Abstract
BACKGROUND
Idiopathic membranous nephropathy, a common form of the nephrotic syndrome, is an antibody-mediated autoimmune glomerular disease. Serologic diagnosis has been elusive because the target antigen is unknown.
METHODS
We performed Western blotting of protein extracts from normal human glomeruli with serum samples from patients with idiopathic or secondary membranous nephropathy or other proteinuric or autoimmune diseases and from normal controls. We used mass spectrometry to analyze the reactive protein bands and confirmed the identity and location of the target antigen with a monospecific antibody.
RESULTS
Serum samples from 26 of 37 patients (70%) with idiopathic but not secondary membranous nephropathy specifically identified a 185-kD glycoprotein in non-reduced glomerular extract. Mass spectrometry of the reactive protein band detected the M-type phospholipase A2 receptor (PLA2R). Reactive serum specimens recognized recombinant PLA2R and bound the same 185-kD glomerular protein as did the monospecific anti-PLA2R antibody. Anti-PLA2R autoantibodies in serum samples from patients with membranous nephropathy were mainly IgG4, the predominant immunoglobulin subclass in glomerular deposits. PLA2R was expressed in podocytes in normal human glomeruli and colocalized with IgG4 in immune deposits in glomeruli of patients with membranous nephropathy. IgG eluted from such deposits in patients with idiopathic membranous nephropathy, but not in those with lupus membranous or IgA nephropathy, recognized PLA2R.
CONCLUSIONS
A majority of patients with idiopathic membranous nephropathy have antibodies against a conformation-dependent epitope in PLA2R. PLA2R is present in normal podocytes and in immune deposits in patients with idiopathic membranous nephropathy, indicating that PLA2R is a major antigen in this disease.
Idiopathic membranous nephropathy, a common cause of the nephrotic syndrome in adults, is an organ-specific autoimmune disease. Despite extensive investigation, a target antigen has been elusive. Studies of membranous nephropathy in a rat model (Heymann’s nephritis) established that the subepithelial immune deposits that characterize the disease are formed in situ, as a result of capping and shedding of the target antigen, megalin, from the basal surface of podocytes when it forms a complex with circulating antimegalin antibodies.1–8 Although megalin is not expressed on human podocytes, we hypothesized that a similar process, albeit with an unknown antigen, is operative in human membranous nephropathy. Additional evidence from the work of Debiec et al.9,10 supports the in situ formation of glomerular immune deposits in patients with membranous nephropathy. They described an alloimmune form of membranous nephropathy in neonates born to mothers with a deficiency of neutral endopeptidase, a protein expressed on podocytes, to which the mothers had been sensitized during previous pregnancies.
To identify the target antigen in patients with idiopathic membranous nephropathy, we used circulating antibodies from adults with this disease to detect normal glomerular proteins by using Western blotting. Subsequent analysis with the use of mass spectrometry and confirmation with the use of protein-specific reagents allowed for the identification and characterization of the predominant protein detected by these circulating antibodies.
METHODS
SERUM AND KIDNEY-TISSUE SAMPLES
We collected and stored serum samples from patients with membranous nephropathy, those with other glomerular or autoimmune disorders, and normal controls (healthy volunteers). The samples were assigned codes to render them anonymous. The institutional review board of the Boston University School of Medicine approved the study, and written informed consent was obtained from all the subjects. In addition, serum specimens from deceased donors with preserved renal function were obtained from the New England Organ Bank, as were kidneys, from deceased donors, that were unsuitable for transplantation. These samples were exempt from the requirement of informed consent, according to the institutional review board’s approved use of organs and tissues from deceased donors for research.
Idiopathic membranous nephropathy was diagnosed by means of renal biopsy in patients who lacked features suggestive of secondary membranous nephropathy, such as antinuclear antibodies or hepatitis B antibodies in the serum (Table 1 in the Supplementary Appendix, available with the full text of this article at NEJM.org).
Glomeruli were isolated from the kidneys that were unsuitable for transplantation with the use of graded sieving,11 and glomerular proteins were extracted in radioimmunoprecipitation-assay buffer (Boston BioProducts). Contaminating IgG was removed through incubation with Immobilized Protein G Plus (Fisher Scientific). Peptide N-glycosidase F (New England BioLabs) was used to de-glycosylate glomerular proteins. Glomerular glycoproteins were partially purified through absorption by immobilized wheat-germ agglutinin (Vector Laboratories) and elution with N-acetyl glucosamine.
WESTERN BLOTTING
Human glomerular extract or recombinant PLA2R expressed by human cells (see the Methods section of the Supplementary Appendix) was electrophoresed under nonreducing conditions and transferred to nitrocellulose membranes, according to standard protocols. Human serum was used as the primary antibody, at a dilution of 1:100, unless otherwise indicated. A guinea pig polyclonal antibody against the M-type phospholipase A2 receptor (hereafter referred to simply as PLA2R) was raised against full-length purified rabbit PLA2R.12 Sheep antibodies against the four IgG subclasses were used as recommended by the manufacturer (Binding Site). Detecting antibodies were peroxidase-conjugated donkey antibodies against human IgG, guinea pig IgG (Jackson ImmunoResearch Laboratories), or sheep IgG (Sigma). Immunoglobulins were acid-eluted from the remnant cores of kidney-biopsy specimens from patients with membranous nephropathy, lupus membranous nephropathy, or IgA nephropathy (see the Methods section of the Supplementary Appendix). This eluted IgG was used to immunoblot the human glomerular extract or recombinant PLA2R directly.
