Abstract
Structural brain abnormalities have been observed in adolescents with alcohol use disorders but less is known about neuropathological brain characteristics of teens with subdiagnostic binge drinking or the common pattern of binge drinking combined with marijuana use. The goal of this study was to examine white matter integrity in adolescents with histories of binge drinking and marijuana use.
Diffusion tensor imaging (DTI) was conducted with 42 adolescents (ages 16−19) classified as controls, binge drinkers, or binge drinkers who are also heavy marijuana users. Tract based spatial analysis identified shared fiber structure across individuals and facilitated voxelwise comparisons of fractional anisotropy (FA) and mean diffusivity (MD) between groups.
Significant between group differences were found in FA in eight white matter regions (ps ≤ .016) between the binge drink-only group and controls, including superior corona radiata, inferior longitudinal fasciculus, inferior fronto-occipital fasciculus, and superior longitudinal fasciculus. Interestingly, in 4 of these same regions, binge drinkers who are also heavy marijuana users had higher FA than binge drinkers who did not use marijuana (ps < .05). MD did not differ between groups.
Findings are largely consistent with research suggesting less neuropathology in adolescents without histories of substance use. However, binge drinkers who also use marijuana did not show as consistent a divergence from non-users as did the binge drink-only group. Detection of white matter alterations may have implications in identifying early cognitive dysfunction in substance using adolescents.
Keywords: Adolescence, Brain Imaging, Marijuana Abuse, Alcohol Abuse, White Matter, Diffusion Tensor Imaging
1. Introduction
Alcohol use in adulthood is associated with neurotoxic molecular and metabolic changes in the brain. Animal and human studies have shown that chronic alcohol exposure leads to structural and chemical brain alterations [24,45,74], functional brain changes [2] and neurocognitive impairments [31,89,96]. Neuroimaging studies have noted prominent macrostructural (e.g., volume) and microstructural (e.g., fiber density, compactness, coherence) white matter changes in adults with alcohol use disorders [70,72], which may predict deteriorating cognitive performance [21,73]. Less is known about the detrimental effects of alcohol use on the adolescent brain.
As in adulthood, heavy alcohol use during adolescence has been linked to abnormalities in white and grey matter tissue volume [26,27,58,59,65], brain function [4,83,91,93], and neuropsychological performance [17,80,92,110]. Adolescent drinking is particularly important given its potential to interfere with maturational processes such as myelination and synaptic organization that continue throughout adolescence and into early adulthood [13,33-35,71].
Few studies have expanded on the macrostructural information provided by structural magnetic resonance imaging (MRI) in adolescent alcohol users. In addition to changes in overall white matter volume, the microstructural properties of white matter tissue at different stages of myelin development are important for the smooth, efficient, and integrated white matter fiber pathways necessary for neuronal transmission. Several studies have found differences in microstructural white matter indices of the corpus callosum between adolescents with and without alcohol use disorders [28,95], but less is known about white matter in adolescents who engage in sub-diagnostic drinking behaviors. Underage drinking is common [47], and most alcohol consumed by teens is in a binge or heavy episodic fashion. The National Institute on Alcohol Abuse and Alcoholism defines binge drinking as a pattern of alcohol consumption that brings blood alcohol concentration levels to .08 or above, which typically corresponds with consuming ≥ 5 drinks or ≥ 4 drinks on an occasion for boys and girls, respectively [66,88,106]. Sub-diagnostic binge drinking can be associated with changes in neurocognition and altered brain structure in youth and adults [41,48,56,77,98].
The second most widely used intoxicant among teens is marijuana [47], which is frequently used in combination with alcohol [61], but the potential additive, interactive, or protective role of concomitant marijuana use on any neural effects of adolescent binge drinking is unknown. Adolescent marijuana use has been linked to abnormalities in neurocognition and brain function [42,57,67,81,94], and the use of both substances appears related to anomalous brain function [59,82]. However, little is known about how the common pattern of use of these two substances together may relate to adolescent white matter development. Examining white matter tissue in adolescents who engage in binge drinking and marijuana use will help us better understand the neuropathological effects of the most common substance intake behaviors of teens.
Despite the documented negative cognitive effects of heavy marijuana use in adolescence [42,50,57], animal studies examining potential neuroprotective effects of cannabinoids (the active ingredient in marijuana) on toxic insults suggest antioxidant properties and reductions in glutamate excitotoxicity in the brain by inhibition of glutamate transmission [37,38,55,102]. As excitotoxicity and oxidative stress are suggested mechanisms of alcohol-related brain injury [24,25], cannabinoid exposure could potentially mitigate ethanol-induced changes[36]. However, a study of neonatal rats found that activation of endocannabinoid CB1 receptor, in combination with alcohol administration, may modulate glutamate and GABAergic neurotransmission, and prime the brain to suffer cell death by suppressing synaptic activity important for developmental processes. This could indicate that alcohol and marijuana use together may lead to worse neural outcomes, specifically in the developing brain [40].
This study expands on the current literature by examining the quality of white matter fiber structure in relation to adolescent binge drinking in the context of marijuana use. We used diffusion tensor imaging (DTI) to evaluate microscopic white matter change in vivo by estimating the diffusion of water molecules in neural tissue. Brain regions that are rich in fiber tracts have greater restriction in the directionality of water molecule movement [51], so diffusion measurements visualize the white matter bundles and describe the microscopic architecture of white matter tissue [9,51]. DTI exams were performed on: (1) adolescents with no substance use history, (2) those with a history of binge drinking, and (3) those with a history of concomitant binge drinking and marijuana use. We compared between groups two common scalar diffusion measurements: fractional anisotropy (FA), which reflects the directionally-dependent movement of water molecules along fiber tracts, and mean diffusivity (MD), reflecting the degree of overall displacement of water molecules in localized tissue [51,75]. Based on literature examining combined effects of alcohol and cannabinoids on neural toxicity in the immature brain [40] and data from our lab showing abnormal brain functioning in abstinent adolescent marijuana users [81,94] and binge drinkers [56], we hypothesized that histories of binge drinking alone and in combination with marijuana would be linked to poorer white matter fiber tract coherence and organization (i.e., lower FA) compared to controls. We further hypothesized that in regions where FA was low in users, MD values would be higher, reflecting tissue loss or potential demyelination.
2. Methods
2.1 Participants
Adolescents ages 16 through 19 years were recruited from local high schools and colleges. Comprehensive screening interviews [53,57] were administered to adolescents and their guardians. Inclusionary criteria required adolescents to have a parent or legal guardian to provide consent and a medical and psychiatric history. Exclusionary criteria were history of: (1) Diagnostic and Statistical Manual for Mental Disorder –Fourth Edition (DSM-IV) [3] Axis I disorder other than alcohol or cannabis use disorder, (2) use of psychoactive medications, (3) chronic medical illness, (4) neurological condition (e.g., migraine), (5) head trauma with loss of consciousness >2 minutes, (6) prenatal alcohol (> 3 drinks in a day or > 6 drinks in a week) or drug exposure, (7) complicated or premature birth (< 33 weeks gestation), (8) learning disability or mental retardation, (9) left handedness, (10) noncorrectable vision, colorblindness or hearing impairments, (11) parental history of bipolar I or psychotic disorder, and (12) non-fluency in English for youth.
