Abstract
Natural killer (NK) cells have a key role in the innate immune response against pathogens because of their cytotoxic properties and production of interferon-gamma (IFN-γ). Some insight into ruminant NK cell biology has been gained through the characterization of bovine NK cells as NKp46+/CD3− cells. However, ovine NK cells have been little studied because of the lack of specific antibodies. Most NK cells in humans and cattle express CD16. We found that an antibody against human CD16 that cross-reacts with bovine NK cells also recognizes cell populations in ovine peripheral blood mononuclear cells. Using double labelling with CD14 revealed the same profile as described in other species, and we identified a putative NK cell population. We therefore sorted this ovine CD16+/CD14− cell population and tested it for NK cell characteristics. More than 80% of sorted CD16+/CD14− cells expressed perforin. After a week of culture in the presence of IL-2 and IL-15, ovine CD16+/CD14− cells had become large cells with intra-cytoplasmic granules containing perforin, and the vast majority displayed an activated CD2−/low/CD25+/CD8+ phenotype, as observed for bovine NKp46+/CD3− cells. Moreover, these cells expressed transcripts for the NKp46 receptor, and were cytotoxic in a CD16-mediated redirected lysis assay against a murine cell line, P815, and in a direct lysis assay against the ovine cell line, IDO5. Finally, ovine CD16+/CD14− cells having expanded for 7 days in culture secreted IFN-γ in response to IL-12 in a dose-dependent manner. Taken together, these findings led us to conclude that the ovine CD16+/CD14− lymphocyte sub-population displays the phenotype and functional characteristics of NK cells.
Keywords: natural killer cell, ovine, perforin, cytotoxicity, interferon-gamma
1. INTRODUCTION
Natural killer (NK) cells are key cells in the innate immune system which provide early resistance to infection and tumour cell invasion through their effector functions that include cytotoxic activity and cytokine production [10, 17, 23]. These cells are widely distributed in the body [22] and during an infection or a tumour invasion, in response to type I interferons and chemokines, they can migrate to damaged tissues where they will kill cells displaying an abnormal expression of MHC class I, and enter the draining lymph nodes where they interact with dendritic cells and T lymphocytes [11, 31, 46]. They thus influence the development of the adaptive immune response and its Th1 orientation with interferon-gamma (IFN-γ) production [12, 35] which is fundamental for the resolution of many infections by intracellular pathogens.
The cytotoxic activity of NK cells and cytotoxic T lymphocytes (CTL) is rapid and efficient and is initiated via two main pathways i.e., those involving the ligation of death receptors or the exocytosis of the granules [9, 13, 42]. Both cell types can release molecules involved in the cytotoxicity process, including perforin, a pore-forming protein, which allows the entrance of different granzymes in the target cell cytoplasm. However, while cytotoxic molecules in CTL are synthesized only after activation following an encounter with their specific antigen [37], cytotoxic granules in NK cells are formed during cell development [25]. Although naive NK cells present these effector molecules, recent studies have shown that they need priming to respond more rapidly and powerfully to infections [7, 18, 28].
The vast majority of studies with NK cells have been performed with human and rodent cells, initially defined as CD3−/CD16 (FcγRIII)+/CD56+ cells and CD3−/NK1.1+ cells, respectively. NK cells have been much less characterized in other species, because of the lack of specific cell markers [8, 16, 34]. The generation of an antibody against the bovine NKp46 receptor [38] has led to significant progress in understanding NK cells in cattle [1, 4, 14]. In small ruminants such as sheep, only certain NK-like properties have been demonstrated in peripheral blood lymphocytes (PBL) and cells from the endometrium [27, 40, 43]. It was shown that ovine PBL display NK-like cytotoxic activity against human K562 target cells [43] and murine YAK cells [27]. Moreover, the lytic activity of ovine PBL and endometrial cells against a canine D17 cell line infected with bovine herpes virus-1 (BHV-1) was suppressed by perforin inhibitors and by an antibody inhibiting a Function Associated Molecule (FAM) expressed on NK cells of several species [5, 24, 26, 40]. However, no FAM+ cells were detected in the peripheral blood of sheep [40], and ovine NK cells have never been isolated and characterized to date.
To fill this gap, we established a strategy based on a combination of surface markers to characterize ovine NK cells. The CD16 (FcγRIII) receptor involved in antibody-dependent cell-mediated cytotoxicity (ADCC) is commonly used to characterize NK cell subsets; two functionally different subsets of NK cells have been defined in humans, according to the expression of CD16 and CD56 on these cells [10]. In cattle, we demonstrated that an anti-human CD16 mAb (KD1 clone) labels part of peripheral blood mononuclear cells (PBMC), and that 94% of NKp46+ cells express CD16 in peripheral blood and 84% in lymph nodes [3]. In the present study, we used this anti-CD16 mAb in combination with other antibodies against different markers, including CD14, to characterize ovine NK cells. Since CD16 is only expressed on CD14+ cells and a small population of CD14− cells, which does not correspond to T, B or γδ-T lymphocytes, we hypothesized that ovine CD16+/CD14− cells may represent NK cells. We compared the properties of these ovine cells with those of bovine NKp46+ cells [38] and found that both populations presented similar morphology, phenotype and effector functions as NK cells.
2. MATERIALS AND METHODS
2.1. Animals
The clinically healthy 4- to 8-month-old Préalpes/Île-de-France ewes and 6- to 10-month-old female prime Holstein cattle used in this study were reared in conventional but protected sanitary facilities (PFIE, INRA, F-37380 Nouzilly, France).
