Abstract
Bone morphogenetic proteins (BMPs) signal through the BMP type I and type II receptors to regulate cellular processes, including embryonic development. The type I BMP receptors activin-like kinase (ALK)3 and ALK6 share a high degree of homology, yet possess distinct signaling roles. Here, we report that although the transforming growth factor (TGF)-β type III receptor (TβRIII) enhanced both ALK3 and ALK6 signaling, TβRIII more potently enhanced ALK6-mediated stimulation of the BMP-responsive promoters XVent2 and 3GC2, and up-regulation of the early response gene Smad6. In contrast, TβRIII specifically enhanced ALK3-mediated up-regulation of the early response gene ID-1. TβRIII associated with ALK3 primarily through their extracellular domains, whereas its interaction with ALK6 required both the extracellular and cytoplasmic domains. TβRIII, along with its interacting scaffolding protein β-arrestin2, induced the internalization of ALK6. In contrast, TβRIII colocalized with and resulted in the cell surface retention of ALK3, independently of β-arrestin2. Although complex formation between TβRIII, ALK6, and β-arrestin2 and TβRIII/ALK6 internalization resulted in maximal BMP signaling, the TβRIII mutant unable to interact with β-arrestin2, TβRIII-T841A, was unable to do so. These studies support a novel role for TβRIII in mediating differential ALK3 and ALK6 subcellular trafficking resulting in distinct signaling downstream of ALK3 and ALK6.
INTRODUCTION
Bone morphogenetic proteins (BMPs), with 20 members, comprise the largest subfamily in the transforming growth factor (TGF)-β superfamily (Griffith et al., 1996; Massague et al., 2000). BMPs regulate many processes, including development, bone formation, and remodeling, as well as cellular proliferation, differentiation, survival, and migration (Massague et al., 2000; Shi and Massague, 2003; Zhao, 2003; Bierie and Moses, 2006; Gordon and Blobe, 2008). BMPs signal through BMP type I (activin-like kinase [ALK]1, ALK2, ALK3, and ALK6) and type II (BMPRII, ActRII, and ActRIIB) cell surface receptors, both of which contain serine/threonine kinases in their intracellular domains (Liu et al., 1995; Yamashita et al., 1995). Signaling in response to BMP begins when BMP-bound type I receptors recruit one of the type II receptors into a heterocomplex (Koenig et al., 1994; Liu et al., 1995). The type II receptor in turn transphosphorylates the type I receptor activating its kinase activity. Subsequently, the type I receptors engage and phosphorylate their intracellular effectors, the BMP-responsive Smads (Smad1, -5, and -8), which upon phosphorylation, complex with Smad4 and accumulate in the nucleus to regulate the transcription of target genes.
Among the BMP type I receptors, ALK3 and ALK6 are notable in that they share significant structural homology, with 85% identity in their kinase domain and 42% identity in their extracellular domain (Ide et al., 1997). In particular, the two receptors possess identical glycine-serine (GS)-rich domains and L45 loops, known structural elements essential for their kinase activation and Smad recognition, respectively. In addition, their comparable ability to activate the BMP Smads and compensate for one another during chondrogenesis suggests a redundant role for ALK3 and ALK6 in BMP-mediated signaling (Kretzschmar et al., 1997; Nishimura et al., 1998; Yoon et al., 2005). However, as would be suggested by the evolution and conservation of similar yet distinct receptors, studies have also defined unique features for these receptors. Importantly, deletion of these receptors in mice reveals unique phenotypes. The ALK3-null mice are embryonic lethal due to defects in epiblast proliferation and gastrulation, whereas ALK6-null mice are viable but have defects in the formation of distal phalanges and in their reproductive tract (Mishina et al., 1995; Ashique et al., 2002; Gaussin et al., 2002). In terms of expression, although ALK3 is ubiquitously expressed, ALK6 expression is more confined, with highest expression in the brain, lung, and ovary (Dewulf et al., 1995; Kawabata et al., 1998; Gouedard et al., 2000). TGF-β superfamily ligands differentially regulate ALK3 and ALK6 expression, with TGF-β1 inhibiting ALK3 expression but promoting ALK6 expression. ALK3 and ALK6 also differ in their ability to bind BMP ligands with ALK6 but not ALK3 able to bind BMP-7, growth and differentiation factor (GDF)-5, and BMP-15, whereas ALK3 preferentially binds BMP-6 (ten Dijke et al., 1994; Rosenzweig et al., 1995; Nishitoh et al., 1996; Ebisawa et al., 1999). Functionally, constitutively active ALK3 drives the preosteoblast 2T3 cells toward adipocyte differentiation, whereas constitutively active ALK6 drives them toward osteoblast differentiation (Chen et al., 1998; Gilboa et al., 2000). Although many functional differences between ALK3 and ALK6 have been characterized, the molecular mechanisms by which these receptors are differentially regulated have remained elusive. For example, aberrant accumulation of ALK3 on the cell surface has been linked to fibrodysplasia ossificans progressiva (FOP), a disorder characterized by the heterotropic ossification of connective tissues due to excessive BMP signaling and ID-1 expression (Harradine and Akhurst, 2006). Defining the mechanistic aspects of ALK3 receptor trafficking would provide valuable insight into the causal role in the development of FOP.
The TGF-β superfamily coreceptor, the TGF-β type III receptor (TβRIII, or betaglycan) is the most abundantly expressed TGF-β receptor in most cell types (Lopez-Casillas et al., 1991; Wang et al., 1991). TβRIII has classically been defined as a ligand-presenting coreceptor, promoting the binding of TGF-β superfamily ligands to their respective signaling receptors (Lopez-Casillas et al., 1993). However, recent studies support essential, nonredundant roles for TβRIII, including the embryonic lethal phenotype of TβRIII-null mice (Stenvers et al., 2003), the increasingly complex roles for TβRIII in regulating TGF-β receptor trafficking (Blobe et al., 2001; Chen et al., 2003; Finger et al., 2008a) and both Smad-dependent (Blobe et al., 2001; You et al., 2007) and Smad-independent (You et al., 2007; Mythreye and Blobe, 2009) signaling, as well as the emerging role of TβRIII as a suppressor of cancer progression in a broad spectrum of human cancers (Bandyopadhyay et al., 2002a, b; Dong et al., 2007; Hempel et al., 2007; Turley et al., 2007; Finger et al., 2008b; Gordon et al., 2008). We recently reported that TβRIII is a cell surface coreceptor for BMP ligands, serving to enhance ligand binding to ALK3 and ALK6 and mediate BMP signaling in a biologically relevant assay (Kirkbride, 2007; Kirkbride et al., 2008). Here, we investigate the role of TβRIII in BMP signaling through ALK3 and ALK6.
MATERIALS AND METHODS
Cell Culture and Constructs
COS-7 and human embryonic kidney (HEK)293 cells were maintained in DMEM with 10% fetal bovine serum (FBS). P19 (derived from mouse embryonic teratocarcinoma) cells were cultured in α-minimal essential medium (MEM) with 7.5% fetal calf serum (FCS) and 2.5% FBS. Hemagglutinin (HA)-tagged wild-type ALK3 and ALK6, as well as constitutively active HA-tagged ALK3 (Gln233 to Asp233) and HA-tagged ALK6 (Gln203 to Asp203) constructs were generous gifts from Dr. Kohei Miyazono (The Cancer Institute, Tokyo, Japan). Xvent2 and 3GC2 luciferase reporter constructs were generous gifts from Dr. Douglas Marchuk (Duke University, Durham, NC). FLAG-tagged β-arrestin2 and green fluorescent protein (GFP)-fused β-arrestin2 were generous gifts from Dr. Robert Lefkowitz (Duke University, Durham, NC). Recombinant BMP-2 (355-BM), along with TβRIII (AF-242), ALK3 (AF-346), and ALK6 (AF-505) goat antibodies were all purchased from R&D Systems (Minneapolis, MN). Fluorescently tagged secondary antibodies were purchased from Invitrogen (Carlsbad, CA).
