Abstract
Background: Childhood obesity has increased significantly in recent decades.
Objective: The objective was to examine the perinatal risk factors related to childhood obesity.
Design: In a prospective study, 89 women with normal glucose tolerance (NGT) or gestational diabetes mellitus (GDM) and their offspring were evaluated at birth and at 8.8 ± 1.8 y. At birth, obstetrical data, parental anthropometric measures, and neonatal body composition were assessed; at follow-up, diet and activity were assessed and laboratory studies were conducted. Weight was classified by using weight for age and sex, and body composition was measured by using dual-energy X-ray absorptiometry. In childhood, data were analyzed as tertiles and prediction models were developed by using logistic and stepwise regression.
Results: No significant differences in Centers for Disease Control and Prevention weight percentiles, body composition, and most metabolic measures were observed between children of mothers with NGT and GDM at follow-up. Children in the upper tertile for weight had greater energy intake (P = 0.02), skinfold thickness (P = 0.0001), and leptin concentrations (P < 0.0001) than did those in tertiles 1 and 2. Children in the upper tertile for percentage body fat had greater waist circumference (P = 0.0001), insulin resistance (P = 0.002), and triglyceride (P = 0.009) and leptin (P = 0.0001) concentrations than did children in tertiles 1 and 2. The correlation between body fat at birth and follow-up was r = 0.29 (P = 0.02). The strongest perinatal predictor for a child in the upper tertile for weight was maternal pregravid body mass index (BMI; kg/m2) >30 (odds ratio: 3.75; 95% CI: 1.39, 10.10; P = 0.009) and for percentage body fat was maternal pregravid BMI >30 (odds ratio: 5.45; 95% CI: 1.62, 18.41; P = 0.006).
Conclusion: Maternal pregravid BMI, independent of maternal glucose status or birth weight, was the strongest predictor of childhood obesity.
INTRODUCTION
Obesity is a major health concern, not only in developed countries but also in developing areas (1). In addition to the significant increase in obesity in the general population over the past decade (2), there has been a significant increase in childhood and adolescent obesity (3). On the basis of the work of Barker et al (4) and Gluckman et al (5), the increase in the obesity epidemic may have its origins in utero, ie, the developmental origins of health and disease or perinatal programming. However, in addition to the original reports linking low birth weight or intrauterine growth restriction as primary risk factors for adult metabolic dysfunctions, such as obesity and the metabolic syndrome, more recent studies have implicated early infant catch-up growth and fetal overgrowth as primary risk factors (6). Accordingly, there have been many reports describing an increase in the percentage of large-for-gestational age neonates as well an increase in overall birth weights in the past 20 y (7, 8).
In addition to genetics and gestational length, many parental factors are associated with in utero fetal growth, most of which are maternal. The strongest correlatives associated with fetal growth, in particular fetal adiposity, are maternal pregravid obesity and diabetes (9, 10). These in utero risk factors for increased fetal adiposity are also risk factors for diabetes. Long-term follow-up studies have reported an increased risk of type 2 diabetes in women with diabetes and/or obesity during pregnancy (11, 12). Therefore, the objective of this study was to assess, the perinatal risk factors associated with childhood obesity in a prospective longitudinal cohort. A secondary objective was to analyze the metabolic status of offspring of lean and obese women with normal glucose tolerance (NGT) and gestational diabetes mellitus (GDM).
SUBJECTS AND METHODS
Subjects (maternal)
The protocol was approved by the hospital Institutional Review Board and the General Clinical Research Center at Case Western Reserve University. Written informed consent was obtained from each subject before evaluation. Women with NGT were recruited from the general population of women obtaining prenatal care at our institution, and women with GDM were recruited from our pregnancy diabetes clinic from 1990 through 1999. All women in these clinics were eligible to participate and were recruited before delivery. The obstetrical record was reviewed, and each woman was interviewed by our research staff. Exclusion criteria included the following: infants of multifetal gestations or with congenital anomalies, infants unable to undergo assessment of body composition within 48 h of birth, and preterm infants (ie, <37 wk gestation). Of the 89 infants enrolled in the study, 8 (9%) were small-for-gestational age (SGA), ie, less than the 10th percentile; 68 (76%) were appropriate weight-for-gestational age (AGA), ie, between the 10th and 90th percentiles; and 13 (15%) were large-for-gestational age (LGA), ie, greater than the 90th percentile. Birth weight percentiles were based on our previously published population data with adjustments for gestational age, race, and sex (13).The results of the initial comparison of growth and body composition at birth between the infants of NGT and GDM mothers and between overweight and average-weight mothers were previously published (9, 10).
