Abstract
Background: Recent studies have examined sugar-sweetened soda consumption in relation to early markers of kidney disease, but to date there have been no investigations of whether sugar-sweetened beverage consumption affects preexistent chronic kidney disease (CKD).
Objective: This prospective cohort study of 447 participants in the Multi-Ethnic Study of Atherosclerosis (MESA) with preexistent CKD examined the association between sugar-sweetened beverage consumption (<1 drink/wk, 1–6 drinks/wk, and ≥1 drink/d) and progression of CKD.
Design: β-Coefficients for continuous outcomes of changes in estimated glomerular filtration rate (eGFR) and urinary albumin to creatinine ratio (UACR) were calculated by using linear regression. Odds ratios for binary outcomes of accelerated decline in eGFR, defined as >2 mL · min−1 · 1.73 m−2 per year, and clinically significant progression of albuminuria (defined as attainment of UACR ≥30 mg/g for participants without microalbuminuria at visit 1 or a ≥25% increase in UACR for participants with baseline microalbuminuria) were evaluated by using logistic regression.
Results: The mean (±SD) baseline eGFR was 52 ± 6 mL · min−1 · 1.73 m−2 per year, and median baseline UACR was 6.3 mg/g (interquartile range: 3.5–17.6). Univariate and multivariate analyses showed no association between sugar-sweetened beverage consumption and rate of eGFR decline or changes in urinary albumin to creatinine ratio. The multivariate odds ratios comparing participants who drank ≥1 sugary beverage daily with those who drank ≤1 beverage weekly were 0.62 (95% CI: 0.27, 1.41) for accelerated eGFR decline and 1.51 (95% CI: 0.49, 4.62) for clinically significant progression of albuminuria.
Conclusion: A higher consumption of sugar-sweetened beverages was not associated with disease progression, on the basis of either eGFR or the urinary albumin to creatinine ratio, in MESA participants with preexistent CKD.
INTRODUCTION
Chronic kidney disease (CKD) is associated with premature mortality, decreased quality of life, and increased health care expenditures. CKD is typically progressive in nature: patients with CKD stage 3, the least advanced stage defined from glomerular filtration rate (GFR) alone, on average experience a yearly decline in GFR of 1 mL · min−1 · 1.73 m−2 (1). However, the progression of CKD often follows an unpredictable course, even when the etiology is well defined, as in diabetic nephropathy (2). Some patients progress very slowly, whereas others progress quite rapidly, and the reasons for this variability are not completely understood.
Treatment factors (eg, successful control of diabetes), genetics (eg, family history of end-stage renal disease), and environmental cues (eg, exposure to toxins) likely play important roles in determining the rate of renal decline, as do immutable factors such as age and sex. Diet may also affect renal function. Nephrologists often recommend salt restriction, which should help with blood pressure and fluid status, and may advocate for protein restriction, although the benefit of this intervention for CKD remains controversial.
Recently, consumption of sugar-sweetened sodas has been linked, by cross-sectional analyses, to elevated serum creatinine (3) and microalbuminuria (4). However, longitudinal studies provide stronger evidence for or against diet-related diseases. We therefore examined the relation between sugar-sweetened beverage consumption and progression of established CKD using data from the Multi-Ethnic Study of Atherosclerosis (MESA). We hypothesized that daily sugar-sweetened beverage consumption is inversely associated with changes in GFR and positively related to changes in urinary albumin excretion in adults with preexistent CKD (estimated GFR <60 mL · min−1 · 1.73 m−2).
SUBJECTS AND METHODS
Participants and study design
MESA was established by the US National Heart, Lung, and Blood Institute to examine the determinants of subclinical cardiovascular disease measures and their associations with cardiovascular disease outcomes. A full description of MESA is available elsewhere (5). A total of 6814 men and women from 4 ethnic groups (white, African American, Hispanic, and Chinese) aged 45–84 y were recruited from 6 field centers: Baltimore, MD; Chicago, IL; Forsyth County, NC; Los Angeles, CA; New York, NY; and St Paul, MN. The MESA protocol was approved by the institutional review boards of all participating centers, and informed consent was obtained from all participants.
Visit 1 (baseline examination) for the MESA cohort took place between July 2000 and September 2002 and was followed by two 18-mo examination periods and an additional 2-y examination period. Data from visits 1, 3, and 4 (≈5-y duration) were used for this analysis. To create a subcohort of subjects with CKD, we included participants whose estimated GFR at visit 1 was <60 mL · min−1 · 1.73 m−2 and remained <60 mL · min−1 · 1.73 m−2 at visit 3 and/or visit 4. This definition for CKD, based on repeated rather than single measurements of creatinine, best captured the K/DOQI (Kidney Disease Outcomes Quality Initiative) guidelines on classifying CKD as a reduced GFR for ≥3 mo (6).
