Abstract
Expression of prohibitin 1 (PHB), a multifunctional protein in the cell, is decreased during inflammatory bowel disease (IBD). Little is known regarding the regulation and role of PHB during intestinal inflammation. We examined the effect of tumor necrosis factor alpha (TNF-α), a cytokine that plays a central role in the pathogenesis of IBD, on PHB expression and the effect of sustained PHB expression on TNF-α activation of nuclear factor-kappa B (NF-κB) and epithelial barrier dysfunction, two hallmarks of intestinal inflammation. We show that TNF-α decreased PHB protein and mRNA abundance in intestinal epithelial cells in vitro and in colon mucosa in vivo. Sustained expression of prohibitin in intestinal epithelial cells in vitro and in vivo (prohibitin transgenic mice, PHB TG) resulted in a marked decrease in TNF-α–induced nuclear translocation of the NF-κB protein p65, NF-κB/DNA binding, and NF-κB–mediated transcriptional activation despite robust IκB-α phosphorylation and degradation and increased cytosolic p65. Cells overexpressing PHB were protected from TNF-α–induced increased epithelial permeability. Expression of importin α3, a protein involved in p50/p65 nuclear import, was decreased in cells overexpressing PHB and in colon mucosa of PHB TG mice. Restoration of importin α3 levels sustained NF-κB activation by TNF-α during PHB transfection. These results suggest that PHB inhibits NF-κB nuclear translocation via a novel mechanism involving alteration of importin α3 levels. TNF-α decreases PHB expression in intestinal epithelial cells and restoration of PHB expression in these cells can protect against the deleterious effects of TNF-α and NF-κB on barrier function.
INTRODUCTION
Prohibitin 1 (PHB) is a ubiquitously expressed, multifunctional protein implicated in cellular processes including mitochondrial function and protein folding (Nijtmans et al., 2000; Artal-Sanz et al., 2003), proliferation control and suppression of oncogenesis (Jupe et al., 1995; McClung et al., 1995; Dell'Dell'Orcoet al., 1996), and transcription regulation (Wang et al., 1999,Wang et al., 2002; Joshi et al., 2007). PHB contains an N-terminal hydrophobic membrane anchoring α1-helix domain and a C-terminal leucine/isoleucine-rich motif that acts as a nuclear export signal (Rastogi et al., 2006; Winter et al., 2007). At the subcellular level, PHB has been localized to the cell and mitochondrial membranes as well as the nucleus (Wang et al., 2002; Bourges et al., 2004; Kolonin et al., 2004; Sharma and Qadri, 2004). Expression of PHB is decreased during ulcerative colitis and Crohn's disease, two forms of inflammatory bowel disease (IBD; Hsieh et al., 2006; Yeo et al., 2006; Theiss et al., 2007a). We recently demonstrated that PHB localizes to the mitochondria in Caco2-BBE cells, a model intestinal epithelial cell line, as well as in native human colonic epithelia and that PHB may play an important role in decreasing the severity of colitis by acting as a regulator of antioxidant response (Theiss et al., 2007a; Theiss et al., 2009). Because our previous studies showed that PHB is decreased in IBD, we assessed the regulation of PHB expression in intestinal epithelial cells.
Tumor necrosis factor alpha (TNF-α) is known to play a central role in the pathogenesis of IBD as evidenced by the successful clinical response of patients treated with anti-TNF-α antibodies (Holtmann and Neurath, 2005). The concentration of TNF-α is increased in serum and stool of IBD patients (Murch et al., 1991; Braegger et al., 1992). The increased level of TNF-α potentiates the production of other proinflammatory cytokines, further promoting the inflammatory process. Mice that overexpress TNF-α due to deletion of a 3′ mRNA destabilizing element (TNFΔARE) develop chronic transmural inflammation of the ileum, cecum, and colon, with features in common with Crohn's disease (Kontoyiannis et al., 1999; Pizarro et al., 2003). It is well established that IBD is associated with a dysfunctional epithelial barrier and that such barrier dysfunction precedes and predicts relapse for IBD patients (Katz and Hollander, 1989; Ma et al., 2004). A major function of the intestinal epithelia is to form a highly effective barrier that limits exposure of luminal antigens to the underlying interstitium. TNF-α has been shown to increase intestinal epithelial cell permeability, which is accompanied by decreased expression and altered localization of the tight junction proteins (Mullin and Snock, 1990; Gitter et al., 2000; Ma et al., 2004). Therefore, TNF-α not only activates proinflammatory signaling, but also contributes to the pathogenesis of IBD by altering intestinal epithelial barrier function.
A major signaling pathway activated by TNF-α is the nuclear factor-kappa B (NF-κB) pathway, which plays a central role in inflammatory responses and also regulates transcription of multiple cytokines including interleukin (IL)-2, IL-6, IL-8, TNF-α, and ICAM-1 (Ali and Mann, 2004), and is required for regulation of apoptosis and proliferation of intestinal epithelia (Steinbrecher et al., 2008). In most cells, the predominant form of NF-κB is comprised of a heterodimer of p50 (NF-κB1) and p65 (Rel-A) that is sequestered in the cytosol complexed to inhibitory IκB proteins, preferentially IκB-α. Activation of the IκB kinase (IKK) complex by various stimuli such as inflammatory cytokines or growth factors, phosphorylates IκB-α at Ser32 and Ser36. IκB-α is subsequently ubiquitinated and degraded, releasing active NF-κB and allowing it to translocate into the nucleus where it alters gene transcription by binding to specific NF-κB–binding sites in gene promoter regions. It was previously shown that TNF-α–induced increase in intestinal epithelial cell permeability is dependent on NF-κB activation (Ma et al., 2004). Given that TNF-α plays a major proinflammatory role during intestinal inflammation, we assessed the regulation of PHB expression by TNF-α and the effect of sustained PHB expression on TNF-α activation of NF-κB and epithelial cell permeability.
MATERIALS AND METHODS
Cell Culture
The Caco2-BBE human intestinal epithelial cell line was utilized as an in vitro model of polarized intestinal epithelium. Caco2-BBE cells were grown as confluent monolayers in DMEM supplemented with penicillin (40 mg/l), streptomycin (90 mg/l), and 10% fetal calf serum. Cells were plated on permeable supports to allow the cells to polarize (pore size, 0.4 μm; Transwell-Clear polyester membranes; Costar Life Sciences, Acton, MA) and cotransfected using LipofectAMINE 2000 (Invitrogen, Carlsbad, CA) with PHB/pcDNA4 (Theiss et al., 2007a) or pcDNA4 vector alone (referred to as vector; Invitrogen). Seventy-two hours after transfection, cells were serum deprived and treated with 10 ng/ml recombinant human TNF-α (R&D Systems, Minneapolis, MN) on the basolateral side of the cell monolayers. This dose was determined to be the minimal dose to decrease PHB levels (see Figure 1), and previous studies have shown that this is the minimal dose to cause a drop in Caco2-BBE transepithelial resistance (TER), indicating barrier dysfunction (Ma et al., 2004). Therefore, in all subsequent experiments TNF-α was used at a dose of 10 ng/ml. All experiments were performed on Caco2-BBE cells between passages 28 and 33.
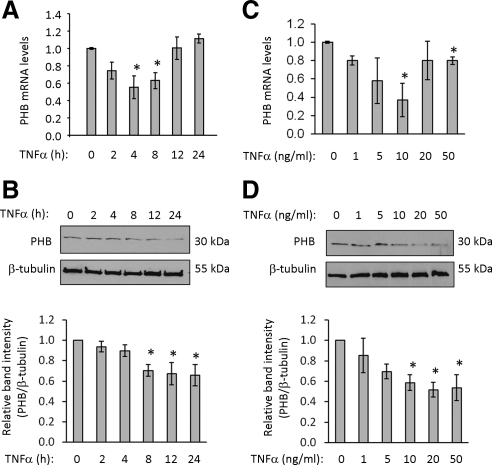
Figure 1.
TNF-α decreases prohibitin mRNA and protein expression in a time- and dose-dependent manner. Quantitative real-time PCR analysis (A) and Western blot (B) from polarized Caco2-BBE cells treated with 10 ng/ml TNF-α for various amounts of time. Histograms show mean ± SEM relative to 0-h time point. *p < 0.05 vs. 0 h; n = 3 per time point. Quantitative real-time PCR analysis (C) and Western blot (D) of Caco2-BBE cells treated with increasing doses of TNF-α for 8 h. Histograms show mean ± SEM relative to 0-h time point. *p < 0.05 vs.0 h; n = 3 per time point.
Animal Studies
All mice were group-housed in standard cages under a controlled temperature (25°C) and photoperiod (12:12-h light-dark cycle) and were allowed standard chow and tap water ad libitum. All procedures using mice were in accordance with the Emory University Institutional Animal Care. Six- to 8-wk-old wild-type (WT) C57BL/6 mice were injected intraperitoneally with 1 μg recombinant murine TNF-α (BioLegend, San Diego, CA) in 200 μl phosphate-buffered saline (PBS) or with PBS alone. Mice were killed by carbon dioxide inhalation 20 h or 48 h after injection, and the colon was removed, opened along the mesenteric border, and stripped of its external muscle by blunt dissection. The mucosa from the colon was collected to measure PHB protein expression.
Generation and characterization of transgenic mice specifically overexpressing prohibitin in intestinal epithelial cells (PHB TG) were previously described (Theiss et al., 2009). PHB TG mice showed a twofold increase in PHB expression in colon mucosa compared with WT mice and were found to be healthy; body weight, breeding, and general appearance were normal. PHB TG mice showed no overt phenotype upon observation of the intestine, including no change in colon length, colon weight, or histology of the colon mucosa. Six- to 8-wk-old WT C57BL/6 and PHB TG littermates were injected intraperitoneally with 1 μg recombinant murine TNF-α in 200 μl PBS or with PBS alone. Mice were killed by carbon dioxide inhalation 3 h after injection, and the colon mucosa was collected and separated into three aliquots for RNA isolation for quantitative real-time PCR, total protein isolation for Western blot, and nuclear/cytosolic protein isolation for electrophoretic mobility shift assay (EMSA).