All participants under 18 years of age and their parents or guardians underwent written informed consent (or assent for minors) in accordance with the University of California, San Diego Human Research Protections Program. Participants over age 18 consented for their own participation, and their parents consented for a collateral interview. Teens were classified as: (1) binge drinkers (BG; n=14) with histories of at least one episode of ≥ 4 drinks on one occasion for females and ≥ 5 drinks for males; (2) binge drinkers and marijuana users (BG+MJ; n=14) with history of lifetime marijuana use between 180 to 1800 times and binge drinking history as above; and (3) control teens with very limited if any substance use history (CON, n=14). Groups were statistically similar on age, gender, household income, general intellect, externalizing and internalizing behaviors, and mood (see Table 1).
All participants received urine toxicology to confirm self-report and parent report of adolescent's abstinence from marijuana and other drugs, and Breathalyzers (Intoximeter, St. Louis, MO) to confirm abstinence from alcohol. BG+MJ teens were abstinent from marijuana at least 23 days (range 23−61) on the day of brain imaging. Histories of marijuana use were reported by six BG (no more than 9 episodes) and three CON (no more than 5 episodes) participants, with 30 to 1076 days since last use. Abstinence from alcohol was an average of 26 days (range 3−45 days) for BG+MJ teens, 30 days (range 13−52 days) for BG teens, and ranged from 90 to 998 days for CON, who had an average of 4 lifetime drinking episodes. Total lifetime drinking episodes in BG and BG+MJ groups ranged from 12 to 405 (see Table 1).
2.2 Measures
Substance use was assessed with the Customary Drinking and Drug Use Record [16], an interview that obtains information on lifetime and past 3-month use of alcohol, marijuana, nicotine, and eight other classes of illicit drugs in addition to DSM-IV abuse and dependence criteria, hangover/withdrawal symptoms, and negative consequences associated with substance use. The Child Behavior Checklist [1] obtains reports from parents regarding child behavior concerns. This measure has shown good reliability and validity on reports of internalizing and externalizing behavior [14]. The Beck Depression Inventory [12] assessed depressive symptoms on the day of scanning. The Wechsler Adult Intelligence Scale, Third Edition (WAIS-III) Vocabulary subtest was administered by trained psychometrists to assess premorbid intellectual functioning [105]. Parental socioeconomic status was assessed with the two-factor Hollingshead scale that combines education and occupation [43]. Family history of psychiatric disorders was assessed with the Family History Assessment Module [76].
2.3 Procedures
2.3.1 DTI Data Acquisition and Processing
All DTI scans were performed on a General Electric 3.0-T magnetic resonance imager at the University of California, San Diego. Data with whole brain coverage were collected through a single-shot dual spin echo excitation with TE = 93.4 ms, TR = 12,400 ms, field of view = 24 cm, slice thickness = 3.0 mm, image matrix = 128 × 128, and b-value = 2000 s/mm2. Diffusion-weighted images were acquired in 15 diffusion directions, in addition to the normalization image with no diffusion encoding (b=0) [32]. Four volumes were acquired and averaged for each direction. Each two-dimensional slice was Fourier transformed and re-gridded to rectilinear space prior to the following processing steps.
Tract-Based Spatial Statistics (TBSS) [85] was used for voxel-wise comparisons of FA and MD values between groups. TBSS attempts to correct for inaccuracies that complicate interpretation of other voxelwise methods (e.g., voxel-based morphometry), such as weaknesses in alignment, smoothing, and localization of brain tissue [85]. Diffusion measurement images (FA and MD) were derived from the 3dDWItoDT program of the Analysis of Functional NeuroImages package (AFNI) [23], which fits the diffusion weighted data to a tensor model using a non-linear least squares approach to compute the three orthogonal eigenvectors and corresponding eigenvalues necessary for calculating the scalar FA and MD indices based on standard formulas [68]. Additional processing procedures used Oxford Centre for Functional Magnetic Resonance Imaging of the Brain (FMRIB) Software Package tools (FSL) [86].
Two pre-alignment processing steps were conducted: a six-degree of freedom affine motion correction for head motion; and a 2D, six degree of freedom alignment to reduce the effects of gradient coil eddy currents (FLIRT) [44]. Each image was inspected for quality, and non-brain voxels were removed from analysis by AFNI 3dAutomask, then manually refined as needed. A representative diffusion image from the control group was selected as the target image for registration. All subjects were aligned by nonlinear transformation to the selected target image and then the whole dataset was affine-aligned to MNI-152 space [30]. These transformed data images (FA and MD) were averaged to create a mean FA or MD image. Next, a white matter tract skeleton was created from the mean FA or MD image to represent the centers of large white matter tracts in the dataset. This skeleton was generated by identifying lines (within white matter tracts) in the mean image with the largest diffusion values. Finally, each participant's transformed FA and MD image was projected onto the diffusion skeleton. This procedure helped achieve alignment between the common skeleton and the tract center of individual FA and MD images. For each point on the skeleton, voxelwise statistics were conducted across individual subjects [85].
2.3.2 Statistical Analysis
A whole brain one-way ANOVA was used to examine the between-group differences in both FA and MD on a voxel-by-voxel basis. Type I error was controlled using intensity and cluster based thresholding (family-wise p<.05). A Monte Carlo simulation with a alpha =.05 and connectivity of 1 mm [85] determined that clusters comprised of at least 27 contiguous voxels would have a 5% chance of occurring under the null hypotheses. Thus, only clusters consisting of ≥27 microliters with each voxel differing at p<.05 were interpreted. Follow-up analyses determined which groups were responsible for significant ANOVAs.
3. Results
3.1 Demographic Information
Groups did not differ on any demographic characteristic (ps > .05, see Table 1). The BG+MJ group had more lifetime drinking episodes than BG or CON groups (p<.005), so this variable was used as a covariate in follow-up analyses. The BG+MJ reported more lifetime illicit drug use episodes (other than alcohol, marijuana, or nicotine) compared to BG and CON (p<.05). In particular, the BG+MJ group reported more lifetime ecstasy use episodes than other groups (see Table 1; p<.05), although no one in any group used it more than once. Groups did not differ on lifetime amphetamine, hallucinogen, barbiturates, benzodiazepine, cocaine, opiate, ketamine, GHB, or inhalant use (ps>.05).
