Abstract
Rab11 is a small GTP-binding protein that in cultured mammalian cells has been shown to be concentrated in the pericentriolar endosomal recycling compartment and to play a key role in passage of the recycling transferrin receptor through that compartment [Ullrich, O., Reinsch, S., Urbé, S., Zerial, M. & Parton, R. G. (1996) J. Cell Biol. 135, 913–924]. To obtain insights into the site(s) of action of rab11 within the recycling pathway, we have now compared the effects on recycling at 37°C of overexpression of wild-type rab11 and various mutant forms of this protein in cells that had been loaded with transferrin at either 37°C or 16°C. We show that incubation at 16°C blocks passage of endocytosed transferrin into the recycling compartment and that, whereas the rab11 dominant negative mutant form (S25N) inhibits transferrin recycling after interiorization at either temperature, the wild-type rab11 and constitutively active mutant (Q70L) have no inhibitory effect on the recycling of molecules that were interiorized at 16°C. This differential inhibitory effect shows that two distinct pathways for recycling are followed by the bulk of the transferrin molecules interiorized at the two different temperatures. The incapacity of the constitutively active form of rab11 (Q70L) to inhibit recycling of molecules interiorized at 16°C is consistent with their recycling taking place directly from sorting endosomes, in a process that does not require hydrolysis of GTP on rab11. The fact that the dominant negative (S25N) form of rab11 inhibits recycling of molecules interiorized at both temperatures indicates that activation of rab11 by GTP is required for exit of transferrin from sorting endosomes, regardless of whether this exit is toward the recycling compartment or directly to the plasma membrane.
Many plasma membrane receptors involved in the uptake of extracellular ligands by endocytosis are capable of undergoing multiple cycles of delivery of their ligands to the endosomal system (1). After their internalization via clathrin-coated pits, all receptor-ligand complexes are delivered to peripheral tubulovesicular sorting endosomes, where dissociation of the ligands from the receptors is induced by the acidic pH of the endosomal lumen (2). The bulk of the dissociated ligands (e.g., low density lipoprotein, α2-macroglobulin) and nonrecycling receptors destined for lysosomal degradation remain in the vesicular portion of the sorting endosome that eventually matures into a late endosome (3). On the other hand, receptors that undergo recycling are concentrated in tubular extensions of the sorting endosome (4). There is evidence (see refs. 5 and 6) that some receptor molecules can be returned directly from the sorting endosome back to the plasma membrane (direct recycling). The bulk of the receptor population, however, before returning to the plasma membrane, is transferred to a distinct recycling compartment, which is not accessible to ligands and receptors destined for lysosomal degradation (2). In many cell types [e.g., BHK and Chinese hamster ovary (CHO) cells], this early endosomal subcompartment consists of a juxtanuclear pericentriolar long-lived collection of membranous tubular elements (5, 7). It appears that not all recycling receptors that reach the pericentriolar compartment proceed from there to the plasma membrane (anterograde pathway) but that some also may be sent back to sorting endosomes (8), from where they could enter the direct recycling pathway. Although the pathways followed by recycling receptors are reasonably well established, the molecular mechanisms that maintain and regulate receptor flow through the various subcompartments of the early endosome system remain to be elucidated.
Small GTPases of the rab family have characteristic subcellular distributions and are known to be regulators of membrane traffic between organelles (reviewed in ref. 9). Specific rab proteins have been implicated in the control of various steps within the endocytic and recycling pathways (see ref. 1). Thus, rab4 is located in early sorting endosomes, and it has been proposed that it may regulate the direct recycling of receptors back to the plasma membrane (1, 6). Rab5 also is associated with sorting endosomes, as well as with the plasma membrane and clathrin-coated vesicles, and it has been shown to regulate endocytosis in vivo, as well as the homotypic fusion between early endosomes in vitro (10, 11). Rab7 is found primarily on late endosomes and is thought to regulate transport from early endosomes to late endosomes and lysosomes (12, 13). Finally, rab9, which is associated with late endosomes and the trans-Golgi network, may control transport of the mannose-6-phosphate receptor (M6PR) from the former back to the latter (14, 15).
Rab11 originally was reported to be associated with Golgi membranes, including the trans-Golgi network and secretory vesicles (16). The demonstration that, in gastrointestinal epithelial cells and in particular in the parietal cells of the stomach, rab11 is concentrated in subapical tubular vesicular structures led to the suggestion that it may play a role in the cycling of proteins through the apical plasma membrane (17, 18). Recently, it was shown that rab11 is highly concentrated in the pericentriolar recycling endosomal compartment in CHO and BHK cells and that it plays a key role in passage of the recycling transferrin receptor through that compartment (19). We have now studied the effect of the expression of rab11 mutants on the transferrin recycling capacity of cells that, in a previous incubation, had been allowed to interiorize transferrin under conditions that normally allow the accumulation of this marker in the recycling compartment or in proximal peripheral endosomes. Our observations suggest that activation of rab11 is required not only for entrance of transferrin into the recycling compartment but also for direct recycling from the sorting endosome itself to the cell surface. On the other hand, GTP hydrolysis on rab11 does not seem to be necessary for the latter step—as it is for transport of transferrin from the pericentriolar recycling endosomes to the plasma membrane.