MASS SPECTROMETRY ANALYSIS AND DATA INTERPRETATION
We excised regions of interest from gels identical to those used for Western blotting and performed in-gel tryptic digestion, as previously described.13 We analyzed the resulting peptides by using a modified version of a method that couples liquid chromatography with tandem mass spectrometry14 and entered the acquired data into existing databases of human proteins to identify relevant proteins in our patients.
IMMUNOPRECIPITATION
Reactive or nonreactive human serum was incubated with human glomerular extract, immunoprecipitated with Immobilized Protein G Plus, electrophoresed in the presence of a reducing agent, and blotted with guinea pig anti-PLA2R antibodies.
IMMUNOHISTOLOGIC ANALYSIS
Cryosections of normal human kidney and kidney-biopsy specimens obtained from patients with membranous nephropathy and made anonymous were fixed in methanol and acetone and blocked with 10% bovine serum albumin. We detected PLA2R by using guinea pig anti-PLA2R antibodies (at a dilution of 1:150), followed by a Cy3-conjugated secondary antibody (see the Supplementary Appendix), and established specificity by preabsorbing the primary antibody with a recombinant fragment of rabbit PLA2R protein15 containing C-type lectinlike domains (CTLDs) 4, 5, and 6. We used a commercial rabbit antibody against human agrin at a dilution of 1:25 (Santa Cruz Biotechnology), followed by an Alexa Fluor 488–conjugated secondary antibody (Invitrogen), to delineate the glomerular basement membrane. To detect podocyte nuclei, we used rabbit antibodies against Wilms’ tumor 1 antigen at a 1:100 dilution (Santa Cruz Biotechnology). We detected IgG4 in immune deposits from patients with membranous nephropathy by using sheep anti-IgG4 antibodies at a dilution of 1:500 (Binding Site), followed by a fluorescein isothiocyanate–conjugated secondary antibody. Cell nuclei were visualized by including a nuclear stain (Hoechst 33342, Molecular Probes) in the mounting medium. Immunofluorescence images were obtained on a microscope (TE-2000, Nikon) linked to a Peltier-cooled CCD camera (CoolSNAP HQ, Photometrics). Confocal microscopy was performed with the use of a spinning-disk confocal instrument (UltraVIEW LCI, PerkinElmer).
STATISTICAL ANALYSIS
Chi-square analysis was performed to compare observed with expected results. The null hypothesis was that a positive signal on Western blotting would occur in a similar proportion of subjects in each group, on the basis of chance alone.
RESULTS
IDENTIFICATION OF A TARGET PROTEIN IN GLOMERULAR EXTRACTS
We performed Western blotting, under nonreducing conditions, of extracts of normal human glomeruli from donor kidneys with serum samples from 37 patients with idiopathic membranous nephropathy. A 185-kD protein band was detected in samples from 26 patients (70%) (Fig. 1). An identical protein band was seen in glomerular extracts prepared from all individual donor kidneys tested thus far. In contrast, serum from 30 normal controls, 15 patients with proteinuric conditions other than membranous nephropathy (diabetic nephropathy or focal and segmental glomerulosclerosis), and 7 patients with other autoimmune disorders did not react with this antigen when assayed under identical conditions (Fig. 1, and Table 1 in the Supplementary Appendix). Moreover, none of the serum samples from eight patients with secondary membranous nephropathy (six with lupus membranous nephropathy and two with hepatitis B–associated membranous nephropathy) were reactive with the 185-kD antigen (Fig. 1B). Chi-square analysis indicated an extremely low probability of such a distribution resulting from chance alone (P = 2×10−12 for the whole series; P = 0.004 for secondary vs. idiopathic membranous nephropathy). Treatment with peptide N-glycosidase F caused a substantial shift in the mobility of this antigen, to approximately 145 kD, indicating that it is heavily glycosylated. All serum samples that were initially reactive with the native 185-kD band also recognized the smaller, deglycosylated band (Fig. 1A).
Figure 1. Results of Western Blotting of Glomerular Proteins with Serum from Patients with Idiopathic Membranous Nephropathy.
The top of Panel A shows the results of Western blotting of extract of human glomerular proteins with serum samples from each of five patients with idiopathic membranous nephropathy (MN1 through MN5) and five patients with other proteinuric conditions (two with focal and segmental glomerulosclerosis [FGS1 and FGS2] and three with diabetic nephropathy [DN1, DN2, and DN3]). Serum samples from the five patients with membranous nephropathy all recognized a band of approximately 185 kD, whereas the samples from the patients with other diseases did not. The bottom of Panel A shows the results of Western blotting, with reactive serum samples from the five patients with membranous nephropathy, of glomerular proteins that were deglycosylated with peptide N-glycosidase F (PNGase F+) or not deglycosylated (PNGase F−). All five samples showed the 185-kD native antigen and a deglycosylated protein of approximately 145 kD. Panel B shows the specificity of the reactivity of serum samples to the 185-kD antigen.
Both the native and N-deglycosylated forms of the antigen could be bound and substantially enriched through absorption by wheat-germ agglutinin (data not shown). The binding of the deglycosylated form may reflect the presence of residual N-linked carbohydrates that are inaccessible to peptide N-glycosidase F under nonreducing conditions. Native and deglycosylated glomerular proteins were eluted from wheat-germ agglutinin and separated by means of gel electrophoresis. The regions corresponding to the 185-kD and 145-kD antigen bands on the Western blots were excised, subjected to in-gel tryptic digestion, and analyzed by means of mass spectrometry. On the basis of the assumption that peptide sequences corresponding to the putative target antigen would be identified in both the 185-kD and 145-kD samples, we generated a working list of candidate proteins (see both Table 2 and Fig. 1 in the Supplementary Appendix). We used available antibodies and recombinant proteins to test candidate antigens and found that anti-bodies against PLA2R identified a glomerular-protein band of the same size as that recognized by the serum from a patient with membranous nephropathy (Fig. 2A).
