Abstract
Mice lacking the purinergic receptors, P2X2 and P2X3 (P2X2/P2X3Dbl−/−), exhibit essentially no tastant-evoked activity in the chorda tympani and glossopharyngeal nerves and substantial loss of tastant-evoked behavior as measured in long-term intake experiments. To assess whether the residual chemically driven behaviors in these P2X2/P2X3Dbl−/− mice were attributable to postingestive detection or oropharyngeal detection of the compounds, we used brief access lickometer tests to assess the behavioral capabilities of the P2X2/P2X3Dbl−/− animals. The P2X2/P2X3Dbl−/− mice showed avoidance to high levels (10 mM quinine and 10–30 mM denatonium benzoate) of classical “bitter”-tasting stimuli in 24-h, 2-bottle preference tests but minimal avoidance of these substances in the lickometer tests, suggesting that the strong avoidance in the intake tests was largely mediated by post-oral chemosensors. Similarly, increases in consumption of 1 M sucrose by P2X2/P2X3Dbl−/− mice in long-term intake tests were not mirrored by increases in consumption of sucrose in lickometer tests, suggesting that sucrose detection in these mice is mediated by postingestive consequences. In contrast, in brief access tests, P2X2/P2X3Dbl−/− mice avoided citric acid and hydrochloric acid at the same concentrations as their wild-type counterparts, indicating that these weak acids activate oropharyngeal chemoreceptors.
Keywords: acid sensing, ATP, feeding, P2X, postingestive effect, taste
Introduction
The gustatory sense is used to assess the chemical composition of potential foods before they are ingested. Yet, it is not the only means by which the chemical composition of foods is evaluated. The oral cavity and pharynx are replete with free nerve endings that mediate the “common chemical sense,” now called chemesthesis (Parker 1912; Keele 1962; Green et al. 1990). Moreover, the trigeminal nerve typically responds to high concentrations of salts, acids, and many other irritant substances such as capsaicin or menthol (e.g., Wang et al. 1993). Once food passes into the esophagus, it can activate a variety of gut chemosensors (Stellar et al. 1954) including epithelial enterochromaffin cells (Sternini et al. 2008) and free nerve endings. Information from each of these chemosensors may be used by the animal to adjust consummatory behavior.
Gustatory-mediated behavior can be divided into 2 broad classes: ingestive (triggered in mammals by the qualities of sweet, umami, and low concentrations of salt) or aversive (including bitter and sour). Taste cells possess receptors or channels appropriate to detect different classes of substances but regardless of transduction mechanism require intact purinergic signaling to transmit taste information to the gustatory nerves (Finger et al. 2005). Mice lacking those purinergic receptors expressed by the gustatory nerves, P2X2 and P2X3, lack essentially all gustatory neural responses in the chorda tympani and glossopharyngeal nerves to stimuli of any taste quality. The similarity in purinergic innervation and presence of ATPases in all taste fields suggests that all gustatory transmission is dependent on purinergic signals, although this has not been tested formally for either the palatal or the laryngeal taste fields. Nonetheless, gustatory-driven behaviors of these P2X2/P2X3Dbl−/− animals are essentially absent except aversion to certain stimuli at moderate concentration, for example, citric acid and caffeine, or to high levels of others, for example, quinine hydrochloride (quinine). Because these substances stimulate non-gustatory nerves as well as taste buds (Pittman and Contreras 1998), we suggested that this residual behavior might be mediated either by postingestive factors or by activation of non-gustatory oral or pharyngeal nerve endings or solitary chemosensory cells. These residual behaviors might also be driven by remnant gustatory function, that is, small responses in populations of gustatory nerve fibers that would not have been revealed in the whole nerve recordings used in the previous study (Finger et al. 2005).
The behavioral assessment in this previous study relied on long-term intake methods (24-h, 2-bottle preference) and so it was impossible to distinguish behaviors evoked by postingestive effects from those attributable to oropharyngeal stimulation. Accordingly, while 24-h, 2-bottle preference tests are useful to demonstrate a lack of taste function, they are not easily interpretable when residual behaviors remain. Indeed, de Araujo et al. (2008) recently showed that mice lacking taste responses to sucrose can learn a 24-h, 2-bottle discrimination on the basis of blood glucose levels within 20 min following ingestion.
In contrast, brief-access taste tests, for example, with a lickometer, permit the animal to sample a stimulus for only a relatively short time. The brief availability precludes substantial postingestive detection on initial stimulus presentations (Breslin et al. 1993). Thus, lickometer tests can be used to distinguish between postingestive detection and oropharyngeal detection of proffered stimuli. Accordingly, we used a brief-access test to test whether the residual chemical responses we observe in P2X2/P2X3Dbl−/− mice were due to oropharyngeal or postingestive detection. If naive P2X2/P2X3Dbl−/− mice still respond to a stimulus in the brief-access test, despite total lack of gustatory nerve responses of these P2X2/P2X3Dbl−/− animals, then they must be using a non-gustatory oropharyngeal modality or a non-purinergic taste function that was undetected in recordings from the chorda tympani and glossopharyngeal nerves. Although it is possible that residual taste abilities are due to remaining gustatory function of palatal or laryngeal taste systems, the taste buds in these areas show similar purinergic traits as do the lingual taste buds (Finger et al. 2005). Nonetheless, a lack of response in the lickometer coupled with a response in 24-h, 2-bottle preference tests likely indicates postingestive detection of the ingested substance.
Materials and methods
Brief-access tests (lickometer)
Subjects
Experiments in this study used B6;129-P2rx2tm1Ckn/P2rx3tm1Ckn, hereafter referred to as P2X2/P2X3Dbl−/− (n = 22) and wild-type (WT) control mice (B6;129 also known as P2X2/P2X3Dbl+/+, n = 22) of both sexes. These mice are on a mixed C57BL/6 and 129Ola background, so there is genetic variability within both P2X2/P2X3Dbl−/− and WT populations. Details about generation of these mice can be found in Cockayne et al. (2005). The animals were individually housed in a vivarium with a 12-h light/dark cycle. Food and water were available ad libitum throughout the course of the experiment except where noted. Mice were placed on a water deprivation schedule 3 days before the training began. During water deprivation, mice were allowed 1 h of water access during a 24-h period. Due to limited supply of P2X2/P2X3Dbl−/− mice, naive mice were not available for every experiment. Mice were never tested with similar tastes (e.g., 2 bitter substances or 2 sweet substances), but some mice were tested with dissimilar tastes (e.g., a sour substance and then a bitter substance). A detailed listing of prior testing experience for the test animals is provided in the Supplementary Table.