Luciferase Assay
P19 cells plated at 20,000 cells/well in 24-well plates were transfected with simian virus 40 (SV40)-Renilla, 3GC2-lux, β-arretin2, ALK3 or ALK6, and TβRIII by using Lipofectamine 2000. In XVent2 experiments, Xvent2 luciferase construct was transfected with constitutively active ALK3 or ALK6, in the presence or absence of wild-type TβRIII. Twenty to 24 h after transfection, the cells were changed to media containing 0.2% FCS and treated with 10 nM BMP-2 overnight. Cells were lysed with 1× passive lysis buffer, and 20 ml of lysate was assayed using dual-luciferase assay (Promega, Madison, WI). Luminescence was determined using a Victor3 1420 multilabel counter (PerkinElmer Life and Analytical Sciences, Boston, MA).
RNA Isolation and Reverse Transcription-Polymerase Chain Reaction (PCR)
Total RNA was isolated using an RNeasy Mini kit (QIAGEN, Valencia, CA) from P19-transfected cells after stimulation with 10 nM recombinant human BMP-2 for 24 h. cDNA was generated with 1 mg of total cellular RNA using 200 U of SuperScript II reverse transcriptase (Invitrogen) with 500 ng of oligo(dT)12–18 in a total volume of 20 ml. PCR was performed using 2 ml of cDNA and 2 U of Taq DNA polymerase (Invitrogen) in a 25-ml final reaction volume. The PCR products were resolved on 2% agarose gels.
Coimmunoprecipitation
HEK293 cells were transiently transfected with the indicated constructs using Lipofectamine 2000 and maintained in Opti-MEM (Invitrogen) until assaying. The cells were lysed 48 h after transfection in lysis buffer (20 mM HEPES, pH 7.4, 0.5% NP-40, 2 mM EDTA, 0.15 M NaCl, and 10% glycerol, wt/vol). The lysates were immunoprecipitated for 4 h at 4°C with specified antibodies followed by three washes. The samples were resolved by SDS-polyacrylamide gel electrophoresis (PAGE) and immunoblotted for the proteins of interest.
Immunofluorescence
HEK293 cells were plated at 50,000 cells/well into six-well dishes containing poly-d-lysine–coated coverslips. After 24 h, the cells were transiently transfected using Lipofectamine 2000 (Invitrogen) with the indicated constructs. The cells were serum starved in Opti-MEM, washed with phosphate-buffered saline (PBS), fixed with 4% paraformaldehyde, permeabilized in 0.1% Triton X-100/PBS, and then blocked with 5% bovine serum albumin in PBS containing 0.05% Triton X-100 for 1 h. ALK3-, ALK6- or TβRIII-specific antibodies were used to probe for transient receptor expression for 1 h. Cells were washed with PBS and incubated with Cy3-conjugated anti-rabbit or fluorescein isothiocyanate-conjugated anti-goat secondary antibodies for 1 h at room temperature, washed, then mounted with Vectashield. Immunofluorescence images were obtained using an LSM-510 laser scanning confocal microscope (Carl Zeiss, Thornwood, NY).
RESULTS
TβRIII Differentially Promotes ALK3 and ALK6 Signaling
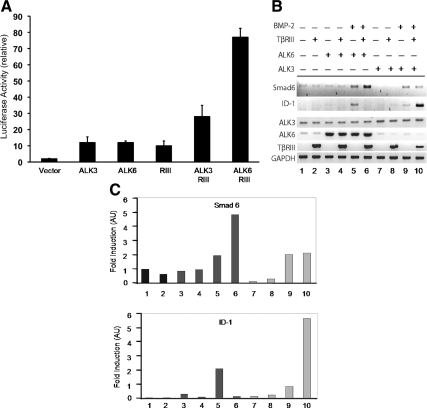
TβRIII functions as a BMP cell surface coreceptor for a number of BMP ligands, including BMP-2, BMP-4, BMP-7, and GDF-5 (Kirkbride, 2007; Kirkbride et al., 2008). In this capacity, TβRIII binds these ligands and enhances their binding to the type I BMP receptors, ALK3 and ALK6 (Kirkbride, 2007; Kirkbride et al., 2008). We reported previously that TβRIII presents BMP-2 to ALK3 and ALK6 to a similar extent (Kirkbride, 2007; Kirkbride et al., 2008). To investigate the effect of TβRIII on downstream BMP signaling, we assessed the effects of TβRIII expression on transcription of a BMP-responsive XVent2 promoter-driven luciferase reporter in the BMP-responsive P19 mouse embryonic carcinoma cells. Although the expression of either TβRIII, constitutively active ALK3 (Gln 233 to Asp233), or constitutively active ALK6 (Gln203 to Asp203) independently increased XVent2 promoter-driven luciferase activity to a similar extent relative to the control (Figure 1A), the expression of TβRIII preferentially enhanced ALK6-mediated luciferase activity, with approximately a sixfold induction for ALK6, relative to a twofold induction for ALK3 (Figure 1A). To further characterize the effect of TβRIII on ALK3 and ALK6 signaling, we examined the message level of the endogenous BMP-responsive genes Smad6 and ID-1 in response to BMP-2 stimulation and TβRIII expression. TβRIII expression specifically enhanced BMP-2–mediated induction of Smad6 transcription in the presence of ALK6 (Figure 1, B and C, lanes 5 and 6) but not in the presence of ALK3 (Figure 1, B and C, lanes 9 and 10). In contrast, TβRIII expression enhanced BMP-2–mediated up-regulation of ID-1 in the presence of ALK3 (Figure 1, B and C, lanes 9 and 10) but not in the presence of ALK6 (Figure 1, B and C, lanes 5 and 6). Together, these data suggest a role for TβRIII in differentially modulating ALK3 and ALK6 downstream signaling, with a preference for enhancing ALK6 signaling to some target genes (i.e., XVent2 and Smad6) and ALK3 signaling to other targets (i.e., ID-1) (Kirkbride, 2007).
Figure 1.
TβRIII modulates distinct ALK3 and ALK6-mediated BMP signaling. (A) P19 cells were transiently transfected with the indicated constructs (vector alone, constitutively active ALK3, ALK6, TβRIII, TβRIII with ALK3, and TβRIII with ALK6) in the presence of the XVent2 luciferase reporter construct. Each luminescence reading was normalized to the cytomegalovirus-driven β-galactosidase activity to account for transfection efficiency. The -fold induction was determined by dividing normalized values to the value of the samples containing only the luciferase promoter and β-galactosidase. Experiments were performed in 24-wells with 20,000 cells, by using 0.5 μg of XVent2, 0.2 μg of TβRIII, and 0.2 μg of ALK3 or ALK6. SE of the mean was calculated for each experimental condition performed in triplicates. (B) P19 cells transiently expressing human TβRIII and either ALK3 or ALK6, as indicated, were treated with 10 nM BMP-2 for 12 h. Total RNA was isolated from these cells and cDNA was made. PCR was performed to look at changes in transcription of the BMP-responsive target gene Smad6 (top) and ID-1 (second panel). Increased expression of ALK3, ALK6, and human TβRIII was verified by RT-PCR (bottom, respectively). Glyceraldehyde-3-phosphate dehydrogenase (GAPDH) served as a total cDNA control. The data represents three independent experiments. (C) Graphical representation of the Smad6 (top) and ID-1 (bottom) bands normalized to the GAPDH housekeeping gene, followed by normalization to empty vector (mock)-transfected cells.
