Abstract
Bone health may be impaired in many patients being treated for cancer. Primary tumors that reside in or form metastases to bone can result in compromised skeletal integrity. It has also been increasingly recognized that patients undergoing therapies for treatment of cancer are at higher risk of bone loss. These include androgen-deprivation therapy for prostate cancer and aromatase inhibitor therapy for breast cancer among others. Hypogonadism induced by many of these cancer treatments results in bone loss and increase the risk of osteoporosis and fractures. Progress has been made in identifying the role of oral and intravenous bisphosphonates to prevent bone loss in these patients. This review will discuss bone loss associated with cancer treatments with a focus on breast cancer, prostate cancer and survivors of childhood malignancies.
Keywords: cancer, osteoporosis, treatment-related bone loss, hypogonadism, bone metastases
I. Introduction
Patients with cancer have an increased incidence of bone disease. Malignant tumors may form osteolytic or osteoblastic bone metastases that result in pathologic fractures, neurologic impairment, hypercalcemia or bone pain. In addition, the therapies administered to treat the underlying malignancy may result in bone loss leading to osteoporosis and fractures (Figure 1).(1) This review will focus on bone diseases related to cancer-treatment.
Figure 1.
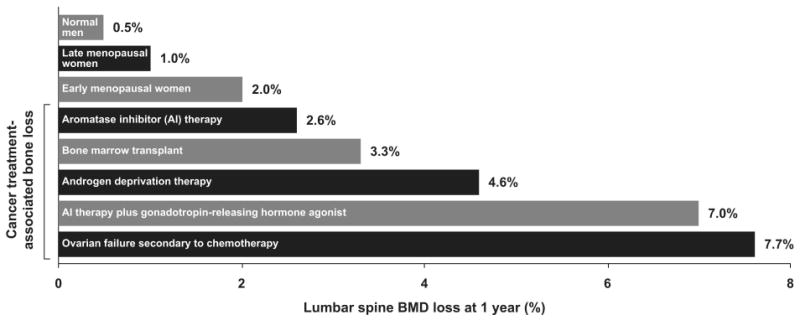
Bone mineral density loss with cancer therapies. Cancer treatment induced bone loss after 1 year often exceeds that seen in normal men and postmenopausal women. BMD=bone mineral density; AI=aromatase inhibitor. Data from Hirbe et al. (1), adapted and reprinted with permission from American Association for Cancer Research, Inc.
II. Mechanism of hypogonadal bone loss
The mechanism by which cancer therapy causes bone loss is primarily through the inducement of hypogonadism but can also include delayed developmental maturation and direct toxic effects on bone (Table 1).(2) Cancer treatments that cause hypogonadism are most often used in the treatment of breast cancer and prostate cancer. In addition, exposure to bone toxic agents, such as glucocorticoids, impact bone health which is of particular concern in survivors of childhood malignancies.
Table 1.
Selected Cancer Therapies Associated With Bone Loss
| Therapy | Tumor |
|---|---|
| Bilateral orchiectomy | Prostate cancer |
| Oophorectomy | Breast cancer |
| GnRH agonists | Breast cancer, Prostate cancer |
| Antandrogens | Prostate cancer |
| Chemotherapy | Various malignancies |
| Cyclophosphamide | Breast cancer |
| Methotrexate/ifosfamide | Osteosarcoma |
| Alkylating agents | Hodgkin's/non-Hodgkin's lymphoma |
| Selective estrogen-receptor modulators | Breast cancer |
| Aromatase inhibitors | Breast cancer |
| Glucocorticoids/cyclosporine | Stem-cell transplantation for various malignancies |
| Radiation therapy | Various malignancies |
Reprinted with permission from American Society of Clinical Oncology.
Bone remodeling in the adult skeleton is a tightly regulated dynamic process and is perturbed in states of hypogonadism. The basic remodeling unit of bone is comprised of three main cell types: bone-forming osteoblasts, bone-resorbing osteoclasts and mechanosensing osteocytes. Osteoblasts are stromal-cell derived cells that may ultimately end up as osteocytes embedded within bone matrix. Osteoclasts are multinucleated hemotopoetic-derived cells designed to hydrolyze bone and efficiently resorb it at designated resorption pits. At the site of bone resorption, osteoclasts are recruited to form a resorption pit which is then filled in by bone-forming osteoblasts. Cross-talk between osteoblasts and cells in the bone microenvironment, most notably osteoclasts, regulates the recruitment and differentiation of cells necessary at a bone remodeling site. Receptor activator of nuclear factor Kappa B-ligand (RANK-L) is a critical factor secreted by osteoblasts in the presence of M-CSF that results in osteoclast differentiation, proliferation and apoptosis. The precise role of osteocytes in calcium and bone metabolism is evolving. Osteocytes may also regulate phosphate and calcium metabolism in addition to their ability to transmit and respond to mechanostimulatory signals through the extensive interconnected network for communication with adjacent osteocytes and cells lining the bone surface.(3)
Hypogonadism disrupts the normal bone remodeling process as estrogen and testosterone have been identified as key determinants of skeletal bone health in both men and women. Circulating testosterone concentrations are independent predictors of fracture risk in both elderly men and postmenopausal women after estradiol concentrations have been taken into account.(4, 5) In addition, the central role of estrogen in mediating hypogonal bone loss is well characterized for women and has been established as an independent determinant for men as well. Male patients with congenital aromatase deficiency and estrogen-receptor mutations have low bone density without suppression of circulating levels of androgens.(6, 7) In addition, medical castration with estrogen in men with prostate cancer does not appear to decrease BMD.(8) Circulating estradiol concentrations have been positively associated with increased BMD in older men.(9)
Classical estrogen signaling through the nuclear receptor superfamily of estrogen receptors (ER-α and ER-β) results in regulation of gene transcription in many tissues.(10) Estrogen receptor dimers may directly or indirectly associate with estrogen-receptor response elements on target gene promoters to control transcriptional activation. Non-genomic actions of estrogen have been identified that result in cellular responses that may include alterations in intracellular calcium, nitrous oxide or kinase activation.(11) These non-genomic actions may occur as a result of interaction with an ER located in or near the plasma membrane or through non-ER estrogen-binding proteins.(11) In bone, ER-α and ER-β have been detected in osteoblasts and osteoclasts.(12-17) ER-α mediates much of estrogen-induced bone formation while the role of ER-β is less clear.(11) Estrogen results in suppression of bone turnover and osteoclast apoptosis.(18) In contrast, bone loss associated with estrogen deficiency, is characterized by increase in bone resorption and a prolongation of the lifespan of the osteoclast. Although there is also a concomitant increase in bone-formation, there overall effect of hypogonadism appears to be an imbalance favoring bone resorption and bone loss.
III. Breast Cancer
A. Therapies and hypogonadism
Breast cancer is estimated to affect 1 in 8 women in their lifetime in the United States.(19) It is the second leading cause of cancer deaths in women in the United States but survival rates have been improving.(19) The Women's Health Initiative Observational Study reported that breast cancer survivors had an increased risk of clinical vertebral fractures, lower arm or wrist fracture and all fractures (excluding hip).(20) A significant portion of breast-cancer is hormone-dependent breast cancer. These cancers have the presence of hormonal receptors such as progesterone or estrogen receptors that are responsive to manipulation. A goal of therapy therefore in this population is to decrease estrogen action at the breast tissue through a variety of mechanisms that result in hypogonadism: selective-estrogen receptor modulators, aromatase inhibitors, oophorectomy or adjuvant gonadotropin-releasing hormone analogue therapy. In addition, chemotherapy may cause direct toxicity to the ovarian follicle and result in hypogonadism in addition to its potential direct toxic effects on bone cells. It is also clear that effects may be dependent on menopausal status and will be discussed in the following section.
B. Normal estrogen production
Estrogen production occurs at multiple sites that varies by menopausal status. In premenopausal women, the ovary is the principal site of circulating estrogen production. In postmenopausal women, muscle and adipose cells are the predominant tissues that convert adrenal androgens to estrogens by the aromatase enzyme (CYP19A1). CYP19A1 catalyzes the conversion of androstenedione to estrone and testosterone to estradiol. The aromatase enzyme is encoded by a single large 75-kb gene on chromosome 15q21.1 and is under differential control at various sites by tissue-specific transcription factors and cytokines. There is also local production of estrogen in multiple organs, including breast and bone, due to the expression of aromatase as well as sulfatase in these estrogen target tissues. In breast tissue, local levels of estradiol and estrone sulfate in breast tissue can be 6- to 10-fold greater respectively due to the combination of local production and concentration of circulating estrogens against a gradient.(21) This local expression of estrogens can be mitogenic to breast tissue, particularly in the significant portion of breast cancers that express estrogen receptors. The characteristics of estrogen action at local breast tissue provide the rationale for the use of therapies directed at blocking estrogen action or production.