All subjects underwent a 1-h 50-g glucose screening test at 24–28 wk gestation. A glucose concentration ≥7.5 mmol/L was considered positive. Subjects with a positive screening test underwent a 3-h 100-g oral-glucose-tolerance test. The subjects with NGT had a 1-h glucose screening test of <7.5 mmol/L, or, if positive, a normal 3-h 100-g oral-glucose-tolerance test according to the National Diabetes Data Group criteria (14). All women with GDM were managed according to our previously published criteria (10). The maternal data obtained included classification of maternal glycemic status and a family history of diabetes. Maternal pregravid overweight/obesity was defined as a pregravid body mass index (BMI), defined as weight (in kg)/ height squared (in m), ≥25; and lean/average was defined as a BMI <25. Maternal age, pregravid weight and height, weight gain during pregnancy, parity, a 1-h glucose screen, and tobacco use were obtained by history at the time of delivery and a review of the maternal antenatal record. Maternal height was measured at the first prenatal visit and pregravid weight attained by history. Maternal weight gain was assessed as the weight at delivery minus the pregravid weight. Information on paternal height, weight, and BMI were also obtained by history.
Subjects (neonates/children)
We measured body composition within 48 h of birth using total-body electrical conductivity (TOBEC). This method was previously described in detail (10). At birth, neonatal data included mode of delivery, length as measured on a measuring board, weight on a calibrated scale, gestational age, sex, fat mass, fat-free mass, percentage body fat, and feeding status (bottle fed or breastfed). Follow-up studies in the children were performed by contacting the mothers and having the follow-up studies performed between 6 and 11 y after birth.
Methods
Women and their children were evaluated in the Clinical Research Unit. Each child was evaluated after a 12-h fast and gave assent to the study protocol. Parents provided written consent. A history and physical exam, including the measurement of blood pressure with a calibrated childhood cuff, was performed to ensure that the child was in good health, had no medical problems, and was not taking medications that could interfere with metabolic function. The parents were previously contacted by the research nutritionist to obtain a 3-d dietary log. The calories, components, and percentage of nutrients in the diet were analyzed by using nutrition software, (Nutritionist 5, version 2.2; First DataBank Inc, San Bruno, CA). Physical activity was quantified by using a validated activity questionnaire (15, 16). Each child was then weighed on a calibrated scale (Mettler Toledo, Worthington, OH) and had height measured with a stadiometer. The 2000 Centers for Disease Control and Prevention (CDC) criteria for weight and BMI for children based on age and sex were used to assign percentiles (17). Anthropometric measurements included triceps, subscapular, midaxillary, flank, thigh, and calf skinfold-thickness measured with a calibrated Harpenden caliper (British Indicators, Sussex, United Kingdom). We calculated a central to peripheral skinfold ratio (subscapular + midaxillary + abdominal + flank)/(triceps + thigh + lower calf) to estimate central compared with peripheral subcutaneous fat distribution. The waist and thigh circumferences, and their ratios, were also measured to assess peripheral and central obesity. Each child was asked to undergo a measure of body composition by dual-energy X-ray absorptiometry (DXA) (series QDR 4500w; Discovery, Bedford, MA). Sixty-three children underwent DXA evaluation: 38 were children of NGT mothers, and 25 were children of GDM mothers.
Each child was asked to have a fasting blood sample collected for the measurement of glucose, insulin, C-peptide, free fatty acids, cholesterol, triglycerides, HDL, LDL, and the cytokines tumor necrosis factor-α (TNF-α) and leptin. Insulin resistance was estimated by using the homeostasis model assessment of insulin resistance (HOMA-IR), ie, fasting plasma glucose × fasting plasma insulin/22.5 (18). Forty-nine children had fasting blood samples collected: 26 were children of NGT mothers, and 23 were children of GDM mothers.
Laboratory analysis
The laboratory studies were performed in the Clinical Research Unit laboratory. Glucose was measured by using the YSI glucose oxidase method (Yellow Springs, OH), and insulin was measured by using a Linco human insulin-specific radioimmunoassay (St Charles, MO). Free fatty acids were measured by using an enzymatic colorimetric method (Wako Chemicals, Richmond, VA). TNF-α was measured with an enzyme-linked immunosorbent assay (R&D Systems, Minneapolis, MN). Plasma leptin was measured by using a human radioimmunoassay (LINCO, St Charles, MO). Cholesterol, triglyceride, HDL, and LDL concentrations were measured in the hospital laboratory by using an enzymatic colorimetric method (Beckman Coulter, Fullerton, CA).