2.2. Antibodies
The monoclonal antibodies (mAbs) used in this study were against the following: human CD16 (clone KD1, IgG2a, [30]; clone GRM1, IgG2a, from SouthernBiotech, Birmingham, USA; clone 3G8, IgG1, from Beckman Coulter, Fort Collins, USA), bovine NKp46 (AKS1; IgG1, [38]), bovine CD5 (CC17; IgG1) and sheep CD8 (38.65; IgG2a) from Serotec (Oxford, UK). Bovine CD25 (CACT116A; IgG1), and CD14 (CAM36A; IgG1), B lymphocytes B-B4 (BAQ155A; IgG1), γδ-T lymphocytes TcR1-N7 (86D; IgG1), CD4 (GC50A1; IgM) and CD2 (MUC2A; IgG2a) cross-reacting with ruminant lymphocytes were all from VMRD (Pullman, USA). The anti-human perforin-FITC kit (δG9; IgG2b) was from BD Biosciences (Le pont de Claix, France). IgG1, IgG2a, IgM mouse isotype controls were from Dako (Golstrup, Denmark). Subtype-specific secondary antibodies conjugated with Tricolor (TC) or R-Phycoerythrin (PE) were from Caltag Laboratories (Cergy Pontoise, France). Goat anti-mouse IgG Fab’ secondary antibodies conjugated with Fluo Probe (FP) 488 were from Fluo Probes (Interchim, Montluçon, France) and Alexa Fluor (AF) 594 from Molecular Probes (Invitrogen, Cergy Pontoise, France).
2.3. Isolation of ovine CD16+/CD14− and bovine NKp46+ cells from PBMC
Blood samples from sheep and cattle were collected on EDTA vacutainers (BD Biosciences). PBMC were isolated as previously described [38] with the modification that ovine blood was diluted threefold with Alsever buffer (0.1 M D-glucose, 0.027 M sodium citrate, 0.07 M NaCl (pH 6.5)) before being layered on a density gradient (Histopaque d = 1.77; Sigma-Aldrich, Lyon, France). Ovine and bovine CD16+/CD14− cells were then isolated using a high speed cell-sorter (MoFlo® Beckman Coulter, Paris, France) after labelling PBMC with mAbs against CD16 and CD14; the labelling was revealed with subtype-specific secondary antibodies conjugated with PE or TC, respectively. The population isolated was 98% pure. Bovine NKp46+ cells were isolated by positive immunomagnetic selection as previously described [14, 38]. The cell purity in all experiments was over 96%.
2.4. Culture of ovine CD16+/CD14− cells and bovine NKp46+ cells
Bovine NKp46+ cells were expanded as previously described [2, 14] in RPMI 1640 medium supplemented with 60 μg/mL penicillin, 100 μg/mL streptomycin, 1 mM sodium pyruvate, non-essential amino acids, 50 μM 2-mercaptoethanol (all from GIBCO, Invitrogen, Eragny-sur-Oise, France), with 10% Foetal Calf Serum (FCS) from Sigma-Aldrich and 100 U/mL recombinant bovine (rb) IL-2 produced as previously described [2]. To expand ovine CD16+/CD14− cells, we tested bovine and human cytokines and found that only the addition of 20 ng/mL recombinant human (rh) IL-2 plus 20 ng/mL rhIL-15, (Immuno Tools Gmbh, Friesoythe, Germany) to the culture medium allowed the expansion of this cell population. Cells were cultured for 7–10 days in the previously described medium.
2.5. Cytospin preparation – Immunohistochemical analysis
About 3–4 × 105 freshly isolated PBMC or in vitro expanded cells were washed twice in PBS buffer and spotted on polylysine slides with a cytocentrifuge (Shandon, Runcorn, Cheshire, UK). Cells were stained with May–Grünwald–Giemsa (kit RAL, CML, Nemours, France). The spotted bovine NK cells were labelled with the AKS1 antibody revealed by a goat anti-mouse antibody conjugated to AlexaFluor 594 or labelled with anti-human perforin (kit BD Biosciences) according to the manufacturer’s protocol.
2.6. Cell labelling and flow cytometry
Single and multiple labelling of surface receptors was performed on ovine PBMC or CD16+/CD14− expanded cells against the molecules: CD16, CD14, CD5, BB4, TcR1-N7, CD4, CD2, CD8 and CD25. Bovine PBMC were double labelled with the anti-CD16 and anti-CD14 mAbs, and bovine CD16+/CD14− expanded cells were further labelled with the anti-NKp46 mAb. Subtype-specific secondary antibodies conjugated with TC or PE were used. Intracellular perforin labelling was performed with the perforin-FITC kit (clone δG9, IgG2b) and the Cytofix/Cytoperm and Permwash solutions from BD Biosciences. The samples were analyzed on a FACSCalibur flow cytometer (Becton Dickinson, Franklin Lakes, USA), equipped with Cellquest™ Pro software. Cell acquisitions were performed on 2–10 × 104 viable cells gated in the forward and side scatter plot for the phenotype characterization and on 1.5–2 × 105 gated ovine lymphocytes for the perforin assay.
2.7. Cytotoxicity assay
For the redirected lysis assay, the cytotoxic activity of ovine CD16+/CD14− expanded cells and bovine NKp46+ expanded cells was tested against a murine tumor cell line P815 which expresses Fcγ-receptors, allowing antibody linking for redirected lysis. The ovine CD16+/CD14− cytotoxic activity was also tested in a direct lysis assay against an ovine fibroblast cell line, IDO5 (initially originating from Rhône-Mérieux, kindly provided by C. Leroux, UMR754 INRA-ENVL-UCBL-EPHE, Lyon, France). Target cells were labelled with 5 μM CFDA-SE (Molecular Probes, Invitrogen) in PBS with 0.2% BSA for 10 min followed by the addition of 5 mL cold RPMI and incubation on ice for 5 min. They were washed three times in RPMI and immediately used in the cytotoxicity assay. For redirected lysis, the target cells were preincubated with undiluted supernatant of the KD1 hybridoma for 5 min. Ovine CD16+/CD14− and bovine NKp46+ cells were seeded in a 96-well micro plate with a constant number of target cells (50 000) over a range of effector:target (E:T) ratios (4:1, 2:1, 1:1, 0.5:1 and 0.25:1). Cells were incubated in a total volume of 200 μL RPMI supplemented with 10% FCS for 1.5 h in a 5% CO2 atmosphere at 37 °C. Cells were then washed with PBS containing 0.2% BSA, and then incubated in the same buffer containing 3 μM propidium iodide (PI) (Molecular Probes, Invitrogen) for 10 min at room temperature in the dark, and analyzed immediately. Target cells incubated without NK cells and stained with PI served as controls to measure basal cell death rate. Cell samples were kept on ice until flow cytometry analysis when 2 × 104 target cells were analyzed for each E:T ratio. The percentage of specific lysis was calculated as follows: percentage of deaths of target cells when co-cultured with NK cells minus percentage of spontaneous deaths of target cells.
