykovskiy et al., 2002; Kiyashchenko et al., 2002; Ohno and Sakurai, 2008). Moreover, in the awake state, versional eye movements are strictly conjugate, which requires tightly linked motor activity of co-acting eye muscles, and also the fine adjustment of muscle activity to obtain a stable retinal image without motion induced blur or double vision (Leigh and Zee, 2006). Recording studies in cat have shown that the discharge pattern of eye muscle motoneurons in the abducens nucleus does not differ between saccades in REM sleep and alertness (Escudero and Marquez-Ruiz, 2008). Similarly, behavioural studies in monkey revealed that rapid eye movements during REM sleep exhibit the same kinematics and temporal synchrony for both eyes as spontaneous saccades in the awake state, and may therefore be generated by the same neuronal circuits. In contrast, binocular coordination of the direction of eye movements is completely lost in REM sleep, when orexin neurons are silent and the rapid eye movements are typically disconjunctive (Zhou and King, 1997). These authors concluded that binocular coordination is an active process related to the attential mechanism associated with alertness. Our findings of a significant orexin-A input on MIF-motoneurons support the concept that eye alignment derives from an active input associated with being awake (Zhou and King, 1997; Lee et al., 2005b). The present data indicate that SIF-motoneurons are not controlled by monosynaptic orexin-A inputs, which is in line with the occurrence of precisely timed eye movements in the awake state and in REM (Zhou and King, 1997; Marquez-Ruiz and Escudero, 2008).
Orexin-A input on motoneurons of the eyelid
In contrast to the relative lack of orexin-A inputs to SIF-motoneurons within the nIII, the LP-motoneurons in the CCN receive a considerable supply of orexin-A-positive boutons. In the awake state, the eyes are kept open by the activation of the LP-motoneurons from an as yet unknown tonical excitatory input (Büttner-Ennever and Horn, 2004; Horn and Büttner-Ennever, 2008). With increasing fatigue, the activation of the LP-motoneurons ceases, and the eyelid lowers without involvement of the orbicularis oculi muscle, which only closes the eye during a blink (Sibony and Evinger, 1998). Orexin-A terminals like those seen in our studies could contribute to the tonic activation of the LP-motoneurons during wakefulness, when orexin-A is released (Lee et al., 2005b; Horn and Büttner-Ennever, 2008). During sleep, the orexin neurons are silent and the lack of activation will support the closure of the eye lids (Vanni-Mercier et al., 1994). This is corroborated by the observation that narcolepsy patients, who lack orexin-producing neurons, can not keep their eyes open during attacks of catalepsy (Peyron et al., 2000; Thannickal et al., 2000; Serra et al., 2008). This fits also well with our observation of a rather strong orexin-A input to the motoneurones in the dorsomedial subgroup of the facial nucleus innervating the frontalis muscle and contributes to eyelid elevation (Skarf, 2005). The orbicularis oculi muscle (OO) provides active eye closure and eye closing during blinks (for review: Skarf, 2005). The modest orexin-A input to OO-motoneurons, similar to that seen in LP-motoneurons, may provide a modulatory excitatory signal that may participate in setting a threshold for activation of the motoneurons during wakefulness.
Orexin-A input on preganglionic neurons of the ciliary ganglion
The preganglionic neurons of the macaque ciliary ganglion are located in the cytoarchitecturally defined EWpg and rostrally adjoining AM (Akert et al., 1980; Ishikawa et al., 1990; May et al., 2008b). Neurons in these areas received the strongest input of orexin-A of all the cell groups of the visuomotor system examined here, implying considerable direct influence over the pupillary and/or accommodation system. The size of the pupil, its responsiveness to light, and the natural occurring fluctuations of its diameter, all vary with the degree of alertness (Wilhelm, 2008), but also with emotional arousal (Bradley et al., 2008). For example the pupillary fluctuations become stronger with increased drowsiness and therefore have been used as an indicator to measure alertness (Lowenstein et al., 1963; Wilhelm et al., 1998; Wilhelm, 2008). In addition, lens accommodation is abolished in the first stage of light sleep, drowsiness. This is when orexin-A neurons become inactive (Henn et al., 1984; Lee et al., 2005b). Interestingly, the naturally appearing fluctuations of the pupil diameter are less pronounced in patients with narcolepsy compared to controls with an intact orexin-system (O'Neill et al., 1998), which supports a direct orexin influence on both muscles controlling pupillary size. In rat there is a similar strong orexin-A innervation of the sympathetic preganglionic neurons in the first thoracic segment of the spinal cord, most of them projecting to the superior cervical ganglion, which in turn mediates the innervation of the dilatator muscle (Llewellyn-Smith et al., 2003). The strong orexin-A input to preganglionic neurons of both, sphincter and dilatator muscle supports the concept that orexin functions as a modulatory system and not as an information signal (Saper et al. 2005); in this case modulating the signal intensities to the pupillary system during wakefulness, and perhaps more indirectly during emotional arousal (Bradley et al., 2008; Tsujino and Sakurai, 2009). Consequently during sleep, the reduction of orexin-A inputs to preganglionic neurons of both pupillary muscles may be the cause of increasing fluctuations of pupil size observed by Wilhelm et al., (1998).