Figure 2. Identification of the 185-kD Antigen in Human Glomeruli.
Panel A shows the results of Western blotting of samples of human glomerular extract (HGE) and recombinant phospholipase A2 receptor (rPLA2R), deglycosylated with peptide N-glycosidase F (PNGase F+) or not deglycosylated (PNGase F−), with either a reactive serum sample from a patient with membranous nephropathy (MN) or a polyclonal antibody raised against the M-type phospholipase A2 receptor (PLA2R). The recombinant protein migrated to a slightly lower position than the native glomerular protein, although deglycosylation with peptide N-glycosidase F caused both the recombinant and native isoforms to migrate to the same position. Panel B shows that immunoprecipitation of native PLA2R occurred in a reactive serum sample from each of five patients with membranous nephropathy (MN1, MN2, MN3, MN6, and MN7) but not in a nonreactive sample (MN8) and not in nonreactive serum samples from normal controls (NC1 and NC2) or in the absence of serum. The immunoprecipitates were then electrophoresed under reducing conditions and subjected to Western blotting with antibodies against PLA2R (top) or against human IgG (bottom). In the reactive samples from all five patients with membranous nephropathy, PLA2R was immunoprecipitated from human glomerular extract, whereas there was no immunoprecipitation in nonreactive samples from one patient and from the controls. No PLA2R was detected when human serum was omitted from the reaction. The bottom image shows that the immunoprecipitates of samples from controls and patients with membranous nephropathy contained at least equivalent amounts of IgG.
ANTI-PLA2R ANTIBODIES IN PATIENTS WITH MEMBRANOUS NEPHROPATHY
Cell-expressed recombinant human PLA2R was immunoblotted with serum from a patient with membranous nephropathy. A distinct band was detected that was slightly smaller than the corresponding band from extracts of normal human glomeruli (Fig. 2A). When both types of samples were deglycosylated, however, they migrated to the same position, suggesting a minor difference in overall glycosylation between the native protein and the recombinant form. Western-blot analysis of the same samples with the use of a monospecific polyclonal antibody against PLA2R revealed an identical pattern (Fig. 2A). The specificity of serum samples from patients with membranous nephropathy for recombinant PLA2R was established by demonstrating that they had no reactivity with extracts of cells transfected with an irrelevant plasmid (Fig. 2A in the Supplementary Appendix). All serum samples from patients with membranous nephropathy that reacted with the 185-kD glycoprotein from human glomeruli also recognized recombinant PLA2R, further suggesting that the 185-kD band from human glomeruli is indeed PLA2R.
We next confirmed that the serum samples from patients with membranous nephropathy and the anti-PLA2R antibody identified the same protein. As shown in Figure 2B (and Fig. 2B in the Supplementary Appendix), immunoprecipitates of human glomerular extract incubated with reactive serum samples from patients with membranous nephropathy yielded a 185-kD protein that was detected by anti-PLA2R antibodies on Western blotting.
None of the positive serum specimens retained reactivity to the 185-kD protein when the glomerular extract was electrophoresed under reducing conditions (Fig. 3A and data not shown). Moreover, recombinant PLA2R and the native glomerular protein share the same reduction-sensitive epitope (Fig. 3A). Both the monospecific anti-PLA2R antibodies and the serum samples from patients with membranous nephropathy strongly detected nonreduced recombinant and native glomerular PLA2R. Under reducing conditions, the polyclonal antibodies still detected both recombinant and native PLA2R (albeit less robustly), but the serum samples from the patients with membranous nephropathy did not. This suggests that autoantibodies in patients with membranous nephropathy uniquely recognize a conformation-dependent epitope in both the native and recombinant antigen.
Figure 3. Characterization of the Antibody Response to Reduced and Nonreduced Phospholipase A2 Receptor (PLA2R).
Panel A shows a Western blot in which equal amounts of human glomerular extract (HGE) and equal amounts of recombinant PLA2R (rPLA2R) were electrophoresed under reducing and nonreducing conditions. Western blotting was performed with the use of a reactive serum sample from a patient with membranous nephropathy (MN5) or a polyclonal anti-PLA2R antibody and detected with appropriate secondary antibodies. Both the serum sample from the patient with membranous nephropathy and the polyclonal anti-PLA2R antibody reacted with the native and recombinant PLA2R in the nonreduced state, but the reduced proteins were reactive with only the anti-PLA2R antibody. Panel B shows the IgG-subclass specificity to PLA2R. HGE was blotted initially with serum samples from six patients with membranous nephropathy (MN1 through MN6), followed by sheep antibodies specific for each human IgG subclass (1 through 4), and was detected with peroxidase-conjugated antisheep IgG antibody. The arrowheads indicate the fully glycosylated native PLA2R. The predominant IgG subclass that reacted with the native antigen was IgG4, with varying amounts of reactivity seen for IgG1, IgG2, and IgG3. Identical results were obtained with the use of recombinant PLA2R instead of HGE (not shown).
The immunoglobulin profile in patients with membranous nephropathy is typical of the type 2 helper T–cell response, with the glomerular immune deposits containing predominantly IgG4-subclass antibodies.16,17 Similarly, serum samples from patients with membranous nephropathy are highly enriched in IgG4 autoantibodies that react with recombinant PLA2R or the native protein in glomerular extract. Although IgG4 is the predominant subclass of anti-PLA2R IgG antibodies, other subclasses are also present in smaller amounts (Fig. 3B).
