Abstract
Background: The skeletal protein osteocalcin is γ-carboxylated by vitamin K. High serum uncarboxylated osteocalcin reflects low vitamin K status. In vitro and animal studies indicate that high uncarboxylated osteocalcin is associated with reduced insulin resistance. However, associations between osteocalcin and measures of insulin resistance in humans are less clear.
Objective: Our aim was to examine cross-sectional and longitudinal associations between circulating forms of osteocalcin (total, uncarboxylated, and carboxylated) and insulin resistance in older men and women.
Design: Cross-sectional associations between serum measures of total osteocalcin, carboxylated osteocalcin, and uncarboxylated osteocalcin and insulin resistance were examined in 348 nondiabetic men and women (mean age: 68 y; 58% female) by using the homeostasis model assessment of insulin resistance (HOMA-IR). Associations between each form of osteocalcin at baseline and 3-y change in HOMA-IR were examined in 162 adults (mean age: 69 y; 63% female) who did not receive vitamin K supplementation.
Results: Lower circulating uncarboxylated osteocalcin was not associated with higher HOMA-IR at baseline or at 3-y follow-up. Those in the lowest tertiles of total osteocalcin and carboxylated osteocalcin at baseline had higher baseline HOMA-IR (P = 0.006 and P = 0.02, respectively). The concentration of carboxylated osteocalcin at baseline was inversely associated with a 3-y change in HOMA-IR (P = 0.002).
Conclusions: In older adults, circulating uncarboxylated osteocalcin was not associated with insulin resistance. In contrast, elevated carboxylated osteocalcin and total osteocalcin were associated with lower insulin resistance, which supports a potential link between skeletal physiology and insulin resistance in humans. The role of vitamin K status in this association remains unclear and merits further investigation. This trial is registered at clinicaltrials.gov as NCT00183001.
INTRODUCTION
Osteocalcin, an abundant noncollagenous protein in bone, may function as a hormone in the regulation of energy metabolism (1, 2). Osteocalcin, which is synthesized by osteoblasts during bone formation, undergoes a posttranslational vitamin K–dependent modification in which 3 glutamic acid residues are carboxylated, which thereby allows the protein to bind calcium. The circulating measure of total osteocalcin, which includes both carboxylated and uncarboxylated forms, is used as a biomarker of bone formation, whereas the percentage of osteocalcin that is uncarboxylated is a measure of the vitamin K status of bone. Serum percentage uncarboxylated osteocalcin increases in response to vitamin K depletion and decreases in response to vitamin K supplementation (3).
Osteocalcin has been reported to influence β cell function, insulin sensitivity, adiponectin production, energy expenditure, and adiposity in animal models (1, 2). In humans, serum osteocalcin is inversely associated with measures of insulin resistance and fat mass and positively associated with adiponectin (4–7). It has been suggested that osteocalcin regulates insulin sensitivity through an effect on adiponectin rather than through a direct effect on insulin (2). Animal and in vitro data suggest that only the uncarboxylated form of osteocalcin functions hormonally in the regulation of glucose homeostasis and energy metabolism (1, 2). This would imply that poor vitamin K status reduces insulin resistance through an increase in the portion of osteocalcin that is not γ-carboxylated. However, vitamin K supplementation for 3 y decreased percentage uncarboxylated osteocalcin and protected against the progression of insulin resistance in older men (13), so the association between uncarboxylated osteocalcin and measures of insulin resistance in humans may differ from animal models.
The primary purpose of these analyses was to examine cross-sectional and longitudinal associations between serum uncarboxylated osteocalcin concentrations and measures of insulin resistance in older men and women not receiving vitamin K supplementation, as estimated by the homeostasis model assessment of insulin resistance (HOMA-IR). We hypothesized that the circulating concentration of uncarboxylated osteocalcin is inversely associated with HOMA-IR.
In secondary analyses, we examined the associations between the other forms of osteocalcin (total osteocalcin, carboxylated osteocalcin, and percentage uncarboxylated osteocalcin) and HOMA-IR. We also explored whether the associations between different forms of osteocalcin and HOMA-IR were dependent on adiponectin.