Human Material
The diagnosis of Crohn's disease was based on clinical, endoscopic, and histologic criteria. Clinical data for Crohn's disease patients were obtained by medical record review. Infectious colitis was ruled out by stool cultures. The collection of samples was approved by the Institutional Review Board of Emory University. Mucosal biopsy specimens were obtained during routine endoscopy that was performed after written informed consent was obtained. Biopsy samples were taken from involved areas of the colon in Crohn's disease patients. Control biopsy samples were collected from volunteers undergoing colonoscopy for colorectal cancer screening who had no overt pathology including polyps. Biopsy specimens were homogenized in lysis buffer containing 1% Triton X-100, 1 Nonidet P-40 (vol/vol), 1 mM disodium ethylenediamine tetraacetate (EDTA), 1 mM sodium orthovanadate, 1 mM sodium fluoride, and 1 μl/ml protease inhibitor mixture III (Boehringer Mannheim, Indianapolis, IN) to obtain protein extracts for Western blotting.
RNA Isolation and Quantitative Real-Time PCR Analysis
Total RNA was isolated from monolayers of Caco2-BBE cells or mouse colon mucosa using TRIzol reagent (Invitrogen). Total RNA was then reverse-transcribed using the Thermoscript RT-PCR system (Invitrogen).Reverse-transcribed cDNA, 50 ng, was amplified by quantitative RT-PCR using 10 μM PHB gene-specific primers and iQ SYBR Green Supermix (Bio-Rad, Hercules, CA) using the following PCR conditions: initial denaturation of one cycle at 95°C for 10 min, followed by amplification at 95°C for 30 s, 55°C for 30 s, and 72°C for 30 s for 35 cycles. Expression level of 18S was used as an internal control. Raw cycle threshold values (Ct values) obtained for TNF-α–treated samples were deducted from the Ct value obtained for internal 18S transcript levels. For graphical representation of quantitative PCR data, the ΔΔCT was calculated as follows: ΔΔCT = (Cttarget − Ctβ-actin)treatment − (Cttarget − Ctβ-actin)nontreatment, with the final graphical data derived from 2−ΔΔCT. The primers utilized for quantitative RT-PCR were designed using human nucleotide sequences available in the GenBank database (Table 1).
Table 1.
Nucleotide sequence of primers used for quantitative real-time PCR analysis
| Name | Forward primer | Reverse primer |
|---|---|---|
| Human PHB | 5′-GGGCACAGAGCTGTCATCTT-3′ | 5′-TGACTGGCACATTACGTGGT-3′ |
| Importin α1 | 5′-GTGTCCTTCTTGGGCAGAAC-3′ | 5′-ATGGCACCTCCATCTACCAC-3′ |
| Importin α3 | 5′-GCTCAGCCACCAGGAAGTTA-3′ | 5′-GCTGGGAAGTGTGAAAGAGC-3′ |
| Importin α4 | 5′-GTCCTTCATCAGTCCCTCCA-3′ | 5′-AGCTGGCAAAATCTCCTGAA-3′ |
| Importin α5 | 5′-AGTAGTGGCCAGGTTTGTGG-3′ | 5′-TGAATCACAATTCGGGTCTG-3′ |
| Importin α6 | 5′-GTGATTGAAACTGGGGCTGT-3′ | 5′-CTGCATTCTGCATTGTCACC-3′ |
| Importin α7 | 5′-ACTCCAAGAGTGGTGGATCG-3′ | 5′-GGTCTGCTGAGAGGTTCCAG-3′ |
| 18S | 5′-CCCCTCGATGCTCTTAGCTGAGTGT-3′ | 5′-CGCCGGTCCAAGAATTTCACCTCT-3′ |
Immunoprecipitation and Western Blot Analysis
Caco2-BBE cells or colon tissue were homogenized in lysis buffer containing 1% Triton X-100, 1% Nonidet P-40 (vol/vol), 1 mM EDTA, 1 mM sodium orthovanadate, 1 mM sodium fluoride, and 1 μl/ml protease inhibitor mixture III to obtain total protein extracts. The samples were separated by SDS-PAGE using Laemmli's 2× SDS sample buffer and 4–20% gradient polyacrylamide gels followed by electrotransfer to nitrocellulose membranes. Membranes were incubated with primary antibodies at 4°C overnight and subsequently were incubated with corresponding peroxidase-conjugated secondary antibodies. Mouse monoclonal PHB antibody was obtained from Lab Vision (Fremont, CA). Mouse monoclonal phospho-IκB-α antibody and rabbit polyclonal total IκB-α antibody were obtained from Cell Signaling Technology (Danvers, MA) and Santa Cruz Biotechnology (Santa Cruz, CA), respectively. Mouse monoclonal p65 antibody was purchased from BD Transduction Laboratories (San Jose, CA). Rat monoclonal importin α1 and importin α3 were obtained from MBL International (Woburn, MA). Mouse monoclonal importin α5 was purchased from Novus Biologicals (Littleton, CO). Immunoreactive proteins were detected using enhanced chemiluminescence (Denville Scientific, South Plainfield, NJ) and exposed to high-performance chemiluminescence film (Denville). Blots were reprobed with anti-β-tubulin (Sigma-Aldrich, St. Louis, MO), anti-β-actin (Sigma) antibody or Nup98, a nucleoporin protein, antibody (for nuclear extracts) or Histone H3 antibody (for nuclear extracts; Upstate Biotechnology, Lake Placid, NY). The Nup98 antibody was a generous gift from Dr. Maureen A. Powers (Emory University, Atlanta, GA), as a loading control. Films were analyzed by densitometry, and signal intensity was quantitated using a gel documentation system (Alpha Innotech, San Leandro, CA).
p65 was immunoprecipitated from 1 mg total cell protein lysates from Caco2-BBE cells with 1 μg mouse anti-p65 antibody (BD Transduction Laboratories) and 30 μl 50% protein A Sepharose beads (GE Healthcare, Piscataway, NJ). Nonspecific proteins were removed by washing two times in cold protein extraction buffer. The samples were separated by SDS-PAGE and transferred to nitrocellulose membranes as described above. Blots were incubated with the rat monoclonal importin α3 antibody and subsequently with the p65 antibody. Omission of primary antibody during the immunoprecipitation was performed as a negative control.
Prohibitin Promoter Reporter Assays
The 5′ flanking region of the human PHB gene was amplified by PCR as described previously (Theiss et al., 2007b). Briefly, human chromosome 17 genomic DNA (clone RP11–1079K10; BacPac Resources, Children's Hospital Oakland Research Institute, Oakland, CA) was used as a template for PCR with the following primers: 5′-GCAAAAGCTTCCTCACAAGTCGGACTCACGC-3′ (underlined nucleotides indicate an HindIII site); 5′-GCAACTCGAGGGAGAAACCCCGTCTCTAC-3′ (underlined nucleotides indicate a XhoI site). After sequence confirmation, the 1192-base pair PCR product was cloned into pGL3 luciferase reporter vector (Promega, Madison, WI) using XhoI and HindIII restriction sites. The DNA sequence of human PHB promoter region has been submitted to GenBank and is available under accession number DQ406856. To determine the importance of the NF-κB site in PHB promoter activity in response to TNF-α, site-specific mutation was introduced into the WT PHB promoter in pGL3 by PCR amplification using the QuikChange II Site-Directed Mutagenesis kit (Stratagene, La Jolla, CA) and sense and antisense primers of the same sequence possessing a mutation in the NF-κB site. the following primers were used: WT sense: 5′-CTGTAATCTCAGCTATTCTGGGAGGGTGAGGCAGGAGAAT-3′; mutant sense: 5′-ATCTCAGCTATTCTGGGAGGAAGAGGCAGGAGAATCGCTT-3′; and mutant antisense: 5′-AAGCGATTCTCCTGCCTCTTCCTCCCAGAATAGCTGAGAT-3′. Nucleotide substitutions are indicated in bold and the WT NF-κB sequence is underlined. The PCR product was sequenced to ensure nucleotide substitution at the NF-κB site.
For reporter gene assays, Caco2-BBE cells were plated onto permeable supports and cotransfected with 1.6 μg of the reporter construct and 20 ng of pRL-CMV (renilla luciferase; Promega) as an internal control. Cells were transfected using LipofectAMINE 2000 (Invitrogen), serum-deprived 72 h after transfection and treated with 10 ng/ml TNF-α for 6 h. Subsets of cells were pretreated with 10, 50, or 100 μM pyrrolidinethiocarbamate (PDTC; Arcos Organics, Morris Plains, NJ), a NF-κB inhibitor, for 30 min before TNF-α treatment. Luciferase activity was measured using the Dual-Luciferase Reporter Assay System (Promega) and a Luminoskan Ascent luminometer (Thermo Electron, Waltham, MA). Relative luciferase was calculated by normalizing firefly luciferase activity to Renilla luciferase activity of the pRL-CMV vector.
EMSA
Nuclear protein extracts were isolated from serum-deprived polarized Caco2-BBE cells treated with 10 ng/ml TNF-α on the basolateral side for 0, 30, 60, or 120 min. Nuclear protein, 10 μg, was assayed for DNA binding to biotin-labeled, double-stranded oligonucleotides corresponding to the putative NF-κB–binding site (5′-GCTATTCTGGGAGGGTGA-3′) in the PHB promoter obtained from Integrated DNA Technologies (Coralville, IA). The putative NF-κB–binding site is underlined.