MATERIALS AND METHODS
Cell Lines.
TRVb cells, a mutant cell line of CHO cells lacking a functional endogenous transferrin receptor, and TRVb-1 cells, a TRVb subline stably expressing the human transferrin receptor (20), were cultured in Ham’s F-12 medium supplemented with 5% fetal bovine serum and antibiotics.
Antibodies.
Rabbit antibodies to rab11 were raised against a peptide (HVPPTTENKPKVQC) within the C-terminal region (residues 199–212) of rab11. The antibodies were affinity-purified by using immobilized recombinant rab11 produced in Escherichia coli. Texas Red-conjugated donkey anti-rabbit IgG was obtained from Jackson ImmunoResearch.
Ligands.
Human holo-transferrin (Sigma) was iodinated (2–6 × 106 cpm/μg) with Iodogen (Pierce) and carrier-free Na125I (NEN). Fluorescein isothiocyanate (FITC)-conjugated human transferrin was obtained from Molecular Probes.
Construction of Expression Plasmids.
The human transferrin receptor (hTfR) cDNA in clone pCDTR1 (21) and the human rab11 cDNA in clone YL8 (22) were subcloned into the vector pcDNA3 (CLONTECH). The following rab11 mutants were constructed with the “Altered Sites” site-directed mutagenesis kit (Promega, Madison, IL) and recloned into pcDNA3: rab11-Q70L, an “active” mutant, which is GTPase defective; rab11-S25N, a dominant negative mutant, which does not bind GTP; rab11-ΔC, in which Asn-211 was changed to a stop codon, which eliminates the six C-terminal amino acid residues and prevents prenylation. All mutations were confirmed by sequencing the entire coding region of each mutant cDNA.
Transfections.
For transferrin receptor recycling assays, TRVb cells grown to 80% confluence in 150-mm diameter dishes were cotransfected with 8 μg of pcDNA3-hTfR and 10 μg of pcDNA3-Rab11 (wild-type or mutant) by using LipofectAmine (Life Technologies, Gaithersburg, MD). Twenty hours posttransfection, the cells were trypsinized and replated into a set of 6-well (35-mm diameter) dishes at the confluent density, to be used for the recycling assays 24 h later. For immunofluorescence microscopy, TRVb-1 cells grown to 70% confluence on coverslips were transfected using LipofectAmine with the pcDNA3 plasmids containing the wild-type or mutant rab11 cDNAs and were processed 40 h post-transfection.
Immunofluorescence Microscopy.
To determine the steady-state distribution of transferrin and rab11 by double-labeling, TRVb-1 cells expressing wild-type or mutant rab11 were grown on coverslips, washed twice with (and preincubated for 60 min at 37°C in) serum-free Ham’s F-12 medium containing 0.1% BSA and 20 mM Hepes (pH 7.4). The coverslips were then washed with Hanks’ balanced salt solution containing 20 mM Hepes (pH 7.4) and incubated for 60 min at 37°C or at 16°C with FITC-conjugated human transferrin (200 μg/ml) in the Hepes-buffered serum-free medium. After three washes in PBS (at 37°C or at 16°C, respectively), the cells were fixed with 3% paraformaldehyde in PBS (pH 7.4) for 60 min, and free aldehyde groups were quenched with 0.05 M NH4 Cl in PBS for 10 min, followed by permeabilization and blocking for 20 min with 0.05% saponin in PBS containing 0.5% BSA. Primary anti-rab11 antibody diluted in the permeabilization buffer was applied to the cells for 6 h at room temperature or overnight at 4°C. The cells were washed and incubated for 90 min at room temperature with Texas Red-conjugated donkey anti-rabbit F(ab′)2 antibody diluted 1:200 in the permeabilization buffer. The coverslips were washed with permeabilization buffer and PBS, mounted in Prolong (Molecular Probes), and examined with a Zeiss Axiophot photomicroscope.
Kinetic Analysis of Transferrin Recycling.
Two days after transfection, TRVb cells grown in 6-well 35-mm dishes were washed twice with and preincubated for 1 h at 37°C in serum-free Ham’s F-12 medium supplemented with 0.2% BSA. They then were incubated for 1 h at 37°C or at 16°C with 3 μg/ml 125I-labeled human transferrin in serum-free Ham’s F-12 medium supplemented with 0.2% BSA and 20 mM Hepes (pH 7.4). The cells then were washed twice with ice-cold Hanks’ balanced salt solution containing 20 mM Hepes (pH 7.4), and surface-bound transferrin was removed by incubation for 15 min at 4°C in 2 ml of 150 mM NaCl, 1 mM CaCl2, and 20 mM sodium acetate buffer (pH 4.6), followed by three washes with ice-cold Hanks’ balanced salt solution containing 20 mM Hepes (pH 7.4) and 100 μM deferoxamine mesylate. They then were incubated in 1 ml of serum-free Ham’s F-12 medium supplemented with 0.2% BSA, 20 mM Hepes (pH 7.4), 100 μg/ml unlabeled human transferrin, and 100 μM deferoxamine mesylate for 5 min at 4°C and then warmed to 37°C to allow internalized transferrin to recycle. At various times, the incubations were stopped by placing the culture dishes on ice and immediately adding 2 ml of ice-cold Hanks’ balanced salt solution to each well. The medium was collected, the cells were solubilized in 1 M NaOH, and the radioactivity in both samples was determined by γ-counting.
