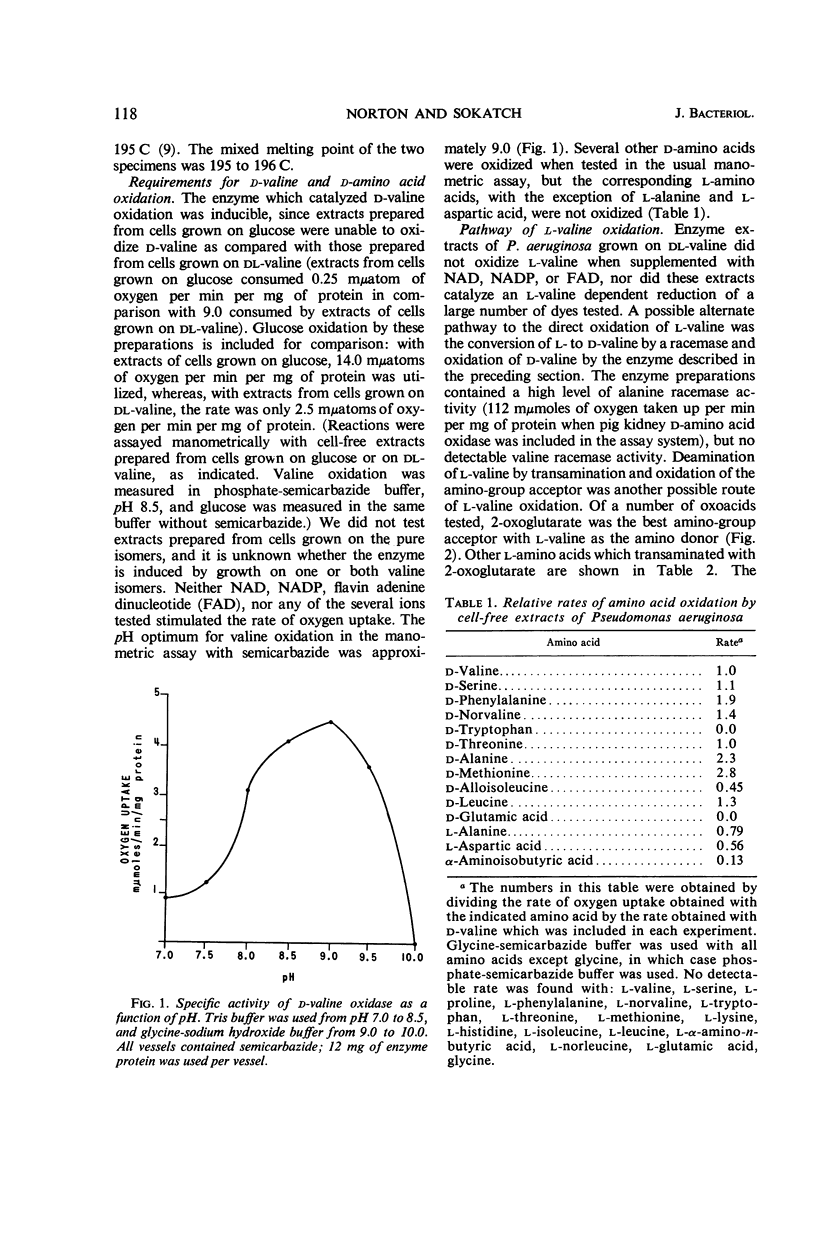
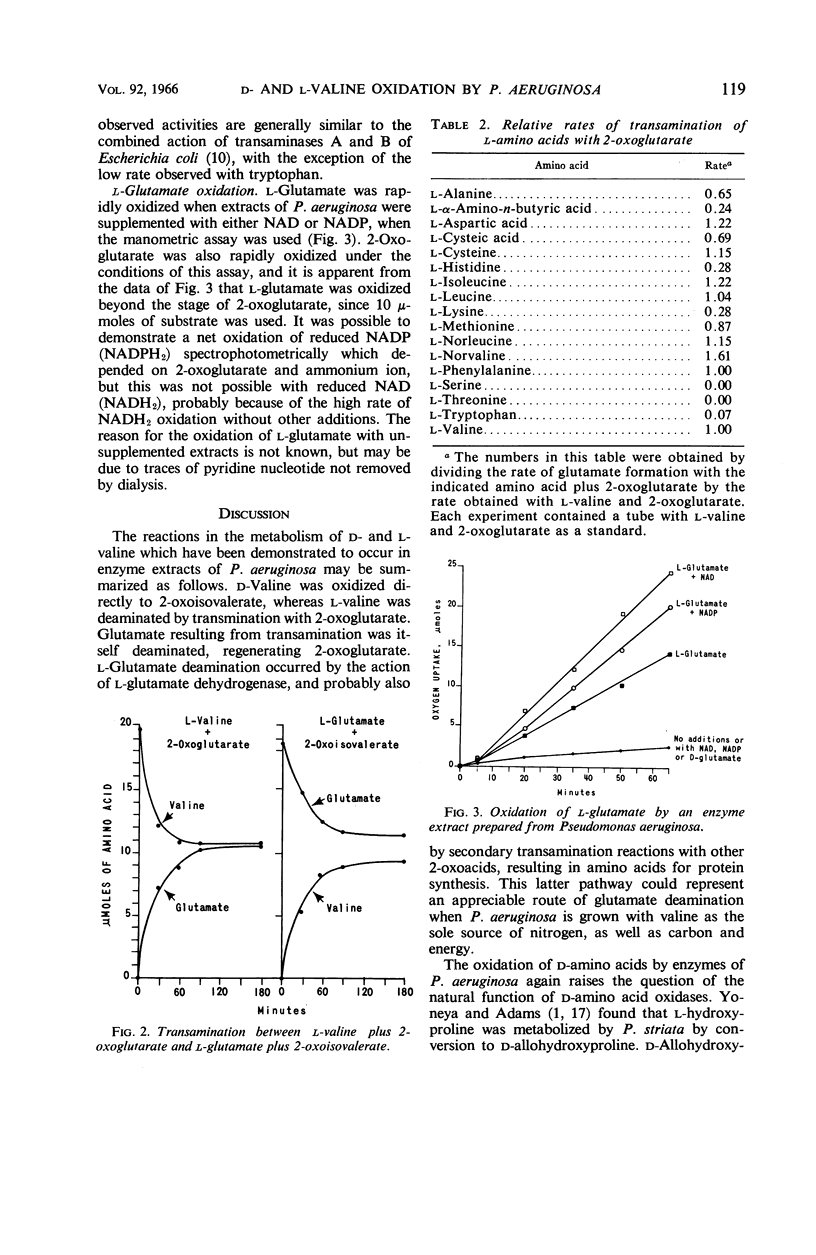
Abstract
Norton, J. E. (University of Oklahoma School of Medicine, Oklahoma City), and J. R. Sokatch. Oxidation of d- and l-valine by enzymes of Pseudomonas aeruginosa. J. Bacteriol. 92:116–120. 1966.—Cell-free extracts prepared from Pseudomonas aeruginosa grown on dl-valine catalyzed the consumption of oxygen with several d-amino acids, but not with the corresponding l-amino acids. The product of d-valine oxidation was identified as 2-oxoisovalerate by the preparation and characterization of 2-oxoisovalerate 2,4-dinitrophenylhydrazone. The enzyme catalyzing d-amino acid oxidation was present in extracts of cells grown on valine, but not on glucose, had a pH optimum of approximately 9.0, consumed 1 atom of oxygen per mole of keto acid produced, and was not stimulated by any of the usual electron transport cofactors. It was not possible to demonstrate either the direct oxidation of l-valine or the conversion of l- to d-valine by these enzyme preparations. However, a possible route of l-valine metabolism by transamination with 2-oxoglutarate with regeneration of the amino group acceptor by glutamate oxidation was established by identification of the transaminase and l-glutamate dehydrogenase in these enzyme preparations.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- ADAMS E. Hydroxyproline metabolism. I. Conversion to alpha-ketoglutarate by extracts of Pseudomonas. J Biol Chem. 1959 Aug;234(8):2073–2084. [PubMed] [Google Scholar]
- HEILMANN J., BARROLLIER J., WATZKE E. Beitrag zur Aminosäurebestimmung auf Papierchromatogrammen. Hoppe Seylers Z Physiol Chem. 1957;309(4-6):219–220. [PubMed] [Google Scholar]
- Halpern Y. S., Lupo M. Glutamate transport in wild-type and mutant strains of Escherichia coli. J Bacteriol. 1965 Nov;90(5):1288–1295. doi: 10.1128/jb.90.5.1288-1295.1965. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MEISTER A. Transamination. Adv Enzymol Relat Subj Biochem. 1955;16:185–246. doi: 10.1002/9780470122617.ch4. [DOI] [PubMed] [Google Scholar]