Here it should be pointed out that although there are similar observations of a rather selective strong expression of the orexin receptor 1 in the Edinger-Westphal nucleus in the rat (Hervieu et al., 2001), these results are related to a very different system from the one in monkey. In the rat, the orexin-A input to the Edinger-Westphal nucleus is thought to be related to feeding behaviour (Willie et al., 2001). In contrast to monkey, in rat the cytoarchitecturally defined Edinger-Westphal nucleus does not contain preganglionic neurons of the ciliary ganglion but represents a cell group expressing the neuropeptide urocortin 1, an endogeneous ligand for the CRF-receptors, which is involved in food intake (Vaughan et al., 1995; Spina et al., 1996; Yamamoto et al., 1998; Weitemier and Ryabinin, 2005; Horn et al., 2009). In monkey, the area containing urocortin-positive forms a cytoarchitecturally inconspicuous cell group next to the EWpg (May et al., 2008a; Horn et al., 2008). We found that it is covered with numerous orexin-A positive fibres and boutons (Horn, personal observation) that were presumably involved in the regulation of the feeding behaviour (Pan and Kastin, 2008).
Orexin-A input to neuronal cell groups of the saccadic system
We did not observe any significant orexin-A input on premotor burst-neurons for vertical saccades in the RIMLF or on the OPNs in the nucleus raphe interpositus (RIP). This finding implies that orexin-A does not exert a direct influence on the immediate premotor cell groups of the saccadic system and or on the execution of saccades per se (Büttner-Ennever et al., 1988; Scudder and Kaneko, 2002). This is in line with the fact that saccadic eye movements occur in alertness, when orexin-A is released, and also during REM sleep, when orexin-A neurons are mostly silent (Lee et al., 2005b). If orexin-A exerts any action on the saccadic system at all, then it must be indirect or through more upstream neurons, e.g. the nucleus raphe magnus, nucleus reticularis pontis oralis or deep layers of the superior colliculus, which all receive orexin-A positive fibres (Peyron et al., 1998; Nunez et al., 2006) and project to omnipause neurons (Langer and Kaneko, 1990; Büttner-Ennever et al., 1999).
Orexin modulates visuomotor function
There are already several lines of evidence from previous tract-tracing experiments that hypothalamic neurons are interconnected with brainstem nuclei involved in visuomotor function: retrograde tract-tracing experiments in monkey revealed neurons in the lateral hypothalamus, which project to the oculomotor complex (Steiger and Büttner-Ennever, 1979). Similarily, experiments with live rabies virus, injected into the lateral rectus muscle of monkey (Ugolini et al., 2006) revealed labelled neurons scattered in the perifornical hypothalamus after 2-3 days survival time (personal observation). In hamster another link between orexin neurons and visuomotor function is implicated by the strong supply with orexin-A positive boutons from the lateral hypothalamus to the intergeniculate leaflet and the medial vestibular nuclei (Horowitz et al., 2005; Vidal et al., 2005). The medial vestibular nuclei, involved in the generation of eye movements during head rotation, have been shown to project to many areas receiving strong orexin-A input including the intergeniculate leaflet (Horowitz et al., 2004). The intergeniculate leaflet of the hamster is the homologue to the pregeniculate nucleus in monkey (Livingston and Mustari, 2000), which corresponds to the ventral lateral geniculate nucleus (Nakamura and Itoh, 2004). The ventral geniculate nucleus has several subdivisions, some of which have been shown to be involved in visuomotor functions (Büttner and Fuchs 1973; Büttner-Ennever et al., 1996b). In our results the medial division of the pregeniculate nucleus received a strong supply of orexin-A boutons as well (own observations), and confirm the hypothesis that orexin modulates visuomotor systems.
Conclusion
Orexin is thought to promote and stabilize wakefulness by its excitatory inputs to monoaminergic nuclei, e.g. locus coeruleus and raphe nuclei, where neurons fire at the highest rates during wakefulness, slow down during NREM sleep, and stop firing during REM sleep (for review: Saper et al., 2005) – the same firing pattern is seen in orexin-A neurons (Lee et al., 2005b). The present study confirms the orexin-A inputs to the locus coeruleus and raphé system in monkey, through which the orexin pathways can certainly modulate the eye movement system indirectly during alertness. In addition our results revealed a considerable input from orexin-A neurons onto motoneurons of LP, OO, MIF-motoneurons and on the preganglionic neurons of the ciliary ganglion. These targets have a high and stable level of activity during wakefulness to keep the eyes open and blinking, provide eye alignment or fixation, and modulate the pupil and lens. Only little orexin-A input was found to target the neurons directly involved in the generation of saccades, and whose activity is stable during wakefulness. These results provide evidence for the functional role of orexin inputs in stabilizing the activity of neuronal systems during wakefulness.
Acknowledgments
We thank Prof. U. Büttner (Department of Neurology, LMU Munich), Dr. S. Yakushin (Mount Sinai Hospital, New York) and Prof. B. Hess (University of Zürich) for their continual support.
Supported by Deutsche Forschungsgemeinschaft DFG HO 1639/4-3, Friedrich-Baur-Stiftung, and National Institutes of Health EY013308; RR00166.