C. Selective estrogen-receptor modulators (SERMs)
Selective estrogen-receptor modulators are agents that have differential effects on modifying estrogen response in target tissues. Selective estrogen-receptor modulators such as tamoxifen have been shown to improve disease-free survival in patients with breast cancer. The first clinical trial in breast cancer was published in 1971(22) and a landmark trial in 1989, the National Surgical Adjuvant Breast and Bowel Project (NSABP), studied adjuvant tamoxifen therapy in pre- and postmenopausal patients with ER-positive, lymph node-negative early disease and reported prolongation of disease-free and overall survival after five years.(23) Subsequent trials have suggested that tamoxifen has favorable effects on reducing invasive breast cancers in patients with DCIS(24), as well as breast cancer prevention in patients at increased risk of breast cancer due to age or high-risk family history or personal history of lobular carcinoma in situ.(25) Therefore tamoxifen has been in standard clinical practice for consideration in women with ER-positive tumors as a 5-year adjuvant therapy following primary surgical and/or chemotherapeutic regimens.
Tamoxifen binds to both ER-α and ER-β and has a partial agonist effect on bone. In vitro and in vivo animal studies of ovariectomized rats, have suggested that tamoxifen has favorable effects, similar to estrogen, on both trabecular and cortical bone preservation.(26, 27) These findings were further supported in human studies of histomorphometric analysis of transiliac crest bone biopsies. Tamoxifen-treated women with breast cancer were found to have similar static bone remodeling indices as women with breast cancer not receiving tamoxifen.(28) There was a trend toward greater connectivity in trabecular parameters despite lower bone formation rates. Taken together, these studies would suggest that tamoxifen does not have a deleterious effect on bone and may be a partial agonist.
The effect of tamoxifen on bone density or fracture risk is different in premenopausal compared to postmenopausal women which may be due to the partial agonist nature of tamoxifen. High-risk premenopausal women treated with tamoxifen for chemoprevention for 3 years had mild decreases in lumbar spine BMD (-1.44% per year) which was significant compared to modest gains in the placebo group (+0.24% per year, P<0.001) while minimal changes in hip BMD occurred in both groups.(29) In contrast, postmenopausal women participating in the same study had mild increases in BMD on tamoxifen at the lumbar spine (+1.17% per year) and the total hip (+1.71% per year) compared to placebo.(29) These discordant responses have been hypothesized to reflect differences in prevailing estrogen levels that affect bone density response. Studies have supported an interaction of menstrual status and BMD response to tamoxifen. A study of premenopausal women with early breast cancer treated with adjuvant chemotherapy compared hormone-receptor positive patients treated with tamoxifen to hormone-receptor negative patients not treated with tamoxifen.(30) Patients who developed chemotherapy-induced amenorrhea had lower BMD than those who continued to menstruate, regardless of tamoxifen status. However, in those women who continued to menstruate, tamoxifen use resulted in a loss of BMD (-4.6% at the spine) compared to a modest gain in the non-tamoxifen treated group. Among women who developed amenorrhea, tamoxifen use was associated with an attenuation of bone loss at the spine (-6.8% loss) when compared to the non-tamoxifen treated group (-9.5% loss). This would suggest that the relative effect tamoxifen has on BMD is related to prevailing estrogen levels in premenopausal women. Small decreases in BMD have also been reported with another SERM, raloxifene, in premenopausal women at increased risk of breast cancer.(31) It is unclear whether these small BMD effects of SERM intervention in premenopausal women result in changes in fracture risk. The NSABP P-1 trial reported a decrease in number of osteoporotic fractures in premenopausal women at high risk for breast cancer treated with tamoxifen for five years compared to placebo.(25)
Postmenopausal women treated with tamoxifen have mild increases in BMD at the spine which is apparent early in clinical trials and tends to stabilize.(32-35) The effect of tamoxifen on fractures in postmenopausal women with breast cancer is not clear. A study in Danish postmenopausal women with high-risk breast cancer randomized to local radiotherapy with or without tamoxifen for one year reported high femoral fractures in the tamoxifen treated patients compared to the control group.(36) Fracture were similar comparing tamoxifen and raloxifene use in high-risk postmenopausal women in the NSABP Study of Tamoxifen and Raloxifene (STAR) P-2.(37)
In summary, tamoxifen use is associated with a modest beneficial effect on BMD in postmenopausal women and has a small decrease in BMD in premenopausal women. It is unclear how these changes in BMD relate to underlying fracture risk in women with breast cancer or at increased risk for breast cancer.
D. Aromatase Inhibitors
Aromatase inhibitors have shown enhanced efficacy compared to tamoxifen and have been supplanting tamoxifen use for adjuvant treatment for postmenopausal women with hormone-receptor positive breast cancer.(38-41) Two classes of aromatase inhibitors are in current use: steroidal (exemestane) and non-steroidal (anastrozole and letrozole). These agents result in significant estrogen deprivation due to the blockade of the aromatase enzyme which is the rate-limiting step in estrogen biosynthesis. The steroidal inhibitor, exemestane, is a suicidal or non-competitive inhibitor. Exemestane is derived from androstenedione and interacts with the substrate binding site of the aromatase enzyme resulting in an irreversible covalent bond. The non-steroidal inhibitors are competitive inhibitors that form a reversible bond to the heme portion of the aromatase enzyme to ultimately block estradiol formation. The enzyme blockade can be overcome by additional enzyme or substrate. Aromatase inhibitors reduce estradiol, estrone and estrogen sulfate by varying amounts: 81-94% by anastrozole, 84-98% by letrozole and 52-72% by exemestane.(42) However, efforts are underway to determine biochemical efficacy based on estrogen suppression in malignant breast tissues rather than circulating levels.(43)
With the increasing use of AIs for the treatment of breast cancer in postmenopausal women, concerns have been raised regarding their bone effects. These agents result in relatively more tissue level estrogen deprivation and therefore have been associated with BMD losses. The Arimidex, Tamoxifen, Alone or in Combination (ATAC) trial in postmenopausal women with hormone receptor-positive disease reported a significantly decreased BMD after 2 years of anastrozole (4.1% median loss at the spine) compared to an increased BMD (2.2% median increase at the spine) in patients taking tamoxifen.(44)
When AIs are used following tamoxifen, the bone loss reported has been more variable. Postmenopausal women taking letrozole following tamoxifen (MA.17 study) had only modest reductions in BMD compared to patients on placebo.(39)
The steroid AI exemestane has also not convincingly been shown to have a protective effect on BMD. Animal models suggest that its androgenic properties might confer a benefit as compared to the nonsteroidal AIs. (45) The 17-hydroexemestane metabolite binds more avidly to the androgen receptor rather than the ER-α receptor.(46) These preclinical observations have largely not been shown to have a protective effect in clinical studies. Postmenopausal women on tamoxifen had a significant reduction in BMD after randomization to exemestane therapy for 2 years whereas the tamoxifen-continuation group had no change in BMD.(47)
Fracture data from the use of AI has been reported as secondary outcomes in limited studies. Fracture rates were higher for postmenopausal women in the ATAC trial receiving anastrozole than for women receiving tamoxifen for five years (incidence rate ratio 1.55, 1.31-1.83 95% confidence interval).(48) This difference in fracture risk was not sustained in the 100-month median post-treatment follow-up period.(48) A trial of exemestane or tamoxifen in postmenopausal women who had already completed 2 or 3 years of adjuvant tamoxifen reported an increase in the odds ratio for fractures in the exemestane group (OR 1.45, 1.13-1.87 95% confidence interval, p=0.003).(41)
E. Other therapies
Bone loss has been reported for patients with breast cancer treated with oophorectomy, chemotherapy or gonadotropin-releasing hormone agonists as well.(49, 50) The Austrian Breast and Colorectal study of premenopausal patients with early breast cancer who were receiving leuteinizing hormone-releasing hormone agonist (goserelin) for 3 years reported severe BMD losses at the lumbar spine of 17.3% in the group that also received anastrozole and losses of 11.6% in the group that also received tamoxifen.(51)
It is clear that patients with breast cancer being treated with therapies that result in hypogonadism need to have their bone health assessed.
