Abstract
Clathrin-coated vesicles play an established role in endocytosis from the plasma membrane, but they are also found on internal organelles. We examined the composition of clathrin-coated vesicles on an internal organelle responsible for osmoregulation, the Dictyostelium discoideum contractile vacuole. Clathrin puncta on contractile vacuoles contained multiple accessory proteins typical of plasma membrane–coated pits, including AP2, AP180, and epsin, but not Hip1r. To examine how these clathrin accessory proteins influenced the contractile vacuole, we generated cell lines that carried single and double gene knockouts in the same genetic background. Single or double mutants that lacked AP180 or AP2 exhibited abnormally large contractile vacuoles. The enlarged contractile vacuoles in AP180-null mutants formed because of excessive homotypic fusion among contractile vacuoles. The SNARE protein Vamp7B was mislocalized and enriched on the contractile vacuoles of AP180-null mutants. In vitro assays revealed that AP180 interacted with the cytoplasmic domain of Vamp7B. We propose that AP180 directs Vamp7B into clathrin-coated vesicles on contractile vacuoles, creating an efficient mechanism for regulating the internal distribution of fusion-competent SNARE proteins and limiting homotypic fusions among contractile vacuoles. Dictyostelium contractile vacuoles offer a valuable system to study clathrin-coated vesicles on internal organelles within eukaryotic cells.
INTRODUCTION
Eukaryotic cells internalize both receptors and nutrients from the plasma membrane through clathrin-coated vesicles. During endocytosis, receptors concentrate within clathrin-coated vesicles via interactions with different clathrin adaptors. In addition to binding specific receptors, clathrin adaptors and accessory proteins also promote clathrin assembly on membranes. After the clathrin coat is assembled, the coated vesicle buds and pinches off from the plasma membrane. The internalized cargo is subsequently transported to endosomes or recycling compartments (reviewed by Kirchhausen, 2000; Brodsky et al., 2001; Mousavi et al., 2004; Royle, 2006).
The physiological contributions of clathrin adaptors and accessory proteins are diverse, and some are well documented. AP2 (a tetrameric AP family member), epsin, and AP180/CALM assemble clathrin triskelia into lattices of hexagons and pentagons on the plasma membrane (Ahle and Ungewickell, 1986; Keen, 1987; Prasad and Lippoldt, 1988; Lindner and Ungewickell, 1992; Ye et al., 1995; Ye and Lafer, 1995; Hirst and Robinson, 1998; Hao et al., 1999; Ford et al., 2001; Ford et al., 2002; Owen, 2004; Edeling et al., 2006; Rodemer and Haucke, 2008). In addition to their clathrin assembly ability, AP2, AP180/CALM, and epsin also have additional distinct roles. AP2 recognizes discrete sorting signals formed from peptide motifs on transmembrane cargo (Ohno et al., 1995; Owen and Evans, 1998; Owen et al., 2001; Boll et al., 2002; Kelly et al., 2008). AP180/CALM is implicated in the efficient assembly of clathrin cages of uniform size (Ahle and Ungewickell, 1986; Prasad and Lippoldt, 1988; Ye and Lafer, 1995; Zhang et al., 1998; Nonet et al., 1999; Ford et al., 2001; Ford et al., 2002). Epsin can induce plasma membrane curvature during vesicle invagination in vitro (Itoh et al., 2001; Ford et al., 2002). Sla2/Hip1 family members also play an important role during clathrin-mediated endocytosis and serve as linkers between clathrin and F-actin (McCann and Craig, 1997; Wesp et al., 1997; Engqvist-Goldstein et al., 1999; Yang et al., 1999; Engqvist-Goldstein et al., 2001; Itoh et al., 2001; Mishra et al., 2001; De Camilli et al., 2002; Henry et al., 2002; Legendre-Guillemin et al., 2004; Brett et al., 2006).
In addition to the plasma membrane, clathrin-coated vesicles are observed on internal organelles, including the trans-Golgi network (TGN) and endosomes (Friend and Farquhar, 1967; Stoorvogel et al., 1996). At the TGN, clathrin-coated vesicles transport lysosomal hydrolases to endosomes (Friend and Farquhar, 1967; von Figura and Weber, 1978; Gonzalez-Noriega et al., 1980). At the endosome, clathrin-coated vesicles are involved in sorting Shiga toxin from late endosomes to the TGN (Lauvrak et al., 2004). Endosome-associated clathrin-coated pits have been shown to function in the recycling of transferrin receptors back to the plasma membrane (van Dam and Stoorvogel, 2002). As with coated pits on the plasma membrane, clathrin-associated proteins also are found on coated vesicles that originate from internal organelles. Monomeric Golgi-localized, Gamma-Ear–containing, ADP-ribosylation factor-binding proteins (GGAs) and the tetrameric AP family member AP1 serve as adaptor proteins and bind sorting signals on TGN membrane receptors (Austin et al., 2002; Crottet et al., 2002; Mishra et al., 2002; Shiba et al., 2002). AP1 also localizes on endosomes when retrograde transport between Golgi apparatus and endosomes is blocked (Mallard et al., 1998). With its ability to promote clathrin assembly, AP1 probably also serves as a clathrin assembly protein on TGN and endosomes (Keen, 1987). To date, how clathrin accessory proteins contribute to coated vesicle function on internal organelles is much less understood than how they contribute to plasma membrane coated pits.
Contractile vacuoles are internal organelles found in protists that are important for osmoregulation (reviewed by Allen and Naitoh, 2002). In Dictyostelium cells, the contractile vacuole is formed from a dynamic labyrinth of membranous tubules and bladders (cisternae) that interconnect in a complex network. In hypo-osmotic conditions, contractile vacuoles collect excess water through tubules which both fuse with each other and round up to form bladders that subsequently fuse with the plasma membrane and contract to expel the water into the extracellular space (Gerisch et al., 2002).
Clathrin puncta have been found on Dictyostelium contractile vacuoles (Patterson, 1980; Heuser, 2006; Stavrou and O'Halloran, 2006). Clathrin also contributes to Dictyostelium contractile vacuole function: clathrin light chain mutant–null cells display abnormally large and dysfunctional contractile vacuoles, whereas clathrin heavy chain mutants contain a dispersed contractile vacuole system (O'Halloran and Anderson, 1992b; Wang et al., 2003). Clathrin assembly proteins, AP180 and AP1, are also linked to contractile vacuole function. AP180 labels Dictyostelium contractile vacuoles, and AP180-null cells display abnormally large contractile vacuoles (Stavrou and O'Halloran, 2006). AP1 was not found on the contractile vacuole, but AP1mu1 subunit–null mutants are osmosensitive (Lefkir et al., 2003). At present it is not clear how these clathrin accessory proteins contribute to contractile vacuole function, or how AP180 and clathrin limit the size of contractile vacuoles.
In this study, we found that clathrin-coated vesicles on contractile vacuole bladders contain adaptor proteins AP180, AP2, and epsin but not Hip1r. We also identified an interaction between AP180 and a contractile vacuole-localized SNARE, Vamp7B. Our results suggest a mechanism for how AP180 and coated vesicles contribute to contractile vacuoles by regulating the internal distribution of fusion-competent SNARE proteins.
MATERIALS AND METHODS
Strains and Cell Culture
Dictyostelium discoideum DH1 wild-type cells and all mutant cells were grown on Petri dishes in HL-5 nutrient media supplemented with 0.6% penicillin-streptomycin (GIBCO BRL, Gaithersburg, MD) at 20°C. Cells expressing plasmids were maintained in HL-5 nutrient media supplemented with 0.6% penicillin-streptomycin and 10 microg/ml G418 (geniticin; GIBCO BRL). All the plasmids in this study were introduced in Dictyostelium cells through electroporation as described before (Brady et al., 2008).
Cloning of the AP2A1 Gene and Generation of the Polyclonal Anti-AP2α Antibody
The AP2A1 gene encoding the gene for the α subunit of AP2 (≈3.1kb) was identified from a Dictyostelium genome database (www.dictybase.org) using BLASTp with the full amino acid sequence of the human AP2α subunit gene. DH1 wild-type cells contain a single copy of the AP2A1 gene. The AP2α gene (AP2A1) was amplified from a cDNA library using the Polymerase Chain Reaction (PCR) with primers selected from the coding region. The 5′ primer, 5′-CGGGGTACCATGAG-TATGAATGTTACAAATC-3′ carried a KpnI site in the beginning while the 3′ primer, 5-CGGGGTACCAGCAGCAGCAGCAGCAGCTTGTAAATGAGAGATTAATAAATT-3′ carried a 6 alanine linker and also a KpnI site. The resulting 3.1-kb fragment was cloned into the pTX-GFP expression vector (Levi et al., 2000) using the KpnI site. The AP2A1 gene was then subcloned from the pTX-GFP vector into the glutathione-S-transferase bacterial expression vector pGEX-2T (Smith and Johnson, 1988) using EcoRI and BamHI sites. GST-AP2A1 was transformed into E. coli BL21 cells, and the expressed protein was purified from bacterial lysates as previously described. (O'Halloran and Anderson, 1992a). The purified protein was sent to Cocalico Biologicals (Reamstown, PA) for immunization and generation of rabbit polyclonal antisera. The resulting anti-AP2α polyclonal antibody specifically recognized AP2α in Western blots of Dictyostelium cells (Supplemental Figure S2B).