Abbreviations
- AM
anteromedian nucleus
- CCN
central caudal nucleus
- ChAT
choline acetyltransferase
- DHA
dorsal hypothalamic area
- DMH
dorsomedial hypothalamus
- DR
dorsal raphe nucleus
- EWpg
Edinger-Westphal nucleus (preganglionic neurons)
- FO
fornix
- H
H field of Forel
- IO
inferior oblique muscle
- IR
inferior rectus muscle
- LHA
lateral hypothalamic area
- LP
levator palpebrae muscle
- MIF
multiply-innervated muscle fibre
- MLF
medial longitudinal fasciculus
- MR
medial rectus muscle
- MT
mamillothalamic tract
- nIII
oculomotor nucleus
- nVII
facial nucleus
- NP-NF
non-phosphorylated neurofilaments
- OO
orbicularis oculi muscle
- OPN
omnipause neurons
- PF
perifornical region
- PV
parvalbumin
- RIMLF
rostral interstitial nucleus of the medial longitudinal fasciculus
- RIP
nucleus raphe interpositus
- SIF
singly-innervated muscle fibre
- SO
superior oblique muscle
- SR
superior rectus muscle
- VMH
ventromedial hypothalamus
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Akert K, Glicksman MA, Lang W, Grob P, Huber A. The Edinger-Westphal nucleus in the monkey. A retrograde tracer study. Brain Res. 1980;184:491–498. doi: 10.1016/0006-8993(80)90816-1. [DOI] [PubMed] [Google Scholar]
- Baker KG, Halliday GM, Halasz P, Hornung JP, Geffen LB, Cotton RGH, Törk I. Cytoarchitecture of serotonin-synthesizing neurons in the pontine tegmentum of the human brain. Synapse. 1991;7:301–320. doi: 10.1002/syn.890070407. [DOI] [PubMed] [Google Scholar]
- Bradley MM, Miccoli L, Escrig MA, Lang PJ. The pupil as a measure of emotional arousal and autonomic activation. Psychophysiol. 2008;45:602–607. doi: 10.1111/j.1469-8986.2008.00654.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown RE, Sergeeva O, Eriksson KS, Haas HL. Orexin A excites serotonergic neurons in the dorsal raphe nucleus of the rat. Neuropharm. 2001;40:457–459. doi: 10.1016/s0028-3908(00)00178-7. [DOI] [PubMed] [Google Scholar]
- Brownstein MJ, Palkovits M, Saavedra JM, Kizer JS. Tryptophan hydroxylase in the rat brain. Brain Res. 1975;97:163–166. doi: 10.1016/0006-8993(75)90925-7. [DOI] [PubMed] [Google Scholar]
- Büttner U, Fuchs AF. Influence of saccadic eye movements on unit activity in simian lateral geniculate and pregeniculate nuclei. J Neurophysiol. 1973;36:127–141. doi: 10.1152/jn.1973.36.1.127. [DOI] [PubMed] [Google Scholar]
- Büttner-Ennever JA. The extraocular motor nuclei: organization and functional neuroanatomy. Prog Brain Res. 2006;151:95–125. doi: 10.1016/S0079-6123(05)51004-5. [DOI] [PubMed] [Google Scholar]
- Büttner-Ennever JA, Cohen B, Horn AKE, Reisine H. Pretectal projections to the oculomotor complex of the monkey and their role in eye movements. J Comp Neurol. 1996;366:348–359. doi: 10.1002/(SICI)1096-9861(19960304)366:2<348::AID-CNE12>3.0.CO;2-L. [DOI] [PubMed] [Google Scholar]
- Büttner-Ennever JA, Cohen B, Horn AKE, Reisine H. Efferent pathways of the nucleus of the optic tract in monkey and their role in eye movements. J Comp Neurol. 1996b;373:90–107. doi: 10.1002/(SICI)1096-9861(19960909)373:1<90::AID-CNE8>3.0.CO;2-8. [DOI] [PubMed] [Google Scholar]
- Büttner-Ennever JA, Cohen B, Pause M, Fries W. Raphe nucleus of the pons containing omnipause neurons of the oculomotor system in the monkey, and its homologue in man. J Comp Neurol. 1988;267:307–321. doi: 10.1002/cne.902670302. [DOI] [PubMed] [Google Scholar]
- Büttner-Ennever JA, Horn AKE, Scherberger H, D'Ascanio P. Motoneurons of twitch and non-twitch extraocular muscle fibers in the abducens, trochlear, and oculomotor nuclei of monkeys. J Comp Neurol. 2001;438:318–335. doi: 10.1002/cne.1318. [DOI] [PubMed] [Google Scholar]
- Büttner-Ennever JA, Gerrits NM. Vestibular System. In: Paxinos G, Mai JK, editors. The human nervous system. Elsevier Academic Press; Amsterdam: 2004. pp. 1212–1240. [Google Scholar]
- Büttner-Ennever JA, Horn AKE. Reticular formation: eye movements, gaze and blinks. In: Paxinos G, Mai JK, editors. The human nervous system. Elsevier Academic Press; Amsterdam: 2004. pp. 479–510. [Google Scholar]
- Büttner-Ennever JA, Horn AKE, Graf W, Ugolini G. Modern concepts of brainstem anatomy. Ann N Y Acad Sci. 2002;956:75–84. doi: 10.1111/j.1749-6632.2002.tb02810.x. [DOI] [PubMed] [Google Scholar]