Outcomes
The outcomes of interest were changes in estimated GFR (eGFR) and albuminuria. eGFR was calculated from serum creatinine concentrations measured at visits 1, 3, and 4 by using the Modification of Diet in Renal Disease Study equation (7):
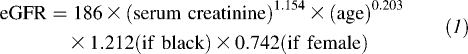 |
All MESA creatinine values were calibrated against the CX3 machine used at the Cleveland Clinic laboratory by using the regression formula y = 0.9954x + 0.0208. We evaluated changes in eGFR as both a continuous outcome and a binary outcome in which accelerated decline of eGFR was defined as >2 mL · min−1 · 1.73 m−2 per year.
Urinary albumin and creatinine concentrations were measured at visit 1 and 3. We evaluated changes in the urinary albumin to creatinine ratio (UACR) as a continuous outcome and as a binary outcome capturing clinically significant progression of albuminuria. We defined this progression as attainment of microalbuminuria (UACR ≥ 30 mg/g) for participants without microalbuminuria at visit 1 or as a ≥25% increase in UACR for participants whose UACR at visit 1 was ≥30 mg/g. For sensitivity analyses, we used an alternative definition for microalbuminuria, using sex-specific cutoffs of ≥17 mg/g in males and ≥25 mg/g in females (8–10).
Predictors
Diet was assessed at the baseline visit via a food-frequency questionnaire asking participants to self-report their usual eating habits over the previous year (11). Sugar-sweetened beverage consumption was quantified from an item listing “regular soft drinks, soda, sweetened mineral water (not diet), nonalcoholic beer.” We were not able to subtract out nonalcoholic beer from this question and therefore assumed that consumption of nonalcoholic beer was negligible. Frequency response options for this questionnaire item were as follows: rare or never, 1–3 per/mo, 1/wk, 2–4/wk, 5–6/wk, 1/d, 2–3/d, 4–5/d, and ≥6/d. According to the exposure distribution, we collapsed these responses into 3 levels: <1 sugar-sweetened beverage/wk, 1–6 sugar-sweetened beverages/wk, and ≥1 sugar-sweetened beverage/d. We felt that these categories captured significant degrees of exposure and consisted of easily communicable levels of beverage intake. For the sensitivity analysis, we also examined sugar-sweetened beverage intake as a binary variable: participants who drank <1 sugary beverage/d compared with participants who drank ≥1 sugary beverage/d. We had no data on sugar-sweetened beverage consumption at points other than the baseline visit.
Other baseline characteristics included demographics (age, sex, race, and education status), lifestyle and dietary characteristics (intentional exercise, tobacco use, daily sodium intake, daily phosphorus intake, daily protein intake, and daily caloric intake), medical history (diabetes, hypertension, and dyslipidemia), physical examination findings (body mass index and systolic and diastolic blood pressure), and laboratory values (LDL and HDL cholesterol, triglycerides, and glucose). Education level was dichotomized by high school graduation status. Smoking was dichotomized by current use. Diabetes was defined by medication use or a fasting glucose concentration ≥126 mg/dL. Hypertension was defined as systolic pressure ≥140 mm Hg, diastolic pressure ≥90 mm Hg, or use of antihypertensive medication. Dyslipidemia was defined as low HDL (<40 mg/dL in men, <50 mg/dL in women), elevated LDL (>130 mg/dL in nonpatients with diabetes, >100 mg/dL in patients with diabetes), elevated triglycerides (>250 mg/dL), or use of lipid-lowering medication.
Statistical analyses
Baseline characteristics by categories of sugar-sweetened beverage consumption were described as mean, median, or percentage values; the distribution of these characteristics was tested by one-factor analysis of variance. Linear regression models were created by using the categories of sugar-sweetened beverage consumption to predict the average annual change in eGFR and UACR (ie, estimated slope from available eGFR and UACR values) for each individual from examinations 1 and 3. Annualized changes in eGFR and UACR were calculated by using a least-squares regression slope. For the dichotomized outcomes of eGFR decline (incorporating data from examinations 1, 3, and 4) and UACR increase, defined above, logistic regression models were used. Covariates were entered into the models as potential confounders if they were identified as antecedents of both kidney disease and sugar-sweetened beverage consumption. Three consecutive models were constructed for each kidney decline endpoint: an unadjusted model; a minimally adjusted model (model 1) including age, sex, race (4 categories), and baseline kidney function; and a fully adjusted model (model 2) including age, sex, race, education, smoking status, BMI, hypertension, diabetes mellitus, dyslipidemia, total caloric intake, total phosphorus intake, total protein intake, and baseline kidney function. Because of its nonparametric distribution, baseline UACR was logarithmically transformed in these analyses.