Generation of Mutant Cell Lines Using Homologous Recombination
To disrupt both AP2A1 (the gene encoding the α subunit of AP2) and clmA (the gene encoding AP180) or epnA (the gene encoding epsin; Stavrou and O'Halloran, 2006; Brady et al., 2008) in Dictyostelium cells, we first disrupted the AP2A1 gene in DH1 wild-type cells. To do so, we amplified a ≈1.08-kb 5′ fragment flanking the coding region (primers 5′-CAAATTCAAAAACAACAAGGAATACCCG-3′ and 5′-GGGTGAAAGATTATCAAATGAATTGCAC-3′) and a ∼1.10 kb 3′ fragment flanking the coding region (primers 5′-TTATAACCACAACTCCCAAATCCTTTTTCAC-3′ and 5′-CCCCAATACCACTTAAATAAATTGTTGC-3′) and subcloned these into the pSP72-pyr plasmid using HindIII/XhoI and EcoRI sites, respectively. pSP72-pyr plasmid is a derivative of pSP72BSR vector (Wang et al., 2002) and has the blasticidin gene replaced by a ∼1.5Kb pyr (pyrimidine biosynthetic) gene from the pRHI30 vector (Insall et al., 1996). The resulting plasmid, pSP72-pyr-ΔAP2A1, was linearized with XhoI and BglII and introduced into wild-type DH1 cells by electroporation (Brady et al., 2008). Each transformation reaction was diluted into FM minimal medium (Formedium, Norwich, United Kingdom) and plated into six 96-well plates. Resulting clonal colonies were expanded and were assessed for the absence of AP2α by Western blot analysis.
To generate the AP2α/AP180 double mutant cell line, we deleted the clmA gene in the AP2α-null cells. To do so, the plasmid pSP72-Bsr-ΔAP180 (Stavrou and O'Halloran, 2006) was linearized with a BamHI and XhoI digestion and transformed into the AP2α-null cells by electroporation. Transformed cells were diluted in FM minimal medium supplemented with 5 μg/ml blasticidin (Bsr) and plated in 96-well plates. Resulting clonal colonies were screened for the absence of both AP180 and AP2α by Western blot analysis.
Using the same pSP72-Bsr-ΔAP180 plasmid, we also generated an AP180-null cell line in the DH1 background as described above. Transformed cells were selected in HL-5 media supplemented with 5 μg/ml blasticidin and verified for the absence of the AP180 protein by Western blot.
To generate the AP2α/epsin double mutant cell line, we deleted the epnA gene in the AP2α-null cells. To do so, pSP72-Bsr-EpsinKO (Brady et al., 2008) was linearized using XhoI/HindIII and EcoRI digestion and transformed in the AP2α-null cells via electroporation. Transformed cells were diluted in FM minimal medium supplemented with 5 microg/ml blasticidin (Bsr) and plated in 96-well plates. Resulting clonal colonies were screened for the absence of both epsin and AP2α by Western blot analysis. The pSP72-Bsr-EpsinKO construct was also used to generate epsin–single null cells in the DH1 background. Transformed cells were selected in HL-5 media supplemented with 5 microg/ml blasticidin and verified for the absence of the epsin protein by Western blot analysis.
Design of Vamp7B and Vti1 GFP-Expression Vectors
The Vamp7B coding region was amplified from a cDNA library using primers designed against sequence DDB_G0277173 from dictyBase. The 5′ primer was 5′-AGGTACC GGATCCATGCCTATTATCTATTCACTTGTAGCAAGAGG-3′ and the 3′ primer was 5′-CTCTAGATTAGGTACCAAATTGGTTTAATAGAGTTTCTAATAATTGT GATGG-3′. The 783-bp product was sequenced and cloned into the BamHI and XbaI sites of pTX-GFP.
The Vti1 coding region was amplified with primers designed against sequence DDB_G0292974 from dictyBase. The 5′ primer was 5′-AGGATCCATGGATGTATT TGAAAGAACCGAACA-3′ and the 3′ primer was 5′-CTCTAGATTATCTTAACCA TTTAAGACAAATGATTAAAGCAATG-3′. The 654-bp product was sequenced and cloned into the BamHI and XbaI sites of pTX-GFP.
Both SNARE constructs result in the expression of proteins tagged with GFP at their N terminus.
Fluorescence Microscopy
Cells in media were settled on coverslips for 15 min. The adhered cells were washed once with PDF buffer (2 mM KCL, 1.1 mM K2HPO4, 1.32 mM KH2PO4, 0.1 mM CaCl2, 0.25 mM MgSO4, pH 6.7), and flattened by overlaying a thin layer of 2% agar (GE Healthcare, Uppsala, Sweden). Then cells were fixed with 1% formaldehyde in 100% methanol at −20°C for 5 min and blocked with 3% BSA (Fisher Scientific, Pittsburgh, PA) in PBS (137 mM NaCl, 2.7 mM KCl, 10 mM Na2HPO4, pH7.4) at 37°C for 15 min. For examining the localization of epsin or Hip1r, cells were fixed following a two-step fixation protocol. Cells were fixed with 2% Formaldehyde and 0.01% Triton X-100 in PDF buffer at room temperature for 15 min and then in 100% methanol at −20°C for 5 min.
Primary antibody was added to the fixed cells and cells were incubated at 37°C for 45 min followed by four washes with PBS. The cells were then incubated with secondary antibody with either a Texas Red tag or a Pacific Blue tag (30 μg/ml; Invitrogen, Carlsbad, CA) at 37°C for 45 min in the dark. After four gentle washes with PBS, the cells were rinsed briefly in distilled water and mounted on microscope slides with mounting media (MOWIOL, Calbiochem, La Jolla, CA).
For immunofluorescence microscopy, anti-AP2α antibody was prepared to reduce background by preabsorption as follows: AP2α-null cells were grown in suspension to a density of 2 × 108cells/ml. Cells were spun down at 1500 rpm for 5 mins, resuspended, and incubated in 2% formaldehyde in PBS for 5 min at room temperature. The cells then were centrifuged at 2000 rpm for 5 min and resuspended in 1% formaldehyde in 100% methanol, and incubated at −20°C for 5 min. Next, the cells were centrifuged at 2000 rpm for 5 min and resuspended in 1.5 ml of 3% BSA in PBS with 0.02% sodium azide. Anti-AP2α antiserum was added to prepared cells at a 1:500 dilution and incubated at 4°C overnight. The supernatant of this antibody–cell mix was added to a new aliquot of prepared AP2α-null cells and incubated at 4°C overnight. This was repeated at least three times. Clathrin light chain antibody was prepared in the same way as anti-AP2α antibody but using clathrin light chain–null cells to preabsorb. Affinity purified Hip1r antibody was prepared according to (Repass et al., 2007). mAb p80 and polyclonal antibody Rh50 were gifts from Dr. Pierre Cosson (University of Geneva, Geneva, Switzerland).
Colocalization of clathrin and accessory proteins was determined by counting puncta in each fluorescence channel. Puncta were defined as foci of 3 to 6 pixels with an intensity 100 times higher than background. Puncta in one channel were considered colocalized with puncta in another channel if the distance between the centers of each was less than one pixel.
Cells were visualized using differential interference contrast microscopy (DIC) and fluorescence microscopy on a NIKON Eclipse TE 200 microscope. Images were acquired on a Photometrics cooled CCD camera, processed using Metamorph 5.0 software (Universal Imaging, Downington, PA). For imaging contractile vacuole dynamics under DIC, cells were allowed to attach in microscopy chambers in nutrient media. Another set of cells was allowed to attach in chambers first in nutrient media and then in distilled water to provide a hypotonic environment. Images of living cells were captured every 3 s for ≈10 min and compiled into quick time movies using Metamorph software played at 6 frames per second. Still images from time lapse microscopy were used to measure the diameter of fully expanded round contractile vacuoles. The maximum diameter size of contractile vacuoles was measured using the 100 × calibrated distance tool in Metamorph software. Cells expressing GFP-CLC and GFP-Vamp7B were imaged in the same way except that low fluorescence media (http:dictybase.org/techniques/media/lowflo_medium.html) was used instead of nutrient HL-5 media.
GST Pull-Down
The Dictyostelium Vamp7B cytosolic domain is found at N-terminal amino acids 1-190 (Bogdanovic et al., 2002). The first 1 to 186 amino acids of this cytosolic domain were amplified by the PCR using the Vamp7B full-length sequence in the pTX-GFP-Vamp7B construct as template and primers selected from the coding region of the gene: 5′ primer, 5′-CGCGGATCCATGCC TATTATCTATTC-3′ carried a BamHI site in the beginning, whereas the 3′ primer, 5′-CGCGGATCCTCATTTCCACCACATTGCAC-3′ carried a stop codon and also a BamHI restriction site. The resulting 558-bp fragment was cloned into the glutathione-S-transferase bacterial expression vector pGEX-2T (Smith and Johnson, 1988) using the EcoRI and BamHI sites. GST-Vamp7B cytosolic domain was transformed into E. coli BL21 cells, and the expressed protein was purified from bacterial lysates as previously described (O'Halloran and Anderson, 1992a).
For doing the GST pull-down, 5 × 106 DH1 wild-type cells expressing pTX-GFP-AP180 (Stavrou and O'Halloran, 2006) were collected and lysed in 1 ml binding buffer (Vithalani et al., 1998). 1 ml cell lysate supernatant was collected and incubated for 2 h at 4°C with 400 microliter Glutathione agarose beads saturated with purified GST-Vamp7B cytosolic domain protein. The beads were then collected and washed 10 times with 1 ml of the same binding buffer at 4°C. The bound fraction was collected by boiling the beads with 400 microliter hot sample buffer for 5 min. Beads saturated with GST protein and DH1 cells expressing only pTX-GFP were used as negative controls.