- Büttner-Ennever JA, Horn AKE, Henn V, Cohen B. Projections from the superior colliculus motor map to omnipause neurons in monkey. J Comp Neurol. 1999;413:55–67. doi: 10.1002/(sici)1096-9861(19991011)413:1<55::aid-cne3>3.0.co;2-k. [DOI] [PubMed] [Google Scholar]
- Büttner-Ennever JA, Tang X, Mustari MJ, Horn AKE. Orexin inputs to oculomotor and Edinger-Westphal cell groups of the brainstem. Soc Neurosci Abstr. 2007;32:718, 16. [Google Scholar]
- Celio MR, Baier W, Schärer L, De Viragh PA, Gerday C. Monoclonal antibodies directed against the calcium binding protein parvalbumin. Cell Calcium. 1988;9:81–86. doi: 10.1016/0143-4160(88)90027-9. [DOI] [PubMed] [Google Scholar]
- Charara A, Parent A. Chemoarchitecture of the primate dorsal raphe nucleus. J Chem Neuroanat. 1998;72:111–127. doi: 10.1016/s0891-0618(98)00036-2. [DOI] [PubMed] [Google Scholar]
- Crosby EC, Woodburne RT. The comparative anatomy of the preoptic area and the hypothalamus. Res Publ Assoc Nerv Ment Dis. 1940;20:52–169. [Google Scholar]
- Date Y, Ueta Y, Yamashita H, Yamaguchi H, Matsukura S, Kangawa K, Sakurai T, Yanagisawa M, Nakazato M. Orexins, orexigenic hypothalamic peptides, interact with autonomic, neuroendocrine and neuroregulatory systems. Proc Natl Acad Sci USA. 1999;96:748–753. doi: 10.1073/pnas.96.2.748. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Lecea L, Kilduff TS, Peyron C, Gao XB, Foye PE, Danielson PE, Fukuhara C, Battenberg ELF, Gautvik VT, Bartlett FS, 2, Frankel WN, van den Pol AN, Bloom FE, Gautvik KM, Sutcliffe JG. The hypocretins: Hypothalamus-specific peptides with neuroexcitatory activity. Proc Natl Acad Sci USA. 1998;95:322–327. doi: 10.1073/pnas.95.1.322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eberhorn AC, Ardelenanu P, Büttner-Ennever JA, Horn AKE. Histochemical differences between motoneurons supplying multiply and singly innervated extraocular muscle fibers. J Comp Neurol. 2005;491:352–366. doi: 10.1002/cne.20715. [DOI] [PubMed] [Google Scholar]
- Escudero M, Marquez-Ruiz J. Tonic inhibition and ponto-geniculo-occipital-related activities shape abducens motoneuron discharge during REM sleep. J Physiol (Lond) 2008;586:3479–3491. doi: 10.1113/jphysiol.2008.153254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goldberg SJ, Clamann HP, McClung JR. Relation between motoneuron position and lateral rectus motor unit contraction speed: an intracellular study in the cat abducens nucleus. Neurosci Lett. 1981;23:49–54. doi: 10.1016/0304-3940(81)90185-3. [DOI] [PubMed] [Google Scholar]
- Hagan JJ, Leslie RA, Patel S, Evans ML, Wattam TA, Holmes S, Benham CD, Taylor SG, Routledge C, Hemmati P, Munton RP, Ashmeade TE, Shah AS, Hatcher JP, Hatcher PD, Jones DNC, Smith MI, Piper DC, Hunter AJ, Porter RA, Upton N. Orexin A activates locus coeruleus cell firing and increases arousal in the rat. Proc Natl Acad Sci USA. 1999;96:10911–10916. doi: 10.1073/pnas.96.19.10911. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harris GC, Aston-Jones G. Arousal and reward: a dichotomy in orexin function. TINS. 2006;29:571–577. doi: 10.1016/j.tins.2006.08.002. [DOI] [PubMed] [Google Scholar]
- Harris GC, Wimmer M, Aston-Jones G. A role for lateral hypothalamic orexin neurons in reward seeking. Nature. 2005;437:556–559. doi: 10.1038/nature04071. [DOI] [PubMed] [Google Scholar]
- Henn V, Baloh RW, Hepp K. The sleep-wake transition in the oculomotor system. Exp Brain Res. 1984;54:166–176. doi: 10.1007/BF00235828. [DOI] [PubMed] [Google Scholar]
- Hervieu GJ, Cluderay JE, Harrison DC, Roberts JC, Leslie RA. Gene expression and protein distribution of the orexin-1 receptor in the rat brain and spinal cord. Neurosci. 2001;103:777–797. doi: 10.1016/s0306-4522(01)00033-1. [DOI] [PubMed] [Google Scholar]
- Holstege G. Some anatomical observations on the projections from the hypothalamus to brainstem and spinal cord: an HRP and autoradiographic tracing study in the cat. J Comp Neurol. 1987;260:98–126. doi: 10.1002/cne.902600109. [DOI] [PubMed] [Google Scholar]
- Horn AK, Büttner-Ennever JA. Brainstem circuits controlling lid-eye coordination in monkey. Prog Brain Res. 2008;171:87–95. doi: 10.1016/S0079-6123(08)00612-2. [DOI] [PubMed] [Google Scholar]