RESULTS
Activation of Rab11 Is Required for Delivery of Transferrin from Sorting Endosomes to the Pericentriolar Recycling Compartment.
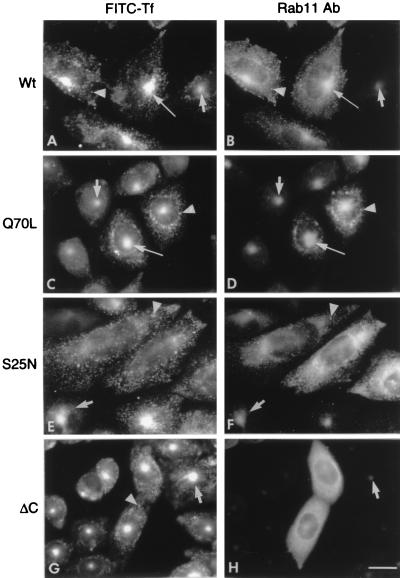
Fluorescently labeled transferrin is a useful marker to monitor the itinerary that a recycling receptor follows during endocytosis and its return to the cell surface (2). To study the role of rab11 in these processes, we examined the effect of the transient expression of wild-type or mutant rab11 proteins on the distribution of FITC–transferrin in TRVb-1 cells that were allowed to endocytose the marker for 1 h at 37°C. TRVb-1 cells are CHO-derived cells that lack the endogenous hamster transferrin receptor but permanently express the human receptor (20). Previous work has shown (see ref. 1) that transferrin recycling in CHO cells proceeds with a t1/2 of ≈20 min so that, in control cells, this loading procedure should yield a nearly steady-state distribution of the labeled ligand within the different subcompartments of the early endosome system. As shown in Fig. 1A, in cells expressing the wild-type rab11, the endocytic marker was highly concentrated in the juxtanuclear region (long arrow) that corresponds to the pericentriolar recycling compartment, which in control cells contains the vast majority of the intracellular receptor molecules (23). FITC–transferrin was also present in many vesicles distributed throughout the cytoplasm (Fig. 1A, arrowhead), which were the only structures labeled within the first few minutes of internalization (not shown) and must correspond to sorting endosomes (3). Confirming the observations of Ullrich et al. (19), in these cells, the pericentriolar compartment also could be labeled intensely with antibodies to rab11 (Fig. 1B, long arrow). Expression of the dominant negative (GDP) form of rab11 (S25N) greatly diminished the accumulation of transferrin in the pericentriolar recycling compartment (Fig. 1E), whereas expression of the constitutively active (GTP) form of rab11 (Q70L) led to a marked accumulation of transferrin in that compartment (Fig. 1C, long arrow). Immunofluorescence with the anti-rab11 antibodies also revealed that the dominant negative (S25N) (Fig. 1F) and constitutively active (Q70L) (Fig. 1D) forms of rab11 themselves had strikingly different distributions, with the former being highly dispersed in the cytoplasm and the latter concentrated in the pericentriolar region (Fig. 1D, long arrow), as in cells expressing the transfected (Fig. 1B, long arrow) or endogenous wild-type rab11 (Fig. 1D, short arrow). A C-terminally deleted form of rab11 (ΔC) that cannot be prenylated and therefore cannot associate with membranes had, as expected, a diffuse cytoplasmic distribution (Fig. 1H). Expression of this mutant did not affect the accumulation of transferrin in the recycling compartment (Fig. 1G). Thus, we conclude, in agreement with Ullrich et al. (19), that an active form of rab11 is required for passage of recycling receptors from peripheral endosomes to the pericentriolar recycling compartment.
Figure 1.
Localization of rab11 in the pericentriolar recycling compartment and the inhibitory effect of the S25N mutant on the accumulation of FITC–transferrin in that compartment. TRVb-1 cells that express the human transferrin receptor were transfected for transient expression with plasmids encoding: (A and B) the wild type (wt); (C and D) constitutively active (Q70L); (E and F) dominant negative (S25N); or (G and H) C-terminally deleted (ΔC) forms of rab11, as indicated on the left side of each pair of panels. Cells on coverslips were incubated with FITC–transferrin (FITC-Tf) for 1 h at 37°C and processed for indirect immunofluorescence with anti-rab11 antibody (rab11 Ab) and Texas Red-conjugated second antibody. Each pair of photographs (A-H) shows, for the same cells, the distribution of FITC–transferrin (left panels) and Texas Red (right panels) fluorescence. All cells express the transferrin receptor, so they all display intense green fluorescence of FITC–transferrin. The long arrows indicate the pericentriolar recycling compartment in the transfected cells, which in the right panels are identified by their intense Texas Red fluorescence. The short arrows point to the pericentriolar compartment in untransfected cells within the same cultures, and the arrowheads point to cytoplasmic vesicles containing both markers. Note that in cultures transfected with the S25N dominant negative rab11 mutant (E and F), the labeling of the pericentriolar compartment is diminished markedly in transfected cells but not in untransfected cells. Cells expressing the ΔC mutant of rab11 show a diffuse distribution of this protein.