SUBJECTS AND METHODS
Study participants
Cross-sectional associations between serum measures of all forms of osteocalcin and HOMA-IR were examined by using baseline measures from nondiabetic men and women participating in a vitamin K supplementation study. Longitudinal associations were examined in those participants who were randomly assigned to the control group and who completed the 3-y intervention. This study was hypothesis-generating because data were obtained from a clinical trial originally designed to study the effect of vitamin K supplementation on bone loss and vascular calcification (8, 9).
The participation criteria of this study have been described elsewhere (8). Briefly, to ascertain the influence of vitamin K supplementation on age-related bone loss and progression of vascular calcification, 452 ambulatory men and postmenopausal women, aged 60–80 y, were randomly assigned to receive a multivitamin that contained either 500 μg multivitamin/d or no phylloquinone plus a daily calcium (600 mg elemental calcium) and vitamin D3 (400 IU) supplement for 3 y (8). Although diagnosed diabetes (defined as fasting plasma glucose ≥126 mg/dL and/or currently taking diabetes medication) was allowed in the original intervention trial, these individuals were excluded from the present analyses (n = 41). All participants signed a written informed consent. This study was approved by the Tufts University Institutional Review Board. Of enrolled participants, there were 421 whites, 14 blacks, 4 Hispanics, 11 Asians, and 2 American Indians.
For cross-sectional analyses, blood samples drawn at the baseline visit before supplement use were used. For longitudinal analyses, blood samples obtained at the end of the 3-y study were limited to those participants in the control group. Those in the vitamin K–supplemented group were excluded only for the longitudinal analysis because there was a treatment effect of vitamin K supplementation on measures of insulin resistance, as previously reported (13).
Biochemical measurements
All blood samples were fasting and were drawn between 0700 and 1000. Dedicated aliquots of plasma and serum were stored at −80 C and protected from the light until the time of analysis. Serum concentrations of uncarboxylated osteocalcin and total osteocalcin were analyzed by using a radioimmunoassay method (10) with an antibody that recognizes both carboxylated osteocalcin and uncarboxylated osteocalcin. Carboxylated osteocalcin was separated from uncarboxylated osteocalcin by adsorption on hydroxyapatite (10). The total CV for the 3 control sera, with an average total osteocalcin of 6.4, 14.7, and 23.8 μg/L, was 8.8, 8.9, and 7.6, respectively. The carboxylated osteocalcin concentration was calculated as the total osteocalcin concentration minus the uncarboxylated osteocalcin concentration, and the percentage uncarboxylated osteocalcin was calculated as (uncarboxylated osteocalcin concentration/total osteocalcin concentration) × 100. Fasting plasma insulin, glucose, and urinary N-telopeptide of collagen type 1 were measured as described previously (11, 12). Plasma concentrations of high-molecular-weight adiponectin, which is considered to be the most active isoform and more predictive of insulin resistance, were measured by using an enzyme-linked immunosorbent assay (Daiichi Pure Chemical, Tokyo, Japan) (total %CV = 12.0%) (13). Plasma 25-hydroxyvitamin D was measured by radioimmunoassay (DiaSorin, Stillwater, MN), and plasma concentrations of phylloquinone were determined by reversed-phase HPLC followed by fluorometric detection, as previously described (8).
Other measurements
Total body fat was measured by using dual-energy X-ray absorptiometry (GE Medical Prodigy, encore 2002, version 6.10.029) and was expressed as percentage of body weight as fat. Body mass index (in kg/m2) was calculated from baseline measures of height and weight. Medical history, medication use, and smoking status were assessed by medical history and examination at baseline. Dietary intakes and physical activity were assessed by using validated surveys (14, 15).
Statistical analyses
All cross-sectional analyses were limited to participants free of diabetes (n = 411) for whom there were measures of osteocalcin, HOMA-IR, adiponectin (n = 55 missing adiponectin measures), and percentage body fat (n = 8 missing measures of percentage body fat) (final n = 348; 206 females, 142 males). Because vitamin K supplementation affects the carboxylation status of osteocalcin, the longitudinal analyses were limited to nondiabetic participants who were randomly assigned to the group that did not receive vitamin K supplementation and for which measures of percentage body fat were available at baseline and follow-up (n = 162, 63% female).
