Abstract
Behavioral genetic studies of humans have associated variation in the DTNBP1 gene with schizophrenia and its cognitive deficit phenotypes. The protein coded for by DTNBP1, dysbindin, is expressed within forebrain glutamatergic neurons, where it interacts with proteins involved in vesicular trafficking and exocytosis. In order to further delineate the cellular, physiological and behavioral phenotypes associated with reduced dysbindin expression, we conducted studies in mice carrying a null mutation within the dtnbp1 gene. Dysbindin mutants exhibited impairments of spatial working memory as compared with wild-type controls; heterozygous mice exhibited intermediate levels of cognitive dysfunction. Deep layer pyramidal neurons recorded in the prefrontal cortex of mutant mice exhibited reductions in paired-pulse facilitation, and evoked and miniature excitatory post-synaptic currents, indicating a difference in the function of pre-synaptic glutamatergic terminals, as well as elevated spike thresholds. Taken together, these data indicate that dysbindin potently regulates excitatory transmission in prefrontal cortex, potentially through a pre-synaptic mechanism, and consequently modulates cognitive functions depending upon this brain region, providing new insights into the molecular mechanisms underlying cortical dysfunction in schizophrenia.
Keywords: working memory, schizophrenia, glutamate, cognition, excitatory, pre-synaptic
Introduction
Emerging evidence suggests that a significant number of the gene mechanisms that contribute to risk for schizophrenia modulate complex cognitive functions that depend upon the prefrontal cortex and medial temporal lobe (Arguello and Gogos, 2006; Cannon, 2005; Harrison and Weinberger, 2005; Weinberger et al, 2001). Indeed, some of these genes, including DISC1, DTNBP1, COMT and NRG1, are expressed normally within the cortical circuitry that subserves cognition (Lipska et al, 2006; Tunbridge et al, 2006; Weickert et al, 2004), and it is thought that polymorphisms within these genes consequently explain variation in cognitive phenotypes, such as declarative memory, attention and executive functions, within the human population. For example, a rare haplotype within the DISC1 gene associates with low prefrontal cortical volume and impairments in cognitive function, specifically working memory (Cannon et al, 2005). These genotype-phenotype relationships further underscore the value of cognitive mechanisms as candidate endophenotypes for schizophrenia and related psychotic disorders.
More recently, attention has focused on DTNBP1, the gene that codes for dystrobrevin-binding protein (dysbindin) and its relationship to cognition (Burdick et al, 2007; Burdick et al, 2006; Donohoe et al, 2007; Luciano et al, 2008). In humans, variation within this gene is associated with risk for developing schizophrenia and impairments in cognitive performance. Moreover, this gene is expressed within cortical neurons, including pyramidal neurons (Talbot et al, 2004), suggesting that it is well-positioned to modulate functions that depend upon cortical excitatory tone (Chen et al, 2008; Numakawa et al, 2004). DTNBP1 haplotypes that represent vulnerability markers for schizophrenia appear to associate with low dysbindin expression (Bray et al, 2005); given the putative role for dysbindin in vesicular trafficking and transmitter exocytosis (Chen et al, 2008; Numakawa et al, 2004; Talbot et al, 2006; Talbot et al, 2004), a reduction in dysbindin expression may lead to a decrease in glutamate output (Numakawa et al, 2004), a mechanism that could explain deviant patterns of prefrontal cortical activty in schizophrenia (Manoach, 2003; Weinberger and Berman, 1988; Williamson, 1987).
Neuronal microcircuits, involving glutamatergic pyramidal neurons and local, inhibitory interneurons, within the prefrontal cortex play essential roles in the encoding and maintenance of information in working memory (Fuster, 2001; Goldman-Rakic, 1995; Miller, 2000). Specifically, neurons within prefrontal cortex exhibit increased activity during maintenance of information within working memory, and this “memory-related” activity has been attributed to glutamate-dependent recurrent excitation within the local circuit (Compte et al, 2000; Compte et al, 2003; Durstewitz and Seamans, 2002). Compromised glutamate release, hypothetically secondary to low dysbindin expression, may be expected to compromise the ability of cortical circuits to maintain recurrent excitation and to “fail” during maintenance of information held in working memory. These alterations in glutamatergic transmission may relate to the altered patterns of prefrontal activity shown by patients with schizophrenia compared with controls across varying degrees of memory load and in particular to the reduced activation of cortical regions during high memory load conditions in these patients (Callicott et al, 2000; Karlsgodt et al, 2007; Karlsgodt et al, 2009; Manoach, 2003).
To examine these relationships more directly, we examined working memory function and excitatory neurotransmission within prefrontal cortex in so-called sandy mice that possess a large deletion contained wholly within the dtnbp1 gene (Li et al, 2003). Several recent studies involving these null mutant mice have shown that loss of dysbindin function impedes excitatory neurotransmission (Chen et al, 2008; Numakawa et al, 2004; Talbot et al, 2006), effects which likely correspond to behavioral abnormalities, including social deficits, enhanced stimulant sensitization and poor performance in certain simple memory tests (Bhardwaj et al, 2008; Cox et al, 2009; Feng et al, 2008; Hattori et al, 2008; Takao et al, 2008). To complement these studies, we sought to gather direct evidence that spatial working memory function, an important endophenotype for schizophrenia (Glahn et al, 2003), is affected by loss of dysbindin, as well as to determine whether physiological changes in the neural circuitry of working memory are a consequence of this mutation. We hypothesized that null mutation of the gene coding for dysbindin would impair working memory and that this cognitive endophenotype would be associated with a decrease in excitatory neurotransmission within circuits involved in this cognitive process.
Materials and Methods
Animals
Dysbindin mutant mouse breeders on the DBA2J background were obtained from Roswell Park Cancer Institute (Conyers GA). Experimental mice were generated by heterozygote crosses, and genotypes were determined by polymerase chain reaction. The wt product [472 bp] was amplified with the following primers: TGAGCCATTAGGAGATAAGAGCA and AGCTCCACCTGCTGAACATT; the dys− product [274 bp] was amplified with the following primers: TCCTTGCTTCGTTCTCTGCT and CTTGCCAGCCTTCGTATTGT). The fragments were separated on a 3% agarose gel.
