Abstract
Leukocyte recruitment to the arterial vessel wall is the first step in the development of atherosclerotic lesions. Leukocyte homing in this event proceeds through a well-defined adhesion cascade, which includes tethering, rolling, adhesion, and transmigration. Selectins, including the P-, E-, and L-selectins, and their ligands mediate the initial tethering and rolling. Interactions between selectins and their ligands serve as a braking system to decelerate fast flowing leukocytes from the central blood stream and enable them to adhere to and transmigrate underneath the activated endothelium. The best characterized ligand for selectins is P-selectin glycoprotein ligand-1 (PSGL-1), an extended homodimeric mucin on leukocytes that binds to all three selectins. Recent studies show that differential expression or glycosylation of PSGL-1 in different leukocytes mediates selective recruitment of different subsets of monocytes or lymphocytes to atherosclerotic arteries.
Introduction
Atherosclerosis is a chronic inflammatory disease characterized by leukocyte infiltration of the arterial vessel wall (Ross 1999, Libby 2002). Recruitment of leukocytes, such as monocytes, lymphocytes, and neutrophils, to the arterial vessel wall is the first step in the initiation of atherosclerosis (Ross 1999, Libby 2002). Leukocyte infiltration into the vessel wall is achieved through the well-defined dynamic adhesion cascade that includes the capture, rolling, slow rolling, firm adhesion, and transmigration of leukocytes. Capture (or tethering) is the first step of leukocyte adhesion, which functions to decelerate fast flowing leukocytes from the central blood stream and enables them to interact closely (rolling) with the activated endothelium and to survey for immobilized chemokines on the surface of activated endothelium. Rolling leukocytes transduce signals from adhesion receptors and chemokine receptors, which activate downstream adhesion molecules that mediate slow rolling and firm adhesion to the endothelium. Adherent leukocytes then infiltrate the arterial vessel wall (Ley et al. 2007). Each of these steps is mediated by the coordinated actions of different adhesion molecules. The interactions of selectins with their ligands mediate capture and most rolling events, whereas the interactions of members of the immunoglobulin family with leukocyte integrins mediate firm adhesion and migration (Ley et al. 2007). Chemokines and chemokine receptors are also important in leukocyte adhesion and migration (Olson et al. 2002, Zernecke et al. 2008). In blood flow conditions, especially arterial flow conditions, the initial capture and rolling steps, which are mediated by selectins and their ligands, serve as an anchoring system to capture flowing leukocytes and initiate rolling on the endothelium for subsequent firm adhesion and transmigration. Recently, studies have demonstrated that selectins and their ligands also play a crucial role in the selective recruitment of certain types of leukocytes into atherosclerotic lesions (An et al. 2008).
Selectin and their common ligands
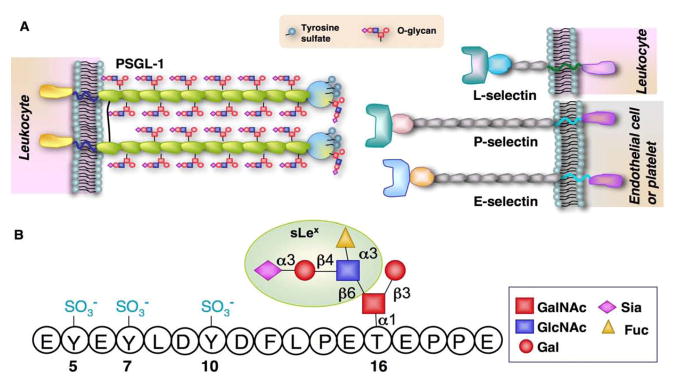
Selectins are a family of three calcium-dependent lectins that consist of P-, E-, and L-selectin. P-selectin and E-selectin are expressed on activated endothelial cells and/or platelets, whereas L-selectin is expressed on leukocytes. Each selectin mediates adhesion in part through interactions of its N-terminal lectin domain with a sialyl Lewis x (sLex) capping structure (NeuAcα2-3Galβ1-3[Fucα1-3]GlcNAcβ1-R) that is found on selectin ligands. All selectin ligands are cell-surface glycoproteins. Among them, P-selectin glycoprotein ligand 1 (PSGL-1) is the only selectin ligand that has been extensively characterized at the molecular, cellular, and functional levels (McEver et al. 1997). PSGL-1 is a transmembrane glycoprotein comprising extracellular, transmembrane, and cytoplasmic domains (McEver et al. 1997). The