For consensus NF-κB binding studies, Caco2-BBE cells were transfected with PHB or vector for 72 h, serum-deprived overnight, and treated with 10 ng/ml TNF-α on the basolateral side for 0, 30, 60, or 120 min. Double-stranded oligonucleotides containing the consensus NF-κB–binding site (5′-AGTTGAGGGGACTTTCCCAGGC-3′) were purchased from Promega. The oligonucleotides were end-labeled using the Biotin 3′ End DNA Labeling kit (Pierce, Rockford, IL) according to manufacturer's instructions. EMSAs were performed using the Lightshift Chemiluminescent EMSA kit (Pierce). Biotin-labeled oligonucleotide, 20 fmol, was incubated with 10 μg nuclear proteins for 20 min at room temperature in binding buffer (50 mM Tris, pH 7.4, 2.5 mM EDTA, 0.25 mg/ml poly(dI/dC), 250 mM NaCl, 2.5 mM DTT, 5 mM MgCl2, and 20% glycerol). Binding was competed by fourfold excess unlabeled NF-κB oligonucleotides (cold). NF-κB-binding complexes were resolved by electrophoresis using 5% TBE Criterion gels (Bio-Rad), transferred to Biodyne B Precut Modified Nylon membranes (Pierce), UV cross-linked, and visualized using the Chemiluminescent Nucleic Acid Detection System (Pierce). For NF-κB supershift, 2 μg p50 antibody (Millipore, Billerica, MA) was added after incubation of the 10 μg nuclear proteins with the biotin-labeled oligonucleotides and was subsequently incubated for 30 min at room temperature. For NF-κB supershift using colon mucosa nuclear extracts, 20 μg of nuclear proteins were incubated with biotin-labeled oligonucleotides and 2.5 μg p50 antibody to visualize the supershift.
Measurement of Macromolecular Permeability
Vector- and PHB-transfected Caco2-BBE cells were allowed to grow to confluency on filters. Cells were treated with 10 ng/ml TNF-α on the basolateral side for 48 h, which was previously shown to significantly increase Caco2 permeability (Ma et al., 2004). Fluorescein isothiocyanate (FITC)-dextran (10 mg/ml; molecular weight 4 kDa; Sigma-Aldrich) was then added to the apical side. The apical and basolateral reservoirs were sampled at 30 and 60 min after the addition of FITC-dextran to the apical side. FITC-dextran concentration was quantified via spectrofluorometry (λex = 492 nm, λem = 510 nm). Values are shown as ng/ml/min FITC-dextran present in the basolateral reservoir. On completion of FITC-dextran translocation experiments, total protein or RNA was collected from the cells to ensure PHB overexpression.
Confocal Microscopy
Caco2-BBE cells grown to confluency on filters were fixed in buffered 4% paraformaldehyde for 20 min, blocked in 2% bovine serum albumin, incubated with mouse monoclonal p65 antibody (BD Transduction Laboratories) overnight at 4°C, washed with PBS, and subsequently incubated with fluoresceinated secondary antibodies for 1 h at room temperature. Cell monolayers were counterstained with rhodamine/phalloidin to visualize actin. Cells were also stained with 1 μM TO-PRO-3 iodine (Molecular Probes, Eugene, OR) to visualize nuclei. Samples were mounted in p-phenylenediamine/glycerol (1:1) and analyzed by confocal microscopy (Zeiss dual-laser confocal microscope) as previously described (Sitaraman et al., 2002).
For computerized quantification of p65 nuclear fluorescence staining, MetaMorph Version 7.1.1.0 (Molecular Devices, Downingtown, PA) image analysis software was used. Merged p65 (FITC) and nuclei (Cy5) images were saved as 24-bit color TIFF format. A minimum of six images of cells (taken at 40× magnification) was analyzed per treatment. Before analysis, each image was converted to a black/white reference image. Nuclear p65-positive pixels were selected and displayed with red pixel overlay using threshold parameters that selectively thresholded all the nuclear-p65–positive pixels without including regions of background. Using the total area filter, every separate cluster of thresholded pixels with a minimal size of 20 pixels was defined as one object. The number of objects (p65-positive nuclei) was measured.
NF-κB-Luciferase Reporter Assays
Caco2-BBE cells plated on permeable supports were cotransfected with 20 ng of pRL-CMV (renilla luciferase), vector, or PHB and pGL3-Luc as an empty vector control or the pNF-κB-Luc plasmid (Clontech Laboratories, Mountain View, CA), which contains multiple copies of the NF-κB consensus sequence fused to a TATA-like promoter region from the herpes simplex virus thymidine kinase promoter upstream of the firefly luciferase reporter gene. The reporter gene is activated when endogenous NF-κB proteins bind the multiple copies of the NF-κB sequence. Cells were serum-deprived 72 h after transfection and treated with 10 ng/ml TNF-α on the basolateral side for 6 h. Luciferase activity was measured as described above.
For cotransfection experiments with the importin α3 plasmid, Caco2-BBE cells plated on permeable supports were cotransfected with 20 ng of pRL-CMV (renilla luciferase), the pNF-κB-Luc plasmid, vector or PHB, and importin α3/pCMV6-XL5 (Imp α3) or empty pCMV6-XL5 vector. The imp α3/pCMV6-XL5 and empty pCMV6-XL5 plasmids were purchased from OriGene Technologies (Rockville, MD). Cells were serum-deprived 72 h after transfection and treated with 10 ng/ml TNF-α on the basolateral side for 6 h. Luciferase activity was measured as described above.
Statistical Analysis
Values are expressed as mean ± SEM. Comparisons between treatments in WT cells or mice were analyzed by unpaired Student's t test for significant effects of treatments versus control. Comparisons between treatments and PHB transfection or PHB transgene expression were analyzed by two-way analysis of variance to test for a significant interaction between TNF-α treatment and PHB overexpression. Subsequent pairwise comparisons used Bonferroni post tests to test for significant differences between two particular groups. p < 0.05 was considered statistically significant in all analyses.
RESULTS
TNF-α Decreases Prohibitin mRNA and Protein Expression in a Time- and Dose-dependent Manner
To determine the effect of TNF-α on PHB expression in intestinal epithelial cells, Caco2-BBE cells were treated with TNF-α for various amounts of time and assayed for PHB mRNA and protein expression. PHB mRNA expression is significantly decreased by TNF-α treatment (10 ng/ml) after 4 and 8 h of treatment and returns to basal levels at 12 h after treatment (Figure 1A). PHB protein expression is significantly decreased after 8 h of TNF-α treatment and is sustained through 24 h (Figure 1B). PHB expression was also measured in Caco2-BBE cells treated with increasing doses of TNF-α for 8 h. Although statistically significant only at 10 ng/ml, TNF-α decreases PHB mRNA (Figure 1C) and protein (Figure 1D) in a dose-dependent manner when using 1, 5, and 10 ng/ml TNF-α, which are doses commonly used for TNF-α treatment of cultured intestinal epithelial cells (Ma et al., 2004; Wang et al., 2005). The effect of 20 or 50 ng/ml TNF-α on PHB mRNA expression is not as maximal as 10 ng/ml TNF-α, whereas PHB protein is decreased to the same magnitude at 10, 20, or 50 ng/ml TNF-α. TNF-α at 10 ng/ml was the minimum dose that gave a maximal response, and therefore, all subsequent experiments were performed using this dose.
TNF-α Decreases PHB Protein Expression in Colon Mucosa
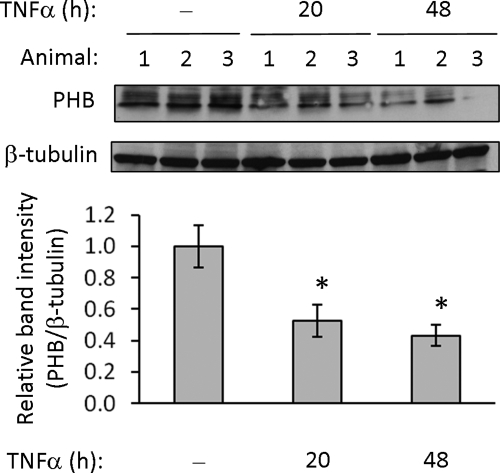
To determine the in vivo effect of increased TNF-α levels on intestinal PHB expression, colon mucosa was collected from mice injected intraperitoneally with TNF-α and assayed for PHB protein expression. Mice injected with TNF-α showed decreased PHB protein expression in colon mucosa compared with mice injected with PBS as a control (Figure 2).
Figure 2.
TNF-α decreases PHB protein expression in colon mucosa. Mice were injected intraperitoneally with 1 μg of recombinant murine TNF-α or PBS. Twenty or 48 h after injection, mice were killed, colon mucosa was collected and total protein was analyzed by immunoblot for PHB and β-tubulin (loading control) expression. Quantitative data shown are mean ± SEM. *p < 0.05; n = 5 per treatment.
TNF-α Decreases PHB Promoter Activation, and NF-κB Is Required for Responsiveness to TNF-α
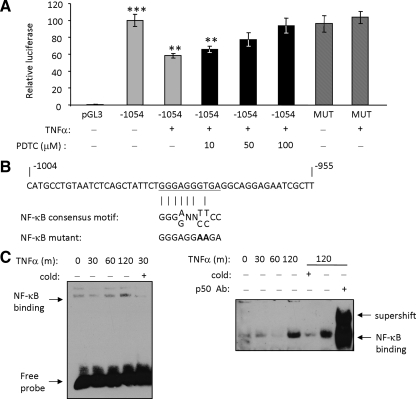
Cells transfected with a full-length PHB promoter luciferase reporter construct as previously described (Theiss et al., 2007b) were treated with TNF-α for 6 h and compared with no-treatment control cells. As shown in Figure 3A, cells treated with TNF-α showed a 45% decrease in promoter activity compared with vehicle-treated cells transfected with full-length promoter (−1054), indicating that TNF-α decreases PHB promoter activation. Because NF-κB is a major downstream signaling molecule of TNF-α (Ali and Mann, 2004), cells were pretreated with various doses of PDTC, an NF-κB inhibitor, for 30 min followed by treatment with TNF-α for 6 h. The TNF-α–induced decrease in PHB promoter activity was dependent on the dose of PDTC used and was completely abolished in cells pretreated with 100 μM PDTC (Figure 3A). These results suggest that the TNF-α–induced decrease in PHB promoter activity is dependent on NF-κB. As shown in Figure 3B, a putative NF-κB–binding site is located in the PHB promoter at −971 to −980 base pairs from the transcription start site (Theiss et al., 2007b). The effect of a mutation introduced in the NF-κB–binding site on promoter activation was assessed by luciferase reporter assays. The TNF-α–induced decrease in PHB promoter activity was abolished when the NF-κB site was mutated (Figure 3A), indicating that the NF-κB site is necessary for PHB promoter responsiveness to TNF-α.
