Abstract
Female cynomolgus monkeys exhibit different degrees of reproductive dysfunction with moderate metabolic and psychosocial stress. When stressed with a paradigm of relocation and diet for 60 days or 2 menstrual cycles, highly stress resilient monkeys (HSR) continued to ovulate during the stress cycles whereas stress sensitive monkeys (SS) did not. After cessation of stress, monkeys characterized as HSR or SS were administered placebo (PL) or S-citalopram (CIT) for 15 weeks at doses that normalized ovarian steroid secretion in the SS animals and that maintained blood citalopram levels in a therapeutic range. After euthanasia, the brain was perfused with 4% paraformaldehyde. The pontine midbrain was blocked and sectioned at 25μm. The expression of 4 genes pivotal to serotonin neural function was assessed in the 4 groups of monkeys (n=4/group). Fev (fifth Ewing variant) ETS transcription factor, tryptophan hydroxylase 2 (TPH2), the serotonin reuptake transporter (SERT), and the 5HT1A autoreceptor were determined at 7–8 levels of the dorsal raphe nucleus with in situ hybridization (ISH) using radiolabeled- and digoxygenin-incorporated riboprobes. Positive pixel area and cell number were measured with Slidebook 4.2 in the digoxigenin assay for Fev. Optical density (OD) and positive pixel area were measured with NIH Image software in the radiolabeled assays for TPH2, SERT and 5HT1A. All data were analyzed with 2-way ANOVA. Stress-sensitive monkeys had significantly fewer Fev-positive cells and lower Fev-positive pixel area in the dorsal raphe than stress-resilient monkeys. Stress-sensitive macaques also had significantly lower levels of TPH2, SERT and 5HT1A mRNAs in the dorsal raphe nucleus than stress-resilient macaques. However, citalopram did not alter the expression of either Fev, TPH2, SERT or 5HT1A mRNAs. These data suggest that stress-sensitive macaques have fewer serotonin neurons than stress-resilient macaques, and that they have deficient Fev expresssion, which in turn, leads to deficient TPH2, SERT and 5HT1A expression. In addition, the therapeutic effect of citalopram is probably achieved through mechanisms other than alteration of serotonin-related gene expression.
Keywords: citalopram, stress, tryptophan hydroxylase, serotonin reuptake transporter, 5HT1A autoreceptor, serotonin, macaques
Introduction
Exposure to stressful stimuli can lead to a variety of secondary diseases such as anxiety, depression, cardiovascular disease, and immune suppression (McEwen, 2002). Reproductive dysfunction has been recently added to this growing list of stress-related disorders (Xiao et al., 1999, Cameron, 2000). Clinically, one of the most common forms of infertility is stress-induced infertility, also called Functional Hypothalamic Amenorrhea (FHA; (Berga and Loucks, 2006)). FHA accounts for as much as 30% of female infertility (Reindollar et al., 1986), and is defined as a sustained absence of normal menstrual cycles despite healthy reproductive organs and the capability of showing normal physiologic functioning (Reifenstein, 1946). Psychometric testing has shown that women with FHA report an increased amount of psychosocial stress in their lives compared to control populations, or women with other forms of infertility, despite experiencing comparable numbers of stressful life events (Giles and Berga, 1993, Fioroni et al., 1994, Marcus et al., 2001). Women with FHA tend to be normal weight and carefully watch their food intake, scoring higher on eating disorder inventories than a control population, but not in the range of having eating disorders (Laughlin et al., 1998, Marcus et al., 2001). They also tend to exercise on a regular basis, often to control life stress (Berga and Girton, 1989, Giles and Berga, 1993, Berga et al., 2003). Thus, FHA appears to result from exposure to a combination of common psychosocial and metabolic life stresses. Clearly, not all women who experience these everyday life stresses develop fertility problems, suggesting that there is a range of sensitivity to developing stress-induced reproductive dysfunction in normal women.
We have developed an experimental nonhuman primate model of FHA in which mild psychosocial stress (relocation) combined with a mild diet and/or a moderate exercise regimen suppresses reproductive function that reverses upon stress removal (Williams et al., 1997, Cameron, 2000, Bethea et al., 2005a, Williams et al., 2007). Using this monkey model, we find that some individuals are sensitive to stress-induced suppression of reproductive hormone secretion, while others are stress-resilient. Female cynomolgus monkeys are either highly stress-resilient (HSR) and maintain normal menstrual cyclicity when exposed to two cycles of combined stress, or medium stress-resilient (MSR) and are ovulatory in the first stress cycle, but anovulatory in the second stress cycle, or stress-sensitive (SS) and become anovulatory as soon as stress is initiated.
We have shown that even in non-stressed conditions there are differences in the functioning of several key neural systems in stress-sensitive vs. stress-resilient monkeys. Stress-sensitive animals chronically have lower release of serotonin (5HT) in response to fenfluramine (Bethea et al., 2005a), and have a down regulation of the central serotonergic system as indicated by less serotonin reuptake transporter (SERT) gene expression, less 5HT-1A receptor gene expression and less expression of the genes that degrade serotonin (MAO-A and MAO-B) in the raphe nucleus (Bethea et al., 2005b). In the hypothalamus, there is an up-regulation of 5HT-2A and –2C receptors (Centeno et al., 2007a), and an increase in GAD67 gene expression (Centeno et al., 2007a) and CRH gene expression (Centeno et al., 2007c), which are inhibitory to the reproductive axis. We also found that stress-sensitive animals have less immunoreactive GnRH in the median eminence, lower pituitary LH and FSH secretion, and lower ovarian estradiol and progesterone secretion compared to more stress-resilient monkeys, when they are not experiencing stress (Centeno et al., 2007b).
Recently, we characterized a new population of monkeys as stress-sensitive and stress-resilient, and then treated them with citalopram or placebo for 15 weeks in the absence of stress (Cameron et al., 2004). We found that there was an increase in estradiol and progesterone secretion only in the SS group treated with citalopram, suggesting that central neural drive to the reproductive axis was improved with citalopram treatment (Cameron et al., 2004).
Citalopram is a selective serotonin uptake inhibitor indicating that the serotonin system was a primary cite of action. In this study, we examined the expression of 4 pivotal genes that regulate the function of the serotonin neural system in the stress-sensitive and stress-resilient animals treated with placebo or citalopram. The genes examined were tryptophan hydroxylase 2 (TPH2) which codes for the rate-limiting enzyme in serotonin synthesis; the serotonin reuptake transporter (SERT) which regulates extracellular serotonin, and the 5HT1A autoreceptor which inhibits serotonin neuron excitation, and Fev, an ETS domain transcription factor that determines whether a neuron is serotonergic, and functions specifically in the differentiation and maintenance of serotonin neurons. Conserved Fev-1 binding sites are present in or near the promoter regions of the human and mouse TPH2, serotonin transporter and 5-HT1A receptor genes (Hendricks et al., 1999, Hendricks et al., 2003). We found that the expression of Fev, TPH2, SERT and 5HT1A mRNAs was compromised in stress-sensitive animals, but that citalopram treatment for 15 weeks did not alter the expression of these genes.
Materials and Methods
Animals and treatments
All studies were reviewed and approved by the Institutional Animal Care and Use Committee of the ONPRC and performed according to federal guidelines. Twenty-five female cynomolgus monkeys started the study, but 16 were utilized for neuroanatomical assays in a 2 × 2 block design with 4 animals per group. They were housed in single cages, in large rooms with other monkeys. Movement of monkeys within the room, and in and out of the room, was limited so that the monkeys had a stable social environment for 4 months. The monkeys were fed commercially available monkey chow (12 pellets of Purina High Protein monkey chow) once a day at about 1100 hours supplemented with fruit. Water was available ad libitum.
Menstrual cyclicity was monitored by daily swabbing of the vaginal area with a cotton-tipped applicator. Blood samples were collected every other day by femoral venipuncture (monkeys were trained to extend a leg for blood sampling) to monitor reproductive hormones throughout the study. To categorize monkeys as highly stress resilient (HSR) or stress sensitive (SS) they were moved to a new housing room, surrounded by unfamiliar monkeys and they were put on a diet change eating 80% of the food they consumed during the control phase of the study. Their menstrual cyclicity and reproductive hormones were evaluated for 2 months after the move/diet. Based upon the menstrual response to this combined psychosocial and metabolic stress, the monkeys were labeled highly stress resilient (HSR, n=8), medium stress resilient (MSR, n=4), stress sensitive (SS, n=7) and very stress sensitive (VSS, n=6). The VSS animals did not exhibit cycles during the 4-month control period indicating they were extremely sensitive to the environmental change after purchase and so they were not moved. However, they did cycle prior to necropsy.
All of the moved animals were then returned to their home cage and allowed to eat ad libitum. Approximately half of each group was treated with vehicle (placebo) and the other half was treated for 15 weeks with 1.2–4.8 mg/kg S-citalopram (b.i.d.) to elevate blood levels of citalopram into the therapeutic range for treating depression. Blood samples were collected twice from representative animals to monitor blood citalopram levels by HPLC and doses were adjusted if necessary to keep levels in the target range of 20–40 ng/ml as observed in depressed patients. After ~1–2 weeks, blood levels of citalopram at 3- and 6-hours after dosing averaged 24.8±5.0 and 13.5±3.6 ng/ml, respectively, and desmethyl-citalopram averaged 12.1±1.7 and 8.7±1.8 ng/ml, respectively (n=5 animals), which required adjustment. After a further 3 weeks, blood levels of citalopram at 3- and 6-hours after dosing averaged 23.7±6.2 and 22.4±5.7 ng/ml, respectively, and desmethyl-citalopram averaged 13.0±3.1 and 13.0±3.1 ng/ml, respectively (n=6 animals). It appeared that the monkeys metabolized s-citalopram faster than reported for women (Sit, et al., 2008). Menstrual cyclicity and reproductive hormones were monitored throughout the citalopram treatment.
For hormone analysis at the end of citalopram treatment, the groups that showed some sensitivity to stress (MSR, SS and VSS groups) were combined (n=17) and compared to the highly stress resilient group (n=8). In stress sensitive monkeys, citalopram treatment significantly increased peak estradiol levels in the follicular phase of the menstrual cycle from 360±67 to 544±82 pg/ml (p < 0.05) and increased peak progesterone levels in the luteal phase of the menstrual cycle from 6.7±1.4 to 11.3± 1.8 ng/ml (p < 0.05). In contrast, vehicle- and citalopram-treated stress-resilient monkeys had similar peak estradiol and peak progesterone, before and after treatment, which averaged (both groups) 575±100 pg/ml and 11.5±3 ng/ml, respectively (Cameron et al., 2004). (For subsequent neuroanatomical analysis only the SS and VSS groups were combined. The MSR animals were not included as explained below.)
Tissue Preparation
The monkeys were euthanized according to the procedures recommended by the Panel on Euthanasia of the American Veterinary Association. Each animal was sedated with ketamine, given an overdose of pentobarbitol (25 mg/kg, i.v.), and exsanguinated by severance of the descending aorta.
