Abstract
Objective
To describe the 9-year incidence of visual impairment, and primary causes of blindness among black participants of the Barbados Eye Studies.
Design
Population-based prospective cohort study.
Participants
The Barbados Eye Studies (BESs) followed a nationally representative cohort selected by simple random sampling, aged 40 to 84 years at baseline, with re-examinations after 4 (Barbados Incidence Study of Eye Diseases: BISED) and 9 years (BISED II). BISED II included 2,793 (81%) of those eligible.
Methods
Cumulative 9-year incidence rates were estimated by the Product-Limit approach. The study was reviewed and approved by the Institutional Review Boards of collaborating institutions.
Main Outcome Measures
Best-corrected visual acuity was assessed by the Ferris-Bailey chart, following a modified Early Treatment of Diabetic Retinopathy Study protocol. Low vision and blindness were defined by World Health Organization (WHO) criteria as visual acuity (VA) <6/18 to 6/120, and <6/120, respectively, in the better eye, and by United States (US) criteria, VA ≤20/40 and ≤20/200, respectively. Vision loss was defined as a decrease of 15 letters or more read correctly in the better eye between baseline and follow-up examinations.
Results
The 9-year incidence was 1.0% and 2.1% for blindness and 6.0% and 9.0% for low vision, by WHO and US criteria, respectively. Older age at baseline was associated with higher incidence of low vision and blindness, reaching 23.0% (95% confidence interval (CI): 18.8, 28.0) and 4.3% (95% CI: 2.7, 6.9) at age 70 years or older, based on WHO criteria. The primary causes of incident bilateral blindness (US criteria) in 126 eyes were age-related cataract (48.3%), open-angle glaucoma (OAG: 14.3%), combined cataract and OAG (6.3%), diabetic retinopathy (DR: 8.7%) and optic atrophy (7.1%). Age-related macular degeneration (2.4%) rarely caused blindness.
Conclusions
Incident visual impairment is exceedingly high in this population. Cataract, OAG and DR remain the major causes of blindness, underpinning the clinical and public health significance of these conditions in this and similar populations.
Visual impairment imposes significant social and economic burdens worldwide. According to estimates based on the 2002 global population, more than 161 million people were visually impaired; 124 million people had low vision and 37 million were blind.1 Although the estimates of blindness are somewhat lower than projections of the 1990s,2 recent estimates of visual impairment are considerably higher. Increasing life expectancy and visual loss due to conditions associated with ageing, underpin the continuing importance of visual impairment and related disability as major global public health concerns.
Visual impairment is more prevalent in black than white populations,3-6 and data from many population-based studies have indicated racial disparities in the distribution of cause-specific blindness.5-13 Early studies highlighted the role of glaucoma and cataract as principal causes of blindness in African-American populations,7,8 in contrast to the burden of visual loss due to age-related macular degeneration (AMD) in white populations. Glaucoma and cataract were confirmed as the principal causes of visual loss in the black population of the Barbados Eye Studies.5 Other population-based studies have highlighted the importance of cataract as a major cause of visual impairment or blindness in rural India,14,15 Singapore,16 Taiwan,17 Beijing, China18 as well as in Latin America.19 Racial variation in the causes of blindness can also be illustrated by the role of degenerative myopia as a major cause of visual loss in Chinese populations.18 Consistent with an inordinately high prevalence, the development of visual impairment in the black population of the Barbados Eye Studies (BESs) after 4 years of follow-up, was also higher than in predominantly European-derived populations studied for similar periods.20-22 There are, however, very limited longer-term data on the development of visual impairment, and an absence of such information from African-origin populations.23-27 The purpose of this report is to describe the nine-year incidence of visual acuity impairment, as well as the primary causes for incident blindness in the black population of the Barbados Eye Studies.
METHODS
The Barbados Eye Studies (BESs) were designed to investigate the prevalence, incidence, progression, and risk factors for major causes of visual loss in a large predominantly African-origin cohort.28-30 The baseline prevalence study, the Barbados Eye Study (BES, 1987-1992) comprised a randomly selected representative sample of Barbados-born citizens aged 40-84 years. A total of 4631 participants (84% participation) completed examinations at the study site; 4314 (93%) were black, 184 (4%) were mixed (black and white), and 133 (3%) were white/other by self-report.28 The cohort was re-examined after baseline examinations ended in 1992, in the Barbados Incidence Study of Eye Diseases (BISED I, 1992-1997) which re-examined 3,427 persons or 85% of the eligible surviving members,29 and the Barbados Incidence Study of Eye Diseases II (BISED II, 1997-2003) which re-examined 2,793 individuals or 81% of the eligible survivors.30 As compared to the prevalence study, BISED I and II, non-participants (mainly due to death and refusals) were older, and more likely to have been hypertensive at baseline. Those who died during the follow up period were more likely to have been male and more frequently reported a history of diabetes. The distribution of self-reported race in the study cohort was similar at baseline and during follow up. Given the low numbers of participants of other groups, this report is based on participants with self-reported African ancestry at baseline.
Standardized protocols used for baseline and both follow-up visits, included various ophthalmic measurements, such as Humphrey perimetry, applanation tonometry, lens gradings (LOCS II31), and color stereo photography, as well as an interview, blood pressure and anthropometric measurements. A systematic 10% sample and participants with specific findings (e.g., best corrected visual acuity <20/30, ocular disease or diabetes history, intraocular pressure >21 mmHg), were referred to the study ophthalmologists for a comprehensive ophthalmologic examination with dilatation. The study was reviewed and approved by the Institutional Review Boards of collaborating institutions and informed consent was obtained from all study participants.