Reagents
All taste stimuli except sucrose were obtained from the Sigma Chemical Co.; sucrose was from Fisher Scientific. Solutions were prepared in distilled water.
Apparatus
A Davis Rig (MS-160; Dilog Instruments and Systems) lictkometer was used for training and brief-access testing of mice. A small fan (8 cm) was placed next to the space between the rack and the chamber to push air past the nose of the mouse to reduce olfactory clues (Glendinning et al. 2002; Taylor-Burds et al. 2004). Black construction paper was placed on the sides of the chamber to reduce possible visual cues.
Procedure
Mice were placed on a water deprivation schedule 3 days before the training began during which mice were allowed 1 h of water access per 24-h period. On the first day of training, water-deprived animals were placed into the lickometer for a 30-min period in which 1 water bottle was available. The shutter remained open for the full 30 min and the rack did not change bottle positions. On the second day of training, 3 bottles containing water were placed into the rack. A presentation sequence was created that randomly presented the 3 bottles at the same frequency. From the initiation of the first lick of a bottle, the shutter would remain open for 5 s to allow licking and then close. Finally, the rack would slide to allow presentation of the next bottle. To allow enough time for the rack to change bottle positions, there was a 7.5-s delay between the end of one presentation and the beginning of the next. All training and testing days lasted 30 min unless a mouse finished 7 complete blocks before the end of the 30-min testing session. After these 2 training days, the mice were water deprived for at least 20 h for all substances except sucrose. On the day immediately before sucrose testing, mice were deprived 16–20 h. A lower state of motivation was necessary for mice to differentially respond to sucrose and water. For the experimental sessions, bottles containing different concentrations of tastant and a water bottle were randomized into blocks. The same presentation timings were used as on the second day of training. If an animal did not complete 3 testing blocks in the 30-min period, the animal was tested on the following day and both days’ data combined, as long as at least 2 blocks were completed on each day. The computer recorded the number of licks per presentation and latency until first contact with the spout.
Data analysis
For aversive compounds, only the initial blocks were analyzed to minimize the chance of behavioral changes reflected from learning about the stimuli with repeated trials (see Results). This change in learned behavior was reflected in the data by changes in variances of latencies (see following). Thus, only 1 block was used for each of denatonium benzoate and quinine hydrochloride, whereas 2 blocks of data were used for citric acid, hydrochloric acid (HCl), and sucrose.
To control for inherent interanimal differences in lick rates, the number of licks per tastant presentation was standardized into a lick ratio with respect to each animal's average lick rate to water. These lick ratios were averaged for each genotype at every concentration of tastant and used for statistical testing and graphical representation. Latency, or the time taken to initiate the first lick of a trial, was also standardized to the average latency for water.
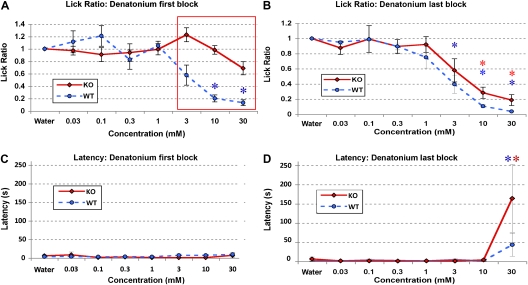
For some tastants, the animals’ licking behavior changed over the course of the experimental testing session with regard to latency (see also Rhinehart-Doty et al. 1994; Dotson et al. 2005). The block-dependent change in latency was significant only for mice tested with high concentrations of denatonium benzoate; mice tested with other tastants showed no significant concentration-dependent change in latency. Lick ratios decreased for P2X2/P2X3Dbl−/− mice at higher concentrations of denatonium in the last block when compared with the first block (Figure 1A,B). This block-dependent increase in avoidance of high concentrations (30 mM) of denatonium benzoate was concurrent with a block-dependent increase in latency (Figure 1C,D). The change in latency suggests that the mice were associating non-oral (e.g., nasal) cues with an aversive consequence of ingestion of that substance, as has been reported previously for 50 mM concentrations of denatonium for PLCβ-KO mice (Dotson et al. 2005).
Figure 1.
Brief-access lickometer taste test (A and B) lick responses of P2X2/P2X3Dbl−/− and WT control mice to denatonium in the first and last block of a testing session and (C and D) time to initiate first lick (latencies). Boxes that outline data points indicate significant differences between KO and WT mice (P < 0.05). Asterisks denote values significantly different from a latency of 0 or preference value of 1.0. (A) P2X2/P2X3Dbl−/− mice (n = 6) do not avoid any concentration of denatonium in the first block of trials (P > 0.05), whereas WT mice (n = 6) avoid concentrations of 10 and 30 mM (P < 0.001). (B) P2X2/P2X3Dbl−/− mice avoid concentrations of 10 and 30 mM denatonium in the last block of trials (P < 0.05). WT mice avoid 3, 10, and 30 mM denatonium in the last block (P < 0.05) similar to their responses in the first block. Values in A and B are presented as the mean standardized lick ratio (± standard error of the mean). (C) Upon the first block of presentations, no significant differences in latency exist for any concentration of denatonium tested with either genotype (ps > 0.05). (D) Both WT and P2X2/P2X3Dbl−/− mice respond with significantly longer latency at 30 mM denatonium compared with baseline (P < 0.05). Latencies in C and D are depicted as the median (± median absolute deviation) and correspond to the lick ratios in A and B, respectively. Concentration is on the x axis and latency (in seconds) is on the y axis. Furthermore, comparing C and D, in the last block tested for each mouse, both P2X2/P2X3Dbl−/− and WT mice respond with a significantly longer latency for 30 mM denatonium in the last block when compared with the first (P < 0.05). The double asterisks in panel B and D indicate that both WT and P2X2/P2X3Dbl−/− mice are significantly different from baseline; a single asterisk indicates that only the WT mice are significantly different from baseline. This figure appears in color in the online version of Chemical Senses.