Sensitivity analyses were performed excluding diabetic subjects, without adjustment for baseline kidney function, and substituting continuous values of systolic and diastolic blood pressure for the binary covariable of hypertensive status. In addition, for comparison and to potentially isolate the effect of the high-fructose corn syrup used to sweeten regular sodas, all analyses were repeated by using diet soda consumption, rather than sugar soda consumption, as the exposure of interest. Diet soda intake was quantified from the food-frequency questionnaire item listing “diet soft drinks, unsweetened mineral water.”
Two-sided hypotheses tests with a 5% type I error were adopted for all statistical inferences. S-Plus (release 8.0; Insightful Inc, Seattle, WA) and SPSS statistical software (release 15.0.1; SPSS Inc, Chicago, IL) were used for the analyses.
RESULTS
Of 6814 MESA participants, 447 met our prespecified criteria for CKD, with a baseline eGFR <60 mL · min−1 · 1.73 m−2 that remained <60 mL · min−1 · 1.73 m−2 at visit 3 and/or visit 4. Of these CKD subjects, 294 (66%) drank <1 sugar-sweetened beverage/wk, 108 (24%) drank between 1 and 6 sweetened beverages/wk, and 45 (10%) drank ≥1 sugar-sweetened beverage/d (Table 1). In comparison, 271 (61%) drank <1 diet soda/wk, 95 (21%) drank between 1 and 6 diet sodas/wk, and 79 (18%) drank ≥1 diet soda/d.
TABLE 1.
Baseline characteristics of the study population1
| Sugar-sweetened beverage intake |
|||
| <1/wk (n = 294) | 1–6/wk (n = 108) | ≥7/wk (n = 45) | |
| Age (y) | 70 ± 82 | 67 ± 9 | 67 ± 9 |
| Female [n (%)] | 195 (66) | 56 (52) | 21 (49) |
| Race-ethnicity [n (%)] | |||
| White | 175 (60) | 55 (51) | 22 (51) |
| Asian | 40 (14) | 8 (7) | 0 (0) |
| Black | 40 (14) | 27 (25) | 9 (21) |
| Hispanic | 39 (13) | 18 (17) | 12 (28) |
| High school education and higher [n (%)] | 242 (83) | 94 (87) | 34 (79) |
| BMI (kg/m2) | 28.6 ± 5.2 | 28.3 ± 5.2 | 29.2 ± 5.8 |
| Hypertension [n (%)]3 | 202 (69) | 68 (63) | 27 (63) |
| SBP (mm Hg) | 136 ± 22 | 132 ± 21 | 137 ± 21 |
| DBP (mm Hg) | 71 ± 10 | 71 ± 11 | 74 ± 11 |
| Diabetes [n (%)] | 54 (18) | 12 (11) | 3 (7) |
| Current smokers [n (%)] | 13 (4) | 12 (11) | 4 (9) |
| Dyslipidemia [n (%)]4 | 213 (72) | 86 (80) | 27 (63) |
| Low-density lipoprotein (mg/dL) | 118 ± 31 | 120 ± 31 | 112 ± 37 |
| High-density lipoprotein (mg/dL) | 53 ± 15 | 50 ± 13 | 45 ± 13 |
| Triglycerides (mg/dL) | 122 (85–170)5 | 129 (88–190) | 135 (100–173) |
| Use of lipid-lowering medication [n (%)] | 86 (29) | 34 (32) | 5 (12) |
| Sodium intake (mg/d) | 1660 (1202–2455) | 2255 (1534–2722) | 2593 (1713–3208) |
| Phosphorus intake (mg/d) | 837 (607, 1224) | 895 (667–1245) | 1069 (827–1402) |
| Protein intake (g/d) | 57 ± 27 | 60 ± 22 | 68 ± 32 |
| Total energy intake (kcal/d) | 1251 (916–1704) | 1570 (1216–1859) | 1926 (1579–2311) |
| Intentional exercise (MET min/wk) | 994 (315–2460) | 1020 (171–1890) | 420 (0–1125) |
| Baseline serum creatinine (mg/dL) | 1.26 ± 0.25 | 1.34 ± 0.28 | 1.33 ± 0.25 |
| Baseline eGFR (mL · minminus1 · 1.73 mminus2) | 53 ± 6 | 53 ± 7 | 53 ± 6 |
| Baseline albumin:creatinine ratio (mg/g) | 6.2 (3.5–16.1) | 7.2 (3.7–21.3) | 4.6 (2.9–30.3) |
SBP, systolic blood pressure; DBP, diastolic blood pressure; MET, metabolic equivalent task; eGFR, estimated glomerular filtration rate.
Mean ± SD (all such values).
Defined as SBP ≥ 140 mm Hg, DBP ≥ 90 mm Hg, or use of blood pressure–lowering medication.