The left ventricle of the heart was cannulated and the head was perfused with 1L of saline (made with DEPC-treated water [0.1% diethyl pyrocarbonate] to minimize RNase contamination) followed by 7 liters of 4% paraformaldehyde in 3.8% borate, pH 9.5 (Berod et al., 1981, Simmons et al., 1989). The brain was removed and dissected. Blocks of tissue containing the pontine midbrain were post-fixed for 3 hours in 4% paraformaldehyde, then washed in 0.02 M potassium phosphate-buffered saline (KPBS) containing first 10% (overnight), then 20% glycerol (48 h), plus 2% dimethyl sulfoxide (DMSO) to cryoprotect the tissue. The blocks were frozen in precooled methylbutane (−55°C) and stored at −80°C for up to 6 months.
Post hoc animal selection for tissue analysis
The eight HSR animals that were treated with placebo or citalopram were all included (n=4/treatment). The four animals that were MSR yielded only 2 each for placebo or citalopram treatment, so they were excluded due to insufficient numbers for statistical analysis. The SS and VSS groups were considered together as highly stress sensitive. Five V/SS animals were treated with placebo, but one animal had a poor perfusion of the brain, so the remaining four animals were included. Eight V/SS animals were treated with citalopram. Four of the eight animals, which never cycled or which exhibited immediate ceasation of ovulation upon moving, were included in the tissue analysis providing an n of four in a 2 × 2 block design. The midbrain blocks containing the dorsal raphe were cut on a sliding microtome at 25 μm, and the sections were mounted on Superfrost Plus Slides (Fisher Scientific, Pittsburgh, PA), dehydrated under vacuum overnight and then stored at −80°C until processing for in situ hybridization (ISH) assays.
Riboprobe
A 268 bp segment of Fev (Accession ID XM_001095962) was amplified by RT-PCR from RNA extracted from rhesus monkey embryonic stem cell derived serotonin neurons (Bethea et al., 2009). The primer sequences were as follows:
F_CAGAAAGGCAGCGGACAGAT (709bp–728bp)
R_CCTGGAAGTCGAAGCGGTAG (976bp–957bp)
The Fev amplicon was inserted into pGEM-T and sequenced with an Applied Biosystems 3730xl with capillary DNA sequencing in the ONPRC Cell and Molecular Biology Core. The Fev sequence was compared to human Fev for sequence identity with BLAST at the NCBI website. For riboprobe production, the Fev cDNA was linearized with NCO1.
A monkey specific partial cDNA clone of TPH2 containing 251 bp of the 5′ region, which has very little homology to TPH1, was previously constructed and the TPH2 riboprobe-incorporating 35S-UTP was synthesized as previously described (Sanchez et al., 2005). A recombinant subclone of the 5′ cytoplasmic domain of human SERT was previously generated with a 253 bp insert. The SERT riboprobe incorporating 35S-UTP was synthesized as previously described (Pecins-Thompson et al., 1998, Bethea et al., 2005b). A monkey specific partial cDNA clone of the 5HT1A autoreceptor was previously constructed that contained 432 bp extending from base 647 to 1078 of the 5HT1A receptor and the 5HT1A riboprobe incorporating 35S-UTP was synthesized as previously described (Pecins-Thompson and Bethea, 1998, Centeno et al., 2007a). This region corresponds to the area between transmembrane domains V and VI and it has the least sequence homology with other 5HT receptors, the β2-adrenergic and the α1-adrenergic receptors that also share sequence homology with the 5HT1A receptor (Julius, 1991).
In situ hybridization (ISH) assays
The non-isotopic in situ hybridization procedure, described in detail elsewhere (Berg-von der Emde et al., 1995) utilizing a digoxygenin-labeled Fev cRNA, was used to determine if Fev mRNA abundance differs between SS and HSR monkeys treated with placebo or citalopram. The cRNA was prepared using the cDNA mentioned above as template and SP6 RNA polymerase to drive the transcription reaction (Berg-von der Emde et al., 1995). Following an overnight hybridization at 55–56°C, the slides were washed and processed for digoxygenin detection of Fev mRNA, as reported (Berg-von der Emde et al., 1995). After developing the digoxygenin/antidigoxygenin-alkaline phosphatase conjugate reaction by an overnight incubation in a 4-nitrobluetetrazolium chloride/5-bromo-4-chloro-3indoyl-phosphate staining solution, the slides were extensively washed in potassium phosphate-sodium chloride buffer pH 7.4, dehydrated in graded ethanol, dried, and coverslipped for microscopic examination. Eight levels of the dorsal raphe were analyzed at 250 μm intervals. The sections were matched anatomically and rostral, medial and caudal sections from all animals were processed together.
The details of the isotopic in situ hybridization assays for TPH2, SERT and 5HT1A have been published (Pecins-Thompson and Bethea, 1998, Pecins-Thompson et al., 1998, Sanchez et al., 2005) in which the prehybridization, hybridization and wash temperatures were empirically optimized for each probe. In brief, raphe sections were removed from frozen storage and post-fixed in 4% paraformaldehyde for 15 min, rinsed in TE (0.1M Tris, 0.05 M EDTA, in DEPC water, pH 8) for 10 min, permeabilized at 37°C with proteinase K (10 μg/ml) in TE for 30 min, then acetylated with 0.2% acetic anhydride in 0.1 M triethanolamine for 10 min, and rinsed in 2X saline sodium citrate (2X SSC: 0.3 M NaCl, 0.03 M sodium citrate) for 4 min. Following rinses and dehydration in ethanol, sections were hybridized overnight at 40°C for TPH2, 50°C for SERT, and at 60°C for 5HT1A. The following morning, sections were washed with 4X saline sodium citrate (SSC), ribonuclease A (RNase A) at 37°C for 30 min, 2X SSC, and 0.1X SSC at a temperature of 60°C for TPH2 and SERT and 70°C for 5HT1A. Sections were dehydrated and apposed to Hyperfilm b-max (Amersham Pharmacia Biotech, Arlington Heights, IL) at room temperature for 5 days for all probes. Negative controls included an RNase pretreatment before hybridization and hybridization with sense riboprobe. Seven levels of the dorsal raphe at 250 μm intervals were examined in each animal. The sections were matched anatomically and rostral, medial and caudal sections were run in separate assays.
Densitometric Analysis
In the digoxigenin ISH for Fev, sections (8 levels/animal) were video-captured with the Marianas Stereology Workstation and Slidebook 4.2. A montage of the dorsal raphe was created by Slidebook, which was subjected to further analysis. For each anatomical level, the largest representation of the dorsal raphe was chosen from amongst all of the animals. A square outline was placed over the chosen dorsal raphe and the exact dimensions were recorded. The same size square was then used for all of the animals at that anatomical level. First, the operator outlined (boxed) the dorsal raphe nucleus and counted the number of Fev-positive neurons by marking each positive cell. The software program tallied the marks and provided the number of cells in the defined area. Then, the image was segmented into positive (stained) and negative (unstained) pixels. Two measurements were generated from the segmented image. The area covered by the Fev-positive pixels was obtained, and called positive pixel area. Then, the intensity of each pixel was obtained and summed for each level. This value was called pixel intensity, which is analogous to optical density in autoradiographic analysis. While these different parameters are related, it is possible for them to change independently depending on Fev expression in individual cells. The same procedure was applied at each anatomical level from the rostral to caudal extent of the dorsal raphe nucleus. The outlined area was also measured to ensure equal areas were analyzed across all animals.
Autoradiographic films from TPH2, SERT and 5HT1A assays were developed, illuminated, and images of sections (7 levels/animal) were video-captured using a CCD video camera module (Sony, Japan). The images were digitized using the NIH Image Program. Two measurements were generated from the autoradiographs that required the operator to outline (box) the dorsal raphe nucleus. For each probe and each anatomical level, the largest representation of the dorsal raphe was chosen from amongst all of the animals. A square outline was placed over the chosen dorsal raphe and the exact dimensions were recorded. The same size square was then used for all of the animals at that anatomical level. The same procedure was applied at each anatomical level from the rostral to caudal extent of the dorsal raphe nucleus.
The first measurement was the average gray scale optical density, obtained by subtracting the background from the optical density of the region encircled. The second measurement yielded the average positive pixel area, obtained by operator thresholding of signal to background (subjectively set to best reflect the signal and then the same setting was used for all animals). This indicates the area covered by the signal.
To verify the linearity of the autoradiographic films and to verify that individual films exhibited similar responsiveness, 14C-autoradiographic standards (Amersham, Arlington Heights, IL) were used which yield an exposure comparable to 35S. The standards are calibrated to reflect rat brain gray matter impregnated with increasing amounts of 14C. The average optical density measured from experimental regions fell within the linear range of the standards and film responses were consistent between different films. The experimental films were exposed for 3 to 14 days depending on the probe.
In the TPH2 assays, initially 9 levels were examined to get an accurate assessment of the morphology of the dorsal and median raphe nuclei. The median raphe emerged irregularly at levels 6–9. One animal appeared to lack a median raphe altogether and in the remaining animals, the anatomy was inconsistent, sometimes consisting of scattered cells and other times forming a dense central nucleus. These anatomical anomalies precluded rigorous analysis of the median raphe.
Statistical Analysis of ISH signals
All measurements were averaged across the levels, generating one overall average for each animal. In addition, the total number of Fev-positive cells and the number of cells per cubic mm was determined. The data were compared with a two-by-two analysis of variance (ANOVA) followed by Bonferroni’s post hoc pairwise comparison. Thus, the variance reflects the difference between animals. All statistical analyses were conducted using the Prism Statistic Program 5.0 (GraphPad, San Diego, CA). A confidence level of p<0.05 was considered significant.
Results
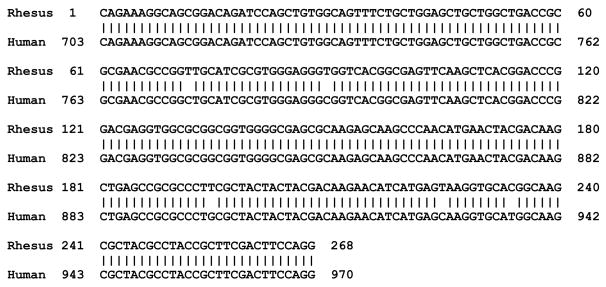
The sequence of the rhesus macaque Fev amplification product was obtained and compared to the human sequence in Figure 1. There is 98% sequence identity between the macaque and human sequences in this region of Fev, which corresponds to nucleotides 121–389 of the coding region. There were 5 nucleotide changes in the 268 bp macaque sequence, which were silent and should not change the protein sequence of the Fev transcription factor.
Figure 1.
The sequence alignment of our rhesus macaque Fev fragment with the same region of the human Fev gene. This region spans bp 121 to 389 from the start codon of the coding region. Translation of the rhesus transcript yields the same amino acid sequence as the human transcript.
One of the blocks from the HSR-citalopram group was apparently poorly perfused and adequate signals were not obtained, which left 3 animals for gene analysis in the HSR+citalopram group and 4 animals in all of the other groups.