- NISMAN B. The Stickland reaction. Bacteriol Rev. 1954 Mar;18(1):16–42. doi: 10.1128/br.18.1.16-42.1954. [DOI] [PMC free article] [PubMed] [Google Scholar]
- NORTON J. E., BULMER G. S., SOKATCH J. R. THE OXIDATION OF D-ALANINE BY CELL MEMBRANES OF PSEUDOMONAS AERUGINOSA. Biochim Biophys Acta. 1963 Oct 8;78:136–147. doi: 10.1016/0006-3002(63)91619-6. [DOI] [PubMed] [Google Scholar]
- RUDMAN D., MEISTER A. Transamination in Escherichia coli. J Biol Chem. 1953 Feb;200(2):591–604. [PubMed] [Google Scholar]
- SANWAL B. D., ZINK M. W. L-Leucine dehydrogenase of Bacillus cereus. Arch Biochem Biophys. 1961 Sep;94:430–435. doi: 10.1016/0003-9861(61)90070-4. [DOI] [PubMed] [Google Scholar]
- Sokatch J. R. Alanine and aspartate formation during growth on valine-C14 by Pseudomonas aeruginosa. J Bacteriol. 1966 Jul;92(1):72–75. doi: 10.1128/jb.92.1.72-75.1966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stickland L. H. Studies in the metabolism of the strict anaerobes (genus Clostridium): The chemical reactions by which Cl. sporogenes obtains its energy. Biochem J. 1934;28(5):1746–1759. doi: 10.1042/bj0281746. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vender J., Jayaraman K., Rickenberg H. V. Metabolism of glutamic acid in a mutant of Escherichia coli. J Bacteriol. 1965 Nov;90(5):1304–1307. doi: 10.1128/jb.90.5.1304-1307.1965. [DOI] [PMC free article] [PubMed] [Google Scholar]
- WOOD W. A., GUNSALUS I. C. D-Alanine formation; a racemase in Streptococcus faecalis. J Biol Chem. 1951 May;190(1):403–416. [PubMed] [Google Scholar]
- YONEYA T., ADAMS E. Hydroxyproline metabolism. V. Inducible allohydroxy-D-proline oxidase of Pseudomonas. J Biol Chem. 1961 Dec;236:3272–3279. [PubMed] [Google Scholar]