Homogeneity of variance was determined using Levene's test (P > 0.05). The variance of the latencies for denatonium across blocks (compare Figure 1C and D) varied significantly (i.e., they were heteroscedastic), and the latency data were also skewed (non-normally distributed). Thus, these latency data were analyzed using Wilcoxin signed-rank tests, and a 0.05 α level was used and corrected for the number of comparisons made. These results showed a significant difference in latencies between water and 30 mM denatonium (but not at any other concentration) for both the KO and WT mice, P < 0.05 (Figure 1). No other tastant showed a concentration-related change in latency variability.
All mice were motivated by water deprivation to maximally lick both water and sucrose (the only hedonically positive compound tested) throughout the first several blocks, leaving it impossible to detect concentration-dependent increases in licking. Therefore, only the last 2 completed blocks of the testing session for each mouse were used for sucrose analyses. In addition, for analysis of the sucrose licking, data were analyzed for changes in lick ratio (as for other tastants) and difference in total licks subtracting the total licks for water from total licks to tastants (see Supplementary Figure 2). The resulting analysis showed essentially identical outcomes.
Three-way analyses of variance (ANOVAs) were conducted on the data where multiple blocks of data were available (denatonium benzoate, citric acid, HCl, and sucrose). Genotype (2 levels; WT and KO) was a between-subjects factor, whereas concentration (5–7 levels depending on the particular tastant) and block (2 levels) were within-subjects factors. Quinine, where only 1 block of data was available, was analyzed with a 2-way ANOVA, with genotype (2 levels) and concentration (7 levels) as the 2 factors. When licks were standardized to 1 block (denatonium benzoate and quinine), ANOVAs did not include the data on water, as these values were all 1.0 and lacked variance. In these cases, a 1-sample 2-tailed t-test was used to test for differences between the standardized water values (all 1.0) and the lick ratio/latency of the tastant. Post-hoc tests were also conducted between genotype (2 levels) at each concentration of tastant, which varied between 5 and 7 depending on the specific experiment. Rejection levels were adjusted with a Bonferroni correction by dividing the standard α level of 0.05 by the number of comparisons made. All statistical analyses were done with SPSS 16.0 (SPSS Inc.) or STATISTICA v5.1 (StatSoft Inc.).
Twenty-four-hour preference tests (2-bottle taste preference)
P2X2/P2X3Dbl−/− (n = 20) and WT control (n = 22) mice were housed as in the aforementioned brief-access experiment except no water deprivation was used. Mice tested with denatonium benzoate, quinine, and sucrose were experimentally naive. Methods were as described previously (Finger et al. 2005). Briefly, mice were given 2 bottles (25 mL each) in their home cage. One bottle contained water and the other water or a taste substance (0.3–30 mM denatonium benzoate, 0.1–10 mM quinine, 10 mM citric acid, or 1–1000 mM sucrose, all presented in an ascending concentration series). After bottles were left in the cage for 48 h for each concentration (with a position switch at 24 h), the volume consumed from each bottle was measured to the nearest 0.5 mL and preference ratios were calculated by dividing the volume of taste substance consumed by the total volume of water and taste substance consumed. Statistics were performed as described for brief access tests. Post-hoc tests were adjusted with a Bonferroni correction to account for the multiple comparisons.
Results
“Bitter” tastants
We previously reported that P2X2/P2X3Dbl−/− mice were largely unresponsive to bitter stimuli in the 24-h, 2-bottle taste preference tests (Finger et al. 2005). Even in that data set, however, a slight avoidance of high concentrations (3.0 mM) of quinine was evident (see Figure 2; Finger et al. 2005). Accordingly, here we tested an extended range of concentrations for the 2 bitter tastants: quinine hydrochloride and denatonium using the same 24 h, 2-bottle taste preference methods (Finger et al. 2005). The P2X2/P2X3Dbl−/− mice avoided 10 mM quinine hydrochloride when compared with baseline levels (P < 0.05), and preference ratios were not significantly different from the WT control mice (P > 0.05) at this concentration (Table 1). The P2X2/P2X3Dbl−/− mice also significantly avoided 30 mM denatonium compared with baseline levels (water; P < 0.05), and they performed similarly to WT mice at these concentrations (P > 0.05; Table 1). In summary, the P2X2/P2X3Dbl−/− mice avoided 2 bitter-tasting substances when presented at high concentrations that were well above the avoidance thresholds for WT controls (Finger et al. 2005).
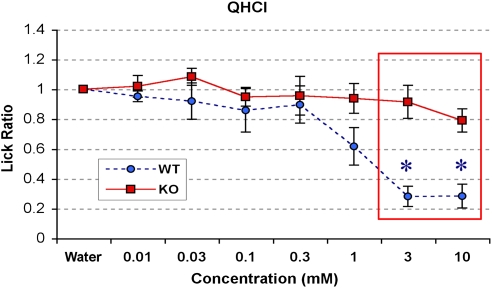
Figure 2.
Brief-access lickometer responses of P2X2/P2X3Dbl−/− and WT control mice to bitter substances. Boxes that outline data points indicate significant differences between KO and WT mice (P < 0.05). Asterisks indicate points significantly (P < 0.05) different from a lick ratio of 1.0. KO mice (n = 10) do not avoid quinine at any of the concentrations tested (P > 0.05), whereas WT mice (n = 8) avoid 3 and 10 mM concentrations (P < 0.05). KO and WT mice differed in their preference for 3 and 10 mM quinine (P < 0.05). QHCl, quinine HCl. This figure appears in color in the online version of Chemical Senses.
Table 1.
Two-bottle taste preference ratios from P2X2/P2X3Dbl−/− mice and WT controls for multiple concentrations of denatonium benzoate and quinine
| Denatonium |
Quinine |
||||
| Concentration (mM) | WT | P2X-KO | Concentration (mM) | WT | P2X-KO |
| 0 | 0.44 ± 0.08 | 0.52 ± 0.08 | 0 | 0.54 ± 0.05 | 0.49 ± 0.03 |
| 0.3 | 0.26 ± 0.08* | 0.64 ± 0.12* | 0.1 | 0.17 ± 0.01* | 0.51 ± 0.03* |
| 3 | 0.12 ± 0.02* | 0.42 ± 0.06* | 0.3 | 0.16 ± 0.03* | 0.51 ± 0.01* |
| 30 | 0.18 ± 0.02 | 0.15 ± 0.02 | 1 | 0.22 ± 0.02* | 0.49 ± 0.02* |
| 3 | 0.17 ± 0.05 | 0.29 ± 0.06 | |||
| 10 | 0.22 ± 0.03 | 0.14 ± 0.02 | |||
A preference ratio of 0.5 indicates that the animals drank equally from the 2 bottles; numbers lower than 0.5 indicate avoidance of the tastant. Results are presented as the mean preference ratio ± standard error of the mean. Significant differences (P < 0.05) between KO and WT controls are indicated by an asterisk. Boldface shows values for KO mice that were significantly different from the non-preferred baseline. Our previous publication on the P2X2/P2X3Dbl−/− mice (Finger et al. 2005) reported intake behaviors only up to a concentration of 3 mM for each substance.