3.2 Between group comparisons
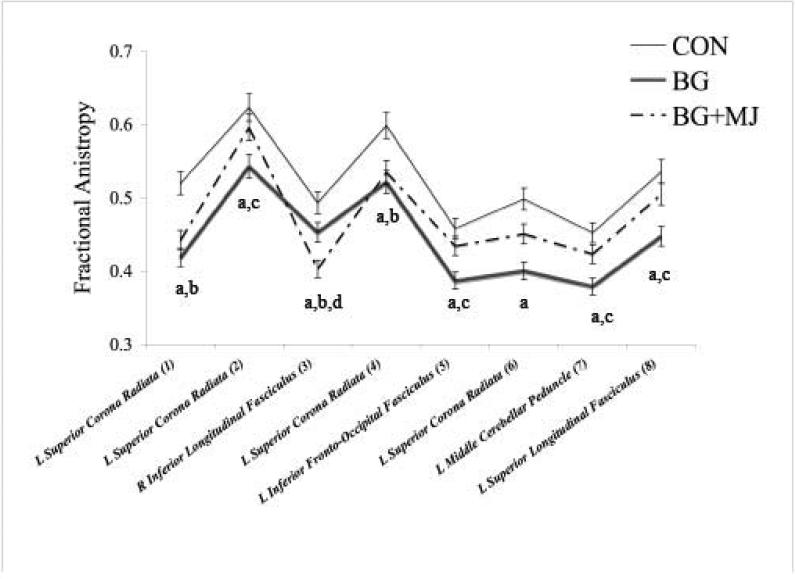
A whole brain ANOVA revealed significant between-group FA differences in 8 separate fiber tract regions: 4 clusters in the left superior corona radiata, 1 cluster in the right inferior longitudinal fasciculus, 1 cluster in the left inferior fronto-occipital fasciculus, 1 cluster in the left middle cerebellar peduncle, and 1 cluster in the left superior longitudinal fasciculus (see Table 2 and Figure 1; clusters ≥ 27 contiguous voxels each at p<.05). Follow-up pair-wise comparisons controlling for lifetime drinking episodes indicated that BG displayed lower FA than CON in all 8 clusters (ps ≤ .016, see Table 2). BG+MJ displayed lower FA than CON in 2 of the corona radiata clusters and in the superior longitudinal fasciculus cluster (ps ≤ .01, see Table 2). Interestingly, BG demonstrated significantly lower FA than the BG+MJ group in 4 of the 8 regions: 1 of the superior corona radiata clusters, inferior fronto-occipital fasciculus, middle cerebellar peduncle, and the superior longitudinal fasciculus (ps .014 to .043). Results were unchanged when lifetime drinking episodes were not controlled for, except that BG+MJ now showed lower FA than CON (p = .029) in the middle cerebellar peduncle as well. Whole brain analysis revealed no significant between group differences for MD.
Figure 1.
Between Group FA Differences, a = CON > BG; b = CON > BG+MJ; c = BG < BG+MJ; d = BG > BG+MJ; all p < .05
Groups did not differ on any other class of illicit substances except lifetime ecstasy use episodes, therefore we conducted pairwise comparisons controlling for reported ecstasy use. Results were unchanged in 6 of the 8 regions, and differed in 2 regions as follows. In 1 of the 4 superior corona radiata regions, the FA difference between BG+MJ and CON no longer reached significance (p = .08), but BG now had lower FA than BG+MJ in this region (p = .03). In the middle cerebellar peduncle, the FA difference between BG and BG+MJ no longer reached significance (p = .16), but BG+MJ now had lower FA than CON (p = .028) after controlling for ecstasy use.
3.3 Bivariate relationships
Relationships between FA in regions that differed by group and measures of lifetime and recent (past 3 month) marijuana and alcohol use were examined among the two user groups (BG and BG+MJ, n = 28). As lifetime marijuana use increased, FA values in two left superior corona radiata clusters also increased (rs = .38 to .41, ps = .03). Further, more marijuana hits in the past three months was linked to higher FA in the superior longitudinal fasciculus (r = .51, p = .006). Similarly, those with more lifetime drinking occasions had higher FA in the superior longitudinal fasciculus (r = .43, p = .02). Lifetime drinking occasions and number of hits smoked in the past three months were not significantly correlated (p>.05). Alcohol drinks consumed in the past three months was not associated with FA in any of the 8 clusters that differed between groups.
4. Discussion
The aim of this study was to look at how adolescent binge drinking behavior affects white matter in the context of marijuana use. White matter integrity, indexed by FA, differed between groups in eight clusters located in both association fiber pathways (e.g., fronto-occipital fasciculus, superior longitudinal fasciculus) as well as the corona radiata. These particular association and projection white matter fiber tracts, often implicated in neurocognitive functioning in both adults and children, continue to develop throughout adolescence and are considered important for connecting sensory structures to the frontal lobes [8,10,52,79,99,103,104].
Overall, we found that teens reporting histories of binge drinking alone and with concomitant marijuana use displayed lower FA compared to controls. Teens reporting binge drinking behaviors alone had significantly lower FA than controls in all eight clusters, whereas teens reporting both binge drinking and marijuana use had lower FA than controls in only three of the eight clusters: two clusters located in the corona radiata and one cluster in the inferior longitudinal fasciculus. In seven clusters (excluding the right inferior longitudinal fasciculus), teens reporting both marijuana and binge drinking had higher FA values than teens who reported binge drinking only (statistically significant in four of the eight clusters: corona radiata, fronto-occipital fasciculus, middle cerebellar peduncle, superior longitudinal fasciculus).
Interestingly, we observed increasing FA values with more marijuana use in white matter fiber tracts such as the left superior longitudinal fasciculus and the left superior corona radiata. In the superior longitudinal fasciculus, FA values and marijuana use episodes in the past three months were positively related. Also in this region, we found an unexpected positive relationship between lifetime drinking occasions and FA values, even though marijuana use and lifetime drinking frequency were not correlated.
No MD differences between groups were seen in any of the 8 clusters that showed between-group FA differences, or anywhere else in the brain, suggesting the potential mechanism of white matter change in this sample may be alterations in the highly organized fiber structure, as compared to tissue loss or demyelination, although this remains uncertain [7,11,54]. Although the pathological relationship between these diffusion indices (e.g., FA and MD) is not entirely understood due to the complicated geometry of white matter tracts in the brain, correlations between MD and FA may point to contributions to the breakdown in microstructural integrity of white matter (e.g., changes in intra/extra cellular fluid, axonal density, disorganization of fiber structure) [72]. Our results propose a relationship between both adolescent binge drinking and marijuana use and subtle white matter tissue microstructural abnormalities in varying projection and association fiber pathways (e.g. superior longitudinal fasciculus) that are important for healthy adolescent neural and cognitive development [19,63,64,87].
Notably, our results highlight the possible neural consequences of subdiagnostic binge drinking behavior in adolescents, independent of marijuana or other drug use. In support of this hypothesis, animal and human studies have shown that the pathophysiological effects of binge drinking include rate changes in excitatory and inhibitory neurotransmitters in many forebrain structures (e.g., hippocampus, nucleus accumbens), neurochemical metabolite changes, and cell death by inflammatory processes [60,69,84,90,97]. Cellular apoptotic death is even suggested to occur with very small doses of alcohol [109]. Recently, Crews and Nixon (2008) found that ethanol intoxication during binge drinking, as opposed to ethanol withdrawal episodes, may lead to proinflammatory cytokines and increases in oxidative stress. Although the mechanism of white matter change in this sample is complex and not entirely understood, we hope that our findings will build on the current literature exploring the neuropathological consequences of binge drinking on adolescent brain tissue.
While the effects of alcohol use independently on the brain are better understood, the effects of marijuana use remain less clear. Some studies have found evidence of structural and functional brain abnormalities [5,6,20], while other neuroimaging studies report no differences in the brains of heavy marijuana users compared to non-using controls [15,22,29,39,49,100,108]. In previous studies, our group has found between-group differences in cognition and brain activity in heavy marijuana using adolescents compared to controls [67,81,94]. In this sample, white matter tracts in adolescents who binge drink and use marijuana were more coherent than in adolescents reporting only binge drinking. These findings are unexpected, as recent animal studies have found that rats receiving the psychoactive principal component of marijuana (THC) along with alcohol show an enhanced susceptibility of the developing brain to incur alcohol-related apoptotic neuronal cell death [40].