RESULTS
Dictyostelium Contractile Vacuole Clathrin Punctae Contain AP2, AP180, and Epsin but not Hip1r
To explore the diversity of clathrin adaptors on the contractile vacuole, we developed reagents that allowed us to determine the distribution of clathrin, AP2, AP180, epsin, and Hip1r in Dictyostelium (Materials and Methods; Stavrou and O'Halloran, 2006; Repass et al., 2007; Brady et al., 2008). To compare the distribution of these clathrin adaptors on the contractile vacuole versus the plasma membrane, we performed double and triple staining immunofluorescence experiments on wild-type Dictyostelium cells (Figure 1). Cells coexpressing red fluorescent protein–tagged clathrin light chain (RFP-CLC) and green fluorescent protein–tagged AP180 (GFP-AP180) were immunostained with our anti-AP2α polyclonal antibody and a secondary antibody conjugated with Pacific Blue. We found that 78% of plasma membrane associated clathrin punctae contained AP2, and 69% contained both AP2 and AP180 (Figure 1A, n = 221 clathrin punctae; 27 cells). This extensive colocalization indicated that the majority of plasma membrane clathrin-coated vesicles have both AP2 and AP180.
Figure 1.
The majority of clathrin punctae on the plasma membrane (A) and the contractile vacuole (B–D) contain AP2, AP180, and epsin, but not Hip1r. (A and B) Wild-type cells expressing GFP-AP180 (green) and RFP-clathrin light chain (red) were immunostained for AP2α (blue). (C) Epsin localizes on GFP-AP180-labeled contractile vacuoles. Wild-type cells expressing GFP-AP180 (green) and epsin-RFP (red) were immunostained with a contractile vacuole marker, Rh50 (blue). (D) Hip1r does not localize on AP180-labeled contractile vacuoles. Wild-type cells expressing GFP-AP180 (green) were immunostained for Hip1r (red). Contractile vacuoles are indicated by arrows. Scale bar, 10 μm.
We compared this distribution with the composition of assembly proteins in clathrin-coated vesicles on Dictyostelium contractile vacuoles (Figure 1B and Supplemental Figure S3). To enhance contractile vacuole activity, we immersed cells in water, and then fixed them for immunofluorescence microscopy. Cells expressing RFP-CLC and GFP-AP180 were fixed and stained with anti-AP2α antibody followed by a secondary antibody conjugated with Pacific Blue. We found that, like clathrin pits on the plasma membrane, most clathrin on contractile vacuoles contained both assembly proteins: 85% of clathrin punctae were labeled with AP2, and 82% of these clathrin punctae contained both AP2 and AP180 (Figure 1B, n = 67 clathrin punctae; 13 contractile vacuoles).
The presence of AP2 and AP180 on clathrin-coated vesicles raised the question of whether clathrin-coated vesicles on the contractile vacuoles contain other clathrin accessory proteins typically found at the plasma membrane. We therefore investigated whether epsin or Hip1r are also on contractile vacuoles (Figure 1, C and D; Supplemental Figure S3). We coexpressed RFP-tagged epsin (RFP-epsin) with GFP-AP180 in wild-type cells and stained these cells with an antibody against the contractile vacuole marker Rh50 (Benghezal et al., 2001). We found that epsin also associated with the contractile vacuole and colocalized with AP180 (Figure 1C). To assess Hip1r localization, we stained wild-type cells expressing GFP-AP180 with an anti-Hip1r polyclonal antibody. In contrast with epsin, Hip1r was absent from contractile vacuoles (Figure 1D and Supplemental Figure S3).
Our data suggested that the contractile vacuole is an unusual organelle in that it contains three of the clathrin adaptors normally associated with the plasma membrane: AP2, AP180, and epsin. In contrast, Hip1r was only on the plasma membrane but not on contractile vacuoles. Thus clathrin-coated vesicles on the contractile vacuoles have a similar, but not identical, composition to coated vesicles on the plasma membrane.
Depletion of AP2 and AP180 Causes Synergistic Defects in Osmoregulation
To investigate whether AP2, AP180, and epsin contribute to contractile vacuole function, we generated single mutants that carried deletions in the AP2α (AP2α−), AP180 (AP180−), and epsin (epsin−) genes. We also generated AP2α/AP180 double mutant (AP2α/AP180 DKO) and AP2α/epsin double mutant (AP2α/epsin DKO) cell lines. The absence of the products of AP2α, AP180 or epsin genes was confirmed using Western blot analysis (Supplemental Figures S1 and S2A). Inspection of these cell lines in a hypotonic environment revealed that they all contained prominent contractile vacuoles. This contrasted with absence of large vacuoles seen in clathrin heavy chain–null cells or AP1mu1 subunit–null cells (O'Halloran and Anderson, 1992b; Lefkir et al., 2003). Thus, while clathrin and AP1 play a role in the biogenesis of the contractile vacuole, clathrin-associated proteins could also contribute distinct functions to contractile vacuoles after they have formed.
Clathrin light chain mutants have enlarged contractile vacuoles (Wang et al., 2003). To investigate whether clathrin associated proteins also influence contractile vacuole function, we measured the size of contractile vacuoles in various mutant cell lines in different osmotic environments (Figure 2B). In isotonic nutrient medium, the average maximum diameter of contractile vacuoles in wild-type cells was 3.39 ± 0.10 μm (n = 32 contractile vacuoles). Epsin-null cells displayed contractile vacuoles similar to the wild-type average maximum size (3.41 ± 0.15 μm, n = 20 contractile vacuoles). In contrast, AP2α-null, AP180-null, and the AP2α/AP180–double null cells had enlarged contractile vacuoles (average maximum diameters, AP2α−, 3.59 ± 0.14 μm; AP180−, 4.05 ± 0.20 μm; AP2α/AP180 DKO, 4.88 ± 0.22 μm; n = 21 to 28 contractile vacuoles for each cell line). Whereas the double AP2α/epsin mutant did not show an enhanced defect in contractile vacuole size, relative to the single mutants, the double AP2α/AP180 mutant cells displayed the largest contractile vacuoles of all mutants (4.88 ± 0.22 μm).
Figure 2.
AP2 and AP180 mutant cell lines have enlarged contractile vacuoles. (A) DIC images of wild-type (WT), epsin-null (epsin−), AP2α-null (AP2α−), AP180-null (AP180−), AP2α/AP180–double null (AP2α/AP180 DKO), AP2α/epsin–double null (AP2α/epsin DKO) cell lines in isotonic medium (top) and in water (bottom). Arrowheads indicate contractile vacuoles. Scale bar, 10 μm. (B) Quantification of contractile vacuole diameters in both isotonic (medium) and hypotonic (H2O) condition. Error bar, SE; n = 20 to 61 contractile vacuoles for each condition. (* p <0.05, ** p <0.005, *** p <0.0001).
In water, cells displayed similar defects in their contractile vacuoles (Figure 2B). Contractile vacuoles in wild-type cell and epsin-null cells reached a similar maximum size (average maximum diameters, wild type, 3.48 ± 0.09 μm; epsin−, 3.36 ± 0.07 μm, n = 57 contractile vacuoles for each mutant). However, contractile vacuoles were larger in AP2 and AP180 mutants (average maximum diameters, AP2α−, 3.99 ± 0.13 μm; AP180−, 4.24 ± 0.09 μm, n = 40 to 61 contractile vacuoles for each line). AP2α/epsin–double null cells contained contractile vacuoles similar in size to AP2α–single null cells (average maximum diameter, 3.91 ± 0.12 μm; n = 45 contractile vacuoles). The double AP2α/AP180 mutants exhibited the largest contractile vacuoles (average maximum diameter, 5.20 ± 0.11 μm, n = 56 contractile vacuoles). Despite having abnormally enlarged contractile vacuoles, all mutant cell lines survived immersion in water for at least 22 h (Supplemental Figure S4).
Thus, among all clathrin adaptors, only the absence of AP2 or AP180 contributed to enlarged contractile vacuoles. The absence of epsin did not affect contractile vacuole size in single or double mutants. Because the AP2α/AP180–double null mutant exhibited an enhanced contractile vacuole size phenotype relative to the single mutants, we postulated that AP180 and AP2 had individual contributions to contractile vacuole size. Therefore, we explored the role of AP2 and AP180 in contractile vacuole function in more detail.
Both AP2 and AP180 Recruit Clathrin onto Contractile Vacuoles
To examine the contribution of AP2 and AP180 to clathrin recruitment onto contractile vacuoles, we imaged living cells in water that expressed GFP-tagged clathrin light chain (GFP-CLC). In wild-type, AP2α-null, and AP180-null cells, we frequently observed punctae of clathrin that outlined the circumference of the bladder and remained until the bladder discharged (Figure 3A). However, clathrin punctae were rarely found on the contractile vacuoles of AP2α/AP180–double null cells. We quantified how many contractile vacuole bladders were labeled with clathrin. We scored a contractile vacuole as clathrin-positive if at least one clathrin punctae associated with the bladder for at least 9 s. The majority of clathrin-positive contractile vacuoles had at least five clathrin punctae, whereas some had as many as fifteen clathrin punctae. In wild-type cells, 56% of contractile vacuoles were clathrin positive. In AP2α-null and AP180-null cells, we observed a decrease in clathrin-labeled contractile vacuoles (30% of the contractile vacuoles in AP2α-null cells and 35% in AP180-null were labeled by clathrin (n = 20 cells) (Figure 3B). In AP2α/AP180–double null cells, clathrin was associated with only 15% of contractile vacuoles (n = 20 cells). These differences in the association of clathrin with contractile vacuoles were not caused by different amounts of GFP-CLC expressed in each line (Figure 3C). The enhanced phenotype in clathrin recruitment in the double mutant suggested that both AP2 and AP180 contribute to the recruitment of clathrin to the contractile vacuole.
Figure 3.