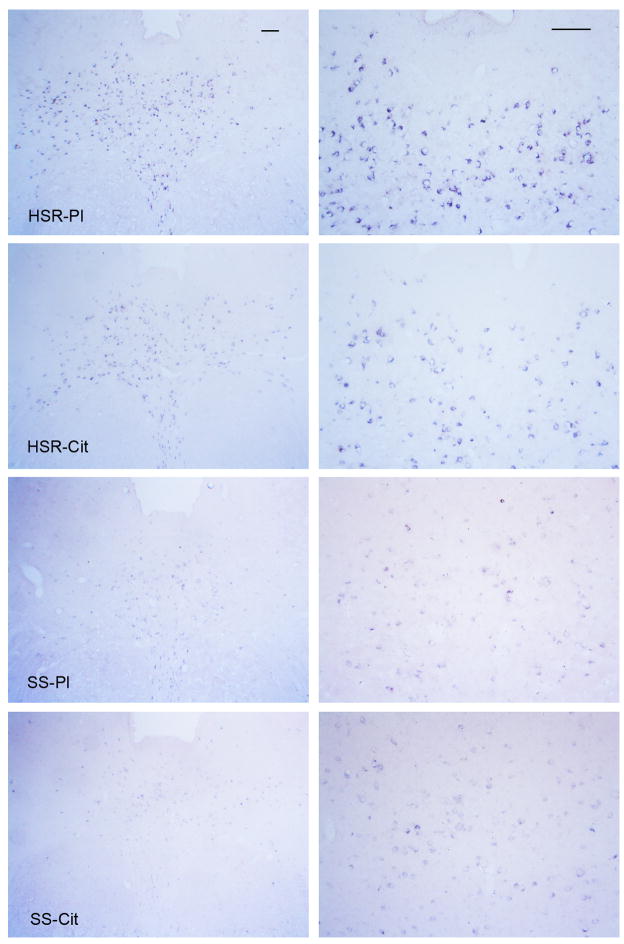
As illustrated in Figure 2, there was robust expression of Fev in the HSR groups compared to the SS groups. Fev expression was restricted to the neurons of the raphe nuclei and the presence of Fev indicates that these neurons are solely serotonergic.
Figure 2.
Illustration of Fev expression in the rostral dorsal raphe in representative monkeys from each treatment group at two magnifications. The scale bar equals 100 μm. There is robust expression of Fev in the HSR-placebo (HSR-Pl) and HSR-citalopram (SHR-Cit) groups, but Fev expression is markedly reduced in the SS-placebo (SS-Pl) and SS-citalopram (SS-Cit) groups.
Figure 3 illustrates the morphological appearance of Fev-positive cells at levels 3, 5 and 7. The change in the presentation and anatomy of the dorsal raphe nucleus from the rostral to caudal levels is apparent. The anatomy of the dorsal raphe changes from a unified mass of Fev-positive cells at the rostral levels to a bifurcated structure at the caudal levels. The entire dorsal raphe nucleus was outlined for analysis excluding the dorsal raphe intrafascicularis.
Figure 3.

Illustration of the anatomical organization of the Fev-positive neurons at 3 of the 8 levels of the dorsal raphe that were subjected to analysis. The anatomy of the dorsal raphe changes from a unified mass of Fev-positive cells at the rostral levels to a bifurcated structure at the caudal levels.
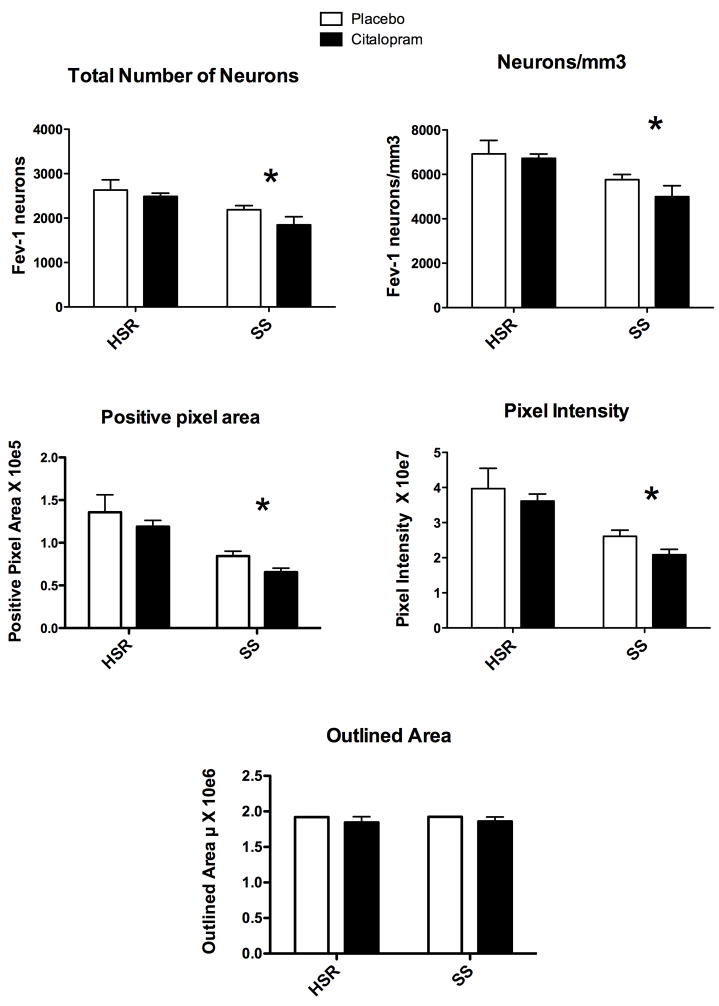
The number of neurons in each level was summed generating the total number of neurons per animal. The mean of the total number of neurons for each group is illustrated in Figure 4, top left panel. Two way ANOVA indicated that there was a significant effect of stress sensitivity (p = 0.008), but no effect of treatment (p= 0.18) and no interaction (p=0.57). Hence, SS animals have significantly fewer Fev-positive serotonin neurons than HSR animals, but citalopram did not change serotonin cell number. The volume of the analyzed region was computed by multiplying the average outlined area of each animal by the total thickness of the sections (200μm). Then, the number of Fev positive serotonin neurons per mm3 volume was calculated and the results are shown in Figure 4, top right panel. Two way ANOVA indicated that there was a significant effect of stress sensitivity (p = 0.008), but no effect of treatment (p= 0.31) and no interaction (p=0.54). Hence, SS animals have significantly fewer Fev-positive serotonin neurons per mm3 than HSR animals, but citalopram treatment did not change serotonin cell number.
Figure 4. Overall averages of several Fev parameters for each group (n=3–4/group).
Top Left - The number of neurons in each level was summed generating the total number of neurons per animal. The mean of the total number of neurons for each group is illustrated. There was a significant effect of stress sensitivity (*p = 0.008), but no effect of treatment (p= 0.18) and no interaction (p=0.57).
Top Right - The number of Fev positive serotonin neurons per mm3 volume is illustrated. There was a significant effect of stress sensitivity (*p = 0.008), but no effect of treatment (p= 0.31) and no interaction (p=0.54).
Middle Left - The average positive pixel area is illustrated. There was a significant effect of stress sensitivity (*p = 0.001), but no effect of treatment (p= 0.167) and no interaction (p=0.938).
Middle Right - The intensity of each positive pixel was obtained and summed for each level. The average of the summed pixel intensity across all 8 levels was obtained for each animal and this was used to obtain the group means of positive pixel intensity as illustrated. There was a significant effect of stress sensitivity (*p = 0.002), but no effect of treatment (p= 0.23) and no interaction (p=0.81).
Bottom - The outlined area was averaged for each animal across all 8 levels and this was used to obtain the group means of the outlined area as illustrated. There was no difference in the outlined area that was analyzed between the groups.
The positive pixel area was obtained after segmentation of the image and computed by Slidebook 4.2. The results are illustrated in Figure 4, middle left panel. Two way ANOVA indicated that there was a significant effect of stress sensitivity (p = 0.001), but no effect of treatment (p= 0.167) and no interaction (p=0.938). Hence, SS animals have significantly fewer Fev-positive pixels than HSR animals, but citalopram did not change the positive pixel area, suggesting that citalopram did not change Fev gene expression. Next, the intensity of each positive pixel was obtained and summed for each level. The average pixel intensity across all 8 levels was obtained for each animal and this was used to obtain the group means of positive pixel intensity. The results are illustrated in Figure 4, middle right panel. Two- way ANOVA indicated that there was a significant effect of stress sensitivity (p = 0.002), but no effect of treatment (p= 0.23) and no interaction (p=0.81). Hence, SS animals have significantly lower Fev-positive pixel intensity than HSR animals, but citalopram treatment did not change the positive pixel intensity further supporting the notion that citalopram did not change Fev gene expression.
The outlined area was averaged for each animal across all 8 levels and this was used to obtain the group means of the outlined area, which was subjected to further analysis. The results are illustrated in Figure 4, bottom panel. There was no difference in the outlined area that was analyzed between the groups.
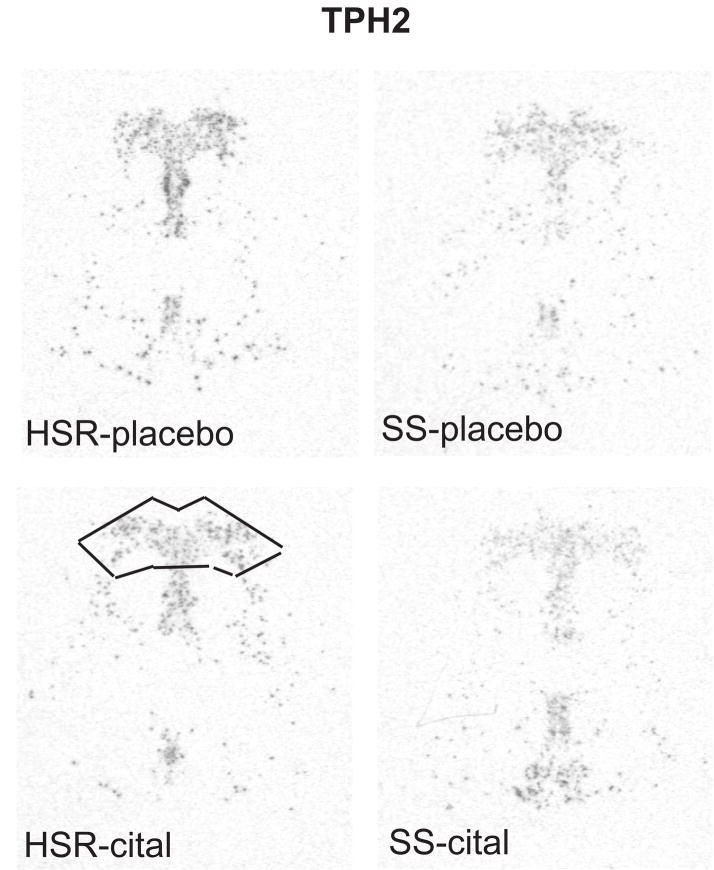
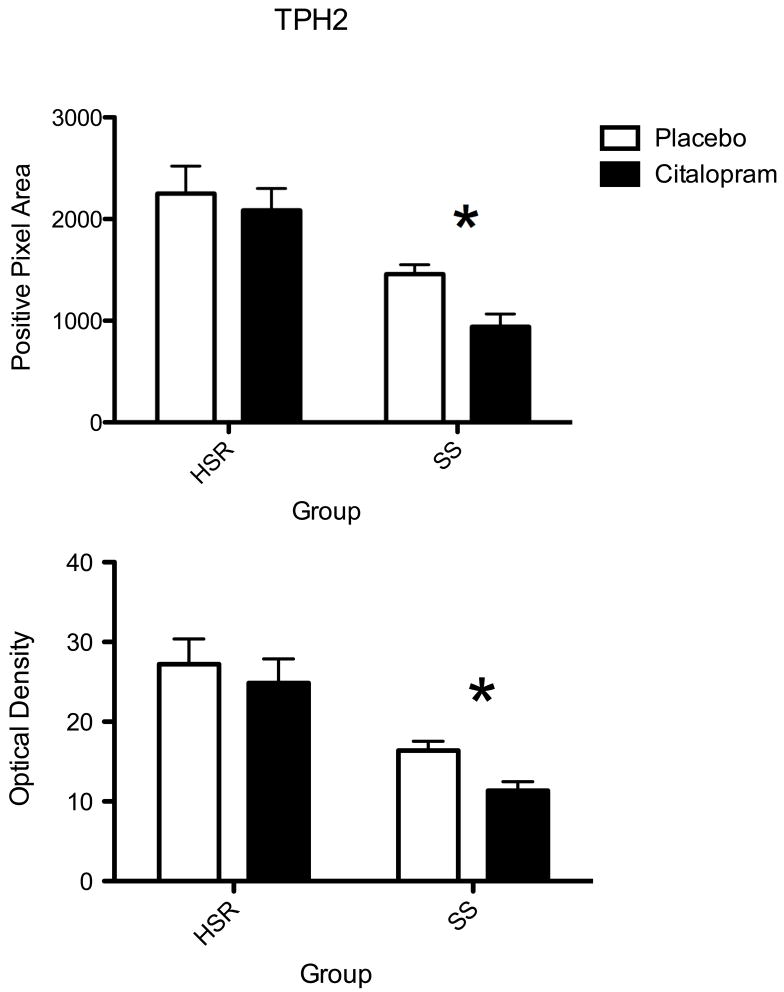
The autoradiographic signal for TPH2 mRNA at a rostral level of the dorsal raphe nucleus is illustrated in Figure 5. TPH2 was robustly expressed in the HSR animals treated with placebo or citalopram. There was an apparent decrease in TPH2 mRNA in the SS animals treated with placebo or citalopram. The optical density of the signal and the pixel area covered by the signal was obtained from 7 levels of the dorsal raphe and the average signal/section was obtained for each animal. The mean ± SEM of each group was compared with a 2-way ANOVA and the results are shown in Figure 6. There was a significant effect of stress sensitivity on optical density (p=0.0002) and on positive pixel area (p=0.0003). However, there was no effect of drug treatment on optical density (p=0.13) or on positive pixel area (p= 0.10) and there was no interaction (p=0.37 for OD; p=0.57 for pixels). Thus, TPH2 gene expression was lower in the SS animals compared to the HSR animals, but citalopram treatment for 15 weeks did not alter TPH2 gene expression.