However, our lab has found similar findings looking at macrostructural changes in adolescent hippocampal volumes. We found reduced hippocampal volumes and abnormal asymmetry in adolescent drinkers compared to controls, but did not observe volume or asymmetry abnormalities in teens reporting both alcohol and marijuana use [59]. It is possible that marijuana may have some neuroprotective properties in mitigating alcohol-related oxidative stress or excitoxic cell death as suggested in animal models of alcohol-induced neuronal death and cell regeneration [24,37,101,102]. Although research on the neuroprotective role of cannabinoids is not completely understood, studies have identified several potential neuroprotective molecular mechanisms. For example, activation of cannabinoid receptors may reduce inflammatory activity and excess glutamate neurotransmission that can lead to toxic cell death due to influx of intracellular calcium ion concentrations. Marijuana may also prevent toxicity through anti-oxidative benefits that prevent damage to cellular lipids and proteins, and which may not require cannabinoid-receptor mediated action [18,36,78]. Research on the role of cannabinoids in animal models of binge drinking has found evidence for cannabinoids as a neuroprotectant against binge alcohol toxicity in hippocampal regions when both substances are administered concurrently [36]. Furthermore, binge alcohol-related modulation of cannabinoid receptors may also contribute to the cellular mechanisms of neuroprotection. Binge alcohol consumption in animals has been found to initially down-regulate CB1 receptors (after 2 days of withdrawal), then up-regulate CB1 receptors later, after 40 days of withdrawal [62]. Given how widely used cannabis and alcohol are alone and in combination, a greater understanding of their interactive, neuroprotective, and neurotoxic effects is important.
Several limitations in this study should to be noted. Although we did not find differences on demographic variables, these three groups of adolescents could differ on a variable not assessed. Since analyses were not longitudinal, we cannot ascertain if changes resulted from substance use or were preexisting. Lastly, we used a nonprobability sampling approach, and our sample was fairly small precluding the ability to examine continuous relationships between FA, alcohol, and marijuana use within each user group separately. Future studies examining the effects of combined alcohol and marijuana use on microstructural integrity of white matter will need larger samples.
In this study, we found that concomitant binge drinking and marijuana use was not associated with better white matter compared to controls, but higher FA values compared to adolescents reporting only binge drinking. Findings suggest that binge drinking may lead to microscopic disruption of white matter fibers. Although not evaluated in this study, alcohol-related diffusion changes in white matter tissue have been linked with altered cognitive performance in adult samples [73]. Studies on cognitive and behavioral consequences of binge drinking have found differences in executive functions including decision-making, which may be related to changes in neural circuitry [46,107]. Gender effects have even been suggested, with female bingers performing worse on neurocognitive tasks compared to male bingers and controls [98]. In future studies, we plan to examine how white matter changes in these adolescents over 18 and 36 month follow-up periods as well as how these changes relate to drug use and risk taking behavior.
Acknowledgements
This research was made possible by funding from the National Institute on Drug Abuse (R01 DA021182, PI: Tapert) and the National Institute on Alcohol Abuse and Alcoholism (R01 AA13419, PI: Tapert).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Conflict of Interest Statement
The authors declare that there are no conflicts of interest.
Portions of this study were presented at the annual meeting of the Research Society on Alcoholism, June 2008, Washington DC.
References
- 1.Achenbach TM, Rescorla LA. Manual for the ASEBA School-Age Forms & Profiles, University of Vermont, Research Center for Children, Youth, & Families. Burlington, VT: 2001. [Google Scholar]
- 2.Akine Y, Kato M, Muramatsu T, Umeda S, Mimura M, Asai Y, Tanada S, Obata T, Ikehira H, Kashima H, Suhara T. Altered brain activation by a false recognition task in young abstinent patients with alcohol dependence. Alcohol Clin Exp Res. 2007;31(9):1589–1597. doi: 10.1111/j.1530-0277.2007.00453.x. [DOI] [PubMed] [Google Scholar]
- 3.American Psychiatric Association . Diagnostic and Statistical Manual of Mental Disorders: DSM-IV. 4th Ed. Washington, DC: 1994. [Google Scholar]
- 4.Anderson KG, Schweinsburg AD, Paulus MP, Brown SA, Tapert SF. Examining personality and alcohol expectancies using functional magnetic resonance imaging (fMRI) with adolescents. J Stud Alcohol. 2005;66(3):323–331. doi: 10.15288/jsa.2005.66.323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Arnone D, Barrick TR, Chenqappa S, MacKay CE, Clark CA, Abou-Saleh MT. Corpus callosum damage in heavy marijuana use: preliminary evidence from diffusion tensor tractography and tract-based spatial statistics. Neuroimage. 2003;41(3):1067–1074. doi: 10.1016/j.neuroimage.2008.02.064. [DOI] [PubMed] [Google Scholar]
- 6.Ashtari M, Cervellione K, Cottone J, Ardekani BA, Kumra S. Diffusion abnormalities in adolescents and young adults with a history of heavy cannabis use. J Psychiatr Res. 2009;43(3):189–204. doi: 10.1016/j.jpsychires.2008.12.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Assaf Y, Pasternak O. Diffusion tensor imaging (DTI)-based white matter mapping in brain research: A review. J Mol Neurosci. 2008;34:51–56. doi: 10.1007/s12031-007-0029-0. [DOI] [PubMed] [Google Scholar]
- 8.Barnea-Goraly N, Menon V, Eckert M, Tamm L, Bammer R, Karchemskiy A, Dant CC, Reiss AL. White matter development during childhood and adolescence: a cross-sectional diffusion tensor imaging study. Cereb Cortex. 2005;15(12):1848–1854. doi: 10.1093/cercor/bhi062. [DOI] [PubMed] [Google Scholar]