2.8. IFN-γ ELISA
Ovine CD16+/CD14− cells were pre-cultured for 7–10 days in the presence of IL-2 and IL-15 to assess their IFN-γ secretion. Triplicates of 105 cells were incubated for 20 h in the presence of different rhIL-12 concentrations (Immuno Tools) (0, 100, 500, 1 000 pg/mL) already tested for bovine NK cells [14]. IFN-γ production in the supernatants was measured by ELISA as previously described [41] using antibody pairs raised against bovine IFN-γ, already reported to recognize ovine IFN-γ [33]. Briefly, Maxisorp plates (NUNC, Roskilde, Denmark) were pre-coated overnight in PBS with an anti-bovine IFN-γ mAb (CC330; IgG1, Serotec), then incubated for 1 h with a blocking buffer containing 0.05% Tween, 1% BSA and 5% sucrose in PBS. A biotinylated anti-bovine IFN-γ mAb (CC302; IgG1, Serotec) was used to detect IFN-γ capture. Concentrations were calculated from a standard dilution curve of purified bovine IFN-γ (Perbio, Brébières, France).
2.9. mRNA extraction and real-time RT-PCR
The expression of NKp46 was detected in ovine cells by real time reverse transcriptase PCR. mRNA was extracted using the Nucleospin RNA II System (Macherey-Nalgen, Düren, Germany) according to the manufacturer’s protocol. The amount of purified mRNA recovered was measured using a NanoDrop spectrophotometer, and then reverse transcribed using oligo (dT) primers and Moloney murine leukaemia virus reverse transcriptase, according to the manufacturer’s instructions (Eurogentec, Angers, France). Synthesized cDNA was then amplified by real-time PCR. Hypoxanthine phosphoribosyltransferase (HPRT) mRNA levels were used to normalize RNA quantification. mRNA levels for the NKp46 gene were quantified by SYBR Green incorporation using an iCycler thermocycler (Bio-rad, Marnes-la-Coquette, France). The primers used for mRNA quantification were as follows: NKp46 forward: 5′-CCTGTGAAGCTCCTGGTCAA-3′ and reverse: 5′-CAGGACAGCCAGGCCAAGC-3′; HPRT forward: 5′-AAACCAAAGATGGTCAAGGT-3′ and reverse: 5′-TCTTAGGCTTTGTATTTTGCTT-3′.
3. RESULTS
3.1. Anti-human CD16 mAb labels three subsets of ovine and bovine PBMC
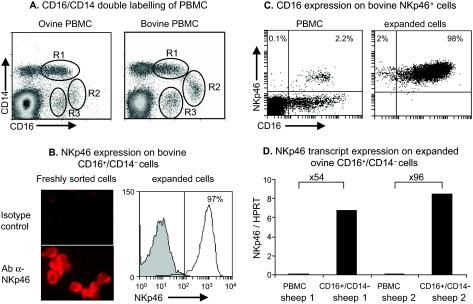
We first analyzed the cross-reactivity of human CD16 mAbs (clones KD1, GRM1 and 3G8) on ovine PBMC and found that only the clone KD1 cross-reacted with ovine PBMC. We found that 7.5–12.6% of ovine PBMC were CD16+ and this range was of the same order of magnitude as that observed with bovine PBMC (8–11%) (Tab. I). To find out which lymphocyte subsets express CD16, we double labelled ovine PBMC with the anti-CD16 mAb and anti T, B and γδ-T lymphocyte markers. Since none of these lymphocyte populations expressed CD16 (Tab. I) we next investigated the expression of CD16 on monocytes that are known to express CD14. Among the CD14-positive cells, two different CD16-positive subsets could be distinguished on both ovine and bovine PBMC. The CD16+/CD14high subset, defined as the R1 region, represented the largest subset (2.4–5.9% of ovine and 4.7–7.0% of bovine PBMC) while the CD16+/CD14low subset (R2 region) represented only 0.4–1.4% of ovine and 0.2–2.2% of bovine PBMC on the dot plots (Fig. 1A). The May–Grünwald–Giemsa staining on sorted cells revealed that both subsets presented monocyte morphology (data not shown). The last population (R3 region) expressed CD16 but not CD14, and represented 0.9–3.5% of ovine and 0.6–2.7% of bovine PBMC (Fig. 1A). Since the percentages of CD16+/CD14− cells were of the same order of magnitude in both species and since it is also reported for humans that the majority of blood NK cells express CD16 [10] but do not express the monocyte marker CD14, we hypothesized that this ovine cell subtype might be NK cells.
Table I.
Expression of CD16 on total ovine blood lymphocytes and different lymphocyte subsets. PBMC were double labelled with anti-human CD16 (clone KD1 antibody) and anti-CD4, pan T cell (-CD5), B cell (-BB4), γδ Tcell (-TcR1-N7) mAbs. N = number of animals investigated.
| CD16+ |
CD16+/CD5+ |
CD16+/BB4+ |
CD16+/TcR1-N7+ |
CD16+/CD4+ |
||
|---|---|---|---|---|---|---|
| Ovine | Bovine | Ovine | ||||
| Median (%) | 9.1 | 10.2 | 0.1 | 0.2 | 0.04 | 0.3 |
| Range | (7.5–12.6) | (8.1–11) | (0.1–0.3) | (0.04–0.4) | (0.00–0.18) | (0.06–0.5) |
| N | 8 | 6 | 6 | 8 | 8 | 8 |
Figure 1.