GLOMERULAR LOCATION OF PLA2R
Although we hypothesized that the target antigen resides on the surface of podocytes, we first discounted the possible formation of circulating immune complexes with soluble PLA2R. Neither serum samples from patients with membranous nephropathy nor serum samples from controls had any detectable PLA2R, even after enrichment by means of lectin binding (Fig. 3A in the Supplementary Appendix). Nor could we detect circulating immune complexes of PLA2R–IgG through either precipitation with polyethylene glycol 600018 or protein G immunoprecipitation of IgG in serum samples from either group (Fig. 3B and 3C in Supplementary Appendix). Conversely, we detected PLA2R in human glomeruli (Fig. 4A) and, more specifically, in podocytes through immunofluorescence microscopy with the monospecific anti-PLA2R antibody (Fig. 4D through 4G). PLA2R expression in podocytes was further confirmed by positive staining with Wilms’ tumor 1 antigen of PLA2R-positive cells in normal human glomeruli (Fig. 4G), as well as by the detection of PLA2R in cultured immortalized human podocytes19 through the polymerase-chain-reaction assay and Western blotting (Fig. 4 in the Supplementary Appendix). The specificity of PLA2R staining was shown by the near-complete inhibition of the glomerular signal after preincubation of anti-PLA2R antibody with a recombinant fragment of PLA2R containing CTLDs 4, 5, and 6 (Fig. 4B, 4E, and 4H). In contrast to podocytes, mesangial cells in normal human kidney tissue did not show staining for PLA2R (Fig. 5 in the Supplementary Appendix).
Figure 4. Expression of the M-Type Phospholipase A2 Receptor (PLA2R) in Normal Kidney Tissue and Glomeruli.
Serial cryosections of the cortex of normal human kidneys were immunostained with anti-PLA2R antibody (1:150 dilution), followed by Cy3-conjugated anti–guinea pig IgG antibody (1:500 dilution). Panel A shows two positively stained glomeruli (arrowheads) and scattered tubular staining (arrow). Panel B shows an adjacent section in which the anti-PLA2R antiserum was preabsorbed with a recombinant fragment of PLA2R containing C-type lectinlike domains (CTLDs) 4, 5, and 6 (Panel H). The glomerular PLA2R staining is completely blocked by the recombinant fragment (arrowheads), whereas the scattered tubular staining (arrow) is not blocked and is therefore nonspecific. Panel C shows a human kidney-tissue specimen treated with nonimmune guinea pig serum (1:200 dilution), followed by Cy3-conjugated anti–guinea pig IgG antibody, and stained with Hoechst 33342 (a nuclear stain); the inset shows a glomerulus exposed instead to anti-PLA2R antibody (1:200 dilution) but otherwise assessed under identical conditions. Next, cryosections of normal human kidney cortex were costained with antiagrin antibody, followed by an Alexa Fluor 488–conjugated antirabbit secondary antibody, to label the glomerular basement membrane (Panels D and E); in addition, PLA2R staining with guinea pig anti-PLA2R antibody was either left unblocked (Panel D) or was blocked (Panel E), as described above. The PLA2R signal is clearly present outside the glomerular basement membrane and localizes to both the cell body and the processes of the podocyte. The PLA2R staining is markedly reduced when the antibody is preabsorbed with the recombinant PLA2R fragment (Panel E). Panel F shows an enlargement of the left lower portion of the glomerulus shown in Panel D, with demarcation of the capillary lumina (asterisks) and urinary space (US) to highlight the location of the PLA2R-stained podocytes. Hoechst-stained nuclei of parietal epithelial cells in the Bowman’s capsule are indicated by the arrow. Panel G shows a portion of a human glomerulus stained with anti-PLA2R antibody (as described above), anti–Wilms’ tumor 1 antibody (WT1) (1:100 dilution), and Alexa Fluor 488–conjugated anti–rabbit IgG antibody (1:500 dilution) to label podocyte nuclei. The inset shows the image of the entire glomerulus from which the enlarged view was taken. Panel H illustrates the domain structure of PLA2R, which is composed of an N-terminal cysteine-rich domain (Cys-R), a fibronectin type II domain (FNII), eight CTLDs, a transmembrane domain (TM), and a short intracellular C-terminal tail (IC). The blocking fragment used in the experiments described above consists of CTLDs 4, 5, and 6 of a recombinant rabbit PLA2R.