A natural log transformation was applied to all forms of osteocalcin, HOMA-IR, fasting insulin, and adiponectin to reduce skewness for formal analyses. However, data in Tables 1 and 2 are presented in the original scale. There was no effect modification by sex with respect to the association of any form of osteocalcin with HOMA-IR, so all analyses were sex pooled. Because 94% of the participants used in these analyses were white, we were unable to examine for effect modification by race, so all analyses are race pooled as well.
TABLE 1.
Baseline characteristics of study subjects1
| Values | |
| Age (y) | 68 ± 62 |
| Female [n (%)] | 206 (59) |
| Uncarboxylated osteocalcin (ng/mL) | 3.6 ± 2.3 |
| Total osteocalcin (ng/mL) | 8.5 ± 3.0 |
| Carboxylated osteocalcin (ng/mL) | 4.9 ± 1.6 |
| Uncarboxylated osteocalcin (%) | 41 ± 16 |
| Fasting insulin (mU/mL) | 10.6 ± 5.4 |
| Fasting glucose (mg/dL) | 94 ± 10 |
| HOMA-IR | 2.4 ± 1.4 |
| Adiponectin (μg/mL) | 4.2 ± 2.8 |
| BMI (kg/m2) | 27.8 ± 5.1 |
| Body fat (%) | 37 ± 9 |
| Physical Activity Score for the Elderly | 127 ± 58 |
| NTX (nmol/L BCE) | 15.3 ± 4.4 |
| 25-Hydroxyvitamin D (ng/mL) | 23.0 ± 8.5 |
| Smoking [n (%)] | 17 (5) |
HOMA-IR, homeostasis model assessment of insulin resistance; NTX, N-telopeptide of collagen type 1; BCE, bone collagen equivalents.
Mean ± SD (all such values).
TABLE 2.
Homeostasis model assessment of insulin resistance (HOMA-IR), circulating insulin, glucose, and adiponectin across tertiles of total osteocalcin in older nondiabetic men and women (n = 348)1
| Total osteocalcin |
||||
| Tertile 1 [mean (range): 5.5 (2.6–6.9) ng/mL] | Tertile 2 [mean (range): 8.1 (7.0–9.2) ng/mL] | Tertile 3 [mean (range): 11.8 (9.3–24.8) ng/mL] | P for trend2 | |
| HOMA-IR | ||||
| Model 1 | 2.7 ± 0.1a,b | 2.5 ± 0.1a | 2.2 ± 0.1b | 0.006 |
| Model 2 | 2.6 ± 0.1a | 2.5 ± 0.1a | 2.2 ± 0.1b | 0.04 |
| Insulin (mU/mL) | ||||
| Model 1 | 11.5 ± 0.5a | 10.8 ± 0.5a,b | 9.5 ± 0.5b | 0.11 |
| Model 2 | 11.3 ± 0.5 | 10.9 ± 0.4 | 9.7 ± 0.5 | 0.33 |
| Glucose (mg/dL) | ||||
| Model 1 | 96 ± 1a | 94 ± 1a,b | 93 ± 1b | 0.04 |
| Model 2 | 96 ± 1 | 94 ± 1 | 93 ± 1 | 0.10 |
| Adiponectin (μg/mL) | ||||
| Model 1 | 3.6 ± 0.3a | 4.3 ± 0.2b | 4.6 ± 0.2b | 0.004 |
All values are least-square (LS) means ± SEMs. LS means are the mean values in the outcomes when individual covariates are held constant: model 1 was adjusted for age, sex, race, Physical Activity Score for the Elderly, smoking, serum 25-hydroxyvitamin D, and percentage body fat; model 2 was adjusted for the same covariates as model 1 and further adjusted for adiponectin. n = 113, 119, and 116 for tertiles 1, 2, and 3, respectively. Values with different superscripts are significantly different, P < 0.05 (Tukey’s honestly significant difference adjustment for multiple comparisons).