To test whether the effects observed in the 24-h, 2-bottle preference tests were due to non-gustatory or postingestive effect, the mice were tested in a brief-access paradigm. The data for denatonium benzoate were analyzed with a 3-way ANOVA. There was no 3-way interaction (P > 0.05), but there was an interaction between genotype and block (P < 0.05), indicating that the 2 genotypes behaved differently across blocks. There were significant main effects of genotype (P < 0.01), concentration (P < 0.001), and block (P < 0.01). Because there was a significant interaction of genotype and block, as well as a main effect of block, data on the first and last blocks are presented separately in Figure 1A and B, respectively. WT mice avoided denatonium at 10 and 30 mM in the first block (P < 0.05). In contrast, the P2X2/P2X3Dbl−/− mice did not avoid denatonium at any concentration tested (P > 0.05) in the first block. In the last block, WT mice again avoided 10 and 30 mM denatonium benzoate but also avoided 3 mM (Ps < 0.01). P2X2/P2X3Dbl−/− mice changed their behavior and avoided 10 and 30 mM denatonium benzoate (P < 0.05) and also marginally avoided 3 mM denatonium benzoate (P = 0.04; the Bonferroni-corrected α level for significance was 0.017). Additionally, post-hoc tests showed that WT animals avoided denatonium benzoate significantly more than the P2X2/P2X3Dbl−/− mice at concentrations of 3 mM and higher (P < 0.05) during the first block (Figure 1A) but that they responded similarly during the last block (Figure 1B).
Mice only finished 1 block of data with quinine, so a 2-way ANOVA was performed on these data. There was a significant interaction between genotype and concentration; F (6, 96) = 3.87, P < 0.01. A significant main effect of genotype was observed; F (1,16) = 9.12, P = 0.008; a main effect of concentration was also observed; F (6, 96) = 10.18, P < 0.001. Post-hoc analyses revealed that WT mice had significantly lower lick ratios when compared to P2X2/P2X3Dbl−/− mice at 3 and 10 mM quinine (P < 0.05). Additionally, WT mice significantly avoided quinine (licked at levels significantly lower than water) at concentrations of 3 and 10 mM (P < 0.001; Figure 2), while they marginally avoided 1 mM quinine (P = 0.019; the Bonferroni corrected α level was 0.017). In contrast, the P2X2/P2X3Dbl−/− mice did not significantly avoid quinine at any concentration tested (P > 0.05; Figure 2). In summary, the P2X2/P2X3Dbl−/− mice did not avoid any concentration of quinine tested in this brief access test. Conversely, in the 24 h, 2-bottle test, both groups equally avoided 10 mM quinine. Together, these results indicate that the avoidance of high concentrations of quinine and denatonium detectable in the 24 h, 2-bottle preference experiments was at least partially due to non-gustatory detection or postingestive effects.
“Sour” tastants
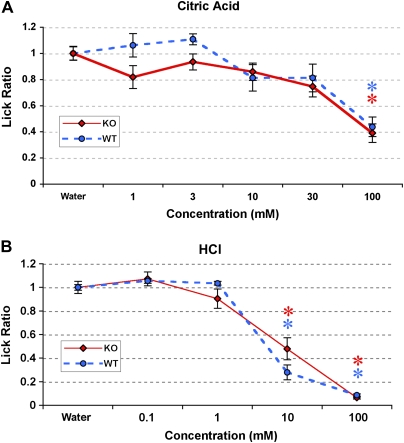
Our previous report (Finger et al. 2005) indicated that in 24-h, 2-bottle preference tests, P2X2/P2X3Dbl−/− mice avoided citric acid over the same concentration range as WT mice despite the lack of gustatory neural responses to this substance. In the current experiment, we tested citric acid in brief-access tests, and also extended the concentration range to include 100 mM. The P2X2/P2X3Dbl−/− and WT mice both avoided 100 mM citric acid when compared with baseline levels (P < 0.05). There was no significant main effect of genotype, F(1, 11) = 2.84, P > 0.10, and no significant main effect of block, F(1, 11) = 0.28, P > 0.50 (Figure 3A). There was a main effect of concentration, F(5, 55) = 13.85, P < 0.001.
Figure 3.
(A and B) Brief-access lickometer responses of P2X2/P2X3Dbl−/− and WT control mice to sour substances. Asterisks denote values significantly different from 1.0. Stacked asterisks indicate that values for both KO and WT are significantly different from 1.0. (A) KO (n = 7) and WT (n = 6) mice avoid citric acid at 100 mM (P < 0.001). (B) Both KO (n = 5) and WT (n = 5) mice avoid HCl at 10 and 100 mM (P < 0.001). This figure appears in color in the online version of Chemical Senses.
We also assessed the reactivity of WT and P2X2/P2X3Dbl−/− mice to 4 different concentrations (0.1–100 mM) of HCl in the lickometer. Both P2X2/P2X3Dbl−/− and WT mice avoided 10 and 100 mM HCl (P < 0.05; Figure 3B) but no other concentration. There was no 3-way interaction between the factors, nor a 2-way interaction between genotype and concentration, nor a significant main effect of genotype, F(1, 8) = 0.086, P = 0.77 (Figure 3B). However, there were main effects of concentration and block (Ps < 0.01), as well as a significant interaction of genotype and block (P < 0.05). Post-hoc tests revealed that only P2X2/P2X3Dbl−/− mice had higher lick ratios during the last block compared with the first block (P < 0.01). These data are divided by block and presented in Supplementary Figure 1. Although the lick ratios for P2X2/P2X3Dbl−/− mice are different across blocks (0.58 for block 1 vs. 0.82 for block 2; P < 0.05), post-hoc tests did not reveal any significant differences across blocks for a given concentration of HCl (Ps > 0.05). This difference seems primarily attributable to a higher intake at 1 mM in the last block, and we feel this is attributable to a type I error rather than learning that this near-threshold concentration is appetitive, although we cannot rule the latter out.
