Abstract
Recent studies have indicated a role for the endocannabinoid system in the behavioral and physiological effects of alcohol (ethanol), particularly ethanol seeking behaviors. However, its role in modulating binge-like intake and/or the mechanism by which it may exert these effects remain poorly understood. The current study used a newly developed strain-specific animal model of binge drinking, dubbed ‘Drinking In the Dark’ (DID), to determine if facilitation of the endocannabinoid system with the synthetic cannabinoid agonist WIN 55–212,2 (WIN) modulates binge-like ethanol intake in male C57BL/6J (B6) mice. Based on the results of these systemic (i.p.) manipulations, and evidence in support of the involvement of subregions of the Ventral Tegmental Area (VTA) in governing self-administration of ethanol (Rodd-Henricks et al., 2000) as well as binge-like intake using the DID model (Moore & Boehm, 2009), we extended these findings to evaluate the role of the endocannabinoid system within the anterior and posterior sub regions of the VTA using site-specific microinjections. Consistent with previous research, the lowest systemic dose of WIN (0.5 mg/kg) significantly increased ethanol intake in the first 30 minutes of access whereas the two highest doses (1 and 2 mg/kg) decreased ethanol intake within this time interval. Intra-pVTA (but not aVTA) microinjections elicited time-dependent and dose-dependent increases (0.25 and 0.5 µg/side) and decreases (2.5 µg/side) in ethanol intake. Importantly, follow-up studies revealed that in some cases alterations in fluid consumption may have been influenced by competing locomotor activity (or inactivity).The present data are consistent with previous research in that agonism of the endocannabinoid system increases ethanol intake in rodents and implicate the pVTA in the modulation of drinking to intoxication. Moreover, the dose-dependent alterations in locomotor activity emphasize the importance of directly assessing multiple (possibly competing) behaviors when evaluating drug effects on voluntary consumption.
One hallmark feature of alcoholism that has recently received much attention is repeatedly drinking to intoxication or ‘binge’ drinking. Binge drinking was recently defined by the National Institute on Alcohol and Alcoholism (NIAAA) as a pattern of alcohol intake that elicits blood ethanol concentrations (BECs) of ≥ 0.08 grams percent (or 80 mg/dl in plasma). For adults this concentration typically occurs after 4 (female) to 5 (male) or more alcoholic beverages within a two-hour window. This pattern of ethanol intake is distinct from ‘risky’ drinking (resulting in BECs ranging from 50–80 mg/dl) and is believed to pose an increased health risk to the individual.
Obvious ethical limitations in studying binge drinking in human populations necessitate the use of valid animal models. Fortunately, a genetic mouse model of binge drinking, dubbed ‘drinking-in- the-dark’ (DID), has recently been described wherein the C57BL6/J (B6) inbred strain of mice voluntarily consume copious amounts of unsweetened ethanol solution over 2 hours (Rhodes et al., 2005; Rhodes et al., 2007). Unlike the traditional two-bottle choice test (Dole & Gentry, 1984), the DID model consistently produces BECs that exceed 80–100 mg/dl, leading to motor impairment as measured by the rotarod and balance beam apparatus (Moore et al., 2007; Rhodes et al., 2007). Importantly, because ethanol access (and consumption) occurs during a two-hour window, the investigator is capable of effectively testing the effects of manipulations at time points conducive to their specific interests. For example, we recently used the DID model to evaluate the role of GABAergic systems in the modulation of binge drinking (Moore & Boehm, 2009; Moore et al., 2007). Others have used the DID model to test the efficacy of systemically administered drugs targeting opioid (Kamdar et al., 2007), CRF (Sparta et al., 2008), and glutamatergic (Gupta et al., 2008) systems, as well as intra-cranially administered urocortin (Ryabinin et al., 2008), to assess the role of these systems in binge-like ethanol intake. Thus, the DID model provides a means by which to investigate the neurobiology of binge drinking and lends itself to the screening of pharmacological agents targeting this strain-specific behavioral phenotype.
Recent literature investigating alcohol (ethanol) consumption and preference in rodents has suggested a role for the endogenous cannabinoid system. Pharmacological and/or genetic manipulation of the endocannabinoid system alters ethanol-related behavioral phenotypes and physiology (Colombo et al., 2007; Colombo et al., 2005). Pharmacological studies using systemically administered cannabinoid 1 receptor (CB1) agonists and antagonists have been shown to consistently alter ethanol consumption and preference in various strains of preferring and non-preferring rats and mice (Colombo et al., 2007; Colombo et al., 2005). For example, the extremely potent synthetic cannabinoid agonist CP 55,940 (CP) has been shown to increase ethanol-maintained responding (Gallate et al., 1999) and 2 bottle choice preference (Colombo et al., 2002) in Wistar and Sardinian ethanol-preferring (sP) rats respectively. Similarly, pharmacological blockade of the activity of the major degradative enzyme of the endogenous cannabinoid ligand anandamide, fatty acid amide hydrolase (FAAH), has been shown to increase 2 bottle choice ethanol preference in several mouse strains (Blednov et al., 2007; Vinod et al., 2008a). On the other hand, in the same way that increasing endocannabinoid activity effectively increases ethanol-seeking behaviors, inhibiting endocannabinoid activity via direct antagonism of CB1 receptors with the CB1 selective antagonist SR141716 (SR(Colombo et al., 2007) or through inhibition of the anandamide transporter (Cippitelli et al., 2007), significantly decreases ethanol-seeking behaviors.
Studies using transgenic mice have yielded similar results. Increases in endocannabinoid activity via genetic knockout of FAAH significantly increases ethanol consumption and preference (Blednov et al., 2007; Vinod et al., 2008a) whereas mutant mice deficient in CB1 receptors display significantly lower levels of alcohol consumption and preference compared to wild-type mice (Hungund et al., 2003; Poncelet et al., 2003; Thanos et al., 2005; Vinod et al., 2008b; Wang et al., 2003). Collectively, these studies suggest that the facilitation or enhancement of endocannabinoid-related processes increase the likelihood of ethanol-seeking behavior whereas the inhibition of these same processes decreases them.
Of the four known endocannabinoid ligands, the two most abundantly expressed within the mammalian brain, anandamide (AEA) and 2-arachidonoylglycerol (2-AG), bind to the Gi/o-protein coupled CB1 receptors. CB1 receptors are found in high concentrations in many regions of the brain including regions of the mesolimbic system thought to be involved in alcohol reward and self-administration (Colombo et al., 2005; Pertwee, 1997; Pertwee & Ross, 2002). However, until very recently, mechanisms by which the cannabinoid system modulates ethanol-seeking behaviors have been largely unknown. The previously mentioned report by Hungund et al., (2003) found that CB1 knockout mice show decreased ethanol intake and preference compared to wildtype mice. Interestingly, in this same study, prototypical dopamine efflux in the Nucleus Accumbens (NAc) following acute ethanol challenge was completely absent in these knockout mice as measured by in vivo microdialysis. Furthermore, this same dopamine response could be greatly reduced in wildtype mice through blockade of the CB1 receptor using systemic administration of the selective CB1 antagonist SR.
Although the mechanisms of such pharmacological and genetic manipulations on ethanol consumption and preference are unclear, there is converging evidence for a distinct relationship between the endocannabinoid system within the Ventral Tegmental Area (VTA) and these behaviors. For example, rats will self-administer ethanol (Rodd-Henricks et al., 2000) and Δ9-THC (Zangen et al., 2006) directly into the posterior portion of the VTA (but not anterior). Similarly, ethanol-maintained responding was recently shown by Parsons et al. (2008) to be dose- dependently decreased through blockade of CB1 receptors within the posterior VTA using SR. This same group also showed discrete changes in endocannabinoid microdialysates (specifically 2-AG) in this region following ethanol intake suggesting the involvement of tonic endocannabinoid regulation. These data along with our recent findings that pharmacological manipulations within subregions of the VTA are capable of modulating binge-like intake using the DID model (Moore & Boehm, 2009) strongly suggests convergence of endocannabinoid receptor regulation in this area in modulation of binge drinking behavior.
The goal of the present work was to evaluate the influence of the cannabinoid system on binge-like ethanol intake in B6 mice using a modified version of the DID model. This model produces consistent binge-like ethanol consumption resulting in physiologically relevant BECs, as well as significant behavioral impairment (Moore et al., 2007). Based on the previously mentioned literature, we hypothesized that administration of the synthetic cannabinoid agonist WIN 55–212,2 (WIN) would dose-dependently increase binge-like ethanol intake while having no effect on water intake. In a follow-up of our systemic manipulations, we then extended these findings to evaluate the role of the anterior and posterior VTA (aVTA and pVTA, respectively) in WIN induced DID modulation using site specific microinjections.