Calculated by using ANCOVA and adjusted for covariates in model 1 and model 2.
Cross-sectional analyses
HOMA-IR, which was calculated as [fasting plasma glucose (mmol/L) × fasting plasma insulin (U/mL)]/22.5, was the primary measure of insulin resistance (16). We also assessed associations with fasting plasma insulin and fasting plasma glucose separately. For primary analyses, we created tertiles of uncarboxylated osteocalcin and used analysis of covariance (PROC GLM SAS version 9.1; SAS Institute Inc, Cary, NC) to determine cross-sectional differences at baseline in HOMA-IR, fasting insulin, and fasting glucose across tertiles of uncarboxylated osteocalcin, which were adjusted for age, sex, race, physical activity, smoking, percentage body fat, and plasma 25-hydroxyvitamin D. These same analyses were repeated to examine the cross-sectional differences at baseline in HOMA-IR, fasting insulin, and fasting glucose across tertiles of the other forms of osteocalcin (total osteocalcin, carboxylated osteocalcin, and percentage uncarboxylated osteocalcin). We subsequently tested whether the association between measures of all forms of osteocalcin and HOMA-IR was dependent on adiponectin by adjusting these cross-sectional models for serum adiponectin. By using this approach, if the association between osteocalcin and HOMA-IR was completely dependent on adiponectin, there would be no association between osteocalcin and HOMA-IR when controlled for adiponectin (17). If the trend for difference in measures of insulin resistance across a tertile of osteocalcin was significant (P < 0.05), differences among individual tertiles, adjusted for multiple comparisons, were tested by using Tukey’s honestly significant difference adjustment.
To determine whether the different forms of osteocalcin were associated with percentage body fat, we compared serum uncarboxylated osteocalcin, total osteocalcin, carboxylated osteocalcin, and percentage uncarboxylated osteocalcin across tertiles of percentage body fat by using analysis of covariance, which was adjusted for sex, age, ethnicity, physical activity, smoking, energy intake, and percentage of energy from fat. To determine whether associations between osteocalcin and other variables were a reflection of associations between body fat and bone turnover, analyses were repeated by using urinary N-telopeptide of collagen type 1, a marker of bone resorption, as the main exposure. All analyses were considered to be statistically significant at P < 0.05.
Longitudinal analyses
Multiple linear regression was used to determine whether baseline total osteocalcin or the uncarboxylated or carboxylated forms of osteocalcin predicted change in HOMA-IR. The 3-y change in HOMA-IR, fasting insulin, or fasting glucose (calculated as the year 3 minus the baseline measure) was the outcome, and baseline osteocalcin form (total osteocalcin, uncarboxylated osteocalcin, carboxylated osteocalcin, percentage uncarboxylated osteocalcin) and concentration were the primary exposures in separate linear models, which were adjusted for the baseline measure of HOMA-IR, fasting insulin, or fasting glucose as well as age, race, smoking, and physical activity. Because there was a statistically significant increase in percentage body fat over 3 y (P = 0.008), these analyses were adjusted for baseline and 3-y change in percentage body fat. Similarly, because all participants received 400 IU vitamin D3 as part of the primary intervention, these analyses were adjusted for baseline and 3-y change in serum 25-hydroxyvitamin D.
RESULTS
As previously reported, baseline characteristics were similar between the 2 treatment groups (12). Participant characteristics at baseline are summarized in Table 1. The overall mean (±SD) percentage body fat was 42 ± 8% in women and 30 ± 7% in men, and 75% were considered overweight on the basis of a body mass index >25.