- 9.Basser PJ, Mattiello J, LeBihan D. MR diffusion tensor spectroscopy and imaging. Biophys J. 1994;66(1):259–267. doi: 10.1016/S0006-3495(94)80775-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Bava S, Frank LR, McQueeny T, Schweinsburg BC, Schweinsburg AD, Tapert SF. Altered white matter microstructure in adolescent substance users. Psychiatry Res. doi: 10.1016/j.pscychresns.2009.04.005. (in press) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Beaulieu C, Allen PS. Determinants of anisotropic water diffusion in nerves. Magn Reson Med. 1994;31:394–400. doi: 10.1002/mrm.1910310408. [DOI] [PubMed] [Google Scholar]
- 12.Beck AT, Ward C, Mendelson M. Beck Depression Inventory (BDI) Arch Gen Psychiatry. 1961;4:561–571. doi: 10.1001/archpsyc.1961.01710120031004. [DOI] [PubMed] [Google Scholar]
- 13.Benes FM, Turtle M, Khan Y, Farol P. Myelination of a key relay zone in the hippocampal formation occurs in the human brain during childhood, adolescence, and adulthood. Arch Gen Psychiatry. 1994;51(6):477–484. doi: 10.1001/archpsyc.1994.03950060041004. [DOI] [PubMed] [Google Scholar]
- 14.Berube RL, Achenbach TM. Bibliography of published studies using ASEBA instruments, 2005 Edition, University of Vermont, Research Center for Children, Youth, & Families. Burlington, VT: 2005. [Google Scholar]
- 15.Block RI, O'Leary DS, Ehrhardt JC, Augustinack JC, Ghoneim MM, Arndt S, Hall JA. Effects of frequent marijuana use on brain tissue volume and composition. Neuroreport. 2000;11(3):491–496. doi: 10.1097/00001756-200002280-00013. [DOI] [PubMed] [Google Scholar]
- 16.Brown SA, Myers MG, Lippke L, Tapert SF, Stewart DG, Vik PW. Psychometric evaluation of the Customary Drinking and Drug Use Record (CDDR): A measure of adolescent alcohol and drug involvement. J Stud Alcohol. 1998;59:427–438. doi: 10.15288/jsa.1998.59.427. [DOI] [PubMed] [Google Scholar]
- 17.Brown SA, Tapert SF, Granholm E, Delis DC. Neurocognitive functioning of adolescents: effects of protracted alcohol use. Alcohol Clin Exp Res. 2000;24(2):164–171. [PubMed] [Google Scholar]
- 18.Campbell VA, Downer EJ. Cannabinoids and Neuroprotection. In: Kofalv A, editor. Cannabinoids and the Brain. Springer; New York: 2008. pp. 317–330. [Google Scholar]
- 19.Cascio CJ, Gerig G, Piven J. Diffusion tensor imaging: Application to the study of the developing brain. J Am Acad Child Adolesc Psychiatry. 2007;46(2):213–223. doi: 10.1097/01.chi.0000246064.93200.e8. [DOI] [PubMed] [Google Scholar]
- 20.Chang L, Chronicle EP. Functional imaging studies in cannabis users. Neuroscientist. 2007;13(5):422–432. doi: 10.1177/1073858406296601. [DOI] [PubMed] [Google Scholar]
- 21.Chanraud S, Martelli C, Delain F, Kostogianni N, Douaud G, Aubin HJ, Reynaud M, Martinot JL. Brain morphometry and cognitive performance in detoxified alcohol-dependents with preserved psychosocial functioning. Neuropsychopharmacology. 2007;32(2):429–438. doi: 10.1038/sj.npp.1301219. [DOI] [PubMed] [Google Scholar]
- 22.Co BT, Goodwin DW, Gado M, Mikhael M, Hill SY. Absence of cerebral atrophy in chronic cannabis users. Evaluation by computerized transaxial tomography. JAMA. 1977;237(12):1229–1230. [PubMed] [Google Scholar]
- 23.Cox R. AFNI: Software for Analysis and Visualization of Functional Magnetic Resonance Neuroimages. Comput Biomed Res. 1996;29:162–173. doi: 10.1006/cbmr.1996.0014. [DOI] [PubMed] [Google Scholar]
- 24.Crews FT, Nixon K. Mechanisms of neurodegeneration and regeneration. Alcohol Alcohol. 2008;0:1–13. doi: 10.1093/alcalc/agn079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Crews FT, Waage HG, Wilkie MB, Lauder JM. Ethanol pretreatment enhances NMDA excitotoxicity in biogenic amine neurons: protection by brain derived neurotrophic factor. Alcohol Clin Exp Res. 1999;23(11):1834–1842. [PubMed] [Google Scholar]
- 26.De Bellis MD, Clark DB, Beers SR, Soloff PH, Boring AM, Hall J, Kersh A, Keshavan MS. Hippocampal volume in adolescent-onset alcohol use disorders. Am J Psychiatry. 2000;157(5):737–744. doi: 10.1176/appi.ajp.157.5.737. [DOI] [PubMed] [Google Scholar]
- 27.De Bellis MD, Narasimhan A, Thatcher DL, Keshavan MS, Soloff P, Clark DB. Prefrontal cortex, thalamus, and cerebellar volumes in adolescents and young adults with adolescent-onset alcohol use disorders and comorbid mental disorders. Alcohol Clin Exp Res. 2005;29(9):1590–1600. doi: 10.1097/01.alc.0000179368.87886.76. [DOI] [PubMed] [Google Scholar]
- 28.De Bellis MD, Van Voorhees E, Hooper SR, Gibler N, Nelson L, Hege SG, Payne ME, MacFall J. Diffusion tensor measures of the corpus callosum in adolescents with adolescent onset alcohol use disorders. Alcohol Clin Exp Res. 2008;32(3):395–404. doi: 10.1111/j.1530-0277.2007.00603.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.De Lisi LE, Bertisch HC, Szulc KU, Majcher M, Brown K, Bappal A, Ardekani BA. A preliminary DTI study showing no brain structural change associated with adolescent cannabis use. Harm Reduct J. 2006;9(3):17. doi: 10.1186/1477-7517-3-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Evans AC, Collins DL, Milner B. An MRI-based stereotactic brain atlas from 300 young normal subjects. Soc Neurosci Abstr. 1992:408. [Google Scholar]
- 31.Fein G, Torres J, Price LJ, Di Sclafani V. Cognitive performance in long-term abstinent alcoholic individuals. Alcohol Clin Exp Res. 2006;30(9):1538–1544. doi: 10.1111/j.1530-0277.2006.00185.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Frank LR. Anisotropy in high angular resolution diffusion-weighted MRI. Magn Reson Med. 2001;45(6):935–939. doi: 10.1002/mrm.1125. [DOI] [PubMed] [Google Scholar]
- 33.Giedd JN. Structural magnetic resonance imaging of the adolescent brain. Ann N Y Acad Sci. 2004;1021:77–85. doi: 10.1196/annals.1308.009. [DOI] [PubMed] [Google Scholar]
- 34.Giedd JN, Blumenthal J, Jeffries NO, Castellanos FX, Liu H, Zijdenbos A, Paus T, Evans AC, Rapoport JL. Brain development during childhood and adolescence: a longitudinal MRI study. Nat Neurosci. 1999;2(10):861–863. doi: 10.1038/13158. [DOI] [PubMed] [Google Scholar]
- 35.Gogtay N, Giedd JN, Lusk L, Hayashi KM, Greenstein D, Vaituzis AC, Nugent TF, 3rd, Herman DH, Clasen LS, Toga AW, Leinbenluft E, Thompson PM, Rapoport JL. Dynamic mapping of human cortical development during childhood through early adulthood. Proc Natl Acad Sci U S A. 2004;101(21):8174–8179. doi: 10.1073/pnas.0402680101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Hamelink C, Hampson A, Wink DA, Eiden LE, Eskay RL. Comparison of cannabidiol, antioxidants, and diuretics in reversing binge ethanol-induced neurotoxicity. J Pharmacol Exp Ther. 2005;314:780–788. doi: 10.1124/jpet.105.085779. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Hampson AJ, Grimaldi M, Axelrod J, Wink D. Cannabidiol and (−)Delta9-tetrahydrocannabinol are neuroprotective antioxidants. Proc Natl Acad Sci U S A. 1998;95(14):8268–8273. doi: 10.1073/pnas.95.14.8268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Hampson AJ, Grimaldi M, Lolic M, Wink D, Rosenthal R, Axelrod J. Neuroprotective antioxidants from marijuana. Ann N Y Acad Sci. 2000;899:274–282. [PubMed] [Google Scholar]