The Constitutively Active (Q70L) and Wild-Type Forms of Rab11, as well as the Dominant Negative Mutant (S25N), Inhibit Recycling of Transferrin Molecules Interiorized for 1 h at 37°C.
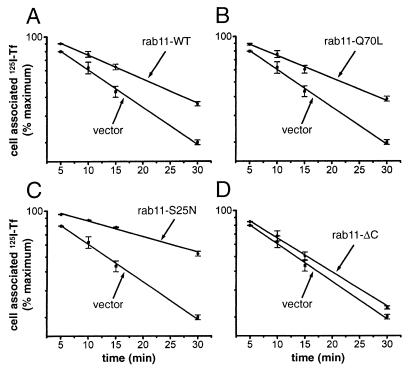
We also examined the effect of the overexpression of the wild-type and rab11 variants on the capacity of cells to recycle interiorized transferrin. For these experiments, we used the TRVb CHO-derived cell line, which lacks the endogenous transferrin receptor and in which the human receptor was expressed transiently together with wild-type rab11 or the individual rab11 mutants. This approach ensured that only those cells that express the exogenous rab11 proteins, whose effects we were attempting to determine, were capable of endocytosis and recycling of transferrin. The doubly transfected cells were allowed to endocytose 125I-labeled human transferrin during a 1-h incubation at 37°C, and then the return of the labeled transferrin into the medium was measured during a subsequent chase incubation at the same temperature (Fig. 2). As mentioned above, under these labeling conditions, the bulk of the ligand and its receptor should be present in the recycling compartment. In cells expressing the transferrin receptor but no exogenous rab11 (Fig. 2; Table 1), transferrin was recycled to the medium with a t1/2 of ≈12 min (Kr = 0.058 ± 0.007 min−1), which agrees with its reported t1/2 for exit from the recycling compartment (see ref. 1). Expression of the wild-type (Fig. 2A) or of the constitutively active (Q70L) (Fig. 2B) or dominant negative (S25N) (Fig. 2C) forms of rab11 each caused a substantial inhibition of transferrin recycling, decreasing the rate constant by 38%, 40% and 59%, respectively (Table 1). It is noteworthy that overexpression of the truncated mutant (rab11-ΔC) that cannot be prenylated had only a negligible effect on transferrin recycling (Fig. 2D), which confirms that membrane association of rab11 is required for it to participate in the molecular interactions that regulate recycling. Immunoblot and immunofluorescence microscopy analyses (not shown) demonstrated that, except for rab11ΔC, which was expressed at 5-fold higher levels, wild-type rab11 and the two other mutants were expressed at comparable levels and that the transfection efficiencies (i.e., percentage of cells expressing the exogenous proteins) were similar (≈35%) for all of the rab11 variants. It can, therefore, be concluded that the differences in transferrin recycling kinetics observed in cells expressing the various rab11 proteins are due to specific effects that they had on the function of the recycling apparatus.
Figure 2.
The constitutively active (Q70L) and wild-type rab11, as well as the dominant negative (S25N) form of this protein, inhibit the recycling of transferrin molecules internalized at 37°C. TRVb cells were cotransfected with pairs of plasmids, one encoding the human transferrin receptor and the other encoding a form of rab11, as indicated in each panel (A, Rab11-wt; B, Rab11-Q70L; C, Rab11-S25N; and D, Rab11-ΔC). The transfected cells were incubated with 125I-transferrin for 1 h at 37°C, to fully load the various intracellular compartments. The cells then were washed, stripped of surface-bound ligand, and incubated at 37°C in medium containing unlabeled transferrin. The extent of release of the internalized marker into the medium was monitored at various times thereafter. The percentage of 125I-transferrin that remains cell-associated in cells expressing each rab11 variant is shown in a semi-log plot as a function of time, together with equivalent data from control cells transfected with the vector lacking a rab11 cDNA insert. Except for rab11-ΔC, the data represent the averages of two separate experiments. The straight lines were obtained by nonlinear curve fitting to the equation y = A* exp (−Kr ⋅ t), where y is the percent of cell-associated 125I-transferrin and Kr the apparent first order recycling rate constant. The rate constants for the different transfected cultures are listed in Table 1.
Table 1.