Best-corrected visual acuity was based on the Ferris-Bailey chart, following a modified Early Treatment of Diabetic Retinopathy Study (ETDRS) protocol.32 Participants were refracted using the Humphrey Automated Refractor #530 to determine refractive status and to obtain the best corrected visual acuity. If the refractor could not be used or if refraction was unreliable, the participant's present correction, if any, was determined with a lensometer (Topcon LM-6, Topcon Corporation, Tokyo, Japan). Testing of all participants began at four meters for the right eye followed by the left eye. Pinhole-correction was applied to either eye when the total number of letters read corresponded to a visual acuity (VA) of 6/12 or worse. If the pinhole-corrected VA in either eye was 6/30 or worse, the VA was also measured at one meter. Those who were unable to read the chart letters at one meter were also tested for ability to count fingers, detect hand motion or light perception. The study ophthalmologists assessed each visually impaired participant to determine the underlying causes and the extent of impairment due to each cause.
Low vision and blindness were determined according to the VA component of the World Health Organization (WHO) criteria2, which defines low vision as a VA better than or equal to 6/120 (20/400) and worse than 6/18, and blindness as a VA worse than 6/120. We also determined visual impairment for comparison purposes, according to criteria used in United States (US) studies33 where low vision is defined as acuity worse than 6/12 (20/40) to better than 6/60 (20/200) and blindness is defined as visual acuity of 6/60 (20/200) or worse.
Nine-year cumulative incidence of visual impairment in the BESs cohort was estimated as the development of low vision or blindness during the 9-year follow-up interval, in persons free of these conditions at baseline. Incidence of unilateral or bilateral visual impairment was evaluated first. Incidence in the population was person-based and defined as low vision or blindness in the better eye. Cumulative incidence of VA loss with doubling of the visual angle was defined as a decrease of 15 letters or more read correctly in the better eye between the baseline and follow-up examinations, e.g., a decrease from baseline to 9-year follow-up from 35 to 20 letters read correctly (corresponding to a change in visual acuity from 6/15 to 6/30). Persons with no light perception at baseline were not included in the group at risk for loss of vision. Progression was defined as a doubling of the visual angle among those who had low vision at baseline. Cumulative 9-year incidence/progression rates were estimated by the product-limit approach,34 which allowed the contribution of data from persons with only 4-years of follow-up. Hazard Ratio (HR) estimates were based on Cox proportional hazards regression models with discrete-time data.35 The Statistical Analysis System (SAS; Institute Inc., Cary, NC) was used to perform the analyses.
RESULTS
Table 1 presents the 9-year population-based incidence of bilateral and unilateral visual impairment. The incidence of bilateral blindness in this population was 1.0% (95% confidence interval (CI): 0.7, 1.4) and 2.1% (95% CI: 1.7, 2.7) according to WHO and US criteria, respectively. The 9-year incidence of combined blindness and low vision in fellow eyes was 2.3% (95% CI: 1.8, 2.9) by WHO criteria and 3.7% (95% CI: 3.1, 4.6) by US criteria. As expected, rates of incident bilateral low vision were even higher and affected 4.5% (95% CI: 3.8, 5.4) and 6.4% (95% CI: 5.6, 7.5) of the cohort, as defined by WHO and US criteria, respectively.
Table 1.
9-year Incidence of unilateral and bilateral visual impairment by World Health Organization (WHO) and United States (US) criteria
| WHO* |
US† |
|||
|---|---|---|---|---|
| No. at risk | Incidence % (95% CI) | No. at risk | Incidence % (95% CI) | |
| Bilateral | ||||
| Bilateral blindness | 3294 | 1.0 (0.7, 1.4) | 3271 | 2.1 (1.7, 2.7) |
| Blind, one eye; low vision in fellow eye | 3269 | 2.3 (1.8, 2.9) | 3225 | 3.7 (3.1, 4.6) |
| Low vision, both eyes | ||||
| 3173 | 4.5 (3.8, 5.4) | 3049 | 6.4 (5.6, 7.5) | |
| Unilateral | ||||
| Blindness, one eye; normal vision in fellow eye | 3100 | 2.0 (1.5, 2.6) | 2962 | 1.7 (1.2, 2.3) |
| Low vision, one eye; normal vision in fellow eye | 2949 | 6.8 (5.9, 7.9) | 2784 | 8.9 (7.8, 10.2) |
CI=confidence interval
WHO criteria: Blindness - visual acuity <6/120; low vision - 6/120 ≤ visual acuity < 6/18
US criteria: Blindness – visual acuity ≤ 6/60; low vision – 6/60 <visual acuity < 6/12
The rate of incident unilateral blindness (normal vision in the fellow eye) was twice as high as new onset bilateral blindness according to WHO criteria (2.0% (95% CI: 1.5, 2.6) vs. 1.0% (95% CI: 0.7, 1.4)). In a similar comparison based on US criteria, the comparable rate of incident unilateral blindness was marginally lower than bilateral blindness (1.7% vs. 2.1%).
As presented in Table 2, older age at baseline was associated with higher rates of low vision and incident blindness over 9 years (P<0.05). None of the persons initially aged 40 to 49 years were observed to develop bilateral blindness during the follow-up period. As compared to incident blindness in those aged 50 to 59 years, and based on WHO and US criteria respectively, there was a 10-fold (HR (95% confidence interval): 9.9 (95% CI: 1.2, 79.5) vs. 5-fold (5.0 (95% CI: 1.7, 14.9) respective increase in blindness in the 60-69 year age group. Similarly, there was a 38-fold (38.4 (95% CI: 5.1, 288.0)) vs. 23-fold (22.6 (95% CI: 8.1, 63.5)) increase in blindness among those aged 70 years and older.
Table 2.