- Horn AK, Eberhorn A, Härtig W, Ardelenanu P, Messoudi A, Büttner-Ennever JA. Perioculomotor cell groups in monkey and man defined by their histochemical and functional properties: reappraisal of the Edinger-Westphal nucleus. J Comp Neurol. 2008;507:1317–1335. doi: 10.1002/cne.21598. [DOI] [PubMed] [Google Scholar]
- Horn AK, Schulze C, Radtke-Schuller S. The Edinger-Westphal nucleus represents different functional cell groups in different species. Ann N Y Acad Sci. 2009 doi: 10.1111/j.1749-6632.2009.03856.x. in press. [DOI] [PubMed] [Google Scholar]
- Horn AKE. The reticular formation. Prog Brain Res. 2006;151:127–155. doi: 10.1016/S0079-6123(05)51005-7. [DOI] [PubMed] [Google Scholar]
- Horn AKE, Büttner-Ennever JA. Premotor neurons for vertical eye-movements in the rostral mesencephalon of monkey and man: the histological identification by parvalbumin immunostaining. J Comp Neurol. 1998;392:413–427. [PubMed] [Google Scholar]
- Horn AKE, Büttner-Ennever JA, Wahle P, Reichenberger I. Neurotransmitter profile of saccadic omnipause neurons in nucleus raphe interpositus. J Neurosci. 1994;14:2032–2046. doi: 10.1523/JNEUROSCI.14-04-02032.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Horowitz SS, Blanchard J, Morin LP. Medial vestibular connections with the hypocretin (orexin) system. J Comp Neurol. 2005;487:127–146. doi: 10.1002/cne.20521. [DOI] [PubMed] [Google Scholar]
- Horowitz SS, Blanchard JH, Morin LP. Intergeniculate leaflet and ventral lateral geniculate nucleus afferent connections: an anatomical substrate for functional input from the vestibulo-visuomotor system. J Comp Neurol. 2004;474:227–245. doi: 10.1002/cne.20125. [DOI] [PubMed] [Google Scholar]
- Ichikawa T, Shimizu T. Organization of choline acetyltransferase-containing structures in the cranial nerve motor nuclei and spinal cord of the monkey. Brain Res. 1998;779:96–103. doi: 10.1016/s0006-8993(97)01090-1. [DOI] [PubMed] [Google Scholar]
- Ishikawa S, Sekiya H, Kondo Y. The center for controlling the near reflex in the midbrain of the monkey: a double labelling study. Brain Res. 1990;519:217–222. doi: 10.1016/0006-8993(90)90080-u. [DOI] [PubMed] [Google Scholar]
- Kiyashchenko LI, Mileykovskiy BY, Maidment N, Lam HA, Wu MF, John J, Peever J, Siegel JM. Release of hypocretin (orexin) during waking and sleep states. J Neurosci. 2002;22:5282–5286. doi: 10.1523/JNEUROSCI.22-13-05282.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Langer TP, Kaneko CR. Brainstem afferents to the oculomotor omnipause neurons in monkey. J Comp Neurol. 1990;295:413–427. doi: 10.1002/cne.902950306. [DOI] [PubMed] [Google Scholar]
- Lee HS, Park SH, Song WC, Waterhouse BD. Retrograde study of hypocretin-1 (orexin-A) projections to subdivisions of the dorsal raphe nucleus in the rat. Brain Res. 2005a;1059:35–45. doi: 10.1016/j.brainres.2005.08.016. [DOI] [PubMed] [Google Scholar]
- Lee MG, Hassani OK, Jones BE. Discharge of identified orexin/hypocretin neurons across the sleep-waking cycle. J Neurosci. 2005b;25:6716–6720. doi: 10.1523/JNEUROSCI.1887-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Le Gros Clark WE. Morphological aspects of the hypothalamus. In: Le Cors Clark WE, Beattie J, Riddoch C, Dott NM, editors. The hypothalamus. Oliver and Boyd; Edinburgh: 1938. pp. 1–68. [Google Scholar]
- Leigh RJ, Zee DS. The Neurology of Eye Movements. Oxford University Press; New York: 2006. [Google Scholar]
- Liu R, van den Pol AN, Aghajanian GK. Hypocretins (orexins) regulate serotonin neurons in the dorsal raphe nucleus by excitatory direct and inhibitory indirect actions. J Neurosci. 2002;22:9453–9464. doi: 10.1523/JNEUROSCI.22-21-09453.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Livingston CA, Mustari MJ. The anatomical organization of the macaque pregeniculate complex. Brain Res. 2000;876:166–179. doi: 10.1016/s0006-8993(00)02647-0. [DOI] [PubMed] [Google Scholar]
- Llewellyn-Smith IJ, Martin CL, Marcus JN, Yanagisawa M, Minson JB, Scammell TE. Orexin-immunoreactive inputs to rat sympathetic preganglionic neurons. Neurosci Lett. 2003;351:115–119. doi: 10.1016/s0304-3940(03)00770-5. [DOI] [PubMed] [Google Scholar]
- Lowenstein O, Feinberg R, Loewenfeld IE. Pupillary movements during acute and chronic fatigue. Invest Ophthalmol. 1963;2:138–157. [Google Scholar]