Group-housed male mice were used in the behavioral and electrophysiological studies. The mice in the behavioral experiments were 60–100 days of age during experimentation, while the mice used in the recording studies were 45–60 days of age. All experimental protocols were approved by the Chancellor’s Animal Research Committee at UCLA or the Medical University of South Carolina Institutional Animal Care and Use Committee.
Working memory testing
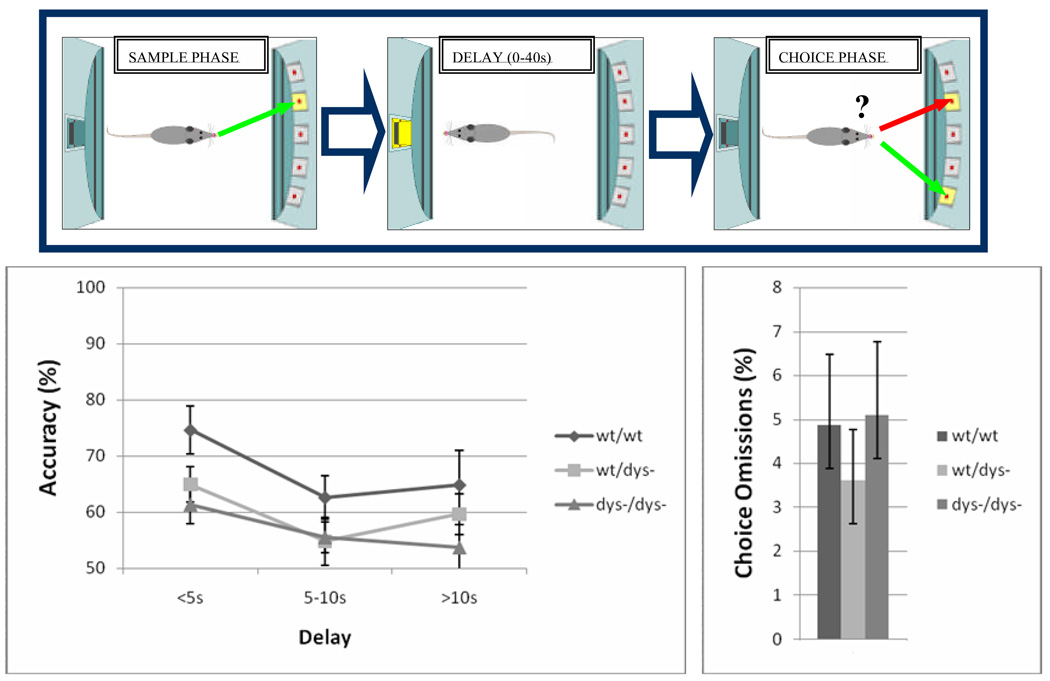
Mice were trained and tested using a delayed non-match-to-position test (Marrs et al. 2005; Aarde and Jentsch 2006) in small aluminum and Plexiglas operant conditioning chambers (Med-Associates Inc., St Albans VT), fitted with a horizontal array of five nose-poke apertures on one-side of the box and a 20-mg pellet delivery magazine on the opposite side. This delayed non-match-to-position task emphasizes retrospective encoding and maintenance of spatial information in a manner analogous to spatial delayed response tests used to measure working memory in rats, monkeys and humans.
Each trial in the delayed non-match to sample task consists of both a sample phase and a choice phase. The inter-trial interval and time-out periods were 5 s and 3 s, respectively. In the sample phase, one of the 5 nose-poke apertures was chosen at random and illuminated for up to 15 s. A response into the illuminated aperture (correct sample-phase response) caused the aperture light to extinguish and the magazine light to be illuminated. The magazine remained lit until an entry into the magazine is detected, after which the choice phase was initiated (see below). In the sample phase, the trial was aborted and a time-out ensues if the mouse makes a response in an unlit nose-poke aperture during stimulus presentation (incorrect sample-phase response) or failed to respond to the illuminated aperture during the 15 s presentation period (sample-phase omission). During the choice phase of the trial, two apertures were illuminated: the sample aperture as well as another, randomly selected aperture (the non-match location). Both of these apertures were illuminated for up to 15 s. A response in any aperture other than that of the non-match location resulted in a time-out, and an incorrect choice-phase response was recorded. Failure to make a response while the apertures were illuminated resulted in a time-out, and a choice-phase omission was recorded. Responses to the non-match location (correct response) triggered magazine illumination and pellet delivery.
During the first 30 days of training, there was no imposed delay, allowing the mice to acquire the task under relatively low memory load conditions. After this initial training, probe sessions were administered in which minimum delay periods were imposed between a correct sample-phase response and the first response into the magazine that initiated the choice phase. The imposed delays were either 0.5, 5 or 10 s and were interpolated in pseudorandom order and at equivalent frequencies across the session. The tasks ended after 75 trials are completed or 60 min passes, whichever comes first.
Incorrect choices and omissions during the sample phase and omissions during the choice phase were calculated and analyzed by one-way analyses of variance, with genotype as the factor. Accuracy of responding during the choice phase was analyzed by repeated measures ANOVA with genotype as the factor and delay length as the repeated measure.
Electrophysiological Recordings
Brain slices were prepared from male wt/wt, wt/dys− and dys−/ dys− mice. Subjects were anesthetized with the inhalant isoflurane (Abbott Laboratories). The brain was then removed and coronal slices were cut at 300 µm thickness in ice-cold high-sucrose solution containing (in mM): sucrose, 200; KCl, 1.9; Na2HPO4, 1.2; NaHCO3, 33; MgCl2, 6; CaCl2, 0.5; glucose, 10; ascorbic acid, 0.4. Slices were incubated at 33°C for at least 1 h before recordings; the incubation medium was an artificial cerebrospinal fluid solution containing (in mM): NaCl, 125; KCl, 2.5; NaH2PO4,1.25; NaHCO3, 25; MgCl, 4, CaCl, 1, d-glucose, 10; sucrose, 15; ascorbic acid, 0.4, continuously aerated with 5%CO2/95%O2. After incubation, slices were transferred to a submerged chamber and superfused with oxygenated artificial cerebrospinal fluid (in mM: 125 NaCl, 2.5 KCl, 25 NaHCO3, 2.0 CaCl2, 1.3 MgCl2, 10 glucose and 0.4 ascorbic acid) at room temperature. Recordings were made using a Multiclamp 700B amplifier (Axon Instruments, CA), connected to a computer running Windows XP and Axograph X® software and later analyzed off-line. All recordings were obtained from neurons in layers V or VI of the prelimbic or infralimbic cortex, identified using infrared-differential interference contrast optics and video-microscopy.