Figure 3.
TNF-α decreases PHB promoter activation and NF-κB is required for responsiveness to TNF-α. (A) Relative luciferase activity was measured in serum-deprived Caco2-BBE cells transfected with pGL3 vector or full-length PHB promoter luciferase reporter (−1054) and treated with TNF-α (10 ng/ml) for 6 h. A subset of cells was treated with various concentrations of pyrrolidinethiocarbamate (PDTC), a NF-κB inhibitor, for 30 min before TNF-α treatment. The NF-κB site in the full-length PHB promoter was mutated by site-directed mutagenesis (MUT) to determine the importance of the NF-κB site in response to TNF-α. All data presented represents the mean ± SEM for firefly luciferase activity normalized to Renilla luciferase activity. ***p < 0.001 vs. pGL3; **p < 0.005 vs. −1054; n = 6 per treatment. (B) The putative NF-κB–binding site in the PHB promoter is underlined and was compared with the consensus NF-κB site. Nucleotides exchanged to mutate the NF-κB site are shown in bold. (C) EMSA showing binding of nuclear proteins from Caco2-BBE cells treated with 10 ng/ml TNF-α for various amounts of time to the NF-κB site in the PHB promoter. Binding is competed by unlabeled NF-κB oligonucleotides (cold) and shifted by the addition of a p50 antibody.
We next performed EMSA to determine whether TNF-α stimulates transcription factor binding to the NF-κB site in the PHB promoter. Nuclear protein extracts show increased binding to the NF-κB site after 2 h of TNF-α treatment (Figure 3C). Binding is competed by unlabeled NF-κB oligonucleotides (cold) and shifted by the addition of a p50 antibody.
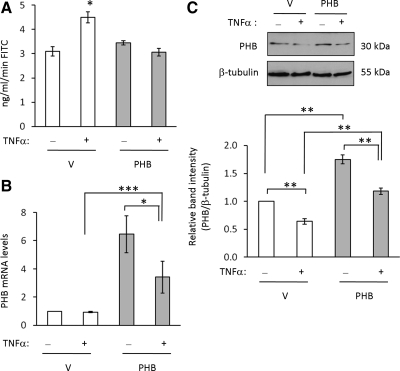
Sustained Expression of PHB Protects against TNF-α–induced Permeability Changes in Intestinal Epithelial Cells
It is widely accepted that TNF-α increases intestinal epithelial cell permeability contributing to mucosal barrier disruption during IBD (Mullin and Snock, 1990; Gitter et al., 2000; Ma et al., 2004). Because our findings showed that TNF-α decreases PHB expression in Caco2-BBE cells, we next determined if restoration of PHB levels by exogenous expression could prevent TNF-α–stimulated increased permeability in Caco2-BBE cells. TNF-α treatment increased permeability compared with untreated control cells in vector-transfected cells (Figure 4A), as shown previously (Ma et al., 2004). PHB-transfected cells were protected from TNF-α–induced increased permeability and showed rates of FITC translocation similar to that of untreated cells (Figure 4A). These data indicate that PHB protects against TNF-α–induced permeability changes in intestinal epithelial cells. To ensure overexpression of PHB by transfection, PHB mRNA (Figure 4B) and protein (Figure 4C) expression were assessed by quantitative real-time PCR analysis and Western immunoblot, respectively. Vector and PHB-overexpressing cells show a very similar fold decrease of PHB protein by TNF-α; vector-transfected cells show a 36% decrease in PHB protein expression when treated with TNF-α (vector = 1.0 ± 0.001; vector + TNF-α = 0.64 ± 0.05; p < 0.01), and PHB-transfected cells show a 33% decrease in PHB protein expression when treated with TNF-α (PHB = 1.75 ± 0.08; PHB + TNF-α = 1.18 ± 0.06; p < 0.01). The total amount of PHB is higher at baseline in PHB-overexpressing cells and therefore the PHB levels in PHB overexpressing cells treated with TNF-α are significantly higher than vector-overexpressing cells treated with TNF-α.
Figure 4.
Sustained expression of PHB protects against TNF-α–induced permeability changes in intestinal epithelial cells. (A) Polarized monolayers of vector- or PHB-transfected Caco2-BBE cells were treated with 10 ng/ml TNF-α on the basolateral side for 48 h. FITC-dextran at 10 mg/ml was then added to the apical side, and the apical and basolateral reservoirs were sampled at 30 and 60 min after the addition of FITC-dextran to the apical side. Histograms show mean ± SEM of ng/ml/min FITC-dextran translocation to the basolateral reservoir. *p = 0.05 vs. no treatment; n = 6 per treatment. (B) To ensure PHB overexpression even during TNF-α treatment, PHB mRNA was measured by quantitative real-time PCR amplification in cells used to assay FITC-dextran translocation 48 h after TNF-α treatment. Histograms show mean ± SEM relative to vector-transfected cells. ***p < 0.001, *p < 0.05; n = 3 per time point performed in duplicate. (C) PHB protein expression was also measured by Western blot to ensure PHB overexpression. Histograms show mean ± SEM relative to vector-transfected cells. **p < 0.01; n = 3 per treatment.
NF-κB Nuclear Translocation, NF-κB/DNA Binding, and NF-κB–mediated Gene Transcription Are Reduced in Caco2-BBE Cells Overexpressing PHB
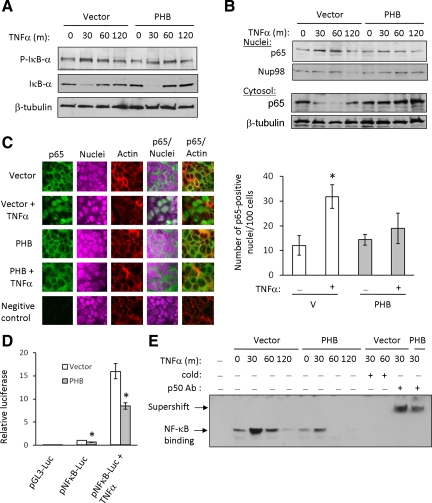
PHB expression is decreased in IBD patients as well as animal models of colitis (Hsieh et al., 2006; Yeo et al., 2006; Theiss et al., 2007a), whereas TNF-α is concomitantly increased (Murch et al., 1991; Braegger et al., 1992). We have shown that TNF-α not only decreases PHB promoter activation, but that NF-κB is essential for this inhibition (Figure 3). We hypothesized that PHB may exert positive feedback regulation on itself at the level of NF-κB signaling. To examine this, we measured NF-κB activation in Caco2-BBE cells overexpressing PHB. Phosphorylation of IκB-α at Ser32 is essential for IκB-α degradation and subsequent NF-κB release. Both vector- and PHB-transfected cells show a marked increase in IκB-α phosphorylation after 30 and 60 min of TNF-α treatment and subsequent IκB-α degradation (Figure 5A). Nuclear and cytosolic extracts were assayed for p65 expression. TNF-α increased nuclear expression and decreased cytosolic expression of p65 in vector-transfected cells, indicating p65 translocation into the nucleus (Figure 5B). Interestingly, PHB-transfected cells show less induction of nuclear p65 expression by TNF-α compared with vector-transfected cells and no decrease of cytosolic p65, suggesting less p65 translocation into the nucleus during PHB overexpression (Figure 5B).
Figure 5.
NF-κB nuclear translocation, NF-κB/DNA binding and NF-κB–mediated gene transcription are reduced in Caco2-BBE cells overexpressing PHB. (A) Representative Western blot of three independent experiments showing phospho-Ser32/36 IκB-α (p-IκB-α), total IκB-α, or β-tubulin (loading control) expression in vector- or PHB-transfected cells treated with 10 ng/ml TNF-α for 0, 30, 60, or 120 min. (B) Nuclear and cytosolic p65 expression in cells transfected with vector or PHB and treated with 10 ng/ml TNF-α. Blots are representative of three independent experiments. (C) Confocal staining of p65 localization (green) in polarized Caco2-BBE cell monolayers. Sections were counterstained with rhodamine/phalloidin and TO-PRO-3 iodine to visualize actin (red) and nuclei (purple), respectively. Vector-transfected cells incubated with normal rabbit serum were used as a negative control. Magnification, ×40. A minimum of six merged p65/nuclear stained images per treatment were analyzed for the number of p65 positive nuclei using computer software. *p = 0.05 vs. all other treatments. (D) Relative luciferase activity of Caco2-BBE cells cotransfected with vector or PHB and pGL3-Luc (empty vector) or the pNF-κB-Luc plasmid. Cells were treated with 10 ng/ml TNF-α for 6 h. *p = 0.05 vs. vector; n = 6. (E) EMSA showing nuclear proteins isolated from TNF-α–treated vector- or PHB-transfected cells binding to a consensus NF-κB site. Binding is competed by unlabeled NF-κB oligonucleotides (cold) and shifted by the addition of a p50 antibody.
To confirm our findings by Western blot, subcellular localization of p65 was assessed by confocal microscopy. Confluent monolayers of Caco2-BBE cells transfected with vector or PHB were treated with TNF-α for 30 min and stained for p65 by immunofluorescence. Control vector- and PHB-transfected cells show predominately cytosolic p65 localization (Figure 5C). Upon TNF-α treatment, p65 rapidly translocates to the nucleus in vector-transfected cells and colocalizes with the nuclear stain; however, in PHB-transfected cells, p65 remains predominately cytosolic after TNF-α treatment. Computer quantification of p65/nuclear-stained merged images show that the number of p65-positive nuclei is increased in vector-transfected cells compared with PHB-overexpressing cells 30 min after TNF-α treatment (Figure 5C).
