Abstract
People are often able to reliably detect a mixture of 2 or more odorants, even if they cannot reliably detect the individual mixture components when presented individually. This phenomenon has been called mixture agonism. However, for some mixtures, agonism among mixture components is greater in barely detectable mixtures than in more easily detectable mixtures (level dependence). Most studies that have used rigorous methods have focused on simple, 2-component (binary) mixtures. The current work takes the next logical step to study detection of 3-component (ternary) mixtures. Psychometric functions were measured for 5 unmixed compounds and for 3 ternary mixtures of these compounds (2 of 5, forced-choice method). Experimenters used air dilution olfactometry to precisely control the duration and concentration of stimuli and used gas chromatography/mass spectrometry to verify vapor-phase concentrations. For 2 of the 3 mixtures, agonism was approximately additive in general agreement with similar work on binary mixtures. A third mixture was no more detectable than the most detectable component, demonstrating a lack of agonism. None of the 3 mixtures showed evidence of level dependence. Agonism may be common in ternary mixtures, but general rules of mixture interaction have yet to emerge. For now, detection of any mixture must be measured empirically.
Keywords: mixture interaction, psychophysics, threshold
Introduction
Detection of odors by humans has both basic and applied significance in clinical investigations, environmental and occupational exposure, olfactory genetics, neuroscience, food science, and cross-species comparisons (Wysocki and Beauchamp 1984; Lawless and Heyman 1998; Laska et al. 2003; Cain et al. 2007; Lötsch et al. 2007; Menashe et al. 2007; Rosenfeld et al. 2007). Detection of mixtures is particularly important because most natural odorants are comprised of more than one compound. Unfortunately, relatively few studies on human odor detection have focused on mixtures.
The majority of work on odor mixtures has focused on perceived intensity of suprathreshold (clearly detectable) mixtures. Excellent discussions of models of suprathreshold mixture interactions are available (e.g., Laffort et al. 1989; Cain et al. 1995; Thomas-Danguin and Chastrette 2002). The current work will focus on detection of odor mixtures in the perithreshold range.
The basic rules that govern detection of odor mixtures remain unclear, but some generalities have emerged regarding detection of mixtures of perithreshold odors. Concentrations of individual compounds in threshold-level mixtures tend to be lower than thresholds for the corresponding compounds when presented alone, a phenomenon sometimes referred to as agonism (Rosen et al. 1962; Baker 1963; Guadagni et al. 1963; Laska and Hudson 1991; Patterson et al. 1993; Cometto-Muñiz et al. 1997). Degree of agonism is commonly assessed with respect to a specific model of additivity, such as dose addition or probability summation (Cometto-Muñiz et al. 2003; Wise et al. 2007; Miyazawa et al. 2008; also see Materials and methods). Approximately additive interactions are common, but subadditivity (agonism, but detection performance that falls below the predictions of additivity models) and synergy (detection performance that exceeds the predictions of additivity models) have also been observed (Wise et al. 2007; Miyazawa et al. 2008). Antagonism, in which a mixture would be less detectable than the most detectable component, remains plausible (see Spehr et al. 2004; Brodin et al. 2009).
Methodological limitations make it difficult to determine whether much of the observed variation in degree of agonism is meaningful. The rigor of stimulus control has seldom been clear, and most of the earlier studies did not verify stimulus concentrations in the vapor phase. Furthermore, most studies only estimated thresholds, so detailed analyses of mixture interactions across a range of concentrations have seldom been possible.
Recent studies of binary (2-component) mixtures have overcome some of these limitations. Cometto-Muñiz et al. (1999, 2003, 2005) measured vapor-phase concentrations of stimuli and measured complete psychometric functions for single (unmixed) compounds. Psychometric functions for unmixed compounds were used to formulate binary mixtures with different levels of predicted detection performance. As in earlier studies, agonism occurred. However, mixtures below the level typically defined as threshold (but detected at greater than chance level) showed a greater degree of agonism than mixtures that were above threshold (but detected <100% of the time). In short, level dependence occurred. Other studies of binary mixtures using rigorous methods not only yielded evidence for both agonism and level dependence but also found that level dependence does not occur for all binary mixtures and that mixture interactions may be related to the molecular properties of the components (Wise et al. 2007; Miyazawa et al. 2008).
This work on binary mixtures constitutes a logical first step, but natural odors are often comprised of many constituent chemicals (Nijssen et al. 1996). Furthermore, some work on the perception of suprathreshold odor mixtures suggests that interactions in complex mixtures may not always be easy to predict based on interactions in simple mixtures (Moskowitz and Barbe 1977; Laing et al. 1994; Wagner et al. 2006; Brossard et al. 2007). To understand the detection of natural odors, we must also study complex mixtures.
Here, we extend a recent series of studies on binary mixtures in the perithreshold range (Wise et al. 2007; Miyazawa et al. 2008) to ternary (3-component) mixtures. To the best of our knowledge, these experiments constitute the first work on detection of ternary mixtures in the perithreshold range that combines precise stimulus control via air dilution olfactometry, vapor-phase calibration of stimuli, and measurement of full detection functions using rigorous psychophysical methods. Psychometric functions were measured for 5 unmixed compounds and for 3 ternary mixtures of those compounds (see Materials, below). One basic question was whether clear agonism occurs in these more complex mixtures and whether degree of agonism varies greatly between mixtures. Another basic question was whether patterns of mixture interaction would depend on overall concentration (whether agonism would differ between the low and high perithreshold levels for a given mixture).