Current Clamp
For current-clamp recordings, thick-walled borosilicate pipettes (3–7 MΩ tip resistance) were filled with (in mM): 125 K+-gluconate, 3 KCl, 2 MgCl2, 10 HEPES, 0.1 EGTA. A series of current steps (1000 ms duration, −100 to +300 pA, at 1 Hz) were injected to evoke spike firing at various steady-state membrane potentials. In between the steps, cells were held as close to −80 mV as possible via DC current injection (referred to as holding current in Table 1). Measures of the intrinsic membrane excitability (rheobase current, holding current, number of evoked spikes, input resistance, action potential threshold, amplitude, and half-width) were compared across genotypes.
Table 1.
Membrane properties of prefrontal cortex neurons recorded in wild-type and heterozygous or homozygous dysbindin null mutant mice.
| genotype | rheobase (pA) | spike threshold (mV) | spike amplitude (mV) | spike half-width (msec) | input resistance (mOhms) | cell capacitance (pF/cm2) | Holding current (pA) |
|---|---|---|---|---|---|---|---|
| wt/wt | 135 +/− 7.2 | −47.2 +/− 2.0 | 79.05 +/− 1.6 | 2.9 +/− 0.1 | 126.7 +/− 10.5 | 165.1 +/− 16.1 | −178.2 +/− 21.5 |
| wt/dys− | 121.4 +/− 22.5* | −49.6 +/− 2.6* | 78.2 +/− 3.9 | 2.2 +/− 0.1 | 118.6 +/− 12.9 | 79.5 +/− 19.8 | −169.7 +/−36.2 |
| dys−/dys− | 67.1 +/− 11.7** | −56.7 +/− 1.5** | 76.3 +/− 5.9 | 2.9 +/− 0.5 | 125 +/− 6.5 | 54.1 +/− 7.4 | −192.4 +/− 40.9 |
Astrices indicate statistically significant differences between wild-type controls and mutant groups.
p<0.05
p<0.01.
Voltage clamp
For voltage-clamp recordings, electrodes (3–7 MΩ resistance in situ) were filled with a solution containing (in mM): 135 CsCl, 10 HEPES, 2 MgCl2, 1 EGTA, 4 NaCl; 2 Na-ATP, 0.3 tris-GTP, 1 QX-314, 10 phosphocreatine; 285 mOsmols. All the voltage-clamp experiments were performed in the presence of 100 µM picrotoxin. Series resistances (10–20 MΩ) and input resistances were continually monitored throughout the experiment via a −1 mV (100 ms) hyperpolarizing pulse. Evoked EPSCs (eEPSCs) were elicited by applying low-intensity, square-wave pulses (50–150 µA; 100 µsec in duration) using a bipolar concentric electrode placed within 200 µm of the recording electrode. The eEPSC amplitude was defined as the mean amplitude during a 1–2 ms window at the peak of the EPSC minus the amplitude during a similar window immediately before the stimulus artifact. Pulses were administered every 30 seconds and peak eEPSC amplitude was measured. Stimulus intensity was gradually increased in order to construct an input-output curve from 10 µA up to 500 µA (in some cases), until maximum amplitude was reached, meaning that response amplitude remained consistent regardless of increasing stimulation intensity. The responses included in the analysis were limited to those measured at 75% of maximum amplitude. Paired pulse stimuli were delivered at a frequency of 0.3Hz, with an ISI of 50 msec.
Miniature EPSCs (mEPSCs) were obtained from 50–200 sweeps per cell; TTX (1 µM) was added to the recording buffer when mEPSCs were assessed. Amplitude and frequencies were calculated using MiniAnalysis software® with a detection threshold of 8 pA, events were then manually checked for accuracy.
Statistical Analysis
Parametric analyses of variance (ANOVA) were used to examine main effects (e.g., genotype) and interactions with repeated measures, when appropriate. Significant main effects or interactions were followed up with post hoc tests. In some cases, paired t-tests were used to evaluate within subject, a priori hypothesized effects. All figures present data as mean ± SEM.
Results
Spatial Working Memory
We first sought to determine whether null mutation of the dtnbp1 gene associates with working memory dysfunction in mice. Working memory performance of wt/wt, wt/dys− and dys−/dys− mice was evaluated using an operant delayed-non-match-to-position task (Figure 1). During each trial in this procedure, mice first respond to a single illuminated aperture (the sample), experience a delay during which no cues are presented and then must make a response into one of two apertures: the sample stimulus and a non-match stimulus. Across an initial 30 day training period in which the conditional rules of the task were trained, all groups acquired and performed the task equivalently, exhibiting above chance levels of choice accuracy. Probe sessions were then delivered in which delays of 0.5–10 s were introduced; as expected, a main effect of delay was found for choice accuracy (F(2,56)=4.9, p=0.01), indicating that performance was sensitive to the length of the retention interval. Considering all completed trials, choice accuracy was significantly affected by genotype (Figure 1; F(2,28)=4.2, p=0.02) but the genotype x delay interaction did not reach significance; post hoc Tukey tests confirmed that mutants exhibited poorer choice accuracy than did controls (p<0.01), but that the difference between wild-type and heterozygous mice only reached the trend level (p=0.07).
Figure 1.