We next measured NF-κB activation using a luciferase reporter construct. Caco2-BBE cells were cotransfected with vector or PHB and pGL3-Luc as an empty vector control or the pNF-κB-Luc plasmid. pNF-κB-Luc contains multiple copies of the NF-κB consensus sequence fused to a region of the herpes simplex virus thymidine kinase promoter upstream of the firefly luciferase reporter gene. Cells transfected with pNF-κB-Luc plasmid show increased luciferase activity compared with cells transfected with pGL3-Luc (Figure 5D). Interestingly, PHB-transfected cells show decreased basal luciferase activity compared with cells transfected with vector (vector = 1.00 ± 0.03 vs. PHB = 0.63 ± 0.06; p < 0.01; Figure 5D). TNF-α increased luciferase activity ∼16-fold in vector-transfected cells and ∼8.5-fold in PHB-transfected cells compared with untreated cells; PHB overexpression caused a 47% reduction in TNF-α–stimulated NF-κB luciferase activity (Figure 5D).
We next performed EMSA to assess TNF-α–induced transcription factor binding to consensus NF-κB–binding site oligonucleotides. PHB overexpression reduces basal nuclear protein/DNA binding compared with vector-transfected cells (0-time point; Figure 5E), similar to basal pNF-κB-Luc activity (Figure 5D). Nuclear protein extracts show increased binding to the consensus NF-κB site 30 and 60 min after TNF-α treatment in vector-transfected cells, and binding is abolished by 120 min after TNF-α treatment (Figure 5D). In PHB-transfected cells however, nuclear protein extracts show a less robust increase in binding to the NF-κB site 30 min after TNF-α treatment compared with vector-transfected cells, and binding is abolished by 60 min after TNF-α treatment. These results suggest that TNF-α-stimulated transcription factor binding to the consensus NF-κB site is reduced in magnitude and in duration during PHB overexpression. Binding is competed by unlabeled NF-κB oligonucleotides (cold) and shifted by the addition of a p50 antibody (Figure 5E). Collectively, these results suggest that PHB decreases NF-κB activation without affecting degradation of IκB-α, which is in contrast to bacterial and pharmacological inhibitors that modulate NF-κB activation by acting on IκB degradation. These results suggest that PHB inhibits NF-κB activation through a novel mechanism, such as modulating p65 import/export from the nucleus.
NF-κB Activation Is Reduced in Colon Mucosa of PHB TG Mice Treated with TNF-α
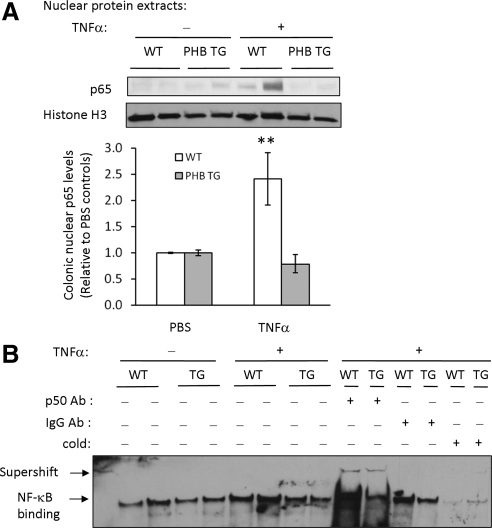
To determine the in vivo effect of PHB overexpression on NF-κB activation, transgenic mice specifically overexpressing prohibitin in intestinal epithelial cells (PHB TG) (Theiss et al., 2009) and WT littermates were injected intraperitoneally with 1 μg recombinant murine TNF-α in 200 μl PBS or with PBS alone and killed 3 h after injection. Colon mucosa from WT and PHB TG mice injected with PBS showed low nuclear expression of p65 (Figure 6A). Administration of TNF-α increased nuclear expression of p65 in colon mucosa of WT mice but not PHB TG mice (Figure 6A).
Figure 6.
NF-κB activation is reduced in colon mucosa of PHB TG mice treated with TNF-α. WT or PHB TG mice were injected intraperitoneally with 1 μg recombinant murine TNF-α or PBS and killed 3 h later to collect colon mucosa. (A) Representative Western blot of p65 expression in nuclear extracts from colon mucosa of WT or PHB TG mice. Blots were reprobed with histone H3 as a loading control. Quantitative data shown are mean ± SEM.**p < 0.01; n = 4 for PBS treatment, n = 5 for TNF-α treatment. (B) EMSA showing binding of 10 μg nuclear proteins isolated from colon mucosa of WT or PHB TG mice to a consensus NF-κB site. Binding is competed by unlabeled NF-κB oligonucleotides (cold) and shifted by the addition of a p50 antibody. To visualize the p50 supershift, double the amount of nuclear protein extract (20 μg) was incubated with NF-κB oligonucleotides and p50 antibody. Extracts incubated with an isotypic control antibody instead of the p65 antibody as a negative control did not supershift (IgG Ab).
We next performed EMSA to assess TNF-α–induced transcription factor binding to NF-κB consensus oligonucleotides. Nuclear extracts isolated from colon mucosa of WT mice injected with TNF-α showed increased binding compared with PBS-injected mice and TNF-α–injected PHB TG mice (Figure 6B). Binding is competed by unlabeled NF-κB oligonucleotides (cold) and shifted by the addition of a p50 antibody (Figure 6B). Extracts incubated with an isotypic control antibody instead of the p50 antibody as a negative control did not supershift (IgG Ab).
PHB Reduces Importin α3 Levels and p65 Association with Importin α3 in Caco2BBE Cells
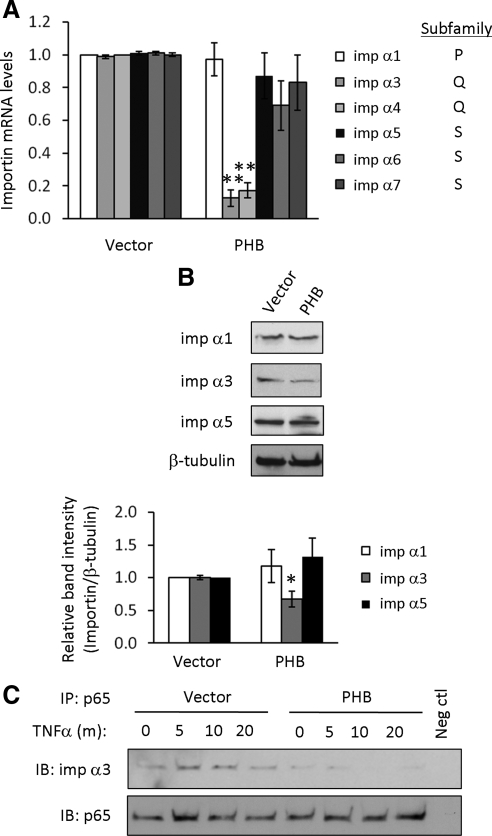
Phosphorylation of NF-κB-bound IκB leads to degradation of IκB and unmasking of the p65 arginine/lysine-rich nuclear localization signal (NLS; Shirakawa and Mizel, 1989). Molecules containing the classical, arginine/lysine-rich NLS are transported into the nucleus by importin α/β heterodimers (Goldfarb et al., 2004). TNF-α–induced nuclear import of p50/p65 heterodimers is mediated by importins α3 and α4, which constitute the Q subfamily of importins based on sequence similarity (Fagerlund et al., 2008). PHB overexpression reduced mRNA expression of importin α3 and α4, but not importin α1, which constitutes the P subfamily, or importin α5, α6, or α7, which constitute the S subfamily (Figure 7A). We pursued importin α3 as a representative importin of the Q subfamily. Cells overexpressing PHB exhibited decreased protein expression of importin α3 compared with cells overexpressing vector but showed no change in importin α1 or α5 protein levels (Figure 7B). To determine the effect of PHB overexpression on the interaction of p65 and importin α3, p65 was immunoprecipitated from total cell lysates from Caco2-BBE cells overexpressing vector or PHB and treated with TNF-α. After immunoprecipitation and SDS-PAGE, membranes were immunoblotted for importin α3 or p65 expression. Less importin α3 coimmunoprecipitated with p65 during basal conditions in cells overexpressing PHB compared with cells overexpressing vector (Figure 7C, top panel, 0-time point), which is likely due to lower levels of importin α3 in cells transfected with PHB. Cells transfected with vector and treated with TNF-α showed increased coimmunoprecipitation of p65/importin α3 after 5 and 10 min of TNF-α treatment, whereas there was no effect of TNF-α treatment on coimmunoprecipitation of p65/importin α3 in cells overexpressing PHB (Figure 7C, top panel). Both vector and PHB-overexpressing cells show increased TNF-α–induced p65 expression as shown by immunoblotting for p65 after immunoprecipitation of p65 (Figure 7C, bottom panel). Collectively, these results suggest that PHB overexpression decreases expression of importin α3 and that TNF-α induces interaction of p65 with importin α3 in vector-transfected cells but not in PHB-transfected cells.
Figure 7.
PHB reduces importin α3 levels and reduces p65 association with importin α3 in Caco2-BBE cells. (A) Quantitative real-time PCR analysis of importin α expression in total RNA isolated from Caco2-BBE cells transfected with vector or PHB for 72 h. Histograms show mean ± SEM. **p < 0.01 vs. vector; n = 3. (B) Representative Western blots showing imp α1, α3, or α5, and β-tubulin (loading control) protein expression in Caco2-BBE cells transfected with PHB or empty vector. Quantitative data shown are mean ± SEM. *p < 0.05; n = 3 per treatment. (C) p65 was immunoprecipitated (IP) from 1 mg total cell lysates from Caco2-BBE cells overexpressing vector or PHB and treated with 10 ng/ml TNF-α. Immunoprecipitates were separated by SDS-PAGE, and membranes were immunoblotted (IB) for imp α3 or p65 expression. Omission of primary antibody during the immunoprecipitation was performed as a negative control.