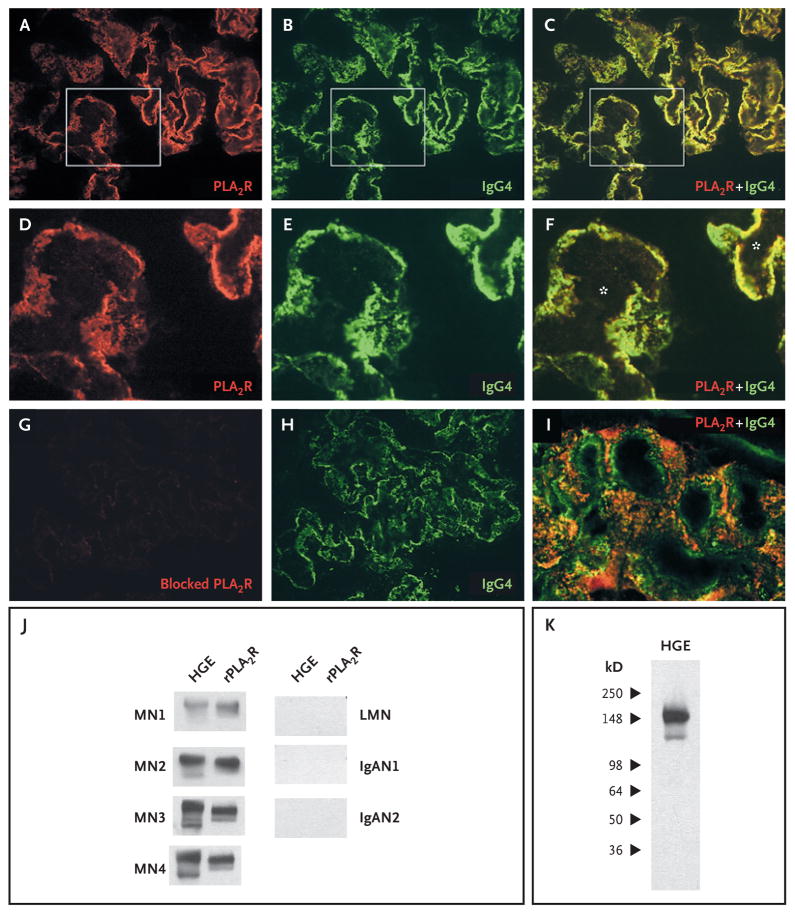
Experimental models of membranous nephropathy have shown capping and shedding of megalin from the surface of podocytes and accumulation of megalin–antimegalin antibody complexes in subepithelial immune deposits. On the basis of these data, we sought evidence that PLA2R and IgG colocalize in the glomerular immune deposits in patients with membranous nephropathy. As shown through confocal analysis of cryosections of kidney-biopsy specimens from patients with membranous nephropathy (Fig. 5A through 5F, with control samples shown in Fig. 6 and 7 in the Supplementary Appendix), PLA2R is present and colocalizes with IgG4 in a fine granular pattern that is typical of membranous nephropathy. As in the normal kidney, the staining of PLA2R could be blocked by the recombinant PLA2R fragment (Fig. 5G). Although the intensity of staining varied among the specimens from eight patients with membranous nephropathy, all revealed the same granular pattern of PLA2R (data not shown). In contrast, PLA2R and IgG4 did not colocalize in a biopsy specimen from a patient with lupus membranous nephropathy, examined under identical conditions (Fig. 5I).
Figure 5. Colocalization of the M-Type Phospholipase A2 Receptor (PLA2R) and IgG4 and Reactivity of Eluted IgG4.
Confocal microscopic analysis of cryosections of a kidney-tissue specimen from a patient with membranous nephropathy revealed the presence of PLA2R (Panel A) and IgG4 (Panel B), which were colocalized in the peripheral capillary walls and the glomerular basement membrane (Panel C). Panels D, E, and F are enlarged images of the boxed areas in Panels A, B, and C, respectively. The asterisks in Panel F indicate the capillary lumina. The anti-PLA2R antibody was blocked with the recombinant fragment (Panel G), with staining virtually eliminated, despite the continued presence of IgG4 (Panel H). Panel I is a confocal image of representative glomerular capillary loops in a biopsy specimen from a patient with lupus membranous nephropathy; PLA2R and IgG4 are not colocalized. IgG4 was detected with sheep anti–human IgG4 antibody (1:500 dilution) and rabbit anti–sheep IgG antibody (1:500 dilution). IgG was eluted from biopsy cores from patients with membranous nephropathy (MN), lupus membranous nephropathy (LMN), or IgA nephropathy (IgAN). This eluted IgG was used to immunoblot human glomerular extract (HGE) or recombinant PLA2R (rPLA2R) (Panel J). Only IgG eluted from the MN samples identified the native and recombinant PLA2R. Panel K shows that the IgG eluted from the MN3 biopsy sample recognized only those bands corresponding to PLA2R.
To confirm that the IgG in the glomeruli from patients with membranous nephropathy was reactive with PLA2R, we eluted IgG from biopsy specimens and used it in Western blotting with native and recombinant PLA2R. IgG was successfully eluted from four biopsy samples from patients with idiopathic membranous nephropathy, one from patients with lupus membranous nephropathy, and two from patients with IgA nephropathy (Fig. 8 in the Supplementary Appendix). The IgG eluted from the samples from patients with idiopathic membranous nephropathy specifically detected the appropriately sized PLA2R bands in human glomerular extract and in cell lysates that were positive for recombinant PLA2R, whereas the IgG eluted from the three other samples from patients with immune-complex glomerular disease did not (Fig. 5J and 5K).
ASSOCIATION WITH DISEASE ACTIVITY
We analyzed serum specimens collected serially from several patients in whom treatment-induced or spontaneous remission occurred. We found that, in general, autoantibodies against PLA2R were present when there was clinically significant disease activity, as measured by urinary protein and serum albumin levels (Fig. 6, and Fig. 9 in the Supplementary Appendix). In the patients with remission, there was a decline or disappearance of anti-PLA2R antibodies before the proteinuria fully resolved.
Figure 6. Antibody against the M-Type Phospholipase A2 Receptor (PLA2R) and Disease Activity in a Patient with Membranous Nephropathy.
Serum samples were collected serially from a patient with membranous nephropathy who had clinical remission after receiving immunosuppressive treatment with cyclophosphamide and prednisone (see the Clinical Vignettes section in the Supplementary Appendix). Panel A shows a decline in the urinary protein level (as measured by the protein-to-creatinine ratio) and an increase in the serum albumin level. The results of Western blotting of serum samples taken from the patient at the same times as the laboratory measurements (Panel B) show reactivity to PLA2R, either the 185-kD native form not deglycosylated by peptide N-glycosidase F (PNGase F−) or the 145-kD deglycosylated form (PNGase F+) in the initial serum sample (from December 2005) only. Equal loading is verified by the nonspecific detection of a 98-kD band. Equal volumes of the four serial serum samples were electrophoresed and blotted with anti–human IgG antibody (bottom), with a progressive increase in total IgG after the patient entered a period of remission from membranous nephropathy.