Analysis of CD16 expression on PBMC and NKp46+ cells, and NKp46 expression on CD16+/CD14− cells. (A) PBMC freshly obtained from sheep and cattle were double labelled with anti-CD16 and anti-CD14 mAbs. Three populations of CD16+ cells were defined: the first expressed CD14 at a high level (R1: CD16+/CD14high), the second expressed a low level of CD14 (R2: CD16+/CD14low), and the last did not express CD14 (R3: CD16+/CD14−). Data shown are from 1 animal representative of 8 sheep and 4 cattle. (B, left) Freshly sorted bovine CD16+/CD14− cells were spotted on slides and labelled with an anti-NKp46 mAb or isotype control mAb. (B, right), bovine expanded CD16+/CD14− cells were labelled with the NKp46 mAb (solid line, empty histogram) or the isotype control (shaded histogram); the plot is representative of 2 animals. (C) Bovine PBMC and sorted then expanded NKp46+cells were double labelled with anti-NKp46 and anti-CD16 mAbs and analyzed by flow cytometry. Numbers indicate percentages of positive cells in each quadrant. Data shown are from 1 animal representative of 4. (D) The NKp46 gene transcripts in ovine CD16+/CD14− cultured cells or PBMC were quantified by real-time RT-PCR. Values are the relative NKp46 mRNA to HPRT mRNA ratios. Increases (-fold) in the level of NKp46 expression between PBMC and CD16+/CD14− cells are indicated above the bars for each animal.
3.2. Ovine CD16+/CD14− cells are large granular lymphocytes expressing NKp46 and perforin
We first verified that freshly sorted bovine CD16+/CD14− cells expressed the NK cell marker NKp46 by immuno-labelling of cytospots (Fig. 1B) and found that more than 95% of the cells expressed this marker. After 7–10 days of expansion, flow cytometry analysis revealed that more than 95% of the cells were still NKp46+ (Fig. 1B). We next analyzed the expression of CD16 on bovine NKp46+ cells among PBMC and in sorted NKp46+ cells expanded in the presence of IL-2. In both cases, more than 95% of the cells expressed CD16 (Fig. 1C), suggesting that bovine NKp46+ cells and CD16+/CD14− cells are two overlapping populations. Since antibodies against NKp46 are not yet available for sheep, we verified that ovine CD16+/CD14− cells expressed NKp46 by real time quantitative RT-PCR. In the two sheep examined, the CD16+/CD14− cells represented 3% and 2% of PBMC and, once isolated and expanded in vitro, these cells expressed 54- and 96-fold more NKp46 transcripts respectively than PBMC (Fig. 1D).
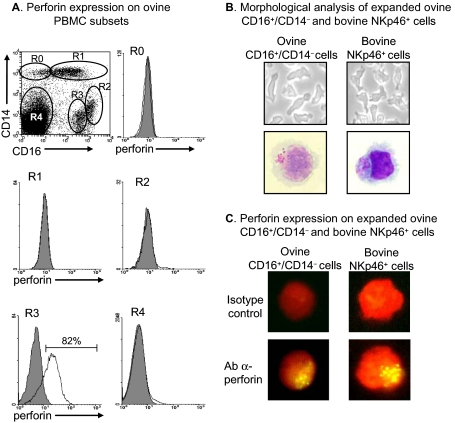
Another characteristic of NK cells is the presence of intracellular granules which contain perforin and granzymes involved in the cytotoxicity process. We therefore analyzed the expression of perforin in different ovine PBMC subsets by performing triple labelling with the anti-CD16, anti-CD14 and anti-perforin mAbs (Fig. 2A). Only the CD16+/CD14− subset (R3 region) presented clear intracellular staining for perforin, with over 80% of positive cells. In a few CD16−/CD14− cells (R4 region), representing less than 2% of this cell population, a lower level of perforin was also detected. We also verified the morphology of ovine CD16+/CD14− cells by comparing them to bovine NKp46+ cells. Ovine CD16+/CD14− cells expanded as non-adherent cells with prominent lamellipodia (Fig. 2B) as do bovine NKp46+ cells. On cytospots stained with May–Grünwald–Giemsa, both ovine CD16+/CD14− cells and bovine NKp46+ cells were large granular lymphocytes containing acidophilic granules co-localized at one pole of the cell (Fig. 2B). We finally demonstrated by immunocytology that, as for bovine NKp46+ cells, the granules of ovine CD16+/CD14− cells contained perforin (Fig. 2C).
Figure 2.
Perforin content and morphology of ovine CD16+/CD14− cells; comparison with bovine NKp46+ cells. (A) PBMC freshly obtained from sheep were triple labelled with the anti-CD16, anti-CD14 and anti-perforin mAbs: five different subsets (R0 to R4) were analyzed for perforin expression according to CD16 and CD14 expression (solid line, empty histogram). Shaded histograms represent labelling with the isotype control. Data shown are from 1 animal representative of 4. (B, top) Phase-contrast microscopy and (B, bottom) May–Grünwald–Giemsa staining of expanded ovine CD16+/CD14− and bovine NKp46+ cells. Pictures shown are from 1 culture representative of 6 for ovine cells, and more than 10 for bovine cells. (C) Perforin labelling of expanded ovine CD16+/CD14− and bovine NKp46+ cells counterstained with Evans blue. The cells shown are from 1 culture representative of 4 for both species.