IV. Prostate Cancer
A. Incidence and Androgen Deprivation Therapies
Prostate cancer is estimated to affect 1 in 6 men in their lifetime and is the third leading cause of cancer death in men in the United States. Growth of normal and malignant prostate cancer cells is dependent on androgens (primarily testosterone and dihydrotestosterone). The testes produce 90-95% of androgens and the remaining 5-10% is predominantly produced by the adrenal glands. Androgen deprivation via surgical orchiectomy has been long recognized as beneficial for patients with metastatic prostate cancer.(52) Studies have subsequently suggested that early hormonal treatment of men with locally advanced prostate cancer may improve survival.(53, 54) These studies have resulted in increasing use of androgen deprivation therapy (ADT) for adjuvant treatment of locally advanced, recurrent or metastatic prostate cancer.(55) ADT can include bilateral orchiectomy, gonadotropin-releasing hormone (GnRH) agonists, antiandrogens (e.g. bicalutamide) and less commonly estrogen therapy. The intended effect of these therapies is testosterone deficiency or blockade of testosterone action.
GnRH agonists work at the level of the hypothalamus to ultimately downregulate luteinzing hormone (LH) and follicle stimulating hormone (FSH) receptors. A decrease in LH results in decreased androgen synthesis primarily in the testes. At the initiation of therapy, the GnRH agonists can result in an initial “flare” of symptoms as the LH/FSH receptor downregulation does not occur immediately.(56) After several weeks of continuous GnRH agonist therapy, circulating testosterone levels are reduced by 90% similar to bilateral orchiectomy. However, estrogen deficiency also results from bilateral orchiectomy and GnRH agonists due to loss of conversion of testosterone to estradiol. This may have important implications for bone health.
Antiandrogen therapy includes nonsteroidal agents (bicalutamide, flutamide) available in the United States and steroidal agents (cyproterone acetate) available in Canada and Europe.(54) The nonsteroidal antiandrogens competitively bind with the androgen receptor to block its activation. With these agents, testosterone levels remains unchanged or even increased.(54) Antiandrogens may be used as monotherapy but is more typically used in combination with GnRH agonist to prevent a “flare” or added to bilateral orchiectomy or other regimen for combined androgen blockade (CAB). Patients with advanced prostate cancer however are increasingly maintained long-term on ADT therapies and effects on bone outcomes have been recognized.
B. Androgen-Deprivation Therapy: Effects on BMD
Men who are undergoing therapy with androgen-deprivation therapy (ADT) are at higher risk for BMD loss and increased incidence of fractures.(57) Substantial BMD losses from ADT have been reported in several trials of 2-4% at the hip and 2-5% at the spine by DXA in one-year after initiation of ADT(58-60) but rates have been reported as high as 8.5% at the spine by QCT.(58) Other studies have reported significant losses at the ultradistal radius (5.3%) after one-year of ADT.(61) These high bone loss rates occur early in the treatment course and rates of bone loss are similar to those seen in early postmenopausal women. This bone loss occurs due to the hypogonadism which is well known to increase bone turnover and thereby increase bone resorption.
Bicalutamide is often used in association with GnRH therapy but can also be used as monotherapy. An open-label study of 52 men with non-metastatic prostate cancer receiving either bicalutamide or a GnRH agonist for one year reported a 2.5% increase in lumbar spine BMD with bicalutamide compared with 2.5% loss in BMD with GnRH agonists.(62) Bicalutamide as monotherapy was associated with a 97% increase in testosterone concentrations and 146% increase in estradiol concentrations in that study. With androgen receptor blockade, LH signaling from the pituitary is intact and results in increased testosterone which can then be converted to estrogen via an intact aromatase enzyme. These differences in effects on hormonal response between bicalutamide and GnRH agonists may explain the difference in BMD responses. However, bicalutamide as monotherapy is associated with gynecomastia and it is unclear whether it has survival advantages and therefore is less often used as monotherapy.
C. Androgen-Deprivation Therapy: Effects on Fracture Risk
Fracture rates are higher in men undergoing androgen deprivation therapy for nonmetastatic prostate cancer when compared to men with prostate cancer not undergoing ADT in analysis of medical claims and Medicare databases.(63-65) Fracture rates were higher for any clinical fracture (RR 1.21, 95% CI 1.09-1.34) as well as hip fractures (RR 1.76, 95% CI 1.33-2.33) for men with prostate cancer receiving GnRH agonist therapy compared to men not receiving GnRH therapy.(65) This risk appears to increase with longer duration of therapy.(63, 64)
V. Survivors of childhood malignancies
It has also been recognized that deficits in bone accrual are a long-term morbidity associated with survival of a childhood malignancy. Low bone mineral density has been shown in studies of adult-survivors of hematologic malignancies (e.g. acute lymphoblastic leukemia) and solid tumors (e.g. brain tumors).(66-68) In this group, alterations in gonadal function occur and growth may be impaired. Peak bone mass may therefore not be fully attained. It has been noted that patients receiving cranial irradation as adjunct therapy for ALL or brain tumors may experience hypothalamic or pituitary deficiencies such growth hormone or gonadotropin deficiency.(69, 70) Both these pituitary hormones are critical to maintenance of normal skeletal accrual during growth. Further, radiation-induced damage and use of alkylating agents may result in gonadal dysfunction. Due to the multitude of potential insults, bone health has therefore become an important aspect to care of survivors of childhood malignancies.
VI. Treatment of Cancer-treatment related bone loss
A. General Guidelines
Although the data are compelling regarding the effect of hypogonadism on inducing bone loss in cancer patients, secondary causes should always be considered in the differential diagnosis and investigated in selected patients. These including hyperthyroidism, hyperparathyroidism (primary or secondary), vitamin D deficiency, celiac disease among others. Each patient should have an assessment of vitamin D and replaced appropriately to attain 25-hydroxyvitamin D concentrations of more than 30 ng/mL. A minimum of 800IU of vitamin D should be given daily in patients with bone loss and dosing could be adjusted higher based on vitamin D concentrations attained. Calcium is best obtained through the diet and targeted total consumption should be 1200-1500mg per day. Weight-bearing exercise is likely to be important to maintain muscle tone and balance although the effects of exercise on bone mineral density are less clear. Osteoporotic fragility fractures occur in association with a fall, therefore patients should be assessed for falls risk and appropriate counseling be provided.
Recent studies have suggested that patients at risk for bone loss are not being identified and therefore not effectively treated. As the patients' malignancy often dominates the focus of discussion, the possibility of silent bone disease may not often be addressed. A study of men with prostate cancer at risk for bone loss due to ADT or orchiectomy found that only 34% had received a recent DXA or pharmacological intervention for osteoporosis prevention or treatment.(71)
Patients should have their BMD assessed when initiating therapy on aromatase inhibitors, GnRH agonists, antiandrogens, or other therapies at high risk of bone loss (Table 2).(72) Consensus groups have formulated guidelines on the approach to bone loss in patients with cancer.(73) The American Society of Clinical Oncology (ASCO) guidelines suggest that women with breast cancer should receive a BMD with additional risk factors of AI therapy, premature ovarian failure, family history of fractures, body weight less than 70kg or prior nontraumatic fracture.(74) Clinical practice guidelines have recommended that men undergoing surgical or chemical castration have a BMD measured at baseline.(75) BMD should be assessed at the hip and spine using dual-energy X-ray absorptiometry (DXA). Those patients who have fragility fractures or osteoporosis by BMD criteria (BMD 2.5 standard deviations or more below a normal young adult) should be offered therapy with anti-osteoporosis agents. BMD should be assessed yearly for patient at risk of bone loss. Patients experiencing significant BMD losses while undergoing cancer therapy may also be candidates for further pharmacotherapies. The most effective agents for fracture risk reduction are bisphosphonate therapies.
Table 2. Management of bone loss in patients with breast or prostate cancer.
| All patients |
|
| Bisphosphonate or other anti-osteoporosis therapy is indicated for |
|
Reprinted with permission from Osteoporosis Reports.
