Abstract
P. falciparum, the most lethal malarial parasite, expresses an ortholog for the protein kinase C (PKC) activator RACK1. However, PKC has not been identified in this parasite, and the mammalian RACK1 can interact with the inositol 1,4,5-trisphosphate receptor (InsP3R). Therefore we investigated whether the Plasmodium ortholog PfRACK also can affect InsP3R-mediated Ca2+ signaling in mammalian cells. GFP-tagged PfRACK and endogenous RACK1 were expressed in a similar distribution within cells. PfRACK inhibited agonist-induced Ca2+ signals in cells expressing each isoform of the InsP3R, and this effect persisted when expression of endogenous RACK1 was reduced by siRNA. PfRACK also inhibited Ca2+ signals induced by photorelease of caged InsP3. These findings provide evidence that PfRACK directly inhibits InsP3-mediated Ca2+ signaling in mammalian cells. Interference with host cell signaling pathways to subvert the host intracellular milieu may be an important mechanism for parasite survival.
Keywords: Plasmodium falciparum, Receptor for ctivated C kinase, InsP3 Receptors, Calcium signaling, primary hepatocytes
Introduction
Malaria is the world's most lethal parasitic disease, causing nearly half a billion infections and up to 2.7 million deaths annually. In Plasmodium, there are several cellular and molecular events activated by Ca2+, including gametocyte exflagellation [1] and movement of the motor complex of P. falciparum, which are modulated by Ca2+ dependent protein kinase (CDPK) [2]. Another example is activation of PfPKB, a Ca2+/calmodulin-regulated protein kinase B [3]. Plasmodium phospholipase C (PLC) participates in these processes because they are inhibited by the PLC inhibitor U73122 [4]. Similarly, the host hormone melatonin increases Ca2+ in Plasmodium, but this is blocked by U73122 [5; 6]. Furthermore Plasmodium creates a microenvironment rich in Ca2+ inside the infected erythrocyte [7] to fully exploit Ca2+ signaling and to enhance survival. Melatonin signaling in Plasmodium involves interaction between Ca2+ and cAMP, including activation of protein kinase A (PKA) [8]. The malaria parasite genome also includes four putative receptors each containing seven transmembrane domains [9].
A question is whether or how Plasmodia manipulate host signaling pathways. Receptor for Activated C Kinases (RACK1) is a protein expressed both in mammalian cells and Plasmodia. One function of mammalian RACK1 is to anchor Protein Kinase C (PKC) [10]. In humans, impairment of both RACK1 and PKC correlate with disorders such as Alzheimer's disease [11], and RACK1 association with PKC increases in bipolar affective disorder [12]. RACK1 also binds to InsP3 receptors and modulates Ca2+ signaling [13]. Plasmodium falciparum expresses an ortholog of RACK1, termed PfRACK [14], which is constitutively expressed throughout the intraerythrocytic cycle and in sporozoites, and is exported into host cells [15]. A canonical PKC protein has not been identified in the Plasmodium genome [16], raising the question of whether PfRACK serves a purpose other than anchoring PKC within the parasite. Here we use a functional genomics approach to investigate whether PfRACK interacts with mammalian InsP3 receptors in order to modulate Ca2+ release from the host cell's endoplasmic reticulum. Such an effect could provide a mechanism for Plasmodia to directly manipulate the Ca2+ signaling machinery of host cells, thus providing a potential route to control host cell processes such as apoptosis and metabolism and availability of nutrients to the intracellular parasite.
Materials and Methods
Codon optimization and gene synthesis
Plasmodium falciparum proteins are transcribed from a distinct genetic code, with 80% AT nucleotides [17]. To transcribe PfRACK properly in a mammalian system, codon optimization of the complete ORF of the gene sequence was performed, which was then commercially synthesized (Genscript Corp, Piscataway, NJ). To improve expression levels, a Kozak consensus sequence (GCCGCC) was added to the 5′ end. The assembled sequence was flanked by EcoRI and Bam-HI restriction sites, used later for N-terminal cloning to fusion with enhanced green fluorescent protein (EGFP) in the pEGFP-N1 plasmid (Clontech, Mountainview, CA).
Cell lines
Four cell lines were used for expression studies. This included the AR4-2J exocrine pancreatic, the HEK293 human embryonic kidney, the Mz-Cha-1 human cholangiocarcinoma, and the PC12 pheochromocytoma cell lines (ATCC; Manassas, VA). In selected studies, isolated rat hepatocytes in primary culture were used.
siRNA constructs
A siRNA conjugated with CY3 dye was developed to knockdown expression of endogenous RACK1 in HEK293 cells. The sequences used for RACK1 were determined from the complete ORF of human and rat RACK1 genes with BLOCK-iT™ RNAi Designer web application (Invitrogen, Carlsbad, CA) and synthesized by Invitrogen. Sequences were: RACK1fw: CCUCAAGGGCCAUAAUGGAUGGGUU; RACK1rv: AACCCAUCCAUUAUGGCCCUUGAGG. Scrambled sequences using the same nucleotide composition were used as negative controls (indicated as ‘control’ in Figure 2h-i): Scrfw: SCCUGGGAUACCGUAAGUAGGACGUU and Scrrv: AACGUCCUACUUACGGUAUCCCAGG. Cells were transfected with up to 100nM siRNA using Lipofectamine 2000 (Invitrogen), and experiments were performed 48hr later.
Fig. 2.