Statistical analysis
Statistical analyses were performed by using either a 2-sample Student's t test or a Mann-Whitney U test to compare the means or medians of the 2 groups depending on the normalcy of the distribution. Comparisons between noncontinuous data were made by using a chi-square test. An analysis of variance for parametric data and a Kruskal-Wallis test for nonparametric data were used to assess the difference between the various tertiles and Fisher's protected least-significant difference was used to assess post hoc analyses between tertiles. Logistic regression and multiple regression analyses were used to develop models predicting the outcome of interest. In the multivariate and stepwise logistic regression analyses, independent variables were entered into the model if the P value was <0.10 using simple regression. For example, the data were entered as 0 if the mother had NGT and as 1 if the mother had GDM; the remaining variables were continuous. We include maternal obstetrical data, paternal anthropometric data, and neonatal birth data to best determine which combination of perinatal factors best modeled the risk of adiposity in the child. Data are expressed as means ± SDs, and a P value <0.05 was considered significant. BMI z scores for the children at follow-up were calculated as follows: BMI of the study subject − mean BMI of the population for age and sex/SD of the population BMI for age and sex. Statistical analyses were performed by using Statistix version 8.0 (Tallahassee, FL) or SAS/StatView (version 5.0.1; Statistical Analysis System, Cary, NC)
The data are presented as tables in the text. Tables 1, 2, and 3 include the information regarding comparisons between the NGT and GDM groups. Tables 4, 5, and 6 include the CDC child weight percentiles as the dependent variable organized as tertiles. Tables 7, 8, and 9 include DXA-estimated percentage body fat as the dependent variables organized as tertiles.
TABLE 1.
Demographic characteristics of subjects with normal glucose tolerance (NGT) and gestational diabetes mellitus (GDM) and their neonates at birth
| NGT (n = 52) | GDM (n = 37) | P value1 | |
| Maternal | |||
| Age (y) | 31.8 ± 3.82 | 28.9 ± 5.9 | 0.008 |
| Height (cm) | 167.4 ± 6.5 | 163.0 ± 7.5 | 0.004 |
| Pregravid weight (kg) | 65.1 ± 13.0 | 80.4 ± 23.9 | 0.0002 |
| BMI (kg/m2) | 23.2 ± 4.4 | 30.2 ± 8.7 | 0.0001 |
| Weight gain (kg) | 14.2 ± 5.7 | 13.1 ± 7.7 | 0.42 |
| Parity (n) | 0.19 | ||
| 0–1 | 31 | 27 | |
| >1 | 21 | 10 | |
| Glucose at screening (mmol/L) | 6.1 ± 1.3 | 8.6 ± 1.2 | 0.0001 |
| Ethnicity (n) | 0.04 | ||
| White | 51 | 30 | |
| African American | 0 | 3 | |
| Hispanic | 1 | 3 | |
| Asian | 0 | 1 | |
| Tobacco use (yes/no) | 4/48 | 8/29 | 0.06 |
| Family history of diabetes (yes/no) | 17/35 | 21/16 | 0.02 |
| Education (n) | 0.0001 | ||
| 0–12 y | 6 | 18 | |
| 13–16 y | 26 | 18 | |
| >16 y | 20 | 1 | |
| Paternal | |||
| Height (cm) | 180.5 ± 7.3 | 177.2 ± 8.3 | 0.06 |
| Weight (kg) | 87.8 ± 13.4 | 86.7 ± 21.4 | 0.78 |
| BMI (kg/m2) | 27.0 ± 4.4 | 27.4 ± 5.3 | 0.71 |
| Neonatal | |||
| Gestational age (wk) | 39.4 ± 1.2 | 38.7 ± 1.3 | 0.01 |
| Male/female sex (n) | 22/30 | 15/22 | 0.87 |
| Weight (kg) | 3.376 ± 0.496 | 3.373 ± 0.532 | 0.97 |
| Length (cm) | 50.5 ± 1.9 | 49.7 ± 2.2 | 0.06 |
| Body composition | |||
| Fat mass (kg) | 0.378 ± 0.189 | 0.438 ± 0.208 | 0.16 |
| Fat-free mass (kg) | 2.998 ± 0.354 | 2.934 ± 0.393 | 0.42 |
| Body fat (%) | 10.8 ± 4.2 | 12.6 ± 4.6 | 0.06 |
Data were analyzed by using Student's t, Mann-Whitney U, and chi-square tests.
Mean ± SD (all such values).
TABLE 2.