General Materials and Methods
Animals
7 week old Male C57BL/6J mice were purchased from Jackson Laboratory (Bar Harbor, ME) or bred in our colony at the Binghamton University animal facility. All breeders in our colony were originally purchased from Jackson Laboratory and care was taken to avoid breeding past 2 generations from these founder mice. All animals were individually housed in standard mouse cages for at least one week prior to allow for acclimation to the housing environment. Lighting was maintained on a 12-hour reverse light/dark cycle with lights out at 12PM and the temperature of the room was maintained at 21±1 degrees Celsius. Animals had ad lib access to food and water except during testing when water bottles were replaced with 20% ethanol solution. At the start of each experiment animals were at least 60 days of age. All procedures were approved by the Binghamton University Institutional Animal Care and Use Committee and conformed to Guidelines for the Care and Use of Mammals in Neuroscience and Behavioral Research (National Academy Press, 2003).
Drugs
Ethanol solution (20% v/v) was made using 200 proof ethanol from Pharmco, Inc. (Brookfiels, CT) and tap water. For systemic administration WIN55–212,2 (WIN) was purchased from Sigma Aldrich (St/ Louis, MO) and suspended in Tween 80 before being dissolved in 0.9% physiological saline. For microinjection studies WIN was suspended in a 1:2:10 Tween 80, Dimethyl Sulfoxide (DMSO), 0.9% physiological saline solution following pilot work from our lab (unpublished data) which confirmed this concentration allowed passage of high concentrations of this hydrophobic drug through the fine gauged microinjectors. For systemic studies, WIN and vehicle doses were injected in a volume of 0.1 ml per 10 g of body weight. For microinjection studies, WIN and vehicle doses were created as a function of WIN concentration such that the same volume was injected per mouse with varying concentrations of drug per brain hemisphere.
Blood Collection and Analysis
Immediately after 2 hour ethanol access on the 7th day, 50µl retro-orbital sinus blood samples were collected for determination of Blood Ethanol Concentration (BEC). Blood plasma was analyzed using an Analox Alcohol Analyzer (Lunenburg, MA) and recorded in mg/dl (mg %).
Experiment 1: Modulation of DID via Systemic WIN55–212,2
Experiment 1 Methods
DID Procedure
The procedural timeline for experiment 1 can be seen in Table 1. On days 1 through 6 all animals were given once daily access to ethanol or tap water according to a slightly modified version of the ‘Drinking in the Dark’ (DID) model (Moore et al., 2007). Animals were assigned to groups receiving either 20% unsweetened ethanol solution or plain tap water (control solution) in a ball-bearing sipper tube for 2 hours, 3 hours into the dark cycle each day. On day 7 all animals were weighed to calculate dosing, and then injected i.p. with one of 4 doses of WIN (0, 0.5, 1, 2 mg/kg). Twenty minutes after WIN injections (3 hours into the dark cycle) animals were given access to their assigned solutions and fluid volume was recorded in 30-minute intervals.
Table 1. Experiment 1 DID Procedure.
Arrows indicate the order in which procedures were performed (top-to-bottom). Animals were given DID access to ethanol or water for 6 habituation days. On day 7 systemic WIN treatments were administered 20 minutes prior to DID access and were immediately followed by blood collection. Animals were given re-habituation to DID access on days 8–13. On day 14 systemic WIN treatments were administered 20 minutes prior to DID access and were immediately followed by blood collection.
| Days 1–6 | Day 7 | Days 8–13 | Day 14 |
|---|---|---|---|
| WIN Treatment | WIN Treatment | ||
| DID Access | DID Access | DID Access | DID Access |
| Blood Samples * | Blood Samples * | ||
Only ethanol assigned animals had blood samples taken on these days.
Home Cage Locomotor Activity
A separate cohort of animals were given access to ethanol using DID procedures and monitored to evaluate the influence of WIN on home cage locomotion. Locomotor activity was detected by a VersaMax Animal Activity Monitoring System (Accuscan Instruments Inc., Columbus, OH) through the interruption of intersecting photocell beams evenly spaced along the walls of the 40×40 cm test chamber. These chambers were situated in sound-attenuating chambers (inside dimensions, 53 cm across × 58 cm deep × 43 cm high) equipped with a fan for ventilation and background noise. Beam break data were then translated into distance traveled in cm by the VersaMax computer program. To avoid interfering with locomotor activity, and because any accidental movement of the home cages could have interfered with photocell beam placement, ethanol sipper tube volumes were only recorded immediately before access and immediately after removal. One hour prior to ethanol access (2 hours into the dark cycle) the home cages of singly housed animals were placed into the activity monitoring chambers. One hour later (3 hours into the dark cycle) all animals received sipper tubes containing 20% ethanol solutions as previously described. After two hours of ethanol access sipper tubes were removed and locomotion was monitored for an additional hour. In sum, animals received 4 hours of locomotor monitoring on every test day with concomitant DID procedures occurring during the 2nd and 3rd hours. This was performed for 6 consecutive habituation days. On the 7th, 8th, and 9th days each animal received one of 3 doses of WIN (0, 0.5, or 2.0 mg/kg) 20 minutes before ethanol access in a counterbalanced order. No blood samples were taken from these animals to avoid interfering with behavior on subsequent days.
Statistical Analysis
Solution intake on habituation days was analyzed using a full repeated measures analysis of variance (ANOVA) with time bins (hours) and days as within subject’s factors. The time-course of consumption on drug challenge days was analyzed by two way ANOVAs with drug dose as the between groups factor and bin (30 minutes) as the within subjects factor. Total 2 hour consumption and BECs were analyzed by one-way ANOVA.
As there was no indication that dose order affected locomotion, these data were collapsed on day and analyzed by one-way ANOVA. Dunnet post-hoc tests were run when appropriate to compare groups receiving WIN injections to the vehicle injected control group.
Experiment 1 Results
Habituation
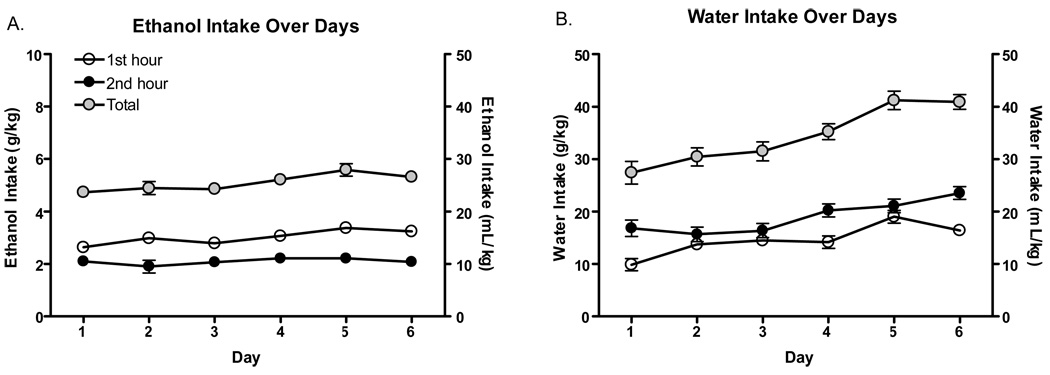
Ethanol and water consumption on habituation days 1–6 can be seen in Figure 1. Analysis of ethanol-consuming animals across all 6 days (with hour bins and days as within subject’s factors) revealed significant main effects of hour time bin [F(1, 44)=31.4 p<.001] and day [F(5, 220)=3.22 p<.01]. Analysis of water-consuming animals revealed significant main effects of hour time bin [F(1, 44)=39.87 p<.001] day [F(1, 220)=16.89 p<.001] and a significant bin*day interaction [F(5, 220)=2.35 p<.05]. However, whereas ethanol intake remained generally stable over days, water-consuming animals progressively drank more fluid on each subsequent day. Additionally, ethanol-consuming animals consumed significantly more fluid during the first half of the testing session (hour 1) compared to the water-consuming animals which drank more during the last half of the session (hour 2). Importantly, there were no significant differences in mean ethanol or water intake between dosing groups on habituation days prior to drug challenge.
Figure 1. Ethanol and water consumption over days (N=45 per group).
A. Mean ethanol intake (g/kg and mL/kg) by 1 hour bin and 2 hour total. B. Mean water intakes (g/kg and mL/kg) by 1 hour bin and 2 hour total.
