Abstract
Background: The optimal combination of diet and exercise that produces the greatest reversal of obesity-related insulin resistance is unknown.
Objectives: We examined the effects of a combined 7-d low–glycemic index (low-GI) diet and exercise training intervention on insulin sensitivity in older obese humans.
Design: Participants [n = 32; mean (±SEM) age: 66 ± 1 y; body mass index (in kg/m2): 33.8 ± 0.7] were randomly assigned to a parallel, double-blind, controlled-feeding trial and underwent supervised aerobic exercise (EX; 60 min/d at 80–85% maximum heart rate) in combination with either a low-GI (LoGI + EX: 41.1 ± 0.4) or a high-GI (HiGI + EX: 80.9 ± 0.6) diet. All meals were provided and were isocaloric to individual energy requirements. Insulin sensitivity and hepatic glucose production were assessed with a 40–mU ⋅ m−2 · min−1 hyperinsulinemic euglycemic clamp combined with a [6,6-2H2]-glucose infusion.
Results: After the intervention, small decreases were observed in body weight (−1.6 ± 0.2 kg; P < 0.0001) and fat mass (−1.7 ± 0.9%; P = 0.004) in both groups. Maximal aerobic capacity (V̇O2max) also improved slightly (0.06 ± 0.02 L/min; P = 0.004). Resting systolic blood pressure, fasting glucose, insulin, triglycerides, and cholesterol all decreased after the study (all P < 0.05). Larger changes in systolic blood pressure and V̇O2max were seen in the LoGI + EX group. Insulin-stimulated glucose disposal (P < 0.001), insulin suppression of hepatic glucose production (P = 0.004), and postabsorptive fat oxidation (P = 0.03) improved equally in both groups after the intervention.
Conclusions: These findings suggest that the metabolic improvements after short-term exercise training in older obese individuals are dependent on increased physical activity and are not influenced by a low-GI diet. However, a low-GI diet has added benefit in alleviating hypertension, thus reducing the risk of diabetic and vascular complications.
INTRODUCTION
The prevalence of obesity and the risk of type 2 diabetes and cardiovascular morbidity are a major health concern in older adults (1, 2). Exercise training is well documented to reduce adiposity, improve insulin sensitivity, and reduce morbidity in all age groups, and it can improve postabsorptive and insulin-stimulated metabolism in the absence of weight loss or improvements in aerobic fitness (3–6). However, the optimal use of diet therapy is not well understood. Recent studies linking dietary glycemic index (GI) to the onset of diabetes has highlighted the GI concept as an important component of nutritional research (7, 8). Evidence indicates that a high-GI diet may be an independent predictor of diabetes risk (9, 10). Further data indicate the advantage of low-GI diets for weight loss and glucose tolerance in overweight adults (11–14). Yet, the supporting literature for GI-induced alterations in insulin sensitivity and substrate metabolism is ambiguous (15–21).
Despite vast knowledge on the effects of exercise training on adiposity, substrate metabolism, and insulin sensitivity, the literature base about exercise training combined with various GI meals is lacking. Previously, our group and others have reported that prior consumption of low-GI meals before an exercise bout improves exercise performance and aerobic capacity, and it elicits increased lipid utilization when compared with high-GI foods (22–26). Therefore, it is reasonable to hypothesize that consumption of low-GI meals may complement exercise training by encouraging optimal nutrient storage or utilization. This may extrapolate to greater exercise-induced improvements in metabolic variables. Recently, we showed that a 1-wk exercise training intervention reduced hepatic glucose production (HGP) and increased insulin-stimulated glucose disposal in obese patients with type 2 diabetes (27). The potential additive effect of low-GI feeding and short-term exercise training on glucose flux and metabolism has not been studied. We hypothesized that a low-GI diet would complement the acute exercise stimulus and further improve metabolism when compared with a high-GI diet.
SUBJECTS AND METHODS
Subjects
Thirty-two older, obese, previously sedentary volunteers [mean (±SEM) age: 66 ± 1 y; body mass index (in kg/m2): 33.8 ± 0.7] were recruited from the local population to undergo a 7-d exercise training and diet intervention (Figure 1). All volunteers underwent a medical history, physical examination, oral-glucose-tolerance test, and complete blood profile (lipid profile and hepatic, renal, hematologic function tests). Medical screening excluded individuals with heart, kidney, liver, thyroid, intestinal, and pulmonary diseases or those taking medications known to affect our outcome variables. Participants' physicians were consulted and approved the withdrawal of antihypertensive and lipid- or glucose-lowering therapy for the duration of the study. Preintervention washout periods were determined from drug half-lives. Screening also excluded participants with any contraindications to physical activity highlighted during a resting 12-lead electrocardiogram and a submaximal exercise stress test. Female subjects were postmenopausal and not using hormone replacement therapy. Prior physical activity levels were recorded with the use of the Minnesota Leisure Time Physical Activity questionnaire (28); volunteers were deemed sedentary if their leisure time activity was <300 kcal/d. Subjects were required to be weight stable for ≥6 mo before study participation. During medical screening, volunteers underwent resting metabolic rate (RMR) measurements by ventilated hood indirect calorimetry (described in full below) to ascertain individual caloric requirements (29). The study was approved by the Institutional Review Board, and all subjects provided signed informed written consent in accordance with our guidelines for the protection of human subjects. Initial subject recruitment began in December 2004; thus, this study was not registered as a clinical trial.
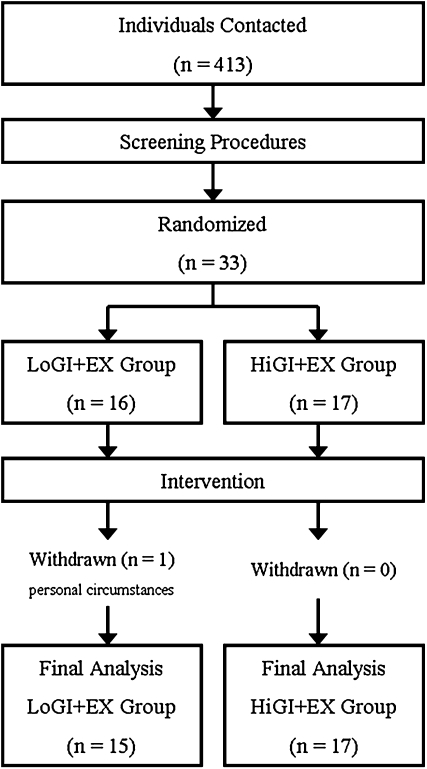
FIGURE 1.