extracellular polypeptide of PSGL-1 is rich in threonines and serines to which O-linked oligosaccharides (O-glycans) are frequently attached (McEver et al. 1997). PSGL-1 interacts with all three selectins (Figure 1A). Its N-terminal region contains tyrosine sulfate residues and a core 2 O-glycan capped with sLex that are essential for binding to selectins (Figure 1B). The O-linked branching enzyme core 2 β1,6-glucosaminyltransferase-I (C2GlcNAcT-I) and a β1,4 galactosyltransferase-I (β1,4GalT-I) are essential for the formation of the branched core 2 O-glycan, while two α1,3-fucosyltransferases, FucT-VII and FucT-IV, and at least two sialyltransferases of the ST3Gal family, one of which is ST3Gal-IV, are required to form the sLex. Antibody-blocking studies and genetic deletion of PSGL-1 indicate that PSGL-1 is the only physiologically relevant ligand for P- and L-selectin on leukocytes. Modification by FucT-VII, C2GlcNAcT-I, β1,4GalT-I and at least one ST3Gal is essential for PSGL1 binding to P- and L-selectin. PSGL-1 is also able to bind to E-selectin, and this activity of PSGL1 requires modifications by FucT-VII and ST3Gal (McEver et al. 1997, Ley et al. 2004).
Figure 1.
A. Scheme of selectins and their common ligand PSGL-1. B. Schematic illustration of the selectin-binding epitopes at the N-termus of the human PSGL-1.
PSGL-1 is constitutively expressed on all leukocytes. However, its expression level and degree of glycosylation differ significantly in different subtypes of leukocytes. The differential expression and glycosylation has been found to contribute to selective recruitment of different types of leukocytes to atherosclerotic lesions (An et al. 2008, Wang et al. 2009).
PSGL-1 is expressed at a high level on Ly-6Chi monocytes, an inflammatory subset of monocytes, and mediates preferential homing of Ly-6Chi monocytes to the atherosclerotic vessel wall
Macrophages are the predominant cellular components in early atherosclerotic lesions (Ross 1999, Libby 2002). Lesion macrophages are differentiated from circulating blood monocytes, and macrophages in lesions behave differently. For example, macrophages adjacent to the cholesterol clefts and within the necrotic cores express high levels of tissue factor, whereas macrophages in the shoulders of lesions produce the enzymes myeloperoxidase and neutrophil elastase. In addition, macrophages within the advanced human atheroma appear to express high levels of apoE (Libby et al. 2008). The diverse activities of macrophages in atherosclerotic lesions suggest a lineal difference for macrophages in atherosclerotic lesions, which may result from the heterogeneity of circulating blood monocytes.
Blood monocytes are heterogeneous (Sunderkotter et al. 2004). Recently two main subsets of monocytes have been defined. Monocytes that are Ly-6Chi in mice and CD14+CD16− in humans are termed inflammatory monocytes. These cells are short-lived and usually home to inflamed tissue, where they release proinflammatory cytokines to initiate inflammation and trigger immune responses. Monocytes that are Ly-6Clo (in mice) and CD14hiCD16+ (in humans) are called resident monocytes. These cells have a longer half-life and home to non-inflamed tissues, where they patrol healthy tissues and differentiate into resident macrophages (Geissmann et al. 2003). Heterogeneous monocytes may not be differentiated from different lineages of myeloid precursors. A few studies have shown that Ly-6Chi monocytes are cells that are newly released from bone marrow. These cells mature in circulating blood and are gradually converted to Ly-6Clo monocytes (Tacke et al. 2007). In wild-type mice, the numbers of Ly-6Chi and Ly-6Clo monocytes are well balanced with a ratio of one to one. Interestingly, inflammatory stimuli, such as hypercholesterolemia, impair the conversion of Ly-6Chi to Ly-6Clo, thus increasing the number of circulating Ly-6Chi monocytes in mice (Tacke et al. 2007, Swirski et al. 2007).