Defined as low HDL cholesterol (<40 mg/dL in men, <50 mg/dL in women), elevated LDL cholesterol (>130 mg/dL in nonpatients with diabetes, >100 mg/dL in patients with diabetes), elevated triglyceride (>250 mg/dL), or use of lipid-lowering medication.
Median; interquartile range (all such values).
Participants who drank ≥1 sugar-sweetened beverage/d, the highest exposure category, were more likely to be male, African American or Hispanic, and current smokers than were participants who drank <1 sugary beverage/wk. Although there was no differences in mean body mass index across exposure groups, participants who consumed more sugar-sweetened beverages had significantly greater sodium, protein, and total calorie intakes. Hypertensive status did not differ between exposure groups, whereas patients with diabetes were most represented in the group with the least amount of sugary beverage consumption. The 3 exposure groups did not significantly differ with regard to baseline kidney function as determined by serum creatinine, eGFR, or UACR.
Change in estimated GFR
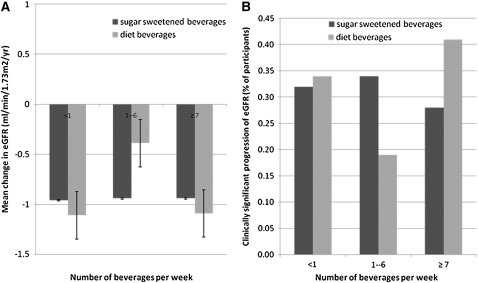
Of all participants identified with CKD, the mean (± SD) baseline eGFR was 52 ± 6 mL · min−1 · 1.73 m−2. The mean (±SD) decline in eGFR during the 5-y study period was 0.95 (±3.12) mL · min−1 · 1.73 m−2 per year for all CKD participants. In univariate and multivariate analyses, the yearly change in eGFR was not related to a subject's degree of sugar-sweetened beverage consumption (Figure 1, Table 2). A binary outcome for accelerated decline of eGFR, defined as >2 mL · min−1 · 1.73 m−2 per year, showed a similar lack of association. With participants who drank <1 sugary beverage/wk as a referent, the multivariate odds ratios for rapid GFR decline were 1.17 (0.70, 1.97; P = 0.6) for participants who drank 1–6 sugary beverages/wk and 0.62 (0.27, 1.41; P = 0.3) for participants who drank ≥1 sugary beverage/d.
FIGURE 1.
Mean changes in estimated glomerular filtration rate (eGFR) (A) and the clinically significant decline in eGFR (B) after sugar-sweetened and diet beverage intakes per week among participants with chronic kidney disease. The data reflect univariate relations; in multivariate analyses, point estimates for diet and regular soda were not significantly different. The bars in panel A reflect 95% CIs.
TABLE 2.
Association of sugar-sweetened beverage intake with rate of decline in kidney function between visits 1, 3, and 41
| Unadjusted |
Model 12 |
Model 23 |
||||
| Kidney function | Value | P | Value | P | Value | P |
| Continuous ΔeGFR4,5 | ||||||
| Sugar-sweetened beverage intake | ||||||
| <1/wk (n = 294) | Reference | Reference | Reference | |||
| 1–6/wk (n = 108) | 0.04 (−0.66, 0.73) | 0.9 | 0.05 (−0.64, 0.73) | 0.9 | −0.06 (−0.75, 0.64) | 0.9 |
| ≥7/wk (n = 45) | 0.03 (−0.97, 1.04) | 0.9 | 0.12 (−0.87, 1.11) | 0.8 | 0.29 (−0.78, 1.37) | 0.6 |
| Rapid decline: ΔeGFR < −2.006 | ||||||
| Sugar-sweetened beverage intake | ||||||
| <1/wk (n = 93) | Reference | Reference | Reference | |||
| 1–6/wk (n = 37) | 1.12 (0.70, 1.79) | 0.6 | 1.07 (0.65, 1.74) | 0.8 | 1.17 (0.70, 1.97) | 0.6 |
| ≥7/wk (n = 12) | 0.83 (0.41, 1.69) | 0.6 | 0.72 (0.34, 1.52) | 0.4 | 0.62 (0.27, 1.41) | 0.3 |
ΔeGFR, change in estimated glomerular filtration rate.
Adjusted for baseline eGFR, age, sex, and race.
Adjusted for baseline eGFR, age, sex, race, education, field center, current smoking, BMI, hypertension, diabetes mellitus, dyslipidemia, phosphorus intake, protein intake, and total calorie intake.
per mL · min−1 · 1.73 m−2 per year.
Values are β-coefficients (95% CIs).
Values are odds ratios (95% CIs).