The association of clathrin at the contractile vacuole is reduced in AP2α-null or AP180-null mutants. (A) Living wild-type, AP2α−, AP180−, and AP2α/AP180 DKO cells expressing GFP-clathrin light chain in hypotonic condition (water) were imaged using fluorescence microscopy. GFP-CLC punctae decorated contractile vacuoles in the four cell lines (arrows). Scale bar, 10 μm. (B) Quantification of clathrin-positive contractile vacuoles in wild-type, AP2α−, AP180−, and AP2α/AP180 DKO cells. Error bar, SE, n = 20 cells for each cell line (* p <0.05, ** p <0.005, *** p <0.0001). (C) Western blots show equivalent expression levels of clathrin in all cell lines. In each lane, whole cell lysates (1 × 106 cells) were blotted with anti-clathrin heavy chain. Anti-Aurora antibody served as a loading control.
Loss of AP180 but not AP2 Causes an Increase in Fusion among Contractile Vacuoles
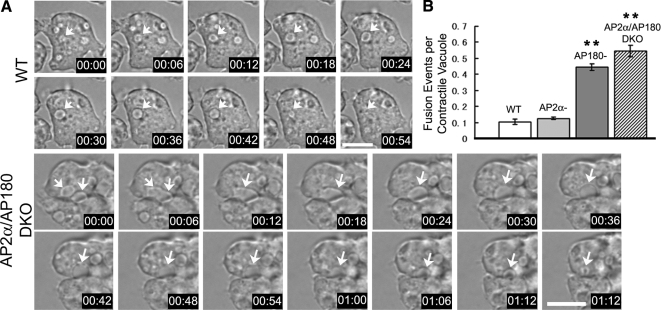
To determine how the absence of AP2 and AP180 influenced contractile vacuoles, we monitored the dynamic behavior of contractile vacuoles in different cell lines by DIC microscopy. In wild-type cells, contractile vacuoles became round as they filled, reached a maximum size, and moved to the membrane to contract and discharge their contents to the extra-cellular space. Occasionally one contractile vacuole would fuse with another and form a single larger contractile vacuole. In mutant cells, contractile vacuoles exhibited similar phases: expansion, contact with the plasma membrane, and discharge. However, in sharp contrast with wild-type contractile vacuoles, we also observed that contractile vacuoles in AP180 mutant cells fused more frequently with each other. This increase in homotypic fusion between contractile vacuoles was observed in AP180–single null cells and in AP2α/AP180–double null cells, but not in AP2α-null cells (Figure 4A, Supplemental Movies S1 and S2).
Figure 4.
Increased fusion of contractile vacuoles in the absence of AP180. (A) Time lapse of living wild-type cells and AP2α/AP180 DKO cells in water. In wild-type cells (WT), a whole life cycle of one contractile vacuole (arrow) is shown, from expansion to contraction. In AP2α/AP180 DKO cells (AP2α/AP180 DKO) two contractile vacuoles (arrows) fuse into a single contractile vacuole (arrow). Scale bar, 10 μm. See supplemental Movies S1 and S2 for the corresponding time lapse movies. (B) Quantification of homotypic fusion of contractile vacuoles in wild-type, AP2α−, AP180−, and AP2α/AP180 DKO cells. Error bar, SE, n = 3 independent experiments, 34 contractile vacuoles for each cell line quantified in each experiment. (*p <0.05, **p <0.005).
Dajumin-GFP is a fluorescent marker that specifically labels both contractile vacuoles bladders and tubules. Using this marker, we observed that wild-type cells displayed a dynamic reticular network of tubules and bladders (Supplemental Movie S3). The tubular network was connected to round bladders, and the tubules were incorporated into the bladders as they expanded. After contraction, bladders collapsed at the plasma membrane. All three mutant cell lines displayed a similar organization of the contractile vacuole network, with bladders and tubules labeled with Dajumin-GFP. In addition, we also observed the increased homotypic fusion among contractile vacuole bladders in the AP180-null and AP2α/AP180–double null cell (Supplemental Movies S4 and S5).
We quantified the fusion frequency by recording how many times two contractile vacuoles fused in each cycle of contractile vacuole expansion and discharge. Because contractile vacuoles were sensitive to UV-light and exhibited altered kinetics, we used time-lapsed DIC images for quantification. In wild-type cells and AP2α–single null cells, the fusion frequency was ≈0.1 event per vacuole lifetime (0.10 ± 0.02 events in wild-type cells, 0.12 ± 0.01 events in AP2α-null cells, n = 3 independent experiments, 34 contractile vacuoles for each cell line). But in AP180 single and AP2α/AP180 double mutant cells, the fusion frequency increased fivefold to ≈0.5 event per vacuole (0.45 ± 0.02 in AP180–single null, 0.55 ± 0.04 in AP2α/AP180 DKO, n = 3 independent experiments, 34 contractile vacuoles for each cell line in each experiment; Figure 4B). These data suggested that the abnormally enlarged contractile vacuoles in AP180-null mutants could be attributed to increased fusion events in contractile vacuoles. In contrast, the enlarged contractile vacuoles in AP2α-null cells were independent of homotypic vacuole fusion.
Loss of AP180 Leads to an Increase in Vamp7B on Contractile Vacuoles
We postulated that the increase in fusion among contractile vacuoles in AP180 mutants could be associated with defects in SNARE protein traffic. Soluble N-ethylmaleimide-sensitive factor attachment protein receptors (SNAREs) are a class of transmembrane proteins that drive fusion between the membranes of vesicles and organelles (Chen and Scheller, 2001). Previous studies have linked AP180 to traffic of the SNARE protein, synaptobrevin. In Drosophila and C.elegans, AP180 is required for recycling synaptobrevin from the plasma membrane to synaptic vesicles (Nonet et al., 1999; Bao et al., 2005). In cultured mammalian cells, CALM, the AP180 nonneuron homologue, is important for the endocytosis of synaptobrevin 2 (Vamp2; Harel et al., 2008). We therefore tested whether the loss of AP180 led to a defect in the traffic of a synaptobrevin-like SNARE on the contractile vacuole.
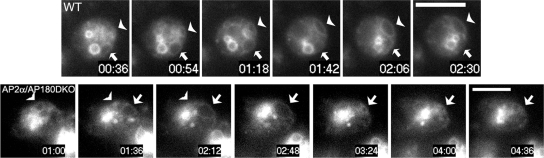
A BLAST search of the Dictyostelium genome with the C. elegans synaptobrevin protein sequence revealed that the closest relative was Dictyostelium SNARE protein Vamp7B (DDB_G0277173). We cloned and expressed a GFP-tagged version of Dictyostelium Vamp7B (GFP-Vamp7B). Inspection of wild-type cells that expressed GFP-tagged Vamp7B revealed that this protein localized on endosomes (Figure 5A), that labeled with the p80 marker (Ravanel et al., 2001; Mercanti et al., 2006). GFP-Vamp7B was also found on the contractile vacuole where it colocalized with clathrin and the contractile vacuole marker Rh50 (Figure 5B). Inspection of living cells that expressed GFP-Vamp7B revealed that Vamp7B was associated throughout the entire cycle of contractile vacuoles in both wild-type and mutant cells, from expansion to contraction, and labeled both tubules and bladders of the organelle (Figure 6; Supplemental Figure S5). In contrast, a related SNARE protein, Vamp7A, was found only on endosomal vesicles but not on the contractile vacuole (Bersuker and De Lozanne, unpublished and (Bennett et al., 2008). Thus, GFP-Vamp7B was a good candidate for a synaptobrevin-related SNARE protein that could be regulated on the contractile vacuole by AP180.
Figure 5.
The SNARE Vamp7B localizes to contractile vacuoles and endosomes and is enriched on the contractile vacuoles of AP180-null cells. (A) Cells expressing GFP-Vamp7B (green) were immunostained with the contractile vacuole marker, Rh50 (red), and the endosomal marker, p80 (blue). Contractile vacuoles are indicated by arrows and endosomes are indicated by arrowheads. Scale bar, 10 μm. (B) Vamp7B-containing contractile vacuoles label with clathrin. Wild-type cells expressing GFP-Vamp7B (green) were immunostained with a contractile vacuole marker, Rh50 (blue), and with anti-clathrin light chain (clathrin) (red). Scale bar, 10 μm. (C) Quantification of Vamp7B-labeled contractile vacuoles in wild-type, AP2α−, AP180−, and AP2α/AP180 DKO cells. Error bar, SE, n = 3 independent experiments. In each experiment 32 to 75 contractile vacuoles in each cell line were scored (*p <0.05, **p <0.005). (D) Western blots show equivalent expression of GFP-Vamp7B. In each lane, whole cell lysates (1 × 106 cells) were stained with anti-GFP antibody. Anti–myosin-heavy-chain antibody was used as a loading control.
Figure 6.
Localization of Vamp7B on contractile vacuoles in living cells. Top row, Images of living wild-type cells in water expressing GFP-Vamp7B. Vamp7B is found on contractile vacuoles as they expand (arrowhead) and as they contract (arrow). See Supplemental Movies S6 and S7. Bottom row, Images of living AP2α/AP180–double null cells in water expressing GFP-Vamp7B. An entire cycle of a contractile vacuole is labeled with GFP-Vamp7B, from expansion to contraction (arrow). The end of a contraction phase of a second contractile vacuole is also labeled with GFP-Vamp7B (arrowhead). Scale bar, 10 μm. See Supplemental Movies S8 and S9.