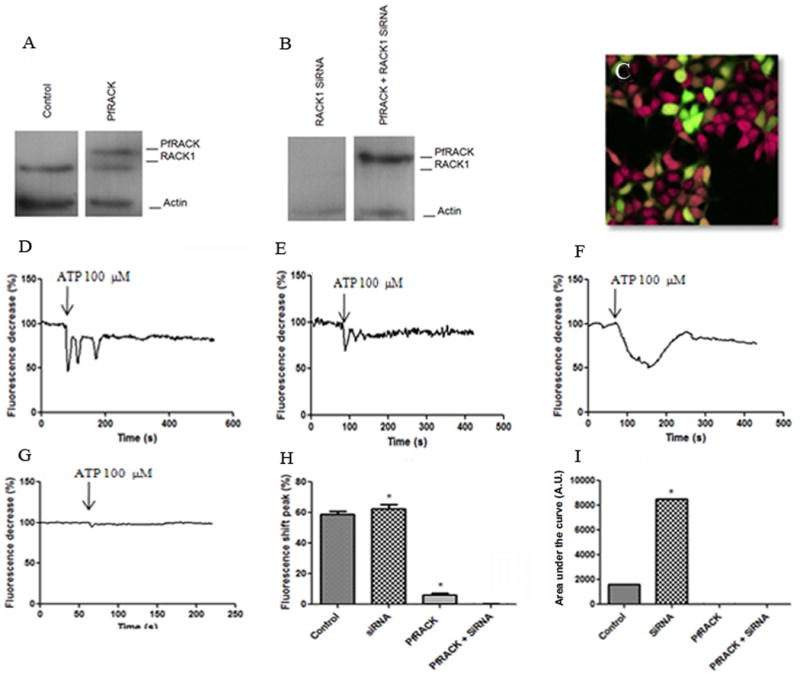
Immunoblot shows (a) (from left to right) a ∼50 Kd band corresponding to RACK1 in control cells, an additional ∼75 Kd band in cells transfected with GFP-PfRACK. Immunoblot shows (b) neither band in cells treated with RACK1 siRNA, and only the ∼75 Kd band in cells treated with RACK1 siRNA and transfected with GFP-PfRACK. (c-i) Confocal image demonstrates fura-red fluorescence (red) monitored in GFP-PfRACK transfected (green) and non-transfected cells simultaneously. Typical tracings of Ca2+ signals in individual (d) non-transfected and (e) transfected cells, each stimulated with ATP (100μM). Fura-red fluorescence decreases when cytosolic Ca2+ increases. Typical tracings of Ca2+ signals in individual cells treated with siRNA to decrease expression of endogenous RACK1 reveal that (f) the ATP-induced Ca2+ signal is more sustained in cells lacking RACK1, but (g) expression of PfRACK suppresses this signal. (h) Summary of peak amplitude of ATP-induced Ca2+ signals shows that loss of RACK1 accentuates the Ca2+ signal, while Ca2+ is markedly decreased by PfRACK expression (*p<0.001). Values are mean±SEM of triplicate measurements made of >40 cells under each condition. (i) Summary of the area under the curve of ATP-induced Ca2+ signals shows that loss of RACK1 accentuates this measure of the Ca2+ signal, while it is markedly decreased by expression of PfRACK. Values are mean±SEM of triplicate measurements made of >40 cells under each condition (*p<0.001). “Control” represents cells transfected with scrambled sequence.
Mammalian cell culture and transfection
Cells were cultured in Dulbecco's modified Eagle's medium (Invitrogen), supplemented with 10% fetal bovine serum (FBS), streptomycin (100μg/ml), penicillin (100U/ml) and L-glutamine (2mM) (Invitrogen), and maintained in a 5%CO2 atmosphere at 37°C. HEK293 cells with stable expression of GFP-PfRACK were also developed. Transfected HEK293 cells were subject to antibiotic selection using G418 600μM (Gibco, Carlsbad, CA). After 7 days of treatment, the cell line stably expressed the construct, evidenced by ubiquitous GFP labeling. Primary hepatocytes were isolated from rat liver by collagenase perfusion as described previously [18]. Hepatocytes were plated at 1×106 cells/cm2 and transfected after 72hr with Lipofectamine 2000. Cells were used 48hr later.
Immunofluorescence
Cells were fixed with 4% paraformaldehyde and confocal immunofluorescence was performed as described [19]. Cells were incubated with anti-RACK1 (BD Biosciences, San Jose, CA) and anti-GFP, 1:100 (MBL, Santa Cruz, CA). This was followed by incubation with an anti-IgG mouse antibody, conjugated with Alexa 555 (1:300, Invitrogen). TO-PRO-3 (1:400, Invitrogen) was used to stain the nucleus. Markers were incubated for 1hr at room temperature. Images were obtained using an LSM 510NLO confocal microscope (Carl Zeiss, Thornwood, NY) using Plan-NeoFluor X40 or X63 objectives. Settings to observe the different fluorescent dyes were described previously [20].
Immunoblotting
Cells were disrupted with lysis buffer (PBS/EDTA 5 mM, Triton X-100 0.5%) including the protease inhibitors leupeptin 5μg/ml, pepstatin A 5μg/ml, antipaine 5μg/ml, quimostatin 5μg/ml, benzamidine, and PMSF 0.2mM for 15min (4°C). Samples were quantified by spectrometry; 30μg of protein were separated in a gradient (5-25%) polyacrylamide gel (Biorad, Hercules, CA). Primary antibodies (1:1000) were incubated overnight (4°C). After washing, blots were incubated for 1hr with secondary HRP-conjugated anti-IgG anti-mouse antibody (1:5000, GE Healthcare). Immunodetection used enhanced chemiluminescence.