Rate constants for transferrin recycling in transfected TRVb cells loaded with the marker at either 37°C or 16°C
| Transfected plasmid | Kr(37°C ➞ 37°C) min−1 | Kr(16°C ➞ 37°C) min−1 |
|---|---|---|
| Vector | 0.058 ± 0.007 | 0.060 ± 0.001 |
| Rab11-WT | 0.036 ± 0.001 | 0.049 ± 0.006 |
| Rab11-Q70L | 0.035 ± 0.002 | 0.053 ± 0.001 |
| Rab11-S25N | 0.024 ± 0.002 | 0.027 ± 0.001 |
| Rab11-ΔC | 0.052 ± 0.004 | 0.051 |
First order rate constants (Kr) for transferrin recycling were determined from the data in Figs. 2 and 4 and are presented as the means ± SE of two separate experiments. The middle column gives the recycling rate constants at 37°C for transferrin molecules interiorized at 37°C [Kr (37°C ➞ 37°C)], and the rightmost column gives the rate constants for molecules interiorized at 16°C [Kr(16°C ➞ 37°C)].
The Recycling of Transferrin Molecules Interiorized During a Prolonged Incubation at 16°C Is Inhibited by Expression of the Dominant Negative Mutant (S25N) but Not by the Wild-Type or Constitutively Active (Q70L) Forms of Rab 11.
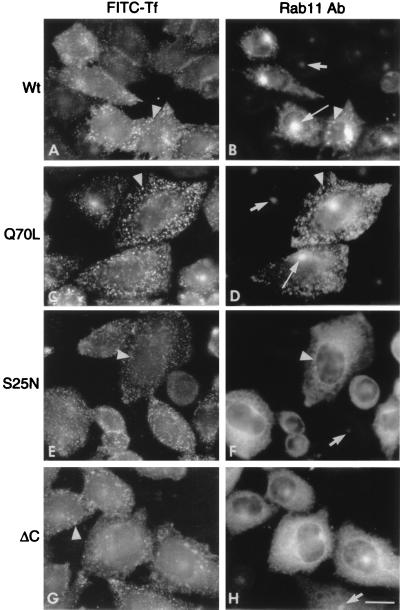
To investigate possible effects of the rab11 mutants on the direct recycling of transferrin from sorting endosomes to the cell surface, we attempted to devise labeling conditions that would preferentially load the earliest subcompartments of the recycling pathway and, hence, increase the fraction of internalized transferrin molecules that could return directly to the cell surface. Temperatures lower than 20°C have been shown to prevent recycling (24) and transcytosis (25, 26), as well as to block progression of endocytosed material along the endocytic pathway (see, e.g., refs. 27 and 28) and the transfer of endocytosed synaptic vesicle proteins to the perinuclear region (29), so we determined whether a low temperature would prevent transport of transferrin receptors from sorting endosomes to the pericentriolar compartment. This prevention was, in fact, observed in untransfected cells, as well as in transfected cells that expressed the wild-type or mutant rab11 proteins and were incubated at 16°C for 1 h with labeled transferrin (Fig. 3). In all cases, the tracer remained dispersed in peripheral endosomes (Fig. 3 A, C, E, and G, arrowheads) and did not reach the pericentriolar recycling compartment. The 16°C incubation, however, did not affect the distributions of the various rab11 proteins (Fig. 3 B, D, F, and H), which were similar to those observed at 37°C: The wild-type (Fig. 3B) and constitutively active (Q70L) (Fig. 3D) rab11 forms were still concentrated in the pericentriolar region, whereas the dominant negative rab11 (S25N) was mainly present in vesicles distributed throughout the cytoplasm (Fig. 3F). The truncated rab11 (ΔC) again had a diffuse cytoplasmic distribution (Fig. 3H).
Figure 3.
FITC–transferrin molecules endocytosed at 16°C do not reach the pericentriolar recycling compartment in which rab11 is concentrated. TRVb-1 cells that express the human transferrin receptor were transfected, as in Fig. 1, with the various rab11 plasmids indicated on the left of each pair of panels. The cells grown on coverslips were allowed to take up FITC–transferrin (FITC-Tf) for 1 h at 16°C, before processing, as in Fig. 1, for double immunofluorescence to localize internalized transferrin (left panels) and rab11 (right panels). The long arrows indicate the pericentriolar recycling compartment identified by the high concentration of rab11. Short arrows point to the recycling compartment in untransfected cells within the same cultures, and the arrowheads point to cytoplasmic vesicles containing both markers. Note that in all cases (left panels), FITC–transferrin is distributed in cytoplasmic vesicles and is not concentrated in the pericentriolar compartment, which in B and D is intensely labeled with anti-rab11 antibodies.
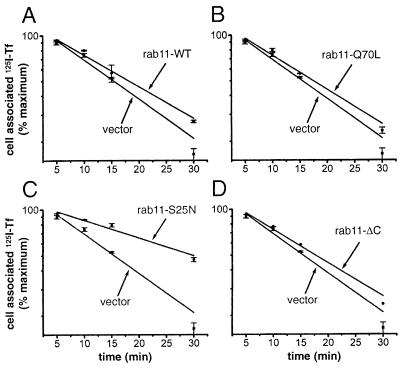
The recycling of transferrin molecules that had been interiorized at 16°C was measured after transfer of the cultures to 37°C (Fig. 4). In cells expressing the GDP form of rab11 (S25N) (Fig. 4C), this recycling was inhibited to the same extent (decrease of Kr by 55%) as in cells that expressed the same mutant but had been loaded at 37°C (Table 1). In striking contrast, however, expression of the wild-type rab11 (Fig. 4A) or of the constitutively active rab11 (Q70L) (Fig. 4B) had only minor inhibitory effects (18% and 12%, respectively, Table 1) on the recycling of transferrin that had been interiorized at 16°C. We, therefore, conclude that the bulk of the interiorized molecules that at 16°C were prevented from reaching the pericentriolar compartment, upon transfer to 37°C, followed a pathway to reach the cell surface that requires activation of rab11 (hence the marked inhibitory effect of rab11-S25N) but not hydrolysis of its bound GTP.