Age specific 9-year incidence of low vision and blindness by World Health Organization (WHO) and United States (US) criteria
| Criteria | Age (years) | Low Vision |
Blindness |
||
|---|---|---|---|---|---|
| N** | % (95% CI) | N** | % (95% CI) | ||
| WHO* | 40-49 | 1062 | 0.9 (0.4, 1.7) | 1065 | 0.0 |
| 50-59 | 878 | 2.8 (1.8, 4.2) | 894 | 0.1 (0.0, 1.0) | |
| 60-69 | 747 | 8.1 (6.2, 10.6) | 772 | 1.2 (0.6, 2.5) | |
| 70+ | 486 | 23.0 (18.8, 28.0) | 563 | 4.3 (2.7, 6.9) | |
| Male | 1311 | 7.1 (5.8, 8.8) | 1369 | 1.1 (0.6, 1.9) | |
| Female | 1862 | 5.2 (4.2, 6.3) | 1925 | 0.9 (0.5, 1.4) | |
| Total | 3173 | 6.0 (5.1, 6.9) | 3294 | 1.0 (0.7, 1.4) | |
| US† | 40-49 | 1054 | 1.6 (1.0, 2.6) | 1065 | 0.0 |
| 50-59 | 871 | 4.1 (2.9, 5.8) | 892 | 0.5 (0.2, 1.3) | |
| 60-69 | 700 | 14.0 (11.4, 171.) | 767 | 2.7 (1.6, 4.3) | |
| 70+ | 424 | 34.0 (28.9, 39.8) | 547 | 9.3 (6.9, 12.6) | |
| Male | 1252 | 9.4 (7.8, 11.3) | 1354 | 2.6 (1.8, 3.8) | |
| Female | 1797 | 8.7 (7.4, 10.2) | 1917 | 1.8 (1.3, 2.5) | |
| Total | 3049 | 9.0 (8.0, 10.2) | 3271 | 2.1 (1.7, 2.7) | |
CI=confidence interval
Low vision = visual acuity worse than 6/18 (20/60) to 6/120 (20/400) or better in the better eye; blindness = visual acuity worse than 6/120 (20/400) in the better eye
Low vision = visual acuity worse than 6/12 (20/40) to better than 6/60 (20/200) in the better eye; blindness = visual acuity 6/60 (20/200) or worse in the better eye
number of persons at risk
New onset of low vision occurred in persons aged a decade younger (40 to 49 years) at baseline. The gradient of increasing visual impairment with older age was however, still evident. As expected, there were comparatively higher overall rates of low vision compared to blindness, 9.0% (95% CI: 8.0, 10.2) vs. 6.0% (95% CI: 5.1, 6.9) by WHO and US criteria, respectively. Slightly higher rates of incident low vision and blindness were observed in men compared to women, although statistically significant differences (P<0.05, adjusting for age) were only found for low vision according to the WHO criteria.
Table 3 presents data on the incidence of vision loss (doubling of the visual angle) among black participants of the BESs. The development of doubling of the visual angle over the 9-year period increased with age (P<0.05), from 1.4% (95% CI: 0.8, 2.4) in the youngest age group (40-49 years) to 17.1% (95% CI: 13.5, 21.5) in the oldest age group. Compared to persons aged 40-49 years at baseline, the gender-adjusted HR was 2.1 (95% CI: 1.1, 4.2) for those aged 50-59 years, 5.8 (95% CI: 3.1, 10.8) for persons aged 60-69 years, and 16.0 (95% CI: 8.8, 29.3) for persons aged 70 years and older. Men tended to experience higher rates of visual loss than did women (6.3% (95% CI: 5.0, 7.9) vs. 4.9% (95% CI: 4.0, 6.1)), although a statistically significant gender difference was found only among those aged 60-69 years at baseline. Further evaluation of the progression of visual impairment among persons with low vision at baseline, according to US criteria (6/60<VA<6/12), was based on the small number of persons at risk (n=220). Overall, 14.3% (95% CI: 9.6, 21.0) of the cohort; 13.8% (95% CI: 7.4, 25.0) of men (n=101) and 14.7% (95% CI: 8.7, 24.3) of women (n=119)) progressed with a doubling of the visual angle after 9 years. No particular patterns in age and gender were observed, possibly due to the small sample size.
Table 3.
Age-sex specific 9-year incidence of vision loss (doubling of the visual angle)* among BESs black participants
| Male |
Female |
Both sexes† |
||||
|---|---|---|---|---|---|---|
| Age group (years) | n | % (95% CI) | n | % (95% CI) | n | % (95% CI) |
| 40-49 | 471 | 1.3 (0.5, 3.0) | 594 | 1.5 (0.8, 3.0) | 1065 | 1.4 (0.8, 2.4) |
| 50-59 | 345 | 3.0 (1.6, 5.7) | 548 | 2.8 (1.6, 4.8) | 893 | 2.9 (1.9, 4.4) |
| 60-69 | 299 | 10.7 (7.3, 15.7) | 470 | 5.9 (3.9, 8.8) | 769 | 7.7 (0.6, 10.2) |
| 70+ | 256 | 19.4 (13.7, 27.1) | 316 | 15.5 (11.3, 21.1) | 572 | 17.1 (13.5, 21.5) |
| Total | 1371 | 6.3 (5.0, 7.9) | 1928 | 4.9 (4.0, 6.1) | 3299 | 5.5 (4.7, 6.4) |
BESs = Barbados Eye Studies. CI=confidence interval
decrease of 15 letters or more read correctly in the better eye
Gender-adjusted Hazard Ratio (compared to persons aged 40-49 years at baseline): 2.1 (95% CI: 1.1, 4.2) for persons aged 50-59 years; 5.8 (95% CI: 3.1, 10.8) for persons aged 60-69 years, and 16.0 (95% CI: 8.8, 29.3) for persons aged 70+ years
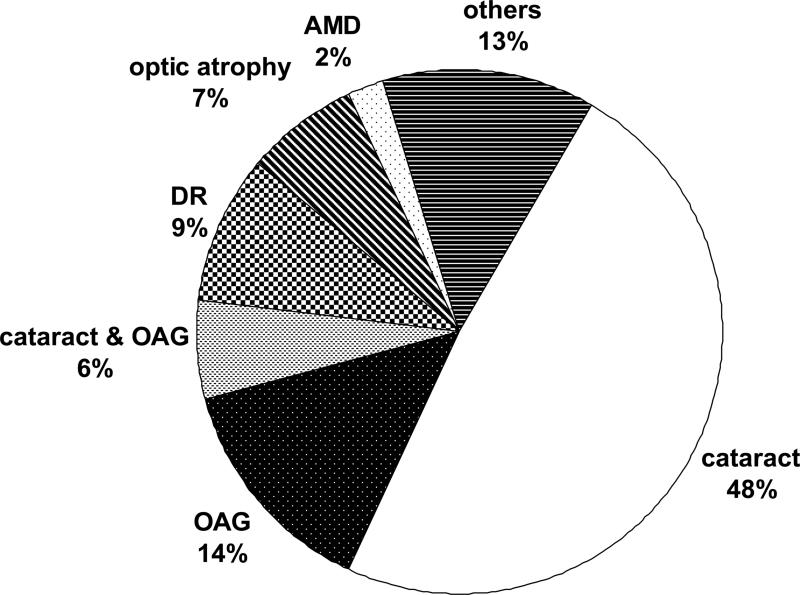