DISCUSSION
Our findings show that PLA2R is a major target antigen in idiopathic membranous nephropathy. Seventy percent of our patients with biopsy-proven idiopathic membranous nephropathy had IgG antibodies (predominantly of the IgG4 subclass) that reacted with a reduction-sensitive epitope present on PLA2R, a glycoprotein constituent of normal human glomeruli. The distribution of PLA2R in normal glomeruli suggests that it is located on podocytes, as are megalin in rat models of membranous nephropathy and neutral endopeptidase in patients with alloimmune neonatal membranous nephropathy. PLA2R colocalizes with IgG4 in immune deposits of kidney-biopsy specimens from patients with idiopathic membranous nephropathy, and IgG eluted from such biopsy specimens is reactive with PLA2R. Moreover, serial studies in a limited number of patients suggest that the presence of circulating anti-PLA2R antibodies corresponds to disease activity. Finally, the specificity of the reactivity of anti-PLA2R antibodies in patients with idiopathic membranous nephropathy, as opposed to secondary forms of membranous nephropathy or other proteinuric diseases, suggests that the antibodies are most likely the cause, rather than a consequence, of podocyte injury and proteinuria.
There are several possible reasons why anti-PLA2R antibodies are not detected in all patients with idiopathic membranous nephropathy. First, there may be target antigens other than PLA2R; however, screening by means of Western blotting revealed no consistent alternative candidates. Second, there may be cryptic epitopes that were not revealed under our extraction and electrophoretic conditions. Third, even though all our patients had some degree of proteinuria, a proportion may have no longer had immunologically active disease at the time of sample collection. Residual proteinuria in patients with membranous nephropathy is most likely due to altered podocyte architecture that results from remodeling of the glomerular basement membrane in the advanced disease state, as shown by the transplantation of kidneys from rats with active Heymann’s nephritis into healthy rats.20 Although proteinuria from the transplanted kidneys lessened in the absence of antimegalin antibodies, a moderate level of residual proteinuria persisted indefinitely in the recipient rats.
Although anti-PLA2R antibodies are predominantly of the IgG4 isotype, which corresponds to the isotype found in immune deposits in patients with idiopathic membranous nephropathy,16,21 the other three IgG subclasses were variably present in serum but in smaller amounts. The presence of anti–neutral endopeptidase IgG1 and IgG4 antibodies, but not IgG4 alone, corresponds to the development of proteinuria in patients with alloimmune membranous nephropathy10; however, we could not correlate the presence or absence of anti-PLA2R IgG1 antibodies with the severity or duration of disease.
PLA2R is a type I transmembrane receptor and one of four mammalian members of the mannose-receptor family.22,23 All four have a conserved domain structure (Fig. 4H) and undergo endocytic recycling,12 which may provide a constant source of accessible PLA2R at the podocyte membrane for immune-complex formation. PLA2R was initially identified as a binding protein for secreted phospholipase A2 (PLA2) and has a high binding affinity for several types of secreted PLA2 in certain species.24 However, human PLA2R has a different affinity for the more common types of secreted PLA2,25 and its exact physiological role remains unclear.24 Human PLA2R was originally cloned from kidney tissue, in which it is expressed at high levels25; our studies suggest that its expression in human kidneys is largely confined to glomerular podocytes. In contrast, PLA2R is weakly expressed in the glomerulus in normal rats and is up-regulated in experimental models of mesangial proliferative glomerulonephritis.26 In addition, we observed that human anti-PLA2R autoantibodies do not detect PLA2R in rodent or rabbit glomerular extract (data not shown).
Anti-PLA2R autoantibodies from unrelated patients react with a similar reduction-sensitive epitope. This commonality implies that the autoantigen exists only when PLA2R is in a configuration dependent on intramolecular disulfide bonds and that the autoantibody response is restricted to this conformation. A similarly restricted immune response to a reduction-sensitive epitope has been described for Goodpasture’s epitope in the noncollagenous domain NC1 in type IV collagen α3.27,28 The finding of such a restricted response in patients with membranous nephropathy has interesting implications for the proximal events that initiate the immune response and limit epitope spreading to the rest of the PLA2R molecule. Since members of the mannose-receptor family exist in both extended and bent conformations that confer distinct ligand binding and multimerization capacities,29 these different conformations may also play a role in exposure of the epitope in PLA2R.
The role of PLA2R in the pathogenesis of membranous nephropathy is currently unknown. The antigen may simply be a target for antibody binding and complement-mediated podocyte injury, as in experimental models of membranous nephropathy.30,31 Alternatively, the antibodies may act as receptor agonists or antagonists, altering podocyte architecture and barrier function. Because PLA2R is also present in the lungs and on leukocytes,32,33 it will be of interest to determine why the pathologic features are limited to the kidney.
Although there is a shorter, alternatively spliced PLA2R transcript that is predicted to encode a soluble form of the receptor,25 we could not detect such a form in the serum samples from either normal controls or patients with membranous nephropathy. We also found no evidence of circulating complexes of soluble or shed PLA2R and anti-PLA2R antibodies. These findings are consistent with many earlier studies in which circulating immune complexes were not detected in patients with idiopathic membranous nephropathy3 and further support the concept that immune deposits form in situ in those with the disease.