Inspection of AP2 and AP180 mutant cells that expressed GFP-Vamp7B demonstrated that the Vamp7B SNARE protein sorted properly to contractile vacuoles, and was found on both tubules and bladders through the entire contractile vacuole cycle (Figures 5A and 6, Supplemental Figure S5). However, we observed an important difference in the intensity of GFP-Vamp7B on the contractile vacuoles of AP180-null cells compared with the intensity of GFP-Vamp7B on the contractile vacuoles of wild-type or AP2α cells. In wild-type cells and in AP2α-null cells, endosomal compartments (p80 positive compartments) were labeled more strongly by GFP-Vamp7B than the contractile vacuole (Figure 5A). In contrast, in cells lacking AP180 (AP180-null and AP2α/AP180–double null mutants), labeling by GFP-Vamp7B was equivalent in the contractile vacuoles and the p80-labeled endosomal compartment (Figure 5A). Not only were contractile vacuoles more strongly labeled with Vamp7B, more contractile vacuoles were labeled. In wild-type cell, GFP-Vamp7B labeled 63 ± 2% of the contractile vacuoles bladders (n = 3 independent experiments. 32 to 75 contractile vacuoles for each cell line). The absence of Vamp7B from a population of contractile vacuoles was not caused by variation between cells expressing Vamp7B as we observed cells that contained two contractile vacuoles, one labeled with Vamp7B and one not labeled with Vamp7B. Thus the variation in Vamp7B-labeling of contractile vacuoles could reflect differences in concentrations of Vamp7B during different stages of the contractile vacuole cycle. Similar to wild-type cells, 56% ± 4% contractile vacuoles in AP2α-null cells were labeled with GFP-Vamp7B. In contrast, contractile vacuoles in AP180 single mutant and the AP2α/AP180–double null cells were more uniformly and were more strongly labeled with Vamp7B. These mutants displayed ≈90% of the contractile vacuoles labeled with GFP-Vamp7B (92 ± 2% in AP180-null, 87 ± 3% in AP2α/AP180 DKO, n = 3 independent experiments; 32 to 75 contractile vacuoles for each cell; Figure 5C). Western blotting of the cells lines demonstrated that the different cell lines expressed similar amounts of GFP-Vamp7B (Figure 5D). The increase in the amount of Vamp7B on the contractile vacuole in the absence of AP180 suggested that AP180 could be important for recycling Vamp7B from the contractile vacuole.
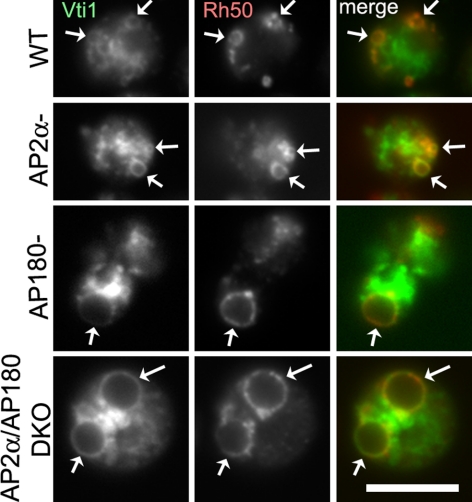
In addition to Vamp7B, we also examined Vti1, a SNARE protein that also localized to the contractile vacuole. This second SNARE allowed us to test whether the requirement for AP180 was specific for Vamp7B, by determining whether the localization of Vti1 required either AP180 or AP2. We found that the distribution of Vti1 in the contractile vacuole of AP180 or AP2α-null cells was indistinguishable from that of wild-type cells (Figure 7). The similar distribution of Vti1 on the contractile vacuoles in wild-type and mutant cells suggested that the defect for Vamp7B in AP180-null cells was specific for Vamp7B and not a general deficit in trafficking contractile vacuole SNARE proteins.
Figure 7.
The SNARE protein Vti1 localizes equivalently on the contractile vacuoles of wild-type and mutant cells. Wild-type, AP2α−, AP180−, and AP2α/AP180 DKO cells expressing GFP-Vti1 (green) were immunostained with the contractile vacuole marker Rh50 (red). In all four cell lines, GFP-Vti1 labels contractile vacuoles (arrows). Scale bar, 10 μm.
Our results suggested that AP180 could participate in the trafficking of Vamp7B perhaps by recycling Vamp7B from the contractile vacuole. To test whether AP180 interacted physically with Vamp7B, we performed a pull-down assay. We used the cytosolic domain of Vamp7B (amino acids 1 to 558) fused to GST (GST-Vamp7B1-558) as a bait to pull-down interacting proteins from Dictyostelium cell lysates. As a control we used lysates from cells expressing GFP alone. We found that GFP-AP180 was specifically pulled down GST-Vamp7B1–558 but not by GST alone, confirming a physical interaction between the two proteins (Figure 8). The interaction between AP180 and Vamp7B was specific: GST-Vamp7B1–558 did not pull down GFP alone, and GST did not pull down GFP-AP180 (Figure 8).
Figure 8.

Interaction of AP180 with Vamp7B. Either the purified GST-Vamp7B cytosolic domain (GST-Vamp7Bctyo) protein or GST (negative control) was incubated with lysates prepared from wild-type cells expressing GFP-AP180. The whole cell lysate (Input) and fractions that did not bind (Unbound; UnB) or that did bind (Bound; B) to glutathione beads were immunoblotted for GFP-AP180, for the AP2 μu2 subunit, or for epsin. To exclude a nonspecific interaction of the GFP tag with the GST-Vamp7B cytosolic domain, the purified GST-Vamp7B cytosolic domain was also incubated with lysates prepared from wild-type cells expressing GFP.
DISCUSSION
Clathrin-coated vesicles were known to localize on the contractile vacuoles of Dictyostelium cells, and clathrin mutants were known to exhibit defects in osmoregulation, yet the composition of proteins in coated vesicles on contractile vacuoles and their functional contribution to vacuole function remained unknown. Our study demonstrated that the clathrin-coated pits of contractile vacuoles contain an array of adaptors remarkably similar to coated pits on the plasma membrane. Two clathrin associated proteins, AP180 and AP2, contribute to the ability of contractile vacuoles to limit their size. The defect in AP180-null cells can be attributed to the mistrafficking of a contractile vacuole SNARE, Vamp7B, which results in excessive homotypic fusion between contractile vacuoles.
AP180 Serves as a Clathrin Adaptor Protein that Retrieves Vamp7B from Contractile Vacuoles
Previous studies have shown that AP180 is required for the proper localization of the SNARE synaptobrevin in metazoans (Nonet et al., 1999; Bao et al., 2005; Harel et al., 2008). We demonstrated that AP180 is required for retrieval of the synaptobrevin homologue, Vamp7B, from Dictyostelium contractile vacuoles. Loss of AP180 resulted in increased labeling of contractile vacuoles with Vamp7B. Concomitant with increased Vamp7B on contractile vacuoles, we also observed increased homotypic fusion among contractile vacuoles, leading to the formation of enlarged vacuoles in AP180 mutant cells. The defect in AP180 mutants was specific for Vamp7B and was not a general SNARE trafficking defect, because another contractile vacuole associated SNARE, Vtil, was not affected in AP180 mutant cells. We demonstrated a physical interaction between AP180 and Vamp7B, as shown by the ability of the GST-Vamp7B cytosolic domain to pull down AP180. The interaction between AP180 and Vamp7B joins a list of other clathrin adaptors and clathrin binding proteins shown to bind SNARE proteins. These include an interaction between the clathrin adaptor epsinR with the endosomal SNARE protein Vti1b, the clathrin-binding ARF-GAP Hrb with SNARE Vamp7, and the clathrin adaptor protein AP1 with the Golgi resident SNARE protein Vamp4 (Peden et al., 2001; Hinners et al., 2003; Miller et al., 2007; Pryor et al., 2008). These interactions between adaptors and SNARES are not mediated by the known linear peptide-binding motifs characteristic of clathrin-adaptor binding, but rather involve interactions between the surfaces of the two proteins (Miller et al., 2007; Pryor et al., 2008). Accordingly, the interaction between Dictyostelium AP180 and Vamp7B is probably also mediated by a surface to surface interaction that remains to be identified.
In wild-type cells, we found that Vamp7B distributes strongly on endosomes labeled with the p80 marker and distributes weakly on the contractile vacuole. What is it doing on these compartments? Mammalian Vamp7 is required for the heterotypic fusion between late endosomes and lysosomes and also during the fusion between lysosomes and the plasma membrane (reviewed by Luzio et al., 2007). By analogy, Dictyostelium Vamp7B could also promote fusion between endosomes or between endosomes and the plasma membrane. The contribution of Vamp7B to contractile vacuole fusion is novel. We speculate that Vamp7B could play a role in mediating homotypic fusion between contractile vacuoles, a known behavior exhibited by contractile vacuoles as they fuse into a dynamic and membranous labyrinth (Clarke et al., 2002). Vamp7B could also promote the heterotypic fusion of Golgi-derived secretory vesicles with the contractile vacuole (Schneider et al., 2000; Gerisch et al., 2004) or the heterotypic fusion of plasma-membrane-derived vesicles that recycle contractile vacuole proteins (Mercanti et al., 2006).
The absence of AP180 was associated with increased labeling of the contractile vacuole by Vamp7B and increased fusion between contractile vacuoles. We propose that AP180 plays a role in recycling Vamp7B from the contractile vacuole. Normally, after secretory or other vesicles fuse with contractile vacuoles, Vamp7B molecules could remain on the contractile vacuoles until NSF (N-ethylmaleimide-sensitive factor) pried apart the resulting SNARE complexes (Sollner et al., 1993). AP180 could bind and recruit Vamp7B into clathrin-coated vesicles to recycle the SNARE back to its originating compartment. Without AP180, Vamp7B would accumulate on contractile vacuoles, resulting in increased homotypic fusion and the formation of abnormally large contractile vacuoles. Thus one of the roles of clathrin vesicles on the contractile vacuole could be recycling SNARE proteins. Given the more severe contractile phenotype of clathrin heavy chain mutant cells, which do not even form contractile vacuoles, there are probably additional functional contributions of clathrin to contractile vacuole physiology.