Ca2+ Imaging
Cells were incubated with cell-permeant Fura-Red (5μM)(Molecular Probes, Eugene, OR) for 30min at 37°C. Cells were transferred to a perfusion chamber on the stage of the LSM510 confocal microscope. Time-lapse images were acquired at 3 frames/sec using a 63× lens. Samples were illuminated at 488nm and fluorescence collected with a 650nm long-pass filter for Fura-Red and a 505-550 bandpass filter for EGFP. HEK293 and PC-12 cells were stimulated with 100μM ATP (Sigma-Aldrich, St. Louis, MO) to release Ca2+ through P2Y receptor-mediated InsP3 formation. AR4-2J and Mz-Cha-1 cells were stimulated with 25μM carbachol (Calbiochem, San Diego, CA) to release Ca2+ through muscarinic receptor-mediated InsP3 formation. In separate studies, HEK293 cells were loaded with caged InsP3, which was photoreleased by two-photon flash photolysis. For these studies, cells were incubated with Fura-Red, then with 3μM caged InsP3 (Calbiochem) for 30min at 37°C. A MaiTai titanium-sapphire laser (SpectraPhysics, Mountainview, CA) tuned to 720nm was used for two-photon flash photolysis. InsP3 was photoreleased by laser excitation for 0.2sec as Fura-Red Ca2+ signals were simultaneously detected by confocal microscopy [21].
Statistics
Fluorescence data and statistical analyses were performed with Prism 5.0 software (GraphPad, La Jolla, CA) using one-way ANOVA and Tukey tests. Data were expressed as mean±SEM, and p<0.05 indicated a significant difference.
Results
Expression of PfRACK in mammalian cells
Codon usage within the Plasmodium falciparum genome is unique in that the guanine nucleotide is predominantly the first base and the thymidine nucleotide is last, although adenine and cytosine nucleotides occur with similar frequency in all positions [22]. To express the correct PfRACK protein in mammalian cells, we transposed the Plasmodium nucleotide sequence to the mammalian one that would correspond to the proper amino acid composition and sequence. The codon-optimized form of the ORF of the sequence encoding the PfRACK gene (gene ID: PF08_0019 deposited at www.plasmodb.org) was then coupled to an expression vector containing EGFP.
PfRACK-EGFP first was expressed in HEK293 cells because they are derived from human tissue and express all three InsP3R isoforms [23]. The subcellular distribution of the expressed protein evolved over time. Confocal immunofluorescence studies compared the distribution of PfRACK to that of endogenous RACK1. While PfRACK-EGFP was present in the nucleus and cytoplasm (Figure 1a), RACK1 was detected primarily in the cytoplasm and near the plasma membrane (Figure 1b). There was partial co-localization of the two proteins in the cytoplasm (Figure 1c). Nuclear localization of PfRACK was confirmed by co-labeling with To-Pro-3 (Figure 1d-f). The same pattern of distribution was observed in rat hepatocytes transfected with PfRACK-EGFP (Figure 1g-i), illustrating that this pattern occurs in both primary cells and cell lines. However, a different PfRACK distribution was seen in Mz-Cha-1 cells. In these cells, the protein was excluded from the nucleus and was more concentrated, albeit in a patchy distribution, near the plasma membrane (Figure 1j). There was partial co-localization of PfRACK with the type III InsP3R (Figure 1k-l), which is the predominant isoform of the InsP3R in these cells [20]. Thus there may be variation in processing or distribution of PfRACK among different cell types.
Fig. 1.
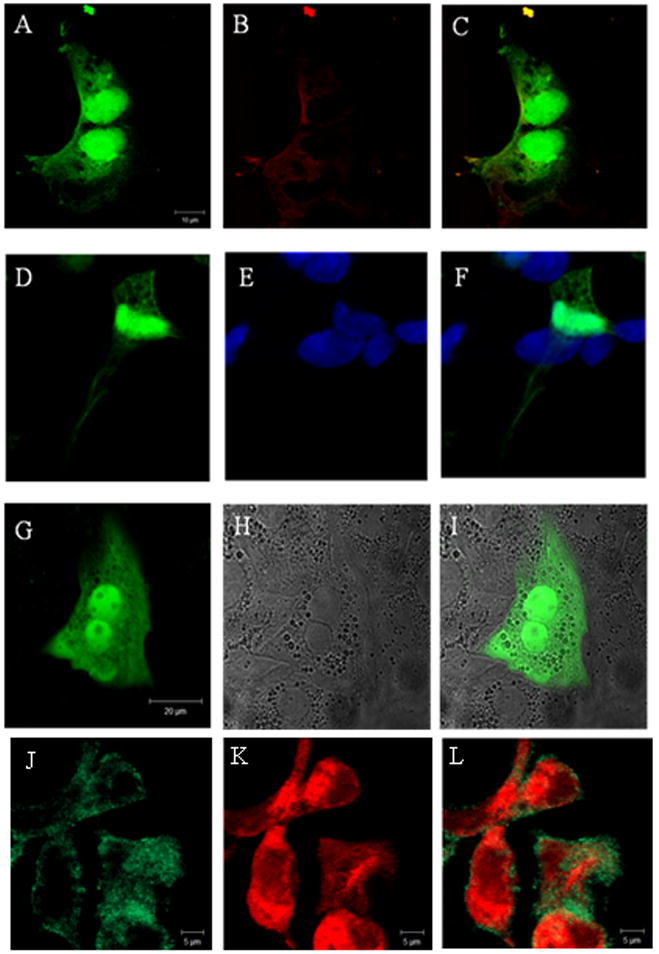
Confocal immunofluorescence shows that (a) PfRACK and (b) RACK1 partially co-localizes in HEK293 cells. (c) Merged image. HEK293 cells (d) expressing GFP-PfRACK and (e) labeled with TO-PRO-3 show that (f) some PfRACK is in the nucleus. Transfection of rat hepatocytes with GFP-PfRACK shows the distribution of this protein is similar in these cells: (g) GFP fluorescence; (h) transmitted light image; (i) merged image. Mz-Cha-1 cells (j) expressing GFP-PfRACK and (k) labeled with an anti- type III InsP3R antibody show that (l) PfRACK only partially co- localizes with this InsP3R isoform.