It appears that there is a distinct difference in the profile of adhesion molecules and chemokine receptors on resident and inflammatory monocytes (Table). For example, mouse Ly-6Chi monocytes are CCR2+, CX3CR1lo, L-selectin+, CD44+, LFA1+, and VLA4+, whereas Ly-6Clo cells are CCR2−, CX3CR1hi, L-selectin−, CD44+, LFA1+, and VLA4+ 13. Ly-6Chi monocytes are preferentially recruited to atherosclerotic plaques and give rise to lipid-laden macrophages in apoE-deficient (ApoE−/−) mice (Swirski et al. 2007). Ly-6Chi monocytes are recognized as the key monocyte subset in the development of atherosclerosis, but the mechanism by which these monocytes selectively accumulate in atherosclerotic lesions is largely unknown. CCR2 is differentially expressed on Ly-6Chi cells and is important for monocytes to enter atherosclerotic lesions, but it does not mediate the early adhesion steps, such as tethering and initial rolling, which are the prerequisite for selective homing of leukocytes (Huo et al. 2001).
Table 1.
Homing molecules expressed on different monocyte subsets
| Monocyte subsets | Human monocyte | Murine monocyte | |||
|---|---|---|---|---|---|
| Adhesion cascade | CD14+ | CD16+ | Ly-6Chi | Ly-6Clo | |
| Tethering/rolling | PSGL-1 | ++ | + | ++ | + |
| L-selectin | + | − | + | − | |
| E-selectin ligand | + | ? | + | ? | |
| Adhesion | Mac-1 | + | + | + | + |
| LFA-1 | + | + | + | + | |
| VLA-1 | + | + | + | + | |
| VLA-4 | + | + | + | + | |
| Activation/migration | CCR1 | + | − | + | − |
| CCR2 | + | − | + | − | |
| CXCR1 | + | − | + | − | |
| CXCR2 | + | − | + | − | |
| CXCR4 | − | + | − | + | |
| CCR7 | + | − | + | − | |
| CX3CR1 | + | ++ | + | ++ | |
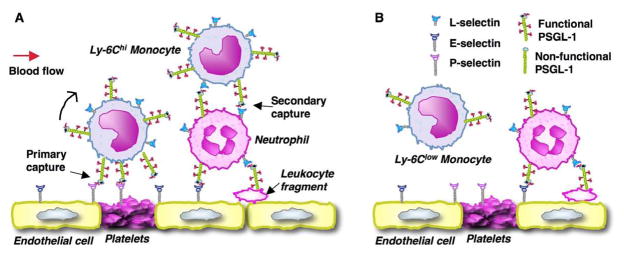
PSGL-1 expressed on monocytes is fully modified by glycosyltransferases and able to interact with selectins. However, recently we have found that Ly-6Chi monocytes express a higher level of PSGL-1 and have enhanced binding to the P-, E-, and L-selectins compared with Ly-6Clo monocytes (An et al. 2008). We demonstrate that PSGL-1 plays a key role in mediating the selective homing of Ly-6Chi to arterial walls in atherosclerosis (Figure 2A). First, PSGL-1 mediates Ly-6Chi monocyte homing via primary tethering. PSGL-1 on Ly-6Chi monocytes interacts with P-selectin either on activated endothelial cells, on neointimal smooth muscle cells (Zeiffer et al. 2004) or on activated platelets adhered to the injured arterial wall (Figure 2A). PSGL-1 may also mediate tethering and rolling on E-selectin on the activated endothelium, either independently or in cooperation with other leukocyte E-selectin ligand(s) (Xia et al. 2002). Second, secondary tethering may serve as another important mechanism for selective Ly-6Chi monocyte homing (Figure 2A). Secondary tethering occurs when a freely flowing leukocyte transiently interacts with a rolling or adherent leukocyte or adherent leukocyte fragments and subsequently rolls on the endothelium (Ley et al. 2007). L-selectin on Ly-6Chi monocytes interacts with PSGL-1 on another leukocyte to mediate Ly-6Chi monocyte secondary tethering. Neutrophils have a high level of PSGL-1 and are able to interact with atherosclerotic arteries (Eriksson et al. 2001). Owing to a much higher number of neutrophils than monocytes in circulation, in vivo there will be a high frequency of secondary tethering of a flowing Ly-6Chi monocyte on neutrophils rolling on or adherent to atherosclerotic arteries. Thus, PSGL-1, through a primary and a possible secondary capturing mechanism, selectively home Ly-6Chi monocytes to atherosclerotic sites. In contrast, Ly-6Clo monocytes express a low level of PSGL-1 and are impaired in these homing mechanisms (Figure 2B). In addition to its capturing function, PSGL-1 can induce leukocyte activation including calcium influx, activation of integrins and slow rolling (Wang HB et al. 2007, Zarbock et al. 2007). These effects may also contribute to PSGL-1 mediated selective recruitment of inflammatory monocytes to atherosclerotic arterial wall.