B. Effectiveness of Bisphosphonate therapy
Bisphosphonate therapy is well-proven for the prevention of bone loss and fracture risk reduction in postmenopausal women with osteoporosis as well as in patients with glucocorticoid-induced osteoporosis. Reductions in vertebral and hip fractures have been reported in healthy postmenopausal women receiving oral alendronate, oral risedronate and intravenous zoledronic acid.(76-78) Oral clodronate and oral ibandronate has reduced vertebral fractures in healthy postmenopausal women.(79, 80) Treatment of skeletal-related complications in cancer, such as bone metastases, has focused primarily on intravenous formulations. In contrast, oral bisphosphonates are the mainstay for treatment of osteoporosis therapies in patients without cancer.
Intravenous bisphosphonates have been effective in patients with prostate cancer undergoing ADT. Pamidronate has been effective at reducing bone loss in GnRH-treated men with locally advanced prostate cancer(58) as well as in men with bone metastases after combined androgen blockade with GnRH agonist and an androgen antagonist.(81)
Zoledronic acid, a more potent bisphosphonate than pamidronate, has been demonstrated to increase BMD above baseline in several trials in non-metastatic prostate cancer. A multicenter randomized, placebo-controlled trial of zoledronic acid (4mg intravenously every 3 months) increased BMD at the hip and spine in men initiating ADT for nonmetastatic prostate cancer (7.8% difference in BMD at the spine between groups, 95% CI 5.6-10.0%).(59) A single yearly infusion of zoledronic acid also increased BMD at one-year of GnRH agonist therapy.(82)
Zoledronic acid has shown promise in preventing AI-related bone loss in breast cancer. It has been shown to significantly decrease BMD losses associated with the use of letrozole, anastrozole and tamoxifen. The optimal timing of administration of zoledronic acid is actively being studied and reports suggest that zoledronic acid given at the initiation of AI therapy is more effective than delayed initiation. Postmenopausal women given adjuvant letrozole for early-stage breast cancer in the Zometa-Femara Adjuvant Synergy Trial (Z-FAST) had higher BMD increases at one year at the spine (4.4%) and total hip (3.3%) when zoledronic acid was given upfront rather than delayed-start.(83) Zoledronic acid prevented bone loss at the spine and hip in premenopausal patients with breast cancer treated with goserelin (GnRH agonist) combined with either anastrozole or tamoxifen.(51)
Oral bisphosphonates are well established to prevent fractures at multiple sites in patients with osteoporosis but have been infrequently studied in patients with cancer.
Men undergoing ADT for prostate cancer given oral alendronate 70mg once weekly for one year had 5.1% greater BMD at the spine and 2.3% greater BMD at the femoral neck compared to placebo.(84) Oral risedronate (35mg once weekly) given to breast cancer survivors with recent chemotherapy-induced menopause resulted in mild improvements in BMD at one year.(85)
C. Safety of Bisphosphonate Therapy
Complications of intravenous bisphosphonate therapy can include acute flu-like reactions, renal insufficiency and hypocalcemia. These complications can be lessened by premedication with anti-pyretics and avoidance of use in patients with known hypocalcemia or significant vitamin D deficiency. In addition, infusion rates have been slowed or doses reduced to decrease the incidence of renal insufficiency.
Concern has been raised regarding osteonecrosis of the jaw (ONJ), a potential complication of bisphosphonate use in patients with cancer. ONJ is generally defined as a non-healing oral ulcer lasting 8 weeks or more usually occurring after invasive dental procedures. Several societies have convened expert panels and issued position papers including the American Academy of Oral and Maxillofacial Pathology(86), American Society of Bone and Mineral Research(87, 88) and the American Dental Association.(89) In the absence of information regarding the pathophysiology and risk factors for this condition, it is recommended that patients being treated with intravenous bisphosphonates undergo a dental exam and invasive procedures should be postponed or completed prior to initiation of therapy. It is hoped that increased awareness and attention to oral hygiene may prevent complications associated with this condition.
D. Additional therapies
Additional therapies for treatment of cancer-related bone loss are often limited. Estrogen may be considered in women without a history of or risk factors for breast cancer who have had premature menopause or other treatment-associated amenorrhea. Postmenopausal women in the Women's Health Initiative who were randomized to conjugated equine estrogen/progesterone combination had a decreased incidence of fracture. This must be weighed against an increased thromboembolism risk, breast cancer risk and unclear cardiovascular protection.
Selective-estrogen receptor modulators (SERMs) may also be appropriate for selective populations. Raloxifene has been approved for the use in postmenopausal osteoporosis as it has been shown to reduce the incidence of spine fractures.(90) Raloxifene has improved BMD in men with prostate cancer at the hip at one-year following GnRH therapy.(91) However, raloxifene is not used following 5-year tamoxifen therapy in women with breast cancer. Teriparatide (recombinant human PTH) has proven fracture reduction efficacy and is used in selected populations for osteoporosis management. However, due to the development of osteosarcoma in rats given lifelong teriparatide therapy, the drug has a black box warning not to be used in patients at increased risk of osteosarcoma such as prior external beam or implant radiation therapy involving the skeleton. It is further recommended that patients with bone metastases or a history of skeletal malignancies should not be given teriparatide. As such, teriparatide is not used in the management of most patients with bone loss due to cancer treatment. Newer therapies for treatment of bone loss include the RANK-L inhibitor, denosumab that results in sustained inhibition of bone turnover. This agent is actively being studied in patients with cancer for both the management of bone loss as well as bone metastases.(92) Early results from a phase III trial reported increases in BMD after 2 years of denosumab (twice yearly subcutaneous dosing) in patients with nonmetastatic breast cancer treated with aromatase inhibitors.(93) Ongoing trials will determine the role of denosumab for management of patients with bone loss. Currently, bisphosphonates are preferred over these agents due to their known protection against hip fractures in addition to vertebral fractures.
Current therapies for bone loss associated with cancer-treatment result in suppression of bone turnover. We have focused on cancer-treatment associated bone loss in this article but it should be noted that suppression of bone turnover may have beneficial effects on the skeletal tumor burden itself. Animal models have suggested that mice with hypogonadism develop metastases from prostate cancer cell inoculation faster than eugonadal mice and this effect can be blocked by the use of the bisphosphonate zoledronic acid.(94) In addition, mice who had PTH-induced increases in bone turnover were found to have increases in prostate cancer metastases to skeletal areas experiencing accelerated bone turnover, the effects of which could be mitigated by zoledronic acid administration.(95) Further support for the role of increased bone resorption in the pathophysiology of bone metastases is gleaned from an exploratory cohort analysis where elevated bone turnover markers were a significant predictor of death in patients with skeletal involvement of myeloma, breast and prostate cancer who were enrolled in phase III clinical trials on the use of zoledronic acid.(96)
VII. Conclusions
It is clear that patients with cancer are at risk for bone loss from treatments that result in hypogonadism. Patients with breast cancer, prostate cancer and survivors of childhood malignancies may be at particular risk but the risk is not limited to those populations. Fortunately, bisphosphonates work well in limiting bone loss associated with many cancer therapies, including AI treatment in breast cancer and ADT therapies in prostate cancer. Patients being treated for malignancies with agents associated with bone loss should undergo a bone health assessment.
Acknowledgments
The authors are grateful for the support from the National Institutes of Health Grants AR051483, CA69158, DK065837; The University of Virginia GCRC RR00847; Aurbach Endowment and Mellon Institute at the University of Virginia; V-Foundation, Mary Kay Ash Foundation, Prostate Cancer Foundation, Department of Defense.