Study flowchart in response to study advertisement. A total of 413 men and women underwent screening. After review of our inclusion-exclusion criteria, 33 were eligible to partake in the 1-wk diet and exercise intervention. These individuals were randomly assigned to an exercise training protocol (EX) accompanied with either a low–glycemic index (GI) diet (LoGI+EX; n = 16) or a high-GI diet (HiGI+EX; n = 17). After baseline testing, one male subject withdrew from the intervention because of personal circumstances. No adverse events were associated with our testing procedures or the intervention. Final statistical analyses were performed on 32 participants (LoGI+EX, n = 15; HiGI+EX, n = 17).
Intervention
All participants undertook 60-min of fully supervised aerobic exercise [treadmill walking and cycle ergometry at ≈80–85% of maximum heart rate (HRmax)] each day for 7 consecutive days, as previously described (27). Compliance to exercise intensity was monitored with the use of a heart rate monitor (Polar Electro Inc, Woodbury, NY). In addition, participants were randomly assigned to receive either a low-GI [LoGI + exercise (EX); n = 15; 7 men, 8 women] or a high-GI (HiGI + EX; n = 17; 8 men, 9 women) diet for the duration of the study. All meals and snacks were prepared in our Metabolic Kitchen and were isocaloric to subjects' individual requirements (screening RMR multiplied by a sedentary activity factor of 1.25). The high- and low-GI diets were formulated with the use of GI data tables from Foster-Powell et al (30). Although macronutrient composition and dietary GI were matched from day to day, each day of the intervention consisted of different foods (a 7-d sample menu is presented under “Supplemental data” in the online issue). The dietary macronutrient composition was matched between groups (LoGI + EX compared with HiGI + EX: 56 ± 1% compared with 57 ± 1% of calories from carbohydrate; 29 ± 1% compared with 30 ± 5% of calories from fat; 18 ± 1% compared with 17 ± 2% of calories from protein). Diets were also matched for fiber between groups (Table 1). Dietary adherence was ensured with food container weigh backs plus counseling by the study dietitian.
TABLE 1.
Dietary intake of study groups1
| LoGI + EX | HiGI + EX | P value2 | |
| EI (kcal/d) | 1795 ± 913 | 1897 ± 104 | 0.47 |
| Carbohydrate (g/d) | 252.6 ± 13.1 | 271.2 ± 16.2 | 0.39 |
| Fat (g/d) | 56.6 ± 2.9 | 61.3 ± 3.1 | 0.28 |
| Protein (g/d) | 79.4 ± 4.0 | 80.5 ± 4.6 | 0.86 |
| Fiber (g/d) | 28.8 ± 1.6 | 28.6 ± 1.8 | 0.95 |
| GI (au) | 41.1 ± 0.4 | 80.9 ± 0.6 | <0.0001 |
| GL (au) | 104.0 ± 5.5 | 219.2 ± 13.0 | <0.0001 |
LoGI, low glycemic index; HiGI, high glycemic index; EX, exercise intervention; EI, energy intake; GI, glycemic index; GL, glycemic load; au, arbitrary units. Study diets were isocaloric and were based on individual energy requirements estimated by indirect calorimetry. Diets were macronutrient matched between the LoGI + EX and HiGI + EX groups and differed only in GI and GL.
Derived by using unpaired between-group t tests.
Mean ± SEM (all such values).
Clinical testing control period
Pre- and postintervention measures were recorded during a 3-d inpatient stay in the Clinical Research Unit at the Cleveland Clinic. During the preintervention inpatient stay, participants received a weight-maintenance isocaloric diet (total kcal/d = RMR × 1.25; 55% carbohydrate, 35% fat, 10% protein). The postintervention in-patient stay incorporated the final 2 d of the GI diet and exercise prescription, so that day 3 of this stay occurred the day after the final exercise session. During the inpatient stay, assessments of body composition, aerobic fitness, insulin sensitivity, and substrate metabolism were conducted before and after the intervention.
Body composition
Height and body weight were measured as previously described (31). Dual-energy X-ray absorptiometry (model iDXA; Lunar, Madison, WI) was used to determine whole-body fat mass. The volunteer lay on the iDXA bed in a supine position for the duration of the whole-body scan. A dual-energy X-ray beam was used to emit alternating high (140 kVp) and low (100 kVp) energy X-rays. After the whole-body scan, a manually determined region of interest that incorporated the first through fourth lumbar vertebral bodies was captured to determine abdominal adiposity (32, 33). Measurements of adiposity were determined by the specific attenuation characteristics associated with the 2 energy levels of X-ray in each type of tissue.
Aerobic fitness and cardiovascular measures
Each participant performed an incremental-graded treadmill exercise test to determine his or her maximal oxygen consumption (V̇O2max), as previously described (31). Expired air was continuously sampled online with the use of an automated system (Jaeger Oxycon Pro; Viasys, Yorba Linda, CA). Because of the acute effects of exercise on insulin sensitivity, the preintervention V̇O2max test was conducted >48 h before metabolic testing and sample collections. The postintervention test was performed the day after the final metabolic tests. All V̇O2max tests were performed in the morning after an overnight fast. Resting systolic blood pressure (SBP) and diastolic blood pressure were measured with a sphygmomanometer; resting and maximal heart rates were also recorded (Polar Electro Inc, Woodbury, NY).