TβRIII Associates with ALK3 and ALK6
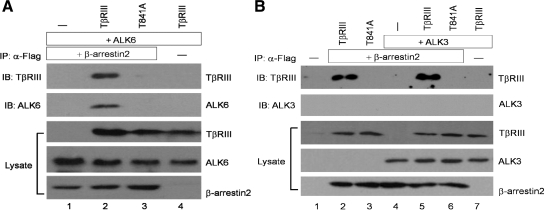
We had established previously that TβRIII serves as a coreceptor, enhancing BMP-2 binding to both ALK3 and ALK6 to an equivalent effect (Kirkbride et al., 2008). To define the mechanism of differential TβRIII-mediated effect on ALK3 and ALK6 signaling, we tested whether TβRIII selectively forms complexes with either ALK3 or ALK6 by coimmunoprecipitation analysis. Full-length TβRIII was able to coimmunoprecipitate both ALK6 (Figure 2A, lane 2) and ALK3 (Figure 2B, lane 2). To define the region of TβRIII facilitating its interaction with ALK3 and ALK6, three mutant TβRIII constructs were used, including TβRIIIΔGAG (TβRIII harboring extracellular point mutations demonstrated previously to abolish glycosaminoglycan [GAG] modification), TβRIIIΔcyto (cytoplasmic domain truncation), and TβRIII-T841A (point mutant demonstrated previously to be unable to bind β-arrestin2; Chen et al., 2003). The extracellular GAG modification of TβRIII had little impact on the interaction between TβRIII and ALK6 (Figure 2A, compare lanes 2 and 3) or ALK3 (Figure 2B, compare lanes 2 and 3), and deletion or mutation of the cytoplasmic domain had little impact on the interaction between TβRIII and ALK3 (Figure 2B, compare lanes 2, 4, and 5). In contrast, deletion or mutation of the cytoplasmic domain significantly decreased the interaction between TβRIII and ALK6 (Figure 2A, compare lanes 2, 4, and 5). These results suggest that the TβRIII extracellular domain largely mediates its association with ALK3, whereas the TβRIII intracellular domain is also required for its efficient interaction with ALK6.
Figure 2.
TβRIII associates with ALK6 and ALK3. COS-7 cells were transiently transfected with 1.5 μg of either vector, wild-type HA-tagged TβRIII, or a mutant HA-TβRIII construct (HA-TβRIII-ΔGAG, HA-TβRIII-ΔcytoΔGAG, and TβRIII-T841A-ΔGAG) along with 1.5 μg of HA-ALK3 or HA-ALK6, as indicated. (A) The lysates were immunoprecipitated with TβRIII-specific antibody that recognizes the extracellular domain of TβRIII, resolved by SDS-PAGE, and immunoblotted with ALK6-specific antibody to detect coprecipitated ALK6 (top; 1:1500 dilution of ALK6 primary antibody), TβRIII antibody for TβRIII (middle; 1:5000 dilution of TβRIII primary antibody), and ALK6-specific antibody for ALK6 expression in the lysate (bottom; 1/20 input of total lysate). (B) The lysates were immunoprecipitated with TβRIII-specific antibody and resolved by SDS-PAGE and immunoblotted with ALK3-specific antibody to detect coprecipitated ALK3 (top; 1:1500 dilution of ALK3 primary antibody), TβRIII antibody for TβRIII (middle; 1:5000 dilution of TβRIII primary antibody), and ALK3 antibody for ALK3 expression in the lysate (bottom; 1/20 of total lysate).
TβRIII Differentially Alters the Subcellular Localization of ALK3 and ALK6
The steady-state levels of both TGF-β and BMP receptors are regulated through a dynamic process, involving constitutive endocytosis and recycling back to the cell surface. BMP receptors have been shown to internalize through both clathrin-dependent and clathrin-independent/lipid raft-mediated pathways (Nohe et al., 2005; Hartung et al., 2006). However, how BMP receptors are localized to their appropriate cellular microenvironment remains to be elucidated. We have reported previously that β-arrestin2 binds TβRIII to mediate the internalization of TβRIII and its associated receptors TβRII and TβRI into endocytic vesicles (Chen et al., 2003). To determine whether TβRIII and/or β-arrestin2 alter the localization of the BMP receptors, we assessed the localization of ALK3 and ALK6 in the presence and absence of TβRIII and GFP-fused β-arrestin2 by using ALK3-, ALK6-, and TβRIII-specific antibodies (see Supplemental Figure 1 for antibody specificity controls). As reported previously (Chen et al., 2003), TβRIII expression alone in HEK293 cells was predominantly plasma membrane-localized (Figure 3O), whereas β-arrestin2-GFP displayed a diffusely localized pattern in the cytoplasm (Figure 3P). Similar to the subcellular distribution of TβRIII, ALK6 expression was largely confined to the plasma membrane when expressed independently (Figure 3A) and colocalized with TβRIII on the cell surface when coexpressed (Figure 3, C–E). In the presence of β-arrestin2-GFP expression, ALK6 remained plasma membrane-localized (Figure 3F), whereas β-arrestin2-GFP remained diffusely cytoplasmic (Figure 3G) with no apparent colocalization (Figure 3H). However, when coexpressed with β-arrestin2-GFP and TβRIII, ALK6 was internalized from the plasma membrane (Figure 3I) at which it colocalized with β-arrestin2 in endocytic vesicles (Figure 3, J and K). As these results suggest that ALK6 internalization is dependent on the interaction of ALK6 with TβRIII and TβRIII with β-arrestin2-GFP, we tested the previously established TβRIII point mutant unable to associate and internalize with β-arrestin2-GFP, TβRIII-T841A(Chen et al., 2003). As expected, in the presence of β-arrestin2-GFP and TβRIII-T841A, ALK6 remained predominantly at the plasma membrane (Figure 3L), whereas β-arrestin2-GFP was diffusely cytoplasmic (Figure 3M) with no apparent colocalization of ALK6 and β-arrestin2-GFP (Figure 3N). Together, these results suggested that TβRIII, β-arrestin2, and ALK6 form a complex, with TβRIII mediating ALK6 internalization into endocytic vesicles in a β-arrestin2–dependent manner.
Figure 3.
β-Arrestin2 induces the internalization of ALK6 in TβRIII-dependent manner. HEK293 cells transiently expressing wild-type HA-tagged ALK6 alone (A) or wild-type HA-tagged ALK3 alone (B) were detected using ALK6- and ALK3-specific antibodies, respectively. HA-tagged TβRIII and GFP-fused β-arrestin2 (β-arrestin2-GFP) expressed independently are shown in O and P. Cells coexpressing HA-ALK6 and HA-TβRIII were probed with ALK-6 and TβRIII-specific antibodies, respectively (C and D), and their merged image is shown (E). Coexpression of HA-ALK6 and β-arrestin2-GFP are shown separately (F and G, and merged image H). HA-ALK6 coexpressed with HA-TβRIII and β-arrestin2-GFP were probed for ALK6 with ALK6-specific antibody (I) and observed for β-arrestin2-GFP distribution (J), with their merged image shown in panel K. HA-ALK6 coexpressed with β-arrestin2-GFP and HA-TβRIII-T841A were probed for ALK6 and β-arrestin2-GFP distribution (L and M, respectively, and merged image N). HA-ALK3 and HA-TβRIII distribution are shown in Q and R, respectively, with their merged image shown in S. HA-ALK3 expressed with β-arrestin2-GFP are shown in T and U, respectively, with their merged image in V. HA-ALK3 coexpressed with β-arrestin2-GFP and HA-TβRIII were probed for ALK3 (W) and observed for β-arrestin2-GFP (X) distribution, with their merged image in Y. The coexpression of HA-ALK3 with HA-TβRIII-T841A is represented in Z and AA, respectively, with their merged image shown in AB. For each experimental condition, HEK293 cells grown on six-well coverslips received a total of 2.5 μg of DNA, comprising 1 μg of each receptor where appropriate, and 0.5 μg of β-arrestin2-GFP.