Figure 1 presents data on primary causes of bilateral incident blindness (US criteria) over 9 years in 126 eyes. Age-related cataract was determined to be the cause in nearly half the eyes affected by new onset bilateral blindness (48%), open-angle glaucoma (OAG: 14%), and combined cataract and OAG (6%) were also major causes of blindness. Diabetic retinopathy and optic atrophy led to blindness in 9% and 7% of affected eyes, respectively, while age-related macular degeneration (2%) was a rare cause of blindness. While rates varied slightly, these conditions also accounted for the principal causes of bilateral blindness according to the WHO criteria, based on 56 eyes. As such, 2 in 5 cases of blindness were due to cataract, 1 in 5 cases were attributable to OAG and 1 in 15 cases were due to both conditions. Among the 43 eyes with incident unilateral blindness (US criteria), similar underlying causes were noted, chiefly age-related cataract (40%), OAG (14%), and diabetic retinopathy (9%).
Figure 1.
Primary causes of incident bilateral blindness (visual acuity ≤20/200) (n=126 eyes). AMD= age-related macular degeneration; DR=diabetic retinopathy; OAG= open-angle glaucoma
There were a total of 413 eyes with low vision among participants with incident low vision (US criteria). Cataract was the principal cause accounting for 65% of visual loss, followed by macular degeneration (7%), diabetic retinopathy (5%), and OAG (4%). Other causes were less frequent, and included optic atrophy (2%), combinations of cataract and other conditions (2%), and all others (3%). Causes were uncertain or unknown for 11%.
DISCUSSION
The nine-year incidence of bilateral blindness in the Barbados Eye Studies was 1.0% and 2.1% by WHO and US criteria, respectively. The comparable incidence of unilateral blindness was twice as high by WHO criteria (2.0%) but similar by US criteria (1.7%) (Table 1). Men tended to experience a higher 9-year incidence of low vision and blindness than did women, and these conditions were both positively associated with older age at baseline (Table 2). Approximately one in twenty persons in the cohort experienced doubling of the visual angle over 9 years (Table 3). The principal causes of incident bilateral blindness based on 126 eyes were age-related cataract (48.3%), and OAG (14.3%), while cataract and OAG accounted for 6.3% of the total. Diabetic retinopathy and optic atrophy led to incident blindness in 8.7% and 7.1% of affected eyes, respectively (Figure1).
Incidence studies of the development and progression of visual impairment provide information relevant to the planning of clinical services and the effectiveness of therapeutic interventions, inform public health policy, and may also allow understanding of etiologic factors leading to population-specific variations in risk and disease burden. Population-based prevalence studies of visual impairment have demonstrated higher rates of blindness and low vision in African-Americans than in predominantly European origin populations.3,4,6,9,13,36 This disparity in the burden of visual impairment was confirmed by the BES, which also documented higher rates of blindness and visual impairment than previously reported.5 Higher rates of incident visual impairment and vision loss also occurred in this Afro-Caribbean population compared to white populations studied for similar periods.20-22 While the reasons for these differences are unclear, socio-economic issues and access to health care must be considered. In this regard, Barbados is one of the most affluent and developed countries in the Caribbean region, with comprehensive public services available at no cost. Genetic and various environmental factors, as well as their interactions, likely underlie the disparity in visual impairment and warrant futher investigation,
To date there are few longitudinal studies that have extended beyond 5 years, and to the best of our knowledge, none have been conducted in predominantly African-origin populations.24-27 Population-based reports are available from the US-based Beaver Dam Eye Study (BDES) at 10 and 15 years24,25 and 2 Italian studies; the Ponza and Priverno Eye Studies.26,27 Table 4 presents 9-year incidence data from the BESs compared to findings from these longer term studies. The rate of incident vision loss (doubling of the vision angle), was higher in Afro-Barbadians at 9 years (5.5%) than observed in the predominantly white BDES population at 10 years (4.8%). These data indicate a higher rate of visual loss in black Barbadians than white Americans studied for a decade; 0.61% vs. 0.48% per year. While the cumulative 15-year incidence of visual loss in the BDES increased to 7.2%, the trajectory remained constant at a rate of 0.48% per year.
Table 4.