Centers for Disease Control and Prevention (CDC) weight percentiles and anthropometric and metabolic measures in children of women with normal glucose tolerance (NGT) and gestational diabetes mellitus (GDM) at follow-up1
| NGT (n = 52) | GDM (n = 37) | P value2 | |
| CDC weight percentile | 60.9 ± 26.43 | 64.1 ± 32.9 | 0.61 |
| Age (y) | 9.0 ± 1.7 | 8.6 ± 1.8 | 0.35 |
| Male/female sex (n) | 22/30 | 15/22 | 0.87 |
| Race (n) | 0.04 | ||
| White | 51 | 30 | |
| African American | 0 | 3 | |
| Hispanic | 1 | 3 | |
| Asian | 0 | 1 | |
| Weight (kg) | 32.0 ± 8.7 | 33.5 ± 12.2 | 0.50 |
| Height (cm) | 134.6 ± 12.2 | 130.8 ± 13.6 | 0.17 |
| BMI z score (%) | 57.2 ± 33.0 | 68.6 ± 30.6 | 0.10 |
| BMI z score | 0.31 ± 1.16 | 0.90 ± 1.40 | 0.03 |
| Activity (h)4 | 291 ± 196 | 266 ± 286 | 0.74 |
| Inactivity (h)5 | 556 ± 208 | 696 ± 419 | 0.15 |
| Total energy (kcal) | 1880 ± 521 | 1830 ± 648 | 0.70 |
| Protein intake (g) | 63 ± 20 | 62 ± 23 | 0.74 |
| Carbohydrate intake (g) | 247 ± 62 | 250 ± 81 | 0.86 |
| Fat intake (g) | 74 ± 29 | 68 ± 31 | 0.38 |
| Waist circumference (mm) | 60.2 ± 8.7 | 62.1 ± 10.5 | 0.36 |
| Thigh circumference (mm) | 42.2 ± 7.2 | 43.9 ± 8.6 | 0.31 |
| Waist:thigh ratio | 1.45 ± 0.26 | 1.43 ± 0.12 | 0.59 |
| Sum of 7 skinfold thicknesses (mm) | 73.8 ± 33.9 | 85.1 ± 41.8 | 0.17 |
| Skinfold thickness ratio | 0.828 ± 0.264 | 0.938 ± 0.363 | 0.13 |
| Systolic BP (mm Hg) | 108 ± 12 | 110 ± 11 | 0.64 |
| Diastolic BP (mm Hg) | 60 ± 8 | 58 ± 7 | 0.23 |
| Fasting glucose (mmol/L)6 | 4.8 ± 0.2 | 4.9 ± 0.3 | 0.77 |
| Fasting insulin (pmol/L)7 | 52 ± 25 | 69 ± 42 | 0.09 |
| HOMA-IR7 | 1.89 ± 0.93 | 2.55 ± 1.58 | 0.08 |
| Fasting FFA (g/L)8 | 0.202 ± 0.045 | 0.235 ± 0.082 | 0.12 |
| Total cholesterol (mmol/L)8 | 4.17 ± 0.62 | 3.86 ± 0.49 | 0.08 |
| Triglyceride (mmol/L)8 | 0.80 ± 0.35 | 0.84 ± 0.69 | 0.88 |
| HDL cholesterol (mmol/L)8 | 1.22 ± 0.26 | 1.22 ± 0.26 | 0.93 |
| LDL cholesterol (mmol/L)9 | 2.69 ± 0.49 | 2.38 ± 0.54 | 0.06 |
| Leptin (ng/mL)6 | 6.4 ± 5.4 | 8.6 ± 7.6 | 0.25 |
| TNF-α (pg/mL)6 | 6.7 ± 7.2 | 7.5 ± 7.0 | 0.70 |
BP, blood pressure; TNF-α, tumor necrosis factor-α; HOMA-IR, homeostasis model assessment of insulin resistance; FFA, free fatty acid.
Data were analyzed by using Student's t, Mann-Whitney U, and chi-square tests.
Mean ± SD (all such values).
For combined NGT and GDM groups: 4n = 45, 5n = 44, 6n = 49, 7n = 47, 8n = 40, 9n = 39.
TABLE 3.