Materials and methods
Subjects
Twelve healthy, nonsmoking adults (7 females) participated. Ages ranged from 22 to 47. Most were employees of the Monell Chemical Senses Center. Other subjects came from the local community. Both employees and outside subjects provided written informed consent on forms approved by the Independent Review Board of the University of Pennsylvania and were financially compensated. All subjects had participated in several experiments using the same procedures and apparatus, so had extensive practice. Previous experiments had also demonstrated that the subjects were able to detect the compounds to be used (see Materials, below) within the range of concentrations used.
Materials
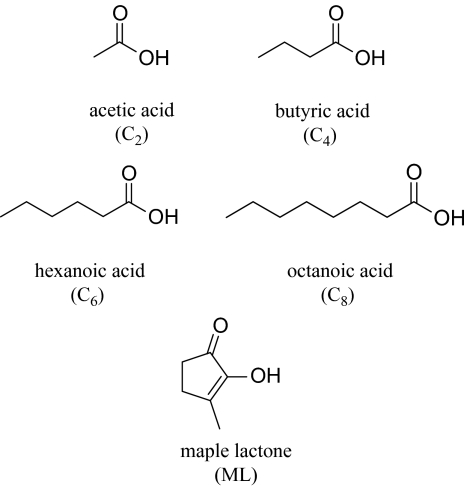
Stimuli included: acetic acid (CAS# 64-19-7; Nagase ChemteX Corporation, 99.7% pure), butyric acid (CAS# 107-92-6; Daicel Chemical Industries, Ltd, 99.6% pure), hexanoic acid (CAS# 142-62-1; Chisso Corporation, 98.5% pure), octanoic acid (CAS# 124-07-2; Inoue Perfumery Co., Ltd, 97.3% pure), and 2-hydroxy-3-methyl-2-cyclopentene-1-one (called maple lactone [ML]; CAS# 80-71-7; Toyotama International Inc., 98.3% pure). Manufacturer claims regarding purity were verified using gas chromatography/mass spectrometry (GC/MS). Regardless, it remains possible (though unlikely) that some trace compounds might have influenced the results.
Subjects received a 6-step dilution series of each unmixed compound (Table 1). Successive concentration steps differed by a factor of about 2.2. Extensive pilot work suggested that the range of concentrations would span a wide range of detection performance for most subjects, with comparable levels of detection at a given step across compounds.
Table 1.
Concentrations of stimuli, log ppm by mass (log mg/m3 in parentheses)
| Compound |
|||||
| Step | C2 | C4 | C6 | C8 | ML |
| 1 | −2.86 (−2.78) | −4.43 (−4.35) | −3.46 (−3.38) | −3.27 (−3.19) | −3.00 (−2.92) |
| 2 | −2.51 (−2.43) | −3.99 (−3.91) | −3.11 (−3.03) | −2.93 (−2.85) | −2.67 (−2.59) |
| 3 | −2.17 (−2.09) | −3.63 (−3.55) | −2.76 (−2.68) | −2.60 (−2.52) | −2.35 (−2.27) |
| 4 | −1.82 (−1.74) | −3.28 (−3.20) | −2.41 (−2.33) | −2.27 (−2.19) | −2.02 (−1.94) |
| 5 | −1.47 (−1.39) | −2.93 (−2.85) | −2.06 (−1.98) | −1.94 (−1.86) | −1.70 (−1.62) |
| 6 | −1.12 (−1.04) | −2.58 (−2.50) | −1.71 (−1.63) | −1.60 (−1.52) | −1.37 (−1.29) |
In addition, subjects received a 6-step dilution series of each of 3 binary mixtures. One mixture (C2 + C4 + C6) included acetic, butyric, and hexanoic acids, closely related compounds which share the same functional group and differ only in the number of methylene units in the base chain (Figure 1). In a second mixture (C2 + C4 + C8), hexanoic acid was replaced with octanoic acid to achieve slightly more structural diversity. In the third mixture (C2 + C4 + ML), hexanoic acid was replaced with ML, which is dissimilar to the acids in both structure and suprathreshold odor character. These ternary mixtures do not allow a thorough structure-activity study. However, manipulating structural similarity within mixtures qualifies as a logical approach to diversify our stimuli in this initial investigation of ternary mixtures.
Figure 1.
Molecular structures of the odor materials used in the experiment.
Concentrations of all compounds were the same in ternary mixtures as when presented alone. For example, the lowest concentration step of C2 + C4 + C6 consisted of the lowest concentration step of C2, the lowest step of C4, and the lowest step of C6. Thus, the concentration ratios of the 3 compounds remained the same as overall concentration of the mixture increased.
Though quality was not systematically examined for any of the stimuli, quality seemed rather indistinct at the perithreshold concentrations at which the single compounds and mixtures were presented. At suprathreshold levels, acetic acid smells like vinegar. Butyric acid smells like rancid fat and is an important note in blue cheese aroma. Hexanoic acid has been described as “goat like,” with a rancid fat note. Octanoic acid also has a rancid fat note. ML is quite different in quality from the carboxylic acids, with a sweet, “maple syrup” aroma.
Olfactometer and calibration
Stimuli were presented using an automated air dilution olfactometer. Nitrogen flowed through odor vessels containing pure chemicals (powdered ML was diluted with MilliQ-filtered water at 0.01 g/mL before being placed in an odor vessel). Odorized nitrogen was mixed with filtered air to create a 6-step dilution series of each stimulus. Chemical mixtures were formed in vapor phase, that is, by combining nitrogen streams from 3 separate odor vessels, before subsequent air dilution. All concentrations were generated continuously and vented out of the room when not presented to subjects. Electronic valves could gate any of the 6 concentrations, or a clean air blank, to a glass cone. Subjects sampled by placing their noses in the cone and sniffing naturally. The olfactometer provided a total flow of 30 L/min at output to allow subjects to sniff without inhaling room air.