Figure 5.
In situ hybridization autoradiograms of TPH2 signal in the dorsal raphe nucleus of representative HSR and SS macaques treated with placebo or citalopram for 15 weeks. Scattered neurons of the rostral median raphe are also visible underneath the decussation of the cerebellar peduncles. The box illustrates the area analyzed at this morphological level. The same box was applied to all sections at this level. Each of the 7 levels had a different sized box that was held constant for all of the sections at that morphological level.
Figure 6.
Histograms illustrating the average positive pixels and optical density of TPH2 signal in the dorsal raphe nucleus of HSR and SS macaques treated with placebo or citalopram for 15 weeks (n=3–4/group). Two-way ANOVA was conducted. There was a significant effect of stress sensitivity on optical density (p=0.0002) and on positive pixel area (p=0.0003). However, there was no effect of drug treatment on optical density (p=0.13) or on positive pixel area (p= 0.10) and there was no interaction (p=0.37 for OD; p=0.57 for pixels).
The autoradiographic signal for SERT mRNA at a rostral level of the dorsal raphe nucleus is illustrated in Figure 7. SERT was robustly expressed in the HSR animals treated with placebo or citalopram. There was an apparent decrease in SERT mRNA in the SS animals treated with placebo or citalopram. The optical density of the signal and the pixel area covered by the signal was obtained from 7 levels of the dorsal raphe and the average signal/section was obtained for each animal. The mean ± SEM of each group was compared with a 2-way ANOVA and the results are shown in Figure 8. There was a significant effect of stress sensitivity on optical density (p=0.005) and on positive pixel area (p=0.001). However, there was no effect of drug treatment on optical density (p=0.27) or on positive pixel area (p= 0.22) and there was no interaction (p=0.66 for OD; p=0.98 for pixels). Thus, SERT gene expression was lower in the SS animals compared to the HSR animals, but citalopram treatment for 15 weeks did not alter SERT gene expression.
Figure 7.
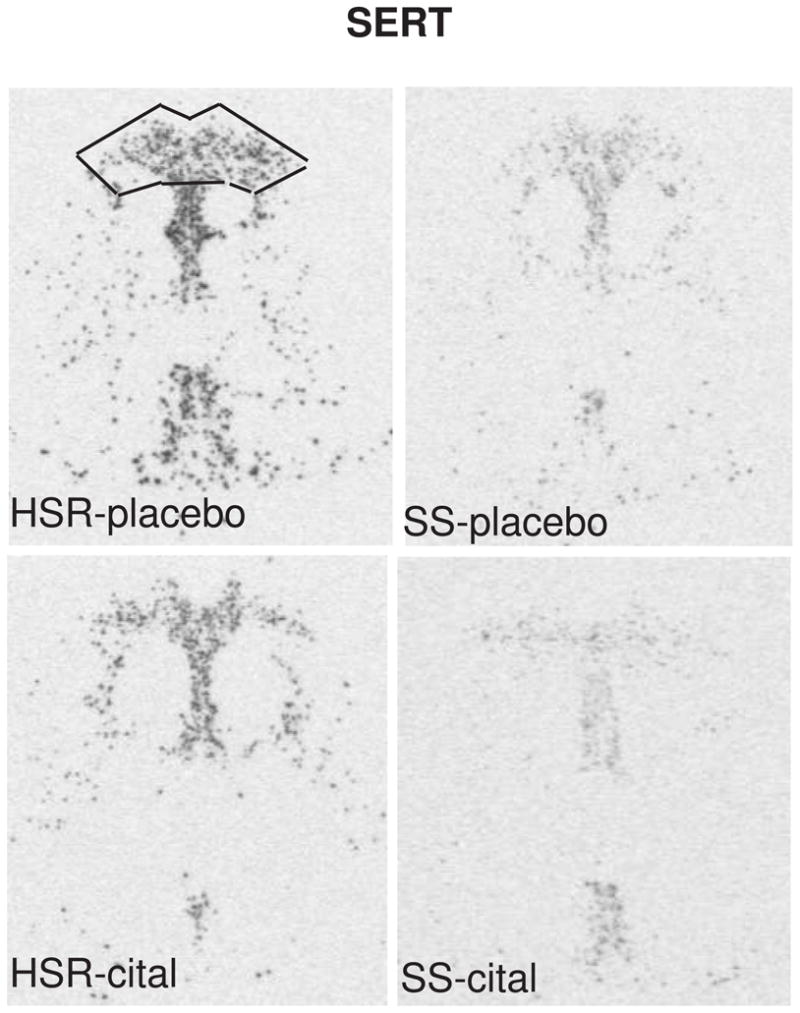
In situ hybridization autoradiograms of SERT signal in the dorsal raphe nucleus of representative HSR and SS macaques treated with placebo or citalopram for 15 weeks. Scattered neurons of the rostral median raphe are also visible underneath the decussation of the cerebellar peduncles. The box illustrates the area analyzed at this morphological level. The same box was applied to all sections at this level. Each of the 7 levels had a different sized box that was held constant for all of the sections at that morphological level.
Figure 8.
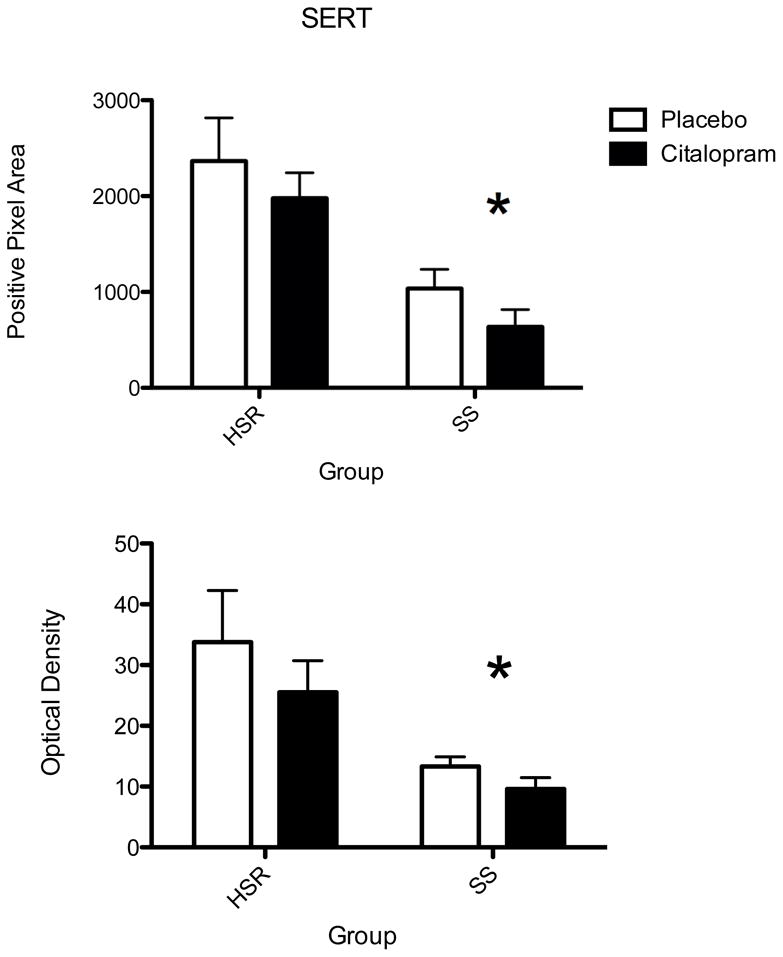
Histograms illustrating the average positive pixels and optical density of SERT signal in the dorsal raphe nucleus of HSR and SS macaques treated with placebo or citalopram for 15 weeks (n=3–4/group). Two-way ANOVA was conducted. There was a significant effect of stress sensitivity on optical density (p=0.005) and on positive pixel area (p=0.001). However, there was no effect of drug treatment on optical density (p=0.27) or on positive pixel area (p= 0.22) and there was no interaction (p=0.66 for OD; p=0.98 for pixels).