To assess whether sensorimotor phenotypes could contribute to the observed impairments in dys−/dys− mice, we examined other aspects of task performance. We first calculated correct responding in the sample phase that only requires animals to respond to a single illuminated light; this measure was not affected by genotype (F(2,28)=0.04, p=0.95), indicating that the two groups were equally able to process the sensory properties of the stimuli and to emit a conditionally appropriate response. Furthermore, omission rates did not differ between the two groups for either the sample (F(2,28)=1.4, p=0.26) or choice phases (Figure 1; F(2.28)=0.3, p=0.7), indicating that both groups were equally motivated to perform the task and were equally capable of responding within the time constraints of the two components of each trial. Therefore, the poor response accuracy in mutants observed during the non-matching phase of the trial appears to depend upon the need to encode and retain information about the sample cue across the delay period.
Physiological Properties of Prefrontal Cortical Pyramidal Neurons
Given the important role for delay-related, glutamate-dependent activity of prefrontal cortical pyramidal neuron networks in the representation of visuospatial information maintained in working memory (Goldman-Rakic, 1995), we next examined whether genetic dysbindin deficiency altered glutamatergic transmission in prefrontal cortex, using in vitro recordings from deep layer pyramidal neurons within the medial frontal cortex in wt/wt, wt/dys− and dys−/dys− mice.
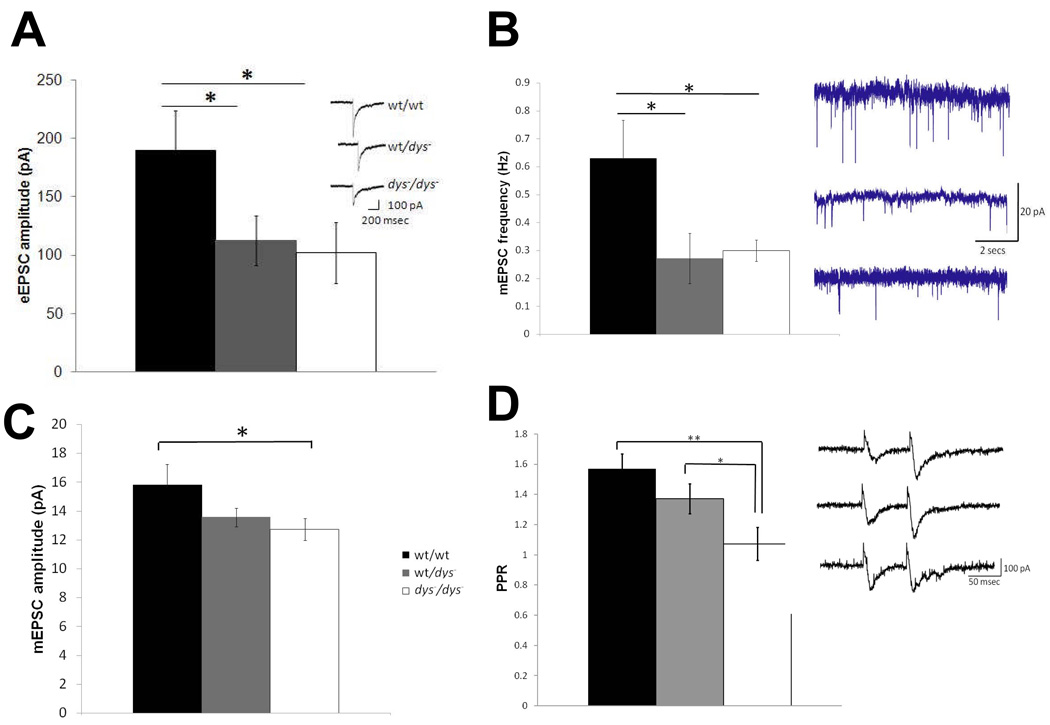
We first measured basic membrane properties in pyramidal neurons from all three genotypes and found that while spike amplitude, spike half-width, input resistance and cell capacitance showed no significant effect of genotype, rheobase and spike threshold were significantly affected (Table 1). Rheobase current was significantly affected by genotype (F(2,24)=5.1; p=0.009, Table 1) with dys−/dys− mice exhibiting an approximately 50% reduction compared to wt/wt. This change in rheobase current can be attributed to a significant reduction in spike threshold in neurons recorded from dys−/dys− mice (−56.7 ± 1.5 mV) compared to wt/wt (−47.2 ± 2.0 mV; F(2,24)=5.6, p=0.006) but not to differences in the amount of holding current injected to maintain a membrane potential of −80 mV (Table 1), suggesting that resting membrane potentials were not different between genotypes. For rheobase and membrane threshold, the corresponding values for heterozygous mice were intermediate between wt/wt and dys−/dys− mice. These data suggest that dysbindin deletion or reduction may increase the excitability of pyramidal neurons.
We next sought to examine whether null mutation of the dtnbp1 gene affected excitatory transmission within the local circuitry of the prefrontal cortex; our a priori hypothesis, stemming from recently published work (Chen et al, 2008; Numakawa et al, 2004; Talbot et al, 2004), was that dysbindin deletion would impair excitatory synaptic transmission. Assessment of eEPSC amplitude showed a significant difference amongst the three genotypes (Figure 2A; F(2,44)=3.2, p<0.05), with a significant reduction in evoked responses in both wt/dys− and dys−/ dys− mice, relative to wt/wt controls (p<0.05). These initial data suggested that evoked synaptic responses were lower in mutant, as compared with wild-type, animals.
Figure 2.
To further investigate changes in synaptic transmission, mEPSCs were recorded from pyramidal neurons in the presence of picrotoxin (100 µM) and TTX (1 µM), and the frequency of mEPSCs across a broad range of amplitudes (8–100 pA) was analyzed. We observed an overall significantly lower frequency of mEPSCs (main effect of genotype: F(2,33)=4.1, p=0.01) in dys−/dys− (0.3 ± 0.03 Hz; n=10) and wt/dys− mice (0.27 ± 0.1 Hz; n=7) animals, compared with wt/wt mice (0.6 ± 0.1 Hz; n=16, Figure 2B); these genotypic differences were most obvious for events in the range of 20–30 pA. Additionally, mEPSC amplitude (F(2,33)=2.2, p=0.04) was also significantly reduced in dys−/dys− (12.7 ± 0.8; n=16) compared to wt/wt neurons (15.8 ± 1.4; n=10; Figure 2C). These data suggested that reduced dysbindin expression leads to significant decreases in excitatory transmission within the prefrontal cortex.