Samples were collected at the output of the olfactometer in Tedlar bags. Samples were quantified using GC/MS. Solid phase micro extraction (SPME) was used to enhance analytical sensitivity. SPME fibers were extended into sample bags for 45 min. After 45 min of collection, the compounds were desorbed in the injection port of the GC/MS system. A liquid dilution series of each compound (in chloroform) provided standards to convert GC area to parts per million (ppm; by mass). Liquid standards were injected into Tedlar bags filled with nitrogen and allowed to evaporate in the bags overnight. The resulting gas-phase standards were sampled using SPME fibers, with the same procedure used to quantify samples from the olfactometer.
A detailed description of the design and calibration of the olfactometer is available in Supplementary material. Calibration yielded 3 important results with respect to interpretation of the psychophysical data. First, 2.2-fold air dilutions in the olfactometer produced 2.2-fold drops in ppm. Second, concentrations were stable, both within and between days. Third, concentrations for a given single compound precisely matched concentrations of that compound when presented in a ternary mixture.
Procedure
Subjects received 2 identical odors and 3 clean air blanks, in random order, during each trail (2 of 5, forced-choice procedure). The 5 samples consisted of 2.5 s odor pulses, with 3 s pauses between pulses. During each pulse, a virtual light on a computer screen lit up under a particular label (“Sample 1,” “Sample 2,” etc.). In addition, a continuous beep accompanied each odor pulse. Subjects could flag each sample as a possible odor by clicking on virtual buttons next to the corresponding label. A mouse click changed the color of a button from green to red and changed text on the button from “blank” to “odor.” The number of samples that subjects could flag as odors was not limited during this initial presentation of the 5 samples.
After the 5 samples were presented, a second screen appeared. The second screen showed a pair of virtual buttons next to each sample label. One button was used to enter responses. The initial states of the response buttons (odor or blank) were set according to the responses during the initial presentation of the 5 samples. The other button next to each label triggered a 2.5 s pulse of the sample in question, so that subjects could resample the 5 stimuli. Subjects were allowed to resample until they were satisfied with their responses. Pilot work had shown that resampling improved detection, so resampling was used to help optimize detection.
Subjects knew that exactly 2 of the 5 stimuli presented each trial were odors. Subjects were required to identify exactly 2 samples as odors, guessing if uncertain. Subject entered their final responses by clicking a virtual “enter” button, and the experimental program would not accept a final response unless exactly 2 samples were flagged as odors. A response counted as correct only if the subject identified both odors. At least 15 s elapsed between trials.
During an experimental session of about 40 min, subjects received 6 presentations of each of 6 concentrations (see Table 1) of a fixed stimulus. The stimulus could be either a pure compound or a ternary mixture (for more detail, see Materials, above). Stimuli were presented in blocked, ascending order of concentration. Subjects received the lowest concentration on the first 3 trials, the next lowest in the next 3 trials, and so forth up to the highest concentration. After a break of at least 5 min, the sequence was repeated, again starting with the lowest concentration. Subjects received each ternary mixture and each single compound in 2 sessions, for a total of 12 trials per condition. The 5 unmixed compounds and 3 ternary mixtures (8 stimuli in total) were tested in blocked, mostly random order (subjects needed to reschedule sessions on occasion, so order was not purely random).
Data analysis
First, data from the 2 replicate sessions for each stimulus condition were pooled to estimate proportion correct (pcorr) for each for each subject in each stimulus condition. Next, a correction for chance (pcorr expected if subjects simply guess) was applied: Chance-corrected pcorr = (pcorr − 0.10)/(1 − 0.10). This transform converts chance-level performance (10% correct) to 0.0 and perfect performance (100% correct) to 1.0, with intermediate values falling between. Next, values of ≤0.0 were converted to 0.005, and values of 1.0 were converted to 0.995 to accommodate a log-odds ratio transform, for which input values of 0 and 1 are undefined (Macmillan and Creelman 1991). The log-odds ratio transform follows: log odds = ln[p/(1 − p)], where p represents chance-corrected pcorr and ln indicates natural log. We chose this transform because pilot work showed that cumulative logistic functions fit detection data better than other sigmoidal functions. Finally, the log-odds ratio of chance-corrected proportion was averaged across subjects for each compound and concentration and plotted against log concentration to form psychometric functions. Transformation made psychometric functions approximately linear, so functions could be fit using least-squares linear regression.
Patterns of mixture interaction were compared with probability summation, that is, the assumption that detecting a ternary mixture equals the probability of detecting one or more of the mixture components: p(ABC) = p(A) + p(B) + p(C) – p(A)p(B) – p(A)p(C) – p(B)p(C) + p(A)p(B)p(C), where p(ABC) represents the predicted probability of detecting a ternary mixture, p(A) represents the observed probability of detecting component A, P(B) represents the observed probability of detecting component B, and p(C) represents the observed probability of detecting component C (Feller 1968). Within the framework of the model, if detection performance for the mixture falls below probability summation, some degree of suppression has occurred relative to statistical independence. If performance falls above probability summation, then some form of mutual enhancement, or synergy, has occurred.