Immunoblots were performed to confirm the expression of PfRACK in HEK293 cells and to determine whether expression could be maintained in cells with reduced expression of the endogenous ortholog RACK1. Cell lysates were probed with a polyclonal antibody directed against the mammalian RACK protein, and with an anti-GFP antibody (Figure 2a). Immunoblots of transfected cells revealed two bands, the lower (∼50 Kd) band corresponding to endogenous RACK1 and the higher (∼75 Kd) band corresponding to GFP-tagged PfRACK. Transfection of the parasite gene did not alter expression of RACK1. To examine the effect of PfRACK independent of endogenous RACK1, siRNA (100 nM) was used to knockdown RACK1 expression. With this approach, expression of PfRACK was detected both before and after knockdown of RACK1 (Figure 2b).
PfRACK impairs Ca2+ signaling in mammalian cells
Ca2+ signaling was monitored in individual cells by confocal microscopy. Cells were loaded with fura-red, because the long emission wavelength does not interfere with fluorescence from GFP-PfRACK [24]. With this approach, agonist-induced Ca2+ signals could be compared in PfRACK-expressing and nonexpressing cells side-by-side (Figure 2c-e). This approach was used rather than comparing cells expressing GFP-PfRACK to cells transfected with GFP alone to minimize variability due to dye loading and culture conditions seen from slide to slide, and because GFP transfection alone does not affect Ca2+ signaling [25]. Both the amplitude and duration of Ca2+ signals were monitored. Under control conditions, ATP (100 μM) induced a 59±2% (mean±SEM) change in fura-red fluorescence, which typically occurred as one or several Ca2+ spikes (Figure 2d). The amplitude of this Ca2+ signal was reduced by 90% to 6.0±0.5% in cells expressing PfRACK (Figure 2h; p<0.0001). The area under the curve (AUC) of the Ca2+ transients was used as an index of the duration of Ca2+ signaling. This value was 2,105±60 in control cells stimulated with ATP and was 0 in cells expressing PfRACK (Figure 2i; p<0.0001). In cells lacking endogenous RACK1 (Figure 2f), there was a small but significant increase in amplitude of ATP-induced signals (62±5%; p<0.001)(Figure 2h), but there was nearly a five-fold increase in signal duration (10,410±3; p<0.001)(Figure 2i). The increase in signal duration reflected that Ca2+ signals typically converted from repetitive Ca2+ spikes to broad, sustained Ca2+ increases in cells treated with RACK1 siRNA (Figure 2f). This would seem to contrast with previous findings that RACK 1 facilitates InsP3-mediated Ca2+ release [26]. The previous study was performed in Ca2+-free medium, and it was recently reported that interactions between RACK1 and InsP3R depend upon association of these proteins with the plasma membrane Ca2+ channel TRPC3 [27], so the effect of RACK1 may depend upon the presence of extracellular Ca2+. Both amplitude and duration of ATP-induced Ca2+ signals was very low in cells expressing PfRACK but lacking RACK1 (Figure 2g-i). These findings demonstrate that expression of PfRACK impairs ATP-induced Ca2+ signals in HEK293 cells.
PfRACK interferes with InsP3R-mediated Ca2+ release
Because RACK1 interacts with the type I InsP3R [13], we investigated whether the effect of PfRACK on Ca2+ signaling reflected an inhibition of InsP3R function. Cells were loaded with a cell-permeant caged InsP3 and then the InsP3 was photo-released in a controlled fashion in individual cells using two-photon flash photolysis (Figure 3a-c). Under control conditions, photo-release of InsP3 induced a 38.9±1.9% change in amplitude in fura-red fluorescence (Figure 3a,c). This was reduced to a 9.1±1.7% change in amplitude in cells expressing PfRACK (Figure 3b,c; p<0.001). These findings suggest that PfRACK inhibits Ca2+ signaling by directly inhibiting InsP3-mediated Ca2+ release.
Fig. 3.
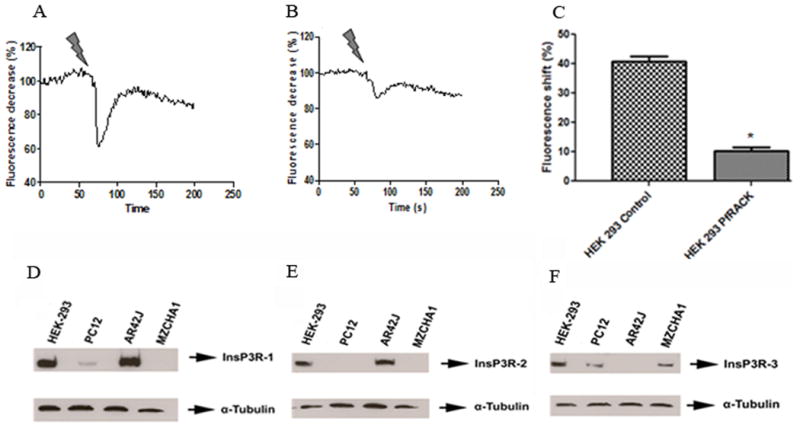
(a-c) Cells were loaded with fura-red and caged InsP3, then monitored by confocal microscopy as InsP3 was uncaged by two-photon flash-photolysis. Typical tracings of the InsP3-induced Ca2+ signal in (a) a non-transfected control cell and (b) a cell expressing GFP-PfRACK. (c) Summary shows the peak amplitude of the InsP3-induced Ca2+ signal is reduced (*p<0.001) in cells expressing GFP-PfRACK. Values are mean±SEM of triplicate measurements made of >40 cells under each condition. (d-f) Expression of InsP3R isoforms in different cell lines. Blots for (d) type I, (e) type II, and (f) type III InsP3R show that PC-12 cells express types I and III but not type II InsP3R, AR4-2J cells express types I and II but not type III InsP3R, and Mz-Cha-1 cells express only type III InsP3R. HEK293 cells express all three isoforms.