Incidence of visual impairment and progression of visual loss in longitudinal prospective studies* of more than 5 years duration.
| Study, Location |
Age (yrs) at baseline |
Duration of follow up |
Visual acuity impairment/vision loss criteria |
No. at risk |
Incidence (%) |
Average/yr (%) |
|---|---|---|---|---|---|---|
| Barbados Eye Studies, Barbados | 40 - 84 | 9 | Vision loss: doubling of the visual angle in the better eye | 3299 | 5.5 | 0.6 |
| Incidence: ≤20/40 in the better eye | 3049 | 10.1 | 1.1 | |||
| |
|
|
Incidence: ≤20/200 in the better eye |
3271 |
2.1 |
0.2 |
| Priverno Eye Study, Italy | 45 - 69 | 7 | Incidence: ≤20/40 in the better eye | 283 | 4.9 | 0.7 |
| |
|
|
Incidence: ≤20/200 in the better eye |
291 |
0.3 |
0.04 |
| Ponza Eye Study, Italy** | 40+ | 12 | Incidence: ≤20/40 in the better eye | 356 | 7.0 | 0.6 |
| |
|
|
Incidence: ≤20/200 in the better eye |
363 |
1.4 |
0.1 |
| Beaver Dam Eye Study, USA | 43 - 86 | 10 | Vision loss: doubling of the visual angle in the better eye | 3568 | 4.8 | 0.5 |
| Incidence: ≤20/40 in the better eye | 3467 | 5.9 | 0.6 | |||
| |
|
|
Incidence: ≤20/200 in the better eye |
3558 |
0.8 |
0.08 |
| Beaver Dam Eye Study, USA | 43 - 86 | 15 | Vision loss: doubling of the visual angle in the better eye | 3941 | 7.2 | 0.5 |
| Incidence: ≤20/40 in the better eye | 3788 | 8.3 | 0.6 | |||
| Incidence: ≤20/200 in the better eye | 3926 | 0.8 | 0.05 |
all studies were based on predominantly European-derived populations except the Barbados Eye Studies which included African-derived participants only
data restricted to persons aged 43 to 74 at baseline
The relationship between older age at baseline and incident visual loss noted at 4-years in BISED,20 was also evident at 9 years. Similar to observations in the BDES population, there was a positive gradient between older age at baseline and incident doubling of the visual angle, with the greatest adverse impact evident at 15 years among those aged 70 years and older at baseline, representing a 9 fold increased likelihood of doubling of the visual angle compared to the youngest group.24,25 Sex differences in vision loss were evident in the BESs participants aged 60-69 years at baseline, with a slightly, although not statistically significant, increased incidence of vision loss among men compared to women overall (6.3% vs. 4.9% ). Comparable rates among men and women in the BDES were similar at 10 (4.9% and 4.7%) and 15 years (7.1% and 7.3%).
Rates of incident visual impairment, defined as VA≤20/40 in the better eye, appeared to be lower in the European-derived populations from the BDES (5.9% and 8.3% at 10 and 15 years, respectively), Ponza (7.0%) and Priverno (4.9%) Eye Studies than in Barbados where the comparable incidence (combined low vision and blindness by US criteria) was further estimated as 10.1% (95% CI: 9.0, 11.3). Rates of severe incident visual impairment in the Beaver Dam population remained constant at 0.8% at both 10 and 15 years follow up, while there was marked variation in the rates reported from the Priverno (0.3%) and Ponza (1.4%) Eye Studies. The latter observation might be partly explained both by the younger population and the shorter period of follow up in Priverno.27 The comparable rates of incident blindness in Barbados were much higher at 2.1%, exceeding rates in white populations studied for more than a decade. While these studies were conducted for differing time periods in different populations, using variable data collection methods, these findings highlight the continued disparity in blindness and visual loss, which demonstrated a tendency to increase over time.3-6,20-22,24,25
Recent WHO estimates indicate that the majority of the burden of global blindness is attributable to cataract (47.8%), glaucoma (12.3%) and age-related macular degeneration (AMD: 8.7%), with corneal opacities and diabetic retinopathy accounting for around 5% of blindness each.1 It is known that AMD is frequently reported as the leading cause of vision loss in white populations,7-9,11-13,22,33 and this observation is supported by longitudinal data from the BDES, in which incident severe visual loss was due to late AMD in over half of affected eyes.25 Primary retinal vein occlusion (central and branch vein) accounted for severe visual impairment in 12% of those affected, while diabetic retinopathy accounted for a lower incidence (3%). In contrast, the Ponza Eye Study reported that cataract was the principal cause of incident blindness per eye (46.2%), with myopia and diabetic retinopathy each being the second ranked causes (11.5%), while glaucoma (OAG), AMD and trauma each accounted for 7.7% of blind eyes.26 While the number of affected eyes was small (26 eyes), the pattern of conditions underpinning visual loss appeared to differ from the experience of other European-derived populations. Cross sectional studies have demonstrated that glaucoma and cataract are the principal causes of blindness in predominantly black populations,5,7,8 an association confirmed by 4-year follow up of the BESs cohort.20 The causes of eye-specific blindness at 9-years in the current study are consistent with data from the 4-year follow up, with cataract being the major cause of blindness, followed by OAG, diabetic retinopathy and co-existing OAG and cataract. Among those with cataract as the primary cause of blindness, this was the only ocular condition in the majority (more than 80%), while 14% had concomitant OAG contributing to visual loss to a lesser degree.
Visual loss is associated with adverse implications for health and well being, including reduced quality of life, functional and cognitive decline, work and academic underperformance, anxiety, depression, reduced social interaction, increased frequency of falls and fractures, loss of independence leading to institutionalization or nursing home admission, and perhaps even higher risk of early mortality.1,25,37-41. It is not often recognized that the magnitude of adverse impact on functional status and well-being due to non-correctable visual loss is comparable to that attributable to major medical conditions.40 Data on presenting refraction, which were only available at the 9-year follow up BESs visit, indicate that most visually impaired participants (81%) had conditions that were not corrected by refraction. These results suggest that under-corrected refractive error in this population is a less significant cause than in the Blue Mountains Eye Study cohort.22
Visual impairment also leads to increased direct and indirect costs at the individual level, as well as to increased costs to healthcare systems,42 and there are clear benefits to interventions that prevent or delay vision loss. Perhaps the most immediate benefits might result from cataract extraction.15,25 The BDES reported that more than 60% of eyes with improved vision by the 15 year review were as a direct result of cataract extraction.25 Afro-Caribbean and similar populations are likely to benefit from efforts at prevention such as optimal diabetic control to reduce retinopathy (which accounted for nearly 9% of incident blindness) and cataract (known to be associated with diabetes in this population).43,44 This intervention is likely to also have positive implications for cardiovascular disease prevention in a population with high diabetes rates.45 Clinical interventions including laser therapy for retinopathy and surgical extraction of cataracts, must however, continue to be key strategies in reducing the associated high burden of blindness.