- 39.Hannerz J, Hindmarsh T. Neurological and neuroradiological examination of chronic cannabis smokers. Ann Neurol. 1983;13(2):207–210. doi: 10.1002/ana.410130219. [DOI] [PubMed] [Google Scholar]
- 40.Hansen HH, Krutz B, Sifringer M, Stefovska V, Bittigau P, Pragst F, Marsicano G, Lutz B, Ikonomidou C. Cannabinoids enhance susceptibility of immature brain to ethanol neurotoxicity. Ann Neurol. 2008;64(1):42–52. doi: 10.1002/ana.21287. [DOI] [PubMed] [Google Scholar]
- 41.Hartley DE, Elsabagh S, File SE. Binge drinking and sex: effects on mood and cognitive function in healthy young volunteers. Pharmacol Biochem Behav. 2004;78(3):611–619. doi: 10.1016/j.pbb.2004.04.027. [DOI] [PubMed] [Google Scholar]
- 42.Harvey MA, Sellman JD, Porter RJ, Framptom CM. The relationship between non-acute adolescent cannabis use and cognition. Drug Alcohol Rev. 2007;26(3):309–319. doi: 10.1080/09595230701247772. [DOI] [PubMed] [Google Scholar]
- 43.Hollingshead AB. Two-factor index of social position. University Press; New Haven, CT: 1965. [Google Scholar]
- 44.Jenkinson M, Bannister P, Brady J, Smith S. Improved optimisation for the robust and accurate linear registration and motion correction of brain images. NeuroImage. 2002;17(2):825–841. doi: 10.1016/s1053-8119(02)91132-8. [DOI] [PubMed] [Google Scholar]
- 45.Jernigan TL, Butters N, DiTraglia G, Schafer K, Smith T, Irwin M, Grant I, Schuckit M, Cermak LS. Reduced cerebral grey matter observed in alcoholics using magnetic resonance imaging. Alcohol Clin Exp Res. 1991;15(3):418–427. doi: 10.1111/j.1530-0277.1991.tb00540.x. [DOI] [PubMed] [Google Scholar]
- 46.Johnson CA, Xiao L, Palmer P, Sun P, Wang Q, Wei Y, Jia Y, Grenard JL, Stacy AW, Bechara A. Affective decision-making deficits, linked to a dysfunctional ventromedial prefrontal cortex, revealed in 10th grade Chinese adolescent binge drinkers. Neuropsychologia. 2007;46:714–726. doi: 10.1016/j.neuropsychologia.2007.09.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Johnston LD, O'Malley PM, Bachman JG, Schulenberg JE. Monitoring the Future national results on adolescent drug use: Overview of key findings. National Institute on Drug Abuse; Bethesda, MD: 2007. [Google Scholar]
- 48.Kokavec A, Crowe SF. A comparison of cognitive performance in binge versus regular chronic alcohol misusers. Alcohol Alcohol. 1999;34(4):601–608. doi: 10.1093/alcalc/34.4.601. [DOI] [PubMed] [Google Scholar]
- 49.Kuehnle J, Mendelson JH, Davis KR, New PF. Computed tomographic examination of heavy marijuana smokers. JAMA. 1977;237(12):1231–1232. [PubMed] [Google Scholar]
- 50.Lane SD, Cherek DR, Tcheremissine OV, Steinberg JL, Sharon JL. Response perseveration and adaptation in heavy marijuana-smoking adolescents. Addict Behav. 2007;32(5):977–990. doi: 10.1016/j.addbeh.2006.07.007. [DOI] [PubMed] [Google Scholar]
- 51.Le Bihan D. Looking into the functional architecture of the brain with diffusion MRI. Nat Rev Neurosci. 2003;4(6):469–480. doi: 10.1038/nrn1119. [DOI] [PubMed] [Google Scholar]
- 52.Lebel C, Walker L, Leemans A, Phillips L, Beaulieu C. Microstructural maturation of the human brain from childhood to adulthood. Neuroimage. 2008;40(3):1044–1055. doi: 10.1016/j.neuroimage.2007.12.053. [DOI] [PubMed] [Google Scholar]
- 53.Lucas CP, Zhang H, Fisher PW, Shaffer D, Regier DA, Narrow WE, Bourdon K, Dulcan MK, Canino G, Rubio-Stipec M, Lahey BB, Friman P. The DISC Predictive Scales (DPS): efficiently screening for diagnoses. J Am Acad Child Adolesc Psychiatry. 2001;40(4):443–449. doi: 10.1097/00004583-200104000-00013. [DOI] [PubMed] [Google Scholar]
- 54.Madler B, Drabycz SA, Kolind SH, Whittall KP, MacKay AL. Is diffusion anisotropy an accurate monitor of myelination? Correlation of multicomponent T2 relaxation and diffusion tensor anisotropy in human brain. Magn Reson Imaging. 2008;26(7):874–888. doi: 10.1016/j.mri.2008.01.047. [DOI] [PubMed] [Google Scholar]
- 55.Marsicano G, Goodenough S, Monory K, Hermann H, Eder M, Cannich A, Azad SC, Cascio MG, Gutierrez SO, van der Stelt M, Lopez-Rodriguez ML, Casanova E, Schutz G, Zieglgansberger W, Di Marzo V, Behl C, Lutz B. CB1 cannabinoid receptors and on-demand defense against excitotoxicity. Science. 2003;302(5642):84–88. doi: 10.1126/science.1088208. [DOI] [PubMed] [Google Scholar]
- 56.McQueeny T, Schweinsburg BC, Schweinsburg AD, Jacobus J, Bava S, Frank LR, Tapert SF. Altered white matter integrity in adolescent binge drinkers. Alcohol Clin Exp Res. 2009 doi: 10.1111/j.1530-0277.2009.00953.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Medina KL, Hanson KL, Schweinsburg AD, Cohen-Zion M, Nagel BJ, Tapert SF. Neuropsychological functioning in adolescent marijuana users: subtle deficits detectable after a month of abstinence. J Int Neuropsychol Soc. 2007;13(5):807–820. doi: 10.1017/S1355617707071032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Medina KL, McQueeny T, Nagel BJ, Hanson KL, Schweinsburg AD, Tapert SF. Prefrontal cortex volumes in adolescents with alcohol use disorders: unique gender effects. Alcohol Clin Exp Res. 2008;32(3):386–394. doi: 10.1111/j.1530-0277.2007.00602.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Medina KL, Schweinsburg AD, Cohen-Zion M, Nagel BJ, Tapert SF. Effects of alcohol and combined marijuana and alcohol use during adolescence on hippocampal volume and asymmetry. Neurotoxicol Teratol. 2007;29(1):141–152. doi: 10.1016/j.ntt.2006.10.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Meyerhoff DJ, Blumenfeld R, Truran D, Lindgren J, Flenniken D, Cardenas V, Chao LL, Rothlind J, Studholme C, Weiner MW. Effects of heavy drinking, binge drinking, and family history of alcoholism on regional brain metabolites. Alcohol Clin Exp Res. 2004;28(4):650–661. doi: 10.1097/01.ALC.0000121805.12350.CA. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Midanik LT, Tam TW, Weisner C. Concurrent and simultaneous drug and alcohol use: results of the 2000 National Alcohol Survey. Drug Alcohol Depend. 2007;90(1):72–80. doi: 10.1016/j.drugalcdep.2007.02.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Mitrirattanakul S, Lopez-Valdes HE, Liang J, Matsuka Y, Mackie K, Faull KF, Spigelman I. Bidirectional alterations of hippocampal cannabinoid 1 receptors and their endogenous ligands in a rat model of alcohol withdrawal and dependence. Alcohol Clin Exp Res. 2007;31(5):855–867. doi: 10.1111/j.1530-0277.2007.00366.x. [DOI] [PubMed] [Google Scholar]