References
- 1.Hirbe A, Morgan EA, Uluckan O, Weilbaecher K. Skeletal complications of breast cancer therapies. Clin Cancer Res. 2006 Oct 15;12(20 Pt 2):6309s–14s. doi: 10.1158/1078-0432.CCR-06-0652. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Pfeilschifter J, Diel IJ. Osteoporosis due to cancer treatment: pathogenesis and management. J Clin Oncol. 2000 Apr;18(7):1570–93. doi: 10.1200/JCO.2000.18.7.1570. [DOI] [PubMed] [Google Scholar]
- 3.Feng JQ, Ward LM, Liu S, Lu Y, Xie Y, Yuan B, et al. Loss of DMP1 causes rickets and osteomalacia and identifies a role for osteocytes in mineral metabolism. Nat Genet. 2006 Nov;38(11):1310–5. doi: 10.1038/ng1905. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Lee JS, Lacroix AZ, Wu L, Cauley JA, Jackson RD, Kooperberg C, et al. Associations of serum sex hormone-binding globulin and sex hormone concentrations with hip fracture risk in postmenopausal women. J Clin Endocrinol Metab. 2008 Mar 11; doi: 10.1210/jc.2007-2358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Meier C, Nguyen TV, Handelsman DJ, Schindler C, Kushnir MM, Rockwood AL, et al. Endogenous sex hormones and incident fracture risk in older men: the Dubbo Osteoporosis Epidemiology Study. Arch Intern Med. 2008 Jan 14;168(1):47–54. doi: 10.1001/archinternmed.2007.2. [DOI] [PubMed] [Google Scholar]
- 6.Carani C, Qin K, Simoni M, Faustini-Fustini M, Serpente S, Boyd J, et al. Effect of testosterone and estradiol in a man with aromatase deficiency. N Engl J Med. 1997 Jul 10;337(2):91–5. doi: 10.1056/NEJM199707103370204. [DOI] [PubMed] [Google Scholar]
- 7.Smith EP, Boyd J, Frank GR, Takahashi H, Cohen RM, Specker B, et al. Estrogen resistance caused by a mutation in the estrogen-receptor gene in a man. N Engl J Med. 1994 Oct 20;331(16):1056–61. doi: 10.1056/NEJM199410203311604. [DOI] [PubMed] [Google Scholar]
- 8.Eriksson S, Eriksson A, Stege R, Carlstrom K. Bone mineral density in patients with prostatic cancer treated with orchidectomy and with estrogens. Calcif Tissue Int. 1995 Aug;57(2):97–9. doi: 10.1007/BF00298427. [DOI] [PubMed] [Google Scholar]
- 9.Greendale GA, Edelstein S, Barrett-Connor E. Endogenous sex steroids and bone mineral density in older women and men: the Rancho Bernardo Study. J Bone Miner Res. 1997 Nov;12(11):1833–43. doi: 10.1359/jbmr.1997.12.11.1833. [DOI] [PubMed] [Google Scholar]
- 10.Couse JF, Korach KS. Estrogen receptor null mice: what have we learned and where will they lead us? Endocr Rev. 1999 Jun;20(3):358–417. doi: 10.1210/edrv.20.3.0370. [DOI] [PubMed] [Google Scholar]
- 11.Deroo BJ, Korach KS. Estrogen receptors and human disease. J Clin Invest. 2006 Mar;116(3):561–70. doi: 10.1172/JCI27987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Arts J, Kuiper GG, Janssen JM, Gustafsson JA, Lowik CW, Pols HA, et al. Differential expression of estrogen receptors alpha and beta mRNA during differentiation of human osteoblast SV-HFO cells. Endocrinology. 1997;138(11):5067–70. doi: 10.1210/endo.138.11.5652. [DOI] [PubMed] [Google Scholar]
- 13.Braidman IP, Davenport LK, Carter DH, Selby PL, Mawer EB, Freemont AJ. Preliminary in situ identification of estrogen target cells in bone. J Bone Miner Res. 1995;10(1):74–80. doi: 10.1002/jbmr.5650100112. [DOI] [PubMed] [Google Scholar]
- 14.Onoe Y, Miyaura C, Ohta H, Nozawa S, Suda T. Expression of estrogen receptor beta in rat bone. Endocrinology. 1997;138(10):4509–12. doi: 10.1210/endo.138.10.5575. [DOI] [PubMed] [Google Scholar]
- 15.Denger S, Reid G, Kos M, Flouriot G, Parsch D, Brand H, et al. ERalpha gene expression in human primary osteoblasts: evidence for the expression of two receptor proteins. Mol Endocrinol. 2001 Dec;15(12):2064–77. doi: 10.1210/mend.15.12.0741. [DOI] [PubMed] [Google Scholar]
- 16.Vidal O, Kindblom LG, Ohlsson C. Expression and localization of estrogen receptor-beta in murine and human bone. J Bone Miner Res. 1999 Jun;14(6):923–9. doi: 10.1359/jbmr.1999.14.6.923. [DOI] [PubMed] [Google Scholar]
- 17.Nakamura T, Imai Y, Matsumoto T, Sato S, Takeuchi K, Igarashi K, et al. Estrogen prevents bone loss via estrogen receptor alpha and induction of Fas ligand in osteoclasts. Cell. 2007 Sep 7;130(5):811–23. doi: 10.1016/j.cell.2007.07.025. [DOI] [PubMed] [Google Scholar]
- 18.Krum SA, Miranda-Carboni GA, Hauschka PV, Carroll JS, Lane TF, Freedman LP, et al. Estrogen protects bone by inducing Fas ligand in osteoblasts to regulate osteoclast survival. EMBO J. 2008 Feb 6;27(3):535–45. doi: 10.1038/sj.emboj.7601984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.SEER Surveillance, Epidemiology, and End Results (SEER) Program. National Cancer Institute, DCCPS, Surveillance Research Program, Cancer Statistics Branch; SEER*Stat Database: Mortality - All COD, Public-Use With State, Total U.S (1969-2004) ( www.seer.cancer.gov) released April 2007. Underlying mortality data provided by NCHS ( www.cdc.gov/nchs). [Google Scholar]
- 20.Chen Z, Maricic M, Bassford TL, Pettinger M, Ritenbaugh C, Lopez AM, et al. Fracture risk among breast cancer survivors: results from the Women's Health Initiative Observational Study. Arch Intern Med. 2005 Mar 14;165(5):552–8. doi: 10.1001/archinte.165.5.552. [DOI] [PubMed] [Google Scholar]
- 21.Chatterton RT, Jr, Geiger AS, Gann PH, Khan SA. Formation of estrone and estradiol from estrone sulfate by normal breast parenchymal tissue. J Steroid Biochem Mol Biol. 2003 Aug;86(2):159–66. doi: 10.1016/s0960-0760(03)00266-8. [DOI] [PubMed] [Google Scholar]
- 22.Cole MP, Jones CT, Todd ID. A new anti-oestrogenic agent in late breast cancer. An early clinical appraisal of ICI46474. Br J Cancer. 1971;25(2):270–5. doi: 10.1038/bjc.1971.33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Fisher B, Costantino J, Redmond C, Poisson R, Bowman D, Couture J, et al. A randomized clinical trial evaluating tamoxifen in the treatment of patients with node-negative breast cancer who have estrogen-receptor-positive tumors. N Engl J Med. 1989;320(8):479–84. doi: 10.1056/NEJM198902233200802. [DOI] [PubMed] [Google Scholar]
- 24.Fisher B, Dignam J, Wolmark N, Wickerham DL, Fisher ER, Mamounas E, et al. Tamoxifen in treatment of intraductal breast cancer: National Surgical Adjuvant Breast and Bowel Project B-24 randomised controlled trial. Lancet. 1999;353(9169):1993–2000. doi: 10.1016/S0140-6736(99)05036-9. [DOI] [PubMed] [Google Scholar]
- 25.Fisher B, Costantino JP, Wickerham DL, Redmond CK, Kavanah M, Cronin WM, et al. Tamoxifen for prevention of breast cancer: report of the National Surgical Adjuvant Breast and Bowel Project P-1 Study. J Natl Cancer Inst. 1998;90(18):1371–88. doi: 10.1093/jnci/90.18.1371. [DOI] [PubMed] [Google Scholar]
- 26.Turner RT, Wakley GK, Hannon KS, Bell NH. Tamoxifen prevents the skeletal effects of ovarian hormone deficiency in rats. J Bone Miner Res. 1987;2(5):449–56. doi: 10.1002/jbmr.5650020513. [DOI] [PubMed] [Google Scholar]