Incident OAG accounted for nearly 30% of incident blindness, either singly or in combination with cataract. In spite of the inordinately high disease prevalence and incidence, as well as understanding of the likely etiological factors described in the BES population,28,29,46-48 there still remains a poor awareness of the disease.49 Topical treatments aimed at reducing intraocular pressure delay the progression of OAG,50,51 and early detection and treatment may benefit groups at high risk of OAG.
The 9-year incidence of blindness due to AMD remains low in this population at 2.5% (US criteria) in stark contrast to the more than 50% of severe vision impairment due to this condition in a white population.25 Optic atrophy accounted for more than 12% of incident blindness, consistent with the relatively high rates seen at baseline and 4-year follow up, although there continues to be a dearth of information about the condition.
Potential limitations such as losses to follow-up and the selective mortality of persons with illnesses associated with the development of visual loss (e.g., diabetes), might possibly have affected study outcomes, biasing results towards an underestimate of the associations. This study however, benefited from adherence to rigorous protocols with ongoing quality assurance procedures used in the diagnosis of the various ocular conditions, as well as ongoing high participation.
Rates of incident visual loss and blindness are exceedingly high in this Afro-Caribbean population. Public health and clinical strategies to reduce the burden of visual loss must be based on education and health promotion with screening, early detection and appropriate treatments principally for cataract, glaucoma, and diabetic retinopathy. Cataract surgery rates were lower in the BESs than the BDES cohorts, when both groups were followed longitudinally, in spite of higher rates of incident cataract in Barbados.20,30,52 Clinical interventions are particularly important as cataract and glaucoma are treatable conditions, while DR is potentially preventable. Appropriate interventions must include diabetes prevention and optimal medical and ocular care, including reduction of associated risks such as hypertension. This study highlights the need for blindness prevention programs to reduce the disease burden in a high risk population; such programs can lead to improved quality of life and reduce healthcare and personal costs from vision loss.
Acknowledgments
Supported by Grants EY07625 and EY07617 from the National Eye Institute, Bethesda, MD.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Conflict of Interest: No conflicting relationship exists.
*For group membership, see Leske MC, Wu SY, Hennis A, et al. Nine-year incidence of age related macular degeneration in the Barbados Eye Studies. Ophthalmology 2006;113:29-35.
REFERENCES
- 1.Resnikoff S, Pascolini D, Etya'ale D, et al. Global data on visual impairment in the year 2002. Bull World Health Organ. 2004;82:844–51. [PMC free article] [PubMed] [Google Scholar]
- 2.Thylefors B, Négrel AD, Pararajasegaram R, Dadzie KY. Global data on blindness. Bull World Health Organ. 1995;73:115–21. [PMC free article] [PubMed] [Google Scholar]
- 3.Tielsch JM, Sommer A, Witt K, et al. Blindness and visual impairment in an American urban population. The Baltimore Eye Survey. Arch Ophthalmol. 1990;108:286–90. doi: 10.1001/archopht.1990.01070040138048. [DOI] [PubMed] [Google Scholar]
- 4.Rubin GS, West SK, Munoz B, et al. A comprehensive assessment of visual impairment in a population of older Americans. The SEE Study. Salisbury Eye Evaluation Project. Invest Ophthalmol Vis Sci. 1997;38:557–68. [PubMed] [Google Scholar]
- 5.Hyman L, Wu SY, Connell AMS, et al. Prevalence and causes of visual impairment in the Barbados Eye Study. Ophthalmology. 2001;108:1751–6. doi: 10.1016/s0161-6420(01)00590-5. [DOI] [PubMed] [Google Scholar]
- 6.Klein R, Klein BE, Linton KL, et al. The Beaver Dam Eye Study: Visual Acuity. Ophthalmology. 1991;98:1310–5. doi: 10.1016/s0161-6420(91)32137-7. [DOI] [PubMed] [Google Scholar]
- 7.Sommer A, Tielsch JM, Katz J, et al. Racial differences in the cause-specific prevalence of blindness in east Baltimore. N Engl J Med. 1991;325:1412–7. doi: 10.1056/NEJM199111143252004. [DOI] [PubMed] [Google Scholar]
- 8.Rahmani B, Tielsch JM, Katz J, et al. The cause-specific prevalence of visual impairment in an urban population: the Baltimore Eye Survey. Ophthalmology. 1996;103:1721–6. doi: 10.1016/s0161-6420(96)30435-1. [DOI] [PubMed] [Google Scholar]
- 9.Klaver CC, Wolfs RC, Vingerling JR, et al. Age-specific prevalence and causes of blindness and visual impairment in an older population. The Rotterdam Study. Arch Ophthalmol. 1998;116:653–8. doi: 10.1001/archopht.116.5.653. [DOI] [PubMed] [Google Scholar]