All analyses were conducted using Statistica software (Version 8.0, Statsoft). Linear (least-squares) regression was used to fit to single-compound psychometric functions for individual subjects. These linear fits in turn were used to generate individual predictions of probability summation for each ternary mixture. Repeated measures analysis of variance (ANOVA) models were used to compare psychometric functions for mixtures to psychometric functions for unmixed compounds. Repeated measure ANOVA models were also used to compare probability summation predictions to observed psychometric functions for ternary mixtures. Initial analyses indicated that some violations of the sphericity occurred, so both univariate analyses with corrected degrees of freedom (Greenhouse and Geisser 1959) and multivariate (multivariate analysis of variance [MANOVA], Wilk’s test) analyses (Gill 2001) were conducted. Because both approaches supported the same conclusions, we only report the MANOVA results below (significance criterion of P < 0.05).
Results
Psychometric functions for unmixed compounds
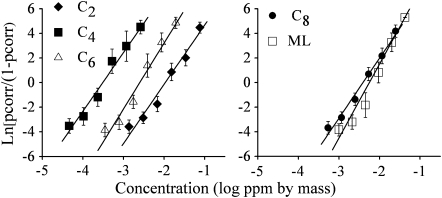
pcorr increased smoothly with concentration, with good linear fits in the coordinate space used (R2 ranged from 0.97 to 0.99, see Figure 2). For linear fits to data from individual subjects, R2 values ranged from 0.51 to 0.99 (mean = 0.80, standard deviation = 0.17). In short, cumulative logistic functions fit the data reasonably well. For average data, detection of all compounds rose from near chance to essentially perfect with an increase in concentration of 1.7 log units (50-fold increase). Slopes of psychometric functions were relatively similar across compounds: In a concentration step × compound ANOVA, the interaction failed to reach significance (P > 0.88). Thresholds, that is, the concentrations that would lead to detection performance halfway between chance level and 100% correct are: −1.96 log ppm by mass (−1.88 log mg/m3) for C2, −3.52 log ppm (−3.44 log mg/m3) for C4, −2.61 log ppm (−2.53 log mg/m3) for C6, −2.40 log ppm (−2.32 log mg/m3) for C8, and −2.20 log ppm (−2.12 log mg/m3) for ML.
Figure 2.
Psychometric functions for single (unmixed) compounds. y axis: the log-odds ratio of chance-corrected pcorr. x axis: stimulus concentration in log ppm (by mass). The lines represent best-fit linear functions (least-squares regression). The functions for ML and octanoic acid (C8) are depicted on a separate graph for clarity.
Detection of ternary mixtures compared with detection of single mixture-components
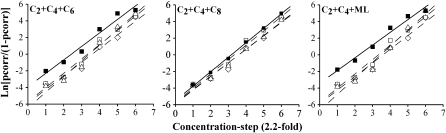
For C2 + C4 + C6 and C2 + C4 + ML, detection of ternary mixtures exceeded detection of the individual mixture components (Figure 3). For C2 + C4 + C8, detection of the ternary mixture was approximately equal to detection of the individual mixture components. Two-way ANOVAs (concentration × mixture condition) that compared detection functions for individual mixture components with corresponding functions for ternary mixtures confirmed these impressions. For C2 + C4 + C6, the detection function for the ternary mixture exceeded the function for C2 (F1,11 = 42.32, P << 0.001), exceeded the function for C4 (F1,11 = 7.61, P < 0.02), and exceeded the function for C6 (F1,11 = 38.27, P << 0.001). For C2 + C4 + C8, the detection function for the ternary mixture failed to exceed the functions for C2, C4, and C8, to a statistically significant degree, though there were marginal trends in this direction for C2, F1,11 = 4.58, P < 0.06, and for C8, F1,11 = 4.46, P < 0.06. For C2 + C4 + ML, the detection function for the ternary mixture exceeded the function for C2 (F1,11 = 71.22, P << 0.001), exceeded the function for C4 (F1,11 = 11.60, P < 0.01), and exceeded the function for ML (F1,11 = 21.06, P < 0.001). Interestingly, the concentration × mixture condition interaction failed to reach statistical significance (P > 0.10) for all comparisons except ML versus C2 + C4 + ML, F5,7 = 5.26, P < 0.02. Accordingly, the analysis provides no strong evidence for concentration dependence.
Figure 3.
Psychometric functions for ternary mixtures (filled squares) and single compounds (open diamonds for C2, open squares for C4, open triangles for the third mixture component). y axis: log-odds ratio of chance-corrected pcorr and x axis: concentration step (2.2-fold dilutions, where the proportions of the 3 compounds in each mixture remain the same as concentration increases). Lines (solid for mixtures, dashed or dotted for single compounds) represent linear fits (least-squares regression). Error bars omitted for clarity (error bars for these functions appear in Figures 2 and 4).
Detection of ternary mixtures compared with additivity predictions
Detection data for all ternary mixtures, together with corresponding predictions based on probability summation of detection data for the unmixed components, were submitted to an ANOVA. Predicted detection of ternary mixtures was close to perfect for the second highest concentrations. Because our methods did not produce data precise enough to discriminate between levels of performance near asymptote, our ability to assess mixture interactions suffered from a ceiling effect. Accordingly, data for the highest concentrations were not analyzed. ANOVA factors follow: concentration step (1–5) × mixture (C2 + C4 + C6, C2 + C4 + C8, C2 + C4 + ML) × data type (observed detection for ternary mixtures vs. predicted values).