Importin α3 Expression Is Decreased in Colon Mucosa of PHB TG Mice
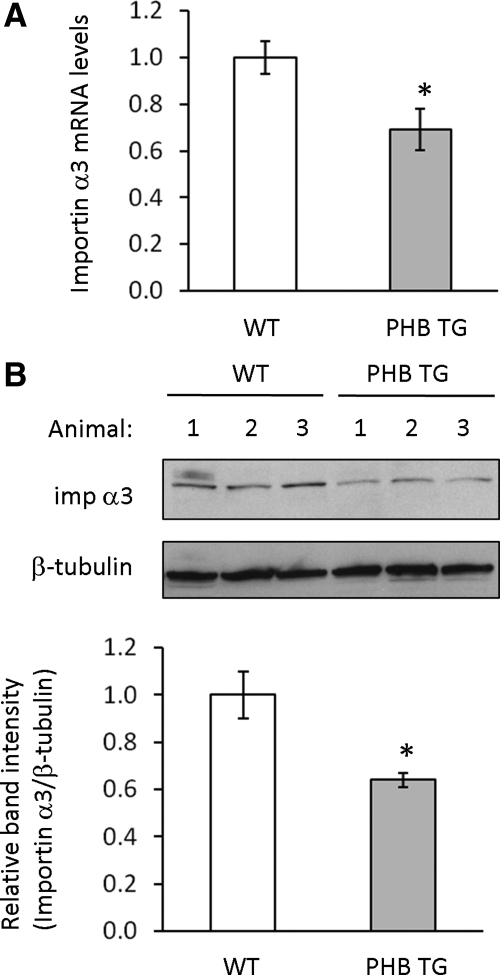
To determine the in vivo effect of PHB overexpression on importin α3 levels, importin α3 mRNA and protein were measured in colon mucosa from WT and PHB TG mice. Importin α3 is decreased in colon mucosa from PHB TG mice compared with WT mice at both the mRNA (Figure 8A) and protein (Figure 8B) levels.
Figure 8.
Importin α3 expression is decreased in colon mucosa of PHB TG mice. (A) Quantitative real-time PCR analysis of importin α3 in total RNA isolated from WT or PHB TG colon mucosa. Histograms show mean ± SEM, *p < 0.05 vs. WT; n = 3. (B) Representative Western blots showing imp α3 and β-tubulin (loading control) protein expression in colon mucosa from three WT mice and three PHB TG mice. Quantitative data shown are mean ± SEM. *p < 0.05; n = 4 per treatment.
Restoration of Importin α3 Levels Reverses the Inhibition of TNF-α–induced NF-κB by PHB
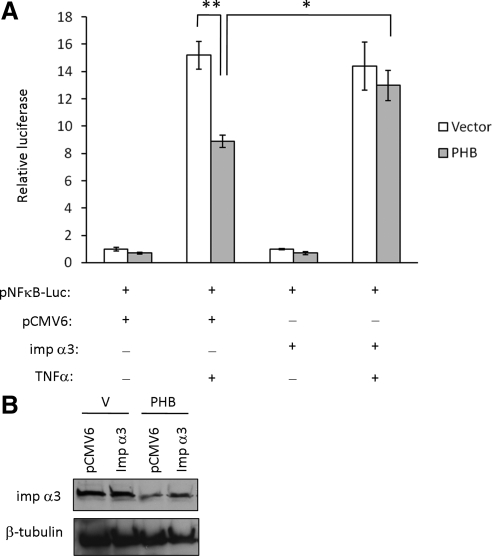
NF-κB–induced luciferase activity was measured in Caco2-BBE cells cotransfected with the pNF-κB-Luc plasmid, vector or PHB, and importin α3/pCMV6-XL5 (imp α3) or empty pCMV6-XL5 vector (pCMV6). Cells were treated with 10 ng/ml TNF-α for 6 h or left untreated. In cells transfected with pCMV6 vector, PHB overexpression decreased basal luciferase activity (vector = 1.00 ± 0.12 vs. PHB = 0.70 ± 0.07; p < 0.05; Figure 9A), which is similar to the results shown in Figure 5D. In cells transfected with pCMV6 vector and treated with TNF-α, PHB overexpression caused a 40% reduction in TNF-α–stimulated NF-κB luciferase activity (Figure 9A), which is also similar to results shown in Figure 5D. In cells transfected with imp α3, PHB overexpression decreased basal luciferase activity similar to pCMV6-transfected cells but the effect was not statistically significant. (vector = 1.00 ± 0.06 vs. PHB = 0.70 ± 0.12; p = 0.08). Interestingly, in cells transfected with Imp α3 and treated with TNF-α, PHB overexpression had no effect on TNF-α–stimulated NF-κB luciferase activity (Figure 9A). These results suggest that restoring importin α3 levels during overexpression of PHB sustains NF-κB activation by TNF-α, indicating that altering the level of importin α3 is the mechanism by which PHB reduces NF-κB activation in intestinal epithelial cells.
Figure 9.
Restoration of importin α3 levels reverses the inhibition of TNF-α–induced NF-κB by PHB. (A) Relative luciferase activity of Caco2-BBE cells cotransfected with the pNF-κB-Luc plasmid, vector, or PHB, and importin α3 (imp α3) or empty pCMV6-XL5 vector. Cells were treated with 10 ng/ml TNF-α for 6 h. Quantitative data shown are mean ± SEM. **p < 0.01, *p < 0.05; n = 3 per treatment. Results are representative of two independent experiments. (B) Importin α3 protein expression was also measured by Western blot to ensure overexpression during PHB transfection.
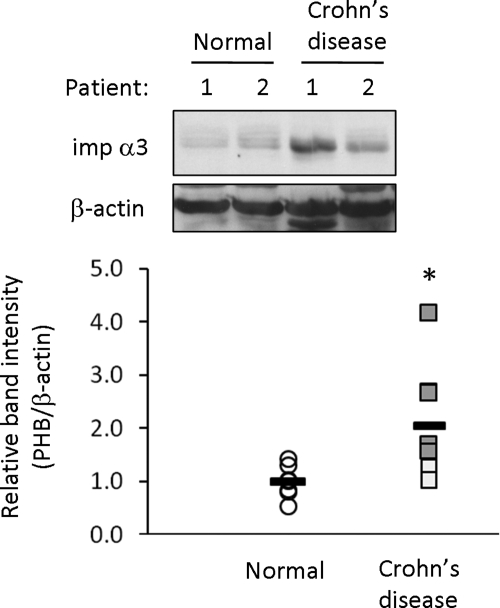
Importin α3 Expression Is Increased in Moderately-to-Severely Inflamed Crohn's Disease Colonic Mucosal Biopsies
We determined whether the decreased expression of PHB in IBD like Crohn's disease correlated with increased importin α3 expression. Importin α3 protein levels are significantly increased in Crohn's disease inflamed mucosal biopsies compared with normal, noninflamed mucosal biopsies (Figure 10). Mildly inflamed biopsies, as determined by the physician during endoscopy, express importin α3 protein levels similar to normal biopsies. Moderately-to-severely inflamed biopsies express high levels of importin α3 compared with normal biopsies, suggesting a direct correlation between severity of inflammation and protein expression of importin α3.
Figure 10.
Importin α3 expression is increased in moderately-to-severely inflamed Crohn's disease colonic mucosal biopsies. Representative Western blots for importin α3 and β-actin (loading control) in total protein extracts isolated from normal colon or Crohn's disease inflamed colon. Mildly inflamed Crohn's disease biopsies are depicted as white boxes; moderately-to-severely inflamed Crohn's disease biopsies are depicted as gray boxes. Each patient biopsy is plotted as an individual dot in the histogram. n = 7 for normal; n = 8 for Crohn's disease; *p < 0.05 vs. normal.
DISCUSSION
PHB is an evolutionarily conserved and multifunctional protein whose expression is decreased during IBD (Hsieh et al., 2006; Yeo et al., 2006; Theiss et al., 2007a). Little is currently known regarding the regulation of PHB expression in the intestine or other tissues. Because TNF-α is known to play a central role in chronic intestinal inflammation (Holtmann and Neurath, 2005), we determined the effect of TNF-α on PHB expression. Here we show that TNF-α decreases prohibitin mRNA and protein expression in a time-dependent manner. TNF-α decreases PHB mRNA and protein in a dose-dependent manner when using 1, 5, and 10 ng/ml TNF-α, which are doses commonly used for TNF-α treatment of cultured intestinal epithelial cells (Ma et al., 2004; Wang et al., 2005), but not at 20 and 50 ng/ml TNF-α. TNF-α is known to exude differing effects at lower (1–10 ng/ml) versus high doses (50 ng/ml), and perhaps at high doses it stimulates posttranscriptional modifications resulting in decreased PHB protein levels without a maximal decrease in mRNA. However, it is not known if TNF-α can induce posttranslational modifications of PHB or if there is a different effect at low versus high doses of TNF-α. We also show that TNF-α reduces PHB promoter activation in cultured intestinal epithelial cells. Further, we demonstrate that the TNF-α–mediated decrease in PHB is transcriptionally mediated through NF-κB, a major downstream signaling molecule of TNF-α. These results demonstrate that TNF-α modulates PHB expression and thus, may contribute to the reduced levels of PHB in the mucosa of IBD patients.
Because we hypothesized that PHB may exert positive feedback regulation on itself, we next addressed whether sustained expression of PHB would diminish the effects of TNF-α on NF-κB activation, which is increased during intestinal inflammation (Rogler et al., 1998) and mediates TNF-α–induced decrease in PHB expression. We therefore measured NF-κB activation in PHB overexpressing cells compared with cells overexpressing vector. Phosphorylation and degradation of IκB-α, an inhibitory protein that sequesters NF-κB in the cytosol, was induced by TNF-α in both vector- and PHB-transfected cells. Conversely, TNF-α caused increased NF-κB activation in vector-transfected cells compared with PHB-transfected cells as shown by nuclear p65 Western blot, nuclear p65 confocal staining, NF-κB-luciferase reporter activation, and nuclear protein/DNA binding to a consensus NF-κB site. Furthermore, EMSA data showed that TNF-α–stimulated transcription factor binding to the consensus NF-κB site is reduced in magnitude and in duration in cells overexpressing PHB. Collectively, these results suggest that PHB decreases NF-κB activation without affecting degradation of IκB-α, which is in contrast to bacterial and pharmacological inhibitors that modulate NF-κB activation by acting on the ubiquitination and degradation of IκB-α.