We next examined the effect of TβRIII and β-arrestin2 on ALK3 subcellular localization. The subcellular distribution of ALK3 was predominantly in endocytic vesicles (Figure 3B). In sharp contrast to that observed for ALK6, ALK3 expression in the presence of TβRIII resulted in its shift from endocytic vesicular localization to the plasma membrane (Figure 3Q), where it colocalized with TβRIII (Figure 3, R and S). When coexpressed with β-arrestin2-GFP, ALK3 remained in endocytic vesicles (Figure 3T), whereas β-arrestin2-GFP remained diffusely cytoplasmic (Figure 3U) with no apparent colocalization (Figure 3V). When coexpressed with β-arrestin2-GFP and TβRIII, the vesicular pattern of ALK3 persisted (Figure 3W), whereas β-arrestin2 was redistributed from diffusely cytoplasmic to endocytic vesicles (Figure 3X). Interestingly, no colocalization was observed between vesicles containing β-arrestin2 and vesicles containing ALK3 (Figure 3Y), suggesting that the alteration in β-arrestin2-GFP localization was due to the previously defined interaction between TβRIII and β-arrestin2 (Chen et al., 2003). Finally, to test whether the apparent effect of TβRIII on the expression and the vesicular to membrane localization of ALK3 was dependent on β-arrestin2-GFP, we expressed the TβRIII-T841A mutant. TβRIII-T841A (Figure 3AA) also resulted in the recruitment of ALK3 to the cell surface (Figure 3Z) where it colocalized with TβRIII-T841A (Figure 3AB), supporting that these effects were independent of the ability of TβRIII to interact with β-arrestin2.
TβRIII Differentially Complexes with ALK3 and ALK6
Our immunofluorescence results revealed altered subcellular distribution of ALK6 and ALK3 on the basis of differential complex formation with TβRIII and β-arrestin2. To determine the specificity of these interactions biochemically, coimmunoprecipitation studies were performed. When ALK6 and β-arrestin2 were coexpressed, β-arrestin2 did not coprecipitate ALK6 (Figure 4A, lane 1). However, when ALK6 and β-arrestin2 were coexpressed in the presence of TβRIII, β-arrestin2 coprecipitated ALK6 and TβRIII (Figure 4 A, lane 2), whereas in the presence of TβRIII-T841A, β-arrestin2 did not coprecipitate ALK6 or TβRIII-T841A (Figure 4A, lane 3). For ALK3, no association was observed between ALK3 and β-arrestin2 (Figure 4B, lane 4). Moreover, ALK3 failed to coprecipitate with β-arrestin2 even when expressed in the presence of TβRIII or TβRIII-T841A (Figure 4B, lanes 5 and 6), consistent with our immunofluorescence studies. Together, these results provide further support for a specific interaction between TβRIII and ALK6, mediated by β-arrestin2.
Figure 4.
β-Arrestin2 associates with ALK6 through TβRIII. (A) HA-ALK6 is expressed in HEK293 cells in the presence of FLAG-tagged β-arrestin2 (lane 1), FLAG-β-arrestin2 and HA-TβRIII (lane 2), FLAG-β-arrestin2 and HA-TβRIII-T841A (lane3), or with HA-TβRIII (lane 4). FLAG-β-arrestin2 was immunoprecipitated using FLAG antibody (10 μg/1 ml lysate) and immunoblotted for coprecipitated TβRIII (top; detected with TβRIII-specific antibody at 1:2000 dilution) and ALK6 (second panel; detected with ALK6-specific antibody at 1:000 dilution). Lysates were probed for TβRIII, ALK6, and β-arrestin2 expression by using TβRIII- and ALK6-specific antibodies, and FLAG antibody (at 1.5 μg/1 ml primary antibody), respectively (third, fourth, and fifth panels, respectively). (B) FLAG-β-arrestin2 is expressed in the presence of wild-type HA-TβRIII (lane 2), HA-TβRIII-T841A (lane 3), HA-ALK3 (lane 4), HA-ALK3 with HA-TβRIII (lane 5), HA-ALK3 with HA-TβRIII-T841A (lane 6). As control, anti-FLAG immunoprecipitation was performed on HEK293 cells transfected with vector alone (lane 1) or cells expressing HA-TβRIII and HA-ALK3 (lane 7). In all conditions, cell lysates were immunoprecipitated for FLAG-β-arrestin2 with FLAG antibody (10 μg/1 ml lysate) and immunoblotted for TβRIII with TβRIII-specific antibody (top; 1:2000 dilution) and ALK3 precipitation (second panel; ALK3-specific antibody at 1:1500 dilution). Cell lysate for each condition (1/20 of total cell lysate) was probed for TβRIII, ALK3, and β-arrestin2 (FLAG antibody at 1.5 μg/1 ml primary antibody) expression with their respective antibodies (third, fourth, and fifth panels, respectively).
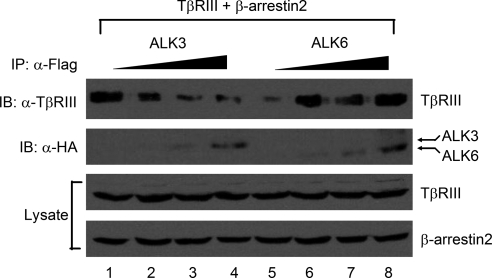
Because TβRIII failed to recruit ALK3 to the cell surface in the presence of β-arrestin2 (Figure 3 W), we hypothesized that ALK3 might be functionally competing with β-arrestin2 for TβRIII. To address this possibility, we expressed a constant level of TβRIII and β-arrestin2 in the presence of increasing expression of either ALK3 or ALK6. Consistent with this hypothesis, the ability of β-arrestin2 to coprecipitate TβRIII decreased with increasing ALK3 expression (Figure 5, lanes 1–4). In contrast, the ability of β-arrestin2 to coprecipitate TβRIII increased with increasing ALK6 expression (Figure 5, lanes 5–8). These results further support a cooperative interaction between TβRIII, ALK6 and β-arrestin2, while supporting mutually exclusive interactions between TβRIII, ALK3 and β-arrestin2.
Figure 5.
β-Arrestin2 modulates the association of TβRIII with ALK6 and ALK3. HA-TβRIII and FLAG-tagged β-arrestin2 were coexpressed in HEK293 cells in the presence of increasing levels of HA-ALK3 (lanes 1–4) or HA-ALK6 (lanes 5–8). FLAG-β-arrestin2 was immunoprecipitated using FLAG antibody (10 μg/1 ml lysate) from cell lysates and immunoblotted for coprecipitated TβRIII (top; TβRIII-specific antibody at 1:2000 dilution), HA-tagged ALK3 or ALK6 with use of HA-antibody at 1.5 μg/1 ml primary dilution (second panel, indicated arrows). The expression of TβRIII and β-arrestin2 levels were assessed with TβRIII-specific antibody (at 1:2000 dilution) and FLAG antibody (1.5 μg/1 ml of primary dilution), respectively (third and fourth panels).