The Role of AP2 in Contractile Vacuoles
Enlarged contractile vacuoles were also associated with the absence of the adaptor protein AP2, but phenotypic differences suggest that AP2 contributes to contractile vacuole function by a different mechanism than AP180. The contractile vacuoles of AP2α-null cells were enlarged only in a hypotonic environment whereas the vacuoles of AP180-null cells were large in both isotonic and hypotonic environments. In contrast with AP180-null cells, AP2α-null cells had a normal distribution of Vamp7B, and the contractile vacuoles of AP2α-null cells did not fuse excessively. The contractile vacuoles of AP2α/AP180 double mutant cells were significantly larger than those of either single mutant, suggesting a cumulative affect. These differences between AP2α-null and AP180-null cells suggest that AP2 contributes differently to contractile vacuole physiology than AP180. One possibility is that AP2 regulates v-ATPase proton pumps.
The v-ATPase proton pump is universally found on acidic vesicles of the endolysosomal pathway. In addition to this location, the Dictyostelium v-ATPase is a major component of contractile vacuole membranes, found on both the bladder and the tubule network (Heuser et al., 1993; Bush et al., 1994; Clarke et al., 2002). The v-ATPase is important to drive water from the cytosol into the contractile vacuoles (Clarke et al., 2002). In mammalian cells, the v-ATPase on the plasma membrane associates with clathrin, and the AP2 mu2 subunit, the cargo-binding subunit of AP2, binds to v-ATPase in vitro (Marquez-Sterling et al., 1991; Myers and Forgac, 1993; Liu et al., 1994). Thus, it is possible that on Dictyostelium contractile vacuoles, AP2, and clathrin-coated vesicles are involved in recycling the v-ATPase from the contractile vacuole membrane to other organelles, such as endosomes. Without AP2, v-ATPase proteins may accumulate on the contractile vacuole resulting in increased water transport into the bladder when the cells are immersed in a hypotonic environment.
The Contractile Vacuole, a Novel System for Studying Clathrin-Mediated Traffic
The contractile vacuole offers a unique system for the study of how clathrin-coated vesicles can remodel the membrane of a particular organelle. In addition to the plasma membrane, clathrin-coated vesicles have been observed in multiple internal organelles including the TGN, endosomes, and lysosomes. Clathrin-coated vesicles have been found on the contractile vacuoles of a wide variety of protists including the alga Vacuolaria virescens (Heywood, 1978; Patterson, 1980). Our identification of a population of functional clathrin-coated vesicles with multiple clathrin accessory proteins on the Dictyostelium contractile vacuoles offers an experimental system to dissect the contribution of individual proteins of coated pits to contractile vacuoles in a genetically tractable system.
Supplementary Material
ACKNOWLEDGMENTS
We thank members of the O'Halloran lab and the De Lozanne lab for support. We are also thankful to Dr. Pierre Cosson for the gifts of p80 antibody and Rh50 antibody. This work is supported by NIH GM48745 (to A.D.), a University of Texas Research Fellowship (to K.B.), and NIH RO1 GM048625 (to T.J.O.).
Abbreviations used:
- RFP
red fluorescent protein
- DKO
double knock out
- CLC
clathrin light chain.
Footnotes
This article was published online ahead of print in MBC in Press (http://www.molbiolcell.org/cgi/doi/10.1091/mbc.E09-03-0243) on August 19, 2009.
REFERENCES
- Ahle S., Ungewickell E. Purification and properties of a new clathrin assembly protein. EMBO J. 1986;5:3143–3149. doi: 10.1002/j.1460-2075.1986.tb04621.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Allen R. D., Naitoh Y. Osmoregulation and contractile vacuoles of protozoa. Int. Rev. Cytol. 2002;215:351–394. doi: 10.1016/s0074-7696(02)15015-7. [DOI] [PubMed] [Google Scholar]
- Austin C., Boehm M., Tooze S. A. Site-specific cross-linking reveals a differential direct interaction of class 1, 2, and 3 ADP-ribosylation factors with adaptor protein complexes 1 and 3. Biochemistry. 2002;41:4669–4677. doi: 10.1021/bi016064j. [DOI] [PubMed] [Google Scholar]
- Bao H., Daniels R. W., MacLeod G. T., Charlton M. P., Atwood H. L., Zhang B. AP180 maintains the distribution of synaptic and vesicle proteins in the nerve terminal and indirectly regulates the efficacy of Ca2+-triggered exocytosis. J. Neurophysiol. 2005;94:1888–1903. doi: 10.1152/jn.00080.2005. [DOI] [PubMed] [Google Scholar]
- Benghezal M., Gotthardt D., Cornillon S., Cosson P. Localization of the Rh50-like protein to the contractile vacuole in Dictyostelium. Immunogenetics. 2001;52:284–288. doi: 10.1007/s002510000279. [DOI] [PubMed] [Google Scholar]
- Bennett N., Letourneur F., Ragno M., Louwagie M. Sorting of the v-SNARE VAMP7 in Dictyostelium discoideum: a role for more than one Adaptor Protein (AP) complex. Exp. Cell Res. 2008;314:2822–2833. doi: 10.1016/j.yexcr.2008.06.019. [DOI] [PubMed] [Google Scholar]
- Bogdanovic A., Bennett N., Kieffer S., Louwagie M., Morio T., Garin J., Satre M., Bruckert F. Syntaxin 7, syntaxin 8, Vti1 and VAMP7 (vesicle-associated membrane protein 7) form an active SNARE complex for early macropinocytic compartment fusion in Dictyostelium discoideum. Biochem. J. 2002;368:29–39. doi: 10.1042/BJ20020845. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boll W., Rapoport I., Brunner C., Modis Y., Prehn S., Kirchhausen T. The mu2 subunit of the clathrin adaptor AP-2 binds to FDNPVY and YppO sorting signals at distinct sites. Traffic. 2002;3:590–600. doi: 10.1034/j.1600-0854.2002.30808.x. [DOI] [PubMed] [Google Scholar]
- Brady R. J., Wen Y., O'Halloran T. J. The ENTH and C-terminal domains of Dictyostelium epsin cooperate to regulate the dynamic interaction with clathrin-coated pits. J. Cell Sci. 2008;121:3433–3444. doi: 10.1242/jcs.032573. [DOI] [PubMed] [Google Scholar]
- Brett T. J., Legendre-Guillemin V., McPherson P. S., Fremont D. H. Structural definition of the F-actin-binding THATCH domain from HIP1R. Nat. Struct. Mol. Biol. 2006;13:121–130. doi: 10.1038/nsmb1043. [DOI] [PubMed] [Google Scholar]
- Brodsky F. M., Chen C. Y., Knuehl C., Towler M. C., Wakeham D. E. Biological basket weaving: formation and function of clathrin-coated vesicles. Annu. Rev. Cell Dev. Biol. 2001;17:517–568. doi: 10.1146/annurev.cellbio.17.1.517. [DOI] [PubMed] [Google Scholar]
- Bush J., Nolta K., Rodriguez-Paris J., Kaufmann N., O'Halloran T., Ruscetti T., Temesvari L., Steck T., Cardelli J. A Rab4-like GTPase in Dictyostelium discoideum colocalizes with V-H(+)-ATPases in reticular membranes of the contractile vacuole complex and in lysosomes. J. Cell Sci. 1994;107:2801–2812. doi: 10.1242/jcs.107.10.2801. [DOI] [PubMed] [Google Scholar]
- Chen Y. A., Scheller R. H. SNARE-mediated membrane fusion. Nat. Rev. Mol. Cell Biol. 2001;2:98–106. doi: 10.1038/35052017. [DOI] [PubMed] [Google Scholar]
- Clarke M., Kohler J., Arana Q., Liu T., Heuser J., Gerisch G. Dynamics of the vacuolar H(+)-ATPase in the contractile vacuole complex and the endosomal pathway of Dictyostelium cells. J. Cell Sci. 2002;115:2893–2905. doi: 10.1242/jcs.115.14.2893. [DOI] [PubMed] [Google Scholar]
- Crottet P., Meyer D. M., Rohrer J., Spiess M. ARF1.GTP, tyrosine-based signals, and phosphatidylinositol 4,5-bisphosphate constitute a minimal machinery to recruit the AP-1 clathrin adaptor to membranes. Mol. Biol. Cell. 2002;13:3672–3682. doi: 10.1091/mbc.E02-05-0309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Camilli P., Chen H., Hyman J., Panepucci E., Bateman A., Brunger A. T. The ENTH domain. FEBS Lett. 2002;513:11–18. doi: 10.1016/s0014-5793(01)03306-3. [DOI] [PubMed] [Google Scholar]
- Edeling M. A., Smith C., Owen D. Life of a clathrin coat: insights from clathrin and AP structures. Nat. Rev. Mol. Cell Biol. 2006;7:32–44. doi: 10.1038/nrm1786. [DOI] [PubMed] [Google Scholar]