Figure 4.
The constitutively active (Q70L) and wild-type rab11 do not inhibit recycling at 37°C of 125I-transferrin molecules internalized during a previous incubation at 16°C, although the dominant negative form (S25N) of rab11 still inhibits recycling. This experiment was carried out as that in Fig. 3, except that the cells were loaded with 125I-transferrin for 1 h by incubation at 16°C rather than at 37°C.
DISCUSSION
We have examined the effects of overexpressing wild-type rab11 or its dominant negative (S25N) and constitutively active (Q70L) mutant forms on the capacity of cells to recycle transferrin molecules that had been interiorized during a previous incubation at either 37°C or 16°C. We found, in agreement with previous observations (2), that transferrin molecules interiorized during 1-h incubation at 37°C are highly concentrated in the pericentriolar recycling compartment, which at a steady-state has been estimated to contain 80% of the interiorized ligand (30). This compartment, however, was not reached by transferrin molecules that were taken into the cell at 16°C, which accumulated in peripheral punctate structures that are likely to correspond to sorting endosomes. Cells expressing the dominant negative (S25N) form of rab11 were impaired substantially in their capacity to recycle at 37°C interiorized transferrin, irrespective of whether the prior interiorization of this marker had taken place at 37°C or at 16°C. On the other hand, cells expressing either the wild-type or the constitutively active (Q70L) form of rab11 were only significantly impaired in their capacity to recycle transferrin molecules that had been interiorized at 37°C and were almost as efficient as control cells in the recycling of molecules that had been taken in at 16°C. These observations provide direct evidence for the existence of two distinct pathways for recycling of interiorized molecules to the cell surface, one that includes passage through the recycling compartment and is followed by the bulk of the molecules interiorized at 37°C and another that involves direct transfer from sorting endosomes to the cell surface and is followed by the bulk of the molecules accumulated at 16°C.
Our findings with CHO cells loaded to the steady-state with transferrin at 37°C are in agreement with those of Ullrich et al. (19), who studied transferrin recycling in BHK cells that had interiorized the marker during a 5-min pulse, followed by a 30-min chase incubation to load the pericentriolar compartment. Like us, these authors observed that the strongest inhibitory effect was caused by the dominant-negative (GDP) mutant form of rab11, which also prevented the marker from reaching the pericentriolar compartment. This result led Ullrich et al. (19) to suggest that rab11-GTP may be required for transfer of receptors from the sorting endosome to the recycling compartment. These authors attributed the inhibitory effect of the constitutively active (GTP) form of rab11 on recycling to its promoting the diversion of transferrin from a fast cycle (direct return from the sorting endosome) to a slower cycle that involves passage through the pericentriolar recycling endosome. We regarded this explanation as problematic because the inhibitory effects of the rab11 wild type and its constitutively active mutant were observed in cells that, before the recycling assay, were submitted to a 30-min chase incubation, so that essentially all of the marker must have been transferred already to the recycling compartment. Although we also conclude that the active form of rab11 is required for the transfer of receptors from sorting endosomes to the recycling compartment, we propose that the constitutively active form of rab11 (Q70L) acts by inhibiting exit of the transferrin receptor from this compartment to the plasma membrane because this step requires hydrolysis of GTP on rab11. The similar inhibitory effect of overexpression of wild-type rab11 could be explained if this protein is also in the GTP configuration due to limiting levels in cellular GAP activity. The residual recycling of transferrin molecules interiorized at 37°C observed in cells expressing the Q70L mutant form (GTP) of rab11 could be caused in part by retrograde transport from recycling to sorting endosomes (8), from where receptors could be delivered to the cell surface.
The fact that expression of neither the constitutively active form nor of the wild-type rab11 caused a significant impairment in the capacity of the cells to recycle transferrin molecules that accumulated in the sorting endosome (whereas the recycling of these molecules was impaired in cells expressing the dominant negative rab11 mutant) suggests that direct transfer from sorting endosomes to the cell surface takes place in a process that, although requiring the active form of rab11, does not require the hydrolysis of its bound GTP. Thus, the inhibitory effects of the dominant negative form of rab11 on the recycling of molecules interiorized at either temperature lead us to conclude that the active form of this rab protein is required for exit from the sorting endosome, irrespective of the direction of transfer, i.e., directly to the cell surface or to the pericentriolar compartment.