- 63.Mukherjee P, Miller JH, Shimony JS, Conturo TE, Lee BC, Almli CR, McKinstry RC. Normal brain maturation during childhood: developmental trends characterized with diffusion-tensor MR imaging. Radiology. 2001;221(2):349–358. doi: 10.1148/radiol.2212001702. [DOI] [PubMed] [Google Scholar]
- 64.Mukherjee P, Miller JH, Shimony JS, Philip JV, Nehra D, Snyder AZ, Conturo TE, Neil JJ, McKinstry RC. Diffusion-tensor MR imaging of gray and white matter development during normal human brain maturation. AJNR Am J Neuroradiol. 2002;23(9):1445–1456. [PMC free article] [PubMed] [Google Scholar]
- 65.Nagel BJ, Schweinsburg AD, Phan V, Tapert SF. Reduced hippocampal volume among adolescents with alcohol use disorders without psychiatric comorbidity. Psychiatry Res. 2005;139(3):181–190. doi: 10.1016/j.pscychresns.2005.05.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.National Institute on Alcohol Abuse and Alcoholism NIAAA council approves definition of binge drinking. NIAAA Newsletter. 2004;3:3. [Google Scholar]
- 67.Padula CB, Schweinsburg AD, Tapert SF. Spatial working memory performance and fMRI activation interaction in abstinent adolescent marijuana users. Psychol Addict Behav. 2007;21(4):478–487. doi: 10.1037/0893-164X.21.4.478. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Parker GJ. Analysis of MR diffusion weighted images. Br J Radiol. 2004;77(2):S176–185. doi: 10.1259/bjr/81090732. [DOI] [PubMed] [Google Scholar]
- 69.Pascual M, Blanco AM, Cauli O, Minarro J, Guerri C. Intermittent ethanol exposure induces inflammatory brain damage and causes long-term behavioral alterations in adolescent rats. Eur J Neurosci. 2007;25(2):541–550. doi: 10.1111/j.1460-9568.2006.05298.x. [DOI] [PubMed] [Google Scholar]
- 70.Pfefferbaum A, Adalsteinsson E, Sullivan EV. Supratentorial profile of white matter microstructural integrity in recovering alcoholic men and women. Biol Psychiatry. 2006;59(4):364–372. doi: 10.1016/j.biopsych.2005.06.025. [DOI] [PubMed] [Google Scholar]
- 71.Pfefferbaum A, Mathalon DH, Sullivan EV, Rawles JM, Zipursky RB, Lim KO. A quantitative magnetic resonance imaging study of changes in brain morphology from infancy to late adulthood. Arch Neurol. 1994;51(9):874–887. doi: 10.1001/archneur.1994.00540210046012. [DOI] [PubMed] [Google Scholar]
- 72.Pfefferbaum A, Sullivan EV. Disruption of brain white matter microstructure by excessive intracellular and extracellular fluid in alcoholism: Evidence from diffusion tensor imaging. Neuropsychopharmacology. 2005;30(2):423–432. doi: 10.1038/sj.npp.1300623. [DOI] [PubMed] [Google Scholar]
- 73.Pfefferbaum A, Sullivan EV, Hedehus M, Adalsteinsson E, Lim KO, Moseley M. In vivo detection and functional correlates of white matter microstructural disruption in chronic alcoholism. Alcohol Clin Exp Res. 2000;24(8):1214–1221. [PubMed] [Google Scholar]
- 74.Pfefferbaum A, Sullivan EV, Mathalon DH, Lim KO. Frontal lobe volume loss observed with magnetic resonance imaging in older chronic alcoholics. Alcohol Clin Exp Res. 1997;21(3):521–529. doi: 10.1111/j.1530-0277.1997.tb03798.x. [DOI] [PubMed] [Google Scholar]
- 75.Pierpaoli C, Basser PJ. Toward a quantitative assessment of diffusion anisotropy. Magn Reson Med. 1996;36(6):893–906. doi: 10.1002/mrm.1910360612. [DOI] [PubMed] [Google Scholar]
- 76.Rice JP, Reich T, Bucholz KK, Neuman RJ, Fishman R, Rochberg N, Hesselbrock VM, Nurnberger JI, Jr., Schuckit MA, Begleiter H. Comparison of direct interview and family history diagnoses of alcohol dependence. Alcohol Clin Exp Res. 1995;19:1018–1023. doi: 10.1111/j.1530-0277.1995.tb00983.x. [DOI] [PubMed] [Google Scholar]
- 77.Sachdev PS, Chen X, Wen W, Anstry KJ. Light to moderate alcohol use is associated with increased cortical gray matter in middle-aged men: A voxel-based morphometric study. Psychiatry Res. 2008;163(1):61–69. doi: 10.1016/j.pscychresns.2007.08.009. [DOI] [PubMed] [Google Scholar]
- 78.Sarne Y, Mechoulam R. Cannabinoids: Between neuroprotection and neurotoxicity. Curr Drug Targets. 2005;4:677–684. doi: 10.2174/156800705774933005. [DOI] [PubMed] [Google Scholar]
- 79.Schmithorst VJ, Wilke M, Dardzinski BJ, Holland SK. Cognitive functions correlate with white matter architecture in a normal pediatric population: a diffusion tensor MRI study. Hum Brain Mapp. 2005;26(2):139–147. doi: 10.1002/hbm.20149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Schulteis G, Archer C, Tapert SF, Frank LR. Intermittent binge alcohol exposure during the periadolescent period induces spatial working memory deficits in young adult rats. Alcohol. 2008;42(6):459–467. doi: 10.1016/j.alcohol.2008.05.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Schweinsburg AD, Nagel BJ, Schweinsburg BC, Park A, Theilmann RJ, Tapert SF. Abstinent adolescent marijuana users show altered fMRI response during spatial working memory. Psychiatry Res. 2008;163(1):40–51. doi: 10.1016/j.pscychresns.2007.04.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Schweinsburg AD, Schweinsburg BC, Cheung EH, Brown GG, Brown SA, Tapert SF. fMRI response to spatial working memory in adolescents with comorbid marijuana and alcohol use disorders. Drug Alcohol Depend. 2005;79(2):201–210. doi: 10.1016/j.drugalcdep.2005.01.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Sher L. Functional magnetic resonance imaging in studies of neurocognitive effects of alcohol use on adolescents and young adults. Int J Adolesc Med Health. 2006;18(1):3–7. doi: 10.1515/ijamh.2006.18.1.3. [DOI] [PubMed] [Google Scholar]
- 84.Smith JE, Co C, McIntosh S, Cunningham CC. Chronic binge-like moderate ethanol drinking in rats results in widespread decreases in brain serotonin, dopamine, and norepinephrine turnover rates reversed by ethanol intake. J Neurochem. 2008 doi: 10.1111/j.1471-4159.2008.05296.x. [DOI] [PubMed] [Google Scholar]