PfRACK interferes with Ca2+ signaling in multiple cell types
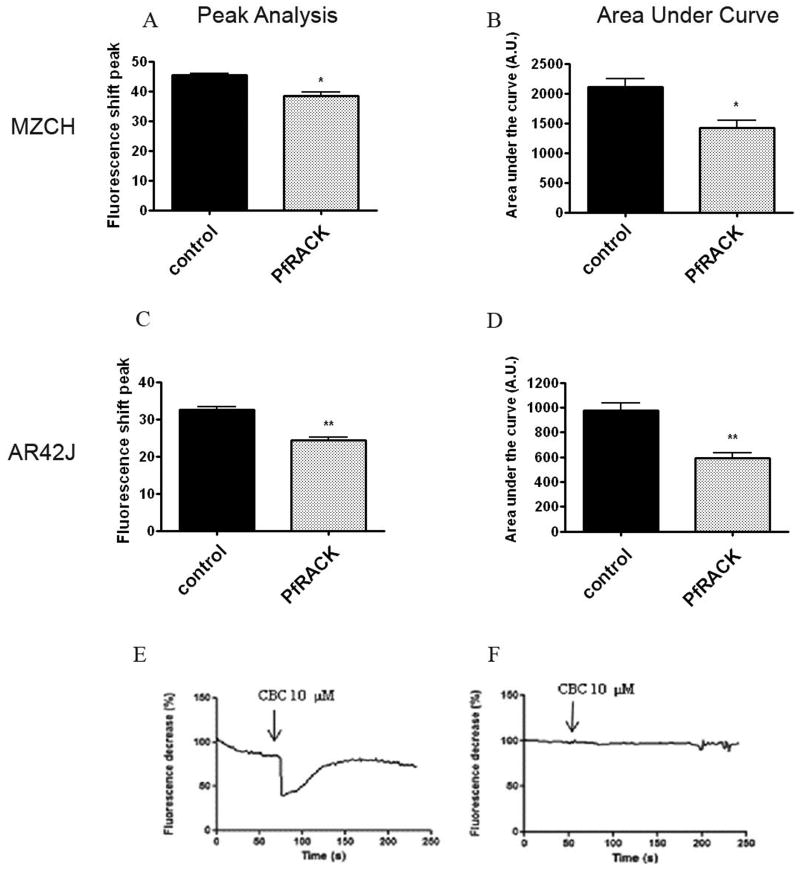
HEK293 cells express all three InsP3R isoforms, so we investigated the effects of PfRACK on Ca2+ signaling in cells expressing different combinations of these isoforms. This included AR4-2J cells, which express only the type I and II isoforms, PC-12 cells, which express only the type I and III isoforms, and Mz-Cha-1 cells, which express only the type III InsP3R (Figure 3d-f). Mz-Cha-1 cells (Figure 4a,b), AR4-2J cells (Figure 4c,d), and PC-12 cells (Figure 4e,f) each were stimulated with carbachol (10 μM). In each case, the amplitude and duration of Ca2+ signals was decreased in cells expressing PfRACK. This suggests that inhibition of InsP3-mediated signaling by PfRACK is not an InsP3 receptor isoform-specific effect.
Fig. 4.
Ca2+ signaling was measured in cells loaded with fura-red and then examined by confocal microscopy. (a-b) Both the amplitude and area under the curve of carbachol (10μM)-induced Ca2+ signals was significantly reduced in Mz-Cha-1 cells transfected with GFP-PfRACK. (c-d) Both the amplitude and area under the curve of carbachol (10μM)-induced Ca2+ signals was significantly reduced in AR4-2J cells transfected with GFP-PfRACK. Values obtained in Mz-Cha-1 and AR4-2J cells are mean±SEM of triplicate measurements made of >40 cells under each experimental condition (*p<0.001). Typical tracings of Ca2+ signals in individual (e) non-transfected and (f) transfected PC-12 cells, each stimulated with carbachol (10μM) shows that PfRACK inhibits Ca2+ signals in these cells as well. Tracings are representative of those observed in >20 cells under each condition.
Discussion
This work provides evidence that PfRACK inhibits InsP3-mediated Ca2+ signaling in mammalian cells. PfRACK inhibited Ca2+ signaling in cells expressing various combinations of InsP3R isoforms, including AR4-2J cells, which have an InsP3R isoform profile similar to hepatocytes [23; 28]. Proteomic analysis of ghosts of infected erythrocytes has identified PfRACK within Maurer's clefts [29], tubulovesicular structures within cytoplasm that are a conduit for Plasmodium proteins to be released into cells [30]. Plasmodium proteins found in Maurer's clefts interact with host cell secretory, metabolic, and signaling pathways [31; 32], and this work provides a potential role for PfRACK released in this fashion as well. Other infectious agents also affect Ca2+ signaling in mammalian cells. For example, Trypanosoma Cruzi expresses oligopeptidase B, a serine hydrolase that induces Ca2+ signals in potential host cells [33; 34]. These signals are responsible for local recruitment of lysosomes to form the parasitophorous vacuole [35-37]. Uropathogenic Escherichia coli secretes alpha-hemolysin, which induces InsP3-mediated Ca2+ oscillations in renal cells [38]. These oscillations induce release of pro-inflammatory cytokines IL-6 and IL-8 [39], a key step in the inflammatory response to infection. These examples illustrate Ca2+ signaling in target cells induced by interaction between the infectious agent and the cell exterior, while Salmonella infections illustrate how infectious agents can interfere more directly with signaling pathways within the target cell. S typhimurium and other strains of Salmonella produce a type III secretion system that allows direct injection of bacterial proteins into the target cell [40]. For example, injection of SopE and SopE2 activates Rho-family GTPases, which in turn remodels the actin cytoskeleton [41]. Our findings are consistent with the idea that, through PfRACK, Plasmodium interferes directly rather than indirectly with host Ca2+ signaling.