Change in urinary albumin excretion
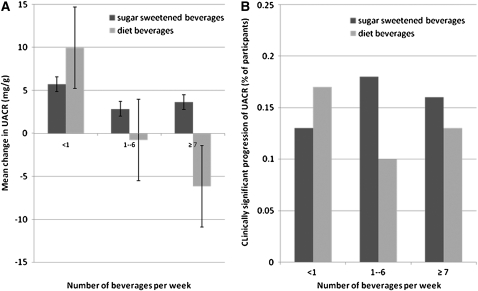
The median baseline UACR for participants with CKD was 6.3 [interquartile range (IQR) = 3.5, 17.6] mg/g. By visit 3, the UACR increased on average by 6.3 ± 94 mg/g for these participants. In univariate and multivariate models, the amount of sugar-sweetened beverage consumption was not associated with changes in UACR (Figure 2, Table 3). A binary outcome for clinically significant progression of albuminuria—defined as attainment of microalbuminuria (UACR ≥ 30 mg/g) for participants without microalbuminuria at visit 1 or as a ≥25% increase in UACR for participants whose UACR at visit 1 was ≥30 mg/g—further confirmed this lack of association. With participants who drank <1 sugary beverage/wk as a referent, the multivariate odds ratios for albuminuria progression was 1.48 (95% CI: 0.73, 3.00; P = 0.3) for participants who drank 1–6 sugary beverages/wk and 1.51 (95% CI: 0.49, 4.62; P = 0.5) for participants who drank ≥1 sugary beverage/d. Sensitivity analyses using sex-specific microalbuminuria cutoffs and excluding diabetic subjects yielded virtually identical estimates. Because UACR was measured only twice in this study, a regression to the mean phenomenon could not be ruled out.
FIGURE 2.
Mean changes in the urinary albumin to creatinine ratio (UACR) (A) and the clinically significant progression of UACR (B) after sugar-sweetened and diet beverage intake per week. Significant progression was defined as a UACR <30 mg/g at examination 1 and ≥30 mg/g at examination 3 or a UACR >30 mg/g at examination 1 with an increase in UACR of ≥25%. The data reflect univariate relations; in multivariate analyses, point estimates for diet and regular soda were not significantly different. The patterns in panel A are highly influenced by comorbidities such as diabetic and hypertensive status. The bars in panel A reflect 95% CIs.
TABLE 3.
Association of sugar-sweetened beverage intake with increase in urinary albumin excretion between visits 1 and 31
| Unadjusted |
Model 12 |
Model 23 |
||||
| UACR | Value | P | Value | P | Value | P |
| Continuous Δ UACR | ||||||
| Sugar-sweetened beverage intake4 | ||||||
| <1/wk (n = 294) | Reference | Reference | Reference | |||
| 1–6/wk (n = 108) | −2.88 (−17.21, 11.44) | 0.7 | −0.98 (−15.65, 13.69) | 0.9 | −0.67 (−15.74, 14.40) | 0.9 |
| ≥7/wk (n = 45) | −2.11 (−22.91, 18.70) | 0.8 | 0.27 (−20.94, 21.49) | 1.0 | −0.39 (−23.60, 22.83) | 1.0 |
| UACR <30 mg/g at exam 1 and ≥30 mg/g at exam 3 or UACR >30 mg/g at exam 1 that increased ≥25%5 | ||||||
| Sugar-sweetened beverage intake | ||||||
| <1/wk (n = 39) | Reference | Reference | Reference | |||
| 1–6/wk (n = 19) | 1.31 (0.71, 2.41) | 0.4 | 1.44 (0.74, 2.81) | 0.3 | 1.48 (0.73, 3.00) | 0.3 |
| ≥7/wk (n = 7) | 1.26 (0.53, 3.03) | 0.6 | 1.28 (0.48, 3.43) | 0.6 | 1.51 (0.49, 4.62) | 0.5 |
UACR, urinary albumin to creatinine ratio; ΔUACR, change in UACR.
Adjusted for baseline UACR, age, sex, race.
Adjusted for baseline UACR, age, sex, race, education, field center, current smoking, BMI, hypertension, diabetes mellitus, dyslipidemia, phosphorus intake, protein intake, and total calorie intake.
Values are β-coefficients (95% CIs).
Values are odds ratios (95% CIs).
DISCUSSION
In this study, we observed no association between a higher consumption of sugar-sweetened beverages and progression of established CKD. In contrast with previously published reports that a high intake of sugar sodas was associated with renal injury, manifested as elevated serum creatinine and presence of albuminuria (3, 4), we found no link between the degree of sugar-sweetened beverage intake and changes in either the estimated GFR or UACR. In fact, sugary beverage consumption showed a lack of association with CKD progression similar to diet soda intake (Figures 1 and 2). Our results suggest that higher consumption of sugar-sweetened drinks may not pose an additional risk to already damaged kidneys.