Because of the extant data indicating that dysbindin is expressed within glutamatergic terminals and interacts with vesicle-trafficking proteins, we hypothesized that the reduction in mEPSCs may result from decreased pre-synaptic release of glutamate in dysbindin mutants. Therefore, we next examined paired-pulse facilitation. The overall ANOVA examining the effects of genotype on the paired-pulse ratio revealed a significant main effect of genotype (F(2,26)=7.4, p=0.0009; Figure 2D) that was largely attributable to a reduction in the paired-pulse ratio in dys−/dys− mice (ratio=1.1 ± 0.06; n=9) compared with wt/wt controls (ratio=1.6 ± 0.1; n=8). These results further support the idea that dysbindin deletion or reduction impairs cortical glutamate release.
Discussion
Null mutation of the gene encoding dysbindin led to specific changes in performance of a delayed non-match to position test of spatial working memory, as well as the associated, underlying neural mechanisms within prefrontal cortex believed to mediate this cognitive function. Although dysbindin null subjects were as capable as wild-type controls in processing and responding in a conditionally appropriate way to visual cues, they were unable to respond correctly in a delayed non-match-to-position task, indicating that they had difficulty encoding or maintaining the item in spatial working memory. These data suggest that poor working memory maintenance is a phenotypic consequence to loss of dysbindin expression.
Encoding and maintenance of visuospatial information in working memory is thought to involve the selective recruitment of prefrontal cortical neuronal circuits, which exhibit persistent activity during the active maintenance of the information (Compte et al, 2000; Compte et al, 2003; Durstewitz et al, 2000; Seamans et al, 2003). Because dysbindin has been shown to modulate glutamate outflow (Chen et al, 2008; Numakawa et al, 2004), we hypothesized that the poor working memory exhibited in dysbindin mice was due to a compromised glutamate transmission. Electrophysiological recordings from deep layer pyramidal neurons in the medial prefrontal cortex confirmed this hypothesis by revealing that the amplitude of eEPSCs and the frequency of miniature EPSCs were decreased and moreover, paired-pulse facilitation was not present in dysbindin mutant mice. Collectively, these data support the conclusion that the pre-synaptic release of glutamate is impaired in dysbindin deficient mice.
Of importance, hippocampal circuits also contribute to performance of delayed match/non-match to sample tasks such as the one used here (Deadwyler et al, 1996; Hampson and Deadwyler, 1996, 2000; Hampson et al, 1993), so neural dysfunction within the hippocampus could also contribute to the working memory phenotype in dysbindin mutant mice. Other behavioral studies in dysbindin null mutant mice have found deficits in tasks that predominantly measure hippocampal function (Bhardwaj et al, 2009; Cox et al, 2009; Takao et al, 2008), so the current behavioral effects may not rely upon prefrontal cortical dysfunction alone. Future studies directly assessing hippocampal circuitry, using electrophysiology and gene expression, are underway.
Relationship to Cognitive Endophenotypes for Schizophrenia
The ability to maintain and manipulate information held in working memory appears to be a quantitative trait, and the heritability of working memory performance amongst unaffected relatives of individuals diagnosed with schizophrenia supports the conclusion that this aspect of cognition is an endophenotype and/or marker of genetic liability for the disorder (Glahn et al, 2002; Glahn et al, 2003). Genetic variation in other schizophrenia risk genes, most notably DISC1, associates with spatial working memory function in humans (Burdick et al, 2005; Cannon et al, 2005; Koike et al, 2006; Kvajo et al, 2008; Li et al, 2007), and studies in mouse genetic models has further supported the linkages between these particular genes and cognitive performance (Cox et al, 2009; Takao et al, 2008). Further to that, the current results support a role for dysbindin in the modulation of spatial working memory and possibly other prefrontal cortical-dependent cognitive functions. Notably, however, dysbindin is expressed in brain regions other than the prefrontal cortex; its considerable expression in hippocampus suggests that it could (through cellular actions similar to those reported here to occur in the prefrontal cortex) strongly affect synaptic plasticity and associated memory functions (Cox et al, 2009; Takao et al, 2008).
Dysbindin and Its Relation to Glutamatergic Mechanisms
Earlier findings regarding the function of dysbindin suggested either no, or an inverse, correlation between levels of dysbindin and VGlutT-1 protein levels in the dentate gyrus of hippocampus in humans (Talbot et al, 2004) and in younger animals (<30 days old) (Chen et al, 2008). However, recent studies using more selective antibodies suggest that there is a positive correlation between dysbindin-1 and VGluT-1 levels in older animals, such as the ones used in this study (45–60 days of age), as well as in humans (Talbot, personal communication). Therefore, it is likely that glutamate release is reduced in the dys−/dys− and wt/dys− animals due to an impairment of the cells’ ability to package glutamate into vesicles; this hypothesis is supported by our observation of reduced amplitude of eEPSCs and frequency of mEPSCs in vitro. Additionally, we observed a significant decrease in the amplitude of mEPSCs in the dys−/dys− animals, suggesting that dysbindin deletion may further impair glutamatergic transmission via postsynaptic mechanisms, as well. This issue requires further investigation in order to specify the relevant mechanisms involved.
Interestingly, although dysbindin deletion appears to decrease glutamate release at the axon terminal, it appears that the absence of dysbindin may also result in an increase in excitability, as shown by a decrease in the amount of intra-somatic current injection required to evoke an action potential (rheobase). When the basic physiological properties of prefrontal cortical pyramidal neurons were examined, significant difference between the genotypes were found in spike threshold and rehobase current, as well as a decrease of more than 50% in cell capacitance in the wt/dys− and dys−/dys− groups. Since C=1/R, a decrease in capacitance will result in an increase in resistance, therefore rendering the cell more excitable. Changes in capacitance may result from a reduction in cell size, neuronal dendritic branching or spine density. Another possibility is that loss of dysbindin may impact GABAergic interneurons, eliciting concomitant reductions in GABA release and resulting in a disinhibition of pyramidal cells, a possibility that deserves future exploration. Regardless of the mechanisms underlying this increase in excitability, it appears that any effects that might result from an increase in the generation of action potentials in the dys−/dys− mice may be counteracted by the relevant impairments in glutamate release. Future experiments will investigate changes in dendritic branching as well as alterations in GABA release and interneuron function in dysbindin deficient mice.