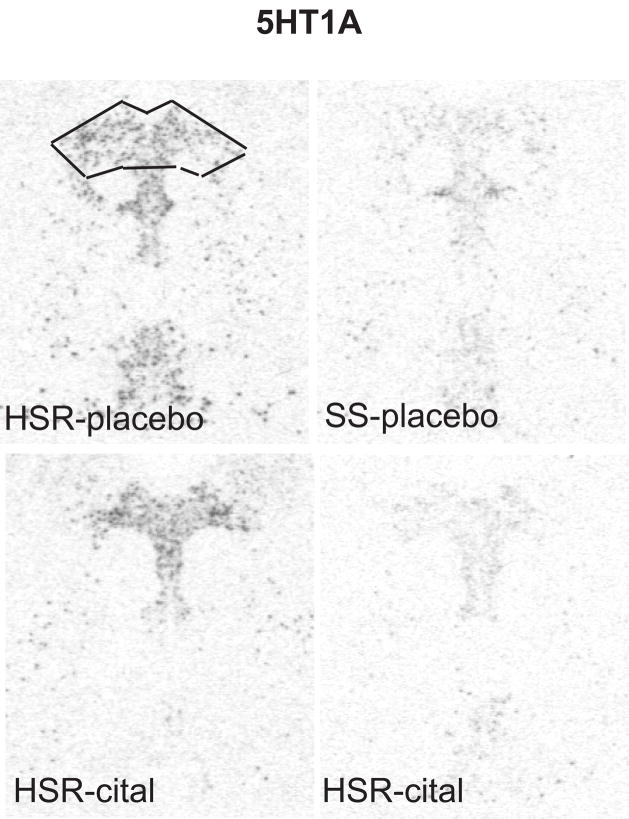
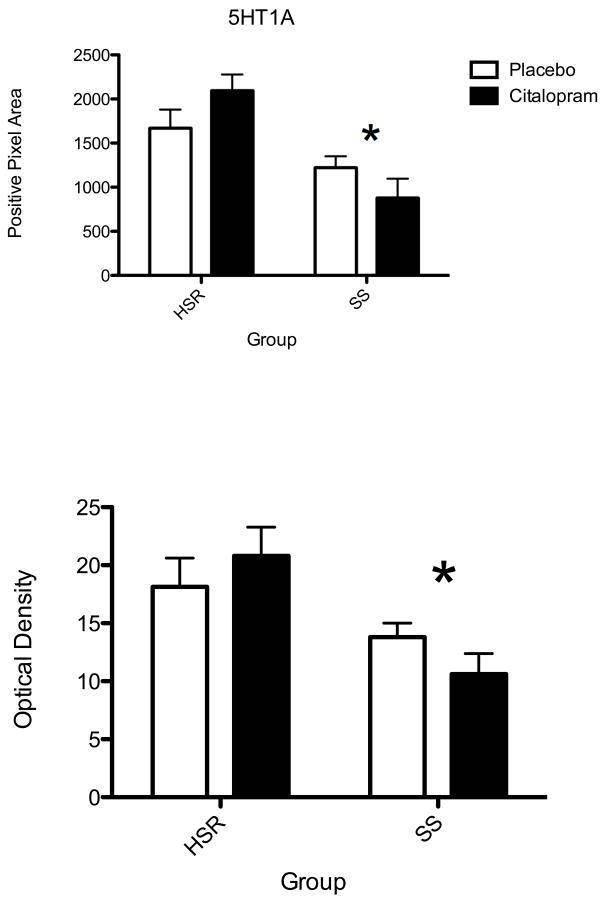
The autoradiographic signal for 5HT1A mRNA at a rostral level of the dorsal raphe nucleus is illustrated in Figure 9. 5HT1A was robustly expressed in the HSR animals treated with placebo or citalopram. There was an apparent decrease in 5HT1A mRNA in the SS animals treated with placebo or citalopram. The optical density of the signal and the pixel area covered by the signal was obtained from 7 levels of the dorsal raphe and the average signal/section was obtained for each animal. The mean ± SEM of each group was compared with a 2-way ANOVA and the results are shown in Figure 10. There was a significant effect of stress sensitivity on optical density (p=0.004) and on positive pixel area (p=0.001). However, there was no effect of drug treatment on optical density (p=0.89) or on positive pixel area (p= 0.83) and there was no interaction (p=0.17 for OD; p=0.072 for pixels). Thus, 5HT1A gene expression was lower in the SS animals compared to the HSR animals, but citalopram treatment for 15 weeks did not alter 5HT1A gene expression.
Figure 9.
In situ hybridization autoradiograms of 5HT1A signal in the dorsal raphe nucleus of representative HSR and SS macaques treated with placebo or citalopram for 15 weeks. Scattered neurons of the rostral median raphe are also visible underneath the decussation of the cerebellar peduncles. The box illustrates the area analyzed at this morphological level. The same box was applied to all sections at this level. Each of the 7 levels had a different sized box that was held constant for all of the sections at that morphological level.
Figure 10.
Histograms illustrating the average positive pixels and optical density of 5HT1A signal in the dorsal raphe nucleus of HSR and SS macaques treated with placebo or citalopram for 15 weeks (n=3–4/group). Two-way ANOVA was conducted. There was a significant effect of stress sensitivity on optical density (p=0.004) and on positive pixel area (p=0.001). However, there was no effect of drug treatment on optical density (p=0.89) or on positive pixel area (p= 0.83) and there was no interaction (p=0.17 for OD; p=0.072 for pixels).
Discussion
A large body of clinical research has found that individuals may be stress sensitive or stress resilient, and that stress sensitive individuals have an increased incidence of mood and anxiety disorders. We used a nonhuman primate model to study stress sensitivity, which was defined as cessation of ovulation due to a combination of psychosocial (relocation) and metabolic (diet) stress. Genetic, environmental, neurobiological and psychosocial factors contribute to stress resilience and stress sensitivity in humans and animal models. Of the neurobiological factors contributing to stress sensitivity, changes in functional aspects of the serotonin system play a leading role.
This study examined the expression of 4 genes that determine a significant portion of serotonin neuronal function, ie, Fev, TPH2, SERT and 5HT1A genes, in stress-sensitive and stress-resilient monkeys treated with citalopram or placebo for 15 weeks in the absence of stress. We found that the expression of each of these genes was significantly reduced in the stress-sensitive animals, but that citalopram treatment did not alter their expression. Blood levels of citalopram were maintained in a therapeutic range and the citalopram treatment was effective in increasing ovarian steroid secretion in the stress- sensitive animals prior to euthanasia, suggesting that the treatment was efficacious (Cameron et al., 2004).
Fev Expression
Fev (a homolog of PET1 in rats) is an ETS domain transcription factor that determines whether a neuron is serotonergic; and it functions specifically in the differentiation and maintenance of serotonin neurons. Fev is absolutely necessary for a neuron to become serotonergic and it is not expressed in any other neurons of the CNS. Indeed, the rodent homolog of Fev, called Pet1, marks and is restricted to, the entire rostral-caudal extent of the rat serotonergic hindbrain raphe complex (Hendricks et al., 1999). Conserved Fev binding sites are present in or near the promoter regions of the human and mouse TPH2, 5-HT1A receptor, serotonin transporter, and aromatic L-amino acid decarboxylase genes, whose expression is characteristic of the serotonergic neuron phenotype (Hendricks et al., 1999, Hendricks et al., 2003).
Fev expression was significantly lower and the number of detectable Fev-positive neurons was reduced in the stress-sensitive monkeys compared to stress-resilient monkeys. Thus, stress-sensitive macaques have reduced expression of the gene that governs serotonin-related gene expression and neuron function. The reduction in Fev-positive cell number strongly suggests that stress-sensitive animals have fewer serotonin neurons. It may be argued that serotonin neurons with little or no Fev expression were missed, but this seems to be a contradiction in terms. Other reports contend that Fev/Pet1 is a key transcriptional regulator of genes required specifically for the terminal serotonergic phenotype (Hendricks et al., 1999). Extending this line of reasoning (with a very sensitive assay), one could deduce that if a neuron does not have detectable Fev expression, then it is unlikely to be a serotonin neuron. This observation explains why stress-sensitive monkeys have lower expression of all of the serotonin-related genes and why, even with lower but adequate levels of ovarian steroids, they have compromised serotonin function. Very likely, this type of deficit has a developmental component.
Tryptophan Hydroxylase 2 Expression
TPH2 expression in stress-sensitive monkeys was significantly lower than in stress-resilient monkeys. This indicates that serotonin synthesis in stress-sensitive monkeys is compromised. TPH2 was discovered after we had exhausted the midbrain sections from the previous cohort of monkeys (Bethea et al., 2005b). Hence, this is the first examination of TPH2 in stress-sensitive versus stress-resilient monkeys. We previously showed that stress-sensitive monkeys secrete less prolactin than stress-resilient monkeys after challenge with fenfluramine, a serotonin releaser and reuptake blocker (Bethea et al., 2005a), indicating that stress-sensitive monkeys have lower available serotonin than stress-resilient monkeys. The lower expression of TPH2 mRNA may provide a molecular basis for the lower availability of serotonin, and together the data indicate that serotonin synthesis is decreased in stress-sensitive individuals. Consistent with these observations, tryptophan depletion and reduction of serotonin causes transient depression only in sensitive individuals (Hasler et al., 2004). The decrease in TPH2 expression is due in part to the reduction in serotonin cell number, but close examination of the autoradiographs also suggests that TPH2 expression is reduced in individual cells.
Serotonin Reuptake Transporter Expression
SERT mRNA expression was also significantly lower in stress-sensitive monkeys compared to stress-resilient monkeys, which independently confirms our earlier report with a different cohort of monkeys (Bethea et al., 2005b). If the lower level of mRNA translates to a lower level of SERT protein expression, then stress-sensitive monkeys would have markedly reduced SERT protein and function. The expression of SERT appears to correlate with the level of serotonin produced. Although somewhat paradoxical, in cases where serotonin is compromised, SERT is also reduced. Several approaches have shown that there is a reduced density of SERT in patients with depression and in the postmortem brain tissue of suicide victims (Lawrence et al., 1990, Drevets et al., 1992, Hrdina et al., 1993, Arango et al., 2002). Much attention has been placed on a polymorphism in the SERT gene, which may lead to a decrease in SERT expression (Bondy et al., 2000). However, cynomolgus macaques do not have the orthologus deletion event. Rather, all cynomolgus macaques have the long allele (Bethea et al., 2005b). Hence, the SERT polymorphism is not responsible for the decrease in SERT mRNA in these stress-sensitive monkeys.
5HT1A Autoreceptor Expression
5HT1A mRNA expression was markedly lower in stress-sensitive compared to stress-resilient monkeys. In our previous study, 5HT1A mRNA expression tended to be lower in stress-sensitive animals, but it did not reach statistical significance probably due to the small sample size and small effect (Bethea et al., 2005b). This study confirms that there is a real deficit in 5HT1A gene expression in stress-sensitive individuals. Indeed, it indicates that in the previous study there was a type II error, in that we did not detect a real difference. Unfortunately, this is a risk in primate studies that are constrained by costs. In a different model, we showed that 5HT1A binding correlated with 5HT1A gene expression (Lu and Bethea, 2002). Hence, the stress-sensitive monkeys probably have lower 5HT1A receptor density. These results are consistent with clinical studies that showed decreased 5HT1A binding in multiple brain regions of patients with depression (Drevets et al., 2000) and 5HT1A subsensitivity has been linked to late luteal phase dysphoric disorder (Yatham, 1993). Subordinate and chronically stressed male tree shrews have lower 5HT1A binding across a variety of brain regions including the hippocampus and frontal cortex (Flugge et al., 1998). Chronic stress also reduced post-synaptic 5HT1A binding in the hippocampus of rats (Watanabe et al., 1993), but there was no effect on 5HT1A autoreceptor binding in the raphe nuclei (Pare and Tejani-Butt, 1996). Paradoxically however, antidepressants are thought to decrease 5HT1A binding (Hjorth et al., 2000) and 5HT1A antagonists are beneficial adjuncts to antidepressant treatment (Singh and Lucki, 1993, Artigas et al., 1994, Romero and Artigas, 1997).