Percentage body fat measured by using dual-energy X-ray absorptiometry and anthropometric and metabolic measures in children of women with normal glucose tolerance (NGT) and gestational diabetes mellitus (GDM) at follow-up1
| NGT (n = 38) | GDM (n = 25) | P value2 | |
| Body fat (%) | 27.8 ± 8.13 | 31.0 ± 9.3 | 0.14 |
| Age (y) | 9.6 ± 1.5 | 9.3 ± 1.8 | 0.50 |
| Male/female sex (n) | 16/22 | 10/15 | 0.87 |
| Race (n) | 0.14 | ||
| White | 37 | 21 | |
| African American | 0 | 1 | |
| Hispanic | 1 | 3 | |
| Asian | 0 | 0 | |
| Height (cm) | 138.9 ± 10.8 | 135.9 ± 13.1 | 0.33 |
| BMI z score | 0.25 ± 0.10 | 0.98 ± 1.22 | 0.02 |
| Fat mass (kg) | 10.0 ± 5.0 | 12.3 ± 6.6 | 0.12 |
| Lean body mass (kg) | 24.5 ± 4.9 | 25.0 ± 6.7 | 0.72 |
| Activity (h)4 | 291 ± 196 | 266 ± 286 | 0.74 |
| Inactivity (h)5 | 556 ± 208 | 696 ± 418 | 0.15 |
| Total energy (kcal) | 1936 ± 515 | 1904 ± 704 | 0.84 |
| Protein intake (g) | 66 ± 21 | 63 ± 24 | 0.60 |
| Carbohydrate intake (g) | 252 ± 57 | 260 ± 84 | 0.66 |
| Fat intake (g) | 76 ± 29 | 70 ± 35 | 0.51 |
| Waist circumference (mm) | 61.6 ± 9.0 | 65.3 ± 10.2 | 0.14 |
| Thigh circumference (mm) | 44.0 ± 7.1 | 47.0 ± 8.0 | 0.12 |
| Waist:thigh ratio | 1.43 ± 0.30 | 1.40 ± 0.12 | 0.65 |
| Sum of 7 skinfold thicknesses (mm) | 77.8 ± 32.3 | 93.2 ± 43.7 | 0.13 |
| Skinfold thickness ratio | 0.843 ± 0.289 | 1.009 ± 0.397 | 0.07 |
| Systolic BP (mm Hg) | 108 ± 9 | 112 ± 11 | 0.13 |
| Diastolic BP (mm Hg) | 60 ± 8 | 59 ± 7 | 0.50 |
| Fasting glucose (mmol/L)6 | 4.8 ± 0.2 | 4.9 ± 0.3 | 0.33 |
| Fasting insulin (pmol/L)7 | 50 ± 24 | 78 ± 43 | 0.02 |
| HOMA-IR7 | 1.81 ± 0.86 | 2.86 ± 1.64 | 0.02 |
| Fasting FFA (g/L)8 | 0.202 ± 0.045 | 0.235 ± 0.085 | 0.13 |
| Total cholesterol (mmol/L)6 | 4.17 ± 0.62 | 3.86 ± 0.52 | 0.09 |
| Triglyceride (mmol/L)6 | 0.80 ± 0.35 | 0.86 ± 0.70 | 0.76 |
| HDL cholesterol (mmol/L)6 | 1.21 ± 0.25 | 1.20 ± 0.26 | 0.88 |
| LDL cholesterol (mmol/L)9 | 2.69 ± 0.49 | 2.38 ± 0.57 | 0.08 |
| Leptin (ng/mL)6 | 6.7 ± 5.8 | 9.8 ± 8.1 | 0.16 |
| TNF-α (pg/mL)6 | 6.9 ± 8.0 | 7.9 ± 7.9 | 0.72 |
BP, blood pressure; TNF-α, tumor necrosis factor-α; HOMA-IR, homeostasis model assessment of insulin resistance; FFA, free fatty acid.
Data were analyzed by using Student's t, Mann-Whitney U, and chi-square tests.
Mean ± SD (all such values).
For combined NGT and GDM groups: 4n = 45, 5n = 44, 6n = 39, 7n = 37, 8n = 9, 9n = 39.
TABLE 4.
Maternal and paternal demographic data from the time of birth in relation to tertiles of Centers for Disease Control and Prevention (CDC) weight percentiles at follow-up1
| Tertile 1 (n = 30) | Tertile 2 (n = 29) | Tertile 3 (n = 30) | P value2 | |
| CDC weight percentile | 29.0 ± 17.73 | 65.5 ± 12.3 | 92.4 ± 4.9 | 0.000145 |
| Maternal | ||||
| Age at delivery (y) | 30.1 ± 5.5 | 31.5 ± 4.0 | 30.2 ± 5.2 | 0.50 |
| Height (cm) | 163.9 ± 6.6 | 166.1 ± 7.0 | 166.8 ± 8.0 | 0.27 |
| Pregravid weight (kg) | 64.2 ± 15.5 | 67.7 ± 13.6 | 82.3 ± 24.0 | 0.000545 |
| Pregravid BMI (kg/m2) | 23.9 ± 5.5 | 24.7 ± 5.6 | 29.8 ± 9.1 | 0.00345 |
| Weight gain (kg) | 13.0 ± 5.1 | 16.4 ± 6.4 | 12.0 ± 7.4 | 0.0256 |
| Parity (n) | 0.48 | |||
| 0–1 | 17 | 20 | 21 | |
| >1 | 13 | 9 | 9 | |
| Glucose (mmol/L) | 7.2 ± 1.9 | 7.0 ± 1.7 | 6.7 ± 1.6 | 0.67 |
| Group (n) | 0.32 | |||
| NGT | 17 | 20 | 15 | |
| GDM | 13 | 9 | 15 | |
| Race (n) | 0.41 | |||
| White | 28 | 26 | 27 | |
| African American | 0 | 2 | 1 | |
| Hispanic | 2 | 0 | 2 | |
| Asian | 0 | 1 | 0 | |
| Tobacco use (yes/no) | 6/24 | 3/26 | 3/27 | 0.44 |
| Family history of diabetes (yes/no) | 8/22 | 14/15 | 16/14 | 0.09 |
| Education (n) | 0.20 | |||
| 0–12 y | 8 | 6 | 10 | |
| 13–16 y | 18 | 12 | 14 | |
| >16 y | 4 | 11 | 6 | |
| Type of feeding (n) | 0.97 | |||
| Bottle | 9 | 8 | 9 | |
| Breast | 21 | 21 | 21 | |
| Paternal | ||||
| Height (cm) | 178.0 ± 7.6 | 179.9 ± 8.6 | 179.7 ± 7.5 | 0.59 |
| Weight (kg) | 82.5 ± 10.3 | 86.0 ± 14.4 | 93.8 ± 22.8 | 0.044 |
| BMI (kg/m2) | 26.1 ± 3.4 | 26.6 ± 4.5 | 28.8 ± 5.8 | 0.074 |
NGT, normal glucose tolerance; GDM, gestational diabetes mellitus.