- 10.Leibowitz HM, Krueger DE, Maunder LR, et al. The Framingham Eye Study Monograph. Surv Ophthalmol. 1980;24(suppl):335–610. [PubMed] [Google Scholar]
- 11.Munoz B, West SK, Rubbin GS, et al. Causes of blindness and visual impairment in a population of older Americans: the Salisbury Eye Evaluation Study. Arch Ophthalmol. 2000;118:819–25. doi: 10.1001/archopht.118.6.819. [DOI] [PubMed] [Google Scholar]
- 12.Van Newkirk MR, Weih L, McCarty CA, Taylor HR. Cause-specific prevalence of bilateral visual impairment in Victoria, Australia: the Visual Impairment Project. Ophthalmology. 2001;108:960–7. doi: 10.1016/s0161-6420(01)00554-1. [DOI] [PubMed] [Google Scholar]
- 13.Attebo K, Mitchell P, Smith W. Visual acuity and the causes of visual loss in Australia. The Blue Mountains Eye Study. Ophthalmology. 1996;103:357–64. doi: 10.1016/s0161-6420(96)30684-2. [DOI] [PubMed] [Google Scholar]
- 14.Thulasiraj RD, Nirmalan PK, Ramakrishnan R, et al. Blindness and vision impairment in a rural south Indian population: the Aravind Comprehensive Eye Survey. Ophthalmology. 2003;110:1491–8. doi: 10.1016/S0161-6420(03)00565-7. [DOI] [PubMed] [Google Scholar]
- 15.Nirmalan PK, Thulasiraj RD, Maneksha V, et al. A population based eye survey of older adults in Tirunelveli district of south India: blindness, cataract surgery, and visual outcomes. Br J Ophthalmol. 2002;86:505–12. doi: 10.1136/bjo.86.5.505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Wong TY, Chong EW, Wong WL, et al. Singapore Malay Eye Study Team. Prevalence and causes of low vision and blindness in an urban malay population: the Singapore Malay Eye Study. Arch Ophthalmol. 2008;126:1091–9. doi: 10.1001/archopht.126.8.1091. [DOI] [PubMed] [Google Scholar]
- 17.Hsu WM, Cheng CY, Liu JH, et al. Prevalence and causes of visual impairment in an elderly Chinese population in Taiwan: the Shihpai Eye Study. Ophthalmology. 2004;111:62–9. doi: 10.1016/j.ophtha.2003.05.011. [DOI] [PubMed] [Google Scholar]
- 18.Xu L, Wang Y, Li Y, et al. Causes of blindness and visual impairment in urban and rural areas in Beijing: the Beijing Eye Study. Ophthalmology. 2006;113:1134.e1–11. doi: 10.1016/j.ophtha.2006.01.035. [DOI] [PubMed] [Google Scholar]
- 19.Limburg H, Barria von-Bischhoffshausen F, Gomez P, et al. Review of recent surveys on blindness and visual impairment in Latin America. Br J Ophthalmol. 2008;92:315–9. doi: 10.1136/bjo.2007.125906. [DOI] [PubMed] [Google Scholar]
- 20.Leske MC, Wu SY, Hyman L, et al. Four-year incidence of visual impairment. Barbados Incidence Study of Eye Diseases. Ophthalmology. 2004;111:118–24. doi: 10.1016/j.ophtha.2003.04.002. [DOI] [PubMed] [Google Scholar]
- 21.Klein R, Klein BE, Lee KE. Changes in visual acuity in a population. The Beaver Dam Eye Study. Ophthalmology. 1996;103:1169–78. doi: 10.1016/s0161-6420(96)30526-5. [DOI] [PubMed] [Google Scholar]
- 22.Foran S, Wang JJ, Mitchell P. Causes of incident visual impairment. The Blue Mountains Eye Study. Arch Ophthalmol. 2002;120:613–9. doi: 10.1001/archopht.120.5.613. [DOI] [PubMed] [Google Scholar]
- 23.Thompson JR, Du L, Rosenthal AR. Recent trends in the registration of blindness and partial sight in Leicestershire. Br J Ophthalmol. 1989;73:95–9. doi: 10.1136/bjo.73.2.95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Klein R, Klein BE, Lee KE, et al. Changes in visual acuity in a population over a 10-year period. The Beaver Dam Eye Study. Ophthalmology. 2001;108:1757–66. doi: 10.1016/s0161-6420(01)00769-2. [DOI] [PubMed] [Google Scholar]
- 25.Klein R, Klein BE, Lee KE, et al. Changes in visual acuity in a population over a 15-year period: the Beaver Dam Eye Study. Am J Ophthalmol. 2006;142:539–49. doi: 10.1016/j.ajo.2006.06.015. [DOI] [PubMed] [Google Scholar]
- 26.Nucci C, Cedrone C, Culasso F, et al. Incidence of visual loss in the Ponza Eye Study, Italy. Eye. 2005;19:175–82. doi: 10.1038/sj.eye.6701444. [DOI] [PubMed] [Google Scholar]
- 27.Cedrone C, Culasso F, Cesareo M, et al. Incidence of blindness and low vision in a sample population: the Priverno Eye Study, Italy. Ophthalmology. 2003;110:584–8. doi: 10.1016/S0161-6420(02)01898-5. [DOI] [PubMed] [Google Scholar]
- 28.Leske MC, Connell AM, Schachat AP, et al. The Barbados Eye Study: Prevalence of open-angle glaucoma. Arch Ophthalmol. 1994;112:821–9. doi: 10.1001/archopht.1994.01090180121046. [DOI] [PubMed] [Google Scholar]
- 29.Leske MC, Connell A, Wu S, et al. Incidence of open-angle glaucoma. Arch Ophthalmol. 2001;119:89–95. [PubMed] [Google Scholar]
- 30.Leske MC, Wu SY, Nemesure B, et al. 9-year Incidence of Lens Opacities in the Barbados Eye Studies. Ophthalmology. 2004;111:483–90. doi: 10.1016/j.ophtha.2003.06.016. [DOI] [PubMed] [Google Scholar]
- 31.Chylack LT, Jr, Leske MC, McCarthy D, et al. Lens Opacities Classification System II (LOCS II). Arch Ophthalmol. 1989;107:991–7. doi: 10.1001/archopht.1989.01070020053028. [DOI] [PubMed] [Google Scholar]