- 85.Smith SM, Jenkinson M, Johansen-Berg H, Rueckert D, Nichols TE, Mackay CE, Watkins KE, Ciccarelli O, Cader MZ, Matthews PM, Behrens TE. Tract-based spatial statistics: voxelwise analysis of multi-subject diffusion data. Neuroimage. 2006;31(4):1487–1505. doi: 10.1016/j.neuroimage.2006.02.024. [DOI] [PubMed] [Google Scholar]
- 86.Smith SM, Jenkinson M, Woolrich MW, Beckmann CF, Behrens TEJ, Johansen-Berg H, Bannister PR, De Luca M, Drobnjak I, Flitney DE, Niazy RK, Saunders J, Vickers J, Zhang Y, De Stefano N, Brady JM, Matthews PM. Advances in functional and structural MR image analysis and implementation as FSL. NeuroImage. 2004;23(S1):208–219. doi: 10.1016/j.neuroimage.2004.07.051. [DOI] [PubMed] [Google Scholar]
- 87.Snook L, Paulson LA, Roy D, Phillips L, Beaulieu C. Diffusion tensor imaging of neurodevelopment in children and young adults. Neuroimage. 2005;26(4):1164–1173. doi: 10.1016/j.neuroimage.2005.03.016. [DOI] [PubMed] [Google Scholar]
- 88.Substance Abuse and Mental Health Services Administration . Results from the 2007 National Survey on Drug Use and Health: National Findings. Rockville, MD: 2008. [Google Scholar]
- 89.Sullivan EV, Rosenbloom MJ, Pfefferbaum A. Pattern of motor and cognitive deficits in detoxified alcoholic men. Alcohol Clin Exp Res. 2000;24(5):611–621. [PubMed] [Google Scholar]
- 90.Szumlinski KK, Diab ME, Friedman R, Henze LM, Lominac KD, Bowers MS. Accumbens neurochemical adaptations produced by binge-like alcohol consumption. Psychopharmacology (Berl) 2007;190(4):415–431. doi: 10.1007/s00213-006-0641-7. [DOI] [PubMed] [Google Scholar]
- 91.Tapert SF, Brown GG, Kindermann SS, Cheung EH, Frank LR, Brown SA. fMRI measurement of brain dysfunction in alcohol-dependent young women. Alcohol Clin Exp Res. 2001;25(2):236–245. [PubMed] [Google Scholar]
- 92.Tapert SF, Brown SA. Substance dependence, family history of alcohol dependence and neuropsychological functioning in adolescence. Addiction. 2000;95(7):1043–1053. doi: 10.1046/j.1360-0443.2000.95710436.x. [DOI] [PubMed] [Google Scholar]
- 93.Tapert SF, Schweinsburg AD, Barlett VC, Brown SA, Frank LR, Brown GG, Meloy MJ. Blood oxygen level dependent response and spatial working memory in adolescents with alcohol use disorders. Alcohol Clin Exp Res. 2004;28(10):1577–1586. doi: 10.1097/01.alc.0000141812.81234.a6. [DOI] [PubMed] [Google Scholar]
- 94.Tapert SF, Schweinsburg AD, Drummond SP, Paulus MP, Brown SA, Yang TT, Frank LR. Functional MRI of inhibitory processing in abstinent adolescent marijuana users. Psychopharmacology (Berl) 2007;194(2):173–183. doi: 10.1007/s00213-007-0823-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Tapert SF, Theilmann RJ, Schweinsburg AD. Reduced fractional anisotropy in the splenium of adolescents with alcohol use disorders. Proc Intl Soc Mag Reson Med. 2003;11:8217. [Google Scholar]
- 96.Tarter RE, Ryan CM. Neuropsychology of alcoholism. Etiology, phenomenology, process, and outcome. Recent Dev Alcohol. 1983;1:449–469. [PubMed] [Google Scholar]
- 97.Tokunaga S, Silvers JM, Matthews DB. Chronic intermittent ethanol exposure during adolescence blocks ethanol-induced inhibition of spontaneously active hippocampal pyramidal neurons. Alcohol Clin Exp Res. 2006;30(1):1–6. doi: 10.1111/j.1530-0277.2006.00020.x. [DOI] [PubMed] [Google Scholar]
- 98.Townshend JM, Duka T. Binge drinking, cognitive performance, and mood in a population of young social drinkers. Alcohol Clin Exp Res. 2005;29(3):317–325. doi: 10.1097/01.alc.0000156453.05028.f5. [DOI] [PubMed] [Google Scholar]
- 99.Turken A, Whitfield-Gabrieli S, Bammer R, Baldo JV, Dronkers NF, Gabrieli JD. Cognitive processing speed and the structure of white matter pathways: Convergent evidence from normal variation and lesion studies. Neuroimage. 2008;42(2):1032–1044. doi: 10.1016/j.neuroimage.2008.03.057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Tzilos GK, Cintron CB, Wood JB, Simpson NS, Young AD, Pope HG, Jr., Yurgelun-Todd DA. Lack of hippocampal volume change in long-term heavy cannabis users. Am J Addict. 2005;14(1):64–72. doi: 10.1080/10550490590899862. [DOI] [PubMed] [Google Scholar]
- 101.van der Stelt M, Veldhuis WB, Bar PR, Veldink GA, Vliegenthart JF, Nicolay K. Neuroprotection by Delta9-tetrahydrocannabinol, the main active compound in marijuana, against ouabain-induced in vivo excitotoxicity. J Neurosci. 2001;21(17):6475–6479. doi: 10.1523/JNEUROSCI.21-17-06475.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.van der Stelt M, Veldhuis WB, Maccarrone M, Bar PR, Nicolay K, Veldink GA, Di Marzo V, Vliegenthart JF. Acute neuronal injury, excitotoxicity, and the endocannabinoid system. Mol Neurobiol. 2002;26(2−3):317–346. doi: 10.1385/MN:26:2-3:317. [DOI] [PubMed] [Google Scholar]
- 103.van Eimeren L, Niogi SN, McCandliss BD, Holloway ID, Ansari D. White matter microstructures underlying mathematical abilities in children. Neuroreport. 2008;19(1):1117–1121. doi: 10.1097/WNR.0b013e328307f5c1. [DOI] [PubMed] [Google Scholar]
- 104.Wakana S, Jiang H, Nagae-Poetscher LM, van Zijl PCM, Mori S. Fiber tract-based atlas of human white matter anatomy. Radiology. 2003;230(1):77–87. doi: 10.1148/radiol.2301021640. [DOI] [PubMed] [Google Scholar]
- 105.Wechsler D. Psychological Corporation. New York: 1997. WAIS-III Manual. [Google Scholar]
- 106.Wechsler H, Nelson TF. Binge drinking and the American college student: What's five drinks? Psychol Addict Behav. 2001;15(4):287–291. doi: 10.1037//0893-164x.15.4.287. [DOI] [PubMed] [Google Scholar]
- 107.Weissenborn R, Duka T. Acute alcohol effects on cognitive function in social drinkers: their relationship to drinking habits. Psychopharmacology (Berl) 2003;165(3):306–312. doi: 10.1007/s00213-002-1281-1. [DOI] [PubMed] [Google Scholar]
- 108.Wert RC, Raulin ML. The chronic cerebral effects of cannabis use. I. Methodological issues and neurological findings. Int J Addict. 1986;21(6):605–628. doi: 10.3109/10826088609027381. [DOI] [PubMed] [Google Scholar]
- 109.Young C, Olney JW. Neuroapoptosis in the infant mouse brain triggered by a transient small increase in blood alcohol concentration. Neurobiol Dis. 2006;22(3):548–554. doi: 10.1016/j.nbd.2005.12.015. [DOI] [PubMed] [Google Scholar]
- 110.Ziegler DW, Wang CC, Yoast RA, Dickinson BD, McCaffree MA, Robinowitz CB, Sterling ML. The neurocognitive effects of alcohol on adolescents and college students. Prev Med. 2005;40(1):23–32. doi: 10.1016/j.ypmed.2004.04.044. [DOI] [PubMed] [Google Scholar]