Nuclear pore complexes (NPC) mediate molecular trafficking through the nuclear envelope between the cytoplasm and nucleus in eukaryotic cells. Large molecules (>25 nm in diameter) require import or export signals to pass through the NPC (Ohno et al., 1998). Molecules containing the classical, arginine/lysine-rich NLS, such as p50 and p65 NF-κB subunits, are transported into the nucleus by importin α/β heterodimers (Goldfarb et al., 2004). Importin α molecules serve as adapters that bind directly to the NLS and link NLS-containing proteins to importin β, which subsequently docks the ternary complex at the cytoplasmic side of the NPC and mediates its translocation into the nucleus (Goldfarb et al., 2004). Six importin α paralogs that show different affinity to particular substrates exist in humans (Kohler et al., 1999). The paralogs are classified into three subfamilies based on sequence similarity: P, importin α1; Q, importin α3 and 4; and S, importin α5, 6, and 7. TNF-α–induced nuclear import of p50/p65 heterodimers is mediated by importins α3 and α4 (Fagerlund et al., 2008). We show here that PHB overexpression in intestinal epithelial cells specifically decreases mRNA expression of importin α3 and 4, the Q subfamily members, without affecting expression of the P or S subfamily members. Expression of importin α3 is decreased during PHB overexpression in vitro and in vivo. In addition, PHB overexpression reduced TNF-α–induced p65/importin α3 association as evidenced by coimmunoprecipitation. Forced expression of importin α3 during PHB transfection restored NF-κB activation by TNF-α as measured by luciferase reporter activation, indicating that altering importin α3 levels is the mechanism by which PHB reduces NF-κB activation in intestinal epithelial cells. Furthermore, importin α3 expression is increased in moderately-to-severely inflamed Crohn's disease colonic mucosal biopsies compared with normal, noninflamed mucosal biopsies. Our previous study showed that PHB levels are decreased in Crohn's disease colonic mucosal biopsies (Theiss et al., 2007a), suggesting an inverse correlation between PHB and importin α3 levels during human intestinal inflammation. Little is known regarding the regulation of importin expression levels. A recent study has shown that alteration of importin expression resulted in physiological changes in neural differentiation due to regulated nuclear import of a specific set of transcription factors (Yasuhara et al., 2007). These results indicate that modulating importin expression can ultimately regulate cell responses. Future studies will elucidate the mechanism by which PHB alters expression of importin α3 in intestinal epithelial cells.
Our study shows that PHB overexpression reduces but does not abolish TNF-α–induced p65 nuclear translocation given that we still see induction of pNF-κB luciferase reporter levels as well as NF-κB/DNA binding by EMSA. With PHB overexpression there is a 40% decrease in importin α3 protein expression both in vitro and in vivo, and therefore, some importin α3 remains to transport p65 into the nucleus which could explain the remaining NF-κB activation. The confocal staining and computer quantitation in Figure 5C shows little nuclear localization of p65 30 min after TNF-α treatment in PHB-transfected cells compared with control cells. Because some NF-κB must enter the nucleus to activate pNF-κB luciferase reporter expression and EMSA binding, perhaps PHB overexpression exports p65 more rapidly and therefore confocal does not show p65 nuclear stain 30 min after TNF-α treatment. Although we focused on the effect of PHB on nuclear import of p65, PHB may also couple this affect with more rapid p65 export to contribute to decreased magnitude and duration of NF-κB activation.
It is well established that NF-κB plays a central role in the pathogenesis of IBD. NF-κB activation is increased in colonic biopsies from IBD patients (Rogler et al., 1998; Schreiber et al., 1998), macrophages and epithelial cells isolated from inflamed IBD tissue have high levels of p65 (Rogler et al., 1998), and the level of activated NF-κB correlates with the severity of disease (Rogler et al., 1998). In epithelial cells, NF-κB not only contributes to the pathogenesis of IBD by altering intestinal barrier function but also activates proinflammatory signaling, potentiating the production of other proinflammatory cytokines by epithelial cells and promoting the inflammatory process. Administration of NF-κB antisense oligonucleotides were reported to improve the severity of colitis in two mouse models of intestinal inflammation (Neurath et al., 1996; Lawrance et al., 2003). Although this would indicate that NF-κB is involved in proinflammatory responses, data from animal models in which NF-κB signaling in intestinal epithelial cells is ablated suggest that complete absence of NF-κB has deleterious effects. For example, specific deletion of intestinal epithelial p65 in mice resulted in deregulated response to injury and inflammation and decreased animal survival (Steinbrecher et al., 2008). Unlike this animal model in which NF-κB signaling is abolished in the intestinal epithelium, we show that PHB overexpression in epithelial cells reduces the magnitude and duration of NF-κB activation by the proinflammatory cytokine TNF-α via decreasing expression of importin α3. In this way, PHB may act to dampen NF-κB signaling in intestinal epithelial cells under normal conditions, whereas this effect is lost during chronic intestinal inflammation when PHB expression is decreased by cytokines such as TNF-α, thereby allowing a heightened NF-κB response.
In conclusion, our results suggest that TNF-α negatively regulates PHB expression in intestinal epithelial cells via NF-κB. Although the most common mechanism of inhibiting NF-κB activation is through the modulation of the IKK complex, we demonstrate that PHB inhibits activation of NF-κB activation via a novel mechanism by decreasing importin α3, a protein involved in p50/p65 nuclear translocation. Reduced levels of PHB during intestinal inflammation may be one underlying factor that leads to TNF-α–induced proinflammatory effects. Along with our recent observation that PHB TG mice are protected from colitis (Theiss et al., 2009), these data suggest that PHB may be a novel therapeutic target for IBD.
ACKNOWLEDGMENTS
This work was supported by a Ruth L. Kirschstein National Research Service Award for Individual Postdoctoral Fellows (F32-DK076243; A.L.T.), Senior Research Award, Crohn's & Colitis Foundation of America Grant 2107 (S.V.S.), The Broad Foundation Grant IBD-0226R (S. V. S.), National Institutes of Health, National Institute of Diabetes and Digestive and Kidney Diseases (NIDDK)Grants R01-DK06411 (S.V.S) and R01-DK061941 (D.M.), and NIDDK research center grant (R24-DK064399).
Abbreviations used:
- IBD
inflammatory bowel disease
- NF-κB
nuclear factor-kappa B
- PHB
prohibitin
- TNF-α
tumor necrosis factor alpha
Footnotes
This article was published online ahead of print in MBC in Press (http://www.molbiolcell.org/cgi/doi/10.1091/mbc.E09-05-0361) on September 2, 2009.
REFERENCES
- Ali S., Mann D. A. Signal transduction via the NF-kappaB pathway: a targeted treatment modality for infection, inflammation and repair. Cell Biochem. Funct. 2004;22:67–79. doi: 10.1002/cbf.1082. [DOI] [PubMed] [Google Scholar]
- Artal-Sanz M., Tsang W. Y., Willems E. M., Grivell L. A., Lemire B. D., van der Spek H., Nijtmans L. G. The mitochondrial prohibitin complex is essential for embryonic viability and germline function in Caenorhabditis elegans. J. Biol. Chem. 2003;278:32091–32099. doi: 10.1074/jbc.M304877200. [DOI] [PubMed] [Google Scholar]
- Bourges I., Ramus C., Mousson de Camaret B., Beugnot R., Remacle C., Cardol P., Hofhaus G., Issartel J. P. Structural organization of mitochondrial human complex I: role of the ND4 and ND5 mitochondria-encoded subunits and interaction with prohibitin. Biochem J. 2004;383:491–499. doi: 10.1042/BJ20040256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Braegger C. P., Nicholls S., Murch S. H., Stephens S., MacDonald T. T. Tumour necrosis factor alpha in stool as a marker of intestinal inflammation. Lancet. 1992;339:89–91. doi: 10.1016/0140-6736(92)90999-j. [DOI] [PubMed] [Google Scholar]
- Dell'Orco R. T., McClung J. K., Jupe E. R., Liu X. T. Prohibitin and the senescent phenotype. Exp. Gerontol. 1996;31:245–252. doi: 10.1016/0531-5565(95)02009-8. [DOI] [PubMed] [Google Scholar]
- Fagerlund R., Melen K., Cao X., Julkunen I. NF-kappaB p52, RelB and c-Rel are transported into the nucleus via a subset of importin alpha molecules. Cell Signal. 2008;20:1442–1451. doi: 10.1016/j.cellsig.2008.03.012. [DOI] [PubMed] [Google Scholar]
- Gitter A. H., Bendfeldt K., Schmitz H., Schulzke J. D., Bentzel C. J., Fromm M. Epithelial barrier defects in HT-29/B6 colonic cell monolayers induced by tumor necrosis factor-alpha. Ann. NY Acad. Sci. 2000;915:193–203. doi: 10.1111/j.1749-6632.2000.tb05242.x. [DOI] [PubMed] [Google Scholar]