Insulin sensitivity
After a 10-h overnight fast, subjects were awakened at 0600 and taken by wheelchair to be weighed. Subjects then reclined for the duration of the procedure. A primed (3.28 mg/kg) continuous (0.036 mg · kg−1 · min−1) infusion of [6,6-2H2]-glucose was begun at t = −120 min. At t = 0 min a hyperinsulinemic (40 mU · m−2 · min−1) euglycemic (90 mg/dL) clamp proceeded as previously described (34). In brief, a 2-h primed insulin infusion began, while a variable rate glucose infusion maintained euglycemia. Infusions were administered into an antecubital vein. The glucose infusate was enriched with [6,6-2H2]-glucose at an enrichment intended to achieve ≈1.0 mol percent excess for all subjects. Blood was sampled from a retrograde line inserted into a warmed (≈60°C) dorsal hand vein. Arterialized venous blood was sampled for glucose concentration measurements at 5-min intervals (YSI 2300; STAT Plus, Yellow Springs, OH). Adjustments to the glucose infusion rate were made according to the calculations of DeFronzo et al (35). Glucose kinetics were calculated according to the equations of Steele (36) modified for variable-rate glucose tracer infusions (37). Rates of glucose appearance and disappearance were calculated as the mean rate obtained during postabsorptive and insulin-stimulated conditions, ie, t = −30 to 0 min and t = 90–120 min. The rate of glucose appearance from endogenous sources was calculated as the difference between the exogenous glucose infusion rate and the tracer-derived estimate of total glucose appearance. We refer to rate of endogenous glucose appearance, the majority of which is derived from the liver, as HGP; whereas we refer to the rate of glucose disappearance as the insulin-stimulated glucose disposal rate (GDR). Pre- to poststudy alterations in postabsorptive HGP will provide an explanation for changes in fasting plasma glucose, whereas changes in insulin suppression of HGP will provide a direct measure of hepatic insulin sensitivity.
Substrate metabolism
Indirect calorimetry measures were performed before (postabsorptive metabolism) and during the final 30 min of the clamp procedure (insulin-stimulated metabolism). Expired air was continuously sampled for 20 min with the use of an automated system (Vmax Encore; Viasys). Air collection proceeded in a semidarkened, thermoneutral (22 ± 1°C) environment under a ventilated hood, as previously described (38). The equations of Weir (29) and Frayn (39) were used to calculate energy expenditure and substrate oxidation rates. In addition, overnight, timed urinary nitrogen excretion measurements (Roche Modular Diagnostics, Indianapolis, IN) were also made to correct our estimates for protein metabolism (39). Nonoxidative glucose metabolism was calculated as GDR minus insulin-stimulated carbohydrate oxidation. Furthermore, pre- and postintervention metabolic flexibility was calculated as the response of postabsorptive substrate metabolism to hyperinsulinemic conditions, eg, insulin-stimulated respiratory exchange ratio (RER) minus postabsorptive RER.
Biochemical assays
Insulin was analyzed by radioimmunoassay (Millipore, Billerica, MA). Triglycerides, total cholesterol, HDL cholesterol, LDL cholesterol, and VLDL cholesterol were analyzed by enzymatic analysis on an automated platform (Roche Modular Diagnostics). Glycated hemoglobin was measured with the use of nonporous ion-exchange HPLC (G7 HPLC Analyzer; Tosoh Bioscience Inc, San Francisco, CA). Plasma samples collected for glucose kinetics analyses were deproteinized, extracted, then derivatized before analysis by gas chromatography–mass spectrometry. First, 1 mL 70% methanol was added to 200 μL plasma and centrifuged at 1000 rpm for 10 min. The supernatant fluid was collected, dried under air, and reconstituted with 200 μL double-distilled H2O. Next, the sample was applied to a glass column containing a cation exchange resin (AG50W-X8 200–400 mesh; Bio-Rad, Hercules, CA), the sample of interest was eluted with 5 mL double-distilled H2O (pH 8.0), and dried (Labconco Corporation, Kansas City, MO). Then, 30 μL pyridine and 15 μL acetic anhydride were added to the dried sample and incubated for 2 h at room temperature. Finally, 400 μL H2O and 400 μL ethyl acetate were added. The sample was centrifuged for 5 min at 1000 rpm, and the upper layer was collected for injection into a Hewlett-Packard 5985A gas chromatograph-mass spectrometer (Hewlett-Packard, Palo Alto, CA). Ions mass-to-charge ratio (m/z) 200 and 202 were selectively monitored. The isotopic enrichment (mole percent excess) of the samples were obtained by comparing their peak area percentage (m/z 202)/(m/z 202 + m/z 200) with that of a standard curve.
Statistics
Between-group (LoGI + EX compared with HiGI + EX) comparisons were analyzed with the use of 2-factor (group × time) repeated-measures analysis of variance (ANOVA), and Bonferroni post hoc tests were applied to significant group × time interactions. Sex was applied as a covariate in the ANOVA analysis. Baseline values for each variable were compared between groups with the use of unpaired t tests. In the event of a significant t statistic, baseline values were used as a covariate in the 2-factor repeated-measures ANOVA. Between-group changes with time were also compared with the use of 1-factor ANOVA and Fisher's least significant differences post hoc tests. In addition, bivariate correlation analyses were used to identify relations between changes in variables. Statistical significance was accepted with P < 0.05. Analyses were carried out with STATVIEW for Windows 5.0.1 (SAS Institute, Cary, NC), and all data are expressed as means ± SEMs.
RESULTS
Dietary intake and exercise training
The LoGI + EX and HiGI + EX groups consumed diets of similar caloric loads and macronutrient compositions yet different GIs (Table 1). There was 100% attendance at exercise sessions, and exercise training intensities were matched between the groups (LoGI + EX: 82.1 ± 0.5% HRmax; HiGI + EX: 81.9 ± 0.9% HRmax; P = 0.57).
Subject characteristics
The changes in body composition, cardiovascular fitness, and blood chemistry across the 7-d intervention are shown in Table 2. Body weight, body mass index, and fat mass were significantly decreased after the intervention. ANOVA showed no group × time or sex interactions in body composition. Fasting measures of plasma glucose, plasma insulin, triglycerides, and cholesterol were all significantly improved after the study. No group effects were noted; yet triglycerides (P for time × sex interaction: 0.006) and cholesterol (P for time × sex interaction: 0.01) showed greater improvements in male subjects. No changes in glycated hemoglobin were noted. Resting SBP and V̇O2max during exhaustive exercise improved after the 7-d intervention. Larger improvements in V̇O2max and SBP were observed in the LoGI + EX group (P for group × time interaction: 0.02, 2-way ANOVA, and P for group × time interaction: 0.04, 2-way ANOVA, for each variable). In addition, improvements in V̇O2max tended to be greater in male subjects (P for time × sex interaction: 0.06).