The proposal that transport to the cell surface from the pericentriolar recycling compartment requires hydrolysis of GTP on rab11 may appear to be in conflict with the observation (31) that recycling of transferrin from the pericentriolar compartment in streptolysin-O-permeabilized CHO cells is not affected by the addition of the nonhydrolyzable GTP analog GTPγS. However, in those experiments, labeled transferrin had been allowed to accumulate in the pericentriolar recycling compartment before permeabilization of the cells and addition of the GTP analog. Therefore, the accumulation must have been promoted by rab11 activated with endogenous GTP, whose hydrolysis supported the exit of transferrin toward the plasma membrane after permeabilization of the cells.
The fact that, in contrast to the insignificant effect of the Q70L mutant, the dominant negative (S25N) form of rab11 substantially inhibited transferrin recycling from the compartment that is loaded during endocytosis at 16°C must be reconciled with the finding by Ullrich et al. (19) that the very early phase of recycling—which occurs during the first few minutes after internalization of surface-bound transferrin—is unaffected by expression of either of the rab11 mutants. We suggest that this extremely rapid recycling (that shows no measurable lag and is independent of rab11) takes place from a very early endosomal compartment, proximal to the sorting endosome—possibly a fraction of uncoated primary endocytic vesicles that at 37°C returns directly to the plasma membrane. During endocytosis at 16°C, however, the internalized ligand must be effectively transferred to sorting endosomes even in the presence of the S25N mutant so that the compartment capable of rapid recycling does not contribute measurably to the recycling that takes place during the subsequent incubation of the cells at 37°C. This argument assumes that fusion of endocytic vesicles with sorting endosomes does not involve rab11. Presumably, that process involves rab5, which has been shown to control fusion between early endosomes and between them and endocytic vesicles (10, 32, 33).
Our observations suggest that the active form of rab11 is required for the formation of vesicles that, emerging from sorting endosomes, are capable of carrying receptors either to the pericentriolar compartment or directly to the plasma membrane. Although the predominant model for rab protein function assigns them a role in vesicle docking and fusion (9), there is also considerable evidence that certain rabs may function to promote vesicle formation in donor compartments (15, 34, 35).
It remains to be explained why GTP hydrolysis on rab11 would be a requisite for the transport of receptors out of the pericentriolar compartment toward the plasma membrane but not from sorting endosomes. It is possible that the active form of rab11 is necessary for the transport of vesicles to the recycling compartment because it promotes their association with microtubules responsible for their concentration in the pericentriolar region. Transport out of this region to the plasma membrane then would involve dissociation from microtubules, and this may require hydrolysis of GTP. Recent work (36) has provided the first example of a direct interaction between a rab protein (rab6) and a kinesin-like microtubule-dependent molecular motor. It is possible that rab11 functions in a similar manner.
Acknowledgments
We acknowledge the technical assistance of H. Plesken, I. Gumper, and J. Culkin and the help of M. Cort and T. Rocha in preparing the manuscript. We thank Dr. T. McGraw for his gift of cell lines. This work was partially supported by a grant from the National Institutes of Health (GM43583).
ABBREVIATIONS
- FITC
fluorescein isothiocyanate
- CHO
Chinese hamster ovary
References
- 1.Mukherjee S, Ghosh R N, Maxfield F R. Physiol Rev. 1997;77:759–803. doi: 10.1152/physrev.1997.77.3.759. [DOI] [PubMed] [Google Scholar]
- 2.Yamashiro D J, Tycko B, Fluss S R, Maxfield F R. Cell. 1984;37:789–800. doi: 10.1016/0092-8674(84)90414-8. [DOI] [PubMed] [Google Scholar]
- 3.Dunn K W, McGraw T E, Maxfield F R. J Cell Biol. 1989;109:3303–3314. doi: 10.1083/jcb.109.6.3303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Geuze H J, Slot J W, Strous G J, Lodish H F, Schwartz A L. Cell. 1983;32:277–287. doi: 10.1016/0092-8674(83)90518-4. [DOI] [PubMed] [Google Scholar]