What is the possible significance of inhibition of mammalian Ca2+ signals by PfRACK? Ca2+ regulates a range of cellular activities. In hepatocytes, where the malaria parasite differentiates, Ca2+ regulates secretion [42], metabolism [43], glucose release [44], and apoptosis [25]. PfRACK also accumulates in the nucleus, where Ca2+ regulates gene transcription [44; 45] and proliferation [46]. Because the InsP3R is the principal Ca2+ release channel in hepatocytes [18], inhibition by PfRACK would provide a potent mechanism to inhibit Ca2+ signaling. Inhibition of Ca2+ signaling would likely inhibit apoptosis [25], which in turn could preserve the intracellular milieu for the parasite. Inhibition of Ca2+ signaling would also likely inhibit cell proliferation [46], which in turn could conserve host cell resources. Additional work will need to delineate downstream effects of the PfRACK-InsP3R interaction, and in particular to define the effect on parasite survival.
Conclusion
PfRACK, the malarial ortholog of RACK1, inhibits InsP3-mediated Ca2+ signaling in mammalian cells. This inhibitory effect occurs in cells expressing each isoform of the InsP3R. This provides a potential mechanism for P. falciparum to hijack signaling pathways in host cells.
Acknowledgments
This work was supported by NIH grants DK45710, DK57751, and DK34989 and by a grant from FAPESP. RS was also a FAPESP fellow.
Abbreviations
- RACK1
Receptor for activated C kinase protein 1
- PfRACK
Plasmodium falciparum Receptor for activated c kinase protein
- InsP3R
Inositol 1,4,5-trisphosphate receptor
- CDPK
Calcium dependent protein kinase
- PfPKB
Plasmodium falciparum protein kinase B
- NCS-1
Neuronal calcium sensor 1
- TRPC3
Transient receptor potential cation channel 3
- CaBP1
Calcium Binding Protein 1
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Contributor Information
Robson Sartorello, Email: sartorello@gmail.com.
Maria Jimena Amaya, Email: mariajimena.gutierrez@yale.edu.
Michael H. Nathanson, Email: michael.nathanson@yale.edu.
Célia R. S. Garcia, Email: cgarcia@usp.br.
References
- 1.Billker O, Dechamps S, Tewari R, Wenig G, Franke-Fayard B, Brinkmann V. Calcium and a calcium-dependent protein kinase regulate gamete formation and mosquito transmission in a malaria parasite. Cell. 2004;117:503–514. doi: 10.1016/s0092-8674(04)00449-0. [DOI] [PubMed] [Google Scholar]
- 2.Green JL, Rees-Channer RR, Howell SA, Martin SR, Knuepfer E, Taylor HM, Grainger M, Holder AA. The Motor Complex of Plasmodium falciparum Phosphorylation by A Calcium-Dependent Protein Kinase. Journal of Biological Chemistry. 2008;283:30980–30989. doi: 10.1074/jbc.M803129200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Vaid A, Sharma P. PfPKB, a protein kinase B-like enzyme from Plasmodium falciparum II. Identification of calcium/calmodulin as its upstream activator and dissection of a novel signaling pathway. Journal of Biological Chemistry. 2006;281:27126–27133. doi: 10.1074/jbc.M601914200. [DOI] [PubMed] [Google Scholar]
- 4.Vaid A, Sharma P. PfPKB, a protein kinase B-like enzyme from Plasmodium falciparum II. Identification of calcium/calmodulin as its upstream activator and dissection of a novel signaling pathway. Journal of Biological Chemistry. 2006;281:27126–27133. doi: 10.1074/jbc.M601914200. [DOI] [PubMed] [Google Scholar]
- 5.Hotta CT, Gazarini ML, Beraldo FH, Varotti FP, Lopes C, Markus RP, Pozzan T, Garcia CRS. Calcium-dependent modulation by melatonin of the circadian rhythm in malarial parasites. Nature Cell Biology. 2000;2:466–468. doi: 10.1038/35017112. [DOI] [PubMed] [Google Scholar]
- 6.Garcia CRS, Markus RP, Madeira L. Tertian and quartan fevers: Temporal regulation in malarial infection. Journal of Biological Rhythms. 2001;16:436–443. doi: 10.1177/074873001129002114. [DOI] [PubMed] [Google Scholar]
- 7.Gazarini ML, Thomas AP, Pozzan T, Garcia CRS. Calcium signaling in a low calcium environment: how the intracellular malaria parasite solves the problem. Journal of Cell Biology. 2003;161:103–110. doi: 10.1083/jcb.200212130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Beraldo FH, Almeida FM, da Silva AM, Garcia CRS. Cyclic AMP and calcium interplay as second messengers in melatonin-dependent regulation of Plasmodium falciparum cell cycle. Journal of Cell Biology. 2005;170:551–557. doi: 10.1083/jcb.200505117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Madeira L, Galante PA, Budu A, Azevedo MF, Malnic B, Garcia CR. Genome-wide detection of serpentine receptor-like proteins in malaria parasites. PLoS One. 2008;3:e1889. doi: 10.1371/journal.pone.0001889. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.McCahill A, Warwicker J, Bolger GB, Houslay MD, Yarwood SJ. The RACK1 scaffold protein: A dynamic cog in cell response mechanisms. Molecular Pharmacology. 2002;62:1261–1273. doi: 10.1124/mol.62.6.1261. [DOI] [PubMed] [Google Scholar]