TABLE 2.
Subject characteristics of study groups1
| LoGI + EX (n = 15) |
HiGI + EX (n = 17) |
2-Factor ANOVA |
||||
| Before | After | Before | After | P for time | P for group × time | |
| Sex (F/M) | 8/7 | — | 9/8 | — | — | — |
| Age (y) | 67 ± 12 | — | 66 ± 1 | — | — | — |
| Weight (kg) | 93.6 ± 4.0 | 91.8 ± 3.8 | 97.8 ± 4.1 | 96.4 ± 4.1 | <0.0001 | 0.42 |
| BMI (kg/m2) | 32.8 ± 1.0 | 32.2 ± 1.0 | 34.6 ± 1.0 | 34.1 ± 1.0 | <0.0001 | 0.40 |
| FM (%) | 41.7 ± 2.2 | 41.4 ± 2.1 | 43.6 ± 1.6 | 43.0 ± 1.6 | 0.004 | 0.41 |
| TFM (kg) | 6.45 ± 0.51 | 6.26 ± 0.48 | 6.28 ± 0.56 | 6.05 ± 0.54 | 0.001 | 0.45 |
| SBP (mm Hg) | 130.9 ± 2.8 | 119.9 ± 2.8 | 133.6 ± 3.9 | 132.2 ± 4.2 | 0.01 | 0.04 |
| DBP (mm Hg) | 78.1 ± 2.6 | 73.3 ± 2.3 | 78.1 ± 2.3 | 77.1 ± 1.9 | 0.10 | 0.29 |
| FPG (mg/dL) | 103.2 ± 2.8 | 99.5 ± 2.4 | 98.3 ± 2.5 | 94.7 ± 2.1 | 0.001 | 0.70 |
| FPI (μU/mL) | 16.8 ± 1.5 | 13.0 ± 1.4 | 13.5 ± 1.0 | 12.0 ± 1.3 | 0.0004 | 0.23 |
| TG (mg/dL) | 165.5 ± 19.3 | 113.5 ± 15.0 | 129.4 ± 13.4 | 82.5 ± 6.0 | <0.0001 | 0.27 |
| Cholesterol (mg/dL) | ||||||
| Total | 203.7 ± 9.1 | 188.1 ± 9.2 | 206.3 ± 5.6 | 190.5 ± 4.8 | 0.0009 | 0.90 |
| LDL | 123.7 ± 8.7 | 118.2 ± 7.6 | 130.7 ± 4.6 | 126.0 ± 4.2 | 0.19 | 0.74 |
| HDL | 49.7 ± 4.3 | 48.9 ± 4.6 | 50.8 ± 4.3 | 49.0 ± 4.0 | 0.009 | 0.41 |
| VLDL | 34.3 ± 4.9 | 23.3 ± 3.1 | 47.4 ± 21.9 | 17.4 ± 1.4 | 0.13 | 0.46 |
| Hb A1c (%) | 5.59 ± 0.20 | 5.58 ± 0.19 | 5.56 ± 0.11 | 5.55 ± 0.09 | 0.80 | 0.47 |
| V̇O2max (L/min) | 2.14 ± 0.14 | 2.25 ± 0.16 | 2.13 ± 0.11 | 2.15 ± 0.12 | 0.004 | 0.02 |
LoGI, low glycemic index; HiGI, high glycemic index; EX, exercise intervention; FM, fat mass; TFM, truncal fat mass; SPB, systolic blood pressure; DBP, diastolic blood pressure; FPG, fasting plasma glucose; FPI, fasting plasma insulin; TG, triglycerides; Hb A1c, glycated hemoglobin; V̇O2max, maximal oxygen uptake. Older obese nondiabetic men and women volunteered to partake in a 7-d exercise training intervention, in combination with either a low-GI diet (LoGI + EX) or a high-GI diet (HiGI + EX). No prestudy group differences existed for any variable (P > 0.05, t test). Body weight, BMI, whole-body FM, and TFM showed small but significant decreases with time independently of the trial. Significant improvements in FPG, FPI, and lipidemia (TG and cholesterol) were identified during the study. These occurred independently of the study group. No changes in Hb A1c were identified. V̇O2max during exhaustive exercise and resting SBP improved after the study. These changes were greater in the LoGI + EX group. No variable was different between groups before the intervention.
Mean ± SEM (all such values).
Insulin sensitivity
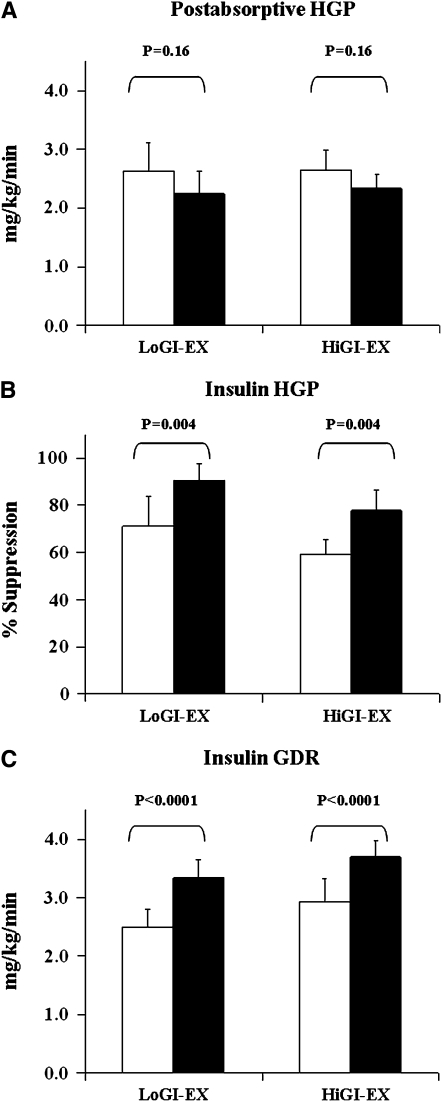
The changes in glucose flux during the study are shown in Figure 2. Postabsorptive HGP showed a nonsignificant decrease after the intervention (Figure 2A; P = 0.16). Before the study, insulin suppressed HGP by 71.3 ± 12.5% and 59.3 ± 6.2%, respectively, in the LoGI + EX and HiGI + EX groups. After the intervention, insulin-suppression of HGP was increased to 90.5 ± 7.2% and 77.9 ± 8.8%, respectively, in the LoGI + EX and HiGI + EX groups (Figure 2B). Insulin-stimulated GDR was increased by 42.1 ± 9.8% and 41.4 ± 11.2%, respectively, in the LoGI + EX and HiGI + EX groups (Figure 2C; P for effect of time <0.0001) after 7 d of exercise training and dietary control. No group × time or sex interactions were present.