- 5.Hopkins C R, Gibson A, Shipman M, Strickland D K, Trowbridge I S. J Cell Biol. 1994;125:1265–1274. doi: 10.1083/jcb.125.6.1265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Daro E, van der Sluijs P, Galli T, Mellman I. Proc Natl Acad Sci USA. 1996;93:9559–9564. doi: 10.1073/pnas.93.18.9559. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Marsh E W, Leopold P L, Jones N L, Maxfield F R. J Cell Biol. 1995;129:1509–1522. doi: 10.1083/jcb.129.6.1509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Ghosh R N, Maxfield F R. J Cell Biol. 1995;128:549–561. doi: 10.1083/jcb.128.4.549. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Novick P, Zerial M. Curr Opin Cell Biol. 1997;9:496–504. doi: 10.1016/s0955-0674(97)80025-7. [DOI] [PubMed] [Google Scholar]
- 10.Gorvel J P, Chavrier P, Zerial M, Gruenberg J. Cell. 1991;64:915–925. doi: 10.1016/0092-8674(91)90316-q. [DOI] [PubMed] [Google Scholar]
- 11.Bucci C, Parton R G, Mather I H, Stunnenberg H, Simons K, Hoflack B, Zerial M. Cell. 1992;70:715–728. doi: 10.1016/0092-8674(92)90306-w. [DOI] [PubMed] [Google Scholar]
- 12.Feng Y, Press B, Wandinger-Ness A. J Cell Biol. 1995;131:1435–1452. doi: 10.1083/jcb.131.6.1435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Meresse S, Gorvel J P, Chavrier P. J Cell Sci. 1995;108:3349–3358. doi: 10.1242/jcs.108.11.3349. [DOI] [PubMed] [Google Scholar]
- 14.Lombardi D, Soldati T, Riederer M A, Goda Y, Zerial M, Pfeffer S R. EMBO J. 1993;12:677–682. doi: 10.1002/j.1460-2075.1993.tb05701.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Riederer M A, Soldati T, Shapiro A D, Lin J, Pfeffer S R. J Cell Biol. 1994;125:573–582. doi: 10.1083/jcb.125.3.573. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Urbe S, Huber L A, Zerial M, Tooze S A, Parton R G. FEBS Lett. 1993;334:175–182. doi: 10.1016/0014-5793(93)81707-7. [DOI] [PubMed] [Google Scholar]
- 17.Goldenring J R, Soroka C J, Shen K R, Tang L H, Rodriguez W, Vaughan H D, Stoch S A, Modlin I M. Am J Physiol. 1994;267:G187–G194. doi: 10.1152/ajpgi.1994.267.2.G187. [DOI] [PubMed] [Google Scholar]
- 18.Goldenring J R, Smith J, Vaughan H D, Cameron P, Hawkins W, Navarre J. Am J Physiol. 1996;270:G515–G525. doi: 10.1152/ajpgi.1996.270.3.G515. [DOI] [PubMed] [Google Scholar]
- 19.Ullrich O, Reinsch S, Urbe S, Zerial M, Parton R G. J Cell Biol. 1996;135:913–924. doi: 10.1083/jcb.135.4.913. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.McGraw T E, Greenfield L, Maxfield F R. J Cell Biol. 1987;105:207–214. doi: 10.1083/jcb.105.1.207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.McClelland A, Kuhn L C, Ruddle F H. Cell. 1984;39:267–274. doi: 10.1016/0092-8674(84)90004-7. [DOI] [PubMed] [Google Scholar]
- 22.Drivas G T, Shih A, Coutavas E E, D’Eustachio P, Rush M G. Oncogene. 1991;6:3–9. [PubMed] [Google Scholar]
- 23.Mayor S, Presley J F, Maxfield F R. J Cell Biol. 1993;121:1257–1269. doi: 10.1083/jcb.121.6.1257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Sipe D M, Jesurum A, Murphy R F. J Biol Chem. 1991;266:3469–3474. [PubMed] [Google Scholar]
- 25.Barroso M, Sztul E S. J Cell Biol. 1994;124:83–100. doi: 10.1083/jcb.124.1.83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Hunziker W, Male P, Mellman I. EMBO J. 1990;9:3515–3525. doi: 10.1002/j.1460-2075.1990.tb07560.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Mueller S C, Hubbard A L. J Cell Biol. 1986;102:932–942. doi: 10.1083/jcb.102.3.932. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Czekay R P, Orlando R A, Woodward L, Lundstrom M, Farquhar M G. Mol Biol Cell. 1997;8:517–532. doi: 10.1091/mbc.8.3.517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Faundez V, Horng J T, Kelly R B. J Cell Biol. 1997;138:505–515. doi: 10.1083/jcb.138.3.505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Gruenberg J, Maxfield F R. Curr Opin Cell Biol. 1995;7:552–563. doi: 10.1016/0955-0674(95)80013-1. [DOI] [PubMed] [Google Scholar]
- 31.Martys J L, Shevell T, McGraw T E. J Biol Chem. 1995;270:25976–25984. doi: 10.1074/jbc.270.43.25976. [DOI] [PubMed] [Google Scholar]
- 32.Horiuchi H, Lippe R, McBride H M, Rubino M, Woodman P, Stenmark H, Rybin V, Wilm M, Ashman K, Mann M, et al. Cell. 1997;90:1149–1459. doi: 10.1016/s0092-8674(00)80380-3. [DOI] [PubMed] [Google Scholar]
- 33.Barbieri M A, Li G, Colombo M I, Stahl P D. J Biol Chem. 1994;269:18720–18722. [PubMed] [Google Scholar]
- 34.Nuoffer C, Davidson H W, Matteson J, Meinkoth J, Balch W E. J Cell Biol. 1994;125:225–237. doi: 10.1083/jcb.125.2.225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Jedd G, Mulholland J, Segev N. J Cell Biol. 1997;137:563–580. doi: 10.1083/jcb.137.3.563. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Echard A, Jollivet F, Martinez O, Lacapere J-J, Rousselet A, Janoueix-Lerosey I, Goud B. Science. 1998;279:580–585. doi: 10.1126/science.279.5350.580. [DOI] [PubMed] [Google Scholar]