- Marquez-Ruiz J, Escudero M. Tonic and phasic phenomena underlying eye movements during sleep in the cat. J Physiol (Lond) 2008;586:3461–3477. doi: 10.1113/jphysiol.2008.153239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- May PJ, Reiner AJ, Ryabinin AE. Comparison of the distributions of Urocortin-containing and cholinergic neurons in the perioculomotor midbrain of the cat and Macaque. J Comp Neurol. 2008a;507:1300–1316. doi: 10.1002/cne.21514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- May PJ, Sun W, Erichsen JT. Defining the pupillary component of the perioculomotor preganglionic population within a unitary primate Edinger-Westphal nucleus. In: Kennard C, Leigh JR, editors. Using eye movements as an experimental probe of brain function. Elsevier; Amsterdam: 2008b. pp. 97–106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McCrea RA, Horn AKE. Nucleus prepositus. Prog Brain Res. 2006;151:205–230. doi: 10.1016/S0079-6123(05)51007-0. [DOI] [PubMed] [Google Scholar]
- Mileykovskiy BY, Kiyashchenko LI, Siegel JM. Behavioral correlates of activity in identified hypocretin/orexin neurons. Neuron. 2005;46:787–798. doi: 10.1016/j.neuron.2005.04.035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mileykovskiy BY, Kiyashchenko LI, Siegel JM. Muscle tone facilitation and inhibition after orexin-A (Hypocretin-1) microinjections into the medial medulla. J Neurophysiol. 2002;87:2480–2489. doi: 10.1152/jn.2002.87.5.2480. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mintz EM, van den Pol AN, Casano AA, Albers HE. Distribution of hypocretin-(orexin) immunoreactivity in the central nervous system of Syrian hamsters (Mesocricetus auratus) J Chem Neuroanat. 2001;21:225–238. doi: 10.1016/s0891-0618(01)00111-9. [DOI] [PubMed] [Google Scholar]
- Nakamura H, Itoh K. Cytoarchitectonic and connectional organization of the ventral lateral geniculate nucleus in the cat. J Comp Neurol. 2004;473:439–462. doi: 10.1002/cne.20074. [DOI] [PubMed] [Google Scholar]
- Nambu T, Sakurai T, Mizukami K, Hosoya Y, Yanagisawa M, Goto K. Distribution of orexin neurons in the adult rat brain. Brain Res. 1999;827:243–260. doi: 10.1016/s0006-8993(99)01336-0. [DOI] [PubMed] [Google Scholar]
- Narita M, Nagumo Y, Hashimoto S, Narita M, Khotib J, Miyatake M, Sakurai T, Yanagisawa M, Nakamachi T, Shioda S, Suzuki T. Direct involvement of orexinergic systems in the activation of the mesolimbic dopamine pathway and related behaviors induced by morphine. J Neurosci. 2006;26:398–405. doi: 10.1523/JNEUROSCI.2761-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nelson JS, Goldberg SJ, McClung JR. Motoneuron electrophysiological and muscle contractile properties of superior oblique motor units in cat. J Neurophysiol. 1986;55:715–726. doi: 10.1152/jn.1986.55.4.715. [DOI] [PubMed] [Google Scholar]
- Nishino S, Ripley B, Overeem S, Lammers GJ, Mignot E. Hypocretin (orexin) deficiency in human narcolepsy. The Lancet. 2000;355:39–40. doi: 10.1016/S0140-6736(99)05582-8. [DOI] [PubMed] [Google Scholar]
- Nieuwenhuys R, Voogd J, van Huijzen C. The Human Central Nervous System. 4th. Springer; Berlin, Heidelberg, New York: 2008. [Google Scholar]
- Nuñez A, Moreno-Balandrán ME, Rodrigo-Angulo ML, Garzón M, De Andrés I. Relationship between the perifornical hypothalamic area and oral pontine reticular nucleus in the rat. Possible implication of the hypocretinergic projection in the control of rapid eye movement sleep. Eur J Neurosci. 2006;24:2834–2842. doi: 10.1111/j.1460-9568.2006.05159.x. [DOI] [PubMed] [Google Scholar]
- O'Neill WD, Oroujeh AM, Merritt SL. Pupil noise is a discriminator between narcoleptics and controls. IEEE Trans Biomed Eng. 1998;45:314–322. doi: 10.1109/10.661156. [DOI] [PubMed] [Google Scholar]
- Ohno K, Sakurai T. Orexin neuronal circuitry: role in the regulation of sleep and wakefulness. Front Neuroendocrin. 2008;29:70–87. doi: 10.1016/j.yfrne.2007.08.001. [DOI] [PubMed] [Google Scholar]
- Pan W, Kastin AJ. Urocortin and the brain. Prog Neurobiol. 2008;84:148–156. doi: 10.1016/j.pneurobio.2007.10.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peyron C, Faraco J, Rogers W, Ripley B, Overeem S, Charnay Y, Nevsimalowa S, Aldrich M, Reynolds D, Albin R, Li R, Hungs M, Pedrazolli M, Padigaru M, Kucherlapati M, Fan J, Maki R, Lammers GJ, Bouras C, Kucherlapati R, Nishino S, Mignot E. A mutation in case of early onset narcolepsy and a generalized absence of hypocretin peptides in human narcoleptic brains. Nat Med. 2000;6:991–997. doi: 10.1038/79690. [DOI] [PubMed] [Google Scholar]