Day 7 Challenge
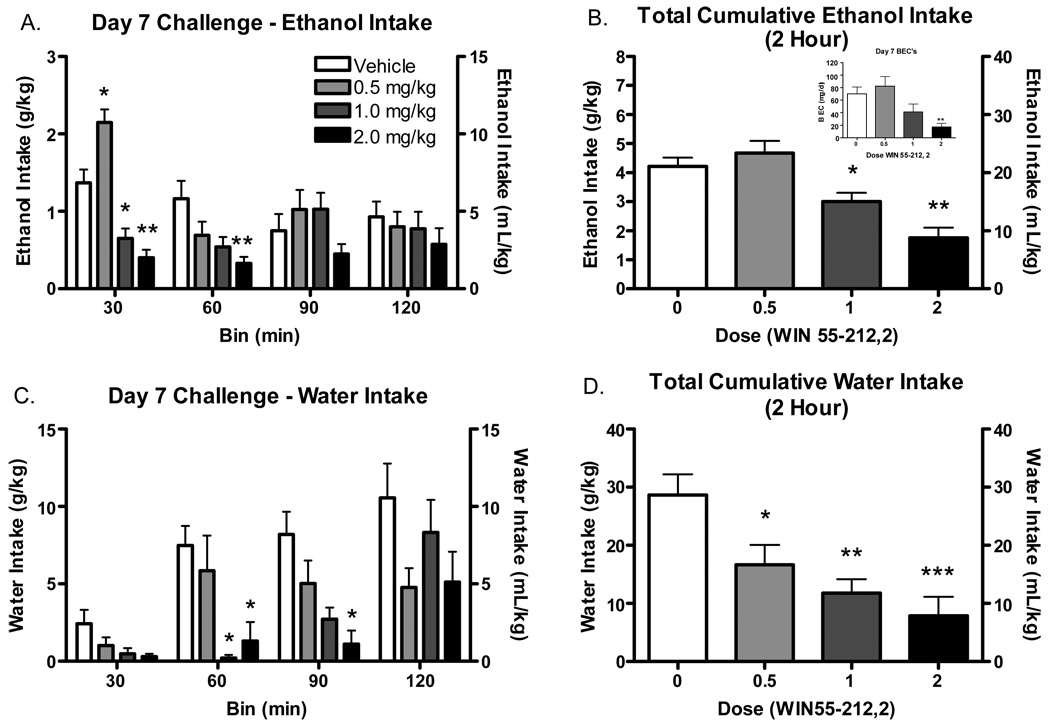
Results of 2 hour ethanol consumption in 30 minute time bins following drug challenge on day 7 can be seen in Figure 2A. Analysis revealed significant main effects of dose [F(3, 41)=13.56 p<.001], bin [F(3, 41)=4.74 p<.01] and a significant dose*bin interaction [F(9, 123)=3.95 p<.001]. The lowest dose (0.5 mg/kg) of WIN elicited a significant increase in ethanol consumption (p<.05) in the first 30 minutes of access whereas the two highest doses (1 and 2 mg/kg) elicited significant decreases in consumption (p<.05) in this time period compared to vehicle injected controls. The decrease in consumption seen in the 2 mg/kg dose group remained significantly different than vehicle injected controls at the 1 hour bin (p<.05).
Figure 2. Day 7 ethanol and water intake over 2 hour access period following WIN55-212,2 Challenge (N=10 per group).
A. Day 7 ethanol intake over 30 min bins. B. 2 hour total ethanol intake on Day 7. Inset reflects mean BECs following 2 hour ethanol intake C. Day 7 water intake over 30 min bins. D. 2 hour total water intake on Day 7. P<.05*, P<.01**, P<.001***.
Results of 2 hour ethanol consumption collapsed over time on day 7 can be seen in Figure 2B. Analysis indicated a significant main effect of dose [F(3, 41)=13.56 p<.001] which post-hoc tests confirmed was due to significant decreases in consumption in the 1 (p<.05) and 2 (p<.001) mg/kg doses compared to vehicle injected controls. Importantly, g/kg ethanol intakes on day 7 were significantly correlated with BECs [R2 = 0.71 (p<.001)] and ranged from 2.9 (effectively zero) to 171 mg/dl.
Results of 2 hour water consumption in 30 minute time bins can be seen in Figure 2C. Analysis revealed significant main effects of dose [F(3, 41)=7.77 p<.001], bin [F(3, 41)=15.04 p<.001] and a significant dose*bin interaction [F(9, 123)=1.96 p<.05]. Post-hoc tests revealed a significant decrease in water consumption at the 60 minute bin for the 1 and 2 mg/kg doses (p<.05) and a continued significant decrease in water consumption at the 90 minute bin for the 2 mg/kg dose (p<.05).
Results of 2 hour water consumption collapsed over time on day 7 can be seen in Figure 2D. Analysis indicated a significant main effect of dose [F(3, 41)=7.77 p<.001] which post-hoc tests confirmed was due to significant decreases in consumption in the 0.5 (p<.05), 1 (p<.01), and 2 mg/kg (p<.001), doses compared to vehicle injected controls.
Home Cage Locomotor Activity
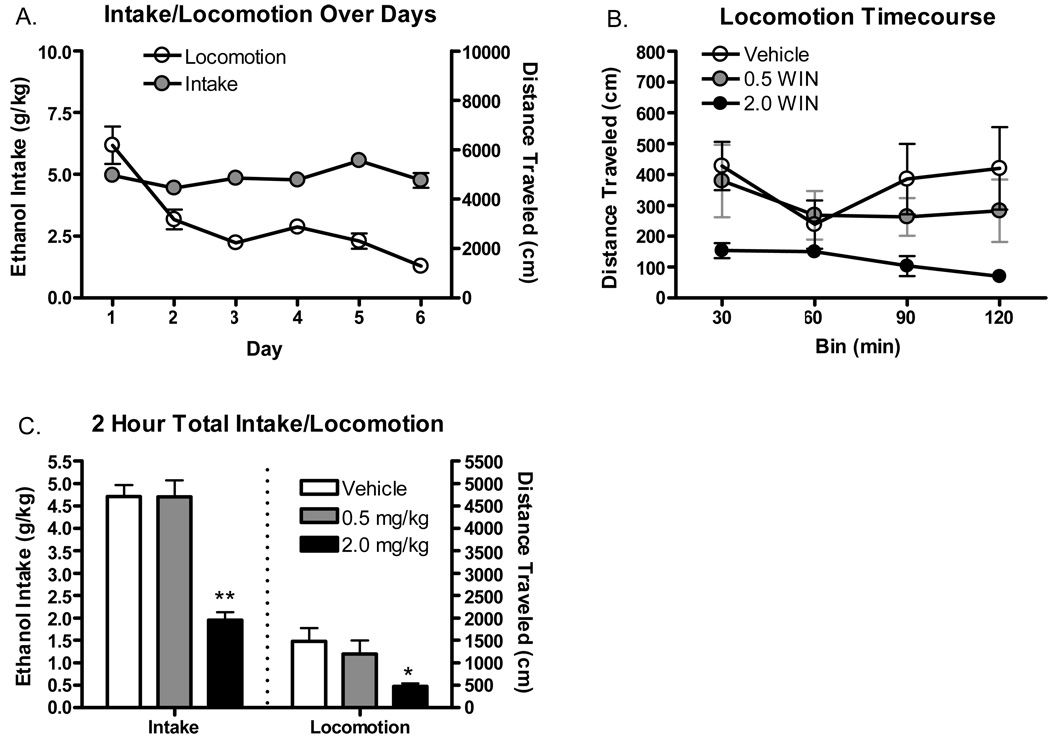
Results of habituation days 1–6 of home cage locomotor monitoring with concomitant DID ethanol access can be seen in Figure 3A. The mean ethanol intake over habituation was 4.9 ± 0.15 g/kg and the mean activity was 3,004 ± 0.69 cm traveled during the 2 hour ethanol access session.
Figure 3. Influence of systemic WIN55-212,2 on home cage locomotion.
A. Total distance traveled (cm) and intake (g/kg) during 2 hour ethanol access on habituation days 1–6. B. Time-course of home cage locomotion in 30 minute bins as a function of dose. C. Total cumulative Intake (g/kg) and distance traveled (cm) as a function of WIN dose. P<.05*, P<.01**, P<.001***.
The time-course of locomotion as a function of WIN dose and concomitant DID ethanol intake can be seen in figure 3B. Analysis revealed a significant main effect of dose [F(2, 135)=4.17 p<.05]. The total ethanol consumed as well as total cumulative distance traveled during the 2 hour sessions can be seen in figure 3C. There was a significant main effect of dose for both ethanol consumption [F(2, 45)=32.6 p<.001] and locomotion [F(2, 45)=4.17 p<.05]. Post-hoc tests confirmed that these effects were due to significant decreases in both ethanol intake (p<.001) and locomotion (p<.05) compared to vehicle injected controls following the highest (2 mg/kg) dose.
Experiment 2: Modulation of DID via Intra-VTA WIN55–212,2
Experiment 2 Methods
Surgery
Animals were surgically implanted with bilateral guide cannulae aimed at the pVTA using a Kopf Model 1900 Stereotaxic Alignment System (Tujunga, CA) equipped with a 1 micron resolution micro manipulator see (Boehm et al., 2002; Moore & Boehm, 2009) for specific procedural details. Briefly, animals were anesthetized using an i.p. injection of a ketamine/xylazine cocktail (0.1ml/10g body weight), implanted bilaterally with stainless steel guide cannulae (15.5 mm long; 25 gauge) using previously published stereotaxic coordinates (from Bregma: lateral 0.5 mm, caudal 3.64 mm, and ventral 2.0 mm), given a subcutaneous injection of 5mg/kg Rimadyl and allowed to recover in a heated bedding lined cage. After recovery from anesthesia animals were singly housed for the remainder of the experiment in large bedding lined rat cages (24 × 47.5 × 20.5 cm) to avoid interference of the cage top with the implanted cannulae and stylets.