“Sweet” tastant
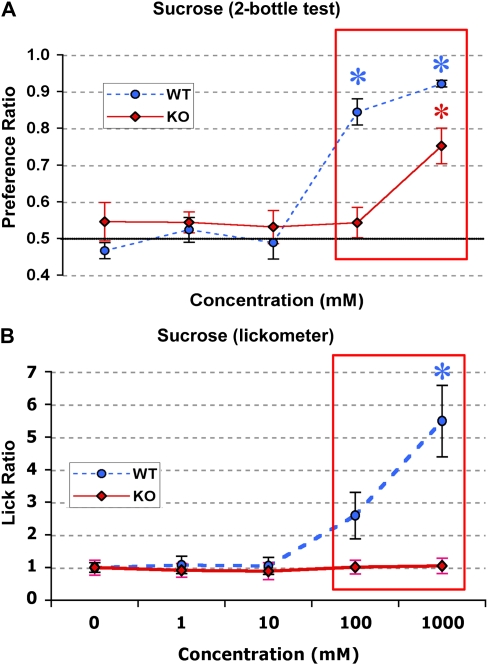
We previously reported that P2X2/P2X3Dbl−/− mice do not prefer sucrose in a 24-h, 2-bottle test at concentrations of 100 mM and lower (Finger et al. 2005). We extended this concentration range to include 1000 mM sucrose (Figure 4A) in a 2-bottle taste preference experiment. There was a significant interaction between genotype and concentration, F(4, 32) = 15.59, P < 0.0001. There was no main effect of genotype, F(1, 8) = 2.67, P > 0.10, but there was a significant main effect of concentration, F(4, 32) = 50.08, P < 0.0001. We also found that P2X2/P2X3Dbl−/− mice have preference ratios above baseline levels at 1000 mM (P < 0.05), albeit still lower than the preferences exhibited by WT mice at this concentration. To test whether this preference at high concentrations is due to non-gustatory or postingestive effect, we used brief-access lickometer tests. There was no 3-way interaction, but there was a significant 2-way interaction between genotype and concentration, F(4, 32) = 4.31, P < 0.01. There was also a significant effect of concentration, F(4, 32) = 4.80, P < 0.01, and a marginally significant effect of genotype, F(1, 8) = 4.87, P = 0.058. WT mice drank significantly more 1000 mM sucrose than water (P < 0.001), whereas P2X2/P2X3Dbl−/− mice did not (P > 0.5; Figure 4B).
Figure 4.
Twenty-four-hour taste preference (A) and brief-access lickometer (B) test responses of P2X2/P2X3Dbl−/− and WT control mice to “sweet” tastants. Boxes outlining points indicate significant differences between KO and WT (P < 0.001). Asterisks denote values significantly different from a preference ratio of 0.5 (2-bottle test) or a lick ratio of 1.0. (A) In a 2-bottle preference test, KO (n = 5) mice prefer sucrose only at 1000 mM, whereas WT mice (n = 5) prefer it at 100 and 1000 mM (P < 0.05). (B) Brief-access taste responses to sucrose. P2X2/P2X3Dbl−/− (n = 4) mice do not prefer sucrose at any concentration (P > 0.50). WT mice (n = 6) prefer sucrose at 1 M (P < 0.05). This figure appears in color in the online version of Chemical Senses.
In summary, despite the lack of gustatory neural responses in P2X2/P2X3Dbl−/− mice, the animals can respond to the presence of certain chemicals in both brief-access and long-term (48 h) intake paradigms. Citric acid and HCl are avoided as in case of WT mice, whereas quinine, denatonium, and sucrose are not detected in a brief-access paradigm at least during the early stages of testing.
Discussion
In a previous study (Finger et al. 2005), we showed that double knockout mice (P2X2/P2X3Dbl-/-), lacking the 2 purinergic receptors (P2X2 and P2X3) expressed by gustatory nerves, exhibit virtually no chorda tympani or glossopharyngeal nerve responses to tastants from all 5 principal qualities (salt, sweet, sour, bitter, umami), although some residual avoidance behavior remained for some taste stimuli, that is, citric acid, caffeine, and high concentrations of denatonium. The original behavioral experiments used a 24-h, 2-bottle access test and then compared the amount of each stimulus consumed at the end of the session. This methodology leaves open the possibility that the mice responded to postingestive cues about the taste stimuli, for example, cues from the gut or from blood levels of metabolites (e.g., Mook 1963; de Araujo et al. 2008; Glendinning et al. 2008), to residual non-purinergic taste functions or to non-gustatory components of the stimuli, for example, irritation as by acid (Pittman and Contreras 1998; Lugaz 2004). The current experiment presented taste stimuli in 5-s blocks and so precluded the use of postingestive cues by mice inexperienced with the proffered stimulus. The brief-access tests we use do not exclude the possibility of non-purinergic–mediated gustatory or non-gustatory cues, for example, trigeminal or olfactory detection.
The WT mice in the current experiment, on a mixed C57BL/6 and 129Ola background, avoided quinine at concentrations (3 mM) similar to quinine-insensitive lines as reported by others (Boughter et al. 2005; Harder and Whitney 1998). Other studies, on C57BL/6 lines, report avoidance at lower concentrations, about 0.1–0.3 mM (Dotson et al. 2005; Glendinning et al. 2005; Damak et al. 2006). The basis for these discrepancies likely relates to the different strains used in the different studies. Our mixed-background animals appear more similar to the quinine-insensitive lines than to the C57BL/6J line (Boughter et al. 2005).
