Abstract
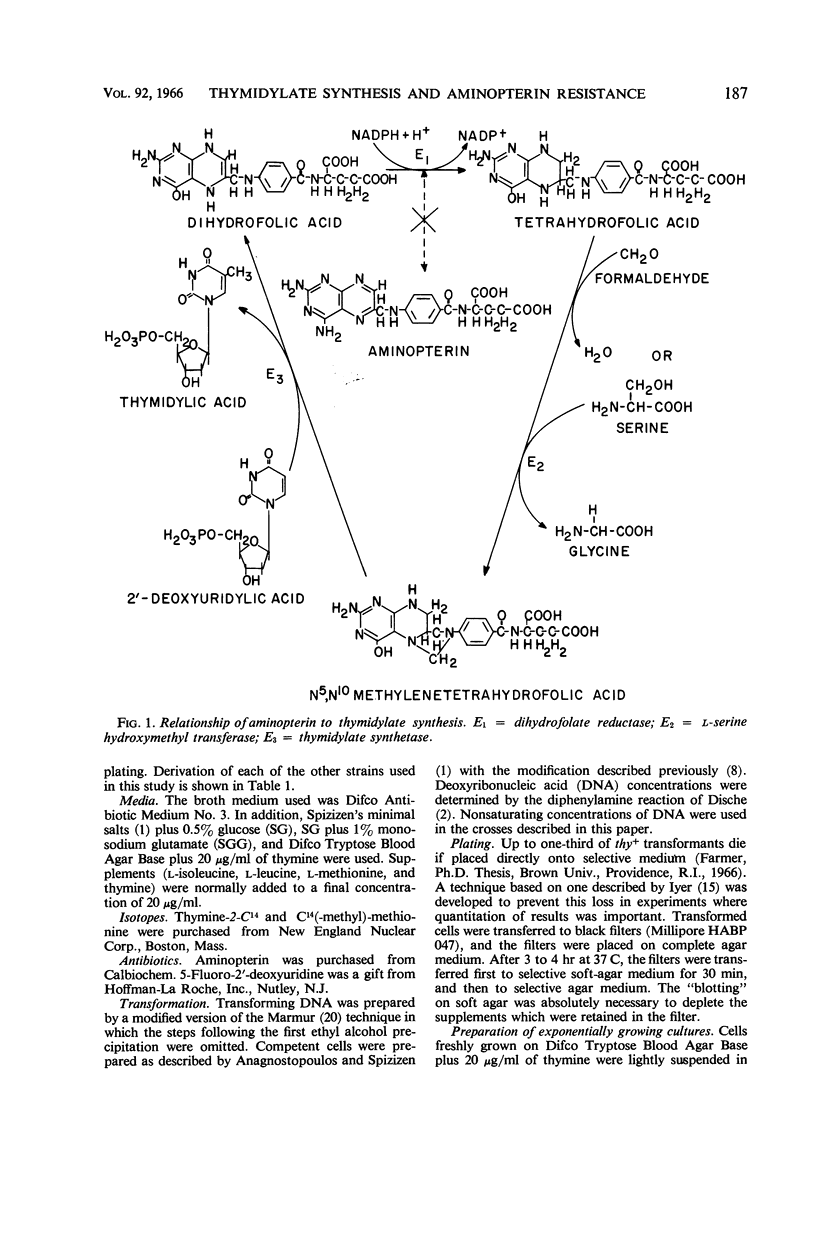
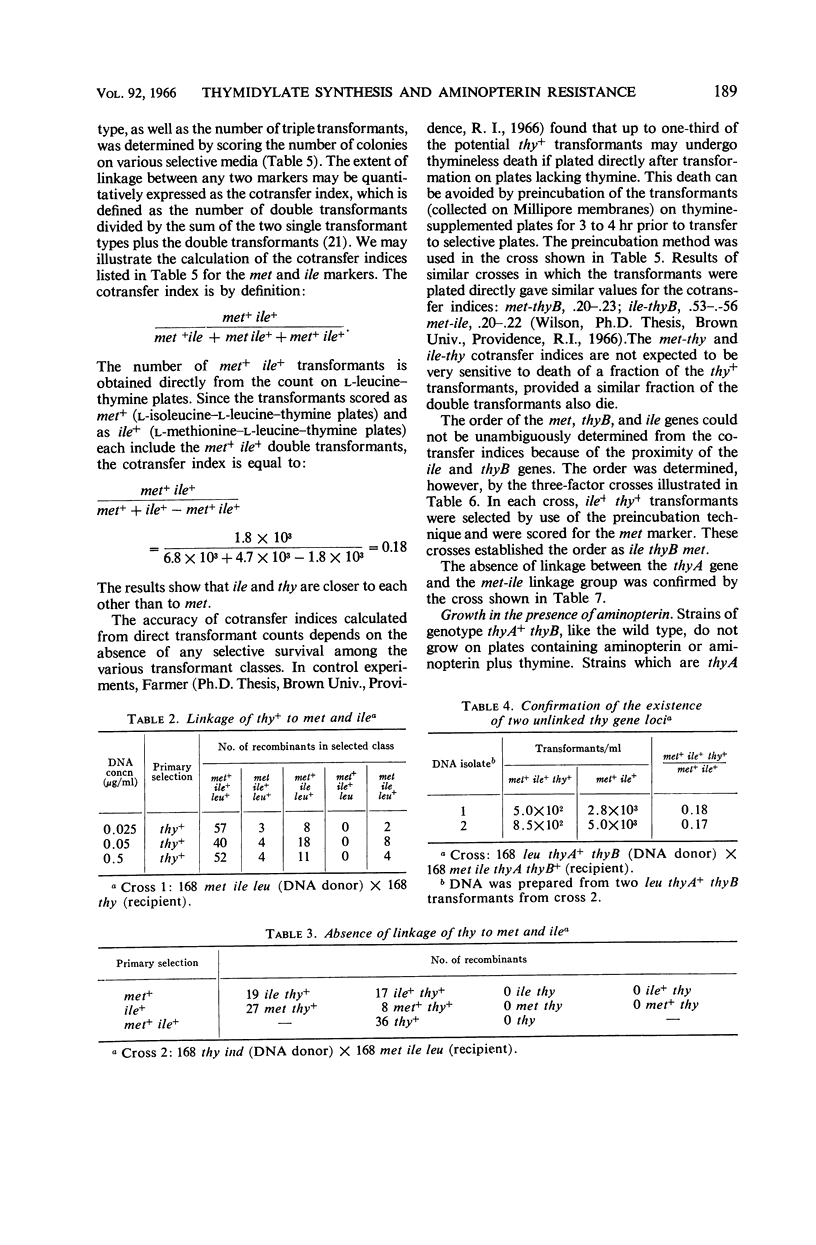
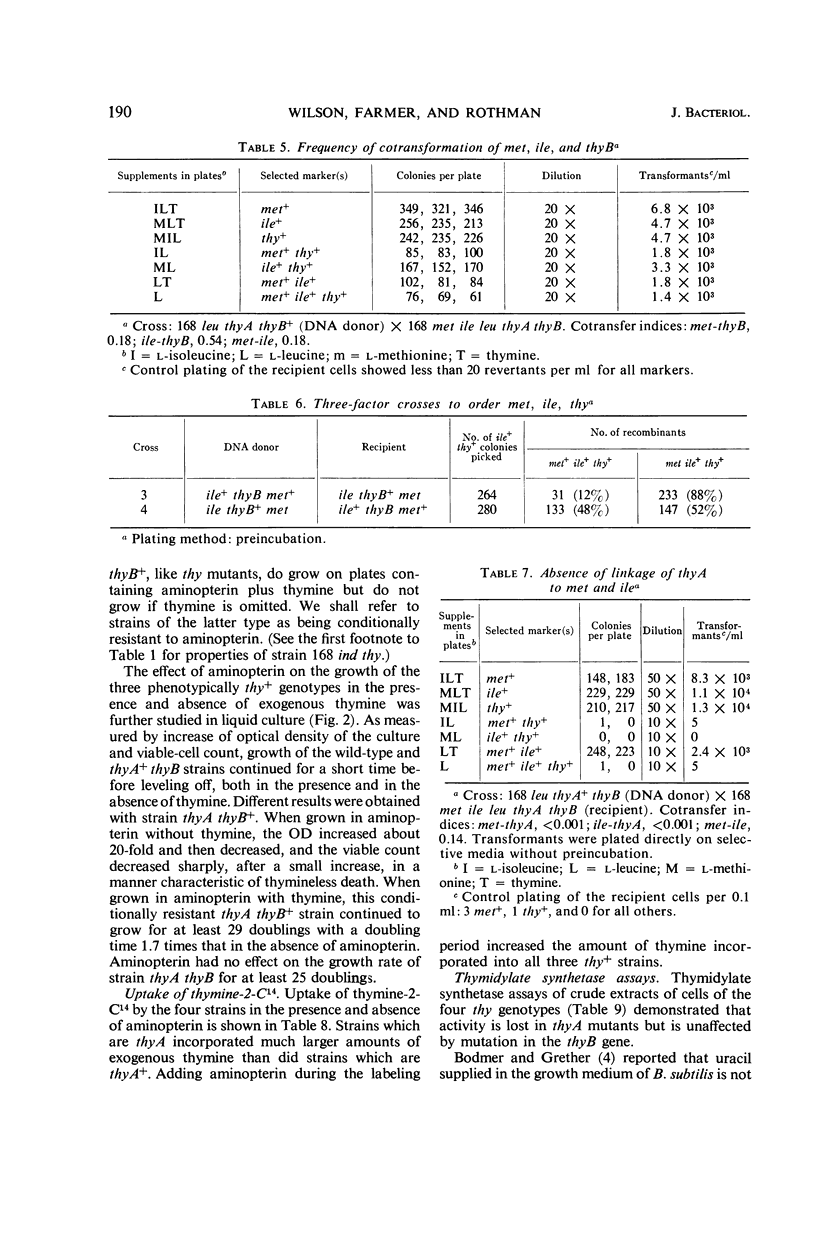
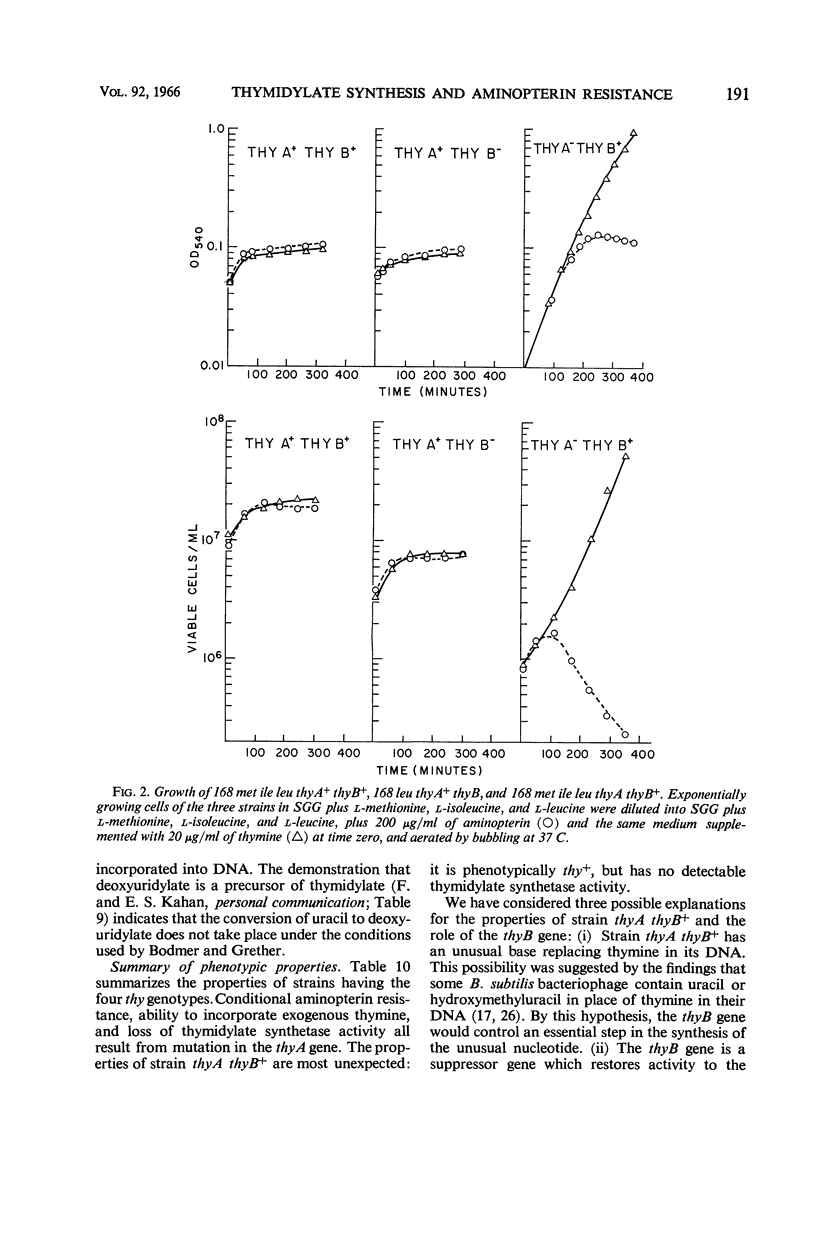
Wilson, Melba Carr (Brown University, Providence, R.I.), James L. Farmer, and Frank Rothman. Thymidylate synthesis and aminopterin resistance in Bacillus subtilis. J. Bacteriol. 92:186–196. 1966.—The thymine-requirement of Bacillus subtilis 168 thy results from mutation in two unlinked genes (i.e., genetic loci) designated thyA and thyB. The thyB gene is located between the met and ile markers. Both thyA+thyB and thyA thyB+ strains are phenotypically thy+. ThyA+thyB strains resemble the wild type in their sensitivity to aminopterin, poor incorporation of exogenous thymine into deoxyribonucleic acid (DNA), and high level of thymidylate synthetase activity in crude extracts. ThyA thyB+ strains are resistant to aminopterin in the presence of thymine, incorporate exogenous thymine into DNA, and have no detectable thymidylate synthetase activity. Experiments designed to elucidate the role of the thyB gene indicate that it specifies an alternate pathway of thymidylate synthesis, similar to thymidylate synthetase but requiring a cofactor other than tetrahydrofolate. The mechanism of selection of thymine-requiring mutants by aminopterin is revealed by these results.