Regulation and Correlations
The lower expression of TPH2, SERT and 5HT1A is likely a consequence of the lower expression of Fev. The TPH2, SERT and 5HT1A promoters contain a Fev binding site and Fev is thought to be the terminal differentiation gene controlling the expression of serotonin specific genes such as TPH2, SERT and the 5HT1A receptor. Therefore, Fev determines whether a neuron is serotonergic or not. Thus, the lower expression of TPH2, SERT and 5HT1A may reflect the lower serotonin cell number as well as lower Fev expression in individual cells in the stress-sensitive animals.
It is noteworthy that in Pet1 knock out mice, the serotonin neurons fail to differentiate and remaining ones show deficient expression of serotonin-related genes. Moreover, these mice show heightened anxiety-like and aggressive behavior as adults (Hendricks et al., 2003). The stress-sensitive monkeys, who have deficient Fev expression, and hence fewer serotonin neurons, as well as reduced expression of the other serotonin–related genes, also show heightened anxiety-like behavior in a temperament test. In parallel studies, we used a series of tests for anxious behavior that were adapted for monkey behavioral testing (Cameron, 2003, Bethea et al., 2004) to assess temperamental characteristics in stress-sensitive vs. stress-resilient monkeys. We found that stress-sensitive monkeys show increased behavioral agitation when placed in a novel situation (Herod et al., 2006), a characteristic associated with low central serotonin release in a number of psychiatric disorders. Behaviors such as excessive worry or fear (Schulkin et al., 1998, Kalin and Shelton, 2003, Myers and Davis, 2007), inhibited approach to novelty/hypoactivity (Biederman et al., 1990, Hirshfeld et al., 1992, Fox, 2004), and increased agitation/hyperactivity in response to novelty (Kelly et al., 1997, Strekalova et al., 2005, Shroff et al., 2006) have all been observed in both clinical tests and animal models of anxiety. The pathophysiologic mechanisms underlying psychomotor agitation include dysregulation of dopaminergic, serotonergic, noradrenergic and GABAergic systems (Lindenmayer, 2000), several of which show differences between stress-sensitive and stress-resilient animals (Bethea et al., 2008).
Effect of Citalopram
Citalopram is a selective serotonin reuptake inhibitor (SSRI) that alleviates many of the symptoms of serotonin deficiency, initially by increasing extracellular serotonin (Smith et al., 2000). SSRIs are also thought to induce neuronal plasticity (Manji et al., 2003) although whether this is a direct effect of the SSRI, or an effect of the elevated extracellular serotonin, has not been fully resolved. Nonetheless, SSRI’s have a delayed efficacy, which has lead to questions of whether changes in gene expression may be involved. In the hippocampus of male tree shrews, chronic stress causes changes in gene expression that are reversed by administration of antidepressants (Alfonso et al., 2004) and microarray analysis found a significant number of gene changes in the dorsal raphe nucleus of naïve rats treated with fluoxetine (Conti et al., 2007). However, an early study in which naive rats were treated with a variety of antidepressants for four weeks reported no significant changes in TPH1, SERT or 5HT1A receptor mRNAs (Spurlock et al., 1994), but it was recently reported that four or eight weeks of fluoxetine (7.5 mg/kg/day) increased TPH2 mRNA in the dorsal raphe nucleus, which correlated with antidepressant action in the forced swim test, but two weeks of treatment had no effect (Shishkina et al., 2007). The same treatment decreased SERT mRNA at all time points. Nonetheless, we observed no effect of 15 weeks of citalopram on Fev, TPH2, SERT or 5HT1A mRNAs in the dorsal raphe nucleus of stress-resilient or stress-sensitive monkeys. Thus, our results are more consistent with the early study of antidepressant effects on gene expression in rats (Spurlock et al., 1994).
The lack of an effect of citalopram on serotonin-related gene expression suggests that changes in expression of Fev, TPH2, SERT or 5HT1A mRNAs in the dorsal raphe were not central to the therapeutic action of citalopram. Moreover, the lack of an effect of citalopram on Fev gene expression may also indicate why antidepressants are frequently required for life. They probably do not cause permanent effects on this pivotal serotonin neuronal determinant. It is well accepted that SSRIs increase extracellular serotonin (Hjorth et al., 2000) and either serotonin and/or antidepressants probably increase pivotal growth factors that stimulate neuronal remodeling (Alfonso et al., 2005, Turner et al., 2006). In our model of hypothalamic amenorrhea, citalopram increased serum estrogen and progesterone concentrations in the stress-sensitive, but not the stress-resilient monkeys.
It may be questioned whether the citalopram-induced increase in estrogen and progesterone could exert feedback regulation on serotonin-related gene expression. In a model of surgical menopause with hormone replacement therapy (HRT), we have shown that estrogen and progesterone increase TPH2 mRNA expression in the dorsal raphe of rhesus macaques (Sanchez et al., 2005). However, in the model of HRT, estradiol increased from <20 pg/ml in the ovariectomized animals to a range of 100–150 pg/ml in the replacement animals, which is much lower than the peak concentrations measured in the stress-sensitive animals treated with placebo. Also, in the model of HRT, progesterone increased from <0.12 ng/ml in the ovariectomized animals to a range of 6–7 ng/ml in the replacement animals, which is comparable to the stress-sensitive animals treated with placebo. So, in the HRT model, the steroid hormone concentrations are adjusted from nearly absent to levels observed in the early to mid-luteal phase, whereas in the stress-sensitive group, the steroid hormones change from adequate (with placebo) to better (with citalopram). The citalopram-induced adjustment does not appear to exert the same kind of dynamic effect on TPH2 expression as the HRT adjustment.
It may be noted that there was a small decrease in TPH2 and 5HT1A expression in the stress sensitive animals treated with citalopram. However, this difference was not significant using the 2 × 2 block design and two–way anova. Power analysis indicates that many more animals are needed to assign signficance to an effect this small. Thus, with 4 animals and a small effect, we run the risk of a false negative conclusion or type II error, which misses a small, but real difference. However, it is reasonable to question the relevance of such a small effect of citalopram given that there is a large effect of stress sensitivity. Indeed, we originally hypothesized that citalopram would normalize gene expression in the SS animals to the levels observed in the HSR animals, and that was clearly not the case. Hence, the small decrease in TPH2 and 5HT1A expression in the stress sensitive animals treated with citalopram animals may be a real populational phenomena or not, but if real, then it is very hard to interpret. How can decreasing the expression of these genes lead to an increase in serotonin and ovarian steroid secretion? We think the small decrease is due to animal variation. Moreover, the alternate interpretation becomes so convoluted that it is safer to accept that there is no major difference in serotonin-related gene expression with citalopram treatment and seek other actions of citalopram that could mediate the increase in ovarian steroid secretion.
We have recently obtained preliminary data showing that citalopram treatment significantly reduces CRF fiber staining in the dorsal raphe nucleus in stress-sensitive animals, but not in stress-resilient animals (unpublished). This correlates closely with the ovarian steroid secretion in response to citalopram. The CRF cell bodies, which innervate the dorsal raphe, are located in the PVN and amygdala; and in a reciprocal fashion, serotonin innervates CRH neurons in the PVN and amygdala (Bassett and Foote, 1992, Laflamme et al., 1999 Lowry, 2000 #3320, Ruggiero et al., 1999, Kirby et al., 2000, Asan et al., 2005, Clark et al., 2007). Our preliminary data indicate that citalopram may act to increase serotonin release in the PVN and amygdala of stress-sensitive animals, which in turn would decrease CRF production. Earlier work demonstrated that CRF plays an anti-reproductive role in macaques (Ferin, 1995) and CRF may directly or indirectly inhibit GnRH neurons in the hypothalamus (Ferin, 1993, Polkowska and Przekop, 1997, Wang and Millam, 1999).
In conclusion, stress-sensitive monkeys have lower expression of the Fev gene in individual neurons, and fewer detectable serotonin neurons than stress-resilient monkeys. This deficit has downstream consequences including lower expression of TPH2, SERT and 5HT1A genes, as well as elevated expression of hypothalamic GAD67 and CRH and finally, decreased transport of LHRH to the median eminence (Bethea et al., 2008). These shifts in CNS systems lead to a decrease in ovarian production of estrogen and progesterone although ovulation was adequate without exogenous stress. Preliminary data indicate that the stress-sensitive animals exhibit higher levels of behavioral agitation/anxiety in temperament tests. Administration of citalopram increases estrogen and progesterone production, but it does not alter gene expression of Fev, TPH2, SERT or 5HT1A in the dorsal raphe nucleus. Rather, we have preliminary evidence that it decreases CRH fiber density in the raphe. This suggests that the mechanism of action of citalopram involves post-translational events in the serotonin system, which may in turn alter the CRH system.