Figure 2.
Model for PSGL-1-mediated selective homing of monocytes. A. Ly-6Chi monocytes that are also PSGL-1hi and L-selectin+ preferentially interact with P- and E-selectin on activated endothelium/adherent platelets (primary tethering) or with L-selectin on a rolling/adherent leukocyte or a leukocyte fragment (a neutrophil in this case, secondary tethering) under flow. B. Ly-6Clo monocytes that are also PSGL-1lo and L-selectin− cannot efficiently interact with activated endothelium under flow because of low-level surface expression of PSGL-1, and, possibly, lack of L-selectin.
Atherosclerotic lesions are prone to develop at bifurcations, branchings, and curvatures where the shear stress ranges from 1 to 3 dyn/cm2 (Pantos et al. 2007). Neointimal lesions after wire injury develop at locations where shear stress can be much higher because of the angioplasty procedure. Thus, the high level of functional PSGL-1 may give Ly-6Chi monocytes a unique homing advantage under arterial flow conditions. Indeed, the lack of PSGL-1 protected ApoE−/− mice from developing severe wire injury–induced neointimal and atherosclerotic plaques, which may be attributable to diminished Ly-6Chi monocyte recruitment. Significantly, the lack of PSGL-1 provides ApoE−/− mice more protection from developing neointimas than atherosclerotic plaques, which supports the contention that PSGL-1 plays a more important role in the recruitment of monocytes in acute lesions than in chronic lesions (An et al. 2008).
Regulated glycosylation of PSGL-1 plays a key role in mediating activated lymphocyte selective homing to atherosclerotic vessels
In humans, T cells have long been found in atherosclerotic plaques (Jonasson et al. 1985). In mice lacking apolipoprotein E or the LDL receptor, T cells, especially CD4 T cells, are the predominant lymphocytes in atherosclerotic lesions (Roselaar et al. 1996). The presence of T cells in atherosclerotic lesions has a causative role in the formation of atherosclerotic lesions in mice. In loss-of-function experiments, mice with a global deficiency of adaptive immunity have decreased atherosclerosis. In contrast, the adoptive transfer of CD4 T cells to those immune-deficient mice accelerates the formation of atherosclerotic lesions (Zhou et al. 2000, Song et al. 2001), demonstrating that CD4 T cells play a pathogenic role in atherosclerosis.
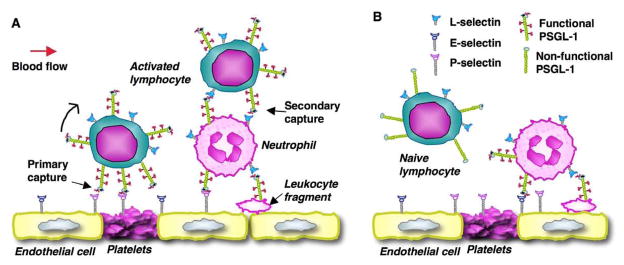
Most T cells found in lesions are effector or memory T cells, and the proportion of the activated T cells increases with the severity of atherosclerosis (Hansson et al. 1989). Naive T cells are rarely found in atherosclerotic plaques. All T cells express a similar level of PSGL-1, yet naïve T cells do not interact with P- and E-selectins on activated endothelium, because their PSGL-1 is not appropriately glycosylated and is thus not functional (Ley et al. 2004). However, naïve T cells can home to regional lymph nodes through PSGL-1-independent mechanism (L-selectin interacting with PNAd on the high endothelium of lymph nodes). In regional lymph nodes, naïve T cells interact with activated dendritic cells (DCs), which emigrated from atherosclerotic lesions (Robertson et al. 2006). The interactions of naïve T cells with DCs and local proinflammatory cytokines regulate glycosyltransferases that are related to selectin-ligand activity. For example, the expression of FucT-VII is upregulated through the T cell receptor, possibly through Ras, and by the ligation of interleukin-12 (IL-12R) (Barry et al. 2003). C2GlcNAcT-I expression is upregulated by IL-12 through a signal transducer and activator of transcription (STAT4) and by IL-2 and IL-4, possibly through STAT5 and STAT6 (Lim et al. 1999), respectively. Increased levels of FucT-VII and C2GlcNAcT-I transform PSGL1 to its functional form that binds to all three selectins. Therefore, regulated glycosylation of PSGl-1 is essential for PSGL-1 to mediate the selective recruitment of activated T cells to atherosclerotic lesions (Figure 3A).