The effect of concentration step reached significance, F4,8 = 197.17, P << 0.0001, demonstrating an expected dose-response relationship. The effect of mixture also reached significance, F2,10 = 9.84, P < 0.01, indicating that not all mixtures were equally detectable overall. Perhaps the more interesting result is a significant interaction between mixture and data type, F2,10 = 4.25, P < 0.05, which indicates that some mixtures match additivity predictions better than others. Other effects failed to reach statistical significance.
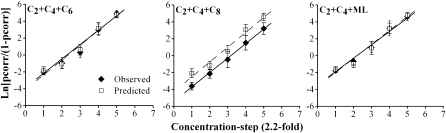
Inspection of the observed and predicted psychometric functions for ternary mixtures suggests that detection for C2 + C4 + C6 and C2 + C4 + ML matched predictions quite well but that detection of C2 + C4 + C8 was uniformly lower than predicted (Figure 4).
Figure 4.
Psychometric functions for 3 ternary mixtures: Acetic, butyric, and hexanoic acids (left); acetic, butyric, and octanoic acids (middle); and acetic acid, butyric acid, and ML (right). y axis: log-odds ratio of chance-corrected pcorr; x axis: concentration step (2.2-fold dilutions, where the proportions of the 3 compounds in each mixture remain the same as concentration increases). Filled symbols (solid lines) represent observed detection data. Open symbols (dashed lines) represent additivity predictions based on detection functions for individual components under an assumption of probability summation. Error bars represent ± standard error of the mean.
Simple concentration × data versus model ANOVAs for individual mixtures confirmed these impressions. For C2 + C4 + C6, both the main effect of data type and the interaction with concentration failed to reach significance (P > 0.30). The same was true for C2 + C4 + ML (P = 0.37). In contrast, for C2 + C4 + C8, the main effect of data type reached significance, F1,11 = 12.55, P < 0.01; the interaction with concentration failed to reach significance (P > 0.76). Thus, the analysis provided no evidence that detection of C2 + C4 + C6 or C2 + C4 + ML deviated from response addition at any of the measured concentrations but did provide evidence that C2 + C4 + C8 showed general (nonconcentration dependent) suppression with respect to response addition.
Discussion
Agonism among mixture components
For 2 of 3 ternary mixtures, clear agonism occurred such that mixtures were easier to detect than individual components. This finding is in good general agreement with a growing body of literature showing that concentrations of individual chemicals in a threshold-level mixture tend to fall below individual threshold concentrations (e.g., Rosen et al. 1962; Baker 1963; Guadagni et al. 1963; Laska and Hudson 1991; Patterson et al. 1993; Cometto-Muñiz et al. 1997, 2003, 2005; Wise et al. 2007; Miyazawa et al. 2008).
Agonism among mixture components also occurs in detection of perithreshold tastes (Stevens 1998). It seems that both chemosensory modalities can function as general detectors of ambient chemicals, at least to some extent. Detector systems that integrate across chemicals could enhance our ability to detect weak signals, at the possible expense of discrimination among compounds. At suprathreshold levels, mutual suppression among mixture components appears to be the general rule for olfaction and often holds even when one accounts for the compressive nature of psychophysical functions (Berglund et al. 1973; Cain 1975; Laing et al. 1984; Cain et al. 1995; Laing 1995; Lawless 1997). Because lateral inhibition in the brain can sharpen the chemical specificity of odor-responsive neurons (Mori and Shepherd 1994; Yokoi et al. 1995; Mori et al. 1999), the olfactory system may sacrifice sensitivity for enhanced discrimination once absolute detection becomes less of a challenge.
From a practical standpoint, mixture agonism has implications for indoor air quality, where analytical studies could fail to find problematic concentrations of any single chemical in air samples taken from clearly problematic environments, as has been discussed for chemical irritation (Schiffman and Williams 2005). In a similar fashion, it could prove very difficult to identify the source of an odor in a food, beverage, or personal product when the target is a mixture whose components have little or no perceptual impact on their own (discussed in Bult et al. 2001).
Differences among mixtures in degree of agonism
The 2 mixtures that showed clear agonism conformed to additivity predictions. However, detection of C2 + C4 + C8 fell below the predictions of additivity. In fact, this mixture was no more detectable than it is most detectable component, demonstrating a complete lack of agonism. Thus, the work has shown that additivity (and mixture agonism in general) may serve as a useful rule of thumb but cannot describe all interactions in detection of complex mixtures.
Interestingly, C2 + C4 + C8 was neither the mixture whose components were the most structurally similar nor the mixture whose components were the least similar. However, because both olfactory receptor neurons and cells in the brain may respond to molecules from more than one chemical family, what appears dissimilar to the eye may not be dissimilar to the olfactory system (Araneda et al. 2000; Haddad et al. 2008). With respect to perceived quality, the longer chain carboxylic acids share a rancid fat note and thus probably smell more similar to each other than does the sweet, maple odor of ML. Thus, C2 + C4 + C8 was probably not the most qualitatively diverse odor triad either. Regardless, until further work more fully elucidates the rules of mixture interactions, detection of any given mixture must be determined empirically.
Of course, mixtures that depart from general patterns, as C2 + C4 + C8 appears to do, might be of special interest as stimuli to neurophysiologists. These and similar experiments can provide stimulus sets that elicit clearly different patterns of response in the whole organism. These data on responses of the intact olfactory system can prove vital in the effort to understand how patterns of interaction observed at various levels of neural processing (e.g., Duchamp-Viret et al. 2003; Kadohisa and Wilson 2006; Mori et al. 2006; Yoshida and Mori 2007; Rospars et al. 2008) interact to shape perception.