Supplementary Material
Acknowledgments
Supported by the Eunice Kennedy Shriver NICHD through cooperative agreement U54 HD 18185 as part of the specialized Cooperative Centers Program in Reproduction and Infertility Research, NIH grant MH62677 to CLB, and NIH RR000163 for the operation of ONPRC
We are very grateful for the dedicated work of J.A. Bytheway, S. Guay, D. Kerr, and N. Rockcastle for the performance of the physiological studies treating monkeys with citalopram. We are also indebted to the collaboration with D.A. Axelson and J.M. Perel in the Dept. of Psychiatry at the University of Pittsburgh who measured blood citalopram levels. We thank S.R. Ojeda (Division of Neuroscience, ONPRC) for his help with the non-isotopic in situ hybridization procedure. Technical assistance from the Primate Core laboratory of the Center for Research in Reproductive Physiology at the University of Pittsburgh was also greatly appreciated. We also thank Yibing Ja, M.S., ONPRC Cell and Molecular Biology Core, for cloning and sequencing of the Fev amplicon.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Literature Cited
- Alfonso J, Frasch AC, Flugge G. Chronic stress, depression and antidepressants: effects on gene transcription in the hippocampus. Rev Neurosci. 2005;16:43–56. doi: 10.1515/revneuro.2005.16.1.43. [DOI] [PubMed] [Google Scholar]
- Alfonso J, Pollevick GD, Van Der Hart MG, Flugge G, Fuchs E, Frasch AC. Identification of genes regulated by chronic psychosocial stress and antidepressant treatment in the hippocampus. Eur J Neurosci. 2004;19:659–666. doi: 10.1111/j.1460-9568.2004.03178.x. [DOI] [PubMed] [Google Scholar]
- Arango V, Underwood MD, Mann JJ. Serotonin brain circuits involved in major depression and suicide. Prog Brain Res. 2002;136:443–453. doi: 10.1016/s0079-6123(02)36037-0. [DOI] [PubMed] [Google Scholar]
- Artigas F, Perez V, Alvarez E. Pindolol induces a rapid improvement of depressed patients treated with serotonin reuptake inhibitors. Arch Gen Psychiatry. 1994;51:248–251. doi: 10.1001/archpsyc.1994.03950030084009. [DOI] [PubMed] [Google Scholar]
- Asan E, Yilmazer-Hanke DM, Eliava M, Hantsch M, Lesch KP, Schmitt A. The corticotropin-releasing factor (CRF)-system and monoaminergic afferents in the central amygdala: investigations in different mouse strains and comparison with the rat. Neuroscience. 2005;131:953–967. doi: 10.1016/j.neuroscience.2004.11.040. [DOI] [PubMed] [Google Scholar]
- Bassett JL, Foote SL. Distribution of corticotropin-releasing factor-like immunoreactivity in squirrel monkey (Saimiri sciureus) amygdala. J Comp Neurol. 1992;323:91–102. doi: 10.1002/cne.903230108. [DOI] [PubMed] [Google Scholar]
- Berg-von der Emde K, Dees WL, Hiney JK, Hill DF, Dissen GA, Costa ME, Moholt-Siebert M, Ojeda SR. Neurotrophins and the neuroendocrine brain: different neurotrophins sustain anatomically and functionally segregated subsets of hypothalamic dopaminergic neurons. J Neurosci. 1995;15:4223–4237. doi: 10.1523/JNEUROSCI.15-06-04223.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berga SL, Girton LG. The psychoneuroendocrinology of functional hypothalamic amenorrhea. Psychiatric Clinics of North America. 1989;12:105–116. [PubMed] [Google Scholar]
- Berga SL, Loucks TL. Use of cognitive behavior therapy for functional hypothalamic amenorrhea. Ann N Y Acad Sci. 2006;1092:114–129. doi: 10.1196/annals.1365.010. [DOI] [PubMed] [Google Scholar]
- Berga SL, Marcus MD, Loucks TL, Hlastala S, Ringham R, Krohn MA. Recovery of ovarian activity in women with functional hypothalamic amenorrhea who were treated with cognitive behavior therapy. Fertil Steril. 2003;80:976–981. doi: 10.1016/s0015-0282(03)01124-5. [DOI] [PubMed] [Google Scholar]
- Berod A, Hartman BK, Pujol JF. Importance of fixation in immunohistochemisty: use of formaldehyde solutions at variable pH for the localization of tyrosine hydroxylase. J Histochem Cytochem. 1981;29:844–850. doi: 10.1177/29.7.6167611. [DOI] [PubMed] [Google Scholar]
- Bethea CL, Centeno ML, Cameron JL. Neurobiology of stress-induced reproductive dysfunction in female macaques. Mol Neurobiol. 2008;38:199–230. doi: 10.1007/s12035-008-8042-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bethea CL, Pau FK, Fox S, Hess DL, Berga SL, Cameron JL. Sensitivity to stress-induced reproductive dysfunction linked to activity of the serotonin system. Fertil Steril. 2005a;83:148–155. doi: 10.1016/j.fertnstert.2004.06.051. [DOI] [PubMed] [Google Scholar]
- Bethea CL, Reddy AP, Pedersen D, Tokuyama Y. Expression profile of differentiating serotonin neurons derived from rhesus embryonic stem cells and comparison to adult serotonin neurons. Gene Expr Patterns. 2009;9:94–108. doi: 10.1016/j.gep.2008.10.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bethea CL, Streicher JM, Coleman K, Pau FK, Moessner R, Cameron JL. Anxious behavior and fenfluramine-induced prolactin secretion in young rhesus macaques with different alleles of the serotonin reuptake transporter polymorphism (5HTTLPR) Behav Genet. 2004;34:295–307. doi: 10.1023/B:BEGE.0000017873.61607.be. [DOI] [PubMed] [Google Scholar]
- Bethea CL, Streicher JM, Mirkes SJ, Sanchez RL, Reddy AP, Cameron JL. Serotonin-related gene expression in female monkeys with individual sensitivity to stress. Neuroscience. 2005b;132:151–166. doi: 10.1016/j.neuroscience.2004.11.022. [DOI] [PubMed] [Google Scholar]
- Biederman J, Rosenbaum JF, Hirshfeld DR, Faraone SV, Bolduc EA, Gersten M, Meminger SR, Kagan J, Snidman N, Reznick JS. Psychiatric correlates of behavioral inhibition in young children of parents with and without psychiatric disorders. Arch Gen Psychiatry. 1990;47:21–26. doi: 10.1001/archpsyc.1990.01810130023004. [DOI] [PubMed] [Google Scholar]
- Bondy B, Erfurth A, De Jonge S, Kruger M, Meyer H. Possible association of the short allele of the serotonin transporter promoter gene polymorphism (5-HTTLPR) with violent suicide. Molecular Psychiatry. 2000;5:193–195. doi: 10.1038/sj.mp.4000678. [DOI] [PubMed] [Google Scholar]
- Cameron JL. Reproductive dysfunction in primates, behaviorally induced. In: Fink G, editor. Encyclopedia of Stress. New York: Academic Press; 2000. pp. 366–372. [Google Scholar]
- Cameron JL. Stress and reproduction. In: Henry HL, Norman AW, editors. The Encyclopedia of Hormones. Elsevier Science; 2003. pp. 433–438. [Google Scholar]
- Cameron JL, Bytheway JA, Guay S, Bethea CL, Kerr DI, Rockcastle N, Perel JM, Axelson DA. Treatment with a serotonin reuptake inhibitor increases reproductive hormone secretion in stress sensitive monkeys. 34th Annual Meeting of the Society for Neuroscience; San Diego, CA. October 23–27; 2004. Program number 192.18. [Google Scholar]
- Centeno ML, Sanchez RL, Cameron JL, Bethea CL. Hypothalamic expression of serotonin 1A, 2A and 2C receptors and GAD67 in female cynomolgus monkeys with different sensitivity to stress. Brain Research. 2007a;1142:1–12. doi: 10.1016/j.brainres.2007.01.056. [DOI] [PubMed] [Google Scholar]
- Centeno ML, Sanchez RL, Cameron JL, Bethea CL. Hypothalamic gonadotrophin-releasing hormone expression in female monkeys with different sensitivity to stress. J Neuroendocrinol. 2007b;19:594–604. doi: 10.1111/j.1365-2826.2007.01566.x. [DOI] [PubMed] [Google Scholar]
- Centeno ML, Sanchez RL, Reddy AP, Cameron JL, Bethea CL. Corticotropin-releasing hormone and pro-opiomelanocortin gene expression in female monkeys with differences in sensitivity to stress. Neuroendocrinology. 2007c;86:277–288. doi: 10.1159/000109877. [DOI] [PubMed] [Google Scholar]
- Clark MS, McDevitt RA, Hoplight BJ, Neumaier JF. Chronic low dose ovine corticotropin releasing factor or urocortin II into the rostral dorsal raphe alters exploratory behavior and serotonergic gene expression in specific subregions of the dorsal raphe. Neuroscience. 2007;146:1888–1905. doi: 10.1016/j.neuroscience.2007.03.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Conti B, Maier R, Barr AM, Morale MC, Lu X, Sanna PP, Bilbe G, Hoyer D, Bartfai T. Region-specific transcriptional changes following the three antidepressant treatments electro convulsive therapy, sleep deprivation and fluoxetine. Mol Psychiatry. 2007;12:167–189. doi: 10.1038/sj.mp.4001897. [DOI] [PubMed] [Google Scholar]
- Drevets WC, Frank E, Price JC, Kupfer DJ, Greer PJ, Mathis C. Serotonin type-1A receptor imaging in depression. Nucl Med Biol. 2000;27:499–507. doi: 10.1016/s0969-8051(00)00119-0. [DOI] [PubMed] [Google Scholar]
- Drevets WC, Videen TO, Price JL, Preskorn SH, Carmichael ST, Raichle ME. A functional anatomical study of unipolar depression. J Neurosci. 1992;12:3628–3641. doi: 10.1523/JNEUROSCI.12-09-03628.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ferin M. Neuropeptides, the stress response, and the hypothalamo-pituitary-gonadal axis in the female rhesus monkey. Ann N Y Acad Sci. 1993;697:106–116. doi: 10.1111/j.1749-6632.1993.tb49927.x. [DOI] [PubMed] [Google Scholar]
- Ferin M. The antireproductive role of corticotropin releasing hormone and interleukin-1 in the female rhesus monkey. Ann Endocrinol (Paris) 1995;56:181–186. [PubMed] [Google Scholar]
- Fioroni L, Fava M, Genazzani AD, Facchinetti F, Genazzani AR. Life events impact in patients with secondary amenorrhoea. J Psychosom Res. 1994;38:617–622. doi: 10.1016/0022-3999(94)90059-0. [DOI] [PubMed] [Google Scholar]
- Flugge G, Kramer M, Rensing S, Fuchs E. 5HT1A-receptors and behaviour under chronic stress: selective counteraction by testosterone. Eur J Neurosci. 1998;10:2685–2693. [PubMed] [Google Scholar]
- Fox NA. Temperament and early experience form social behavior. Ann N Y Acad Sci. 2004;1038:171–178. doi: 10.1196/annals.1315.025. [DOI] [PubMed] [Google Scholar]
- Giles DE, Berga SL. Cognitive and psychiatric correlates of functional hypothalamic amenorrhea: a controlled comparison. Fertility & Sterility. 1993;60:486–492. [PubMed] [Google Scholar]
- Hasler G, Drevets WC, Manji HK, Charney DS. Discovering endophenotypes for major depression. Neuropsychopharmacol. 2004;29:1765–1781. doi: 10.1038/sj.npp.1300506. [DOI] [PubMed] [Google Scholar]
- Hendricks T, Francis N, Fyodorov D, Deneris ES. The ETS domain factor Pet-1 is an early and precise marker of central serotonin neurons and interacts with a conserved element in serotonergic genes. J Neurosci. 1999;19:10348–10356. doi: 10.1523/JNEUROSCI.19-23-10348.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hendricks TJ, Fyodorov DV, Wegman LJ, Lelutiu NB, Pehek EA, Yamamoto B, Silver J, Weeber EJ, Sweatt JD, Deneris ES. Pet-1 ETS gene plays a critical role in 5-HT neuron development and is required for normal anxiety-like and aggressive behavior. Neuron. 2003;37:233–247. doi: 10.1016/s0896-6273(02)01167-4. [DOI] [PubMed] [Google Scholar]