In conclusion, we identified circulating autoantibodies against PLA2R in a large proportion of patients with idiopathic membranous nephropathy whom we examined. This protein is expressed on normal glomerular podocytes and is present in the glomerular immune deposits in patients with the disease. These findings and the characteristics of PLA2R strongly suggest that it is a major target antigen in patients with idiopathic membranous nephropathy. Moreover, the specificity of these findings for idiopathic membranous nephropathy, in contrast to secondary forms of membranous nephropathy or other proteinuric kidney diseases, may have implications for the diagnosis and treatment of the disease.
Supplementary Material
Acknowledgments
Supported by grants from the National Institute of Diabetes and Digestive and Kidney Diseases (DK067658 and DK30932, to Dr. Salant, and DK076743, to Dr. Powell), Amgen (to Dr. Beck), the Halpin Foundation (to Dr. Beck), Centre National de la Recherche Scientifique and Association pour la Recherche sur le Cancer (to Dr. Lambeau), and the Department of Veterans Affairs (to Dr. Klein).
Dr. Beck reports receiving grant support from Amgen and having a patent pending for a diagnostic immunoassay to detect anti-PLA2R antibodies in membranous nephropathy; Dr. Lambeau, holding patents related to the therapeutic use of secretory PLA2 proteins and their inhibitors; and Dr. Salant, receiving consulting fees from Questcor Pharmaceuticals, Cormedix, and DiObix and having a patent pending for a diagnostic immunoassay to detect anti-PLA2R antibodies in membranous nephropathy. No other potential conflict of interest relevant to this article was reported.
We thank Kathleen Dashner for help with tissue processing for immunofluorescence microscopy, Dr. Eric Boilard for help in preparing the human recombinant PLA2R and rabbit PLA2R fragment consisting of CTLDs 4 through 6, the New England Organ Bank for providing human kidneys for research purposes, the families of the organ donors, and the patients and volunteers who provided us with their serum for these studies.
References
- 1.Van Damme BJ, Fleuren GJ, Bakker WW, Vernier RL, Hoedemaeker PJ. Experimental glomerulonephritis in the rat induced by antibodies directed against tubular antigens. V. Fixed glomerular antigens in the pathogenesis of heterologous immune complex glomerulonephritis. Lab Invest. 1978;38:502–10. [PubMed] [Google Scholar]
- 2.Couser WG, Steinmuller DR, Stilmant MM, Salant DJ, Lowenstein LM. Experimental glomerulonephritis in the isolated perfused rat kidney. J Clin Invest. 1978;62:1275–87. doi: 10.1172/JCI109248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Couser WG, Salant DJ. In situ immune complex formation and glomerular injury. Kidney Int. 1980;17:1–13. doi: 10.1038/ki.1980.1. [DOI] [PubMed] [Google Scholar]
- 4.Kerjaschki D, Farquhar MG. The pathogenic antigen of Heymann nephritis is a membrane glycoprotein of the renal proximal tubule brush border. Proc Natl Acad Sci U S A. 1982;79:5557–61. doi: 10.1073/pnas.79.18.5557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Idem. Immunocytochemical localization of the Heymann nephritis antigen (GP330) in glomerular epithelial cells of normal Lewis rats. J Exp Med. 1983;157:667–86. doi: 10.1084/jem.157.2.667. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Makker SP, Singh AK. Characterization of the antigen (gp600) of Heymann nephritis. Lab Invest. 1984;50:287–93. [PubMed] [Google Scholar]
- 7.Raychowdhury R, Niles JL, McCluskey RT, Smith JA. Autoimmune target in Heymann nephritis is a glycoprotein with homology to the LDL receptor. Science. 1989;244:1163–5. doi: 10.1126/science.2786251. [DOI] [PubMed] [Google Scholar]
- 8.Saito A, Pietromonaco S, Loo AK, Farquhar MG. Complete cloning and sequencing of rat gp330/“megalin,” a distinctive member of the low density lipoprotein receptor gene family. Proc Natl Acad Sci U S A. 1994;91:9725–9. doi: 10.1073/pnas.91.21.9725. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Debiec H, Guigonis V, Mougenot B, et al. Antenatal membranous glomerulonephritis due to anti-neutral endopeptidase antibodies. N Engl J Med. 2002;346:2053–60. doi: 10.1056/NEJMoa012895. [DOI] [PubMed] [Google Scholar]
- 10.Debiec H, Nauta J, Coulet F, et al. Role of truncating mutations in MME gene in fetomaternal alloimmunisation and antenatal glomerulopathies. Lancet. 2004;364:1252–9. doi: 10.1016/S0140-6736(04)17142-0. [DOI] [PubMed] [Google Scholar]
- 11.Salant DJ, Cybulsky AV. Experimental glomerulonephritis. Methods Enzymol. 1988;162:421–61. doi: 10.1016/0076-6879(88)62096-9. [DOI] [PubMed] [Google Scholar]
- 12.Zvaritch E, Lambeau G, Lazdunski M. Endocytic properties of the M-type 180-kDa receptor for secretory phospholipases A2. J Biol Chem. 1996;271:250–7. doi: 10.1074/jbc.271.1.250. [DOI] [PubMed] [Google Scholar]