Full text
PDF



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Anagnostopoulos C., Spizizen J. REQUIREMENTS FOR TRANSFORMATION IN BACILLUS SUBTILIS. J Bacteriol. 1961 May;81(5):741–746. doi: 10.1128/jb.81.5.741-746.1961. [DOI] [PMC free article] [PubMed] [Google Scholar]
- BODMER W. F., GRETHER S. UPTAKE AND INCORPORATION OF THYMINE, THYMIDINE, URACIL, URIDINE, AND 5-FLUOROURACIL INTO THE NUCLEIC ACIDS OF BACILLUS SUBTILIS. J Bacteriol. 1965 Apr;89:1011–1014. doi: 10.1128/jb.89.4.1011-1014.1965. [DOI] [PMC free article] [PubMed] [Google Scholar]
- CRAWFORD L. V. Thymine metabolism in strains of Escherichia coli. Biochim Biophys Acta. 1958 Nov;30(2):428–429. doi: 10.1016/0006-3002(58)90071-4. [DOI] [PubMed] [Google Scholar]
- Cohen S. S., Barner H. D. STUDIES ON UNBALANCED GROWTH IN ESCHERICHIA COLI. Proc Natl Acad Sci U S A. 1954 Oct;40(10):885–893. doi: 10.1073/pnas.40.10.885. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cohen S. S., Flaks J. G., Barner H. D., Loeb M. R., Lichtenstein J. THE MODE OF ACTION OF 5-FLUOROURACIL AND ITS DERIVATIVES. Proc Natl Acad Sci U S A. 1958 Oct 15;44(10):1004–1012. doi: 10.1073/pnas.44.10.1004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- FARMER J. L., ROTHMAN F. TRANSFORMABLE THYMINE-REQUIRING MUTANT OF BACILLUS SUBTILS. J Bacteriol. 1965 Jan;89:262–263. doi: 10.1128/jb.89.1.262-263.1965. [DOI] [PMC free article] [PubMed] [Google Scholar]