- 27.Arnett TR, Lindsay R, Kilb JM, Moonga BS, Spowage M, Dempster DW. Selective toxic effects of tamoxifen on osteoclasts: comparison with the effects of oestrogen. J Endocrinol. 1996 Jun;149(3):503–8. doi: 10.1677/joe.0.1490503. [DOI] [PubMed] [Google Scholar]
- 28.Wright CD, Garrahan NJ, Stanton M, Gazet JC, Mansell RE, Compston JE. Effect of long-term tamoxifen therapy on cancellous bone remodeling and structure in women with breast cancer. J Bone Miner Res. 1994;9(2):153–9. doi: 10.1002/jbmr.5650090204. [DOI] [PubMed] [Google Scholar]
- 29.Powles TJ, Hickish T, Kanis JA, Tidy A, Ashley S. Effect of tamoxifen on bone mineral density measured by dual-energy x-ray absorptiometry in healthy premenopausal and postmenopausal women. J Clin Oncol. 1996 Jan;14(1):78–84. doi: 10.1200/JCO.1996.14.1.78. [DOI] [PubMed] [Google Scholar]
- 30.Vehmanen L, Elomaa I, Blomqvist C, Saarto T. Tamoxifen treatment after adjuvant chemotherapy has opposite effects on bone mineral density in premenopausal patients depending on menstrual status. J Clin Oncol. 2006 Feb 1;24(4):675–80. doi: 10.1200/JCO.2005.02.3515. [DOI] [PubMed] [Google Scholar]
- 31.Eng-Wong J, Reynolds JC, Venzon D, Liewehr D, Gantz S, Danforth D, et al. Effect of raloxifene on bone mineral density in premenopausal women at increased risk of breast cancer. J Clin Endocrinol Metab. 2006 Oct;91(10):3941–6. doi: 10.1210/jc.2005-2827. [DOI] [PubMed] [Google Scholar]
- 32.Kristensen B, Ejlertsen B, Dalgaard P, Larsen L, Holmegaard SN, Transbol I, et al. Tamoxifen and bone metabolism in postmenopausal low-risk breast cancer patients: a randomized study. J Clin Oncol. 1994 May;12(5):992–7. doi: 10.1200/JCO.1994.12.5.992. [DOI] [PubMed] [Google Scholar]
- 33.Love RR, Mazess RB, Barden HS, Epstein S, Newcomb PA, Jordan VC, et al. Effects of tamoxifen on bone mineral density in postmenopausal women with breast cancer. N Engl J Med. 1992 Mar 26;326(13):852–6. doi: 10.1056/NEJM199203263261302. [DOI] [PubMed] [Google Scholar]
- 34.Grey AB, Stapleton JP, Evans MC, Tatnell MA, Ames RW, Reid IR. The effect of the antiestrogen tamoxifen on bone mineral density in normal late postmenopausal women. Am J Med. 1995 Dec;99(6):636–41. doi: 10.1016/s0002-9343(99)80251-4. [DOI] [PubMed] [Google Scholar]
- 35.Love RR, Barden HS, Mazess RB, Epstein S, Chappell RJ. Effect of tamoxifen on lumbar spine bone mineral density in postmenopausal women after 5 years. Arch Intern Med. 1994 Nov 28;154(22):2585–8. [PubMed] [Google Scholar]
- 36.Kristensen B, Ejlertsen B, Mouridsen HT, Andersen KW, Lauritzen JB. Femoral fractures in postmenopausal breast cancer patients treated with adjuvant tamoxifen. Breast Cancer Res Treat. 1996;39(3):321–6. doi: 10.1007/BF01806160. [DOI] [PubMed] [Google Scholar]
- 37.Vogel VG, Costantino JP, Wickerham DL, Cronin WM, Cecchini RS, Atkins JN, et al. Effects of tamoxifen vs raloxifene on the risk of developing invasive breast cancer and other disease outcomes: the NSABP Study of Tamoxifen and Raloxifene (STAR) P-2 trial. JAMA. 2006 Jun 21;295(23):2727–41. doi: 10.1001/jama.295.23.joc60074. [DOI] [PubMed] [Google Scholar]
- 38.Howell A, Cuzick J, Baum M, Buzdar A, Dowsett M, Forbes JF, et al. Results of the ATAC (Arimidex, Tamoxifen, Alone or in Combination) trial after completion of 5 years' adjuvant treatment for breast cancer. Lancet. 2005 Jan 1-7;365(9453):60–2. doi: 10.1016/S0140-6736(04)17666-6. [DOI] [PubMed] [Google Scholar]
- 39.Perez EA, Josse RG, Pritchard KI, Ingle JN, Martino S, Findlay BP, et al. Effect of letrozole versus placebo on bone mineral density in women with primary breast cancer completing 5 or more years of adjuvant tamoxifen: a companion study to NCIC CTG MA.17. J Clin Oncol. 2006 Aug 1;24(22):3629–35. doi: 10.1200/JCO.2005.05.4882. [DOI] [PubMed] [Google Scholar]
- 40.Coates AS, Keshaviah A, Thurlimann B, Mouridsen H, Mauriac L, Forbes JF, et al. Five years of letrozole compared with tamoxifen as initial adjuvant therapy for postmenopausal women with endocrine-responsive early breast cancer: update of study BIG 1-98. J Clin Oncol. 2007 Feb 10;25(5):486–92. doi: 10.1200/JCO.2006.08.8617. [DOI] [PubMed] [Google Scholar]
- 41.Coleman RE, Banks LM, Girgis SI, Kilburn LS, Vrdoljak E, Fox J, et al. Skeletal effects of exemestane on bone-mineral density, bone biomarkers, and fracture incidence in postmenopausal women with early breast cancer participating in the Intergroup Exemestane Study (IES): a randomised controlled study. Lancet Oncol. 2007 Feb;8(2):119–27. doi: 10.1016/S1470-2045(07)70003-7. [DOI] [PubMed] [Google Scholar]
- 42.Geisler J, Haynes B, Anker G, Dowsett M, Lonning PE. Influence of letrozole and anastrozole on total body aromatization and plasma estrogen levels in postmenopausal breast cancer patients evaluated in a randomized, cross-over study. J Clin Oncol. 2002 Feb 1;20(3):751–7. doi: 10.1200/JCO.2002.20.3.751. [DOI] [PubMed] [Google Scholar]
- 43.Lonning PE, Geisler J. Aromatase inhibitors: Assessment of biochemical efficacy measured by total body aromatase inhibition and tissue estrogen suppression. J Steroid Biochem Mol Biol. 2008 Feb;108(35):196–202. doi: 10.1016/j.jsbmb.2007.09.017. [DOI] [PubMed] [Google Scholar]
- 44.Eastell R, Hannon RA, Cuzick J, Dowsett M, Clack G, Adams JE. Effect of an aromatase inhibitor on BMD and bone turnover markers: 2-year results of the Anastrozole, Tamoxifen, Alone or in Combination (ATAC) trial (18233230) J Bone Miner Res. 2006 Aug;21(8):1215–23. doi: 10.1359/jbmr.060508. [DOI] [PubMed] [Google Scholar]
- 45.Goss PE, Qi S, Cheung AM, Hu H, Mendes M, Pritzker KP. Effects of the steroidal aromatase inhibitor exemestane and the nonsteroidal aromatase inhibitor letrozole on bone and lipid metabolism in ovariectomized rats. Clin Cancer Res. 2004 Sep 1;10(17):5717–23. doi: 10.1158/1078-0432.CCR-04-0438. [DOI] [PubMed] [Google Scholar]
- 46.Ariazi EA, Leitao A, Oprea TI, Chen B, Louis T, Bertucci AM, et al. Exemestane's 17-hydroxylated metabolite exerts biological effects as an androgen. Mol Cancer Ther. 2007 Nov;6(11):2817–27. doi: 10.1158/1535-7163.MCT-07-0312. [DOI] [PubMed] [Google Scholar]
- 47.Gonnelli S, Cadirni A, Caffarelli C, Petrioli R, Montagnani A, Franci MB, et al. Changes in bone turnover and in bone mass in women with breast cancer switched from tamoxifen to exemestane. Bone. 2007 Jan;40(1):205–10. doi: 10.1016/j.bone.2006.06.027. [DOI] [PubMed] [Google Scholar]
- 48.Forbes JF, Cuzick J, Buzdar A, Howell A, Tobias JS, Baum M. Effect of anastrozole and tamoxifen as adjuvant treatment for early-stage breast cancer: 100-month analysis of the ATAC trial. Lancet Oncol. 2008 Jan;9(1):45–53. doi: 10.1016/S1470-2045(07)70385-6. [DOI] [PubMed] [Google Scholar]
- 49.Saarto T, Blomqvist C, Valimaki M, Makela P, Sarna S, Elomaa I. Chemical castration induced by adjuvant cyclophosphamide, methotrexate, and fluorouracil chemotherapy causes rapid bone loss that is reduced by clodronate: a randomized study in premenopausal breast cancer patients. J Clin Oncol. 1997;15(4):1341–7. doi: 10.1200/JCO.1997.15.4.1341. [DOI] [PubMed] [Google Scholar]