The P2X2/P2X3Dbl−/− mice exhibited avoidance in long-term intake tests to bitter-tasting stimuli only at the highest concentrations tested and well above the avoidance thresholds for WT mice. The degree of loss of taste avoidance to quinine and denatonium in long-term tests of the P2X2/P2X3Dbl−/− mice is roughly equivalent to the losses reported in similar tests following genetic elimination of elements of the bitter transduction cascade including α-gustducin (Glendinning et al. 2005), PLCβ2 (Dotson et al. 2005), IP3R3 (Hisatsune et al. 2007), and Trpm5 (Damak et al. 2006). In all cases, the mice fail to avoid quinine at 1 mM but do avoid it at concentrations higher than 3 mM. Similarly, the various KO mice do not avoid denatonium at 1 mM (which is strongly aversive to WT mice) but do avoid it at 10 mM. In contrast, in brief-access tests, the P2X2/P2X3Dbl−/− mice did not significantly avoid initial presentations of high concentrations of bitter tastants, whereas all the mice with genetic ablation of elements of the bitter transduction cascade (α-gustducin, PLCβ2, and IP3R3, Trpm5 KO mice) do (Dotson et al. 2005; Glendinning et al. 2005; Damak et al. 2006). Similarly, the transduction cascade KO mice exhibit residual, albeit greatly diminished, gustatory nerve responses to these tastants, but P2X2/P2X3Dbl−/− mice do not. These results suggest that the transduction KO mice may have some residual taste functions, as suggested in the original reports. However, other investigators’ results suggest that transduction cascade KO mice do not have such residual taste function. Similar to our findings in P2X KO mice, Zhang et al. (2003) report that genetic elimination of either Trpm5 or PLCβ2 totally eliminates both gustatory neural responses and short-term taste avoidance behavior. Why the Zhang et al. KO animals exhibit total taste loss, whereas similar KO mice in other laboratories do not, is unclear.
The change in lick latency of P2X2/P2X3Dbl−/− mice during the 30-min test sessions for bitter compounds suggests that the mice learned during the session to associate a postingestive negative hedonic sensation with a nasally detected cue as also reported previously for PLCβ-KO mice (Dotson et al. 2005). We suggest that the strong solutions containing the “bitter-tasting” substances produce irritation (or other negative hedonic experience) of the oropharynx or upper gastrointestinal (GI) tract, as suggested previously by several investigators (Glendinning et al. 2008; Hao et al. 2009). The mice then learn to associate that experience with the odor of the solution in the lick tube. After several repetitions, the mice learn to avoid, or at least delay contact with, the proffered lick tube containing the relevant solution. The fact that there is no delay in licking for these strong solutions upon initial presentation indicates that the odor of the substance itself does not carry a negative hedonic value to a naive animal. The odor of the solution then becomes the conditioned stimulus after repeated contact.
Several systems may mediate a residual chemosensory response including remaining taste functions, for example, as mediated by the greater superficial petrosal (GSP) nerve, oropharyngeal free nerve endings, laryngeal solitary chemoreceptor cells (SCC) innervated by the superior laryngeal branch of the vagus nerve (Finger et al. 2005), or enterochromaffin cells (ECs) of the gut (Bezencon et al. 2007) innervated by the vagus nerve. The likelihood that palatal taste buds innervated by the GSP operate substantially differently from all other taste buds seems remote. The palatal taste buds express the same hallmarks of purinergic signaling as do taste buds in all other gustatory fields and are innervated by P2X2/3-expressing nerve fibers. But until GSP nerve recordings are undertaken in the P2X2/P2X3Dbl−/− animals, we cannot formally exclude this possibility. The SCCs and ECs express bitter (T2R) taste receptors and other components of the bitter taste cascade. The SCCs are innervated by fibers lacking the P2X receptors (whether the same holds true for ECs is not determined; Finger et al. 2005). Presumably, such systems would be intact in P2X2/P2X3Dbl−/− animals used in this study. In hamsters, the superior laryngeal nerve exhibits robust responses to acids and hypertonic solutions, and lower responsiveness to several bitter-tasting substances including quinine (Dickman and Smith 1988). Other investigators have attributed this chemosensitivity of the larynx to the taste buds housed there, but it is possible that the numerous laryngeal SCCs (Sbarbati et al. 2004) contribute significantly to the response to substances that activate the T2R (bitter taste) receptors of the SCCs. The fact that laryngeal taste buds, like their lingual and palatal counterparts, are innervated by nerve fibers expressing P2X receptors (Finger et al. 2005) suggests that these laryngeal taste buds are impaired in the P2X2/P2X3Dbl−/− mice, whereas the SCCs, which are innervated by non-P2X2–expressing fibers of the same nerve, may not be.
P2X2/P2X3Dbl−/− mice largely responded to citric acid similar to WT controls despite a pronounced lack of gustatory nerve activity to this stimulus in P2X2/P2X3Dbl−/− mice. The trigeminal nerve responds to citric acid near concentrations commonly used for taste testing (Pittman and Contreras 1998; Lugaz 2004) and detection by the trigeminal system may underlie the residual acid responsiveness of the P2X2/P2X3Dbl−/− animals (Lugaz 2004). Citric acid is one of the substances that highly activates the superior laryngeal branch of the vagus nerve (Smith and Hanamori 1991; Dickman and Smith 1988), possibly via a non-gustatory mechanism, and could explain the similarity between P2X2/P2X3Dbl−/− and WT mice. This finding is of further import in that it implies that investigators should not expect an obvious behavioral phenotype for genetic elimination of any putative sour taste receptors. The P2X2/P2X3Dbl−/− mice substantially lack gustatory neural responses to the acids tested and yet they retain essentially normal behavioral avoidance, possibly via non-gustatory oropharyngeal detection systems. Conversely, the P2X2/P2X3Dbl−/− may have residual gustatory functions that were not observed in our whole nerve recordings (Finger et al. 2005). Indeed, some minor responsiveness could be seen in the glossopharyngeal nerve records, and these relatively small nerve responses may represent substantial activity in a small number of fibers, which could be meaningful to the animal. Similarly, substantial function might remain in the gustatory nerves, which we did not examine, that is, the GSP and superior laryngeal nerves.