Figure 3.
Model for PSGL-1-mediated selective homing of lymphocytes. A. Activated lymphocytes use mechanisms similar to that of Ly-6Chi monocytes to selectively interact with inflamed vessel wall. D. Naïve lymphocytes express a similar level, yet non-functional form of PSGL-1, thus do not interact with selectins.
Activated CD4 memory T cells of the T helper 1 and 2 (TH1, TH2)-cell types are found in atherosclerotic lesions (Roselaar et al. 1996). During early lesion formation, TH1 cells predominate over the TH2-cell subset, secrete IFNγ cytokine, and propagate adaptive immune reactivity through the induction of MHC class II on antigen-presenting cells and the stimulation of smooth muscle cell proliferation in vivo (Robertson et al. 2006). Deficiency of T-bet, a TH1-cell-associated transcription factor, leads to attenuated atherosclerosis, further demonstrating the proatherogenic effect of TH1 (Buono et al. 2005). With the progression of atherosclerosis, TH-cells in plaque diametrically balance, shifting from a TH1-cell type to a TH2-cell type. The latter produces cytokines, such as IL-4 and IL-10, and increases antibodies against oxLDL, which consequently suppresses atherosclerosis (Robertson et al. 2006). Fully glycosylated PSGL-1 on TH1 or TH2 cells interacts with the P- and E-selectins on atherosclerotic endothelium or on platelets or platelet microparticles adhering to the endothelium and mediates the selective recruitment of these cells to atherosclerotic lesions (Ley et al. 2004). In addition, neutrophils, monocytes, and their particles adhering to atherosclerotic endothelium present their PSGL-1 to T cells. Thus, PSGL-1 can interact with L-selectin on T cells to mediate T cell homing to atherosclerotic arteries (Ley et al. 2004) (Figure 3). ApoE−/− mice lacking C2GlcNAcT-I have a defect in PSGL-1 function. As a result, the number of T cells infiltrated in atherosclerotic lesions is dramatically decreased (Wang et al. 2008).
PSGL-1 in homing of other leukocytes to atherosclerotic arteries
Neutrophils and mast cells have been found in atherosclerotic lesions, and these cells contribute to the formation of atherosclerotic lesions (Weber et al. 2008). In atherosclerotic mice, immunostaining with antibodies specific to neutrophils has shown the presence of neutrophils in the luminal-plaque regions and also in the adventitia. Long-term disruption of the interactions between CXCR4 and its ligand CXCL12 (SDF1α) results in the homeostatic expansion of neutrophils in the circulation. This leads to an increase of neutrophil infiltration in atherosclerotic lesions and the aggravation of atherosclerosis. A neutrophil-neutralizing antibody reduced the circulating neutrophil number and consequently suppressed the exacerbation of atherosclerosis induced by CXCR4 deficiency (Zernecke et al. 2008). Thus, a role for neutrophils in the formation of atherosclerotic lesions is indicated. In ApoE−/− mice that are treated with cytokine TNFα or ILβ, neutrophils interact with the endothelium of large arteries or atherosclerotic arteries under intravital microscopy. The major types of interactions are capture and rolling, which are dependent on P- and E-selectins (Eriksson et al. 2000). Since PSGL-1 is the major ligand for the P- and E-selectins, PSGL-1 is presumed to be critically involved in neutrophil interactions with atherosclerotic arteries.
Mast cells in atherosclerotic lesions release TNFα and IL-6 and consequently contribute to the formation of atherosclerotic lesion and elastase-induced aortic aneurysms. In mast-cell-deficient mice, atherosclerosis is significantly suppressed (Sun et al. 2007). The molecular mechanism for mast cell homing to atherosclerotic arteries in vivo remains to be investigated. However, under in vitro flow conditions, cultured mast cells interact with the selectin-coated surfaces or activated endothelial monolayers through a PSGL-1-dependent mechanism (Steegmaier et al. 1997), indicating that mast cells may also use PSGL-1 to home to atherosclerotic arteries.