This study, to our knowledge, is the first to examine whether heavy consumption of sugar-sweetened beverages is an important determinant of how renal function progresses in CKD. This question is important for 2 principal reasons. First, given the variable and unpredictable course of CKD, dietary factors that can aggravate preexisting renal injuries should clearly be avoided in this disease state. The findings of this study suggest that sugar-sweetened beverages do not fit this bill. This was an unexpected result, not only because of the findings of previous studies on sugary beverage intake and kidney disease, but also because of presumed associations between regular soda use and higher rates of obesity, hypertension, and diabetes (12–14).
Second, in examining whether sugar-sweetened beverages influence the rate of CKD progression, we also indirectly examined the role of high-fructose corn syrup, the sweetener for these beverages, in such progression. The controversy over the potential dangers of high-fructose corn syrup has been playing out not only in the medical literature (15–20), but also in the mainstream media, including advertising campaigns funded by the corn-producing industry (www.sweetsurprise.com). Opponents of high-fructose corn syrup voice concern over fructose's unique metabolism driving the synthesis of uric acid. In animal models, fructose-associated hyperuricemia produces a metabolic syndrome associated with glomerular hypertension, renal hypertrophy, cortical vasoconstriction, and arteriolopathy of renal vasculature (21, 22). Defenders of high-fructose corn syrup point out that this sweetener consists of ≈40–55% fructose (the other components being glucose and readily hydrolyzable polymers of glucose); therefore, findings from animal and human studies that use 100% fructose formulations are not applicable to high-fructose corn syrup (23). Indeed, in our study, sugar-sweetened beverages showed the same lack of association with CKD progression as diet sodas, which argues against a causative role for high-fructose corn syrup.
Our results must be interpreted with caution, however. The lack of association seen in this study may be due to our starting population, and the findings of this study should not be extrapolated to the non-CKD population. Whereas we showed that sugar-sweetened beverages do not seem to influence the progression of preexistent CKD in this specific subcohort, these results should not be viewed as definitive evidence that these beverages do not pose any risk to kidney function. The previous studies on soda consumption and renal dysfunction cited above could, in light of our current findings, be interpreted as indicative of the harms of sugary soda being limited to incipient kidney disease, whereas our results show that these sweetened beverages do not pose additional risk in already dysfunctional kidneys. In short, once the kidney has suffered a chronic insult, a salubrious intervention such as soda restriction may not be able to yield any substantial benefit. In addition, our analysis only looked at kidney function per se and not the consequences of reduced kidney function, such as anemia, abnormal bone and mineral metabolism, premature cardiovascular disease, and reduced quality of life, which theoretically could be affected by soda intake independent of its effects on kidney function.
The strengths of our study include its use of a racially diverse, community-based population that should permit generalizability to a wide array of patients. The cohort, on average, experienced a decrease in eGFR of ≈1 mL · min−1 · 1.73 m−2 per year, which likely places this group of individuals in the healthier spectrum of patients treated in routine clinical practice. MESA collected detailed characteristics of its participants, including baseline risk factors and comorbidities, which allowed for appropriate statistical adjustments and, again, confirmed that its participants were comparable with patients in the general population. Notably, MESA collected data on total calorie intake and physical activity—confounding variables that have been postulated as the true causes of the epidemic of obesity in the high-fructose corn syrup era (24). We also believe that our definition of CKD, based on multiple serum creatinine measurements, ensured that our subjects truly represented patients with renal dysfunction. This makes our results particularly applicable for practicing nephrologists, who generally see patients once kidney injury is established. Therefore, dietary interventions that slow the progression of established CKD, rather than prevent incident CKD, are more pragmatic lifestyle modifications for nephrologists to stress on their patients.
This study had many limitations. First, our exposure of interest was based on participants' dietary recall; thus, measurement error was inevitable. For example, the daily sodium intakes reported by these same participants (Table 1) suggest underreporting of salt consumption, particularly among participants who drank <1 sugary beverage/wk; this may portend similar underreporting of sugary beverage intake and a potential bias toward a null effect. In addition, our exposure is based on a survey question asking about “regular soft drinks, soda, sweetened mineral water (not diet), nonalcoholic beer.” We were not able to subtract out from this category consumption of nonalcoholic beer and therefore may have overestimated the degree of sugar-sweetened beverage intake for some subjects. It should be noted that our exposure category of ≥1 sugar-sweetened beverage/d is slightly lower than the highest category of exposure used in the analysis by Shoham et al (4), which was ≥2 sugary drinks/d. There were too few participants in the MESA cohort with this level of exposure to permit this categorization.