FIGURE 2.
Mean (±SEM) changes in glucose flux of 32 older, obese, and previously sedentary nondiabetic individuals who underwent a 7-d aerobic exercise training intervention (EX) combined with either a low–glycemic index (GI) diet (LoGI-EX; n = 15) or a high-GI diet (HiGI-EX; n = 17). White bars represent prestudy data; black bars represent poststudy data. A: Postabsorptive hepatic glucose production (HGP) showed a nonsignificant decline after the intervention (P = 0.16). B: The percentage of suppression of HGP by insulin increased after the study (P = 0.004). C: Insulin-stimulated glucose disposal rates (GDR) were significantly elevated after the intervention (P < 0.0001). These changes were not different between study groups, nor was there an effect of sex.
Substrate metabolism
Postabsorptive substrate metabolism is shown in Table 3. RER tended to decrease (P = 0.11) after the exercise and diet intervention, indicating an increase in postabsorptive fat oxidation (P = 0.03). RER and metabolic flexibility were not altered by the intervention (both P > 0.05). However, after the study, there was an increase in nonoxidative glucose metabolism of 1.23 ± 0.68 mg · kg−1 · min−1 and 0.76 ± 0.36 mg · kg−1 · min−1, respectively, in the LoGI + EX and HiGI + EX groups (P for effect of time: 0.01, ANOVA). No group or sex effects were observed.
TABLE 3.
Substrate metabolism before and after intervention1
| LoGI + EX |
HiGI + EX |
2-Factor ANOVA |
||||
| Before | After | Before | After | P for time | P for group × time | |
| RER (au) | 0.84 ± 0.02 | 0.82 ± 0.01 | 0.86 ± 0.02 | 0.83 ± 0.01 | 0.11 | 0.75 |
| REE (kcal/min) | 1.05 ± 0.05 | 1.03 ± 0.05 | 1.11 ± 0.06 | 1.13 ± 0.07 | 0.97 | 0.37 |
| Fox (mg/min) | 42.2 ± 8.8 | 54.3 ± 7.8 | 39.8 ± 6.8 | 56.7 ± 9.3 | 0.03 | 0.71 |
| MetFlex (au) | 0.02 ± 0.01 | 0.04 ± 0.01 | 0.03 ± 0.01 | 0.04 ± 0.01 | 0.21 | 0.72 |
| NonOx GDR (mg · kg−1 · min−1) | 0.90 ± 0.35 | 2.13 ± 0.64 | 1.33 ± 0.42 | 2.09 ± 0.23 | 0.01 | 0.54 |
All values are means ± SEMs. LoGI, low glycemic index; HiGI, high glycemic index; EX, exercise intervention; RER, respiratory exchange ratio; REE, resting energy expenditure; Fox, fat oxidation; MetFlex, metabolic flexibility; au, arbitrary units; NonOx GDR, nonoxidative glucose disposal. No prestudy group differences existed for any variable (P > 0.05, t test). Rates of Fox (P = 0.03) and NonOx GDR (P = 0.01) increased after the 7-d exercise and diet intervention. RERs showed a nonsignificant trend toward decline after the study (P = 0.11). No significant changes were noted for other variables.
Correlation analyses
Intervention-induced changes in HGP were not related to changes in body weight (r = −0.05, P = 0.87), fat mass (r = 0.42, P = 0.16), or V̇O2max (r = −0.38, P = 0.20). Additional analyses showed that changes in insulin-stimulated GDR were also not correlated with alterations in body weight (r = −0.01, P = 0.96), fat mass (r = −0.07, P = 0.71), or V̇O2max (r = 0.07, P = 0.72).
DISCUSSION
This investigation shows that dietary GI has no influence on the improvement in postabsorptive or insulin-stimulated glucose metabolism and insulin sensitivity after a short-term (7-d) aerobic exercise training stimulus in previously sedentary, older, obese men and women. These changes were shown to be independent of alterations in body composition and aerobic capacity, and they highlight the effect of increasing physical activity on metabolism in individuals at high risk of developing further metabolic and cardiovascular disease.
Exercise, diet, and behavioral counseling are first-line therapeutic interventions for diabetes and obesity (40). These are the first data to investigate the combined effects of short-term exercise training and alterations in dietary GI on metabolism in older obese individuals. Previous data investigating short-term interventions with high- compared with low-GI diets have shown conflicting results (9–12, 15–21); whereas short-term exercise training studies have shown consistent improvement in metabolic variables (3–6). Our data indicate the powerful acute stimulus exercise provides to up-regulate whole-body substrate oxidation, suppress HGP, increase peripheral tissue glucose disposal, and improve circulatory lipemia, key aspects in the development of metabolic disease and vascular deterioration. Previous studies have noted that consumption of low-GI diets before a single aerobic exercise bout may decrease the reliance on intramuscular glycogen for carbohydrate oxidation, thus allowing a greater proportion of energy expenditure during exercise to be derived from fat oxidation (24–26). One may hypothesize that prolonged consumption of a low-GI diet in addition to exercise training may extrapolate to decreased whole-body fat mass and thus greater improvements in insulin sensitivity. Here, we have shown that altering dietary GI has no additional effect on improvement in substrate oxidation or insulin sensitivity. This alternative finding to our hypothesis may be due to the different study designs used in previous studies. Prior work has only investigated single exercise bouts in lean healthy or endurance-trained individuals. Our data present a 1-wk diet and exercise stimulus which may overwhelm the responses to a single bout of activity or single GI-controlled meal. We, and others, have previously shown that a short-term (typically 7–10 d) exercise training stimulus is able to suppress HGP and to improve insulin sensitivity in obese patients with type 2 diabetes, probably by alterations in GLUT4 expression or AMPK activity (27, 41–43). Such acute exercise interventions were designed to investigate the direct effects of muscular contraction on metabolism, independent of weight loss and improvements in aerobic capacity. If a low-GI diet is able to complement an exercise training regimen, it may be that a longer term intervention is required to see the effects. Recent data from Botero et al (44) have shown that a 1-wk low-GI diet reduces oxidative stress more so than a high-GI diet, yet the data fail to differentially alter insulin sensitivity, suggesting that indeed longer term interventions are required.