3.3. The majority of the expanded CD16+/CD14− ovine cells present a CD2low CD8+ CD25+ phenotype
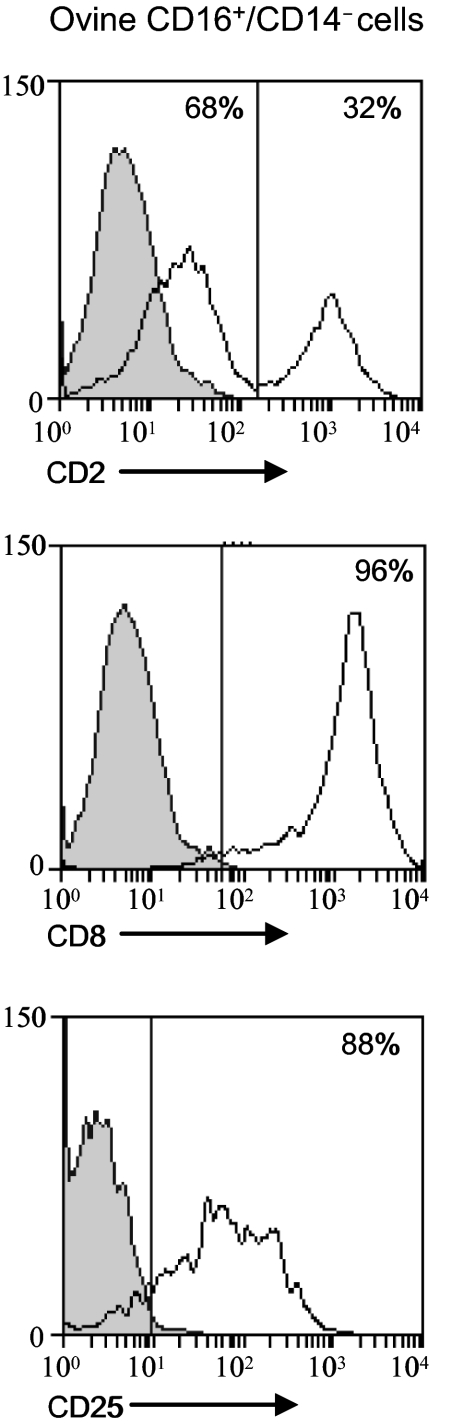
We analyzed the expression of the CD2 and CD25 markers related to proliferation and activation on the expanded ovine CD16+/CD14− cells. Two distinct CD2low and CD2+ cell populations were observed, with a majority of CD2low cells. Moreover, about 95% of the cells were CD8+ and 90% CD25+ (Fig. 3).
Figure 3.
Phenotype of expanded ovine CD16+/CD14− cells. Ovine CD16+/CD14− cells expanded in the presence of IL-2 and IL-15 were labelled with anti-CD2, -CD8 or -CD25 mAbs (solid lines and white histograms) or with isotype control mAbs (shaded histograms). Data shown are from 1 animal representative of 4. Numbers at the top of histograms indicate the percentages of cells labelled with the antibody indicated among the PBMC in the quadrants.
3.4. Ovine CD16+/CD14− cells have cytotoxic properties in direct and redirected lysis assays
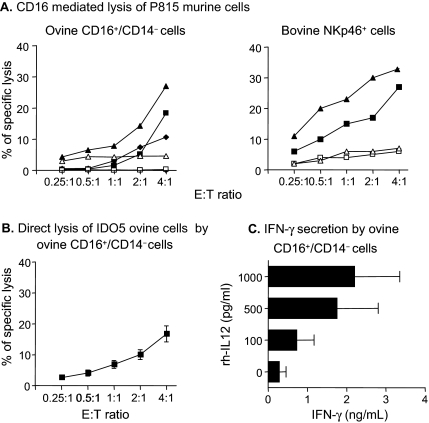
The cytotoxic activity of expanded ovine CD16+/CD14− cells was investigated by performing a redirected lysis assay using the anti-CD16 antibody and P815 murine target cells which bear an Fc receptor. Both CD16+/CD14− ovine cells and NKp46+ bovine cells displayed cytotoxic activity against P815 target cells related to the effector to target cell ratio (Fig. 4A). No or low levels of lysis were observed in the presence of the isotype control, indicating the specific involvement of the CD16 receptor in this test.
Figure 4.
Functional properties of ovine CD16+/CD14− cells. (A) CD16-mediated lysis of murine P815 target cells bearing an Fcγ receptor by ovine CD16+/CD14− cells or by bovine NKp46+ cells in the presence of an anti-human CD16 mAb (KD1 clone) (filled symbols) or its isotype control (open symbols). Data presented are from 3 sheep and 2 cattle. Each symbol represents the cytotoxicity of NK cells from 1 animal. (B) Direct lysis of ovine fibroblast IDO5 target cells by ovine CD16+/CD14− cells. Data presented are means ± SEM from 8 sheep. (C) IFN-γ release in the supernatants of the cultured CD16+/CD14− cells after 20 h of stimulation with different levels of rhIL-12. Data shown are means ± SEM from 6 sheep.
To study the cytotoxicity of CD16+/CD14− ovine cells under more normal physiological conditions, we used a direct lysis assay against the ovine epithelial cell line IDO5. Ovine CD16+/CD14− cells displayed cytotoxic activity which increased with the effector to target cell ratio (Fig. 4B).
3.5. Ovine CD16+/CD14− cells produce IFN-γ in an IL-12 dose-dependent manner
In addition to cytotoxicity, the production of IFN-γ is another characteristic of NK cells [19]. We therefore assessed the IFN-γ-production by ovine CD16+/CD14− cells in the absence or presence of different concentrations of rhIL-12. As shown in Figure 4C, CD16+/CD14− cells produced IFN-γ in an IL-12 dose-dependent manner.