- FLEISSNER E., BOREK E. A new enzyme of RNA synthesis: RNA methylase. Proc Natl Acad Sci U S A. 1962 Jul 15;48:1199–1203. doi: 10.1073/pnas.48.7.1199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- FRIEDKIN M. ENZYMATIC ASPECTS OF FOLIC ACID. Annu Rev Biochem. 1963;32:185–214. doi: 10.1146/annurev.bi.32.070163.001153. [DOI] [PubMed] [Google Scholar]
- GOLD M., HURWITZ J., ANDERS M. The enzymatic methylation of RNA and DNA. Biochem Biophys Res Commun. 1963 Apr 23;11:107–114. doi: 10.1016/0006-291x(63)90075-5. [DOI] [PubMed] [Google Scholar]
- Gore I. Y., Popják G. Sterol biosynthesis in neoplastic cells: utilization of [C]acetate and of [2-C]mevalonate. Biochem J. 1962 Jul;84(1):93–99. doi: 10.1042/bj0840093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- HAKALA M. T., WELCH A. D. A polyglutamate form of citrovorum factor synthesized by Bacillus subtilis. J Bacteriol. 1957 Jan;73(1):35–41. doi: 10.1128/jb.73.1.35-41.1957. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Iyer V. N. APPLICATION OF THE MEMBRANE FILTER FOR THE QUANTITATIVE STUDY OF TRANSFORMATIONS WITH PARTICULAR REFERENCE TO PHENOTYPIC EXPRESSION OF AN ERYTHROMYCIN-RESISTANCE MUTATION. J Bacteriol. 1962 Aug;84(2):326–330. doi: 10.1128/jb.84.2.326-330.1962. [DOI] [PMC free article] [PubMed] [Google Scholar]