Previous short-term training interventions have not reported improvement in body composition and aerobic fitness, whereas in this study it appears that subjects show significant improvements in body weight, fat mass, and V̇O2max. However, these improvements are small and, in terms of percentage of change, are comparable to previous literature (≈1.5% weight loss and a 2.5% improvement in V̇O2max). We would therefore consider these changes to be statistically, but not clinically, significant. Therefore, with minor alterations in body composition and fitness, we show large increases (>40%) in insulin-stimulated peripheral tissue glucose disposal and significant decreases in fasting glucose, insulin, and lipemia. Alterations in glucose independent of body composition and aerobic fitness are further highlighted by the lack of correlation found between changes in such variables. These improvements in glucose disposal are therefore indicative of the muscle contraction-mediated effects on metabolism. The evidence base indicates that short-term aerobic training can increase myocellular GLUT4 protein content (45), endothelial lipoprotein lipase activity (46, 47), and intracellular lipid metabolism (6); improve hepatic cholesterol metabolism; and reverse cholesterol transport (48). This mechanistic literature provides some insight into the elevated peripheral tissue glucose uptake, the decrease in circulating triglycerides, and the more favorable cholesterol subfractions seen after acute training in this study. It appears from our data that when strict control of diet is combined with a 7-d aerobic exercise training program, there are clearly similar improvements in metabolism between the 2 GI study groups. These findings suggest that short-term exercise training has a more pronounced effect over the potential for modification in dietary carbohydrate quality to induce further effects on insulin sensitivity.
A novel insight from this study is that the improvement in SBP was more pronounced in the LoGI + EX group. The effects of short-term exercise training and a low glycemic diet on blood pressure in older individuals at risk of developing future diabetes or other vascular events have not been previously reported. Here, we have shown improvement in an independent marker of cardiovascular disease risk in an “at risk” cohort. Lower blood pressure is probably produced by a reduction in vascular tone as a result of exercise training, as has been previously shown (49, 50); however the effects of a low-GI diet in combination with an exercise stimulus requires further exploration. Previous work has shown that a low-GI diet may be more advantageous to the suppression of mean arterial pressure in overweight-obese young adults (51). Improvements in such variables may be related to reductions in day-long insulinemia induced by low-GI foods. Indeed, Modan et al (52) has shown a strong relation between hyperinsulinemia and hypertension in humans (52). Insulin is known to have direct effects on sodium-potassium transport mechanisms (53); thus, the lower insulinemic concentrations induced by a low-GI diet may have favorable consequences on blood pressure by such mechanisms. These findings highlight the potential therapeutic effect of low-GI foods on elevated arterial pressure.
This study reports that the improvements in glucose flux after 7 d of exercise training are not influenced by dietary GI. In addition, we have also confirmed the importance of physical activity for the improvement of hyperglycemia, hyperinsulinemia, dyslipidemia, and elevated blood pressure in older obese men and women at risk of developing future diabetic or cardiovascular complications. Although a low-GI diet did not have any additional benefit on HGP or insulin sensitivity over that of a high-GI diet, it did show a more favorable outcome on SBP. This is an important component of cardiometabolic risk that must be addressed by treatment courses. Thus, besides increments in a patient's physical activity, it would be sensible to additionally advise or prescribe a low-GI diet to address this component of metabolic disease that has an independent contribution toward vascular dysfunction.
Supplementary Material
Acknowledgments
We thank the research volunteers for their outstanding dedication and effort; the nursing and nutrition staffs of the Clinical Research Unit; and Christine Marchetti, Latina Brooks, and students who helped with the implementation of the study and assisted with data collection. We also thank our clinical research coordinator, Julianne Filion, for her excellent nursing and organizational assistance.
The authors' responsibilities were as follows—JPK and HB: conception and design; TPJS, JMH, KRK, MDC, M Riccardi, M Rocco, HB, and JPK: data acquisition; TPS, JMH, KRK, M Riccardi, SRK, HB, and JPK: analysis and interpretation of data; TPJS drafting of the manuscript; JMH, KRK, SRK, HB, and JPK: critical revision of the manuscript; TPJS: statistical analysis; JPK: obtaining funding; MDC and M Riccardi: technical support; M Riccardi and HB: dietary support; M Rocco and SRK: clinical support; and HB and JPK: supervision. TPJS had full access to all of the data in the study and takes responsibility for the integrity of the data and the accuracy of the data analysis. None of the authors had a personal or financial conflict of interest.