- Engqvist-Goldstein A. E., Kessels M. M., Chopra V. S., Hayden M. R., Drubin D. G. An actin-binding protein of the Sla2/Huntingtin interacting protein 1 family is a novel component of clathrin-coated pits and vesicles. J. Cell Biol. 1999;147:1503–1518. doi: 10.1083/jcb.147.7.1503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Engqvist-Goldstein A. E., Warren R. A., Kessels M. M., Keen J. H., Heuser J., Drubin D. G. The actin-binding protein Hip1R associates with clathrin during early stages of endocytosis and promotes clathrin assembly in vitro. J. Cell Biol. 2001;154:1209–1223. doi: 10.1083/jcb.200106089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ford M. G., Mills I. G., Peter B. J., Vallis Y., Praefcke G. J., Evans P. R., McMahon H. T. Curvature of clathrin-coated pits driven by epsin. Nature. 2002;419:361–366. doi: 10.1038/nature01020. [DOI] [PubMed] [Google Scholar]
- Ford M. G., Pearse B. M., Higgins M. K., Vallis Y., Owen D. J., Gibson A., Hopkins C. R., Evans P. R., McMahon H. T. Simultaneous binding of PtdIns(4,5)P2 and clathrin by AP180 in the nucleation of clathrin lattices on membranes. Science. 2001;291:1051–1055. doi: 10.1126/science.291.5506.1051. [DOI] [PubMed] [Google Scholar]
- Friend D. S., Farquhar M. G. Functions of coated vesicles during protein absorption in the rat vas deferens. J. Cell Biol. 1967;35:357–376. doi: 10.1083/jcb.35.2.357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gerisch G., Benjak A., Kohler J., Weber I., Schneider N. GFP-golvesin constructs to study Golgi tubulation and post-Golgi vesicle dynamics in phagocytosis. Eur J. Cell Biol. 2004;83:297–303. doi: 10.1078/0171-9335-00393. [DOI] [PubMed] [Google Scholar]
- Gerisch G., Heuser J., Clarke M. Tubular-vesicular transformation in the contractile vacuole system of Dictyostelium. Cell Biol. Int. 2002;26:845–852. doi: 10.1006/cbir.2002.0938. [DOI] [PubMed] [Google Scholar]
- Gonzalez-Noriega A., Grubb J. H., Talkad V., Sly W. S. Chloroquine inhibits lysosomal enzyme pinocytosis and enhances lysosomal enzyme secretion by impairing receptor recycling. J. Cell Biol. 1980;85:839–852. doi: 10.1083/jcb.85.3.839. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hao W., Luo Z., Zheng L., Prasad K., Lafer E. M. AP180 and AP-2 interact directly in a complex that cooperatively assembles clathrin. J. Biol. Chem. 1999;274:22785–22794. doi: 10.1074/jbc.274.32.22785. [DOI] [PubMed] [Google Scholar]
- Harel A., Wu F., Mattson M. P., Morris C. M., Yao P. J. Evidence for CALM in directing VAMP2 trafficking. Traffic. 2008;9:417–429. doi: 10.1111/j.1600-0854.2007.00694.x. [DOI] [PubMed] [Google Scholar]
- Henry K. R., D'Hondt K., Chang J., Newpher T., Huang K., Hudson R. T., Riezman H., Lemmon S. K. Scd5p and clathrin function are important for cortical actin organization, endocytosis, and localization of sla2p in yeast. Mol. Biol. Cell. 2002;13:2607–2625. doi: 10.1091/mbc.E02-01-0012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heuser J. Evidence for recycling of contractile vacuole membrane during osmoregulation in Dictyostelium amoebae–a tribute to Gunther Gerisch. Eur J. Cell Biol. 2006;85:859–871. doi: 10.1016/j.ejcb.2006.05.011. [DOI] [PubMed] [Google Scholar]
- Heuser J., Zhu Q., Clarke M. Proton pumps populate the contractile vacuoles of Dictyostelium amoebae. J. Cell Biol. 1993;121:1311–1327. doi: 10.1083/jcb.121.6.1311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heywood P. Osmoregulation in the alga Vacuolaria virescens. Structure of the contractile vacuole and the nature of its association with the Golgi apparatus. J. Cell Sci. 1978;31:213–224. doi: 10.1242/jcs.31.1.213. [DOI] [PubMed] [Google Scholar]
- Hinners I., Wendler F., Fei H., Thomas L., Thomas G., Tooze S. A. AP-1 recruitment to VAMP4 is modulated by phosphorylation-dependent binding of PACS-1. EMBO Rep. 2003;4:1182–1189. doi: 10.1038/sj.embor.7400018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hirst J., Robinson M. S. Clathrin and adaptors. Biochim. Biophys. Acta. 1998;1404:173–193. doi: 10.1016/s0167-4889(98)00056-1. [DOI] [PubMed] [Google Scholar]
- Insall R. H., Borleis J., Devreotes P. N. The aimless RasGEF is required for processing of chemotactic signals through G-protein-coupled receptors in Dictyostelium. Curr. Biol. 1996;6:719–729. doi: 10.1016/s0960-9822(09)00453-9. [DOI] [PubMed] [Google Scholar]
- Itoh T., Koshiba S., Kigawa T., Kikuchi A., Yokoyama S., Takenawa T. Role of the ENTH domain in phosphatidylinositol-4,5-bisphosphate binding and endocytosis. Science. 2001;291:1047–1051. doi: 10.1126/science.291.5506.1047. [DOI] [PubMed] [Google Scholar]
- Keen J. H. Clathrin assembly proteins: affinity purification and a model for coat assembly. J. Cell Biol. 1987;105:1989–1998. doi: 10.1083/jcb.105.5.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kelly B. T., McCoy A. J., Spate K., Miller S. E., Evans P. R., Honing S., Owen D. J. A structural explanation for the binding of endocytic dileucine motifs by the AP2 complex. Nature. 2008;456:976–979. doi: 10.1038/nature07422. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kirchhausen T. Clathrin. Annu Rev. Biochem. 2000;69:699–727. doi: 10.1146/annurev.biochem.69.1.699. [DOI] [PubMed] [Google Scholar]
- Lauvrak S. U., Torgersen M. L., Sandvig K. Efficient endosome-to-Golgi transport of Shiga toxin is dependent on dynamin and clathrin. J. Cell Sci. 2004;117:2321–2331. doi: 10.1242/jcs.01081. [DOI] [PubMed] [Google Scholar]
- Lefkir Y., de Chassey B., Dubois A., Bogdanovic A., Brady R. J., Destaing O., Bruckert F., O'Halloran T. J., Cosson P., Letourneur F. The AP-1 clathrin-adaptor is required for lysosomal enzymes sorting and biogenesis of the contractile vacuole complex in Dictyostelium cells. Mol. Biol. Cell. 2003;14:1835–1851. doi: 10.1091/mbc.E02-10-0627. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Legendre-Guillemin V., Wasiak S., Hussain N. K., Angers A., McPherson P. S. ENTH/ANTH proteins and clathrin-mediated membrane budding. J. Cell Sci. 2004;117:9–18. doi: 10.1242/jcs.00928. [DOI] [PubMed] [Google Scholar]
- Levi S., Polyakov M., Egelhoff T. T. Green fluorescent protein and epitope tag fusion vectors for Dictyostelium discoideum. Plasmid. 2000;44:231–238. doi: 10.1006/plas.2000.1487. [DOI] [PubMed] [Google Scholar]
- Lindner R., Ungewickell E. Clathrin-associated proteins of bovine brain coated vesicles. An analysis of their number and assembly-promoting activity. J. Biol. Chem. 1992;267:16567–16573. [PubMed] [Google Scholar]
- Liu Q., Feng Y., Forgac M. Activity and in vitro reassembly of the coated vesicle (H+)-ATPase requires the 50-kDa subunit of the clathrin assembly complex AP-2. J. Biol. Chem. 1994;269:31592–31597. [PubMed] [Google Scholar]
- Luzio J. P., Pryor P. R., Bright N. A. Lysosomes: fusion and function. Nat. Rev. Mol. Cell Biol. 2007;8:622–632. doi: 10.1038/nrm2217. [DOI] [PubMed] [Google Scholar]
- Mallard F., Antony C., Tenza D., Salamero J., Goud B., Johannes L. Direct pathway from early/recycling endosomes to the Golgi apparatus revealed through the study of shiga toxin B-fragment transport. J. Cell Biol. 1998;143:973–990. doi: 10.1083/jcb.143.4.973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marquez-Sterling N., Herman I. M., Pesacreta T., Arai H., Terres G., Forgac M. Immunolocalization of the vacuolar-type (H+)-ATPase from clathrin-coated vesicles. Eur J. Cell Biol. 1991;56:19–33. [PubMed] [Google Scholar]
- McCann R. O., Craig S. W. The I/LWEQ module: a conserved sequence that signifies F-actin binding in functionally diverse proteins from yeast to mammals. Proc. Natl. Acad Sci. USA. 1997;94:5679–5684. doi: 10.1073/pnas.94.11.5679. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mercanti V., Blanc C., Lefkir Y., Cosson P., Letourneur F. Acidic clusters target transmembrane proteins to the contractile vacuole in Dictyostelium cells. J. Cell Sci. 2006;119:837–845. doi: 10.1242/jcs.02808. [DOI] [PubMed] [Google Scholar]
- Miller S. E., Collins B. M., McCoy A. J., Robinson M. S., Owen D. J. A SNARE-adaptor interaction is a new mode of cargo recognition in clathrin-coated vesicles. Nature. 2007;450:570–574. doi: 10.1038/nature06353. [DOI] [PubMed] [Google Scholar]
- Mishra S. K., Agostinelli N. R., Brett T. J., Mizukami I., Ross T. S., Traub L. M. Clathrin- and AP-2-binding sites in HIP1 uncover a general assembly role for endocytic accessory proteins. J. Biol. Chem. 2001;276:46230–46236. doi: 10.1074/jbc.M108177200. [DOI] [PubMed] [Google Scholar]