Cross-sectional results
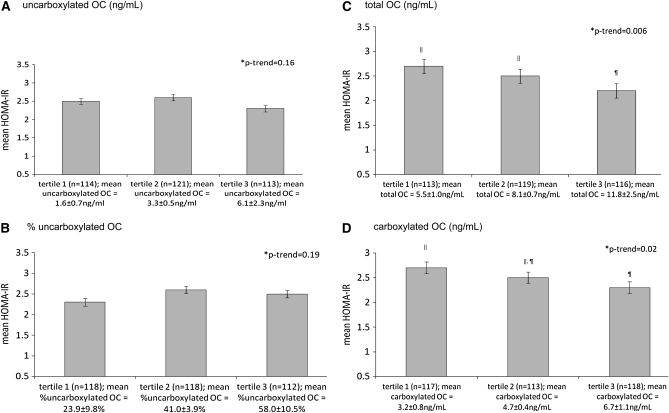
There was no difference in HOMA-IR across tertiles of uncarboxylated osteocalcin (Figure 1A) (P = 0.16). However, HOMA-IR was lower across the higher tertiles of total osteocalcin and carboxylated osteocalcin (Figure 1, C and D) (P for trend = 0.006 and 0.02, respectively). Those in the higher tertiles of total osteocalcin had significantly lower fasting glucose and higher adiponectin (P for trend = 0.04 and 0.004, respectively) (Table 2). Similarly, those in the higher tertiles of carboxylated osteocalcin had lower fasting glucose and higher adiponectin (both P for trend’s = 0.03). Neither the concentration of circulating uncarboxylated osteocalcin nor the percentage uncarboxylated osteocalcin was associated with fasting insulin, glucose, or adiponectin (all P ≥ 0.08).
FIGURE 1.
Mean (±SD) homeostasis model assessment of insulin resistance (HOMA-IR) according to tertiles of (A) uncarboxylated osteocalcin (OC), (B) percentage uncarboxylated OC, (C) total OC, and (D) carboxylated OC in men and women. *P for trend on the basis of ANCOVA and adjusted for age, sex, race, Physical Activity Score for the Elderly, smoking, serum 25-hydroxyvitamin D, and percentage body fat. ‖,¶Tertiles with different superscripts are significantly different, P < 0.05 (Tukey’s honestly significant difference adjustment for multiple comparisons).
When adiponectin was accounted for in the associations between measures of osteocalcin and HOMA-IR, the significant differences in HOMA-IR across higher tertiles of total osteocalcin and carboxylated osteocalcin were attenuated. The association between total osteocalcin and HOMA-IR remained significant (P = 0.04), but the association between carboxylated osteocalcin and HOMA-IR did not (P = 0.11). In these models, adiponectin was significantly inversely associated with HOMA-IR, fasting insulin, and glucose (partial r2 ranged from −0.12 to −0.29; all P < 0.03). There were no differences in HOMA-IR or percentage body fat across tertiles of urinary N-telopeptide of collagen type 1 (all P ≥ 0.12).
Longitudinal results
Among the 162 participants included in the longitudinal analyses, HOMA-IR increased by 0.34 (14%, P = 0.02) over 3 y. The concentration of uncarboxylated osteocalcin and the percentage uncarboxylated osteocalcin did not change significantly. In contrast, the total osteocalcin decreased by 0.88 ng/mL (10%; P < 0.001 on the basis of a paired-sample t test), and carboxylated osteocalcin decreased by 0.67 ng/mL (13%; P < 0.001). The mean percentage body fat increased by 0.5% (P = 0.008). There was no association between baseline uncarboxylated osteocalcin concentration and the 3-y change in HOMA-IR (Table 3). However, a higher concentration of carboxylated osteocalcin at baseline predicted less change in HOMA-IR (P = 0.002), whereas a lower baseline percentage undercarboxylated osteocalcin predicted a greater increase in HOMA-IR (P = 0.02).
TABLE 3.