REFERENCES
- 1.Ogden CL, Carroll MD, Curtin LR, McDowell MA, Tabak CJ, Flegal KM. Prevalence of overweight and obesity in the United States, 1999-2004. JAMA 2006;295:1549–55 [DOI] [PubMed] [Google Scholar]
- 2.Cowie CC, Rust KF, Byrd-Holt DD, et al. Prevalence of diabetes and impaired fasting glucose in adults in the U.S. population: National Health and Nutrition Examination Survey 1999-2002. Diabetes Care 2006;29:1263–8 [DOI] [PubMed] [Google Scholar]
- 3.Black SE, Mitchell E, Freedson PS, Chipkin SR, Braun B. Improved insulin action following short-term exercise training: role of energy and carbohydrate balance. J Appl Physiol 2005;99:2285–93 [DOI] [PubMed] [Google Scholar]
- 4.Arciero PJ, Vukovich MD, Holloszy JO, Racette SB, Kohrt WM. Comparison of short-term diet and exercise on insulin action in individuals with abnormal glucose tolerance. J Appl Physiol 1999;86:1930–5 [DOI] [PubMed] [Google Scholar]
- 5.Bloem CJ, Chang AM. Short-term exercise improves beta-cell function and insulin resistance in older people with impaired glucose tolerance. J Clin Endocrinol Metab 2008;93:387–92 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Berggren JR, Boyle KE, Chapman WH, Houmard JA. Skeletal muscle lipid oxidation and obesity: influence of weight loss and exercise. Am J Physiol Endocrinol Metab 2008;294:E726–32 [DOI] [PubMed] [Google Scholar]
- 7.Pawlak DB, Ebbeling CB, Ludwig DS. Should obese patients be counselled to follow a low-glycaemic index diet? Yes. Obes Rev 2002;3:235–43 [DOI] [PubMed] [Google Scholar]
- 8.Raben A. Should obese patients be counselled to follow a low-glycaemic index diet? No. Obes Rev 2002;3:245–56 [DOI] [PubMed] [Google Scholar]
- 9.Salmeron J, Ascherio A, Rimm EB, et al. Dietary fiber, glycemic load, and risk of NIDDM in men. Diabetes Care 1997;20:545–50 [DOI] [PubMed] [Google Scholar]
- 10.Salmeron J, Manson JE, Stampfer MJ, Colditz GA, Wing AL, Willett WC. Dietary fiber, glycemic load, and risk of non-insulin-dependent diabetes mellitus in women. JAMA 1997;277:472–7 [DOI] [PubMed] [Google Scholar]
- 11.Bouche C, Rizkalla SW, Luo J, et al. Five-week, low-glycemic index diet decreases total fat mass and improves plasma lipid profile in moderately overweight nondiabetic men. Diabetes Care 2002;25:822–8 [DOI] [PubMed] [Google Scholar]
- 12.Slabber M, Barnard HC, Kuyl JM, Dannhauser A, Schall R. Effects of a low-insulin-response, energy-restricted diet on weight loss and plasma insulin concentrations in hyperinsulinemic obese females. Am J Clin Nutr 1994;60:48–53 [DOI] [PubMed] [Google Scholar]
- 13.Pittas AG, Das SK, Hajduk CL, et al. A low-glycemic load diet facilitates greater weight loss in overweight adults with high insulin secretion but not in overweight adults with low insulin secretion in the CALERIE Trial. Diabetes Care 2005;28:2939–41 [DOI] [PubMed] [Google Scholar]
- 14.Wolever TM, Gibbs AL, Mehling C, et al. The Canadian Trial of Carbohydrates in Diabetes (CCD), a 1-y controlled trial of low-glycemic-index dietary carbohydrate in type 2 diabetes: no effect on glycated hemoglobin but reduction in C-reactive protein. Am J Clin Nutr 2008;87:114–25 [DOI] [PubMed] [Google Scholar]
- 15.Diaz EO, Galgani JE, Aguirre CA. Glycaemic index effects on fuel partitioning in humans. Obes Rev 2006;7:219–26 [DOI] [PubMed] [Google Scholar]
- 16.Frost G, Leeds A, Trew G, Margara R, Dornhorst A. Insulin sensitivity in women at risk of coronary heart disease and the effect of a low glycemic diet. Metabolism 1998;47:1245–51 [DOI] [PubMed] [Google Scholar]
- 17.Jarvi AE, Karlstrom BE, Granfeldt YE, Bjorck IE, Asp NG, Vessby BO. Improved glycemic control and lipid profile and normalized fibrinolytic activity on a low-glycemic index diet in type 2 diabetic patients. Diabetes Care 1999;22:10–8 [DOI] [PubMed] [Google Scholar]
- 18.Kiens B, Richter EA. Types of carbohydrate in an ordinary diet affect insulin action and muscle substrates in humans. Am J Clin Nutr 1996;63:47–53 [DOI] [PubMed] [Google Scholar]
- 19.Rizkalla SW, Taghrid L, Laromiguiere M, et al. Improved plasma glucose control, whole-body glucose utilization, and lipid profile on a low-glycemic index diet in type 2 diabetic men: a randomized controlled trial. Diabetes Care 2004;27:1866–72 [DOI] [PubMed] [Google Scholar]
- 20.Das SK, Gilhooly CH, Golden JK, et al. Long-term effects of 2 energy-restricted diets differing in glycemic load on dietary adherence, body composition, and metabolism in CALERIE: a 1-y randomized controlled trial. Am J Clin Nutr 2007;85:1023–30 [DOI] [PubMed] [Google Scholar]
- 21.Mosdol A, Witte DR, Frost G, Marmot MG, Brunner EJ. Dietary glycemic index and glycemic load are associated with high-density-lipoprotein cholesterol at baseline but not with increased risk of diabetes in the Whitehall II study. Am J Clin Nutr 2007;86:988–94 [DOI] [PubMed] [Google Scholar]
- 22.Kirwan JP, O'Gorman D, Evans WJ. A moderate glycemic meal before endurance exercise can enhance performance. J Appl Physiol 1998;84:53–9 [DOI] [PubMed] [Google Scholar]
- 23.Kirwan JP, Cyr-Campbell D, Campbell WW, Scheiber J, Evans WJ. Effects of moderate and high glycemic index meals on metabolism and exercise performance. Metabolism 2001;50:849–55 [DOI] [PubMed] [Google Scholar]
- 24.Stevenson EJ, Williams C, Mash LE, Phillips B, Nute ML. Influence of high-carbohydrate mixed meals with different glycemic indexes on substrate utilization during subsequent exercise in women. Am J Clin Nutr 2006;84:354–60 [DOI] [PubMed] [Google Scholar]
- 25.Febbraio MA, Keenan J, Angus DJ, Campbell SE, Garnham AP. Preexercise carbohydrate ingestion, glucose kinetics, and muscle glycogen use: effect of the glycemic index. J Appl Physiol 2000;89:1845–51 [DOI] [PubMed] [Google Scholar]