- Peyron C, Tighe DK, van den Pol AN, de Lecea L, Heller HC, Sutcliffe JG, Kilduff TS. Neurons containing hypocretin (orexin) project to multiple neuronal systems. J Neurosci. 1998;18:9996–10015. doi: 10.1523/JNEUROSCI.18-23-09996.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Porter JD, Burns LA, May P. Morphological substrate for eyelid movements: innervation and structure of primate levator palpebrae superioris and orbicularis oculi muscles. J Comp Neurol. 1989;287:64–81. doi: 10.1002/cne.902870106. [DOI] [PubMed] [Google Scholar]
- Sakurai T. The neural circuit of orexin (hypocretin): maintaining sleep and wakefulness. Nature Rev Neurosci. 2007;8:171–181. doi: 10.1038/nrn2092. [DOI] [PubMed] [Google Scholar]
- Sakurai T, Amemiya A, Ishii M, Matsuzaki I, Chemelli RM, Tanaka H, Williams SC, Richardson JA, Kozlowski GP, Wilson S, Arch JRS, Buckingham RE, Haynes AC, Carr SA, Annan RS, McNulty DE, Liu WS, Terrett JA, Elshourbagy NA, Bergsma DJ, Yanagisawa M. Orexins and orexin receptors: A family of hypothalamic neuropeptides and G protein-coupled receptors that regulate feeding behavior. Cell. 1998;92:573–585. doi: 10.1016/s0092-8674(00)80949-6. [DOI] [PubMed] [Google Scholar]
- Saper CB. Hypothalamus. In: Paxinos G, Mai JK, editors. The Human Nervous System. 2nd. Elsevier; Amsterdam, Boston, Heidelberg: 2004. pp. 513–550. [Google Scholar]
- Saper CB, Scammell TE, Lu J. Hypothalamic regulation of sleep and circadian rhythms. Nature. 2005;437:1257–1263. doi: 10.1038/nature04284. [DOI] [PubMed] [Google Scholar]
- Schmidtke K, Büttner-Ennever JA. Nervous control of eyelid function - a review of clinical, experimental and pathological data. Brain. 1992;115:227–247. doi: 10.1093/brain/115.1.227. [DOI] [PubMed] [Google Scholar]
- Scudder CA, Kaneko CRS. The brainstem burst generator for saccadic eye movements. Exp Brain Res. 2002;142:439–462. doi: 10.1007/s00221-001-0912-9. [DOI] [PubMed] [Google Scholar]
- Serra L, Montagna P, Mignot E, Lugaresi E, Plazzi G. Cataplexy features in childhood narcolepsy. Mov Disord. 2008;23:858–865. doi: 10.1002/mds.21965. [DOI] [PubMed] [Google Scholar]
- Shall MS, Goldberg SJ. Extraocular motor units - type classification and motoneuron stimulation frequency-muscle unit force relationships. Brain Res. 1992;587:291–300. doi: 10.1016/0006-8993(92)91010-c. [DOI] [PubMed] [Google Scholar]
- Sibony PA, Evinger C. Normal and abnormal eyelid function. In: Miller NR, Newman NJ, editors. Clinical Neuro-ophthalmology. Williams and Wilkins; Baltimore: 1998. pp. 1509–1592. [Google Scholar]
- Skarf B. Normal and abnormal eyelid function. In: Miller NR NR, Newman NJ, Biousse V, Kersing W, editors. Walsh and Hoyt's Clinical Neuro-Ophthalmology. Lippincott Williams and Wilkins; Philadelphia: 2005. pp. 1177–1229. [Google Scholar]
- Spencer RF, Porter JD. Biological organization of the extraocular muscles. Prog Brain Res. 2006;151:43–80. doi: 10.1016/S0079-6123(05)51002-1. [DOI] [PubMed] [Google Scholar]
- Spina MG, Merrlo-Pich E, Chan RK, Basso AM, Rivier J, Vale W, Koob GF. Appetite-suppressing effects of urocortin, a CRF-related neuropeptide. Science. 1996;273:1561–1564. doi: 10.1126/science.273.5281.1561. [DOI] [PubMed] [Google Scholar]
- Steiger HJ, Büttner-Ennever JA. Oculomotor nucleus afferents in the monkey demonstrated with horseradish peroxidase. Brain Res. 1979;160:1–15. doi: 10.1016/0006-8993(79)90596-1. [DOI] [PubMed] [Google Scholar]
- Taheri S, Zeitzer JM, Mignot E. The role of hypocretins (orexins) in sleep regulation and narcolepsy. Ann Rev Neurosci. 2002;25:283–313. doi: 10.1146/annurev.neuro.25.112701.142826. [DOI] [PubMed] [Google Scholar]