ANOVA, Kruskal-Wallis test, and chi-square test.
Mean ± SD (all such values).
Significant difference between tertiles 1 and 3, P < 0.05 (Fisher's protected least-significant difference).
Significant difference between tertiles 2 and 3, P < 0.05 (Fisher's protected least-significant difference).
Significant difference between tertiles 1 and 2, P < 0.05 (Fisher's protected least-significant difference).
TABLE 5.
Neonatal morphometric measures at birth in relation to tertiles of Centers for Disease Control and Prevention (CDC) weight percentiles at follow-up
| Tertile 1 (n = 30) | Tertile 2 (n = 29) | Tertile 3 (n = 30) | P value1 | |
| CDC weight percentile | 29.03 ± 17.72 | 65.5 ± 12.3 | 92.4 ± 4.9 | 0.00013–5 |
| Gestational age at delivery (wk) | 39.0 ± 1.0 | 39.4 ± 1.3 | 38.9 ± 1.4 | 0.28 |
| Male/female sex (n) | 11/19 | 10/19 | 16/14 | 0.27 |
| Birth weight (kg) | 3.197 ± 0.457 | 3.518 ± 0.462 | 3.414 ± 0.559 | 0.045 |
| Birth length (cm) | 49.6 ± 1.9 | 50.4 ± 1.9 | 50.5 ± 2.2 | 0.16 |
| Fat mass (kg) | 0.334 ± 0.201 | 0.461 ± 0.180 | 0.416 ± 0.198 | 0.045 |
| Fat-free mass (kg) | 2.863 ± 0.313 | 3.057 ± 0.359 | 2.998 ± 0.415 | 0.12 |
| Body fat (%) | 10.0 ± 4.9 | 12.8 ± 4.0 | 11.8 ± 4.0 | 0.045 |
ANOVA, Kruskal-Wallis test, and chi-square test.
Mean ± SD (all such values).
Significant difference between tertiles 1 and 3, P < 0.05 (Fisher's protected least-significant difference).
Significant difference between tertiles 2 and 3, P < 0.05 (Fisher's protected least-significant difference).
Significant difference between tertiles 1 and 2, P < 0.05 (Fisher's protected least-significant difference).
TABLE 6.