- JONES K. M., GUEST J. R., WOODS D. D. Folic acid and the synthesis of methionine by extracts of Escherichia coli. Biochem J. 1961 Jun;79:566–574. doi: 10.1042/bj0790566. [DOI] [PMC free article] [PubMed] [Google Scholar]
- KALLEN R. G., SIMON M., MARMUR J. The new occurrence of a new pyrimidine base replacing thymine in a bacteriophage DNA:5-hydroxymethyl uracil. J Mol Biol. 1962 Aug;5:248–250. doi: 10.1016/s0022-2836(62)80087-4. [DOI] [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- MANTSAVINOS R., ZAMENHOF S. Pathways for the biosynthesis of thymidylic acid in bacterial mutants. J Biol Chem. 1961 Mar;236:876–882. [PubMed] [Google Scholar]
- Nester E W, Schafer M, Lederberg J. Gene Linkage in DNA Transfer: A Cluster of Genes Concerned with Aromatic Biosynthesis in Bacillus Subtilis. Genetics. 1963 Apr;48(4):529–551. doi: 10.1093/genetics/48.4.529. [DOI] [PMC free article] [PubMed] [Google Scholar]
- OKADA T., HOMMA J., SONOHARA H. Improved method for obtaining thymineless mutants of Escherichia coli and Salmonella typhimurium. J Bacteriol. 1962 Sep;84:602–603. doi: 10.1128/jb.84.3.602-603.1962. [DOI] [PMC free article] [PubMed] [Google Scholar]