- Thannickal TC, Moore RY, Nienhuis R, Ramanathan L, Gulyani S, Aldrich M, Cornford M, Siegel JM. Reduced number of hypocretin neurons in human narcolepsy. Neuron. 2000;27:469–474. doi: 10.1016/s0896-6273(00)00058-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Torterolo P, Sampogna S, Morales FR, Chase MH. MCH-containing neurons in the hypothalamus of the cat: searching for a role in the control of sleep and wakefulness. Brain Res. 2006;1119:101–14. doi: 10.1016/j.brainres.2006.08.100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsang YM, Chiong F, Kuznetsov D, Kasarskis E, Geula C. Motor neurons are rich in non-phosphorylated neurofilaments: cross-species comparison and alterations in ALS. Brain Res. 2000;861:45–58. doi: 10.1016/s0006-8993(00)01954-5. [DOI] [PubMed] [Google Scholar]
- Tsujino N, Sakurai T. Orexin/Hypocretin: a neuropeptide at the interface of sleep, energy homeostasis, and reward system. Pharmacol Rev. 2009;61:162–76. doi: 10.1124/pr.109.001321. [DOI] [PubMed] [Google Scholar]
- Ugolini G, Klam F, Doldan Dans M, Dubayle D, Brandi AM, Büttner-Ennever JA, Graf W. Horizontal eye movement networks in primates as revealed by retrograde transneuronal transfer of rabies virus: Differences in monosynaptic input to “slow” and “fast” abducens motoneurons. J Comp Neurol. 2006;498:762–785. doi: 10.1002/cne.21092. [DOI] [PubMed] [Google Scholar]
- Vanni-Mercier G, Pelisson D, Goffart L, Sakai K, Jouvet M. Eye saccade dynamics during paradoxical sleep in the cat. Eur J Neurosci. 1994;6:1298–1306. doi: 10.1111/j.1460-9568.1994.tb00320.x. [DOI] [PubMed] [Google Scholar]
- Vaughan J, Donaldson C, Bittencourt JC, Perrin MH, Lewis K, Sutton S, Chan R, Turnbull A, Lovejoy D, Rivier C, Rivier J, Sawchenko PE, Vale W. Urocortin, a mammalian neuropeptide related to fish urotensin I and to corticotropin-releasing factor. Nature. 1995;378:287–292. doi: 10.1038/378287a0. [DOI] [PubMed] [Google Scholar]
- Vidal L, Blanchard J, Morin LR. Hypothalamic and zona incerta neurons expressing hypocretin, but not melanin concentrating hormone, project to the hamster intergeniculate leaflet. Neurosci. 2005;134:1081–1090. doi: 10.1016/j.neuroscience.2005.03.062. [DOI] [PubMed] [Google Scholar]
- Wasicky R, Horn AKE, Büttner-Ennever JA. Twitch and non-twitch motoneuron subgroups of the medial rectus muscle in the oculomotor nucleus of monkeys receive different afferent projections. J Comp Neurol. 2004;479:117–129. doi: 10.1002/cne.20296. [DOI] [PubMed] [Google Scholar]
- Weitemier AZ, Ryabinin AE. Lesions of the Edinger-Westphal nucleus alter food and water consumption. Behav Neurosci. 2005;119:1235–1243. doi: 10.1037/0735-7044.119.5.1235. [DOI] [PubMed] [Google Scholar]
- Welt C, Abbs JH. Musculotopic organisation of the facial motor nucleus in macaca fascicularis: A morphometric and retrograde tracing study with cholera toxin B-HRP. J Comp Neurol. 1990;291:621–636. doi: 10.1002/cne.902910409. [DOI] [PubMed] [Google Scholar]
- Wilhelm B, Wilhelm H, Lüdtke H, Streicher P, Adler M. Pupillographic assessment of sleepiness in sleep-deprived healthy subjects. Sleep. 1998;21:258–265. [PubMed] [Google Scholar]
- Wilhelm H. The pupil. Curr Opin Neurol. 2008;21:36–42. doi: 10.1097/WCO.0b013e3282f39173. [DOI] [PubMed] [Google Scholar]
- Willie JT, Chemelli RM, Sinton CM, Yanagisawa M. To eat or to sleep? Orexin in the regulation of feeding and wakefulness. Ann Rev Neurosci. 2001;24:429–458. doi: 10.1146/annurev.neuro.24.1.429. [DOI] [PubMed] [Google Scholar]
- Yamada H, Okumura T, Motomura W, Kobayashi Y, Kohgo Y. Inhibition of food intake by central injection of anti-orexin antibody in fasted rats. Biochem Biophys Res Communic. 2000;267:527–531. doi: 10.1006/bbrc.1999.1998. [DOI] [PubMed] [Google Scholar]
- Yamamoto H, Maeda T, Fujimura M, Fujimiya M. Urocortin-like immunoreactivity in the substantia nigra, ventral tegmental area and Edinger-Westphal nucleus of rat. Neurosci Lett. 1998;243:21–24. doi: 10.1016/s0304-3940(98)00071-8. [DOI] [PubMed] [Google Scholar]
- Zhang JH, Sampogna S, Morales FR, Chase MH. Orexin (hypocretin)-like immunoreactivity in the cat hypothalamus: a light and electron microscopic study. Sleep. 2001;24:67–76. doi: 10.1093/sleep/24.1.67. [DOI] [PubMed] [Google Scholar]
- Zhang J, Sampogna S, Morales FR, Chase MH. Distribution of hypocretin (orexin) immunoreactivity in the feline pons and medulla. Brain Res. 2004;995:205–217. doi: 10.1016/j.brainres.2003.10.004. [DOI] [PubMed] [Google Scholar]
- Zhou W, King WM. Binocular eye movements not coordinated during REM sleep. Exp Brain Res. 1997;117:153–160. doi: 10.1007/s002210050209. [DOI] [PubMed] [Google Scholar]