Anatomical control groups with cannulae aimed at the anterior VTA (aVTA) were included in a follow-up DID study to determine site-specificity of drug action. Surgical procedures for these animals were identical to those for pVTA studies with the exception of the location of the implanted guide cannulae in the caudal axis (from Bregma: 3.16 mm).
Microinjection and DID
The procedural timeline for experiment 2 can be seen in Table 3. Twenty-four hrs after recovery from surgery (or post-surgery day 2) stylets were removed from guide cannulae and immediately replaced to ensure that they remained patent. This manipulation occurred during the ‘ON’ phase of the light/dark cycle. Daily DID procedures were initiated starting 48 hrs after surgery (post-surgery day 3); water bottles were replaced with sipper tubes containing 20% unsweetened ethanol solution and fluid volumes were recorded immediately before and just after ethanol presentation each day. However, immediately prior to ethanol access on post-surgery days 3–6, stylets were removed and animals were restrained for 60s (days 3 and 4) and 90s (days 5 and 6). The goal of this restraint was to begin habituating the animals to the microinjection procedure. On post-surgery days 7 and 8, animals received “mock” microinjections and 90s of restraint prior to ethanol presentation. These mock microinjections served to further acclimate the animals to the challenge day microinjection procedures (day 7); microinjectors were inserted, the tips of which only went 1mm past the tip of the guide cannulae. No fluid was infused during mock microinjection procedures. On post-surgery day 7 animals were microinjected with one of three doses of WIN (0.25, 0.5, or 1 µg/side) or vehicle and immediately given access to ethanol solution. Microinjections were given in volumes of 200nL/side/mouse, so fluid volume was kept constant and concentration of WIN in the solution was manipulated. Based on previously mentioned microinjection work from our lab (Boehm et al., 2002; Moore & Boehm, 2009) and data confirming that the highly lipophilic nature of cannabinoids dramatically limits the distance they travel in extracellular fluid (Kreitzer et al., 2002), we were confident that drug action was limited to the regions of interest. On days 6 and 7 fluid volumes were recorded in 30-minute intervals to determine drug/intake related temporal relationships. Immediately following 2 hour ethanol access on day 7 animals were sampled for determination of BECs. Anatomical (aVTA) control groups were treated identically to the pVTA groups with the exception of the intermediate (0.5 µg/side) dose group which was not included.
Table 3. Experiment 2 Microinjection/DID Procedure.
Arrows indicate the order in which procedures were performed (top-to-bottom). Animals were bilaterally implanted with guide cannulae aimed at the pVTA. The following day stylets were removed and replaced to assure they remained patent. On days 3–4 stylets were again manipulated, animals were restrained for 60s, and were then immediately given access to ethanol using DID procedures. On days 5–6 animals underwent the same procedure except restraint was for 90s prior to DID access. On days 7–8 animals had mock microinjections and 90s restraint prior to DID. The final day (9) animals were microinjected with WIN or vehicle and given access to DID.
| Days 1 | Day 2 | Days 3–4 | Days 5–6 | Days 7–8 | Day 9 |
|---|---|---|---|---|---|
| Surgery | Stylet Removal | Stylet Removal | Stylet Removal | Stylet Removal | Stylet Removal |
| (Light) | (3hrs into Dark) | (3hrs into Dark) | (3hrs into Dark) | (3hrs into Dark) | |
| Mock Inj. | WIN Inj. | ||||
| 60s Restraint | 90s Restraint | 90s Restraint | 90s Restraint | ||
| 2hr DID | 2hr DID | 2hr DID | 2hr DID | ||
Microinjection and Locomotor Activity
To determine if the results of pVTA microinjections on ethanol intake could have been influenced by locomotor activation, separate groups of animals were tested in the previously described locomotor monitoring apparatus 3 hours into the dark cycle. However, these animals were not tested in their home (rat) cages as these cages were too large to fit into our locomotor monitoring apparatus. Animals were instead placed directly into Plexiglas chambers as is common for assessment of locomotor activity in our lab. Following surgery animals were allowed to rest for 4 days during which stylets were removed daily to keep patent. On the 6th day animals were restrained for 90 seconds (1 ½ minutes) and then immediately placed directly into the locomotor monitors for 2 hours. The 7th and 8th days served to acclimate the animals to the microinjector procedure. Animals were treated identically to day 6 except mock microinjectors were dropped 1mm past the tips of the guide cannulae into the brain to acclimate the animals to the microinjection procedure as previously described. On the 9th day animals were given microinjections of one of 2 doses of WIN (0.25 or 2.5 µg/side) or vehicle after which locomotion was immediately assessed for two hours.
Histology
To verify placement of microinjectors animals were sacrificed by cervical dislocation, brains were rapidly removed and snap frozen in sub-zero degree C methylbutane, and then stored in −80 °C for later sectioning. Frozen brains were sectioned in 40 µm slices on a Microm HM505E cryostat (Walldorf, Germany), thaw mounted onto glass slides and allowed to dry for 24 hours prior to thionin staining. Stained slides were cover slipped and microinjector tracts/tips were examined under a microscope to determine placement.
Statistical Analysis
Ethanol intake on habituation days (1–6) were analyzed using a repeated measures analysis of variance (ANOVA) with dose as the between subjects factor and day as the within subject’s factor. The time-course of consumption and locomotor activity on drug challenge days was analyzed by two way ANOVAs with drug dose as the between groups factor and bin (30 or 5 minutes, respectively) as the within subjects factor. Total 2 hour consumption, BECs, and locomotor activity were analyzed by one-way ANOVA. Dunnet post-hoc tests were run when appropriate to compare groups receiving WIN injections to the vehicle injected control group.
Experiment 2 Results
Attrition
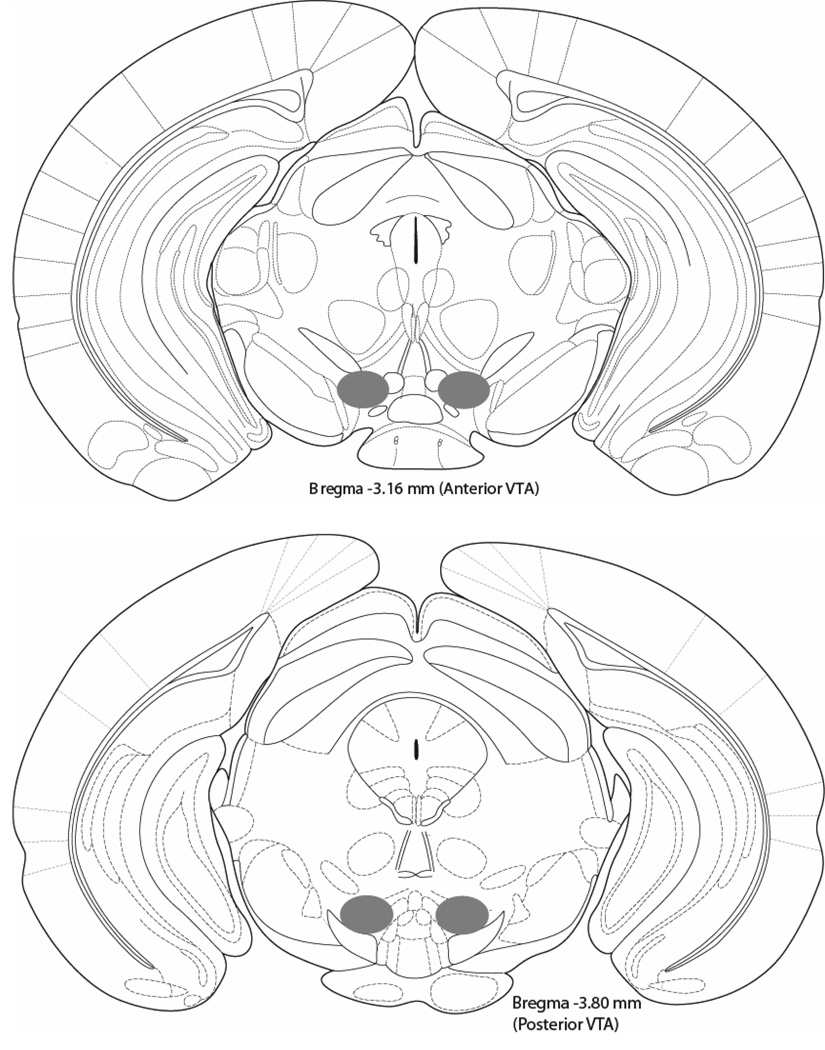
Some animals had to be eliminated due to off-target microinjections (‘misses’) or because of other procedural complications. A group of animals had to be sacrificed after skull caps had fallen off (N=4) or because they failed to display consistent ethanol intakes on the first two days of the procedure (N=5). The two day time interval was chosen based on previous pilot work which indicated that animals that failed to consume appreciable amounts (>1 g/kg in two hours) of ethanol on these two days would never do so given additional days of access (unpublished observation). One additional animal had to be eliminated due to microinfusion pump malfunction. Because pVTA misses were either completely off target or only one of the two microinjectors were successfully placed, none of these groups could be used as position controls. However, as previously mentioned, follow-up aVTA injected animals served as anatomical control groups for the DID intake study. Figure 4 illustrates successful pVTA and aVTA microinjector placements based on (Franklin and Paxinos, 1997). In total, out of the 97 animals that had successful intra-VTA microinjections, 12 had to be eliminated due to off target microinjector tip placements (hit ratio = 82.5%).