Second, we focused on the baseline intake of sugar-sweetened beverages and were not able to evaluate whether participants' intake of these beverages changed during the study period. Theoretically, this could have biased results toward the null if the group with the highest exposure cut down their beverage intake as a result of participation in this study. Other variables that may have changed during the study period and that could have affected renal function include blood pressure and diabetes status, use of medications (particularly renin-angiotensin system blocking drugs), and changes in salt intake. In this relatively healthy cohort, we assumed that access to health care and use of appropriate therapeutic interventions to address these factors was relatively equal across exposure groups.
Third, MESA did not measure uric acid concentrations to allow an exploration of whether sugary beverage intake led to a higher risk of hyperuricemia, as was suggested recently in a cross-sectional analysis of data from the third National Health and Nutrition Examination Survey (25). Given recent reports that elevated uric acid concentrations are an independent risk factor for incident CKD and progression of established CKD (26–28), we may have been able to see an association between sugar-sweetened beverage consumption and CKD progression in subgroup analyses focused on uric acid concentrations.
Finally, despite a relatively large starting population of almost 7000 participants, our strict definition for CKD reduced our study sample to 447 subjects, limiting our power to detect statistically significant differences among groups. In post hoc power analysis, however, we still had 80% power to detect a change of 1.5 mL · min−1 · 1.73 m−2 in eGFR and a change of 12.5 mg/g in UACR over the study period. We do not feel that the lack of association shown here was due to type II error. Our point estimates show relative concordance between univariate and multivariate analyses as well as an absence of dose response. In addition, the results of our varied sensitivity analyses showed essentially an identical lack of association.
We believe that our results should be primarily viewed as a call for repeated investigations into the role of sugar soda in the development and progression of CKD, ideally studies using larger starting populations with varying degrees of kidney function. We do not, by any means, intend these findings to be a justification for unbridled consumption of sugar sodas by individuals with and without CKD. Rather, we intend our study to be an important addition to the large and still-growing body of literature surrounding the potential health consequences of sugar soda and high-fructose corn syrup, which is based principally on observational studies such as this one. Our negative study results—subject to the same potential sources of error (bias, chance, and confounding) as other positive study results—should be used to further inform, rather than end, the heated debate regarding this important public health issue.
In conclusion, sugar-sweetened beverage consumption was not associated with disease progression among adults with preexistent CKD in this multiethnic cohort. The lack of association between sugary beverage consumption and CKD progression, similar to that seen with diet soda intake, does not support the hypothesis that the fructose used to sweeten these drinks poses an additional risk to already damaged kidneys.
Acknowledgments
We thank the other investigators, the staff, and the participants of the MESA study for their valuable contributions. A full list of participating MESA investigators and institutions can be found at http://www.mesa-nhlbi.org.
The authors' responsibilities were as follows—ASB, RK, KH, and PJK: study design; ASB and RK: data analysis; and ASB, RK, KH, DAS, GLB, and PJK: writing and editing of the manuscript. None of the authors had a conflict of interest.
REFERENCES
- 1.Eriksen BO, Ingebretsen OC. The progression of chronic kidney disease: a 10-year population-based study of the effects of gender and age. Kidney Int 2006;69:375–82 [DOI] [PubMed] [Google Scholar]
- 2.Hunsicker LG, Adler S, Caggiula A, et al. Predictors of the progression of renal disease in the Modification of Diet in Renal Disease Study. Kidney Int 1997;51:1908–19 [DOI] [PubMed] [Google Scholar]
- 3.Saldana TM, Basso O, Darden R, Sandler DP. Carbonated beverages and chronic kidney disease. Epidemiology 2007;18:501–6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Shoham DA, Durazo-Arvizu R, Kramer H, et al. Sugary soda consumption and albuminuria: results from the National Health and Nutrition Examination Survey, 1999-2004. PLoS One 2008;3:e3431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Bild DE, Bluemke DA, Burke GL, et al. Multi-ethnic study of atherosclerosis: objectives and design. Am J Epidemiol 2002;156:871–81 [DOI] [PubMed] [Google Scholar]