- Herod SM, Bytheway J, Dolney R, Cameron JL. Female monkeys who are sensitive to stress-induced reproductive dysfunction show increases in certain forms of anxious behavior. 36th Annual Meeting of the Society for Neuroscience; Atlanta, GA. October 14,18; 2006. Program number 257.22. [Google Scholar]
- Hirshfeld DR, Rosenbaum JF, Biederman J, Bolduc EA, Faraone SV, Snidman N, Reznick JS, Kagan J. Stable behavioral inhibition and its association with anxiety disorder. J Am Acad Child Adolesc Psychiatry. 1992;31:103–111. doi: 10.1097/00004583-199201000-00016. [DOI] [PubMed] [Google Scholar]
- Hjorth S, Bengtsson HJ, Kullberg A, Carlzon D, Peilot H, Auerbach SB. Serotonin autoreceptor function and antidepressant drug action. J Psychopharmacol. 2000;14:177–185. doi: 10.1177/026988110001400208. [DOI] [PubMed] [Google Scholar]
- Hrdina PD, Demeter E, Vu TB, Sotonyi P, Palkovits M. 5-HT uptake sites and 5-HT2 receptors in brain of antidepressant-free suicide victims/depressives: increase in 5-HT2 sites in cortex and amygdala. Brain Res. 1993;614:37–44. doi: 10.1016/0006-8993(93)91015-k. [DOI] [PubMed] [Google Scholar]
- Julius D. Molecular biology of serotonin receptors. Ann Rev Neuroscience. 1991;14:335–360. doi: 10.1146/annurev.ne.14.030191.002003. [DOI] [PubMed] [Google Scholar]
- Kalin NH, Shelton SE. Nonhuman primate models to study anxiety, emotion regulation, and psychopathology. Ann N Y Acad Sci. 2003;1008:189–200. doi: 10.1196/annals.1301.021. [DOI] [PubMed] [Google Scholar]
- Kelly JP, Wrynn AS, Leonard BE. The olfactory bulbectomized rat as a model of depression: an update. Pharmacol Ther. 1997;74:299–316. doi: 10.1016/s0163-7258(97)00004-1. [DOI] [PubMed] [Google Scholar]
- Kirby LG, Rice KC, Valentino RJ. Effects of corticotropin-releasing factor on neuronal activity in the serotonergic dorsal raphe nucleus. Neuropsychopharmacol. 2000;22:148–162. doi: 10.1016/S0893-133X(99)00093-7. [DOI] [PubMed] [Google Scholar]
- Laflamme N, Feuvrier E, Richard D, Rivest S. Involvement of serotonergic pathways in mediating the neuronal activity and genetic transcription of neuroendocrine corticotropin-releasing factor in the brain of systemically endotoxin-challenged rats. Neuroscience. 1999;88:223–240. doi: 10.1016/s0306-4522(98)00369-8. [DOI] [PubMed] [Google Scholar]
- Laughlin GA, Dominguez CE, Yen SS. Nutritional and endocrine-metabolic aberrations in women with functional hypothalamic amenorrhea. J Clin Endocrinol Metabol. 1998;83:25–32. doi: 10.1210/jcem.83.1.4502. [DOI] [PubMed] [Google Scholar]
- Lawrence KM, De Paermentier F, Cheetham SC, Crompton MR, Katona CL, Horton RW. Brain 5-HT uptake sites, labelled with [3H]paroxetine, in antidepressant-free depressed suicides. Brain Res. 1990;526:17–22. doi: 10.1016/0006-8993(90)90244-6. [DOI] [PubMed] [Google Scholar]
- Lindenmayer JP. The pathophysiology of agitation. J Clin Psychiatry. 2000;61 Suppl 14:5–10. [PubMed] [Google Scholar]
- Lu NZ, Bethea CL. Ovarian steroid regulation of 5HT1A receptor binding and G protein activation in female monkeys. Neuropsychopharmacol. 2002;27:12–24. doi: 10.1016/S0893-133X(01)00423-7. [DOI] [PubMed] [Google Scholar]
- Manji HK, Quiroz JA, Sporn J, Payne JL, Denicoff K, Gray AN, Zarate CA, Jr, Charney DS. Enhancing neuronal plasticity and cellular resilience to develop novel, improved therapeutics for difficult-to-treat depression. Biol Psychiatry. 2003;53:707–742. doi: 10.1016/s0006-3223(03)00117-3. [DOI] [PubMed] [Google Scholar]
- Marcus MD, Loucks TL, Berga SL. Psychological correlates of functional hypothalamic amenorrhea. Fertil Steril. 2001;76:310–316. doi: 10.1016/s0015-0282(01)01921-5. [DOI] [PubMed] [Google Scholar]
- McEwen BS. The End of Stress As We Know It. Washington, DC: Joseph Henry Press; 2002. [Google Scholar]
- Myers KM, Davis M. Mechanisms of fear extinction. Mol Psychiatry. 2007;12:120–150. doi: 10.1038/sj.mp.4001939. [DOI] [PubMed] [Google Scholar]
- Pare WP, Tejani-Butt SM. Effect of stress on the behavior and 5-HT system in Sprague-Dawley and Wistar Kyoto rat strains. Integr Physiol Behav Sci. 1996;31:112–121. doi: 10.1007/BF02699783. [DOI] [PubMed] [Google Scholar]
- Pecins-Thompson M, Bethea CL. Ovarian steroid regulation of 5HT1A autoreceptor messenger ribonucleic acid expression in the dorsal raphe of rhesus macaques. Neuroscience. 1998;89:267–277. doi: 10.1016/s0306-4522(98)00326-1. [DOI] [PubMed] [Google Scholar]
- Pecins-Thompson M, Brown NA, Bethea CL. Regulation of serotonin re-uptake transporter mRNA expression by ovarian steroids in rhesus macaques. Mol Brain Research. 1998;53:120–129. doi: 10.1016/s0169-328x(97)00286-6. [DOI] [PubMed] [Google Scholar]
- Polkowska J, Przekop F. The effect of corticotropin-releasing factor (CRF) on the gonadotropin hormone releasing hormone (GnRH) hypothalamic neuronal system during preovulatory period in the ewe. Acta Neurobiol Exp (Wars) 1997;57:91–99. doi: 10.55782/ane-1997-1216. [DOI] [PubMed] [Google Scholar]
- Reifenstein ECJ. Psychogenic or “hypothalamic” amenorrhea. Med Clin North Am. 1946;30:1103–1114. doi: 10.1016/s0025-7125(16)35908-9. [DOI] [PubMed] [Google Scholar]
- Reindollar RH, Novak M, Tho SP, McDonough PG. Adult-onset amenorrhea: a study of 262 patients. Am J Obstet Gynecol. 1986;155:531–543. doi: 10.1016/0002-9378(86)90274-7. [DOI] [PubMed] [Google Scholar]
- Romero L, Artigas F. Preferential potentiation of the effects of serotonin uptake inhibitors by 5HT1A receptor antagonists in the dorsal raphe pathway: role of somatodendritic autoreceptors. J Neurochem. 1997;68:2593–2603. doi: 10.1046/j.1471-4159.1997.68062593.x. [DOI] [PubMed] [Google Scholar]
- Ruggiero DA, Underwood MD, Rice PM, Mann JJ, Arango V. Corticotropic-releasing hormone and serotonin interact in the human brainstem: behavioral implications. Neuroscience. 1999;91:1343–1354. doi: 10.1016/s0306-4522(98)00703-9. [DOI] [PubMed] [Google Scholar]
- Sanchez RL, Reddy AP, Centeno ML, Henderson JA, Bethea CL. A second tryptophan hydroxylase isoform, TPH-2 mRNA, is increased by ovarian steroids in the raphe region of macaques. Brain Res Mol Brain Res. 2005;135:194–203. doi: 10.1016/j.molbrainres.2004.12.011. [DOI] [PubMed] [Google Scholar]
- Schulkin J, Gold PW, Mcewan BS. Induction of corticotropin-releasing hormone gene expression by glucocorticoids: implication for understanding the states of fear and anxiety and allostatic load. Psychoneuroendocrinol. 1998;23:219–243. doi: 10.1016/s0306-4530(97)00099-1. [DOI] [PubMed] [Google Scholar]
- Shishkina GT, Kalinina TS, Dygalo NN. Up-regulation of tryptophan hydroxylase-2 mRNA in the rat brain by chronic fluoxetine treatment correlates with its antidepressant effect. Neuroscience. 2007;150:404–412. doi: 10.1016/j.neuroscience.2007.09.017. [DOI] [PubMed] [Google Scholar]
- Shroff H, Reba L, Thornton LM, Tozzi F, Klump KL, Berrettini WH, Brandt H, Crawford S, Crow S, Fichter MM, Goldman D, Halmi KA, Johnson C, Kaplan AS, Keel P, LaVia M, Mitchell J, Rotondo A, Strober M, Treasure J, Woodside DB, Kaye WH, Bulik CM. Features associated with excessive exercise in women with eating disorders. Int J Eat Disord. 2006;39:454–461. doi: 10.1002/eat.20247. [DOI] [PubMed] [Google Scholar]
- Simmons DM, Arriza JL, Swanson LW. A complete protocol for in situ hybridization of messenger RNAs in brain and other tissues with radio-labeled single-stranded RNA probes. The J Histotechnol. 1989;12:169–181. [Google Scholar]
- Singh A, Lucki I. Antidepressant-like activity of compounds with varying efficacy at 5HT1A receptors. Neuropharmacology. 1993;32:331–340. doi: 10.1016/0028-3908(93)90153-t. [DOI] [PubMed] [Google Scholar]
- Sit DK, Perel JM, Helsel JC, Wisner KL. Changes in antidepressant metabolism and dosing across pregnancy and early postpartum. J Clin Psychiatry. 2008;69:652–658. doi: 10.4088/jcp.v69n0419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith TD, Kuczenski R, George-Griedman K, Malley JD, Foote SL. In vivo microdialysis assessment of extracellular serotonin and dopamine levels in awake monkeys during sustained fluoxetine administration. Synapse. 2000;38:460–470. doi: 10.1002/1098-2396(20001215)38:4<460::AID-SYN11>3.0.CO;2-D. [DOI] [PubMed] [Google Scholar]
- Spurlock G, Buckland P, O’Donovan M, McGuffin P. Lack of effect of antidepressant drugs on the levels of mRNAs encoding serotonergic receptors, synthetic enzymes and 5HT transporter. Neuropharmacology. 1994;33:433–440. doi: 10.1016/0028-3908(94)90073-6. [DOI] [PubMed] [Google Scholar]
- Strekalova T, Spanagel R, Dolgov O, Bartsch D. Stress-induced hyperlocomotion as a confounding factor in anxiety and depression models in mice. Behav Pharmacol. 2005;16:171–180. doi: 10.1097/00008877-200505000-00006. [DOI] [PubMed] [Google Scholar]
- Turner CA, Akil H, Watson SJ, Evans SJ. The fibroblast growth factor system and mood disorders. Biol Psychiatry. 2006;59:1128–1135. doi: 10.1016/j.biopsych.2006.02.026. [DOI] [PubMed] [Google Scholar]
- Wang R, Millam JR. Corticotropin-releasing hormone-immunopositive nerve elements in apposition to chicken gonadotropin-releasing hormone I-containing perikarya in Japanese quail (Coturnix coturnix japonica) brain. Cell Tissue Res. 1999;297:223–228. doi: 10.1007/s004410051350. [DOI] [PubMed] [Google Scholar]
- Watanabe Y, Sakai RR, McEwen BS, Mendelson S. Stress and antidepressant effects on hippocampal and cortical 5-HT1A and 5-HT2 receptors and transport sites for serotonin. Brain Res. 1993;615:87–94. doi: 10.1016/0006-8993(93)91117-b. [DOI] [PubMed] [Google Scholar]
- Williams NI, Berga SL, Cameron JL. Mild metabolic stress potentiates the suppressive effect of psychological stress on reproductive function in female cynomolgus monkeys. 79th Annual Meeting of the Endocrine Society; Minneapolis, MN. June 11–14; 1997. Abstract PI-367. [Google Scholar]
- Williams NI, Berga SL, Cameron JL. Synergism between psychosocial and metabolic stressors: impact on reproductive function in cynomolgus monkeys. Am J Physiol Endocrinol Metab. 2007;293:E270–276. doi: 10.1152/ajpendo.00108.2007. [DOI] [PubMed] [Google Scholar]
- Xiao E, Xia-Zhang L, Ferin M. Stress and the menstrual cycle: short- and long-term response to a five-day endotoxin challenge during the luteal phase in the rhesus monkey. J Clin Endocrinol Metabol. 1999;84:623–626. doi: 10.1210/jcem.84.2.5448. [DOI] [PubMed] [Google Scholar]
- Yatham LN. Is 5HT1A receptor subsensitivity a trait marker for late luteal phase dysphoric disorder? A pilot study. Canadian J Psychiatry. 1993;38:662–664. doi: 10.1177/070674379303801007. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.