Figure 4. Injection site locations within the anterior (top) and posterior (bottom) portions of the Ventral Tegmental Area (VTA).
Drawing is an adaptation from the (Franklin and Paxinos, 1997) Mouse Brain Atlas. Only animals that had accurate bilateral microinjector placements were included in statistical analysis.
Habituation
Mean ethanol intakes during habituation days 1–6 for pVTA injected animals were as follows: Day 1, 1.95 ± 0.2 g/kg; Day 2, 2.46 ± .18 g/kg; Day 3, 2.35 ± .22 g/kg; Day 4, 2.78 ± .22 g/kg; Day 5. 2.06 ± .18 g/kg; Day 6, 2.61 ± .18 g/kg. Mean ethanol intake during habituation days 1–6 for anatomical control groups (aVTA) were as follows: Day 1, 3.35 ± 0.3 g/kg; Day 2, 3.33 ± .26 g/kg; Day 3, 3.93 ± .20 g/kg; Day 4, 4.27 ± .23 g/kg; Day 5. 2.66 ± .27 g/kg; Day 6, 3.20 ± .22 g/kg. Statistical analysis indicated a significant main effect of day for both pVTA [F(5, 165)=4.1 p<.01] and anatomical (aVTA) control groups [F(5, 110)=6.4 p<.001]. Because there were no significant differences in intake as a function of dose group (p>.05) across the 6 habituation days, the previously reported means reflect values collapsed on this factor. In this way no differences in drug response should be due to group differences in previous ‘basal’ intakes or relative ethanol exposure.
Day 7 Challenge
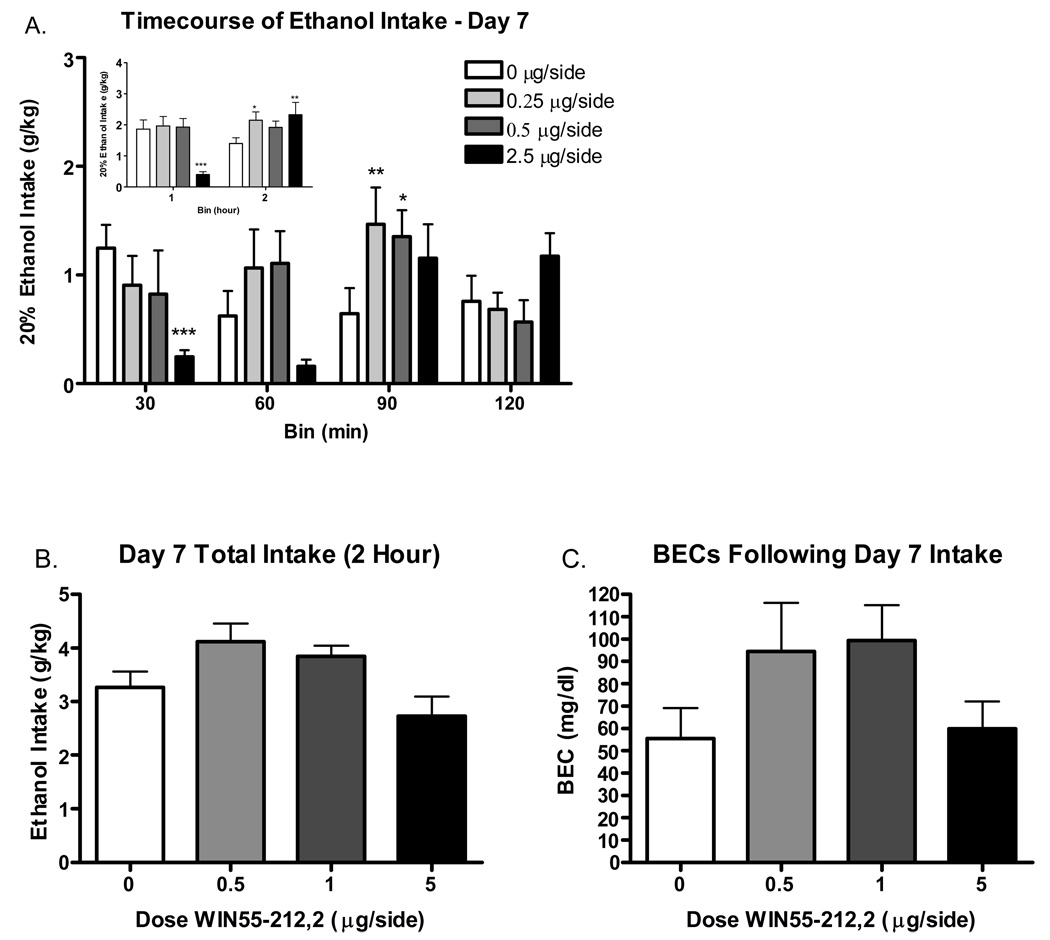
The results of day 7 pVTA WIN microinjections can be seen in Figure 5. Analysis revealed a significant main effect of dose [F(3, 96)=4.1 p<.05] as well as a significant dose*bin interaction [F(9, 96)=2.0 p<.05]. Post-hoc tests confirmed decreased consumption in the highest dose group (2.5 µg/side) at the 30 minute bin (p<.001) compared to vehicle controls in addition to significant increases in intake at the two lowest doses (0.25 and 0.5 µg/side) at the 90 minute bin (p<.05) compared to controls. As there were no significant differences between the 30 and 60 minute bins or the 90 and 120 minute bins, data were collapsed on this factor for graphical presentation (see inset). When data were separated by hour as mentioned, statistics revealed a significant main effect of dose [F(3, 32)=4.08 p<.05] and a significant dose*bin interaction [F(3, 32)=5.82 p<.01]. Post-hoc tests confirmed that these results were due to decreases in intake at the highest dose (2.5 µg/side) in the first hour and significant increases in intake in the second hour at this same dose as well as the lowest dose (0.25 µg/side).
Figure 5. Ethanol intake following bilateral microinjections of WIN55-212,2 into the pVTA (N=9 per group).
A. Data reflect g/kg intak in 30 min bins. Inset reflects timecourse of consumption seperated into hour bins. B. Total (2 hour) ethanol intake following microinjections. C. BECs following ethanol intake. P<.05*, P<.01**, P<.001***.
Analysis of 2 hour intake revealed a significant main effects of dose [F(3, 32)=4.08 p<.05]. However, there were no significant differences between the vehicle injected controls or any of the three doses. There were also no significant differences (p=.14) in BECs immediately following 2 hour intake. Ethanol intakes (g/kg) were significantly correlated with BEC on day 7 [R2 = 0.59 (p<.001)] and ranged from 3.4 (effectively zero) to 179 mg/dl
There were no significant differences in the timecourse or total 2 hour ethanol intakes between any of the 3 anatomical control groups (p>.05). Mean day 7 ethanol intakes over the 4, 30 minute time bins for vehicle (N=10), 0.25 (N=7), and 2.5 (N=8) µg/side dose groups was as follows: 0.82 ± .21 g/kg, 1.1 ± .43 g/kg, and 0.32 ± .13 g/kg (30 min bin); 0.99 ± .34 g/kg, 0.46 ± .19 g/kg, and 0.82 ± .21 g/kg (60 min bin); 0.58 ± .17 g/kg, 0.27 ± .11 g/kg, and 0.84 ± .33 g/kg (90 min bin); 1.35 ± .29 g/kg, 0.56 ± .31 g/kg, and 0.9 ± .32 g/kg (120 min bin). Mean day 7 total 2 hour ethanol intake for the vehicle, 0.25, and 2.5µg/side dose groups was 3.7 ± .37 g/kg, 2.4 ± .47 g/kg, and 2.9 ± .57 g/kg respectively.
Locomotor Activity
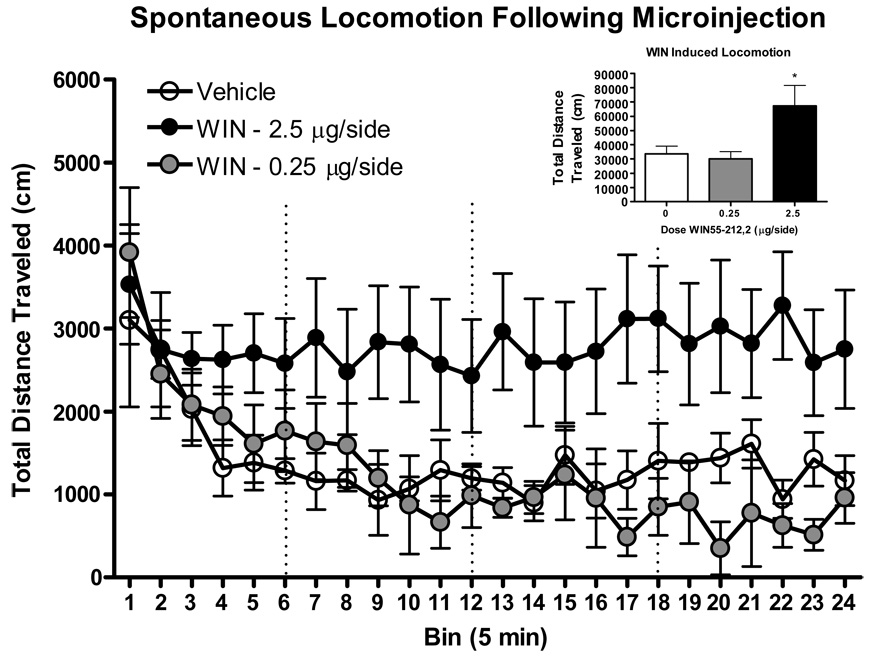
The results of WIN microinjections on locomotor activity can be seen in Figure 6. Analysis revealed a significant main effect of bin [F(23, 345)=1.4 p<.001] as well as a significant dose*bin interaction [F(46, 345)=3.9 p<.05]. However, post-hoc tests did not reveal any significant 5 minute bin differences compared to vehicle injected controls.