4. DISCUSSION
NK cells have never been characterized in small ruminants. Since antibodies specifically directed against sheep NK cells are not yet available, only a few studies have reported on NK-like functional cell properties in PBL or uterine mucosal lymphocytes [15, 27, 40, 43]. The aim of the study presented here was to find a method to purify ovine NK cells from PBMC based on the combination of cell surface markers. In cattle, NK cells have been described as NKp46+/CD3− [38], the majority of which are CD16+ cells [3]. We tested the cross-reactivity of three human CD16 (FcγRIII) antibodies with ovine PBMC and found that only the mAb used to identify CD16 on bovine cells (clone KD1) also cross-reacted with ovine PBMC, and the patterns of labelling for ovine cells were similar to those of bovine cells. Indeed, CD16 (FcγRIII) is an important receptor expressed on NK cells in several species. In human blood, about 90% of NK cells express CD16 [10]. CD16 is one of the markers used to define NK cells in the pig [21], and the anti-human CD16 mAb (KD1 clone) identifies around 95% of NK cells from peripheral blood in cattle [3]. However, monocytes can also express this marker at different intensities according to their degree of maturity. Two monocyte subsets, CD16+/CD14high and CD16+/CD14low, corresponding to maturing and mature monocytes, respectively, have been described in humans [44], and a subset homologous to the human CD16+/CD14+ population has been identified in the pig and mouse [20, 36, 39]. We performed double labelling with anti-CD16 and anti-CD14 mAbs to distinguish monocytes expressing CD16 from putative NK cells, and this allowed us to identify three different populations. The CD16+/CD14high and CD16+/CD14low cells had a monocyte morphology on May–Grünwald–Giemsa staining, and most probably correspond to maturing and mature monocytes, respectively, as described in humans by Ziegler-Heitbrock et al. [44, 45]. The third population consisted of CD16+/CD14− cells which expressed NKp46 transcripts and was present at a similar proportion as the NKp46+ subset in bovine PBMC.
To define the ovine CD16+/CD14− population more precisely, we compared its properties with those of CD16+/CD14− cells and NKp46+ cells isolated from cattle blood. We found that freshly sorted bovine CD16+/CD14− cells were all NKp46+. This was also the case after 7–10 days’ expansion of the bovine cells, and there were neither CD4+ nor γδ+ T lymphocytes in the culture of CD16+/CD14− ovine cells (data not shown). Moreover, the expanded ovine CD16+/CD14− and bovine NKp46+ cells had the same morphological characteristics; the cells were large granular lymphocytes which grew in culture as non-adherent cells with prominent lamellipodia, as already described [38]. In addition, more than 80% of CD16+/CD14− cells expressed perforin, an important component for NK cell cytotoxic properties. Two percent of the CD16−/CD14− population also contained perforin; it cannot be excluded that NK cells were present in this population, though in other species these cells are considered to be most likely memory [32] or activated [37] CD8+ T lymphocytes. Ovine CD16+/CD14− cells expressed perforin after in vitro expansion, and most of the cells presented a CD2low/CD8+/CD25+ phenotype, in agreement with our previous studies on bovine NK cells [2, 3, 14]. Taken together, this first set of findings led us to conclude that the CD16+/CD14− population had the morphology and phenotype characteristics of NK cells.
We examined the cytotoxicity of the putative ovine NK cells by two methods. The direct lysis assay evaluates the natural cytotoxicity of the cells against allogenic targets even though the receptors involved have not been studied. The redirected lysis assay, in which the triggering of NK cells is induced by antibodies against specific receptors, describes the ability of the involved receptor to activate the lysis and the ability of the cells to kill the targets; this test can be more powerful than the direct lysis, depending on the engaged receptor [2, 29, 30]. In the present study, the CD16+/CD14− population exhibited cytotoxic activity that was dependent on the effector to target cell ratio, both in a CD16-mediated redirected lysis assay against a murine cell line and in a direct lysis assay against the IDO5 ovine cell line. These results were in line with results previously reported for bovine NK cells [2, 3, 14]. Nevertheless, the activation of a natural cytotoxicity receptor (NCR) such as NKp46 leads to a stronger cytotoxicity than the CD16 receptor activation [3]. However, the level of cytotoxicity of the ovine CD16+/CD14− cells did not seem very high, which could be explained by a low expression of receptors such as NCR and (in the case of a direct allogenic lysis) by the lineage of the target line (ovine IDO5 fibroblasts).
NK cells produce IFN-γ in response to infection, and this secretion may be dependent on stimulation by cytokines such as IL-12 produced by dendritic cells [19]. We found that ovine CD16+/CD14− cells produced IFN-γ in an IL-12 dose-dependent manner. However, we noted that in the same conditions of IL-12 stimulation ovine cells produced about 10-fold less IFN-γ than previously reported for bovine NKp46+ cells [14]. The difference observed might be due to the cytokines used to expand the cells (IL-2 for bovine cells versus IL-2 plus IL-15 for ovine cells). Indeed, in a previous study we showed that bovine NKp46+ cells expanded in the presence of IL-15 produced no or very little IFN-γ, whatever the IL-12 concentration used to stimulate them, while IL-2-expanded bovine NKp46+ cells produced large amounts of IFN-γ in an IL-12 dose-dependent manner [14]. We compared the IFN-γ production by ovine cells expanded in the presence of IL-2 alone or IL-2 plus IL-15 for the last three days but we could not detect any difference (data not shown). Another possibility could also be a difference in sensitivity of the ELISA quantification because of the use of antibodies produced against bovine IFN-γ to detect ovine IFN-γ or the use of heterologous rhIL-12.
In conclusion, we show that ovine CD16+/CD14− cells have morphological and functional characteristics similar to those of bovine NK cells. The possibility of isolating and growing pure ovine NK cell cultures provides an important opportunity to study the involvement of ovine NK cells in emerging infectious diseases such as Bluetongue [6]. Moreover, this strategy for isolation of NK cells might be applied to other species for which NK antibodies are not yet available.