- 6.K/DOQI clinical practice guidelines for chronic kidney disease: evaluation, classification, and stratification. Am J Kidney Dis 2002;39:S1–266 [PubMed] [Google Scholar]
- 7.Levey AS, Bosch JP, Lewis JB, Greene T, Rogers N, Roth D. A more accurate method to estimate glomerular filtration rate from serum creatinine: a new prediction equation. Modification of Diet in Renal Disease Study Group. Ann Intern Med 1999;130:461–70 [DOI] [PubMed] [Google Scholar]
- 8.Connell SJ, Hollis S, Tieszen KL, McMurray JR, Dornan TL. Gender and the clinical usefulness of the albumin: creatinine ratio. Diabet Med 1994;11:32–6 [DOI] [PubMed] [Google Scholar]
- 9.Warram JH, Gearin G, Laffel L, Krolewski AS. Effect of duration of type I diabetes on the prevalence of stages of diabetic nephropathy defined by urinary albumin/creatinine ratio. J Am Soc Nephrol 1996;7:930–7 [DOI] [PubMed] [Google Scholar]
- 10.Mattix HJ, Hsu CY, Shaykevich S, Curhan G. Use of the albumin/creatinine ratio to detect microalbuminuria: implications of sex and race. J Am Soc Nephrol 2002;13:1034–9 [DOI] [PubMed] [Google Scholar]
- 11.Mayer-Davis EJ, Vitolins MZ, Carmichael SL, et al. Validity and reproducibility of a food frequency interview in a Multi-Cultural Epidemiology Study. Ann Epidemiol 1999;9:314–24 [DOI] [PubMed] [Google Scholar]
- 12.He FJ, Marrero NM, MacGregor GA. Salt intake is related to soft drink consumption in children and adolescents: a link to obesity? Hypertension 2008;51:629–34 [DOI] [PubMed] [Google Scholar]
- 13.Palmer JR, Boggs DA, Krishnan S, Hu FB, Singer M, Rosenberg L. Sugar-sweetened beverages and incidence of type 2 diabetes mellitus in African American women. Arch Intern Med 2008;168:1487–92 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Assy N, Nasser G, Kamayse I, et al. Soft drink consumption linked with fatty liver in the absence of traditional risk factors. Can J Gastroenterol 2008;22:811–6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Anderson GH. Much ado about high-fructose corn syrup in beverages: the meat of the matter. Am J Clin Nutr 2007;86:1577–8 [DOI] [PubMed] [Google Scholar]
- 16.Forshee RA, Storey ML, Allison DB, et al. A critical examination of the evidence relating high fructose corn syrup and weight gain. Crit Rev Food Sci Nutr 2007;47:561–82 [DOI] [PubMed] [Google Scholar]
- 17.Johnson RJ, Segal MS, Sautin Y, et al. Potential role of sugar (fructose) in the epidemic of hypertension, obesity and the metabolic syndrome, diabetes, kidney disease, and cardiovascular disease. Am J Clin Nutr 2007;86:899–906 [DOI] [PubMed] [Google Scholar]
- 18.Neilson EG. The fructose nation. J Am Soc Nephrol 2007;18:2619–21 [DOI] [PubMed] [Google Scholar]
- 19.Johnson RJ, Gower T. The sugar fix: the high-fructose fallout that is making you fat and sick. Emmaus, PA: Rodale, 2008 [Google Scholar]
- 20.Choi ME. The not-so-sweet side of fructose. J Am Soc Nephrol 2009;20:457–9 [DOI] [PubMed] [Google Scholar]
- 21.Nakagawa T, Hu H, Zharikov S, et al. A causal role for uric acid in fructose-induced metabolic syndrome. Am J Physiol Renal Physiol 2006;290:F625–31 [DOI] [PubMed] [Google Scholar]
- 22.Sánchez-Lozada LG, Tapia E, Jiménez A, et al. Fructose-induced metabolic syndrome is associated with glomerular hypertension and renal microvascular damage in rats. Am J Physiol Renal Physiol 2007;292:F423–9 [DOI] [PubMed] [Google Scholar]
- 23.White JS. Straight talk about high-fructose corn syrup: what it is and what it ain't. Am J Clin Nutr 2008;88:1716S–21S [DOI] [PubMed] [Google Scholar]
- 24.Sun SZ, Empie MW. Lack of findings for the association between obesity risk and usual sugar-sweetened beverage consumption in adults–a primary analysis of databases of CSFII-1989-1991, CSFII-1994-1998, NHANES III, and combined NHANES 1999-2002. Food Chem Toxicol 2007;45:1523–36 [DOI] [PubMed] [Google Scholar]
- 25.Choi JW, Ford ES, Gao X, Choi HK. Sugar-sweetened soft drinks, diet soft drinks, and serum uric acid level: the Third National Health and Nutrition Examination Survey. Arthritis Rheum 2008;59:109–16 [DOI] [PubMed] [Google Scholar]
- 26.Weiner DE, Tighiouart H, Elsayed EF, Griffith JL, Salem DN, Levey AS. Uric acid and incident kidney disease in the community. J Am Soc Nephrol 2008;19:1204–11 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Obermayr RP, Temml C, Gutjahr G, Knechtelsdorfer M, Oberbauer R, Klauser-Braun R. Elevated uric acid increases the risk for kidney disease. J Am Soc Nephrol 2008;19:2407–13 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Chonchol M, Shlipak MG, Katz R, et al. Relationship of uric acid with progression of kidney disease. Am J Kidney Dis 2007;50:239–47 [DOI] [PubMed] [Google Scholar]