Associations between baseline measures of circulating osteocalcin and 3-y change in the homeostasis model assessment of insulin resistance (HOMA-IR) in older nondiabetic men and women (n = 162)1
| Baseline (ln) uncarboxylated osteocalcin | Baseline percentage uncarboxylated osteocalcin | Baseline (ln) total osteocalcin | Baseline (ln) carboxylated osteocalcin | |
| 3-y change in HOMA-IR | 0.20 ± 0.19 | 0.01 ± 0.01 | −0.23 ± 0.39 | −0.91 ± 0.29 |
| P value | 0.29 | 0.02 | 0.56 | 0.002 |
| 3-y change in insulin (mU/mL) | 0.70 ± 0.65 | 0.05 ± 0.02 | −0.82 ± 1.33 | −2.99 ± 1.02 |
| P value | 0.28 | 0.03 | 0.54 | 0.004 |
| 3-y change in glucose (mg/dL) | −0.32 ± 1.26 | 0.04 ± 0.05 | −2.70 ± 2.59 | −4.24 ± 1.99 |
| P value | 0.80 | 0.34 | 0.30 | 0.03 |
All values are mean ± SE unstandardized B-coefficients and are calculated on the basis of multiple linear regression, adjusted for sex, age, race, baseline HOMA-IR, insulin or glucose, smoking, physical activity, percentage body fat at baseline, 3-y change in percentage body fat, serum 25-hydroxyvitamin D at baseline, and 3-y change in serum 25-hydroxyvitamin D.
DISCUSSION
In this study, older men and women with high serum carboxylated osteocalcin concentrations at baseline had less increase in HOMA-IR over a 3-y period of follow-up. In contrast, the serum concentration of uncarboxylated osteocalcin was not associated with HOMA-IR in older men and women free of diabetes, nor did it predict a 3-y change in HOMA-IR.
Total osteocalcin serum concentrations reflect the amount of the protein synthesized in bone. The percentage of osteocalcin that is γ-carboxylated (percentage uncarboxylated osteocalcin) depends on the availability of vitamin K. It has been suggested that only the uncarboxylated form of osteocalcin influences glucose homeostasis in mice (1, 2). However, our results suggest that the uncarboxylated fraction of osteocalcin is not associated with insulin resistance in older humans. Rather, serum carboxylated osteocalcin concentrations were inversely associated with HOMA-IR, both cross-sectionally and longitudinally. The divergent findings may reflect species differences in osteocalcin and its putative role in glucose metabolism. Alternatively, differences in findings may reflect the imprecision of HOMA-IR as a surrogate measure of insulin resistance.
Our results are in agreement with others who have recently reported inverse associations between insulin resistance and circulating total osteocalcin in humans (4–7). This study is unique in its measurement of the carboxylated and uncarboxylated forms of osteocalcin in circulation. The mean HOMA-IR was significantly higher among the men and women in the lowest tertiles of total osteocalcin and carboxylated osteocalcin, which suggests that total osteocalcin and carboxylated osteocalcin may have a protective effect on insulin resistance in older age. It is also plausible that osteocalcin is a marker of the general role that osteoblastic activity may have in insulin resistance. Because an HOMA-IR ≥2.6 has been proposed as a threshold to indicate insulin resistance (16, 18), our outcomes suggest that the nondiabetic older adults in the lower tertiles of total osteocalcin and carboxylated osteocalcin may be at increased risk of clinically significant insulin resistance.
Adiponectin, an adipocyte-derived, insulin-sensitizing hormone, was reported to mediate the role of osteocalcin in the regulation of insulin sensitivity in rodent models (1, 2). Reports of associations between adiponectin and osteocalcin specifically in humans are limited (4, 19). In our study, adiponectin was positively associated with total osteocalcin and carboxylated osteocalcin. When adiponectin was held constant in the associations between circulating osteocalcin and HOMA-IR, the strength of the association between total osteocalcin and carboxylated osteocalcin with HOMA-IR was somewhat attenuated, which suggests that the association of total osteocalcin and carboxylated osteocalcin with HOMA-IR may depend partially on adiponectin (17).
The concentration of carboxylated osteocalcin was inversely associated with a 3-y change in HOMA-IR, fasting insulin, and glucose, whereas the percentage uncarboxylated osteocalcin, which is increased when vitamin K status is low, was positively associated with a change in HOMA-IR and fasting insulin. Taken together, these outcomes may suggest a novel role for vitamin K in the protection against age-related impairments in glucose homeostasis, which merits additional investigation. Although the results of our cross-sectional analyses indicated an inverse association between total osteocalcin and fat mass, as has been reported by others (4, 6, 7), the association between baseline carboxylated osteocalcin and percentage uncarboxylated osteocalcin and a change in HOMA-IR was independent of changes in body fat. The baseline concentrations of uncarboxylated osteocalcin and total osteocalcin were not associated with a change in any measure of insulin resistance in our study.