TβRIII and β-Arrestin2 Preferentially Mediate ALK6 Signaling
The internalization of the BMP-receptors has been shown to mediate BMP signaling (Hartung et al., 2006). Having established that the subcellular distribution of ALK3 and ALK6 are differentially altered by either TβRIII and/or β-arrestin2, we next assessed the impact of their internalization on downstream signaling via a BMP-responsive luciferase reporter, 3GC2, in BMP-responsive P19 cells. Compared with the 3GC2 vector alone, the expression of TβRIII, β-arrestin2, or TβRIII with β-arrestin2 yielded modest one- to twofold increases in basal luciferase activity, with minor responses to exogenous BMP stimulation (Figure 6A, lanes 1–4). Likewise, ALK3 expression resulted in relatively weak basal and BMP-induced responses, even in the presence of TβRIII and β-arrestin2 (Figure 6A, lanes 5–7). In contrast, expression of ALK6 or ALK6 with β-arrestin2 in P19 cells resulted in a fourfold induction in BMP-induced luciferase signal (Figure 6A, lanes 8 and 9), and expression of TβRIII, β-arrestin2 and ALK6 resulted in a fivefold increase in basal luciferase activity along with a sevenfold induction after BMP-2 stimulation (Figure 6A, lane 10), supporting a role for TβRIII and β-arrestin2 in specifically enhancing ALK6 signaling through regulation of ALK6 trafficking (Kirkbride, 2007). To determine whether the interaction between ALK6 and the TβRIII/β–arrestin2 complex and the resulting alterations in ALK6 trafficking were necessary for the observed effects on BMP signaling, TβRIII-T841A was expressed in place of wild-type TβRIII in P19 cells. As predicted, compared with ALK6 coexpressed with TβRIII and β-arrestin2, ALK6 in the presence of TβRIII-T841A and β-arrestin2 yielded reduced levels of luciferase activity, both in the presence and absence of ligand (Figure 6B, lanes 2 and 3). These results support the specific interaction of ALK6 and TβRIII, mediated by β-arrestin2 and resulting in ALK6 internalization as a mechanism for TβRIII-mediated activation of ALK6 signaling.
Figure 6.
TβRIII/β-arrestin2 complex enhances ALK6-mediated BMP-signaling. (A and B) P19 cells were transfected (20,000 cells in 24-well) with the BMP-responsive promoter, 3GC2-lux (0.5 μg), the indicated receptor constructs (0.2 μg each), β-arrestin2 (0.1 μg), and SV40-Renilla transfection control (10 ng). Cells were treated for 16–24 h with 10 nM BMP-2 followed by luminescence reading. The luciferase readings were normalized to SV40-Renilla readings followed by normalization of the readings to samples that were transfected with luciferase and Renilla reporters alone or empty vector to determine -fold induction. SE of the mean was calculated for each experimental condition performed in triplicates.
DISCUSSION
The response of cells to extracellular signals can be modulated by regulating the steady-state cell surface levels of receptors for these signals, including regulating biosynthesis and trafficking to the cell surface, stability on the cell surface, internalization, degradation, and recycling back to the cell surface. For BMP receptors, recent studies support regulated internalization as a major mechanism for regulating their cell surface expression. Both BMPRII and ALK3 interact with caveolin-1, a major component of lipid rafts and clathrin-independent internalization, which results in inhibition of BMP signaling (Nohe et al., 2005). BMP ligand decreases the interaction of these BMP receptors with caveolin. Although ALK3 associates with lipid rafts, ALK6 associates with clathrin-rich regions (Nohe et al., 2005; Hartung et al., 2006). Inhibiting clathrin-mediated endocytosis decreases BMP-mediated activation of BMP-responsive transcriptional elements. These data indicate that BMP-activated pathways are differentially regulated by the mode of endocytosis. However, the precise mechanisms by which BMP receptor endocytosis is regulated remains largely undefined. Because TβRIII and the TβRIII-interacting protein β-arrestin2 have been demonstrated to regulate the internalization of associated receptors (Chen et al., 2003), and we had recently demonstrated the functional interaction of TβRIII with the BMP receptors (Kirkbride et al., 2008), we explored the role of TβRIII and β-arrestin2 in ALK3 and ALK6 internalization and signaling. Surprisingly, although we had demonstrated previously that TβRIII presents BMP-2 to both ALK3 and ALK6 to an equivalent extent (Kirkbride et al., 2008), TβRIII preferentially activated ALK6 signaling to BMP-responsive promoters (Figures 1A and 6A) and stimulated ALK6-mediated transcription of the BMP-responsive gene Smad6 (Figure 1, B and C), whereas preferentially stimulating ALK3-mediated transcription of the BMP-responsive gene, ID-1 (Figure 1, B and C). TβRIII also differentially regulated ALK3 and ALK6 receptor trafficking. For ALK3, TβRIII stabilized ALK3 at the cell surface, potentially by inhibiting ALK3 internalization in a β-arrestin2–independent manner (Figure 3). In addition, ALK3 and β-arrestin2 seem to compete for the extracellular and intracellular domains, of TβRIII, respectively (Figure 5, lanes 1–4). One explanation for their ability to compete through mutually exclusive interactions may involve conformational changes induced by the extracellular interaction between TβRIII and ALK3, which disrupts β-arrestin2 binding to the intracellular domain. Alternatively, the competitive interaction may be mediated by other proteins known to associate with TβRIII, including GAIP-interacting protein, C terminus (GIPC), a TβRIII membrane-anchoring adaptor protein (Blobe et al., 2001). One intriguing possibility is that TβRIII–ALK3 complex enhances the recruitment of GIPC to the cell surface, stabilizing TβRIII and ALK3 while minimizing β-arrestin2 interaction. This notion is especially appealing because β-arrestin2 and GIPC target largely the same C-terminal tail segment of TβRIII but possess distinct trafficking roles in the spatial context of TβRIII (Blobe et al., 2001, Chen et al., 2003). The coordinated role of GIPC and β-arrestin2 in regulating TβRIII and associated TGF-β superfamily receptors is currently under investigation.
In contrast, TβRIII formed a complex with ALK6 and cointernalized ALK6 in a β-arrestin2–dependent manner (Figure 3). The complex of TβRIII, ALK6 and β-arrestin2 was necessary for maximal BMP signaling (Figure 6A), and the inability of the TβRIII mutant unable to interact with β-arrestin2, TβRIII-T841A, to mediate this effect (Figure 6B), or for TβRIII and β-arrestin2 to stimulate ALK3 signaling (Figure 6A), supports the effect of TβRIII and β-arrestin2 on ALK6 internalization as the mechanism for the differential effects of TβRIII on ALK6- and ALK3-mediated BMP signaling. Because β-arrestin2 associates with components of the clathrin-dependent endocytic pathway, the specific interaction of ALK6 with TβRIII and β-arrestin2 also likely represents the mechanism by which ALK6 specifically associates with clathrin-coated pits, as reported previously (Gruenberg, 2001; Di Guglielmo et al., 2003).