- 26.Wee SL, Williams C, Gray S, Horabin J. Influence of high and low glycemic index meals on endurance running capacity. Med Sci Sports Exerc 1999;31:393–9 [DOI] [PubMed] [Google Scholar]
- 27.Kirwan JP, Solomon TP, Wojta DM, Staten MA, Holloszy JO. Effects of 7 days of exercise training on insulin sensitivity and responsiveness in type 2 diabetes mellitus. Am J Physiol Endocrinol Metab 2009;297:E151–6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Taylor HL, Jacobs DR, Jr, Schucker B, Knudsen J, Leon AS, Debacker G. A questionnaire for the assessment of leisure time physical activities. J Chronic Dis 1978;31:741–55 [DOI] [PubMed] [Google Scholar]
- 29.Weir JBV. New methods for calculating metabolic rate with special reference to protein metabolism. J Physiol 1949;109:1–9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Foster-Powell K, Holt SH, Brand-Miller JC. International table of glycemic index and glycemic load values: 2002. Am J Clin Nutr 2002;76:5–56 [DOI] [PubMed] [Google Scholar]
- 31.O'Leary VB, Marchetti CM, Krishnan RK, Stetzer BP, Gonzalez F, Kirwan JP. Exercise-induced reversal of insulin resistance in obese elderly is associated with reduced visceral fat. J Appl Physiol 2006;100:1584–9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Park YW, Heymsfield SB, Gallagher D. Are dual-energy X-ray absorptiometry regional estimates associated with visceral adipose tissue mass? Int J Obes Relat Metab Disord 2002;26:978–83 [DOI] [PubMed] [Google Scholar]
- 33.Glickman SG, Marn CS, Supiano MA, Dengel DR. Validity and reliability of dual-energy X-ray absorptiometry for the assessment of abdominal adiposity. J Appl Physiol 2004;97:509–14 [DOI] [PubMed] [Google Scholar]
- 34.Solomon TP, Sistrun SN, Krishnan RK, et al. Exercise and diet enhance fat oxidation and reduce insulin resistance in older obese adults. J Appl Physiol 2008;104:1313–9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.DeFronzo RA, Tobin JD, Andres R. Glucose clamp technique: a method for quantifying insulin secretion and resistance. Am J Physiol 1979;237:E214–23 [DOI] [PubMed] [Google Scholar]
- 36.Steele R. Influences of glucose loading and of injected insulin on hepatic glucose output. Ann N Y Acad Sci 1959;82:420–30 [DOI] [PubMed] [Google Scholar]
- 37.Finegood DT, Bergman RN, Vranic M. Estimation of endogenous glucose production during hyperinsulinemic-euglycemic glucose clamps. Comparison of unlabeled and labeled exogenous glucose infusates. Diabetes 1987;36:914–24 [DOI] [PubMed] [Google Scholar]
- 38.Williamson DL, Kirwan JP. A single bout of concentric resistance exercise increases basal metabolic rate 48 hours after exercise in healthy 59-77-year-old men. J Gerontol A Biol Sci Med Sci 1997;52:M352–5 [DOI] [PubMed] [Google Scholar]
- 39.Frayn KN. Calculation of substrate oxidation rates in vivo from gaseous exchange. J Appl Physiol 1983;55:628–34 [DOI] [PubMed] [Google Scholar]
- 40.American Diabetes Association Nutrition Recommendations and Interventions for Diabetes: a position statement of the American Diabetes Association. Diabetes Care 2008;31(suppl):S61–78 [DOI] [PubMed] [Google Scholar]
- 41.Gulve EA, Spina RJ. Effect of 7-10 days of cycle ergometer exercise on skeletal muscle GLUT-4 protein content. J Appl Physiol 1995;79:1562–6 [DOI] [PubMed] [Google Scholar]
- 42.O'Gorman DJ, Karlsson HK, McQuaid S, et al. Exercise training increases insulin-stimulated glucose disposal and GLUT4 (SLC2A4) protein content in patients with type 2 diabetes. Diabetologia 2006;49:2983–92 [DOI] [PubMed] [Google Scholar]
- 43.Carlson CL, Winder WW. Liver AMP-activated protein kinase and acetyl-CoA carboxylase during and after exercise. J Appl Physiol 1999;86:669–74 [DOI] [PubMed] [Google Scholar]
- 44.Botero D, Ebbeling CB, Blumberg JB, et al. Acute effects of dietary glycemic index on antioxidant capacity in a nutrient-controlled feeding study. Obesity 2009(Epub ahead of print; DOI:10.1038/oby.2009.203) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Dela F, Ploug T, Handberg A, et al. Physical training increases muscle GLUT4 protein and mRNA in patients with NIDDM. Diabetes 1994;43:862–5 [DOI] [PubMed] [Google Scholar]
- 46.Seip RL, Mair K, Cole TG, Semenkovich CF. Induction of human skeletal muscle lipoprotein lipase gene expression by short-term exercise is transient. Am J Physiol 1997;272:E255–61 [DOI] [PubMed] [Google Scholar]
- 47.Duncan GE, Perri MG, Theriaque DW, Hutson AD, Eckel RH, Stacpoole PW. Exercise training, without weight loss, increases insulin sensitivity and postheparin plasma lipase activity in previously sedentary adults. Diabetes Care 2003;26:557–62 [DOI] [PubMed] [Google Scholar]
- 48.Leaf DA. The effect of physical exercise on reverse cholesterol transport. Metabolism 2003;52:950–7 [DOI] [PubMed] [Google Scholar]
- 49.Baynard T, Carhart RL, Jr, Ploutz-Snyder LL, Weinstock RS, Kanaley JA. Short-term training effects on diastolic function in obese persons with the metabolic syndrome. Obesity (Silver Spring) 2008;16:1277–83 [DOI] [PubMed] [Google Scholar]
- 50.Rogers MA, Yamamoto C, Hagberg JM, Martin WH, III, Ehsani AA, Holloszy JO. Effect of 6 d of exercise training on responses to maximal and sub-maximal exercise in middle-aged men. Med Sci Sports Exerc 1988;20:260–4 [DOI] [PubMed] [Google Scholar]
- 51.Pereira MA, Swain J, Goldfine AB, Rifai N, Ludwig DS. Effects of a low-glycemic load diet on resting energy expenditure and heart disease risk factors during weight loss. JAMA 2004;292:2482–90 [DOI] [PubMed] [Google Scholar]
- 52.Modan M, Halkin H, Almog S, et al. Hyperinsulinemia. A link between hypertension obesity and glucose intolerance. J Clin Invest 1985;75:809–17 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Moore RD. Effects of insulin upon ion transport. Biochim Biophys Acta 1983;737:1–49 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.