As previously reported, vitamin K supplementation for 3 y protected against an increase in insulin resistance in men in this cohort (13). Unfortunately, we were not able to further explore the longitudinal associations between the 3 forms of osteocalcin and HOMA-IR in the vitamin K-supplemented group because there were sex-specific differences in the response of HOMA-IR to vitamin K supplementation. With 162 participants in the placebo group, the statistical power to appropriately reject the null hypothesis of no association between baseline carboxylated osteocalcin and a 3-y change in HOMA-IR, at the 0.05 level of significance, was 88%. To reject the same null hypothesis separately in the 75 men and 101 women in the vitamin K–supplemented group at the 0.05 level of significance, our statistical power was reduced to 54% and 68% for men and women, respectively.
There are several limitations to consider in the interpretation of these findings. Baseline measures were cross-sectional. Although the outcomes of our longitudinal analyses suggest that elevated carboxylated osteocalcin and a lower percentage uncarboxylated osteocalcin may protect against insulin resistance, these results are based on secondary analyses from a clinical trial designed to test a different hypothesis in a relatively small sample. Thus, additional larger clinical trials are warranted to better elucidate the influence of carboxylated osteocalcin on changes in insulin resistance and development of type 2 diabetes. Use of more sensitive measures of insulin sensitivity such as the hyperinsulinemic euglycemic clamp or minimal model should be considered to further elucidate components of insulin resistance that are influenced by osteocalcin and vitamin K. In addition, our sample is older, primarily white, and generally healthy, so the generalizability of our findings to other age and racial groups or individuals, especially those at increased risk of diabetes, needs to be determined.
Balanced against these limitations is that this report is the first longitudinal assessment of osteocalcin and insulin resistance in humans in which study participants had assessments of carboxylated and uncarboxylated forms of osteocalcin and body composition. Although the influence of uncarboxylated osteocalcin on insulin resistance has been systematically investigated in in vitro and animal studies, reports of associations of uncarboxylated osteocalcin and insulin resistance in humans are lacking. Although others have suggested that uncarboxylated osteocalcin is the form of osteocalcin that confers protection against insulin resistance in animal models (1, 2), our data do not support this hypothesis in humans.
In summary, our data suggest that osteocalcin may function as a link between skeletal metabolism and glucose metabolism in older men and women. The uncarboxylated form of osteocalcin does not appear to influence HOMA-IR in humans. However, more studies are needed to determine whether this function is contingent on the vitamin K–dependent carboxylation status of the protein.
Acknowledgments
The authors’ responsibilities were as follows—SLB: designed the study and contributed to the design of the analyses, the interpretation of the data, and the writing of the manuscript; MKS: performed the statistical analyses and drafted the manuscript; and CMG, JBM, GED, ES, MY, and PFJ: contributed to the design of the analyses, the interpretation of the data, and the writing of the manuscript. JBM currently has research grants from GlaxoSmithKline and Sanofi-Aventis and serves on consultancy boards for Eli Lilly, Interleukin Genetics, Kalypsis, and Outcomes Sciences. MKS, CMG, ES, PFJ, MY, GED, and SLB had no conflicts of interest.