Figure 6. Timecourse of locomotor activity over 2 hours following bilateral microinjections of WIN55-212,2 into the pVTA (N=8 per group).
Data reflect total distance traveled in cm. Inset reflects mean distance traveled during 2 hour session. P<.05*.
Analysis of 2 hour total distance traveled indicated a significant main effect of dose [F(2, 25)=4.08 p<.05]. Post-hoc tests confirmed that these results were due to increased locomotion at the highest dose (2.5 µg/side) compared to vehicle injected controls (p<.05).
Discussion
The present data illustrate that the pattern of binge-like DID ethanol intake is modulated in ethanol-preferring C57BL/6J mice using the synthetic cannabinoid agonist WIN55–212,2. Our finding that the lowest systemic dose of WIN enhanced binge-like intake at the 30 minute time bin is consistent with literature showing that CB1 receptor agonists increase ethanol consumption in ethanol-preferring animals (Colombo et al., 2002). Our findings that higher systemic doses of WIN decrease locomotion is consistent with literature suggesting induction of catalepsy and associated hypolocomotion at higher doses of this compound (Carriero et al., 1998; Cosenza et al., 2000; Gifford et al., 1999).
Our intra-pVTA microinjection data indicating alterations in the time-course of binge-like DID intake is consistent with the notion that endogenous cannabinoid system within this area is capable of modulating ethanol-seeking behavior (Malinen & Hyytia, 2008; Parsons et al., RSA Abstract 2008). Moreover, increases in locomotor activity at high doses of WIN within the pVTA are consistent with recent literature implicating this region in the psychomotor effects induced by CB1 receptor activation via Δ9-THC (Zangen et al., 2006).
Experiment 1 – Systemic WIN
Although the systemic data are in line with recent research implicating the endocannabinoid system in the modulation of ethanol intake and ethanol-seeking behaviors, there are several details worth noting. First, although water may effectively control for general fluid-seeking behavior (thirst), it is likely that the animals were more motivated to drink the ethanol solution than the plain tap water as evidenced by greater 20% ethanol intakes in the first hour of the DID session (see figure 1). Using a more palatable solution such as sucrose or saccharin may aid in evaluating drug effects on motivation to consume more ‘salient’ reinforcers. However, these types of solutions do not lead to similar post-absorptive effects (such as intoxication), nor do they necessarily equate on a volume or concentration basis. Indeed, data from our lab (Moore & Boehm, 2009) and others (Kamdar et al., 2007; Sparta et al., 2008) have consistently shown much larger volume and g/kg sugar water intakes than 20% ethanol intakes using the DID model. More work is needed to find a qualitatively similar non-alcoholic palatable reinforcer to better address the specificity of drug actions on ethanol reinforcement.
Additionally, whereas the lowest dose of WIN increased consumption within the first 30 minute time interval, there were no significant differences in overall intake compared to vehicle injected controls following 2 hours of access in this dosing group. As B6 mice voluntarily consume large quantities of alcohol using these procedures, it is possible that a limit was reached whereby further increases in ethanol intake were not possible (ceiling effect). However, the observation that the lowest dose of WIN increased ethanol consumption during the first 30 minutes of the 2 hours session (a time when vehicle injected animals display the majority of their intake) argues against such an effect. An alternative explanation might relate to the pharmacokinetics of the low (0.5 mg/kg) dose of WIN. That is, the lack of group differences at 2 hours may be due to dissipation of drug over time. Injection of WIN closer in time to the beginning of the ethanol access period may be one way to assess this possibility. Nevertheless, the marked increase in low-dose WIN-induced ethanol consumption earlier in the session is consistent with data implicating the endocannabinoid system in enhanced ethanol-seeking behavior in rodents.
Finally, although animals that received the highest 2 doses of WIN displayed significantly decreased intake early in the DID session, follow-up home cage locomotor monitoring data with concomitant DID ethanol access confirm that these higher doses (at least the 2mg/kg dose) significantly decrease locomotion; a behavioral effect which likely competes with voluntary ethanol consumption. Indeed, the observed decrease in water intake at these higher doses also provides support for non-selective (motor) drug effects. These findings are in line with other research using this compound (Cosenza et al., 2000; Gifford et al., 1999) and emphasize the importance of evaluating possible competing locomotor behaviors when using models of voluntary consumption such as those described here.
Experiment 2 –Intra-VTA WIN
The results of the intra-pVTA microinjection studies were in line with our systemic manipulations. Based on recent literature implicating the endocannabinoid system within sub regions of the VTA in ethanol-seeking behaviors (see introduction), we hypothesized that microinjection of WIN into the pVTA would dose-dependently alter ethanol consumption. However, as with the systemic manipulations, there were no significant differences in overall intake or BECs following 2 hours of access at any of the doses used. On the other hand, microinjections of WIN changed the timecourse of consumption such that there were significant increases only later in the 2 hour session. This was evident only at the two lowest doses (0.25 and 0.5 µg/side) and only at the 90 minute bin. Significant decreases in consumption occurred at the highest dose of WIN (2.5 µg/side) at the 30 minute bin and/or first hour of the session. However, as previously mentioned, neither of these effects remained for the duration of the session. We believe that whereas the lack of significant increases in overall (total 2 hour) consumption may be reflective of a ceiling effect, the decreases may reflect competing behavioral activation as a direct result of intra-VTA WIN, or its interactions with the ethanol consumed.
Our decision to directly evaluate the extent to which competing locomotor behaviors may have contributed to our DID results following intra-pVTA microinjections stemmed from 1) our initial observations of prolonged stimulant response following the highest dose, and 2) recent data implicating the pVTA in activational effects elicited by the exogenous cannabinoid agonist Δ9-THC (Zangen et al., 2006). Consistent with this notion, the highest dose of WIN significantly increased locomotor activity for the duration of the 2 hour session. This was not the case for the lowest dose group which did not significantly differ from vehicle injected controls.
Inspection of the intra-pVTA DID and locomotor datasets led to some surprising conclusions. The lowest dose of WIN, while not significantly altering locomotor activity, increased ethanol intake both in the 90 minute bin and the second half of the session when broken down by 1 hour bins. On the other hand, WIN-induced increases in locomotor activity at the highest dose would have been expected to decrease ethanol intake. However, the data did not necessarily support this view. For example, the significant decreases in drinking at the highest WIN dose observed at the 30 minute bin occurred despite little to no obvious locomotor differences during this time period. Furthermore, if increased locomotor activity were predictive of the reductions in intake than a greater drinking impairment should have emerged later in the drinking session when locomotor behavior was clearly divergent (see figure 6). Therefore, it appears as if animals that received the highest dose of WIN were reliably consuming ethanol despite seemingly prolonged competitive behavioral activation. One caveat to this interpretation is that the locomotor activating effects experienced in the home cage may be qualitatively different than those in a relatively novel Plexiglas chamber. Additionally, since animals were consuming ethanol during the DID intake study, there may have been effects of ethanol alone or in combination with WIN (particularly within the pVTA) on locomotor activity. In support of this possibility, recent evidence suggests that the CB1 receptor system is capable of influencing the sedative effects of ethanol (Vinod et al., 2008b).
Conclusion
The present systemic and intra-VTA data support the notion that agonism of the CB1 receptor system with the cannabinoid agonist WIN 55–212,2 alters voluntary binge-like ethanol consumption in rodents. Furthermore, these data add to the growing literature suggesting the VTA, via the endocannabinoid system, is a structure mediating ethanol reinforcement and ethanol-seeking behaviors (Malinen & Hyytia, 2008; Parsons et al., RSA Abstract 2008).