Anthropometric and metabolic measures in children in relation to Centers for Disease Control and Prevention (CDC) weight percentiles at follow-up1
| Tertile 1 (n = 30) | Tertile 2 (n = 29) | Tertile 3 (n = 30) | P value2 | |
| CDC weight percentile | 29.0 ± 17.7 | 65.5 ± 12.3 | 92.4 ± 4.9 | 0.00013–5 |
| Age (y) | 8.6 ± 1.8 | 8.9 ± 1.9 | 9.0 ± 1.6 | 0.59 |
| Height (cm) | 126.5 ± 13.0 | 134.5 ± 12.1 | 138.1 ± 11.1 | 0.00135 |
| Weight (kg) | 24.94 ± 5.73 | 31.28 ± 7.59 | 41.61 ± 9.30 | 0.00013–5 |
| BMI z score | −0.45 ± 0.60 | 0.14 ± 0.76 | 1.97 ± 0.93 | 0.00013–5 |
| Activity (h)6 | 293 ± 280 | 305 ± 179 | 246 ± 221 | 0.79 |
| Inactivity (h)7 | 655 ± 232 | 440 ± 225 | 690 ± 413 | 0.104 |
| Total energy (kcal) | 1787 ± 456 | 1692 ± 422 | 2101 ± 738 | 0.0234 |
| Protein intake (g) | 55 ± 18 | 64 ± 19 | 70 ± 24 | 0.023 |
| Carbohydrate intake (g) | 248 ± 59 | 224 ± 52 | 271 ± 89 | 0.054 |
| Fat intake (g) | 66 ± 25 | 63 ± 22 | 84 ± 37 | 0.0234 |
| Waist circumference (cm) | 53.3 ± 3.7 | 59.1 ± 7.4 | 70.6 ± 6.9 | 0.00013–5 |
| Thigh circumference (cm) | 38.4 ± 5.4 | 41.9 ± 6.6 | 48.4 ± 8.0 | 0.000134 |
| Waist:thigh ratio | 1.40 ± 0.15 | 1.42 ± 0.13 | 1.50 ± 0.32 | 0.22 |
| Sum of 7 skinfold thicknesses (mm) | 53.8 ± 14.7 | 74.4 ± 31.4 | 113.7 ± 31.2 | 0.00013–5 |
| Skinfold thickness ratio | 0.704 ± 0.106 | 0.837 ± 0.225 | 1.123 ± 0.404 | 0.000134 |
| Systolic BP (mm Hg) | 104 ± 7 | 111 ± 13 | 112 ± 12 | 0.00635 |
| Diastolic BP (mm Hg) | 56 ± 6 | 60 ± 9 | 61 ± 8 | 0.063 |
| Fasting glucose (mmol/L)8 | 4.8 ± 0.3 | 4.9 ± 0.2 | 4.9 ± 0.2 | 0.26 |
| Fasting insulin (pmol/L)9 | 39 ± 13 | 60 ± 32 | 81 ± 38 | 0.00043 |
| HOMA-IR9 | 1.39 ± 0.53 | 2.19 ± 1.25 | 2.96 ± 1.43 | 0.00053 |
| Fasting FFA (g/L)10 | 0.234 ± 0.080 | 0.220 ± 0.064 | 0.202 ± 0.053 | 0.41 |
| Cholesterol (mmol/L)10 | 4.12 ± 0.62 | 4.07 ± 0.72 | 3.91 ± 0.44 | 0.57 |
| Triglyceride (mmol/L)10 | 0.64 ± 0.29 | 0.77 ± 0.41 | 1.02 ± 0.71 | 0.15 |
| HDL (mmol/L)10 | 1.37 ± 0.19 | 1.20 ± 0.26 | 1.06 ± 0.24 | 0.0023 |
| LDL (mmol/L)11 | 2.54 ± 0.54 | 2.67 ± 0.62 | 2.49 ± 0.49 | 0.71 |
| Leptin (ng/mL)8 | 2.9 ± 1.1 | 6.0 ± 4.6 | 12.7 ± 7.1 | 0.000134 |
| TNF-α (pg/mL)8 | 8.4 ± 6.1 | 5.8 ± 6.8 | 6.6 ± 8.2 | 0.57 |
All values are means ± SDs. BP, blood pressure; TNF-α, tumor necrosis factor-α; HOMA-IR, homeostasis model assessment of insulin resistance; FFA, free fatty acid.
ANOVA, Kruskal-Wallis test, and chi-square test.
Significant difference between tertiles 1 and 3, P < 0.05 (Fisher's protected least-significant difference).
Significant difference between tertiles 2 and 3, P < 0.05 (Fisher's protected least-significant difference).
Significant difference between tertiles 1 and 2, P < 0.05 (Fisher's protected least-significant difference).
For combined tertiles 1–3: 6n = 45, 7n = 44, 8n = 49, 9n = 47, 10n = 40, 11n = 39.
TABLE 7.