The effect of concentration on degree of agonism
The first studies that combined gas-phase calibration of stimuli and intensive psychophysical methods to examine mixture detection at different concentrations, using binary mixtures as model stimuli, suggested that agonism is more complete in the low perithreshold range than in the high perithreshold range (Cometto-Muñiz et al. 2003, 2005). Subsequent work showed that patterns of agonism also depended on the compounds that comprise the mixture but found a similar pattern of level dependence for one pair of mixtures (Wise et al. 2007). In contrast, the current study found no evidence of level dependence. Agonism was either additive (2 mixtures) or nonadditive (one mixture) to the same degree across the full range of concentrations studied. Further studies, using additional model mixtures, can test the hypothesis that level dependence does not hold for more complex mixtures. If level dependence does not occur in more complex mixtures, then this result would indicate at least one clear difference between the rules that govern detection of binary mixtures and those that govern detection of more complex mixtures.
Future directions
More studies using a wider range of compounds are needed. Detailed structure-activity studies in which molecular properties vary systematically might yield further insights. Furthermore, the current experiment included only balanced mixtures whose components roughly matched with respect to detection probability. Other research suggests that relative proportion of components in mixtures can affect degree of agonism (Miyazawa et al. 2008). Furthermore, use of 3-component mixtures is a logical next step beyond work on 2-component mixtures. However, to understand processing of many natural odors, we must eventually study even more complex mixtures.
It would also be interesting to investigate how the perceived quality of mixture components is related to patterns of mixture interaction. Of course, quality itself will be constrained by molecular properties, but quality can also be shaped by learning and experience (Stevenson and Boakes 2003; Wilson and Stevenson 2003). At the suprathreshold level, cognitive factors such as experience and processing strategy can influence perception of mixtures (Mandairon et al. 2006; Le Berre et al. 2008).
To the best of our knowledge, the effects of cognitive factors and experience have not been investigated in detection of perithreshold odor mixtures. However, studies of cross-modal integration imply that cognitive factors play a role in how perithreshold tastes and smells cooperate to facilitate detection (Dalton et al. 2000). Perceptually congruent stimuli, like sucrose and cherry odor, may cooperate whereas incongruent stimuli may not. Congruence may come from learned associations between stimuli from repeated coexposure (Diamond et al. 2005). This idea is consistent with the suggestion that strong agonism might be more likely in naturally-occurring, biologically relevant odor mixtures (Laska et al. 1990). In short, a full understanding of mixture detection may require studies of not only the properties of the stimulus but also how experience and cognitive factors influence how the organism processes the stimulus.
Funding
Kraft Foods through the Term Chair in Chemosensory Psychophysics at Monell (to P.M.W.); National Institute of Environmental Health Sciences (grant 5R03ES013969 to P.M.W.); National Institute on Deafness and Other Communication Disorders (grant 2 T32 DC 00014 to M.G.).
Supplementary material
Supplementary material can be found at http://www.chemse.oxfordjournals.org/
References
- Araneda RC, Kini AD, Firestein S. The molecular receptive range of an odorant receptor. Nat Neurosci. 2000;3:1248–1255. doi: 10.1038/81774. [DOI] [PubMed] [Google Scholar]
- Baker RA. Odor effects of aqueous mixtures of organic chemicals. J Water Pollut Control. 1963;35:728–741. [Google Scholar]
- Berglund B, Berglund T, Lindvall T, Svensson LT. A quantitative principle of perceived intensity summation in odor mixtures. J Exp Psychol. 1973;100:29–38. doi: 10.1037/h0035435. [DOI] [PubMed] [Google Scholar]
- Brodin M, Laska M, Olsson MJ. Odor interaction between Bourgeonal and its antagonist undecanal. Chem Senses. 2009 doi: 10.1093/chemse/bjp044. doi: 10.1093/chemse/bjp044. [DOI] [PubMed] [Google Scholar]
- Brossard C, Rousseau F, Dumont J-P. Perceptual interactions between characteristic notes smelled above aqueous solutions of odorant mixtures. Chem Senses. 2007;32:319–327. doi: 10.1093/chemse/bjm002. [DOI] [PubMed] [Google Scholar]
- Bult JH, Schifferstein HN, Roozen JP, Voragen AG, Kroeze JH. The influence of olfactory concept on the probability of detecting sub- and peri-threshold components in a mixture of odorants. Chem Senses. 2001;26:459–469. doi: 10.1093/chemse/26.5.459. [DOI] [PubMed] [Google Scholar]
- Cain WS. Odor intensity: mixtures and masking. Chem Senses Flav. 1975;1:339–352. [Google Scholar]