PSGL-1 as a therapeutic target for atherosclerosis and arterial injury
It has been demonstrated that a single injection of anti-PSGL-1-blocking antibody is able to dramatically inhibit the growth of arterial neointima following wire-induced arterial injury in ApoE−/− mice (Phillips et al. 2003). An even greater protection from arterial neointima formation after arterial injury is seen in ApoE/PSGL-1 double-deficient mice in our recent experiment (An et al. 2008). In addition, inhibiting the glycosylation of PSGL-1 by deleting FucT or C2GlcNAcT-I also ameliorates atherosclerosis and arterial neointima formation after arterial injury. FucT-IV deficiency results in a modest decrease in monocyte P-selectin ligand activity and is associated with a subtle decrement in atherosclerosis. FucT-VII deficiency substantially decreases the interactions of monocytes with selectin-coated surfaces under flow conditions and the size of atherosclerotic lesions in ApoE−/− mice (Homeister et al. 2004). C2GlcNAcT-I deficiency almost completely abrogates leukocyte binding to P- and E-selectin and decreases Ly-6C(hi) monocyte interactions with atherosclerotic arteries under physiological flow conditions. In ApoE−/− mice, the lack of C2GlcNAcT-I results in fewer and smaller atherosclerotic lesions in mouse aortas and a smaller arterial neointima after arterial injury compared with lesions and arterial neointima in control mice (Wang et al. 2008, 2009). PSGL-1 is also highly expressed on human CD14+CD16− monocytes (An et al. 2008). The glycosylation pathways are identical in humans and mice. In addition to its capturing and signaling functions, PSGL-1 also mediates chemokine presentation and further affect leukocyte recruitment (Veerman et al. 2007). Thus, PSGL-1 blockade and/or therapeutic interventions of glycosylation pathways represent potential therapeutic approaches for the treatment of atherosclerosis or restenosis after angioplasty.
Conclusion
Leukocyte recruitment to the arterial vessel wall initiates and mediates the progression of atherosclerosis. Recent studies have identified the involvement of different leukocyte subsets in the pathology of this disease. The selectin ligand PSGL-1 initiates leukocyte-endothelial interactions for all leukocytes. In vivo studies reveal that PSGL-1 plays a crucial role in the recruitment of pro-atherogenic leukocyte subsets to the arterial wall in mice, which is prerequisite in the formation and progression of atherosclerotic lesions. Thus, the inhibition of PSGL-1 function is a potential therapeutic approach to the treatment of atherosclerosis and arterial restenosis.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Reference List
- An G, Wang H, Tang R, et al. P-selectin glycoprotein ligand-1 is highly expressed on Ly-6Chi monocytes and a major determinant for Ly-6Chi monocyte recruitment to sites of atherosclerosis in mice. Circulation. 2008;117:3227–3237. doi: 10.1161/CIRCULATIONAHA.108.771048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barry SM, Zisoulis DG, Neal JW, et al. Induction of FucT-VII by the Ras/MAP kinase cascade in Jurkat T cells. Blood. 2003;102:1771–8. doi: 10.1182/blood-2002-11-3551. [DOI] [PubMed] [Google Scholar]
- Buono C, Binder CJ, Stavrakis G, et al. T-bet deficiency reduces atherosclerosis and alters plaque antigen-specific immune responses. Proc Natl Acad Sci U S A. 2005;102:1596–601. doi: 10.1073/pnas.0409015102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eriksson EE, Xie X, Werr J, et al. Direct viewing of atherosclerosis in vivo: plaque invasion by leukocytes is initiated by the endothelial selectins. FASEB J. 2001;15:1149–1157. doi: 10.1096/fj.00-0537com. [DOI] [PubMed] [Google Scholar]
- Geissmann F, Jung S, Littman DR. Blood monocytes consist of two principal subsets with distinct migratory properties. Immunity. 2003;19:71–82. doi: 10.1016/s1074-7613(03)00174-2. [DOI] [PubMed] [Google Scholar]