- 50.Prior JC, Vigna YM, Wark JD, Eyre DR, Lentle BC, Li DK, et al. Premenopausal ovariectomy-related bone loss: a randomized, double-blind, one-year trial of conjugated estrogen or medroxyprogesterone acetate. J Bone Miner Res. 1997;12(11):1851–63. doi: 10.1359/jbmr.1997.12.11.1851. [DOI] [PubMed] [Google Scholar]
- 51.Gnant MF, Mlineritsch B, Luschin-Ebengreuth G, Grampp S, Kaessmann H, Schmid M, et al. Zoledronic acid prevents cancer treatment-induced bone loss in premenopausal women receiving adjuvant endocrine therapy for hormone-responsive breast cancer: a report from the Austrian Breast and Colorectal Cancer Study Group. J Clin Oncol. 2007 Mar 1;25(7):820–8. doi: 10.1200/JCO.2005.02.7102. [DOI] [PubMed] [Google Scholar]
- 52.Huggins C, Hodges CV. Studies on prostatic cancer. I. The effect of castration, of estrogen and androgen injection on serum phosphatases in metastatic carcinoma of the prostate. CA Cancer J Clin. 1972 Jul-Aug;22(4):232–40. doi: 10.3322/canjclin.22.4.232. [DOI] [PubMed] [Google Scholar]
- 53.Messing EM, Manola J, Sarosdy M, Wilding G, Crawford ED, Trump D. Immediate hormonal therapy compared with observation after radical prostatectomy and pelvic lymphadenectomy in men with node-positive prostate cancer. N Engl J Med. 1999 Dec 9;341(24):1781–8. doi: 10.1056/NEJM199912093412401. [DOI] [PubMed] [Google Scholar]
- 54.Singer EA, Golijanin DJ, Miyamoto H, Messing EM. Androgen deprivation therapy for prostate cancer. Expert Opin Pharmacother. 2008 Feb;9(2):211–28. doi: 10.1517/14656566.9.2.211. [DOI] [PubMed] [Google Scholar]
- 55.Mason M. What implications do the tolerability profiles of antiandrogens and other commonly used prostate cancer treatments have on patient care? J Cancer Res Clin Oncol. 2006 Aug;132 1:S27–35. doi: 10.1007/s00432-006-0134-4. [DOI] [PubMed] [Google Scholar]
- 56.Waxman J, Man A, Hendry WF, Whitfield HN, Besser GM, Tiptaft RC, et al. Importance of early tumour exacerbation in patients treated with long acting analogues of gonadotrophin releasing hormone for advanced prostatic cancer. Br Med J (Clin Res Ed) 1985 Nov 16;291(6506):1387–8. doi: 10.1136/bmj.291.6506.1387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Smith MR. Androgen deprivation therapy for prostate cancer: new concepts and concerns. Curr Opin Endocrinol Diabetes Obes. 2007 Jun;14(3):247–54. doi: 10.1097/MED.0b013e32814db88c. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Smith MR, McGovern FJ, Zietman AL, Fallon MA, Hayden DL, Schoenfeld DA, et al. Pamidronate to prevent bone loss during androgen-deprivation therapy for prostate cancer. N Engl J Med. 2001 Sep 27;345(13):948–55. doi: 10.1056/NEJMoa010845. [DOI] [PubMed] [Google Scholar]
- 59.Smith MR, Eastham J, Gleason DM, Shasha D, Tchekmedyian S, Zinner N. Randomized controlled trial of zoledronic acid to prevent bone loss in men receiving androgen deprivation therapy for nonmetastatic prostate cancer. J Urol. 2003 Jun;169(6):2008–12. doi: 10.1097/01.ju.0000063820.94994.95. [DOI] [PubMed] [Google Scholar]
- 60.Maillefert JF, Sibilia J, Michel F, Saussine C, Javier RM, Tavernier C. Bone mineral density in men treated with synthetic gonadotropin-releasing hormone agonists for prostatic carcinoma. J Urol. 1999 Apr;161(4):1219–22. [PubMed] [Google Scholar]
- 61.Mittan D, Lee S, Miller E, Perez RC, Basler JW, Bruder JM. Bone loss following hypogonadism in men with prostate cancer treated with GnRH analogs. J Clin Endocrinol Metab. 2002 Aug;87(8):3656–61. doi: 10.1210/jcem.87.8.8782. [DOI] [PubMed] [Google Scholar]
- 62.Smith MR, Goode M, Zietman AL, McGovern FJ, Lee H, Finkelstein JS. Bicalutamide monotherapy versus leuprolide monotherapy for prostate cancer: effects on bone mineral density and body composition. J Clin Oncol. 2004 Jul 1;22(13):2546–53. doi: 10.1200/JCO.2004.01.174. [DOI] [PubMed] [Google Scholar]
- 63.Shahinian VB, Kuo YF, Freeman JL, Goodwin JS. Risk of fracture after androgen deprivation for prostate cancer. N Engl J Med. 2005 Jan 13;352(2):154–64. doi: 10.1056/NEJMoa041943. [DOI] [PubMed] [Google Scholar]
- 64.Smith MR, Lee WC, Brandman J, Wang Q, Botteman M, Pashos CL. Gonadotropin-releasing hormone agonists and fracture risk: a claims-based cohort study of men with nonmetastatic prostate cancer. J Clin Oncol. 2005 Nov 1;23(31):7897–903. doi: 10.1200/JCO.2004.00.6908. [DOI] [PubMed] [Google Scholar]
- 65.Smith MR, Boyce SP, Moyneur E, Duh MS, Raut MK, Brandman J. Risk of clinical fractures after gonadotropin-releasing hormone agonist therapy for prostate cancer. J Urol. 2006 Jan;175(1):136–9. doi: 10.1016/S0022-5347(05)00033-9. [DOI] [PubMed] [Google Scholar]
- 66.Kelly J, Damron T, Grant W, Anker C, Holdridge S, Shaw S, et al. Cross-sectional study of bone mineral density in adult survivors of solid pediatric cancers. J Pediatr Hematol Oncol. 2005 May;27(5):248–53. doi: 10.1097/01.mph.0000162526.77400.78. [DOI] [PubMed] [Google Scholar]
- 67.Kaste SC, Jones-Wallace D, Rose SR, Boyett JM, Lustig RH, Rivera GK, et al. Bone mineral decrements in survivors of childhood acute lymphoblastic leukemia: frequency of occurrence and risk factors for their development. Leukemia. 2001 May;15(5):728–34. doi: 10.1038/sj.leu.2402078. [DOI] [PubMed] [Google Scholar]
- 68.Strauss AJ, Su JT, Dalton VM, Gelber RD, Sallan SE, Silverman LB. Bony morbidity in children treated for acute lymphoblastic leukemia. J Clin Oncol. 2001 Jun 15;19(12):3066–72. doi: 10.1200/JCO.2001.19.12.3066. [DOI] [PubMed] [Google Scholar]
- 69.Hoorweg-Nijman JJ, Kardos G, Roos JC, van Dijk HJ, Netelenbos C, Popp-Snijders C, et al. Bone mineral density and markers of bone turnover in young adult survivors of childhood lymphoblastic leukaemia. Clin Endocrinol (Oxf) 1999 Feb;50(2):237–44. doi: 10.1046/j.1365-2265.1999.00654.x. [DOI] [PubMed] [Google Scholar]
- 70.Vassilopoulou-Sellin R, Brosnan P, Delpassand A, Zietz H, Klein MJ, Jaffe N. Osteopenia in young adult survivors of childhood cancer. Med Pediatr Oncol. 1999 Apr;32(4):272–8. doi: 10.1002/(sici)1096-911x(199904)32:4<272::aid-mpo6>3.0.co;2-g. [DOI] [PubMed] [Google Scholar]
- 71.Yee EF, White RE, Murata GH, Handanos C, Hoffman RM. Osteoporosis management in prostate cancer patients treated with androgen deprivation therapy. J Gen Intern Med. 2007 Sep;22(9):1305–10. doi: 10.1007/s11606-007-0291-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Brown SA, Guise TA. Cancer-associated bone disease. Curr Osteoporos Rep. 2007 Sep;5(3):120–7. doi: 10.1007/s11914-007-0027-8. [DOI] [PubMed] [Google Scholar]
- 73.Body JJ, Bergmann P, Boonen S, Boutsen Y, Devogelaer JP, Goemaere S, et al. Management of cancer treatment-induced bone loss in early breast and prostate cancer --a consensus paper of the Belgian Bone Club. Osteoporos Int. 2007 Nov;18(11):1439–50. doi: 10.1007/s00198-007-0439-4. [DOI] [PubMed] [Google Scholar]