REFERENCES
- 1.Ferron M, Hinoi E, Karsenty G, Ducy P. Osteocalcin differentially regulates beta cell and adipocyte gene expression and affects the development of metabolic diseases in wild-type mice. Proc Natl Acad Sci USA 2008;105:5266–70 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Lee NK, Sowa H, Hinoi E, et al. Endocrine regulation of energy metabolism by the skeleton. Cell 2007;130:456–69 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Booth SL, Lichtenstein AH, O’Brien-Morse M, et al. Effects of a hydrogenated form of vitamin K on bone formation and resorption. Am J Clin Nutr 2001;74:783–90 [DOI] [PubMed] [Google Scholar]
- 4.Fernandez-Real JM, Izquierdo M, Ortega F, Gorostiaga E, Gomez-Ambrosi J, Moreno-Navarrete JM, et al. The relationship of serum osteocalcin concentration to insulin secretion, sensitivity and disposal with hypocaloric diet and resistance training. J Clin Endocrinol Metab 2008;94:237–45 [DOI] [PubMed] [Google Scholar]
- 5.Im JA, Yu BP, Jeon JY, Kim SH. Relationship between osteocalcin and glucose metabolism in postmenopausal women. Clin Chim Acta 2008;396:66–9 [DOI] [PubMed] [Google Scholar]
- 6.Kindblom JM, Ohlsson C, Ljunggren O, Karlsson MK, Tivesten A, Smith U, et al. Plasma osteocalcin is inversely related to fat mass and plasma glucose in elderly Swedish men. J Bone Miner Res 2009;24:785–91 [DOI] [PubMed] [Google Scholar]
- 7.Pittas AG, Harris SS, Eliades M, Stark P, Dawson-Hughes B. Association between serum osteocalcin and markers of metabolic phenotype. J Clin Endocrinol Metab 2009;94:827–32 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Booth SL, Dallal G, Shea MK, Gundberg C, Peterson JW, Dawson-Hughes B. Effect of vitamin K supplementation on bone loss in elderly men and women. J Clin Endocrinol Metab 2008;93:1217–23 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Shea MK, O’Donnell CJ, Hoffmann U, et al. Vitamin K supplementation and progression of coronary artery calcium in older men and women. Am J Clin Nutr 2009;89:1799–807 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Gundberg CM, Nieman SD, Abrams S, Rosen H. Vitamin K status and bone health: an analysis of methods for determination of undercarboxylated osteocalcin. J Clin Endocrinol Metab 1998;83:3258–66 [DOI] [PubMed] [Google Scholar]
- 11.Shea MK, Dallal GE, Dawson-Hughes B, et al. Vitamin K, circulating cytokines, and bone mineral density in older men and women. Am J Clin Nutr 2008;88:356–63 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Yoshida M, Jacques PF, Meigs JB, et al. Effect of vitamin K supplementation on insulin resistance in older men and women. Diabetes Care 2008;31:2092–6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Ebinuma H, Miyazaki O, Yago H, Hara K, Yamauchi T, Kadowaki T. A novel ELISA system for selective measurement of human adiponectin multimers by using proteases. Clin Chim Acta 2006;372:47–53 [DOI] [PubMed] [Google Scholar]
- 14.Washburn RA, Smith KW, Jette AM, Janney CA. The Physical Activity Scale for the Elderly (PASE): development and evaluation. J Clin Epidemiol 1993;46:153–62 [DOI] [PubMed] [Google Scholar]
- 15.Willett WC, Sampson L, Stampfer MJ, et al. Reproducibility and validity of a semiquantitative food frequency questionnaire. Am J Epidemiol 1985;122:51–65 [DOI] [PubMed] [Google Scholar]
- 16.Matthews DR, Hosker JP, Rudenski AS, Naylor BA, Treacher DF, Turner RC. Homeostasis model assessment: insulin resistance and beta-cell function from fasting plasma glucose and insulin concentrations in man. Diabetologia 1985;28:412–9 [DOI] [PubMed] [Google Scholar]
- 17.Baron RM, Kenny DA. The moderator-mediator variable distinction in social psychological research: conceptual, strategic, and statistical considerations. J Pers Soc Psychol 1986;51:1173–82 [DOI] [PubMed] [Google Scholar]
- 18.Hanley AJ, Williams K, Stern MP, Haffner SM. Homeostasis model assessment of insulin resistance in relation to the incidence of cardiovascular disease: the San Antonio Heart Study. Diabetes Care 2002;25:1177–84 [DOI] [PubMed] [Google Scholar]
- 19.Tamura T, Yoneda M, Yamane K, et al. Serum leptin and adiponectin are positively associated with bone mineral density at the distal radius in patients with type 2 diabetes mellitus. Metabolism 2007;56:623–8 [DOI] [PubMed] [Google Scholar]