Recordings during performance of delayed alternation tasks have shown an increase in pyramidal cell firing during the delay period of the task (Fuster and Alexander, 1971; Goldman-Rakic, 1995; Miller, et al, 1996). This recurrent excitatory activity has been proposed to be the cellular correlate for working memory, suggesting that decreases in glutamatergic transmission, such as the decreases reported here in the dys−/dys− and wt/dys− mice, may underlie the cognitive deficits observed in some neuropsychiatric disorders such as schizophrenia and bipolar disorder. A promising new avenue for pharmacological treatments is aimed at targeting modulators of NMDA receptors, such as the glycine transporter, or more recently, positive allosteric modulators of the mGluR5 glutamate receptor. Several studies have shown that mGluR5 antagonists not only decrease burst firing of PFC pyramidal neurons but also impair spatial working memory (Ballard et al, 2005; Homayoun and Moghaddam, 2006; Homayoun et al, 2004; Locchi et al, 2007), suggesting that mGluR5 agonists could restore normal levels of activity in dysfunctional cortical circuits affected in schizophrenia.
Acknowledgements
This work was supported by PHS Grants MH-83269 (TC,JDJ,AL) and K12-GM081265 (HTD) and a NARSAD Distinguished Investigator Award (TC).
Footnotes
Disclosure/Conflict of Interest
Dr. Jentsch has received compensation as a consultant for Merck Research Laboratories. The other author(s) declare that, except for income received from my primary employer, no financial support or compensation has been received from any individual or corporate entity over the past three years for research or professional service and there are no personal financial holdings that could be perceived as constituting a potential conflict of interest.
References
- Arguello PA, Gogos JA. Modeling madness in mice: one piece at a time. Neuron. 2006;52(1):179–196. doi: 10.1016/j.neuron.2006.09.023. [DOI] [PubMed] [Google Scholar]
- Ballard TM, Woolley ML, Prinssen E, Huwyler J, Porter R, Spooren W. The effect of the mGlu5 receptor antagonist MPEP in rodent tests of anxiety and cognition: a comparison. Psychopharmacology (Berl) 2005;179(1):218–229. doi: 10.1007/s00213-005-2211-9. [DOI] [PubMed] [Google Scholar]
- Bhardwaj SK, Baharnoori M, Sharif-Askari B, Kamath A, Williams S, Srivastava LK. Behavioral characterization of dysbindin-1 deficient sandy mice. Behavioural brain research. 2008 doi: 10.1016/j.bbr.2008.10.011. [DOI] [PubMed] [Google Scholar]
- Bhardwaj SK, Baharnoori M, Sharif-Askari B, Kamath A, Williams S, Srivastava LK. Behavioral characterization of dysbindin-1 deficient sandy mice. Behav Brain Res. 2009;197(2):435–441. doi: 10.1016/j.bbr.2008.10.011. [DOI] [PubMed] [Google Scholar]
- Bray NJ, Preece A, Williams NM, Moskvina V, Buckland PR, Owen MJ, et al. Haplotypes at the dystrobrevin binding protein 1 (DTNBP1) gene locus mediate risk for schizophrenia through reduced DTNBP1 expression. Human molecular genetics. 2005;14(14):1947–1954. doi: 10.1093/hmg/ddi199. [DOI] [PubMed] [Google Scholar]
- Burdick KE, Goldberg TE, Funke B, Bates JA, Lencz T, Kucherlapati R, et al. DTNBP1 genotype influences cognitive decline in schizophrenia. Schizophrenia research. 2007;89(1–3):169–172. doi: 10.1016/j.schres.2006.09.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burdick KE, Hodgkinson CA, Szeszko PR, Lencz T, Ekholm JM, Kane JM, et al. DISC1 and neurocognitive function in schizophrenia. Neuroreport. 2005;16(12):1399–1402. doi: 10.1097/01.wnr.0000175248.25535.f6. [DOI] [PubMed] [Google Scholar]
- Burdick KE, Lencz T, Funke B, Finn CT, Szeszko PR, Kane JM, et al. Genetic variation in DTNBP1 influences general cognitive ability. Human molecular genetics. 2006;15(10):1563–1568. doi: 10.1093/hmg/ddi481. [DOI] [PubMed] [Google Scholar]
- Callicott JH, Bertolino A, Mattay VS, Langheim FJ, Duyn J, Coppola R, et al. Physiological dysfunction of the dorsolateral prefrontal cortex in schizophrenia revisited. Cereb Cortex. 2000;10(11):1078–1092. doi: 10.1093/cercor/10.11.1078. [DOI] [PubMed] [Google Scholar]
- Cannon TD. The inheritance of intermediate phenotypes for schizophrenia. Current opinion in psychiatry. 2005;18(2):135–140. doi: 10.1097/00001504-200503000-00005. [DOI] [PubMed] [Google Scholar]
- Cannon TD, Hennah W, van Erp TG, Thompson PM, Lonnqvist J, Huttunen M, et al. Association of DISC1/TRAX haplotypes with schizophrenia, reduced prefrontal gray matter, and impaired short- and long-term memory. Archives of general psychiatry. 2005;62(11):1205–1213. doi: 10.1001/archpsyc.62.11.1205. [DOI] [PubMed] [Google Scholar]
- Chen XW, Feng YQ, Hao CJ, Guo XL, He X, Zhou ZY, et al. DTNBP1, a schizophrenia susceptibility gene, affects kinetics of transmitter release. J Cell Biol. 2008;181(5):791–801. doi: 10.1083/jcb.200711021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Compte A, Brunel N, Goldman-Rakic PS, Wang XJ. Synaptic mechanisms and network dynamics underlying spatial working memory in a cortical network model. Cereb Cortex. 2000;10(9):910–923. doi: 10.1093/cercor/10.9.910. [DOI] [PubMed] [Google Scholar]