- OKADA T., YANAGISAWA K., RYAN F. J. A method for securing thymineless mutants from strains of E. coli. Z Vererbungsl. 1961;92:403–412. doi: 10.1007/BF00890061. [DOI] [PubMed] [Google Scholar]
- OKADA T., YANAGISAWA K., RYAN F. J. Elective production of thymine-less mutants. Nature. 1960 Oct 22;188:340–341. doi: 10.1038/188340a0. [DOI] [PubMed] [Google Scholar]
- SAITO H., MIURA K. I. PREPARATION OF TRANSFORMING DEOXYRIBONUCLEIC ACID BY PHENOL TREATMENT. Biochim Biophys Acta. 1963 Aug 20;72:619–629. [PubMed] [Google Scholar]
- TAKAHASHI I., MARMUR J. Replacement of thymidylic acid by deoxyuridylic acid in the deoxyribonucleic acid of a transducing phage for Bacillus subtilis. Nature. 1963 Feb 23;197:794–795. doi: 10.1038/197794a0. [DOI] [PubMed] [Google Scholar]
- Wachsman J. T., Kemp S., Kogg L. Thymineless death in Bacillus megaterium. J Bacteriol. 1964 May;87(5):1079–1086. doi: 10.1128/jb.87.5.1079-1086.1964. [DOI] [PMC free article] [PubMed] [Google Scholar]
- YOSHIKAWA H., SUEOKA N. Sequential replication of Bacillus subtilis chromosome. I. Comparison of marker frequencies in exponential and stationary growth phases. Proc Natl Acad Sci U S A. 1963 Apr;49:559–566. doi: 10.1073/pnas.49.4.559. [DOI] [PMC free article] [PubMed] [Google Scholar]
- ZAKRZEWSKI S. F. Studies on the substrate specificity of folic acid reductase. J Biol Chem. 1960 Jun;235:1780–1784. [PubMed] [Google Scholar]