- Hansson GK, Holm J, Jonasson L. Detection of activated T lymphocytes in the human atherosclerotic plaque. Am J Pathol. 1989;135:169–75. [PMC free article] [PubMed] [Google Scholar]
- Homeister JW, Daugherty A, Lowe JB. Alpha(1,3)fucosyltransferases FucT-IV and FucT-VII control susceptibility to atherosclerosis in apolipoprotein E−/− mice. Arterioscler Thromb Vasc Biol. 2004;24:1897–903. doi: 10.1161/01.ATV.0000141844.28073.df. [DOI] [PubMed] [Google Scholar]
- Huo Y, Weber C, Forlow SB, et al. The chemokine KC, but not monocyte chemoattractant protein-1, triggers monocyte arrest on early atherosclerotic endothelium. J Clin Invest. 2001;108:1307–14. doi: 10.1172/JCI12877. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jonasson L, Holm J, Skalli O, et al. Expression of class II transplantation antigen on vascular smooth muscle cells in human atherosclerosis. J Clin Invest. 1985;76:125–31. doi: 10.1172/JCI111934. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ley K, Laudanna C, Cybulsky MI, et al. Getting to the site of inflammation: the leukocyte adhesion cascade updated. Nat Rev Immunol. 2007;7:678–89. doi: 10.1038/nri2156. [DOI] [PubMed] [Google Scholar]
- Ley K, Kansas GS. Selectins in T-cell recruitment to non-lymphoid tissues and sites of inflammation. Nat Rev Immunol. 2004;4:325–35. doi: 10.1038/nri1351. [DOI] [PubMed] [Google Scholar]
- Libby P. Inflammation in atherosclerosis. Nature. 2002;420:868–74. doi: 10.1038/nature01323. [DOI] [PubMed] [Google Scholar]
- Libby P, Nahrendorf M, Pittet MJ, et al. Diversity of denizens of the atherosclerotic plaque: not all monocytes are created equal. Circulation. 2008;117:3168–70. doi: 10.1161/CIRCULATIONAHA.108.783068. [DOI] [PubMed] [Google Scholar]
- Lim YC, Henault L, Wagers AJ, et al. Expression of functional selectin ligands on Th cells is differentially regulated by IL-12 and IL-4. J Immunol. 1999;162:3193–201. [PubMed] [Google Scholar]
- McEver RP, Cummings RD. Role of psgl-1 binding to selectins in leukocyte recruitment. [review] [70 refs] J Clin Invest. 1997;100:S97–S103. [PubMed] [Google Scholar]
- Olson TS, Ley K. Chemokines and chemokine receptors in leukocyte trafficking. Am J Physiol Regul Integr Comp Physiol. 2002;283:R7–R28. doi: 10.1152/ajpregu.00738.2001. [DOI] [PubMed] [Google Scholar]
- Pantos I, Patatoukas G, Efstathopoulos EP, et al. In vivo wall shear stress measurements using phase-contrast MRI. Expert Rev Cardiovasc Ther. 2007;5:927–38. doi: 10.1586/14779072.5.5.927. [DOI] [PubMed] [Google Scholar]
- Phillips JW, Barringhaus KG, Sanders JM, et al. Single injection of P-selectin or P-selectin glycoprotein ligand-1 monoclonal antibody blocks neointima formation after arterial injury in apolipoprotein E-deficient mice. Circulation. 2003;107:2244–9. doi: 10.1161/01.CIR.0000065604.56839.18. [DOI] [PubMed] [Google Scholar]
- Robertson AK, Hansson GK. T cells in atherogenesis: for better or for worse? Arterioscler Thromb Vasc Biol. 2006;26:2421–32. doi: 10.1161/01.ATV.0000245830.29764.84. [DOI] [PubMed] [Google Scholar]
- Roselaar SE, Kakkanathu PX, Daugherty A. Lymphocyte populations in atherosclerotic lesions of apoE −/− and LDL receptor −/− mice. Decreasing density with disease progression. Arterioscler Thromb Vasc Biol. 1996;16:1013–8. doi: 10.1161/01.atv.16.8.1013. [DOI] [PubMed] [Google Scholar]
- Ross R. Atherosclerosis--an inflammatory disease [see comments] N Engl J Med. 1999;340:115–26. doi: 10.1056/NEJM199901143400207. [DOI] [PubMed] [Google Scholar]
- Song L, Leung C, Schindler C. Lymphocytes are important in early atherosclerosis. J Clin Invest. 2001;108:251–9. doi: 10.1172/JCI11380. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Steegmaier M, Blanks JE, Borges E, et al. P-selectin glycoprotein ligand-1 mediates rolling of mouse bone marrow-derived mast cells on p-selectin but not efficiently on e- selectin. Eur J Immunol. 1997;27:1339–45. doi: 10.1002/eji.1830270607. [DOI] [PubMed] [Google Scholar]