Acknowledgments
We thank T. Chaumeil, R. Gelineau, D. Leguéré, M. Maillon, C. Mouazé, D. Serreau (INRA PFIE) for the blood samples, D. Raine (Dorking, Surrey, UK) for revising the manuscript, and D. Kerboeuf (INRA, Cytometry laboratory) for advice. We thank H. Watier (Faculty of medicine, Tours, France) for providing antibody samples. This work was supported by INRA and Région Centre (fellowship to J. Elhmouzi-Younes) and EGIDE (Aurora project 15824YG and 181604/V11).
References
- 1.Boysen P., Klevar S., Olsen I., Storset A.K., The protozoan Neospora caninum directly triggers bovine NK cells to produce gamma interferon and to kill infected fibroblasts, Infect. Immun. (2006) 74:953–960 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Boysen P., Olsen I., Berg I., Kulberg S., Johansen G.M., Storset A.K., Bovine CD2−/NKp46+ cells are fully functional natural killer cells with a high activation status, BMC Immunol. (2006) 7:10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Boysen P., Gunnes G., Pende D., Valheim M., Storset A.K., Natural killer cells in lymph nodes of healthy calves express CD16 and show both cytotoxic and cytokine-producing properties, Dev. Comp. Immunol. (2008) 32:773–783 [DOI] [PubMed] [Google Scholar]
- 4.Boysen P., Storset A.K., Bovine natural killer cells, Vet. Immunol. Immunopathol. (2009) 130:163–177 [DOI] [PubMed] [Google Scholar]
- 5.Camenisch T.D., Jaso-Friedmann L., Evans D.L., Harris D.T., Expression of a novel function-associated molecule on cells mediating spontaneous cytolysis in swine, Dev. Comp. Immunol. (1993) 17:277–282 [DOI] [PubMed] [Google Scholar]
- 6.Carpenter S., Wilson A., Mellor P.S., Culicoides and the emergence of Bluetongue virus in northern Europe, Trends Microbiol. (2009) 17:172–178 [DOI] [PubMed] [Google Scholar]
- 7.Chaix J., Tessmer M.S., Hoebe K., Fuseri N., Ryffel B., Dalod M., et al. , Cutting edge: priming of NK cells by IL-18, J. Immunol. (2008) 181:1627–1631 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Charley B., Fradelizi D., Differential effects of human and porcine interleukin 2 on natural killing (NK) activity of newborn piglets and adult pigs lymphocytes, Ann. Rech. Vet. (1987) 18:227–232 [PubMed] [Google Scholar]
- 9.Chavez-Galan L., Arenas-Del Angel M.C., Zenteno E., Chavez R., Lascurain R., Cell death mechanisms induced by cytotoxic lymphocytes, Cell Mol. Immunol. (2009) 6:15–25 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Cooper M.A., Fehniger T.A., Caligiuri M.A., The biology of human natural killer-cell subsets, Trends Immunol. (2001) 22:633–640 [DOI] [PubMed] [Google Scholar]
- 11.Cooper M.A., Fehniger T.A., Fuchs A., Colonna M., Caligiuri M.A., NK cell and DC interactions, Trends Immunol. (2004) 25:47–52 [DOI] [PubMed] [Google Scholar]
- 12.Corthay A., A three-cell model for activation of naive T helper cells, Scand. J. Immunol. (2006) 64:93–96 [DOI] [PubMed] [Google Scholar]
- 13.Cullen S.P., Martin S.J., Mechanisms of granule-dependent killing, Cell Death Differ. (2008) 15:251–262 [DOI] [PubMed] [Google Scholar]
- 14.Elhmouzi-Younes J., Storset A.K., Boysen P., Laurent F., Drouet F., Bovine neonate natural killer cells are fully functional and highly responsive to interleukin-15 and to NKp46 receptor stimulation, Vet. Res. (2009) 40:54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Entrican G., Wheelhouse N.M., Immunity in the female sheep reproductive tract, Vet. Res. (2006) 37:295–309 [DOI] [PubMed] [Google Scholar]
- 16.Evans D.L., Jaso-Friedmann L., Natural killer (NK) cells in domestic animals: phenotype, target cell specificity and cytokine regulation, Vet. Res. Commun. (1993) 17:429–447 [DOI] [PubMed] [Google Scholar]
- 17.Farag S.S., Caligiuri M.A., Human natural killer cell development and biology, Blood Rev. (2006) 20:123–137 [DOI] [PubMed] [Google Scholar]
- 18.Fehniger T.A., Cai S.F., Cao X., Bredemeyer A.J., Presti R.M., French A.R., Ley T.J., Acquisition of murine NK cell cytotoxicity requires the translation of a pre-existing pool of granzyme B and perforin mRNAs, Immunity (2007) 26:798–811 [DOI] [PubMed] [Google Scholar]
- 19.Ferlazzo G., Pack M., Thomas D., Paludan C., Schmid D., Strowig T., et al. , Distinct roles of IL-12 and IL-15 in human natural killer cell activation by dendritic cells from secondary lymphoid organs, Proc. Natl. Acad. Sci. USA (2004) 101:16606–16611 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Geissmann F., Jung S., Littman D.R., Blood monocytes consist of two principal subsets with distinct migratory properties, Immunity (2003) 19:71–82 [DOI] [PubMed] [Google Scholar]
- 21.Gerner W., Kaser T., Saalmuller A., Porcine T lymphocytes and NK cells – an update, Dev. Comp. Immunol. (2009) 33:310–320 [DOI] [PubMed] [Google Scholar]
- 22.Gregoire C., Chasson L., Luci C., Tomasello E., Geissmann F., Vivier E., Walzer T., The trafficking of natural killer cells, Immunol. Rev. (2007) 220:169–182 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Hamerman J.A., Ogasawara K., Lanier L.L., NK cells in innate immunity, Curr. Opin. Immunol. (2005) 17:29–35 [DOI] [PubMed] [Google Scholar]