How does the differential trafficking of ALK3 and ALK6 result in different signaling outputs? As mentioned, it is well established that the spatial regulation of BMP receptors modulates distinct downstream signaling. For example, although ALK6 activates Smad1/5 signaling at the plasma membrane, its signal propagation to elicit a transcriptional response requires clathrin-mediated endocytosis of the receptor (Hartung et al., 2006). In addition, it is now known that Smad-independent BMP-2–mediated induction of alkaline phosphatase is initiated from distinct cholesterol-enriched membrane microdomains (Hartung et al., 2006). Given these findings, it is possible that transcription of ID-1 requires cell surface retention of the receptor, whereas Smad6 requires internalization. Alternatively, the accumulation of Smad6 expression may trigger ALK6 down-regulation as result of a negative feedback mechanism. Although the current studies place TβRIII and β-arrestin2 in the pathway for regulating not only TGF-β, but also BMP receptor trafficking and resulting signaling, the precise mechanism by which receptor trafficking is linked to signaling outcomes in TGF-β superfamily signaling pathways remains to be explored. Based on our data, TβRIII facilitates BMP binding to both ALK3 and ALK6 and results in unique physiological outcomes downstream of both receptors. These unique responses are likely a result of distinct interactions between the receptors, either solely extracellular (as with TβRIII and ALK3) or through both the extracellular domain and the cytoplasmic tail of TβRIII (as with TβRIII and ALK6; Figure 2).
In addition to forming a complex together, ALK6 also seems to promote the interaction between TβRIII and β-arrestin2 (Figure 5). This finding suggests that ALK6 facilitates the heteromeric stability via a favorable protein–protein interaction, perhaps via phosphorylation events. Indeed, we previously determined that the TβRIII–β-arrestin2 interaction is mediated by the phosphorylation of threonine 841 in the cytoplasmic domain of TβRIII by TβRII (Chen et al., 2003). Whether TβRIII phosphorylation can also be mediated by ALK6 and/or other BMP receptors, and the impact of these interactions on TβRIII function and TGF-β superfamily signaling is currently under investigation. In addition, we observed that increased expression of ALK3 could compete with β-arrestin2 for binding to TβRIII, suggesting that TGF-β superfamily receptors may alter not only TβRIII function through phosphorylation, but signaling of other superfamily receptors by altering the ability of β-arrestin2 to bind to the cytoplasmic tail of TβRIII.
The role of TβRIII in BMP signaling and BMP-mediated biology is an emerging area. Evidence for a direct physiological association between TβRIII and the BMP receptors is limited. Available data suggests that TβRIII, ALK3 and ALK6 are all essential for proper development and alterations in any one of these components of the signaling pathway will alter proper tissue development and homeostasis. Loss of TβRIII in mice results in an arrest in the development of the skeletal system at embryonic day 14.5. These mice display reduced ossification and size, a phenotype that does not mimic the loss of TGF-β2, a ligand dependent on TβRIII for signaling (Stenvers et al., 2003). These data suggest the possibility that loss of TβRIII may alter BMP signaling during skeletal development. An exact role for TβRIII in ALK3- and/or ALK6-mediated effects on skeletal development remains to be determined. Based on our data, we suggest that during development TβRIII differentially regulates ALK6 and ALK3 signaling. The presence TβRIII may potentially explain the mechanism by which constitutively active ALK3 and ALK6 have unique roles in the differentiation of the preosteoblast cell line, 2T3 (Chen et al., 1998; Gilboa et al., 2000). A role for a BMP coreceptor in shifting the use of cell surface receptors is supported by the role of DRAGON, a GPI-linked BMP coreceptor, in enhancing BMP signaling through the use of the activin receptor type IIA (ActRIIA) by endogenous BMP-2 and BMP-4 in pulmonary artery smooth muscle cells in the absence of BMPII, normally the preferred BMP type II signaling receptor (Xia et al., 2007). These data suggest that regulation of BMP signaling by coreceptor expression is critical for enhanced BMP signaling by ensuring the utilization of all functional BMP receptors.
Aberrant subcellular localization of type I BMP receptors have biological implications, as evidenced by misregulated localization of ALK3 being linked to FOP (Gordon and Blobe, 2008). Patients with FOP display heterotropic ossification of connective tissues due to excessive BMP signaling and ID-1 expression. The increase in BMP signaling is due to a sixfold increase in ALK3 on the cell surface (Nohe et al., 2005; Hartung et al., 2006). These data support the possibility that cell surface expression of ALK3 is necessary for ID-1 expression. Our data demonstrate that TβRIII dramatically increases ALK3 on the cell surface and downstream ID-1 expression. Whether increased TβRIII expression or mutations in β-arrestin2 are linked to FOP remains to be explored.
We have shown that loss of TβRIII expression is associated with tumor progression (Dong et al., 2007). We have also shown that BMP-mediated invasion of pancreatic cells is blocked by maintaining TβRIII expression (Gordon et al., 2008). Data presented here suggest several potential mechanisms by which TβRIII abrogates BMP-mediated cancer cell invasion: 1) TβRIII alters the subcellular localization of ALK3 altering downstream signaling; 2) the extracellular domains of ALK3 and TβRIII interact, resulting in a conformational change in the cytoplasmic tail of ALK3 that prevents downstream signaling or 3) TβRIII increases ALK3 on the cell surface where their interaction prevents TβRIII from associating with β-arrestin2 and enhancing ALK6 signaling; and 4) ALK6-mediated signaling enhanced by TβRIII is responsible for controlling BMP signaling via Smad6 feedback and loss of this regulation results in aberrant BMP signaling. In addition, altered expression of both ALK6 and TβRIII has been associated with breast cancer. Loss of either TβRIII or ALK6 correlates with a poor prognosis (Dong et al., 2007; Bokobza et al., 2009); however, the consequence of loss of both receptors remains to be established. Based on data presented here, loss of TβRIII during carcinogenesis may be critical for shifting the downstream signaling of both ALK3 and ALK6, resulting in an alteration in BMP signaling and function during carcinogenesis and cancer progression. Investigating the role of TβRIII in these BMP functions during cancer progression is currently being explored.
Supplementary Material
ACKNOWLEDGMENTS
This work was supported by a predoctoral fellowship from the Department of Defense Breast Cancer Research Program (to K.C.K.) and by a National Institutes of Health National Cancer Institute grants R01-CA106307 and R01-CA136786 (to G.C.B.).
Abbreviations used:
- ALK
activin-like kinase
- BMP
bone morphogenetic protein
- TGF
transforming growth factor
- TβRIII
type III transforming growth factor-β receptor
- TβRIII-T841A
TβRIII with a point mutant at Thr 841.
Footnotes
This article was published online ahead of print in MBC in Press (http://www.molbiolcell.org/cgi/doi/10.1091/mbc.E09-07-0539) on September 2, 2009.