Although the exact mechanisms by which the VTA (pVTA specifically) may contribute to ethanol-seeking behaviors is not fully understood, its unique interactions with various components of the mesolimbic dopamine pathway are of much interest. Both ethanol (Howard et al., 2008; Hungund et al., 2003; Weiss et al., 1993) and cannabinoids (Chen et al., 1991; Szabo et al., 1999) cause increases in DA within the NAc following peripheral administration. Also, as might be expected, both ethanol (Brodie et al., 1999; Brodie et al., 1990; Gessa et al., 1985) and cannabinoids (Diana et al., 1998; French, 1997; Gessa et al., 1998) facilitate DA neuron firing within the VTA. However, because CB1 receptors are not located on the VTA DA neurons themselves (Herkenham et al., 1991a; Herkenham et al., 1991b), there must be some other mechanism by which this occurs. Indeed, CB1 receptors are believed to be localized to the axon terminals of GABAergic interneurons within the VTA (Lupica et al., 2004) and upon stimulation release the tonic GABAergic inhibitory control regulating DA release in the NAc. Given these data, the direct facilitation of pVTA CB1 receptors in the present study likely leads to dose dependent alterations in NAc DA efflux. Certainly this mechanism would explain 1) the strong and prolonged stimulant response elicited by the highest WIN dose, and 2) the alterations in ethanol intake following perturbation of this mesolimbic network.
In summary, the results of this work suggest that the endogenous cannabinoid receptor system is involved in binge-like ethanol intake in B6 mice and that the pVTA (but not aVTA) may participate in the modulation of mesolimbic circuitry involved in such behavior. However, further work using selective antagonists and/or endogenous cannabinoid degraders in combination with in vivo microdialysis within the mesolimbic system are needed to better understand the exact role the cannabinoid system has on binge-like ethanol intake.
Table 2. Experiment 1 Home Cage Locomotion Procedure.
Arrows indicate the order in which procedures were performed (top-to-bottom). Animals were put into activity monitors and then given DID access to ethanol for 6 habituation days. On days 7–9 systemic WIN treatments were administered 20 minutes prior to DID access.
| Days 1–6 | Day 7 | Day 8 | Day 9 |
|---|---|---|---|
| Activity Monitor | Activity Monitor | Activity Monitor | Activity Monitor |
| WIN treatment 1 | WIN treatment 2 | WIN treatment 3 | |
| DID | DID | DID | DID |
Table 4. Experiment 2 Microinjection/Locomotor activity Procedure.
Arrows indicate the order in which procedures were performed (top-to-bottom). Animals were bilaterally implanted with guide cannulae aimed at the pVTA and allowed to rest for 5 days during which stylets were removed and replaced daily. On day 6 stylets were removed animals were restrained for 90s and were immediately placed in the activity monitors for 2 hours. On days 7–8 animals were given mock microinjections and immediately placed in the activity monitors for 2 hours. On the 9th day animals were given microinjections of WIN or vehicle after which they were monitored for 2 hours.
| Days 1 | Day 2–5 | Day 6 | Days 7–8 | Days 9 |
|---|---|---|---|---|
| Surgery | Stylet Removal | Stylet Removal | Stylet Removal | Stylet Removal |
| (Light) | (3hrs into Dark) | (3hrs into Dark) | (3hrs into Dark) | |
| 90s Restraint | Mock Inj. (90s) | WIN Inj. (90s) | ||
| Activity Monitor | Activity Monitor | Activity Monitor | ||
Acknowledgments
This work was supported by grants from Integrative Neuroscience Initiative on Alcoholism (INIA) - West and NIAAA # AA015434
Abbreviations
- WIN
WIN55-212, 2
- VTA
Ventral Tegmental Area
- pVTA
Posterior Ventral Tegmental Area
- aVTA
Anterior Ventral Tegmental Area
- DID
Drinking In the Dark
- Δ9-THC
delta9 Tetrahydrocannabinol
- DMSO
Dimethyl Sulfoxide
- 2-AG
2-Arachidonoylglycerol
- AEA
Anandamide
- CB1
Cannabinoid 1 Receptor
- BEC
Blood Ethanol Concentration
- CP
CP55-940
- FAAH
Fatty Acid Amide Hydrolase
- SR
SR141716
- B6
C57BL/6J
- NIAAA
National Institute on Alcohol and Alcoholism
- NAc
Nucleus Accumbens
- sP
Sardinian ethanol-preferring rats
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Blednov YA, Cravatt BF, Boehm SL, 2nd, Walker D, Harris RA. Role of endocannabinoids in alcohol consumption and intoxication: Studies of mice lacking fatty acid amide hydrolase. Neuropsychopharmacology. 2007;32(7):1570–1582. doi: 10.1038/sj.npp.1301274. [DOI] [PubMed] [Google Scholar]
- Boehm SL, 2nd, Piercy MM, Bergstrom HC, Phillips TJ. Ventral tegmental area region governs gaba(b) receptor modulation of ethanol-stimulated activity in mice. Neuroscience. 2002;115(1):185–200. doi: 10.1016/s0306-4522(02)00378-0. [DOI] [PubMed] [Google Scholar]
- Brodie MS, Pesold C, Appel SB. Ethanol directly excites dopaminergic ventral tegmental area reward neurons. Alcohol Clin Exp Res. 1999;23(11):1848–1852. [PubMed] [Google Scholar]
- Brodie MS, Shefner SA, Dunwiddie TV. Ethanol increases the firing rate of dopamine neurons of the rat ventral tegmental area in vitro. Brain Res. 1990;508(1):65–69. doi: 10.1016/0006-8993(90)91118-z. [DOI] [PubMed] [Google Scholar]
- Carriero D, Aberman J, Lin SY, Hill A, Makriyannis A, Salamone JD. A detailed characterization of the effects of four cannabinoid agonists on operant lever pressing. Psychopharmacology (Berl) 1998;137(2):147–156. doi: 10.1007/s002130050604. [DOI] [PubMed] [Google Scholar]
- Chen JP, Paredes W, Lowinson JH, Gardner EL. Strain-specific facilitation of dopamine efflux by delta 9-tetrahydrocannabinol in the nucleus accumbens of rat: An in vivo microdialysis study. Neurosci Lett. 1991;129(1):136–180. doi: 10.1016/0304-3940(91)90739-g. [DOI] [PubMed] [Google Scholar]
- Cippitelli A, Bilbao A, Gorriti MA, Navarro M, Massi M, Piomelli D, et al. The anandamide transport inhibitor am404 reduces ethanol self-administration. Eur J Neurosci. 2007;26(2):476–486. doi: 10.1111/j.1460-9568.2007.05665.x. [DOI] [PubMed] [Google Scholar]
- Colombo G, Orru A, Lai P, Cabras C, Maccioni P, Rubio M, et al. The cannabinoid cb1 receptor antagonist, rimonabant, as a promising pharmacotherapy for alcohol dependence: Preclinical evidence. Mol Neurobiol. 2007;36(1):102–112. doi: 10.1007/s12035-007-0017-y. [DOI] [PubMed] [Google Scholar]
- Colombo G, Serra S, Brunetti G, Gomez R, Melis S, Vacca G, et al. Stimulation of voluntary ethanol intake by cannabinoid receptor agonists in ethanol-preferring sp rats. Psychopharmacology (Berl) 2002;159(2):181–187. doi: 10.1007/s002130100887. [DOI] [PubMed] [Google Scholar]
- Colombo G, Serra S, Vacca G, Carai MA, Gessa GL. Endocannabinoid system and alcohol addiction: Pharmacological studies. Pharmacol Biochem Behav. 2005;81(2):369–380. doi: 10.1016/j.pbb.2005.01.022. [DOI] [PubMed] [Google Scholar]
- Cosenza M, Gifford AN, Gatley SJ, Pyatt B, Liu Q, Makriyannis A, et al. Locomotor activity and occupancy of brain cannabinoid cb1 receptors by the antagonist/inverse agonist am281. Synapse. 2000;38(4):477–482. doi: 10.1002/1098-2396(20001215)38:4<477::AID-SYN13>3.0.CO;2-Y. [DOI] [PubMed] [Google Scholar]
- Diana M, Melis M, Muntoni AL, Gessa GL. Mesolimbic dopaminergic decline after cannabinoid withdrawal. Proc Natl Acad Sci U S A. 1998;95(17):10269–10273. doi: 10.1073/pnas.95.17.10269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dole VP, Gentry RT. Toward an analogue of alcoholism in mice: Scale factors in the model. Proc Natl Acad Sci U S A. 1984;81(11):3543–3546. doi: 10.1073/pnas.81.11.3543. [DOI] [PMC free article] [PubMed] [Google Scholar]
- French ED. Delta9-tetrahydrocannabinol excites rat vta dopamine neurons through activation of cannabinoid cb1 but not opioid receptors. Neurosci Lett. 1997;226(3):159–162. doi: 10.1016/s0304-3940(97)00278-4. [DOI] [PubMed] [Google Scholar]