- 11.Battaini F, Pascale A. Protein kinase C signal transduction regulation in physiological and pathological aging. Reversal of Aging: Resetting the Pineal Clock. 2006;1057:177–192. doi: 10.1196/annals.1356.011. [DOI] [PubMed] [Google Scholar]
- 12.Wang HY, Friedman E. Increased association of brain protein kinase C with the receptor for activated C kinase-1 (RACK1) in bipolar affective disorder. Biological Psychiatry. 2001;50:364–370. doi: 10.1016/s0006-3223(01)01147-7. [DOI] [PubMed] [Google Scholar]
- 13.Patterson RL, van Rossum DB, Barrow RK, Snyder SH. RACK1 binds to inositol 1,4,5-trisphosphate receptors and mediates Ca2+ release. Proc Natl Acad Sci U S A. 2004;101:2328–32. doi: 10.1073/pnas.0308567100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Madeira L, DeMarco R, Gazarini ML, Verjovski-Almeida S, Garcia CR. Human malaria parasites display a receptor for activated C kinase ortholog. Biochem Biophys Res Commun. 2003;306:995–1001. doi: 10.1016/s0006-291x(03)01074-x. [DOI] [PubMed] [Google Scholar]
- 15.Lanzer M, Wickert H, Krohne G, Vincensini L, Breton CB. Maurer's clefts: A novel multifunctional organelle in the cytoplasm of Plasmodium falciparum-infected erythrocytes. International Journal for Parasitology. 2006;36:23–36. doi: 10.1016/j.ijpara.2005.10.001. [DOI] [PubMed] [Google Scholar]
- 16.Carlton JM, Escalante AA, Neafsey D, Volkman SK. Comparative evolutionary genomics of human malaria parasites. Trends in Parasitology. 2008;24:545–550. doi: 10.1016/j.pt.2008.09.003. [DOI] [PubMed] [Google Scholar]
- 17.Saul A, Battistutta D. Codon Usage in Plasmodium-Falciparum. Molecular and Biochemical Parasitology. 1988;27:35–42. doi: 10.1016/0166-6851(88)90022-9. [DOI] [PubMed] [Google Scholar]
- 18.Hirata K, Pusl T, O'Neill AF, Dranoff JA, Nathanson MH. The type II inositol 1,4,5-trisphosphate receptor can trigger Ca2+ waves in rat hepatocytes. Gastroenterology. 2002;122:1088–100. doi: 10.1053/gast.2002.32363. [DOI] [PubMed] [Google Scholar]
- 19.Echevarria W, Leite MF, Guerra MT, Zipfel WR, Nathanson MH. Regulation of calcium signals in the nucleus by a nucleoplasmic reticulum. Nat Cell Biol. 2003;5:440–446. doi: 10.1038/ncb980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Minagawa N, Kruglov EA, Dranoff JA, Robert ME, Gores GJ, Nathanson MH. The anti-apoptotic protein Mcl-1 inhibits mitochondrial Ca2+ signals. J Biol Chem. 2005;280:33637–44. doi: 10.1074/jbc.M503210200. [DOI] [PubMed] [Google Scholar]
- 21.Echevarria W, Leite MF, Guerra MT, Zipfel WR, Nathanson MH. Regulation of calcium signals in the nucleus by a nucleoplasmic reticulum. Nat Cell Biol. 2003;5:440–6. doi: 10.1038/ncb980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Saul A, Battistutta D. Codon Usage in Plasmodium-Falciparum. Molecular and Biochemical Parasitology. 1988;27:35–42. doi: 10.1016/0166-6851(88)90022-9. [DOI] [PubMed] [Google Scholar]
- 23.Wojcikiewicz RJ. Type I, II, and III inositol 1,4,5-trisphosphate receptors are unequally susceptible to down-regulation and are expressed in markedly different proportions in different cell types. J Biol Chem. 1995;270:11678–83. doi: 10.1074/jbc.270.19.11678. [DOI] [PubMed] [Google Scholar]
- 24.O'Brien EM, Gomes DA, Sehgal S, Nathanson MH. Hormonal regulation of nuclear permeability. J Biol Chem. 2007;282:4210–7. doi: 10.1074/jbc.M606300200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Pusl T, Wu JJ, Zimmerman TL, Zhang L, Ehrlich BE, Berchtold MW, Hoek JB, Karpen SJ, Nathanson MH, Bennett AM. Epidermal growth factor-mediated activation of the ETS domain transcription factor Elk-1 requires nuclear calcium. J Biol Chem. 2002;277:27517–27. doi: 10.1074/jbc.M203002200. [DOI] [PubMed] [Google Scholar]
- 26.Patterson RL, van Rossum DB, Barrow RK, Snyder SH. RACK1 binds to inositol 1,4,5-trisphosphate receptors and mediates Ca2+ release. Proc Natl Acad Sci U S A. 2004;101:2328–2332. doi: 10.1073/pnas.0308567100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Bandyopadhyay BC, Ong HL, Lockwich TP, Liu X, Paria BC, Singh BB, Ambudkar IS. TRPC3 controls agonist-stimulated intracellular Ca2+ release by mediating the interaction between inositol 1,4,5-trisphosphate receptor and RACK1. J Biol Chem. 2008;283:32821–32830. doi: 10.1074/jbc.M805382200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Hernandez E, Leite MF, Guerra MT, Kruglov EA, Bruna-Romero O, Rodrigues MA, Gomes DA, Giordano FJ, Dranoff JA, Nathanson MH. The spatial distribution of inositol 1,4,5-trisphosphate receptor isoforms shapes Ca2+ waves. J Biol Chem. 2007 doi: 10.1074/jbc.M700746200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Vincensini L, Richert S, Blisnick T, Van Dorsselaer A, Leize-Wagner E, Rabilloud T, Breton CB. Proteomic analysis identifies novel proteins of the Maurer's clefts, a secretory compartment delivering Plasmodium falciparum proteins to the surface of its host cell. Molecular & Cellular Proteomics. 2005;4:582–593. doi: 10.1074/mcp.M400176-MCP200. [DOI] [PubMed] [Google Scholar]