P2X2/P2X3Dbl−/− mice appeared relatively insensitive to sucrose in brief-access tests but showed a strong preference for high levels of sucrose in the 24-h intake tests. The preference for sucrose in the long-term tests is especially noteworthy in that the gustatory nerves of the P2X2/P2X3Dbl−/− mice do not respond to sucrose stimulation at the highest concentrations tested (Finger et al. 2005). That is, the behavior of the P2X2/P2X3Dbl−/− mice persists in the absence of apparent gustatory input. The ability of P2X2/P2X3Dbl−/− mice to show an intake preference for a solution that they cannot detect by taste suggests postingestive factors. Trpm5 KO mice similarly do not exhibit gustatory neural responses to sucrose at concentrations of 300 mM and less but do exhibit consummatory behavioral responses in 2-bottle preference tests to sucrose concentrations of 160 mM and higher (Damak et al. 2006). These behavioral responses are likely attributable to a postingestive rise in blood glucose as has been described by several investigators (Sclafani and Glendinning 2003, 2005; de Araujo et al. 2008). A difference between the de Araujo et al. (2008) study and the studies by Damak et al. (2006) and ourselves was that in the former work, mice were trained to associate the sipper tube on 1 side with the presence of the sucrose solution, that is, they were exposed to only 1 tube at a time during training and that tube remained in a fixed position. In both the Damak study and ours, 2 sipper tubes were present at all times and so the mouse had to make the association of the rise in blood glucose with the position of the tube sipped from. Because the rise in blood glucose can occur within 5–10 min after ingestion (Louis-Sylvestre 1976; Sumiyoshi et al. 2006), this implies that mice may drink predominantly from 1 tube for a few minutes before switching to the other tube. If the animals would have drunk alternately from the 2 tubes over a relatively short time period, then it would have been impossible to make the association in the absence of gustatory or other consummatory cues. Alternatively, the mice may be using “sweet”-responsive sensors of the GI tract to provide information about a recently ingested substance (Margolskee et al. 2007). In that case, the association of a sipper tube with ingested sucrose could occur faster than the 5- to 10-min period necessary to effect a rise in blood glucose. Another possibility is that postingestive effects would be associated with any novel stimulus presented. Thus, the postingestive rise in glucose levels would be more likely associated with the novel stimulus (sucrose) than the familiar one (water).
In sum, these results extend the previous findings on P2X2/P2X3Dbl−/− mice, which have a profound deficiency in terms of gustatory neural responses to all taste qualities (Finger et al. 2005). Despite the lack of gustatory input, these P2X2/P2X3Dbl−/− mice are able to respond to acids essentially identically as do WT mice. The P2X2/P2X3Dbl−/− mice can avoid high concentrations of “bitter”-tasting stimuli but appear to do so primarily using non-gustatory or postingestive cues. Primary conclusions are as follows: 1) mice can use oropharyngeal and/or gut sensors to detect and avoid high concentrations of many so-called tastants, even with minimal or no gustatory function, and 2) consummatory behavior to high concentrations of sucrose, and avoidance of high concentrations of denatonium, can be mediated by postingestive systems.
Supplementary material
Supplementary material can be found at http://www.chemse.oxfordjournals.org/
Funding
National Institute on Deafness and Other Communication Disorders at the National Institutes of Health [R01 DC06070, R01 DC00766, and P30 DC04657 to T.E.F. and Diego Restrepo (University of Colorado Denver School of Medicine)].
Acknowledgments
The authors are grateful to Debra Cockayne and Roche Palo Alto for providing the P2X2/P2X3Dbl−/− mice and their WT controls. They also thank Debra Cockayne, Jennifer Stratford, Eugene Delay, and Wayne Silver for comments on earlier drafts of the manuscript. They especially thank the anonymous reviewers whose constructive critiques greatly improved the analysis of these data.
References
- Bezencon C, le Coutre J, Damak S. Taste-signaling proteins are coexpressed in solitary intestinal epithelial cells. Chem Senses. 2007;32:41–49. doi: 10.1093/chemse/bjl034. [DOI] [PubMed] [Google Scholar]
- Boughter JD, Jr, Raghow S, Nelson TM, Munger SD. Inbred mouse strains C57BL/6J and DBA/2J vary in sensitivity to a subset of bitter stimuli. BMC Genet. 2005;6:36. doi: 10.1186/1471-2156-6-36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Breslin PA, Kaplan JM, Spector AC, Zambito CM, Grill HJ. Lick rate analysis of sodium taste-state combinations. Am J Physiol. 1993;264:R312–R318. doi: 10.1152/ajpregu.1993.264.2.R312. [DOI] [PubMed] [Google Scholar]
- Cockayne DA, Dunn PM, Zhong Y, Rong W, Hamilton SG, Knight GE, Ruan HZ, Ma B, Yip P, Nunn P, et al. P2X2 knockout mice and P2X2/P2X3 double knockout mice reveal a role for the P2X2 receptor subunit in mediating multiple sensory effects of ATP. J Physiol. 2005;567(Pt 2):621–639. doi: 10.1113/jphysiol.2005.088435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Damak S, Rong M, Yasumatsu K, Kokrashvili Z, Pérez CA, Shigemura N, Yoshida R, Mosinger B, Jr, Glendinning JI, Ninomiya Y, et al. Trpm5 null mice respond to bitter, sweet, and umami compounds. Chem Senses. 2006;31:253–264. doi: 10.1093/chemse/bjj027. [DOI] [PubMed] [Google Scholar]
- de Araujo IE, Oliveira-Maia AJ, Sotnikova TD, Gainetdinov RR, Caron MG, Nicolelis MA, Simon SA. Food reward in the absence of taste receptor signaling. Neuron. 2008;57:930–941. doi: 10.1016/j.neuron.2008.01.032. [DOI] [PubMed] [Google Scholar]
- Dickman JD, Smith DV. Response properties of fibers in the hamster superior laryngeal nerve. Brain Res. 1988;450:25–38. doi: 10.1016/0006-8993(88)91541-7. [DOI] [PubMed] [Google Scholar]