- Cain WS, Schiet FT, Olsson MJ, de Wijk RA. Comparison of models of odor interaction. Chem Senses. 1995;20:625–637. doi: 10.1093/chemse/20.6.625. [DOI] [PubMed] [Google Scholar]
- Cain WS, Schmidt R, Jalowayski AA. Odor and chemesthesis from exposures to glutaraldehyde vapor. Int Arch Occup Environ Health. 2007;80:721–731. doi: 10.1007/s00420-007-0185-0. [DOI] [PubMed] [Google Scholar]
- Cometto-Muñiz JE, Cain WS, Abraham MH. Dose-addition of individual odorants in the odor detection of binary mixtures. Behav Brain Res. 2003;138:95–105. doi: 10.1016/s0166-4328(02)00234-6. [DOI] [PubMed] [Google Scholar]
- Cometto-Muñiz JE, Cain WS, Abraham MH. Odor detection of single chemicals and binary mixtures. Behav Brain Res. 2005;156:115–123. doi: 10.1016/j.bbr.2004.05.014. [DOI] [PubMed] [Google Scholar]
- Cometto-Muñiz JE, Cain WS, Abraham MH, Gola JMR. Chemosensory detectability of 1-butanol and 2-heptanone singly and in binary mixtures. Physiol Behav. 1999;67:269–276. doi: 10.1016/s0031-9384(99)00074-8. [DOI] [PubMed] [Google Scholar]
- Cometto-Muñiz JE, Cain WS, Hudnell HK. Agonistic sensory effects of airborne chemicals in mixtures: odor, nasal pungency, and eye irritation. Percept Psychophys. 1997;59:665–674. doi: 10.3758/bf03206014. [DOI] [PubMed] [Google Scholar]
- Dalton P, Doolittle N, Nagata H, Breslin PAS. The merging of the senses: integration of subthreshold taste and smell. Nat Neurosci. 2000;3:431–432. doi: 10.1038/74797. [DOI] [PubMed] [Google Scholar]
- Diamond J, Breslin PAS, Doolittle N, Nagata H, Dalton P. Flavor processing: perceptual and cognitive factors in multi-modal integration. Chem Senses. 2005;30(Suppl 1):i232–i233. doi: 10.1093/chemse/bjh199. [DOI] [PubMed] [Google Scholar]
- Duchamp-Viret P, Duchamp A, Chaput MA. Single olfactory sensory neurons simultaneously integrate the components of an odour mixture. Eur J Neurosci. 2003;18:2690–2696. doi: 10.1111/j.1460-9568.2003.03001.x. [DOI] [PubMed] [Google Scholar]
- Feller W. An introduction to probability theory and its applications. New York: John Wiley & Sons; 1968. [Google Scholar]
- Gill J. Generalized linear models: a unified approach. Thousand Oaks (CA): Sage Publications; 2001. [Google Scholar]
- Greenhouse SW, Geisser S. On methods in the analysis of profile data. Psychometrika. 1959;24:95–112. [Google Scholar]
- Guadagni DG, Buttery RG, Okano S, Burr HK. Additive effect of sub-threshold concentrations of some organic compounds associated with food aromas. Nature. 1963;200:1288–1289. doi: 10.1038/2001288a0. [DOI] [PubMed] [Google Scholar]
- Haddad R, Khan R, Takahashi YK, Mori K, Harel D, Sobel N. A metric for odorant comparison. Nat Methods. 2008;5:425–429. doi: 10.1038/nmeth.1197. [DOI] [PubMed] [Google Scholar]
- Kadohisa M, Wilson DA. Olfactory cortical adaptation facilitates detection of odors against background. J Neurophysiol. 2006;95:1888–1896. doi: 10.1152/jn.00812.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laffort P, Etcheto M, Patte F, Marfaing P. Implications of power law exponent in synergy and inhibition of olfactory mixtures. Chem Senses. 1989;14:11–23. [Google Scholar]
- Laing DG. Perception of odor mixtures. In: Doty RL, editor. Handbook of olfaction and gustation. New York: Marcel Dekker; 1995. pp. 283–297. [Google Scholar]
- Laing DG, Eddy A, Best DJ. Perceptual characteristics of binary, trinary, and quaternary odor mixtures consisting of unpleasant constituents. Physiol Behav. 1994;56(1):81–93. doi: 10.1016/0031-9384(94)90264-x. [DOI] [PubMed] [Google Scholar]
- Laing DG, Panhuber H, Wilcox ME, Pittman EA. Quality and intensity of binary odor mixtures. Physiol Behav. 1984;33:309–319. doi: 10.1016/0031-9384(84)90118-5. [DOI] [PubMed] [Google Scholar]
- Laska M, Hofmann M, Simon Y. Olfactory sensitivity for aliphatic aldehydes in squirrel monkeys and pigtail macaques. J Comp Physiol A Neuroethol Sens Neural Behav Physiol. 2003;189:263–271. doi: 10.1007/s00359-003-0395-1. [DOI] [PubMed] [Google Scholar]
- Laska M, Hudson R. A comparison of the detection thresholds of odour mixtures and their components. Chem Senses. 1991;16:651–652. [Google Scholar]
- Laska M, Hudson R, Distel H. Olfactory sensitivity to biologically relevant odours may exceed the sum of component thresholds. Chemoecology. 1990;1:139–141. [Google Scholar]
- Lawless HT. Olfactory psychophysics. In: Beauchamp G, Bartoshuk L, editors. Tasting and smelling. San Diego (CA): Academic Press; 1997. pp. 125–168. [Google Scholar]
- Lawless HT, Heyman H. Sensory evaluation of food: principles and practices. New York: Chapman & Hall; 1998. [Google Scholar]
- Le Berre E, Thomas-Danguin T, Béno N, Coureaud G, Etiévant P, Prescott J. Perceptual processing strategy and exposure influence the perception of odor mixtures. Chem Senses. 2008;33:193–199. doi: 10.1093/chemse/bjm080. [DOI] [PubMed] [Google Scholar]