- Gallate JE, Saharov T, Mallet PE, McGregor IS. Increased motivation for beer in rats following administration of a cannabinoid cb1 receptor agonist. Eur J Pharmacol. 1999;370(3):233–240. doi: 10.1016/s0014-2999(99)00170-3. [DOI] [PubMed] [Google Scholar]
- Gessa GL, Melis M, Muntoni AL, Diana M. Cannabinoids activate mesolimbic dopamine neurons by an action on cannabinoid cb1 receptors. Eur J Pharmacol. 1998;341(1):39–44. doi: 10.1016/s0014-2999(97)01442-8. [DOI] [PubMed] [Google Scholar]
- Gessa GL, Muntoni F, Collu M, Vargiu L, Mereu G. Low doses of ethanol activate dopaminergic neurons in the ventral tegmental area. Brain Res. 1985;348(1):201–203. doi: 10.1016/0006-8993(85)90381-6. [DOI] [PubMed] [Google Scholar]
- Gifford AN, Bruneus M, Gatley SJ, Lan R, Makriyannis A, Volkow ND. Large receptor reserve for cannabinoid actions in the central nervous system. J Pharmacol Exp Ther. 1999;288(2):478–483. [PubMed] [Google Scholar]
- The National Academic Press; 2003. Guidelines for the Care and Use of Mammals in Neuroscience and Behavioral Research. [PubMed] [Google Scholar]
- Gupta T, Syed YM, Revis AA, Miller SA, Martinez M, Cohn KA, et al. Acute effects of acamprosate and mpep on ethanol drinking-in-the-dark in male c57bl/6j mice. Alcohol Clin Exp Res. 2008 doi: 10.1111/j.1530-0277.2008.00787.x. [DOI] [PubMed] [Google Scholar]
- Herkenham M, Lynn AB, de Costa BR, Richfield EK. Neuronal localization of cannabinoid receptors in the basal ganglia of the rat. Brain Res. 1991a;547(2):267–274. doi: 10.1016/0006-8993(91)90970-7. [DOI] [PubMed] [Google Scholar]
- Herkenham M, Lynn AB, Johnson MR, Melvin LS, de Costa BR, Rice KC. Characterization and localization of cannabinoid receptors in rat brain: A quantitative in vitro autoradiographic study. J Neurosci. 1991b;11(2):563–583. doi: 10.1523/JNEUROSCI.11-02-00563.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Howard EC, Schier CJ, Wetzel JS, Duvauchelle CL, Gonzales RA. The shell of the nucleus accumbens has a higher dopamine response compared with the core after non-contingent intravenous ethanol administration. Neuroscience. 2008;154(3):1042–1053. doi: 10.1016/j.neuroscience.2008.04.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hungund BL, Szakall I, Adam A, Basavarajappa BS, Vadasz C. Cannabinoid cb1 receptor knockout mice exhibit markedly reduced voluntary alcohol consumption and lack alcohol-induced dopamine release in the nucleus accumbens. J Neurochem. 2003;84(4):698–704. doi: 10.1046/j.1471-4159.2003.01576.x. [DOI] [PubMed] [Google Scholar]
- Kamdar NK, Miller SA, Syed YM, Bhayana R, Gupta T, Rhodes JS. Acute effects of naltrexone and gbr 12909 on ethanol drinking-in-the-dark in c57bl/6j mice. Psychopharmacology (Berl) 2007 doi: 10.1007/s00213-007-0711-5. [DOI] [PubMed] [Google Scholar]
- Kreitzer AC, Carter AG, Regehr WG. Inhibition of interneuron firing extends the spread of endocannabinoid signaling in the cerebellum. Neuron. 2002;34(5):787–796. doi: 10.1016/s0896-6273(02)00695-5. [DOI] [PubMed] [Google Scholar]
- Lupica CR, Riegel AC, Hoffman AF. Marijuana and cannabinoid regulation of brain reward circuits. Br J Pharmacol. 2004;143(2):227–234. doi: 10.1038/sj.bjp.0705931. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Malinen H, Hyytia P. Ethanol self-administration is regulated by cb1 receptors in the nucleus accumbens and ventral tegmental area in alcohol-preferring aa rats. Alcohol Clin Exp Res. 2008 doi: 10.1111/j.1530-0277.2008.00786.x. [DOI] [PubMed] [Google Scholar]
- Moore EM, Boehm SL. Site-specific microinjection of baclofen into the anterior ventral tegmental area reduces binge-like ethanol intake in male c57bl/6j mice. Behav Neurosci. 2009;123(3):555–563. doi: 10.1037/a0015345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moore EM, Serio KM, Goldfarb KJ, Stepanovska S, Linsenbardt DN, Boehm SL., 2nd Gabaergic modulation of binge-like ethanol intake in c57bl/6j mice. Pharmacol Biochem Behav. 2007;88(1):105–113. doi: 10.1016/j.pbb.2007.07.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pertwee RG. Pharmacology of cannabinoid cb1 and cb2 receptors. Pharmacol Ther. 1997;74(2):129–180. doi: 10.1016/s0163-7258(97)82001-3. [DOI] [PubMed] [Google Scholar]
- Pertwee RG, Ross RA. Cannabinoid receptors and their ligands. Prostaglandins Leukot Essent Fatty Acids. 2002;66(2–3):101–121. doi: 10.1054/plef.2001.0341. [DOI] [PubMed] [Google Scholar]
- Poncelet M, Maruani J, Calassi R, Soubrie P. Overeating, alcohol and sucrose consumption decrease in cb1 receptor deleted mice. Neurosci Lett. 2003;343(3):216–218. doi: 10.1016/s0304-3940(03)00397-5. [DOI] [PubMed] [Google Scholar]
- Rhodes JS, Best K, Belknap JK, Finn DA, Crabbe JC. Evaluation of a simple model of ethanol drinking to intoxication in c57bl/6j mice. Physiol Behav. 2005;84(1):53–63. doi: 10.1016/j.physbeh.2004.10.007. [DOI] [PubMed] [Google Scholar]
- Rhodes JS, Ford MM, Yu CH, Brown LL, Finn DA, Garland T, Jr, et al. Mouse inbred strain differences in ethanol drinking to intoxication. Genes Brain Behav. 2007;6(1):1–18. doi: 10.1111/j.1601-183X.2006.00210.x. [DOI] [PubMed] [Google Scholar]
- Rodd-Henricks ZA, McKinzie DL, Crile RS, Murphy JM, McBride WJ. Regional heterogeneity for the intracranial self-administration of ethanol within the ventral tegmental area of female wistar rats. Psychopharmacology (Berl) 2000;149(3):217–224. doi: 10.1007/s002139900347. [DOI] [PubMed] [Google Scholar]
- Ryabinin AE, Yoneyama N, Tanchuck MA, Mark GP, Finn DA. Urocortin 1 microinjection into the mouse lateral septum regulates the acquisition and expression of alcohol consumption. Neuroscience. 2008;151(3):780–790. doi: 10.1016/j.neuroscience.2007.11.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sparta DR, Sparrow AM, Lowery EG, Fee JR, Knapp DJ, Thiele TE. Blockade of the corticotropin releasing factor type 1 receptor attenuates elevated ethanol drinking associated with drinking in the dark procedures. Alcohol Clin Exp Res. 2008;32(2):259–265. doi: 10.1111/j.1530-0277.2007.00575.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Szabo B, Muller T, Koch H. Effects of cannabinoids on dopamine release in the corpus striatum and the nucleus accumbens in vitro. J Neurochem. 1999;73(3):1084–1089. doi: 10.1046/j.1471-4159.1999.0731084.x. [DOI] [PubMed] [Google Scholar]
- Thanos PK, Dimitrakakis ES, Rice O, Gifford A, Volkow ND. Ethanol self-administration and ethanol conditioned place preference are reduced in mice lacking cannabinoid cb1 receptors. Behav Brain Res. 2005;164(2):206–213. doi: 10.1016/j.bbr.2005.06.021. [DOI] [PubMed] [Google Scholar]
- Vinod KY, Sanguino E, Yalamanchili R, Manzanares J, Hungund BL. Manipulation of fatty acid amide hydrolase functional activity alters sensitivity and dependence to ethanol. J Neurochem. 2008a;104(1):233–243. doi: 10.1111/j.1471-4159.2007.04956.x. [DOI] [PubMed] [Google Scholar]
- Vinod KY, Yalamanchili R, Thanos PK, Vadasz C, Cooper TB, Volkow ND, et al. Genetic and pharmacological manipulations of the cb(1) receptor alter ethanol preference and dependence in ethanol preferring and nonpreferring mice. Synapse. 2008b;62(8):574–581. doi: 10.1002/syn.20533. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang L, Liu J, Harvey-White J, Zimmer A, Kunos G. Endocannabinoid signaling via cannabinoid receptor 1 is involved in ethanol preference and its age-dependent decline in mice. Proc Natl Acad Sci U S A. 2003;100(3):1393–1398. doi: 10.1073/pnas.0336351100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weiss F, Lorang MT, Bloom FE, Koob GF. Oral alcohol self-administration stimulates dopamine release in the rat nucleus accumbens: Genetic and motivational determinants. J Pharmacol Exp Ther. 1993;267(1):250–258. [PubMed] [Google Scholar]
- Zangen A, Solinas M, Ikemoto S, Goldberg SR, Wise RA. Two brain sites for cannabinoid reward. J Neurosci. 2006;26(18):4901–4907. doi: 10.1523/JNEUROSCI.3554-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]