- Compte A, Constantinidis C, Tegner J, Raghavachari S, Chafee MV, Goldman-Rakic PS, et al. Temporally irregular mnemonic persistent activity in prefrontal neurons of monkeys during a delayed response task. J Neurophysiol. 2003;90(5):3441–3454. doi: 10.1152/jn.00949.2002. [DOI] [PubMed] [Google Scholar]
- Cox MM, Tucker AM, Tang J, Talbot K, Richer DC, Yeh L, et al. Neurobehavioral abnormalities in the dysbindin-1 mutant, sandy, on a C57BL/6J genetic background. Genes Brain Behav. 2009 doi: 10.1111/j.1601-183X.2009.00477.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deadwyler SA, Bunn T, Hampson RE. Hippocampal ensemble activity during spatial delayed-nonmatch-to-sample performance in rats. Journal of Neuroscience. 1996;16(1):354–372. doi: 10.1523/JNEUROSCI.16-01-00354.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Donohoe G, Morris DW, Clarke S, McGhee KA, Schwaiger S, Nangle JM, et al. Variance in neurocognitive performance is associated with dysbindin-1 in schizophrenia: a preliminary study. Neuropsychologia. 2007;45(2):454–458. doi: 10.1016/j.neuropsychologia.2006.06.016. [DOI] [PubMed] [Google Scholar]
- Durstewitz D, Seamans JK. The computational role of dopamine D1 receptors in working memory. Neural Netw. 2002;15(4–6):561–572. doi: 10.1016/s0893-6080(02)00049-7. [DOI] [PubMed] [Google Scholar]
- Durstewitz D, Seamans JK, Sejnowski TJ. Dopamine-mediated stabilization of delay-period activity in a network model of prefrontal cortex. J Neurophysiol. 2000;83(3):1733–1750. doi: 10.1152/jn.2000.83.3.1733. [DOI] [PubMed] [Google Scholar]
- Feng YQ, Zhou ZY, He X, Wang H, Guo XL, Hao CJ, et al. Dysbindin deficiency in sandy mice causes reduction of snapin and displays behaviors related to schizophrenia. Schizophrenia research. 2008;106(2–3):218–228. doi: 10.1016/j.schres.2008.07.018. [DOI] [PubMed] [Google Scholar]
- Fuster JM. The prefrontal cortex - An update. Neuron. 2001;30:319–333. doi: 10.1016/s0896-6273(01)00285-9. [DOI] [PubMed] [Google Scholar]
- Glahn DC, Kim J, Cohen MS, Poutanen VP, Therman S, Bava S, et al. Maintenance and manipulation in spatial working memory: dissociations in the prefrontal cortex. NeuroImage. 2002;17(1):201–213. doi: 10.1006/nimg.2002.1161. [DOI] [PubMed] [Google Scholar]
- Glahn DC, Therman S, Manninen M, Huttunen M, Kaprio J, Lonnqvist J, et al. Spatial working memory as an endophenotype for schizophrenia. Biological psychiatry. 2003;53(7):624–626. doi: 10.1016/s0006-3223(02)01641-4. [DOI] [PubMed] [Google Scholar]
- Goldman-Rakic PS. Cellular basis of working memory. Neuron. 1995;14(3):477–485. doi: 10.1016/0896-6273(95)90304-6. [DOI] [PubMed] [Google Scholar]
- Hampson RE, Deadwyler SA. Ensemble codes involving hippocampal neurons are at risk during delayed performance tests. Proceedings of the National Academy of Sciences of the United States of America. 1996;93(24):13487–13493. doi: 10.1073/pnas.93.24.13487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hampson RE, Deadwyler SA. Cannabinoids reveal the necessity of hippocampal neural encoding for short-term memory in rats. Journal of Neuroscience. 2000;20(23):8932–8942. doi: 10.1523/JNEUROSCI.20-23-08932.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hampson RE, Heyser CJ, Deadwyler SA. Hippocampal cell firing correlates of delayed-match-to-sample performance in the rat. Behavioral Neuroscience. 1993;107(5):715–739. doi: 10.1037//0735-7044.107.5.715. [DOI] [PubMed] [Google Scholar]
- Harrison PJ, Weinberger DR. Schizophrenia genes, gene expression, and neuropathology: on the matter of their convergence. Molecular psychiatry. 2005;10(1):40–68. doi: 10.1038/sj.mp.4001558. image 45. [DOI] [PubMed] [Google Scholar]
- Hattori S, Murotani T, Matsuzaki S, Ishizuka T, Kumamoto N, Takeda M, et al. Behavioral abnormalities and dopamine reductions in sdy mutant mice with a deletion in Dtnbp1, a susceptibility gene for schizophrenia. Biochemical and biophysical research communications. 2008;373(2):298–302. doi: 10.1016/j.bbrc.2008.06.016. [DOI] [PubMed] [Google Scholar]
- Homayoun H, Moghaddam B. Bursting of prefrontal cortex neurons in awake rats is regulated by metabotropic glutamate 5 (mGlu5) receptors: rate-dependent influence and interaction with NMDA receptors. Cereb Cortex. 2006;16(1):93–105. doi: 10.1093/cercor/bhi087. [DOI] [PubMed] [Google Scholar]
- Homayoun H, Stefani MR, Adams BW, Tamagan GD, Moghaddam B. Functional Interaction Between NMDA and mGlu5 Receptors: Effects on Working Memory, Instrumental Learning, Motor Behaviors, and Dopamine Release. Neuropsychopharmacology. 2004;29(7):1259–1269. doi: 10.1038/sj.npp.1300417. [DOI] [PubMed] [Google Scholar]
- Karlsgodt KH, Glahn DC, van Erp TG, Therman S, Huttunen M, Manninen M, et al. The relationship between performance and fMRI signal during working memory in patients with schizophrenia, unaffected co-twins, and control subjects. Schizophr Res. 2007;89(1–3):191–197. doi: 10.1016/j.schres.2006.08.016. [DOI] [PubMed] [Google Scholar]