- 13.Powell DW, Rane MJ, Joughin BA, et al. Proteomic identification of 14-3-3zeta as a mitogen-activated protein kinase-activated protein kinase 2 substrate: role in dimer formation and ligand binding. Mol Cell Biol. 2003;23:5376–87. doi: 10.1128/MCB.23.15.5376-5387.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Powell DW, Weaver CM, Jennings JL, et al. Cluster analysis of mass spectrometry data reveals a novel component of SAGA. Mol Cell Biol. 2004;24:7249–59. doi: 10.1128/MCB.24.16.7249-7259.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Lambeau G, Ancian P, Barhanin J, Lazdunski M. Cloning and expression of a membrane receptor for secretory phospholipases A2. J Biol Chem. 1994;269:1575–8. [PubMed] [Google Scholar]
- 16.Doi T, Mayumi M, Kanatsu K, Suehiro F, Hamashima Y. Distribution of IgG subclasses in membranous nephropathy. Clin Exp Immunol. 1984;58:57–62. [PMC free article] [PubMed] [Google Scholar]
- 17.Kuroki A, Iyoda M, Shibata T, Sugisaki T. Th2 cytokines increase and stimulate B cells to produce IgG4 in idiopathic membranous nephropathy. Kidney Int. 2005;68:302–10. doi: 10.1111/j.1523-1755.2005.00415.x. [DOI] [PubMed] [Google Scholar]
- 18.Brandslund I, Siersted HC, Jensenius JC, Svehag SE. Detection and quantitation of immune complexes with a rapid polyethylene glycol precipitation complement consumption method (PEG-CC) Methods Enzymol. 1981;74:551–71. doi: 10.1016/0076-6879(81)74039-4. [DOI] [PubMed] [Google Scholar]
- 19.Saleem MA, O’Hare MJ, Reiser J, et al. A conditionally immortalized human podocyte cell line demonstrating nephrin and podocin expression. J Am Soc Nephrol. 2002;13:630–8. doi: 10.1681/ASN.V133630. [DOI] [PubMed] [Google Scholar]
- 20.Makker SP, Kanalas JJ. Course of transplanted Heymann nephritis kidney in normal host: implications for mechanism of proteinuria in membranous glomerulonephropathy. J Immunol. 1989;142:3406–10. [PubMed] [Google Scholar]
- 21.Haas M. IgG subclass deposits in glomeruli of lupus and nonlupus membranous nephropathies. Am J Kidney Dis. 1994;23:358–64. doi: 10.1016/s0272-6386(12)80997-8. [DOI] [PubMed] [Google Scholar]
- 22.East L, Isacke CM. The mannose receptor family. Biochim Biophys Acta. 2002;1572:364–86. doi: 10.1016/s0304-4165(02)00319-7. [DOI] [PubMed] [Google Scholar]
- 23.Lambeau G, Lazdunski M. Receptors for a growing family of secreted phospholipases A2. Trends Pharmacol Sci. 1999;20:162–70. doi: 10.1016/s0165-6147(99)01300-0. [DOI] [PubMed] [Google Scholar]
- 24.Rouault M, Le Calvez C, Boilard E, et al. Recombinant production and properties of binding of the full set of mouse secreted phospholipases A2 to the mouse M-type receptor. Biochemistry. 2007;46:1647–62. doi: 10.1021/bi062119b. [DOI] [PubMed] [Google Scholar]
- 25.Ancian P, Lambeau G, Mattéi MG, Lazdunski M. The human 180-kDa receptor for secretory phospholipases A2: molecular cloning, identification of a secreted soluble form, expression, and chromosomal localization. J Biol Chem. 1995;270:8963–70. doi: 10.1074/jbc.270.15.8963. [DOI] [PubMed] [Google Scholar]
- 26.Beck S, Beck G, Ostendorf T, et al. Up-regulation of group IB secreted phospholipase A(2) and its M-type receptor in rat ANTI-THY-1 glomerulonephritis. Kidney Int. 2006;70:1251–60. doi: 10.1038/sj.ki.5001664. [DOI] [PubMed] [Google Scholar]
- 27.Hellmark T, Burkhardt H, Wieslander J. Goodpasture disease: characterization of a single conformational epitope as the target of pathogenic autoantibodies. J Biol Chem. 1999;274:25862–8. doi: 10.1074/jbc.274.36.25862. [DOI] [PubMed] [Google Scholar]
- 28.Hudson BG. The molecular basis of Goodpasture and Alport syndromes: beacons for the discovery of the collagen IV family. J Am Soc Nephrol. 2004;15:2514–27. doi: 10.1097/01.ASN.0000141462.00630.76. [DOI] [PubMed] [Google Scholar]
- 29.Llorca O. Extended and bent conformations of the mannose receptor family. Cell Mol Life Sci. 2008;65:1302–10. doi: 10.1007/s00018-007-7497-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Nangaku M, Shankland SJ, Couser WG. Cellular response to injury in membranous nephropathy. J Am Soc Nephrol. 2005;16:1195–204. doi: 10.1681/ASN.2004121098. [DOI] [PubMed] [Google Scholar]
- 31.Cybulsky AV, Quigg RJ, Salant DJ. Experimental membranous nephropathy redux. Am J Physiol Renal Physiol. 2005;289:F660–F671. doi: 10.1152/ajprenal.00437.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Granata F, Petraroli A, Boilard E, et al. Activation of cytokine production by secreted phospholipase A2 in human lung macrophages expressing the M-type receptor. J Immunol. 2005;174:464–74. doi: 10.4049/jimmunol.174.1.464. [DOI] [PubMed] [Google Scholar]
- 33.Silliman CC, Moore EE, Zallen G, et al. Presence of the M-type sPLA(2) receptor on neutrophils and its role in elastase release and adhesion. Am J Physiol Cell Physiol. 2002;283:C1102–C1113. doi: 10.1152/ajpcell.00608.2001. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.