REFERENCES
- Ashique A. M., Fu K., Richman J. M. Signalling via type IA and type IB bone morphogenetic protein receptors (BMPR) regulates intramembranous bone formation, chondrogenesis and feather formation in the chicken embryo. Int. J. Dev. Biol. 2002;46:243–253. doi: 10.1387/ijdb.011535. [DOI] [PubMed] [Google Scholar]
- Bandyopadhyay A., Lopez-Casillas F., Malik S. N., Montiel J. L., Mendoza V., Yang J., Sun L. Z. Antitumor activity of a recombinant soluble betaglycan in human breast cancer xenograft. Cancer Res. 2002a;62:4690–4695. [PubMed] [Google Scholar]
- Bandyopadhyay A., Zhu Y., Malik S. N., Kreisberg J., Brattain M. G., Sprague E. A., Luo J., Lopez-Casillas F., Sun L. Z. Extracellular domain of TGFbeta type III receptor inhibits angiogenesis and tumor growth in human cancer cells. Oncogene. 2002b;21:3541–3551. doi: 10.1038/sj.onc.1205439. [DOI] [PubMed] [Google Scholar]
- Bierie B., Moses H. L. Tumour microenvironment: TGFbeta: the molecular Jekyll and Hyde of cancer. Nat. Rev. Cancer. 2006;6:506–520. doi: 10.1038/nrc1926. [DOI] [PubMed] [Google Scholar]
- Blobe G. C., Liu X., Fang S. J., How T., Lodish H. F. A novel mechanism for regulating transforming growth factor beta (TGF-beta) signaling. Functional modulation of type III TGF-beta receptor expression through interaction with the PDZ domain protein, GIPC. J. Biol. Chem. 2001;276:39608–39617. doi: 10.1074/jbc.M106831200. [DOI] [PubMed] [Google Scholar]
- Bokobza S. M., Ye L., Kynaston H. E., Mansel R. E., Jiang W. G. Reduced expression of BMPR-IB correlates with poor prognosis and increased proliferation of breast cancer cells. Cancer Genomics Proteomics. 2009;6:101–108. [PubMed] [Google Scholar]
- Chen D., Ji X., Harris M. A., Feng J. Q., Karsenty G., Celeste A. J., Rosen V., Mundy G. R., Harris S. E. Differential roles for bone morphogenetic protein (BMP) receptor type IB and IA in differentiation and specification of mesenchymal precursor cells to osteoblast and adipocyte lineages. J. Cell Biol. 1998;142:295–305. doi: 10.1083/jcb.142.1.295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen W., Kirkbride K. C., How T., Nelson C. D., Mo J., Frederick J. P., Wang X. F., Lefkowitz R. J., Blobe G. C. beta-Arrestin 2 mediates endocytosis of type III TGF-beta receptor and down-regulation of its signaling. Science. 2003;301:1394–1397. doi: 10.1126/science.1083195. [DOI] [PubMed] [Google Scholar]
- Dewulf N., Verschueren K., Lonnoy O., Moren A., Grimsby S., Vande Spiegle K., Miyazono K., Huylebroeck D., Ten Dijke P. Distinct spatial and temporal expression patterns of two type I receptors for bone morphogenetic proteins during mouse embryogenesis. Endocrinology. 1995;136:2652–2663. doi: 10.1210/endo.136.6.7750489. [DOI] [PubMed] [Google Scholar]
- Di Guglielmo G. M., Le Roy C., Goodfellow A. F., Wrana J. L. Distinct endocytic pathways regulate TGF-beta receptor signalling and turnover. Nat. Cell Biol. 2003;5:410–421. doi: 10.1038/ncb975. [DOI] [PubMed] [Google Scholar]
- Dong M., How T., Kirkbride K. C., Gordon K. J., Lee J. D., Hempel N., Kelly P., Moeller B. J., Marks J. R., Blobe G. C. The type III TGF-beta receptor suppresses breast cancer progression. J. Clin Invest. 2007;117:206–217. doi: 10.1172/JCI29293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ebisawa T., Tada K., Kitajima I., Tojo K., Sampath T. K., Kawabata M., Miyazono K., Imamura T. Characterization of bone morphogenetic protein-6 signaling pathways in osteoblast differentiation. J. Cell Sci. 1999;112:3519–3527. doi: 10.1242/jcs.112.20.3519. [DOI] [PubMed] [Google Scholar]
- Finger E. C., Lee N. Y., You H. J., Blobe G. C. Endocytosis of the type III TGF-beta receptor through the clathrin-independent/lipid raft pathway regulates TGF-beta signaling and receptor downregulation. J. Biol. Chem. 2008a;283:34808–34818. doi: 10.1074/jbc.M804741200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Finger E. C., Turley R. S., Dong M., How T., Fields T. A., Blobe G. C. TbetaRIII suppresses non-small cell lung cancer invasiveness and tumorigenicity. Carcinogenesis. 2008b;29:528–535. doi: 10.1093/carcin/bgm289. [DOI] [PubMed] [Google Scholar]
- Gaussin V., Van de Putte T., Mishina Y., Hanks M. C., Zwijsen A., Huylebroeck D., Behringer R. R., Schneider M. D. Endocardial cushion and myocardial defects after cardiac myocyte-specific conditional deletion of the bone morphogenetic protein receptor ALK3. Proc. Natl. Acad. Sci. USA. 2002;99:2878–2883. doi: 10.1073/pnas.042390499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gilboa L., Nohe A., Geissendorfer T., Sebald W., Henis Y. I., Knaus P. Bone morphogenetic protein receptor complexes on the surface of live cells: a new oligomerization mode for serine/threonine kinase receptors. Mol. Biol. Cell. 2000;11:1023–1035. doi: 10.1091/mbc.11.3.1023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gordon K. J., Blobe G. C. Role of transforming growth factor-beta superfamily signaling pathways in human disease. Biochim. Biophys. Acta. 2008;1782:197–228. doi: 10.1016/j.bbadis.2008.01.006. [DOI] [PubMed] [Google Scholar]
- Gordon K. J., Dong M., Chislock E. M., Fields T. A., Blobe G. C. Loss of type III transforming growth factor beta receptor expression increases motility and invasiveness associated with epithelial to mesenchymal transition during pancreatic cancer progression. Carcinogenesis. 2008;29:252–262. doi: 10.1093/carcin/bgm249. [DOI] [PubMed] [Google Scholar]
- Gouedard L., Chen Y. G., Thevenet L., Racine C., Borie S., Lamarre I., Josso N., Massague J., di Clemente N. Engagement of bone morphogenetic protein type IB receptor and Smad1 signaling by anti-Mullerian hormone and its type II receptor. J. Biol. Chem. 2000;275:27973–27978. doi: 10.1074/jbc.M002704200. [DOI] [PubMed] [Google Scholar]
- Griffith D. L., Keck P. C., Sampath T. K., Rueger D. C., Carlson W. D. Three-dimensional structure of recombinant human osteogenic protein 1, structural paradigm for the transforming growth factor beta superfamily. Proc. Natl. Acad. Sci. USA. 1996;93:878–883. doi: 10.1073/pnas.93.2.878. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gruenberg J. The endocytic pathway: a mosaic of domains. Nat. Rev. Mol. Cell Biol. 2001;2:721–730. doi: 10.1038/35096054. [DOI] [PubMed] [Google Scholar]
- Harradine K. A., Akhurst R. J. Mutations of TGFbeta signaling molecules in human disease. Ann. Med. 2006;38:403–414. doi: 10.1080/07853890600919911. [DOI] [PubMed] [Google Scholar]
- Hartung A., Bitton-Worms K., Rechtman M. M., Wenzel V., Boergermann J. H., Hassel S., Henis Y. I., Knaus P. Different routes of bone morphogenic protein (BMP) receptor endocytosis influence BMP signaling. Mol. Cell. Biol. 2006;26:7791–7805. doi: 10.1128/MCB.00022-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hempel N., How T., Dong M., Murphy S. K., Fields T. A., Blobe G. C. Loss of betaglycan expression in ovarian cancer: role in motility and invasion. Cancer Res. 2007;67:5231–5238. doi: 10.1158/0008-5472.CAN-07-0035. [DOI] [PubMed] [Google Scholar]