- Goldfarb D. S., Corbett A. H., Mason D. A., Harreman M. T., Adam S. A. Importin alpha: a multipurpose nuclear-transport receptor. Trends Cell Biol. 2004;14:505–514. doi: 10.1016/j.tcb.2004.07.016. [DOI] [PubMed] [Google Scholar]
- Holtmann M. H., Neurath M. F. Anti-TNF strategies in stenosing and fistulizing Crohn's disease. Int. J. Colorectal Dis. 2005;20:1–8. doi: 10.1007/s00384-004-0634-0. [DOI] [PubMed] [Google Scholar]
- Hsieh S. Y., Shih T. C., Yeh C. Y., Lin C. J., Chou Y. Y., Lee Y. S. Comparative proteomic studies on the pathogenesis of human ulcerative colitis. Proteomics. 2006;6:5322–5331. doi: 10.1002/pmic.200500541. [DOI] [PubMed] [Google Scholar]
- Joshi B., Rastogi S., Morris M., Carastro L. M., DeCook C., Seto E., Chellappan S. P. Differential regulation of human YY1 and caspase 7 promoters by prohibitin through E2F1 and p53 binding sites. Biochem J. 2007;401:155–166. doi: 10.1042/BJ20060364. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jupe E. R., Liu X. T., Kiehlbauch J. L., McClung J. K., Dell'Orco R. T. Prohibitin antiproliferative activity and lack of heterozygosity in immortalized cell lines. Exp. Cell Res. 1995;218:577–580. doi: 10.1006/excr.1995.1194. [DOI] [PubMed] [Google Scholar]
- Katz K. D., Hollander D. Intestinal mucosal permeability and rheumatological diseases. Baillieres Clin. Rheumatol. 1989;3:271–284. doi: 10.1016/s0950-3579(89)80021-4. [DOI] [PubMed] [Google Scholar]
- Kohler M., Speck C., Christiansen M., Bischoff F. R., Prehn S., Haller H., Gorlich D., Hartmann E. Evidence for distinct substrate specificities of importin alpha family members in nuclear protein import. Mol. Cell Biol. 1999;19:7782–7791. doi: 10.1128/mcb.19.11.7782. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kolonin M. G., Saha P. K., Chan L., Pasqualini R., Arap W. Reversal of obesity by targeted ablation of adipose tissue. Nat. Med. 2004;10:625–632. doi: 10.1038/nm1048. [DOI] [PubMed] [Google Scholar]
- Kontoyiannis D., Pasparakis M., Pizarro T. T., Cominelli F., Kollias G. Impaired on/off regulation of TNF biosynthesis in mice lacking TNF AU-rich elements: implications for joint and gut-associated immunopathologies. Immunity. 1999;10:387–398. doi: 10.1016/s1074-7613(00)80038-2. [DOI] [PubMed] [Google Scholar]
- Lawrance I. C., Wu F., Leite A. Z., Willis J., West G. A., Fiocchi C., Chakravarti S. A murine model of chronic inflammation-induced intestinal fibrosis down-regulated by antisense NF-kappa B. Gastroenterology. 2003;125:1750–1761. doi: 10.1053/j.gastro.2003.08.027. [DOI] [PubMed] [Google Scholar]
- Ma T. Y., Iwamoto G. K., Hoa N. T., Akotia V., Pedram A., Boivin M. A., Said H. M. TNF-alpha-induced increase in intestinal epithelial tight junction permeability requires NF-kappa B activation. Am. J. Physiol. Gastrointest. Liver Physiol. 2004;286:G367–G376. doi: 10.1152/ajpgi.00173.2003. [DOI] [PubMed] [Google Scholar]
- McClung J. K., Jupe E. R., Liu X. T., Dell'Orco R. T. Prohibitin: potential role in senescence, development, and tumor suppression. Exp. Gerontol. 1995;30:99–124. doi: 10.1016/0531-5565(94)00069-7. [DOI] [PubMed] [Google Scholar]
- Mullin J. M., Snock K. V. Effect of tumor necrosis factor on epithelial tight junctions and transepithelial permeability. Cancer Res. 1990;50:2172–2176. [PubMed] [Google Scholar]
- Murch S. H., Lamkin V. A., Savage M. O., Walker-Smith J. A., MacDonald T. T. Serum concentrations of tumour necrosis factor alpha in childhood chronic inflammatory bowel disease. Gut. 1991;32:913–917. doi: 10.1136/gut.32.8.913. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Neurath M. F., Pettersson S., Meyer zum Buschenfelde K. H., Strober W. Local administration of antisense phosphorothioate oligonucleotides to the p65 subunit of NF-kappa B abrogates established experimental colitis in mice. Nat. Med. 1996;2:998–1004. doi: 10.1038/nm0996-998. [DOI] [PubMed] [Google Scholar]
- Nijtmans L. G., de Jong L., Artal Sanz M., Coates P. J., Berden J. A., Back J. W., Muijsers A. O., van der Spek H., Grivell L. A. Prohibitins act as a membrane-bound chaperone for the stabilization of mitochondrial proteins. EMBO J. 2000;19:2444–2451. doi: 10.1093/emboj/19.11.2444. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ohno M., Fornerod M., Mattaj I. W. Nucleocytoplasmic transport: the last 200 nanometers. Cell. 2008;92:327–336. doi: 10.1016/s0092-8674(00)80926-5. [DOI] [PubMed] [Google Scholar]
- Pizarro T. T., Arseneau K. O., Bamias G., Cominelli F. Mouse models for the study of Crohn's disease. Trends Mol. Med. 2003;9:218–222. doi: 10.1016/s1471-4914(03)00052-2. [DOI] [PubMed] [Google Scholar]
- Rastogi S., Joshi B., Fusaro G., Chellappan S. Camptothecin induces nuclear export of prohibitin preferentially in transformed cells through a CRM-1-dependent mechanism. J. Biol. Chem. 2006;281:2951–2959. doi: 10.1074/jbc.M508669200. [DOI] [PubMed] [Google Scholar]
- Rogler G., Brand K., Vogl D., Page S., Hofmeister R., Andus T., Knuechel R., Baeuerle P. A., Scholmerich J., Gross V. Nuclear factor kappaB is activated in macrophages and epithelial cells of inflamed intestinal mucosa. Gastroenterology. 1998;115:357–369. doi: 10.1016/s0016-5085(98)70202-1. [DOI] [PubMed] [Google Scholar]
- Schreiber S., Nikolaus S., Hampe J. Activation of nuclear factor kappa B inflammatory bowel disease. Gut. 1998;42:477–484. doi: 10.1136/gut.42.4.477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sharma A., Qadri A. Vi polysaccharide of Salmonella typhi targets the prohibitin family of molecules in intestinal epithelial cells and suppresses early inflammatory responses. Proc. Natl. Acad. Sci. USA. 2004;101:17492–17497. doi: 10.1073/pnas.0407536101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shirakawa F., Mizel S. B. In vitro activation and nuclear translocation of NF-kappa B catalyzed by cyclic AMP-dependent protein kinase and protein kinase C. Mol. Cell. Biol. 1989;9:2424–2430. doi: 10.1128/mcb.9.6.2424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sitaraman S. V., Wang L., Wong M., Bruewer M., Hobert M., Yun C. H., Merlin D., Madara J. L. The adenosine 2b receptor is recruited to the plasma membrane and associates with E3KARP and Ezrin upon agonist stimulation. J. Biol. Chem. 2002;277:33188–33195. doi: 10.1074/jbc.M202522200. [DOI] [PubMed] [Google Scholar]
- Steinbrecher K. A., Harmel-Laws E., Sitcheran R., Baldwin A. S. Loss of epithelial RelA results in deregulated intestinal proliferative/apoptotic homeostasis and susceptibility to inflammation. J. Immunol. 2008;180:2588–2599. doi: 10.4049/jimmunol.180.4.2588. [DOI] [PubMed] [Google Scholar]
- Theiss A. L., Idell R. D., Srinivasan S., Klapproth J. M., Jones D. P., Merlin D., Sitaraman S. V. Prohibitin protects against oxidative stress in intestinal epithelial cells. FASEB J. 2007a;21:197–206. doi: 10.1096/fj.06-6801com. [DOI] [PubMed] [Google Scholar]
- Theiss A. L., Obertone T. S., Merlin D., Sitaraman S. V. Interleukin-6 transcriptionally regulates prohibitin expression in intestinal epithelial cells. J. Biol. Chem. 2007b;282:12804–12812. doi: 10.1074/jbc.M609031200. [DOI] [PubMed] [Google Scholar]
- Theiss A. L., Vijay-Kumar M., Obertone T. S., Jones D. P., Hansen J. M., Gewirtz A. T., Sitaraman S. V., Merlin D. Prohibitin (PHB) is a novel regulator of antioxidant response that attenuates colonic inflammation in mice. Gastroenterology. 2009;137:199–208. doi: 10.1053/j.gastro.2009.03.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang F., Graham W. V., Wang Y., Witkowski E. D., Schwarz B. T., Turner J. R. Interferon-gamma and tumor necrosis factor- alpha synergize to induce intestinal epithelial barrier dysfunction by up- regulating myosin light chain kinase expression. Am. J. Pathol. 2005;166:409–419. doi: 10.1016/s0002-9440(10)62264-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang S., Fusaro G., Padmanabhan J., Chellappan S. P. Prohibitin co-localizes with Rb in the nucleus and recruits N-CoR and HDAC1 for transcriptional repression. Oncogene. 2002;21:8388–8396. doi: 10.1038/sj.onc.1205944. [DOI] [PubMed] [Google Scholar]
- Wang S., Nath N., Adlam M., Chellappan S. Prohibitin, a potential tumor suppressor, interacts with RB and regulates E2F function. Oncogene. 1999;18:3501–3510. doi: 10.1038/sj.onc.1202684. [DOI] [PubMed] [Google Scholar]
- Winter A., Kamarainen O., Hofmann A. Molecular modeling of prohibitin domains. Proteins. 2007;68:353–362. doi: 10.1002/prot.21355. [DOI] [PubMed] [Google Scholar]
- Yasuhara N., Shibazaki N., Tanaka S., Nagai M., Kamikawa Y., Oe S., Asally M., Kamachi Y., Kondoh H., Yoneda Y. Triggering neural differentiation of ES cells by subtype switching of importin-alpha. Nat. Cell Biol. 2007;9:72–79. doi: 10.1038/ncb1521. [DOI] [PubMed] [Google Scholar]
- Yeo M., Kim D. K., Park H. J., Oh T. Y., Kim J. H., Cho S. W., Paik Y. K., Hahm K. B. Loss of transgelin in repeated bouts of ulcerative colitis–induced colon carcinogenesis. Proteomics. 2006;6:1158–1165. doi: 10.1002/pmic.200500390. [DOI] [PubMed] [Google Scholar]