- 32.Diabetic retinopathy study Report Number 6. Design, methods, and baseline results. Report Number 7. A modification of the Airlie House classification of diabetic retinopathy. Prepared by the Diabetic Retinopathy Study Research Group. Invest Ophthalmol Vis Sci. 1981;21:1–226. [PubMed] [Google Scholar]
- 33.Congdon N, O'Colmain B, Klaver CC, et al. Causes and prevalence of visual impairment among adults in the United States. Arch Ophthalmol. 2004;122:477–85. doi: 10.1001/archopht.122.4.477. [DOI] [PubMed] [Google Scholar]
- 34.Kaplan EL, Meier P. Nonparametric estimation from incomplete observations. J Am Stat Assoc. 1958;53:457–81. [Google Scholar]
- 35.Cox DR. Regression models and life-tables (with discussion). J R Stat Soc. 1972;B34:187–220. [Google Scholar]
- 36.Taylor HR, Livingston PM, Stanislavsky YL, et al. Visual impairment in Australia: distance visual acuity, near vision, and visual field findings of the Melbourne Visual Impairment Project. Am J Ophthalmol. 1997;123:328–37. doi: 10.1016/s0002-9394(14)70128-x. [DOI] [PubMed] [Google Scholar]
- 37.Wu SY, Hennis A, Nemesure B, et al. Impact of glaucoma, lens opacities, and cataract surgery on visual functioning and related quality of life: the Barbados Eye Studies. Invest Ophthalmol Vis Sci. 2008;49:1333–8. doi: 10.1167/iovs.07-1252. [DOI] [PubMed] [Google Scholar]
- 38.West SK, Munoz B, Rubin GS, et al. Function and visual impairment in a population-based study of older adults. The SEE project. Salisbury Eye Evaluation. Invest Ophthalmol Vis Sci. 1997;38:72–82. [PubMed] [Google Scholar]
- 39.Klein BE, Moss SE, Klein R, et al. Associations of visual function with physical outcomes and limitations 5 years later in an older population: the Beaver Dam eye study. Ophthalmology. 2003;110:644–50. doi: 10.1016/S0161-6420(02)01935-8. [DOI] [PubMed] [Google Scholar]
- 40.Chia EM, Wang JJ, Rochtchina E, et al. Impact of bilateral visual impairment on health-related quality of life: the Blue Mountains Eye Study. Invest Ophthalmol Vis Sci. 2004;45:71–6. doi: 10.1167/iovs.03-0661. [DOI] [PubMed] [Google Scholar]
- 41.Berdeaux G, Brézin AP, Fagnani F, et al. Self-reported visual impairment and mortality: a French nationwide perspective. Epidemiol. 2007;14:80–7. doi: 10.1080/09286580600899691. [DOI] [PubMed] [Google Scholar]
- 42.Bramley T, Peeples P, Walt JG, et al. Impact of vision loss on costs and outcomes in medicare beneficiaries with glaucoma. Arch Ophthalmol. 2008;126:849–56. doi: 10.1001/archopht.126.6.849. [DOI] [PubMed] [Google Scholar]
- 43.Leske MC, Wu SY, Hyman L, et al. Diabetic retinopathy in a black population. The Barbados Eye Study. Ophthalmology. 1999;106:1893–9. doi: 10.1016/s0161-6420(99)90398-6. [DOI] [PubMed] [Google Scholar]
- 44.Leske MC, Wu S-Y, Hennis A, et al. Diabetes, hypertension and central obesity as cataract risk factors in a black population: The Barbados Eye Study. Ophthalmology. 1999;106:35–41. doi: 10.1016/s0161-6420(99)90003-9. [DOI] [PubMed] [Google Scholar]
- 45.Hennis A, Wu SY, Nemesure B, et al. Diabetes in a Caribbean Population: Epidemiologic Profile and Implications. Int J Epidemiol. 2002;31:234–9. doi: 10.1093/ije/31.1.234. [DOI] [PubMed] [Google Scholar]
- 46.Leske MC, Connell AM, Wu SY, et al. Risk factors for open-angle glaucoma: The Barbados Eye Study. Arch Ophthalmol. 1995;113:918–24. doi: 10.1001/archopht.1995.01100070092031. [DOI] [PubMed] [Google Scholar]
- 47.Leske MC, Wu S-Y, Honkanen R, et al. 9-Year Incidence of Open-Angle Glaucoma in the Barbados Eye Studies. Ophthalmology. 2007;114:1058–64. doi: 10.1016/j.ophtha.2006.08.051. [DOI] [PubMed] [Google Scholar]
- 48.Leske MC, Wu S-Y, Hennis A, et al. Risk Factors for Incident Open-Angle Glaucoma – The Barbados Eye Studies. Ophthalmology. 2008;115:85–93. doi: 10.1016/j.ophtha.2007.03.017. [DOI] [PubMed] [Google Scholar]
- 49.Hennis A, Wu S-Y, Nemesure B, et al. Awareness of incident open-angle glaucoma in a population study: the Barbados Eye Studies. Ophthalmology. 2007;114:1016–22. doi: 10.1016/j.ophtha.2007.06.013. [DOI] [PubMed] [Google Scholar]
- 50.Leske MC, Heijl A, Hussein M, et al. Factors for glaucoma progression and the effect of treatment: the early manifest glaucoma trial. Arch Ophthalmol. 2003;121:48–56. doi: 10.1001/archopht.121.1.48. [DOI] [PubMed] [Google Scholar]
- 51.Gordon MO, Beiser JA, Brandt JD, et al. The Ocular Hypertension Treatment Study: baseline factors that predict the onset of primary open-angle glaucoma. Arch Ophthalmol. 2002;120:714–20. doi: 10.1001/archopht.120.6.714. [DOI] [PubMed] [Google Scholar]
- 52.Klein BE, Klein R, Moss SE. Incident cataract surgery: the Beaver Dam eye study. Ophthalmology. 1997;104:573–80. doi: 10.1016/s0161-6420(97)30267-x. [DOI] [PubMed] [Google Scholar]