- Dotson CD, Roper SD, Spector AC. PLCbeta2-independent behavioral avoidance of prototypical bitter-tasting ligands. Chem Senses. 2005;30:593–600. doi: 10.1093/chemse/bji053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Finger TE, Danilova V, Barrows J, Bartel DL, Vigers AJ, Stone L, Hellekant G, Kinnamon SC. ATP signaling is crucial for communication from taste buds to gustatory nerves. Science. 2005;310:1495–1499. doi: 10.1126/science.1118435. [DOI] [PubMed] [Google Scholar]
- Glendinning JI, Bloom LD, Onishi M, Zheng KH, Damak S, Margolskee RF, Spector AC. Contribution of alpha-gustducin to taste-guided licking responses of mice. Chem Senses. 2005;30:299–316. doi: 10.1093/chemse/bji025. [DOI] [PubMed] [Google Scholar]
- Glendinning JI, Gresack J, Spector AC. A high-throughput screening procedure for identifying mice with aberrant taste and oromotor function. Chem Senses. 2002;27:461–474. doi: 10.1093/chemse/27.5.461. [DOI] [PubMed] [Google Scholar]
- Glendinning JI, Yiin Y-M, Ackroff K, Sclafani A. Intragastric infusion of denatonium conditions flavor aversions and delays gastric emptying in rodents. Physiol Behav. 2008;93:757–765. doi: 10.1016/j.physbeh.2007.11.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Green BG, Mason JR, Kare MR. In: Chemical Senses. 2. Irritation. Green BG, Mason JR, Kare MR, editors. New York: Marcel Dekker Inc; 1990. p. 361. [Google Scholar]
- Hao S, Dulake M, Espero E, Sternini C, Raybould HE, Rinaman L. Central Fos expression and conditioned flavor avoidance in rats following intragastric administration of bitter taste receptor ligands. Am J Physiol Regul Integr Comp Physiol. 2009;296(3):R528–R536. doi: 10.1152/ajpregu.90423.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harder DB, Whitney G. A common polygenic basis for quinine and PROP avoidance in mice. Chem Senses. 1998;23:327–332. doi: 10.1093/chemse/23.3.327. [DOI] [PubMed] [Google Scholar]
- Hisatsune C, Yasumatsu K, Takahashi-Iwanaga H, Ogawa N, Kuroda Y, Yoshida R, Ninomiya Y, Mikoshiba K. Abnormal taste perception in mice lacking the type 3 inositol 1,4,5-trisphosphate receptor. J Biol Chem. 2007;282:37225–37231. doi: 10.1074/jbc.M705641200. [DOI] [PubMed] [Google Scholar]
- Keele CA. The common chemical sense and its receptors. Arch Int Pharmacodyn Ther. 1962;139:547–557. [PubMed] [Google Scholar]
- Louis-Sylvestre J. Preabsorptive insulin release and hypoglycemia in rats. Am J Physiol. 1976;230:56–60. doi: 10.1152/ajplegacy.1976.230.1.56. [DOI] [PubMed] [Google Scholar]
- Lugaz O. Convergence des sensations gustatives et somesth_siques: cas particulier des acides. Orsay. Universite De Paris-Sud 11: France; 2004. p. 209. [Google Scholar]
- Margolskee RF, Dyer J, Kokrashvili Z, Salmon KS, Ilegems E, Daly K, Maillet EL, Ninomiya Y, Mosinger B, Shirazi-Beechey SP. T1R3 and gustducin in gut sense sugars to regulate expression of Na+-glucose cotransporter 1. Proc Natl Acad Sci U S A. 2007;104:15075–15080. doi: 10.1073/pnas.0706678104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mook DG. Oral and postingestional determinants of the intake of various solutions in rats with esophageal fistulas. J Comp Physiol Psychol. 1963;56:645–659. [Google Scholar]
- Parker GH. The reactions of smell, taste, and the common chemical sense in vertebrates. J Acad Nat Sci Philadelphia. 1912;15:221–234. [Google Scholar]
- Pittman DW, Contreras RJ. Responses of single lingual nerve fibers to thermal and chemical stimulation. Brain Res. 1998;790:224–235. doi: 10.1016/s0006-8993(98)00059-6. [DOI] [PubMed] [Google Scholar]
- Rhinehart-Doty JA, Schumm J, Smith JC. A non-taste cue of sucrose in short-term intake tests in rats. Chem Senses. 1994;19:425–431. doi: 10.1093/chemse/19.5.425. [DOI] [PubMed] [Google Scholar]
- Sbarbati A, Merigo F, Benati D, Tizzano M, Bernardi P, Osculati F. Laryngeal chemosensory clusters. Chem Senses. 2004;29:683–692. doi: 10.1093/chemse/bjh071. [DOI] [PubMed] [Google Scholar]
- Sclafani A, Glendinning JI. Flavor preferences conditioned in C57BL/6 mice by intragastric carbohydrate self-infusion. Physiol Behav. 2003;79:783–788. doi: 10.1016/s0031-9384(03)00174-4. [DOI] [PubMed] [Google Scholar]
- Sclafani A, Glendinning JI. Sugar and fat conditioned flavor preferences in C57BL/6J and 129 mice: oral and postoral interactions. Am J Physiol. 2005;289:R712–R720. doi: 10.1152/ajpregu.00176.2005. [DOI] [PubMed] [Google Scholar]
- Smith DV, Hanamori T. Organization of gustatory sensitivities in hamster superior laryngeal nerve fibers. J Neurophysiol. 1991;65:1098–1114. doi: 10.1152/jn.1991.65.5.1098. [DOI] [PubMed] [Google Scholar]
- Stellar E, Hyman R, Samet S. Gastric factors controlling water-and-salt-solution-drinking. J Comp Physiol Psychol. 1954;47:220–226. doi: 10.1037/h0063148. [DOI] [PubMed] [Google Scholar]
- Sternini C, Anselmi L, Rozengurt E. Enteroendocrine cells: a site of ‘taste’ in gastrointestinal chemosensing. Curr Opin Endocrinol Diabetes Obes. 2008;15:73–78. doi: 10.1097/MED.0b013e3282f43a73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sumiyoshi M, Sakanaka M, Kimura Y. Chronic intake of high-fat and high-sucrose diets differentially affects glucose intolerance in mice. J Nutr. 2006;136:582–587. doi: 10.1093/jn/136.3.582. [DOI] [PubMed] [Google Scholar]
- Taylor-Burds CC, Westburg AM, Wifall TC, Delay ER. Behavioral comparisons of the tastes of L-alanine and monosodium glutamate in rats. Chem Senses. 2004;29:807–814. doi: 10.1093/chemse/bjh246. [DOI] [PubMed] [Google Scholar]
- Wang Y, Erickson RP, Simon SA. Selectivity of lingual nerve fibers to chemical stimuli. J Gen Physiol. 1993;101:843–866. doi: 10.1085/jgp.101.6.843. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang Y, Hoon MA, Chandrashekar J, Mueller KL, Cook B, Wu D, Zuker CS, Ryba NJ. Coding of sweet, bitter, and umami tastes: different receptor cells sharing similar signaling pathways. Cell. 2003;112:293–301. doi: 10.1016/s0092-8674(03)00071-0. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.