- 24.Harris D.T., Jaso-Friedmann L., Devlin R.B., Koren H.S., Evans D.L., Identification of an evolutionarily conserved, function-associated molecule on human natural killer cells, Proc. Natl. Acad. Sci. USA (1991) 88:3009–3013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Kam C.M., Hudig D., Powers J.C., Granzymes (lymphocyte serine proteases): characterization with natural and synthetic substrates and inhibitors, Biochim. Biophys. Acta (2000) 1477:307–323 [DOI] [PubMed] [Google Scholar]
- 26.Kapur R., Evans D.L., Harris D.T., Evolutionary conservation of a human function-associated molecule on murine natural killer cells: expression and function, Scand. J. Immunol. (1994) 40:50–56 [DOI] [PubMed] [Google Scholar]
- 27.Liu W.J., Hansen P.J., Effect of the progesterone-induced serpin-like proteins of the sheep endometrium on natural killer cell activity in sheep and mice, Biol. Reprod. (1993) 49:1008–1014 [DOI] [PubMed] [Google Scholar]
- 28.Lucas M., Schachterle W., Oberle K., Aichele P., Diefenbach A., Dendritic cells prime natural killer cells by trans-presenting interleukin 15, Immunity (2007) 26:503–517 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Melioli G., Prigione I., Merli A., Cantoni C., Chen Q., Machi A.M., Ferrini S., Induction of functional activities of human lymphocytes by monoclonal antibodies, Ann. Ist. Super. Sanita (1991) 27:79–85 [PubMed] [Google Scholar]
- 30.Moretta A., Tambussi G., Ciccone E., Pende D., Melioli G., Moretta L., CD16 surface molecules regulate the cytolytic function of CD3CD16+ human natural killer cells, Int. J. Cancer (1989) 44:727–730 [DOI] [PubMed] [Google Scholar]
- 31.Moretta A., Natural killer cells and dendritic cells: rendezvous in abused tissues, Nat. Rev. Immunol. (2002) 2:957–964 [DOI] [PubMed] [Google Scholar]
- 32.Niiya H., Sakai I., Lei J., Azuma T., Uchida N., Yakushijin Y., et al. , Differential regulation of perforin expression in human CD4+ and CD8+ cytotoxic T lymphocytes, Exp. Hematol. (2005) 33:811–818 [DOI] [PubMed] [Google Scholar]
- 33.Pedersen L.G., Castelruiz Y., Jacobsen S., Aasted B., Identification of monoclonal antibodies that cross-react with cytokines from different animal species, Vet. Immunol. Immunopathol. (2002) 88:111–122 [DOI] [PubMed] [Google Scholar]
- 34.Piriou-Guzylack L., Salmon H., Membrane markers of the immune cells in swine: an update, Vet. Res. (2008) 39:54. [DOI] [PubMed] [Google Scholar]
- 35.Raulet D.H., Interplay of natural killer cells and their receptors with the adaptive immune response, Nat. Immunol. (2004) 5:996–1002 [DOI] [PubMed] [Google Scholar]
- 36.Sanchez C., Domenech N., Vazquez J., Alonso F., Ezquerra A., Dominguez J., The porcine 2A10 antigen is homologous to human CD163 and related to macrophage differentiation, J. Immunol. (1999) 162:5230–5237 [PubMed] [Google Scholar]
- 37.Shresta S., Pham C.T., Thomas D.A., Graubert T.A., Ley T.J., How do cytotoxic lymphocytes kill their targets?, Curr. Opin. Immunol. (1998) 10:581–587 [DOI] [PubMed] [Google Scholar]
- 38.Storset A.K., Kulberg S., Berg I., Boysen P., Hope J.C., Dissen E., NKp46 defines a subset of bovine leukocytes with natural killer cell characteristics, Eur. J. Immunol. (2004) 34:669–676 [DOI] [PubMed] [Google Scholar]
- 39.Sunderkotter C., Nikolic T., Dillon M.J., Van Rooijen N., Stehling M., Drevets D.A., Leenen P.J., Subpopulations of mouse blood monocytes differ in maturation stage and inflammatory response, J. Immunol. (2004) 172:4410–4417 [DOI] [PubMed] [Google Scholar]
- 40.Tekin S., Hansen P.J., Natural killer-like cells in the sheep: functional characterization and regulation by pregnancy-associated proteins, Exp. Biol. Med. (Maywood) (2002) 227:803–811 [DOI] [PubMed] [Google Scholar]
- 41.Tourais-Esteves I., Bernardet N., Lacroix-Lamande S., Ferret-Bernard S., Laurent F., Neonatal goats display a stronger TH1-type cytokine response to TLR ligands than adults, Dev. Comp. Immunol. (2008) 32:1231–1241 [DOI] [PubMed] [Google Scholar]
- 42.Trapani J.A., Smyth M.J., Functional significance of the perforin/granzyme cell death pathway, Nat. Rev. Immunol. (2002) 2:735–747 [DOI] [PubMed] [Google Scholar]
- 43.Tuo W., Ott T.L., Bazer F.W., Natural killer cell activity of lymphocytes exposed to ovine, type I, trophoblast interferon, Am. J. Reprod. Immunol. (1993) 29:26–34 [DOI] [PubMed] [Google Scholar]
- 44.Ziegler-Heitbrock H.W., Fingerle G., Strobel M., Schraut W., Stelter F., Schutt C., et al. , The novel subset of CD14+/CD16+ blood monocytes exhibits features of tissue macrophages, Eur. J. Immunol. (1993) 23:2053–2058 [DOI] [PubMed] [Google Scholar]
- 45.Ziegler-Heitbrock L., The CD14+ CD16+ blood monocytes: their role in infection and inflammation, J. Leukoc. Biol. (2007) 81:584–592 [DOI] [PubMed] [Google Scholar]
- 46.Zitvogel L., Dendritic and natural killer cells cooperate in the control/switch of innate immunity, J. Exp. Med. (2002) 195:F9–F14 [DOI] [PMC free article] [PubMed] [Google Scholar]