- 74.Hillner BE, Ingle JN, Chlebowski RT, Gralow J, Yee GC, Janjan NA, et al. American Society of Clinical Oncology 2003 update on the role of bisphosphonates and bone health issues in women with breast cancer. J Clin Oncol. 2003 Nov 1;21(21):4042–57. doi: 10.1200/JCO.2003.08.017. [DOI] [PubMed] [Google Scholar]
- 75.Theriault RL, Biermann JS, Brown E, Brufsky A, Demers L, Grewal RK, et al. NCCN Task Force Report: Bone Health and Cancer Care. J Natl Compr Canc Netw. 2006 May;4 2:S1–20. [PubMed] [Google Scholar]
- 76.Bauer DC, Black DM, Garnero P, Hochberg M, Ott S, Orloff J, et al. Change in bone turnover and hip, non-spine, and vertebral fracture in alendronate-treated women: the fracture intervention trial. J Bone Miner Res. 2004 Aug;19(8):1250–8. doi: 10.1359/JBMR.040512. [DOI] [PubMed] [Google Scholar]
- 77.Wells G, Cranney A, Peterson J, Boucher M, Shea B, Robinson V, et al. Risedronate for the primary and secondary prevention of osteoporotic fractures in postmenopausal women. Cochrane Database Syst Rev. 2008;(1):CD004523. doi: 10.1002/14651858.CD004523.pub3. [DOI] [PubMed] [Google Scholar]
- 78.Black DM, Delmas PD, Eastell R, Reid IR, Boonen S, Cauley JA, et al. Once-yearly zoledronic acid for treatment of postmenopausal osteoporosis. N Engl J Med. 2007 May 3;356(18):1809–22. doi: 10.1056/NEJMoa067312. [DOI] [PubMed] [Google Scholar]
- 79.McCloskey E, Selby P, Davies M, Robinson J, Francis RM, Adams J, et al. Clodronate reduces vertebral fracture risk in women with postmenopausal or secondary osteoporosis: results of a double-blind, placebo-controlled 3-year study. J Bone Miner Res. 2004 May;19(5):728–36. doi: 10.1359/JBMR.040116. [DOI] [PubMed] [Google Scholar]
- 80.Delmas PD, Recker RR, Chesnut CH, 3rd, Skag A, Stakkestad JA, Emkey R, et al. Daily and intermittent oral ibandronate normalize bone turnover and provide significant reduction in vertebral fracture risk: results from the BONE study. Osteoporos Int. 2004 Oct;15(10):792–8. doi: 10.1007/s00198-004-1602-9. [DOI] [PubMed] [Google Scholar]
- 81.Diamond TH, Winters J, Smith A, De Souza P, Kersley JH, Lynch WJ, et al. The antiosteoporotic efficacy of intravenous pamidronate in men with prostate carcinoma receiving combined androgen blockade: a double blind, randomized, placebo-controlled crossover study. Cancer. 2001 Sep 15;92(6):1444–50. doi: 10.1002/1097-0142(20010915)92:6<1444::aid-cncr1468>3.0.co;2-m. [DOI] [PubMed] [Google Scholar]
- 82.Michaelson MD, Kaufman DS, Lee H, McGovern FJ, Kantoff PW, Fallon MA, et al. Randomized controlled trial of annual zoledronic acid to prevent gonadotropin-releasing hormone agonist-induced bone loss in men with prostate cancer. J Clin Oncol. 2007 Mar 20;25(9):1038–42. doi: 10.1200/JCO.2006.07.3361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Brufsky A, Harker WG, Beck JT, Carroll R, Tan-Chiu E, Seidler C, et al. Zoledronic acid inhibits adjuvant letrozole-induced bone loss in postmenopausal women with early breast cancer. J Clin Oncol. 2007 Mar 1;25(7):829–36. doi: 10.1200/JCO.2005.05.3744. [DOI] [PubMed] [Google Scholar]
- 84.Greenspan SL, Nelson JB, Trump DL, Resnick NM. Effect of once-weekly oral alendronate on bone loss in men receiving androgen deprivation therapy for prostate cancer: a randomized trial. Ann Intern Med. 2007 Mar 20;146(6):416–24. doi: 10.7326/0003-4819-146-6-200703200-00006. [DOI] [PubMed] [Google Scholar]
- 85.Greenspan SL, Bhattacharya RK, Sereika SM, Brufsky A, Vogel VG. Prevention of bone loss in survivors of breast cancer: A randomized, double-blind, placebo-controlled clinical trial. J Clin Endocrinol Metab. 2007 Jan;92(1):131–6. doi: 10.1210/jc.2006-1272. [DOI] [PubMed] [Google Scholar]
- 86.Woo SB, Hellstein JW, Kalmar JR. Narrative [corrected] review: bisphosphonates and osteonecrosis of the jaws. Ann Intern Med. 2006 May 16;144(10):753–61. doi: 10.7326/0003-4819-144-10-200605160-00009. [DOI] [PubMed] [Google Scholar]
- 87.Shane E, Goldring S, Christakos S, Drezner M, Eisman J, Silverman S, et al. Osteonecrosis of the jaw: more research needed. J Bone Miner Res. 2006 Oct;21(10):1503–5. doi: 10.1359/jbmr.060712. [DOI] [PubMed] [Google Scholar]
- 88.Khosla S, Burr D, Cauley J, Dempster DW, Ebeling PR, Felsenberg D, et al. Bisphosphonate-associated osteonecrosis of the jaw: report of a task force of the American Society for Bone and Mineral Research. J Bone Miner Res. 2007 Oct;22(10):1479–91. doi: 10.1359/jbmr.0707onj. [DOI] [PubMed] [Google Scholar]
- 89.American Dental Association Council on Scientific Affairs. Dental management of patients receiving oral bisphosphonate therapy: expert panel recommendations. JADA. 2006;137:1144–50. doi: 10.14219/jada.archive.2006.0355. [DOI] [PubMed] [Google Scholar]
- 90.Ettinger B, Black DM, Mitlak BH, Knickerbocker RK, Nickelsen T, Genant HK, et al. Reduction of vertebral fracture risk in postmenopausal women with osteoporosis treated with raloxifene: results from a 3-year randomized clinical trial. Multiple Outcomes of Raloxifene Evaluation (MORE) Investigators. Jama. 1999 Aug 18;282(7):637–45. doi: 10.1001/jama.282.7.637. [DOI] [PubMed] [Google Scholar]
- 91.Smith MR, Fallon MA, Lee H, Finkelstein JS. Raloxifene to prevent gonadotropin-releasing hormone agonist-induced bone loss in men with prostate cancer: a randomized controlled trial. J Clin Endocrinol Metab. 2004 Aug;89(8):3841–6. doi: 10.1210/jc.2003-032058. [DOI] [PubMed] [Google Scholar]
- 92.Lipton A, Steger GG, Figueroa J, Alvarado C, Solal-Celigny P, Body JJ, et al. Randomized active-controlled phase II study of denosumab efficacy and safety in patients with breast cancer-related bone metastases. J Clin Oncol. 2007 Oct 1;25(28):4431–7. doi: 10.1200/JCO.2007.11.8604. [DOI] [PubMed] [Google Scholar]
- 93.Ellis G, Bone H, Chlebowski R, Paul D, Spadafora S, Smith J, et al. A phase 3 study of the effect of denosumab therapy on bone mineral density in women receiving aromatase inhibitors for non-metastatic breast cancer. Abstract 47 presented at the 30th Annual San Antonio Breat Cancer Symposium; December 14, 2007; San Antonio, TX. 2007. [Google Scholar]
- 94.Padalecki SS, Carreon MR, Grubbs B, Cui Y, Guise TA. Androgen deprivation causes bone loss and increased prostate cancer metastases to bone: prevention by zoledronic acid. J Bone Miner Res. 2002;17(Suppl 1):S310. [Google Scholar]
- 95.Schneider A, Kalikin LM, Mattos AC, Keller ET, Allen MJ, Pienta KJ, et al. Bone turnover mediates preferential localization of prostate cancer in the skeleton. Endocrinology. 2005 Apr;146(4):1727–36. doi: 10.1210/en.2004-1211. [DOI] [PubMed] [Google Scholar]
- 96.Coleman RE, Major P, Lipton A, Brown JE, Lee KA, Smith M, et al. Predictive value of bone resorption and formation markers in cancer patients with bone metastases receiving the bisphosphonate zoledronic acid. J Clin Oncol. 2005 Aug 1;23(22):4925–35. doi: 10.1200/JCO.2005.06.091. [DOI] [PubMed] [Google Scholar]