- Mishra S. K., Keyel P. A., Hawryluk M. J., Agostinelli N. R., Watkins S. C., Traub L. M. Disabled-2 exhibits the properties of a cargo-selective endocytic clathrin adaptor. EMBO J. 2002;21:4915–4926. doi: 10.1093/emboj/cdf487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mousavi S. A., Malerod L., Berg T., Kjeken R. Clathrin-dependent endocytosis. Biochem. J. 2004;377:1–16. doi: 10.1042/BJ20031000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Myers M., Forgac M. The coated vesicle vacuolar (H+)-ATPase associates with and is phosphorylated by the 50-kDa polypeptide of the clathrin assembly protein AP-2. J. Biol. Chem. 1993;268:9184–9186. [PubMed] [Google Scholar]
- Nonet M. L., Holgado A. M., Brewer F., Serpe C. J., Norbeck B. A., Holleran J., Wei L., Hartwieg E., Jorgensen E. M., Alfonso A. UNC-11, a Caenorhabditis elegans AP180 homologue, regulates the size and protein composition of synaptic vesicles. Mol. Biol. Cell. 1999;10:2343–2360. doi: 10.1091/mbc.10.7.2343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O'Halloran T. J., Anderson R. G. Characterization of the clathrin heavy chain from Dictyostelium discoideum. DNA Cell Biol. 1992a;11:321–330. doi: 10.1089/dna.1992.11.321. [DOI] [PubMed] [Google Scholar]
- O'Halloran T. J., Anderson R. G. Clathrin heavy chain is required for pinocytosis, the presence of large vacuoles, and development in Dictyostelium. J. Cell Biol. 1992b;118:1371–1377. doi: 10.1083/jcb.118.6.1371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ohno H., Stewart J., Fournier M. C., Bosshart H., Rhee I., Miyatake S., Saito T., Gallusser A., Kirchhausen T., Bonifacino J. S. Interaction of tyrosine-based sorting signals with clathrin-associated proteins. Science. 1995;269:1872–1875. doi: 10.1126/science.7569928. [DOI] [PubMed] [Google Scholar]
- Owen D. J. Linking endocytic cargo to clathrin: structural and functional insights into coated vesicle formation. Biochem. Soc. Trans. 2004;32:1–14. doi: 10.1042/bst0320001. [DOI] [PubMed] [Google Scholar]
- Owen D. J., Evans P. R. A structural explanation for the recognition of tyrosine-based endocytotic signals. Science. 1998;282:1327–1332. doi: 10.1126/science.282.5392.1327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Owen D. J., Setiadi H., Evans P. R., McEver R. P., Green S. A. A third specificity-determining site in mu 2 adaptin for sequences upstream of Yxx phi sorting motifs. Traffic. 2001;2:105–110. doi: 10.1034/j.1600-0854.2001.020205.x. [DOI] [PubMed] [Google Scholar]
- Patterson D. J. Contractile vacuoles and associated structures: their organization and function. Bio Rev. 1980;55:1–46. [Google Scholar]
- Peden A. A., Park G. Y., Scheller R. H. The Di-leucine motif of vesicle-associated membrane protein 4 is required for its localization and AP-1 binding. J. Biol. Chem. 2001;276:49183–49187. doi: 10.1074/jbc.M106646200. [DOI] [PubMed] [Google Scholar]
- Prasad K., Lippoldt R. E. Molecular characterization of the AP180 coated vesicle assembly protein. Biochemistry. 1988;27:6098–6104. doi: 10.1021/bi00416a040. [DOI] [PubMed] [Google Scholar]
- Pryor P. R., Jackson L., Gray S. R., Edeling M. A., Thompson A., Sanderson C. M., Evans P. R., Owen D. J., Luzio J. P. Molecular basis for the sorting of the SNARE VAMP7 into endocytic clathrin-coated vesicles by the ArfGAP Hrb. Cell. 2008;134:817–827. doi: 10.1016/j.cell.2008.07.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ravanel K., de Chassey B., Cornillon S., Benghezal M., Zulianello L., Gebbie L., Letourneur F., Cosson P. Membrane sorting in the endocytic and phagocytic pathway of Dictyostelium discoideum. Eur J. Cell Biol. 2001;80:754–764. doi: 10.1078/0171-9335-00215. [DOI] [PubMed] [Google Scholar]
- Repass S. L., Brady R. J., O'Halloran T. J. Dictyostelium Hip1r contributes to spore shape and requires epsin for phosphorylation and localization. J. Cell Sci. 2007;120:3977–3988. doi: 10.1242/jcs.011213. [DOI] [PubMed] [Google Scholar]
- Rodemer C., Haucke V. Clathrin/AP-2-dependent endocytosis: a novel playground for the pharmacological toolbox? Handb. Exp. Pharmacol. 2008:105–122. doi: 10.1007/978-3-540-72843-6_5. [DOI] [PubMed] [Google Scholar]
- Royle S. J. The cellular functions of clathrin. Cell Mol. Life Sci. 2006;63:1823–1832. doi: 10.1007/s00018-005-5587-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schneider N., Schwartz J. M., Kohler J., Becker M., Schwarz H., Gerisch G. Golvesin-GFP fusions as distinct markers for Golgi and post-Golgi vesicles in Dictyostelium cells. Biol. Cell. 2000;92:495–511. doi: 10.1016/s0248-4900(00)01102-3. [DOI] [PubMed] [Google Scholar]
- Shiba Y., Takatsu H., Shin H. W., Nakayama K. Gamma-adaptin interacts directly with Rabaptin-5 through its ear domain. J. Biochem. (Tokyo) 2002;131:327–336. doi: 10.1093/oxfordjournals.jbchem.a003107. [DOI] [PubMed] [Google Scholar]
- Smith D. B., Johnson K. S. Single-step purification of polypeptides expressed in Escherichia coli as fusions with glutathione S-transferase. Gene. 1988;67:31–40. doi: 10.1016/0378-1119(88)90005-4. [DOI] [PubMed] [Google Scholar]
- Sollner T., Bennett M. K., Whiteheart S. W., Scheller R. H., Rothman J. E. A protein assembly-disassembly pathway in vitro that may correspond to sequential steps of synaptic vesicle docking, activation, and fusion. Cell. 1993;75:409–418. doi: 10.1016/0092-8674(93)90376-2. [DOI] [PubMed] [Google Scholar]
- Stavrou I., O'Halloran T. J. The monomeric clathrin assembly protein, AP180, regulates contractile vacuole size in Dictyostelium discoideum. Mol. Biol. Cell. 2006;17:5381–5389. doi: 10.1091/mbc.E06-06-0531. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stoorvogel W., Oorschot V., Geuze H. J. A novel class of clathrin-coated vesicles budding from endosomes. J. Cell Biol. 1996;132:21–33. doi: 10.1083/jcb.132.1.21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Dam E. M., Stoorvogel W. Dynamin-dependent transferrin receptor recycling by endosome-derived clathrin-coated vesicles. Mol. Biol. Cell. 2002;13:169–182. doi: 10.1091/mbc.01-07-0380. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vithalani K. K., Parent C. A., Thorn E. M., Penn M., Larochelle D. A., Devreotes P. N., De Lozanne A. Identification of darlin, a Dictyostelium protein with Armadillo-like repeats that binds to small GTPases and is important for the proper aggregation of developing cells. Mol. Biol. Cell. 1998;9:3095–3106. doi: 10.1091/mbc.9.11.3095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- von Figura K., Weber E. An alternative hypothesis of cellular transport of lysosomal enzymes in fibroblasts. Effect of inhibitors of lysosomal enzyme endocytosis on intra- and extra-cellular lysosomal enzyme activities. Biochem. J. 1978;176:943–950. doi: 10.1042/bj1760943. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang J., Virta V. C., Riddelle-Spencer K., O'Halloran T. J. Compromise of clathrin function and membrane association by clathrin light chain deletion. Traffic. 2003;4:891–901. doi: 10.1046/j.1600-0854.2003.00144.x. [DOI] [PubMed] [Google Scholar]
- Wang N., Wu W. I., De Lozanne A. BEACH family of proteins: phylogenetic and functional analysis of six Dictyostelium BEACH proteins. J. Cell. Biochem. 2002;86:561–570. doi: 10.1002/jcb.10254. [DOI] [PubMed] [Google Scholar]
- Wesp A., Hicke L., Palecek J., Lombardi R., Aust T., Munn A. L., Riezman H. End4p/Sla2p interacts with actin-associated proteins for endocytosis in Saccharomyces cerevisiae. Mol. Biol. Cell. 1997;8:2291–2306. doi: 10.1091/mbc.8.11.2291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang S., Cope M. J., Drubin D. G. Sla2p is associated with the yeast cortical actin cytoskeleton via redundant localization signals. Mol. Biol. Cell. 1999;10:2265–2283. doi: 10.1091/mbc.10.7.2265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ye W., Ali N., Bembenek M. E., Shears S. B., Lafer E. M. Inhibition of clathrin assembly by high affinity binding of specific inositol polyphosphates to the synapse-specific clathrin assembly protein AP-3. J. Biol. Chem. 1995;270:1564–1568. [PubMed] [Google Scholar]
- Ye W., Lafer E. M. Bacterially expressed F1–20/AP-3 assembles clathrin into cages with a narrow size distribution: implications for the regulation of quantal size during neurotransmission. J. Neurosci. Res. 1995;41:15–26. doi: 10.1002/jnr.490410104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang B., Koh Y. H., Beckstead R. B., Budnik V., Ganetzky B., Bellen H. J. Synaptic vesicle size and number are regulated by a clathrin adaptor protein required for endocytosis. Neuron. 1998;21:1465–1475. doi: 10.1016/s0896-6273(00)80664-9. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.