- Karlsgodt KH, Sanz J, van Erp TG, Bearden CE, Nuechterlein KH, Cannon TD. Re-evaluating dorsolateral prefrontal cortex activation during working memory in schizophrenia. Schizophr Res. 2009;108(1–3):143–150. doi: 10.1016/j.schres.2008.12.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koike H, Arguello PA, Kvajo M, Karayiorgou M, Gogos JA. Disc1 is mutated in the 129S6/SvEv strain and modulates working memory in mice. Proc Natl Acad Sci U S A. 2006;103(10):3693–3697. doi: 10.1073/pnas.0511189103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kvajo M, McKellar H, Arguello PA, Drew LJ, Moore H, MacDermott AB, et al. A mutation in mouse Disc1 that models a schizophrenia risk allele leads to specific alterations in neuronal architecture and cognition. Proc Natl Acad Sci U S A. 2008;105(19):7076–7081. doi: 10.1073/pnas.0802615105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li W, Zhang Q, Oiso N, Novak EK, Gautam R, O'Brien EP, et al. Hermansky-Pudlak syndrome type 7 (HPS-7) results from mutant dysbindin, a member of the biogenesis of lysosome-related organelles complex 1 (BLOC-1) Nature genetics. 2003;35(1):84–89. doi: 10.1038/ng1229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li W, Zhou Y, Jentsch JD, Brown RA, Tian X, Ehninger D, et al. Specific developmental disruption of disrupted-in-schizophrenia-1 function results in schizophrenia-related phenotypes in mice. Proc Natl Acad Sci U S A. 2007;104(46):18280–18285. doi: 10.1073/pnas.0706900104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lipska BK, Peters T, Hyde TM Halim N, Horowitz C, Mitkus S, et al. Expression of DISC1 binding partners is reduced in schizophrenia and associated with DISC1 SNPs. Human molecular genetics. 2006;15(8):1245–1258. doi: 10.1093/hmg/ddl040. [DOI] [PubMed] [Google Scholar]
- Locchi F, Dall'Olio R, Gandolfi O, Rimondini R. Water T-maze, an improved method to assess spatial working memory in rats: Pharmacological validation. Neurosci Lett. 2007;422(3):213–216. doi: 10.1016/j.neulet.2007.06.023. [DOI] [PubMed] [Google Scholar]
- Luciano M, Miyajima F, Lind PA, Bates TC, Horan M, Harris SE, et al. Variation in the Dysbindin gene and normal cognitive function in three independent population samples. Genes Brain Behav. 2008 doi: 10.1111/j.1601-183X.2008.00462.x. [DOI] [PubMed] [Google Scholar]
- Manoach DS. Prefrontal cortex dysfunction during working memory performance in schizophrenia: reconciling discrepant findings. Schizophrenia research. 2003;60(2–3):285–298. doi: 10.1016/s0920-9964(02)00294-3. [DOI] [PubMed] [Google Scholar]
- Miller EK. The prefrontal cortex and cognitive control. Nature Reviews Neuroscience. 2000;1:59–65. doi: 10.1038/35036228. [DOI] [PubMed] [Google Scholar]
- Numakawa T, Yagasaki Y, Ishimoto T, Okada T, Suzuki T, Iwata N, et al. Evidence of novel neuronal functions of dysbindin, a susceptibility gene for schizophrenia. Human molecular genetics. 2004;13(21):2699–2708. doi: 10.1093/hmg/ddh280. [DOI] [PubMed] [Google Scholar]
- Seamans JK, Nogueira L, Lavin A. Synaptic basis of persistent activity in prefrontal cortex in vivo and in organotypic cultures. Cereb Cortex. 2003;13(11):1242–1250. doi: 10.1093/cercor/bhg094. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takao K, Toyama K, Nakanishi K, Hattori S, Takamura H, Takeda M, et al. Impaired long-term memory retention and working memory in sdy mutant mice with a deletion in Dtnbp1, a susceptibility gene for schizophrenia. Mol Brain. 2008;1(1):11. doi: 10.1186/1756-6606-1-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Talbot K, Cho DS, Ong WY, Benson MA, Han LY, Kazi HA, et al. Dysbindin-1 is a synaptic and microtubular protein that binds brain snapin. Human molecular genetics. 2006;15(20):3041–3054. doi: 10.1093/hmg/ddl246. [DOI] [PubMed] [Google Scholar]
- Talbot K, Eidem WL, Tinsley CL, Benson MA, Thompson EW, Smith RJ, et al. Dysbindin-1 is reduced in intrinsic, glutamatergic terminals of the hippocampal formation in schizophrenia. The Journal of clinical investigation. 2004;113(9):1353–1363. doi: 10.1172/JCI20425. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tunbridge EM, Weickert CS, Kleinman JE, Herman MM, Chen J, Kolachana BS, et al. Catechol-o-Methyltransferase Enzyme Activity and Protein Expression in Human Prefrontal Cortex across the Postnatal Lifespan. Cereb Cortex. 2006 doi: 10.1093/cercor/bhl032. [DOI] [PubMed] [Google Scholar]
- Weickert CS, Straub RE, McClintock BW, Matsumoto M, Hashimoto R, Hyde TM, et al. Human dysbindin (DTNBP1) gene expression in normal brain and in schizophrenic prefrontal cortex and midbrain. Archives of general psychiatry. 2004;61(6):544–555. doi: 10.1001/archpsyc.61.6.544. [DOI] [PubMed] [Google Scholar]
- Weinberger DR, Berman KF. Speculation on the meaning of cerebral metabolic hypofrontality in schizophrenia. Schizophrenia bulletin. 1988;14(2):157–168. doi: 10.1093/schbul/14.2.157. [DOI] [PubMed] [Google Scholar]
- Weinberger DR, Egan MF, Bertolino A, Callicott JH, Mattay VS, Lipska BK, et al. Prefrontal neurons and the genetics of schizophrenia. Biological psychiatry. 2001;50(11):825–844. doi: 10.1016/s0006-3223(01)01252-5. [DOI] [PubMed] [Google Scholar]
- Williamson P. Hypofrontality in schizophrenia: a review of the evidence. Canadian journal of psychiatry. 1987;32(5):399–404. doi: 10.1177/070674378703200516. [DOI] [PubMed] [Google Scholar]