- Ide H., Katoh M., Sasaki H., Yoshida T., Aoki K., Nawa Y., Osada Y., Sugimura T., Terada M. Cloning of human bone morphogenetic protein type IB receptor (BMPR-IB) and its expression in prostate cancer in comparison with other BMPRs. Oncogene. 1997;14:1377–1382. doi: 10.1038/sj.onc.1200964. [DOI] [PubMed] [Google Scholar]
- Kawabata M., Imamura T., Miyazono K. Signal transduction by bone morphogenetic proteins. Cytokine Growth Factor Rev. 1998;9:49–61. doi: 10.1016/s1359-6101(97)00036-1. [DOI] [PubMed] [Google Scholar]
- Kirkbride K. C. Durham, NC: Duke University; 2007. Elucidating The Role of the Transforming Growth Factor-beta Type III Receptor in Bone Morphogenetic Protein Signaling. Ph.D. Thesis. [Google Scholar]
- Kirkbride K. C., Townsend T. A., Bruinsma M. W., Barnett J. V., Blobe G. C. Bone morphogenetic proteins signal through the transforming growth factor-beta type III receptor. J. Biol. Chem. 2008;283:7628–7637. doi: 10.1074/jbc.M704883200. [DOI] [PubMed] [Google Scholar]
- Koenig B. B., et al. Characterization and cloning of a receptor for BMP-2 and BMP-4 from NIH 3T3 cells. Mol. Cell. Biol. 1994;14:5961–5974. doi: 10.1128/mcb.14.9.5961. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kretzschmar M., Liu F., Hata A., Doody J., Massague J. The TGF-beta family mediator Smad1 is phosphorylated directly and activated functionally by the BMP receptor kinase. Genes Dev. 1997;11:984–995. doi: 10.1101/gad.11.8.984. [DOI] [PubMed] [Google Scholar]
- Liu F., Ventura F., Doody J., Massague J. Human type II receptor for bone morphogenic proteins (BMPs): extension of the two-kinase receptor model to the BMPs. Mol. Cell. Biol. 1995;15:3479–3486. doi: 10.1128/mcb.15.7.3479. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lopez-Casillas F., Cheifetz S., Doody J., Andres J. L., Lane W. S., Massague J. Structure and expression of the membrane proteoglycan betaglycan, a component of the TGF-beta receptor system. Cell. 1991;67:785–795. doi: 10.1016/0092-8674(91)90073-8. [DOI] [PubMed] [Google Scholar]
- Lopez-Casillas F., Wrana J. L., Massague J. Betaglycan presents ligand to the TGF beta signaling receptor. Cell. 1993;73:1435–1444. doi: 10.1016/0092-8674(93)90368-z. [DOI] [PubMed] [Google Scholar]
- Massague J., Blain S. W., Lo R. S. TGFbeta signaling in growth control, cancer, and heritable disorders. Cell. 2000;103:295–309. doi: 10.1016/s0092-8674(00)00121-5. [DOI] [PubMed] [Google Scholar]
- Mishina Y., Suzuki A., Ueno N., Behringer R. R. Bmpr encodes a type I bone morphogenetic protein receptor that is essential for gastrulation during mouse embryogenesis. Genes Dev. 1995;9:3027–3037. doi: 10.1101/gad.9.24.3027. [DOI] [PubMed] [Google Scholar]
- Mythreye K., Blobe G. C. The type III TGF-beta receptor regulates epithelial and cancer cell migration through beta-arrestin2-mediated activation of Cdc42. Proc. Natl. Acad. Sci. USA. 2009;106:8221–8226. doi: 10.1073/pnas.0812879106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nishimura R., Kato Y., Chen D., Harris S. E., Mundy G. R., Yoneda T. Smad5 and DPC4 are key molecules in mediating BMP-2-induced osteoblastic differentiation of the pluripotent mesenchymal precursor cell line C2C12. J. Biol. Chem. 1998;273:1872–1879. doi: 10.1074/jbc.273.4.1872. [DOI] [PubMed] [Google Scholar]
- Nishitoh H., Ichijo H., Kimura M., Matsumoto T., Makishima F., Yamaguchi A., Yamashita H., Enomoto S., Miyazono K. Identification of type I and type II serine/threonine kinase receptors for growth/differentiation factor-5. J. Biol. Chem. 1996;271:21345–21352. doi: 10.1074/jbc.271.35.21345. [DOI] [PubMed] [Google Scholar]
- Nohe A., Keating E., Underhill T. M., Knaus P., Petersen N. O. Dynamics and interaction of caveolin-1 isoforms with BMP-receptors. J. Cell Sci. 2005;118:643–650. doi: 10.1242/jcs.01402. [DOI] [PubMed] [Google Scholar]
- Rosenzweig B. L., Imamura T., Okadome T., Cox G. N., Yamashita H., ten Dijke P., Heldin C. H., Miyazono K. Cloning and characterization of a human type II receptor for bone morphogenetic proteins. Proc. Natl. Acad. Sci. USA. 1995;92:7632–7636. doi: 10.1073/pnas.92.17.7632. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shi Y., Massague J. Mechanisms of TGF-beta signaling from cell membrane to the nucleus. Cell. 2003;113:685–700. doi: 10.1016/s0092-8674(03)00432-x. [DOI] [PubMed] [Google Scholar]
- Stenvers K. L., Tursky M. L., Harder K. W., Kountouri N., Amatayakul-Chantler S., Grail D., Small C., Weinberg R. A., Sizeland A. M., Zhu H. J. Heart and liver defects and reduced transforming growth factor beta2 sensitivity in transforming growth factor beta type III receptor-deficient embryos. Mol. Cell. Biol. 2003;23:4371–4385. doi: 10.1128/MCB.23.12.4371-4385.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- ten Dijke P., Yamashita H., Sampath T. K., Reddi A. H., Estevez M., Riddle D. L., Ichijo H., Heldin C. H., Miyazono K. Identification of type I receptors for osteogenic protein-1 and bone morphogenetic protein-4. J. Biol. Chem. 1994;269:16985–16988. [PubMed] [Google Scholar]
- Turley R. S., Finger E. C., Hempel N., How T., Fields T. A., Blobe G. C. The type iii transforming growth factor-{beta} receptor as a novel tumor suppressor gene in prostate cancer. Cancer Res. 2007;67:1090–1098. doi: 10.1158/0008-5472.CAN-06-3117. [DOI] [PubMed] [Google Scholar]
- Wang X. F., Lin H. Y., Ng-Eaton E., Downward J., Lodish H. F., Weinberg R. A. Expression cloning and characterization of the TGF-beta type III receptor. Cell. 1991;67:797–805. doi: 10.1016/0092-8674(91)90074-9. [DOI] [PubMed] [Google Scholar]
- Xia Y., Yu P. B., Sidis Y., Beppu H., Bloch K. D., Schneyer A. L., Lin H. Y. epulsive guidance molecule RGMa alters utilization of bone morphogenetic protein (BMP) type II receptors by BMP2 and BMP4. J. Biol. Chem. 2007;282:18129–18140. doi: 10.1074/jbc.M701679200. [DOI] [PubMed] [Google Scholar]
- Yamashita H., ten Dijke P., Huylebroeck D., Sampath T. K., Andries M., Smith J. C., Heldin C. H., Miyazono K. Osteogenic protein-1 binds to activin type II receptors and induces certain activin-like effects. J. Cell Biol. 1995;130:217–226. doi: 10.1083/jcb.130.1.217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yoon B. S., Ovchinnikov D. A., Yoshii I., Mishina Y., Behringer R. R., Lyons K. M. Bmpr1a and Bmpr1b have overlapping functions and are essential for chondrogenesis in vivo. Proc. Natl. Acad. Sci. USA. 2005;102:5062–5067. doi: 10.1073/pnas.0500031102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- You H. J., Bruinsma M. W., How T., Ostrander J. H., Blobe G. C. The type III TGF-beta receptor signals through both Smad3 and the p38 MAP kinase pathways to contribute to inhibition of cell proliferation. Carcinogenesis. 2007;28:2491–2500. doi: 10.1093/carcin/bgm195. [DOI] [PubMed] [Google Scholar]
- Zhao G. Q. Consequences of knocking out BMP signaling in the mouse. Genesis. 2003;35:43–56. doi: 10.1002/gene.10167. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.