- Sun J, Sukhova GK, Wolters PJ, et al. Mast cells promote atherosclerosis by releasing proinflammatory cytokines. Nat Med. 2007;13:719–24. doi: 10.1038/nm1601. [DOI] [PubMed] [Google Scholar]
- Sunderkotter C, Nikolic T, Dillon MJ, et al. Subpopulations of mouse blood monocytes differ in maturation stage and inflammatory response. J Immunol. 2004;172:4410–7. doi: 10.4049/jimmunol.172.7.4410. [DOI] [PubMed] [Google Scholar]
- Swirski FK, Libby P, Aikawa E, et al. Ly-6Chi monocytes dominate hypercholesterolemia-associated monocytosis and give rise to macrophages in atheromata. J Clin Invest. 2007;117:195–205. doi: 10.1172/JCI29950. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tacke F, Alvarez D, Kaplan TJ, et al. Monocyte subsets differentially employ CCR2, CCR5, and CX3CR1 to accumulate within atherosclerotic plaques. J Clin Invest. 2007;117:185–94. doi: 10.1172/JCI28549. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Veerman KM, Williams MJ, Uchimura K, et al. Interaction of the selectin ligand PSGL-1 with chemokines CCL21 and CCL19 facilitates efficient homing of T cells to secondary lymphoid organs. Nat Immunol. 2007;8:532–9. doi: 10.1038/ni1456. [DOI] [PubMed] [Google Scholar]
- Wang HB, Wang JT, Zhang L, et al. P-selectin primes leukocyte integrin activation during inflammation. Nat Immunol. 2007;8:882–892. doi: 10.1038/ni1491. [DOI] [PubMed] [Google Scholar]
- Wang H, Tang R, Zhang W, et al. Core2 1-6-N-Glucosaminyltransferase-I Is Crucial for the Formation of Atherosclerotic Lesions in Apolipoprotein E-Deficient Mice. Arterioscler Thromb Vasc Biol. 2008;29:180–7. doi: 10.1161/ATVBAHA.108.170969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang H, Zhang W, Tang R, et al. Core2 1-6-N-glucosaminyltransferase-I deficiency protects injured arteries from neointima formation in apoE-deficient mice. Arterioscler Thromb Vasc Biol. 2009;29:1053–9. doi: 10.1161/ATVBAHA.109.187716. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weber C, Zernecke A, Libby P. The multifaceted contributions of leukocyte subsets to atherosclerosis: lessons from mouse models. Nat Rev Immunol. 2008;8:802–15. doi: 10.1038/nri2415. [DOI] [PubMed] [Google Scholar]
- Xia L, Sperandio M, Yago T, et al. P-selectin glycoprotein ligand-1-deficient mice have impaired leukocyte tethering to E-selectin under flow. J Clin Invest. 2002;109:939–50. doi: 10.1172/JCI14151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zarbock A, Lowell CA, Ley K. Spleen tyrosine kinase Syk is necessary for E-selectin-induced alpha (L)beta(2) integrin-mediated rolling on intercellular adhesion molecule-1. Immunity. 2007;26:773–783. doi: 10.1016/j.immuni.2007.04.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zeiffer U, Schober A, Lietz M, et al. Neointimal smooth muscle cells display a proinflammatory phenotype resulting in increased leukocyte recruitment mediated by P-selectin and chemokines. Circ Res. 2004;94:776–784. doi: 10.1161/01.RES.0000121105.72718.5C. [DOI] [PubMed] [Google Scholar]
- Zernecke A, Shagdarsuren E, Weber C, et al. Chemokines in atherosclerosis: an update. Arterioscler Thromb Vasc Biol. 2008;28:1897–908. doi: 10.1161/ATVBAHA.107.161174. [DOI] [PubMed] [Google Scholar]
- Zernecke A, Bot I, Djalali-Talab Y, et al. Protective role of CXC receptor 4/CXC ligand 12 unveils the importance of neutrophils in atherosclerosis. Circ Res. 2008;102:209–17. doi: 10.1161/CIRCRESAHA.107.160697. [DOI] [PubMed] [Google Scholar]
- Zhou X, Nicoletti A, Elhage R, et al. Transfer of CD4(+) T cells aggravates atherosclerosis in immunodeficient apolipoprotein E knockout mice. Circulation. 2000;102:2919–22. doi: 10.1161/01.cir.102.24.2919. [DOI] [PubMed] [Google Scholar]