- Lötsch J, Reichmann H, Hummel T. Different odor tests contribute differently to the evaluation of olfactory loss. Chem Senses. 2007;33:17–21. doi: 10.1093/chemse/bjm058. [DOI] [PubMed] [Google Scholar]
- Macmillan NA, Creelman CD. Detection theory: a user's guide. Cambridge: Cambridge University Press; 1991. [Google Scholar]
- Mandairon N, Stack C, Linster C. Olfactory enrichment improves the recognition of individual components in mixtures. Physiol Behav. 2006;89:379–384. doi: 10.1016/j.physbeh.2006.07.013. [DOI] [PubMed] [Google Scholar]
- Menashe I, Abaffy T, Hasin Y, Goshen S, Yahalom V, Luetje CW, Lancet D. Genetic elucidation of human hyperosmia to isovaleric acid. PLoS Biol. 2007;5:e284. doi: 10.1371/journal.pbio.0050284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miyazawa T, Gallagher M, Preti G, Wise PM. Synergistic mixture interactions in detection of peri-threshold odors by humans. Chem Senses. 2008;33(4):363–369. doi: 10.1093/chemse/bjn004. [DOI] [PubMed] [Google Scholar]
- Mori K, Nagao H, Yoshihara Y. The olfactory bulb: coding and processing of odor molecule information. Science. 1999;286:711–715. doi: 10.1126/science.286.5440.711. [DOI] [PubMed] [Google Scholar]
- Mori K, Shepherd GM. Emerging principles of molecular signal processing by mitral/tufted cells in the olfactory bulb. Semin Cell Biol. 1994;5:65–74. doi: 10.1006/scel.1994.1009. [DOI] [PubMed] [Google Scholar]
- Mori K, Takahashi YK, Igarashi KM, Yamaguchi M. Maps of odorant molecular features in the mammalian olfactory bulb. Physiol Rev. 2006;86:409–433. doi: 10.1152/physrev.00021.2005. [DOI] [PubMed] [Google Scholar]
- Moskowitz HR, Barbe CD. Profiling of odor components and their mixtures. Sens Processes. 1977;1(3):212–226. [PubMed] [Google Scholar]
- Nijssen LM, Visscher CA, Maarse H, Willemsen LC, Boelens MH. Volatile compounds in food: qualitative and quantitative data. 7th ed. Zeist, The Netherlands: TNO Nutrition and Food Research Institute; 1996. [Google Scholar]
- Patterson MQ, Stevens JC, Cain WS, Cometto-Muñiz JE. Detection thresholds for an olfactory mixture and its three constituent compounds. Chem Senses. 1993;18:723–734. [Google Scholar]
- Rosen AA, Peter JB, Middleton FM. Odor thresholds of mixed organic chemicals. J Water Pollut Control. 1962;35:7–14. [Google Scholar]
- Rosenfeld PE, Clark JJ, Hensley AR, Suftet IH. The use of an odour wheel classification for the evaluation of human health risk criteria for compost facilities. Water Sci Technol. 2007;55:345–357. doi: 10.2166/wst.2007.197. [DOI] [PubMed] [Google Scholar]
- Rospars JP, Lansky P, Chaput M, Duchamp-Viret P. Competitive and noncompetitive odorant interactions in the early neural coding of odorant mixtures. J Neurosci. 2008;28:2659–2666. doi: 10.1523/JNEUROSCI.4670-07.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schiffman SS, Williams CM. Science of odor as a potential health issue. J Environ Qual. 2005;34:129–138. [PubMed] [Google Scholar]
- Spehr M, Schwane K, Heilmann S, Gisselmann G, Hummel T, Hatt H. Dual capacity of a human olfactory receptor. Curr Biol. 2004;14:R832–R833. doi: 10.1016/j.cub.2004.09.034. [DOI] [PubMed] [Google Scholar]
- Stevens JC. Detection of very complex taste mixtures. Ann N Y Acad Sci. 1998;855:831–833. doi: 10.1111/j.1749-6632.1998.tb10670.x. [DOI] [PubMed] [Google Scholar]
- Stevenson RJ, Boakes RA. A mnemonic theory of odor perception. Psychol Rev. 2003;110:340–364. doi: 10.1037/0033-295x.110.2.340. [DOI] [PubMed] [Google Scholar]
- Thomas-Danguin T, Chastrette M. Odor intensity of binary mixtures of odorous compounds. C R Biol. 2002;325:767–772. doi: 10.1016/s1631-0691(02)01485-3. [DOI] [PubMed] [Google Scholar]
- Wagner M, Sudhoff H, Zamelczyk-Pajewska M, Kosmider J, Linder R. A computer-based approach to assess the perception of composite odour intensity: a step towards automated olfactometry calibration. Physiol Meas. 2006;27:1–12. doi: 10.1088/0967-3334/27/1/001. [DOI] [PubMed] [Google Scholar]
- Wilson DA, Stevenson RJ. The fundamental role of memory in olfactory perception. Trends Neurosci. 2003;26:243–247. doi: 10.1016/S0166-2236(03)00076-6. [DOI] [PubMed] [Google Scholar]
- Wise PM, Miyazawa T, Gallagher M, Preti G. Human odor detection of homologous carboxylic acids and their binary mixtures. Chem Senses. 2007;32(5):475–482. doi: 10.1093/chemse/bjm016. [DOI] [PubMed] [Google Scholar]
- Wysocki CJ, Beauchamp GK. Ability to smell androstenone is genetically determined. Proc Natl Acad Sci USA. 1984;81:4899–4902. doi: 10.1073/pnas.81.15.4899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yokoi M, Mori K, Nakanishi S. Refinement of odor molecule tuning by dendrodendritic synaptic inhibition in the olfactory bulb. Proc Natl Acad Sci U S A. 1995;92:3371–3375. doi: 10.1073/pnas.92.8.3371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yoshida I, Mori K. Odorant category profile selectivity of olfactory cortex neurons. J Neurosci. 2007;27:9105–9114. doi: 10.1523/JNEUROSCI.2720-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.