- 30.Lanzer M, Wickert H, Krohne G, Vincensini L, Breton CB. Maurer's clefts: A novel multifunctional organelle in the cytoplasm of Plasmodium falciparum-infected erythrocytes. International Journal for Parasitology. 2006;36:23–36. doi: 10.1016/j.ijpara.2005.10.001. [DOI] [PubMed] [Google Scholar]
- 31.Lanzer M, Wickert H, Krohne G, Vincensini L, Breton CB. Maurer's clefts: A novel multifunctional organelle in the cytoplasm of Plasmodium falciparum-infected erythrocytes. International Journal for Parasitology. 2006;36:23–36. doi: 10.1016/j.ijpara.2005.10.001. [DOI] [PubMed] [Google Scholar]
- 32.Carlton JM, Escalante AA, Neafsey D, Volkman SK. Comparative evolutionary genomics of human malaria parasites. Trends in Parasitology. 2008;24:545–550. doi: 10.1016/j.pt.2008.09.003. [DOI] [PubMed] [Google Scholar]
- 33.Garcia CRS, de Azevedo MF, Wunderlich G, Budu A, Young JA, Bannister L. Plasmodium in the postgenomic era: New insights into the molecular cell biology of malaria parasites. International Review of Cell and Molecular Biology. 2008;266:85–+. doi: 10.1016/S1937-6448(07)66003-1. [DOI] [PubMed] [Google Scholar]
- 34.Burleigh BA, Caler EV, Webster P, Andrews NW. Cytosolic serine endopeptidase from Trypanosoma cruzi is required for the generation of Ca2+ signaling in mammalian cells. J Cell Biol. 1997;136:609–620. doi: 10.1083/jcb.136.3.609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Caler EV, de Avalos SV, Haynes PA, Andrews NW, Burleigh BA. Oligopeptidase B-dependent signaling mediates host cell invasion by Trypanosoma cruzi. Embo Journal. 1998;17:4975–4986. doi: 10.1093/emboj/17.17.4975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Tardieux I, Webster P, Ravesloot J, Boron W, Lunn JA, Heuser JE, Andrews NW. Lysosome Recruitment and Fusion Are Early Events Required for Trypanosome Invasion of Mammalian-Cells. Cell. 1992;71:1117–1130. doi: 10.1016/s0092-8674(05)80061-3. [DOI] [PubMed] [Google Scholar]
- 37.Tardieux I, Nathanson MH, Andrews NW. Role in host cell invasion of Trypanosoma cruzi-induced cytosolic-free Ca2+ transients. J Exp Med. 1994;179:1017–1022. doi: 10.1084/jem.179.3.1017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Uhlen P, Laestadius A, Jahnukainen T, Soderblom T, Backhed F, Celsi G, Brismar H, Normark S, Aperia A, Richter-Dahlfors A. alpha-Haemolysin of uropathogenic E-coli induces Ca2+ oscillations in renal epithelial cells. Nature. 2000;405:694–697. doi: 10.1038/35015091. [DOI] [PubMed] [Google Scholar]
- 39.Uhlen P, Laestadius A, Jahnukainen T, Soderblom T, Backhed F, Celsi G, Brismar H, Normark S, Aperia A, Richter-Dahlfors A. alpha-Haemolysin of uropathogenic E-coli induces Ca2+ oscillations in renal epithelial cells. Nature. 2000;405:694–697. doi: 10.1038/35015091. [DOI] [PubMed] [Google Scholar]
- 40.Galan JE, Wolf-Watz H. Protein delivery into eukaryotic cells by type III secretion machines. Nature. 2006;444:567–573. doi: 10.1038/nature05272. [DOI] [PubMed] [Google Scholar]
- 41.Patel JC, Galan JE. Differential activation and function of Rho GTPases during Salmonella-host cell interactions. Journal of Cell Biology. 2006;175:453–463. doi: 10.1083/jcb.200605144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Nathanson MH, Gautam A, Bruck R, Isales CM, Boyer JL. Effects of Ca2+ agonists on cytosolic Ca2+ in isolated hepatocytes and on bile secretion in the isolated perfused rat liver. Hepatology. 1992;15:107–116. doi: 10.1002/hep.1840150119. [DOI] [PubMed] [Google Scholar]
- 43.Hajn¢czky G, Robb-Gaspers LD, Seitz MB, Thomas AP. Decoding of cytosolic calcium oscillations in the mitochondria. Cell. 1995;82:415–424. doi: 10.1016/0092-8674(95)90430-1. [DOI] [PubMed] [Google Scholar]
- 44.Nathanson MH, Rios-Velez L, Burgstahler AD, Mennone A. Communication via gap junctions modulates bile secretion in the isolated perfused rat liver. Gastroenterology. 1999;116:1176–1183. doi: 10.1016/s0016-5085(99)70021-1. [DOI] [PubMed] [Google Scholar]
- 45.Hardingham GE, Chawla S, Johnson CM, Bading H. Distinct functions of nuclear and cytoplasmic calcium in the control of gene expression. Nature. 1997;385:260–265. doi: 10.1038/385260a0. [DOI] [PubMed] [Google Scholar]
- 46.Rodrigues MA, Gomes DA, Leite MF, Grant W, Zhang L, Lam W, Cheng YC, Bennett AM, Nathanson MH. Nucleoplasmic calcium is required for cell proliferation. J Biol Chem. 2007;282:17061–8. doi: 10.1074/jbc.M700490200. [DOI] [PMC free article] [PubMed] [Google Scholar]