Maternal and paternal demographic data from the time of birth in relation to tertiles of percentage body fat at follow-up1
| Tertile 1 (n = 21) | Tertile 2 (n = 21) | Tertile 3 (n = 21) | P value2 | |
| Child body fat by DXA (%) | 19.7 ± 2.63 | 28.2 ± 2.6 | 39.3 ± 4.3 | 0.00014–6 |
| CDC weight percentile | 39.8 ± 27.5 | 66.0 ± 19.1 | 88.0 ± 11.4 | 0.00014–6 |
| Maternal | ||||
| Age at delivery (y) | 30.7 ± 3.8 | 29.8 ± 5.2 | 31.6 ± 4.6 | 0.46 |
| Height (cm) | 166.6 ± 6.3 | 166.2 ± 7.0 | 165.7 ± 8.8 | 0.93 |
| Pregravid weight (kg) | 64.8 ± 15.0 | 66.2 ± 13.0 | 84.4 ± 26.2 | 0.00245 |
| Pregravid BMI (kg/m2) | 23.5 ± 6.1 | 23.9 ± 4.0 | 30.8 ± 9.3 | 0.00145 |
| Weight gain (kg) | 14.2 ± 6.9 | 14.3 ± 5.7 | 11.6 ± 7.6 | 0.35 |
| Parity (n) | 0.62 | |||
| 0–1 | 12 | 13 | 15 | |
| >1 | 9 | 8 | 6 | |
| Glucose screen (mmol/L) | 6.6 ± 2.1 | 6.8 ± 1.4 | 7.3 ± 1.4 | 0.48 |
| Group (n) | 0.28 | |||
| NGT | 13 | 15 | 10 | |
| GDM | 8 | 6 | 11 | |
| Race (n) | 0.62 | |||
| White | 18 | 20 | 20 | |
| African American | 1 | 0 | 0 | |
| Hispanic | 2 | 1 | 1 | |
| Asian | 0 | 0 | ||
| Tobacco use (yes/no) | 2/19 | 2/19 | 1/20 | 0.80 |
| Family history of diabetes (yes/no) | 6/15 | 7/14 | 14/7 | 0.02 |
| Education (n) | 0.64 | |||
| 0–12 y | 5 | 2 | 5 | |
| 13–16 y | 11 | 11 | 11 | |
| >16 y | 5 | 8 | 5 | |
| Type of feeding (n) | 0.99 | |||
| Bottle | 5 | 5 | 5 | |
| Breast | 16 | 16 | 16 | |
| Paternal | ||||
| Height (cm) | 180.2 ± 7.5 | 180.3 ± 7.9 | 179.4 ± 7.4 | 0.93 |
| Weight (kg) | 86.0 ± 10.0 | 85.4 ± 13.8 | 92.1 ± 24.1 | 0.39 |
| BMI (kg/m2) | 26.5 ± 2.8 | 26.3 ± 4.1 | 28.4 ± 5.8 | 0.27 |
CDC, Centers for Disease Control and Prevention; DXA, dual-energy X-ray absorptiometry; NGT, normal glucose tolerance; GDM, gestational diabetes mellitus.
ANOVA, Kruskal-Wallis test, and chi-square test.
Mean ± SD (all such values).
Significant difference between tertiles 1 and 3, P < 0.05 (Fisher's protected least-significant difference).
Significant difference between tertiles 2 and 3, P < 0.05 (Fisher's protected least-significant difference).
Significant difference between tertiles 1 and 2, P < 0.05 (Fisher's protected least-significant difference).
TABLE 8.
Neonatal morphometric measures at birth in relation to tertiles of percentage body fat at follow-up1
| Tertile 1 (n = 21) | Tertile 2 (n = 21) | Tertile 3 (n = 21) | P value2 | |
| Child body fat by DXA (%) | 19.7 ± 2.63 | 28.2 ± 2.6 | 39.3 ± 4.3 | 0.00014–6 |
| CDC weight percentile | 39.8 ± 27.5 | 66.0 ± 19.1 | 88.0 ± 11.4 | 0.00014–6 |
| Gestational age at delivery (wk) | 39.3 ± 1.1 | 39.4 ± 1.2 | 38.6 ± 1.5 | 0.105 |
| Male/female sex (n) | 11/10 | 8/13 | 7/14 | 0.43 |
| Birth weight (kg) | 3.279 ± 0.450 | 3.540 ± 0.617 | 3.402 ± 0.548 | 0.30 |
| Birth length (cm) | 50.6 ± 2.2 | 50.3 ± 2.1 | 50.3 ± 2.3 | 0.87 |
| Fat mass (kg) | 0.322 ± 0.161 | 0.444 ± 0.233 | 0.430 ± 0.201 | 0.11 |
| Fat-free mass (kg) | 2.957 ± 0.341 | 3.096 ± 0.445 | 2.973 ± 0.405 | 0.47 |
| Body fat (%) | 9.5 ± 4.0 | 12.0 ± 5.1 | 12.2 ± 4.2 | 0.094 |
CDC, Centers for Disease Control and Prevention; DXA, dual-energy X-ray absorptiometry.
ANOVA, Kruskal-Wallis test, and chi-square test.
Mean ± SD (all such values).
Significant difference between tertiles 1 and 3, P < 0.05 (Fisher's protected least-significant difference).
Significant difference between tertiles 2 and 3, P < 0.05 (Fisher's protected least-significant difference).
Significant difference between tertiles 1 and 2, P < 0.05 (Fisher's protected least-significant difference).
TABLE 9.
Anthropometric and metabolic measures in children in relation to percentage body fat at follow-up1
| Tertile 1 (n = 21) | Tertile 2 (n = 21) | Tertile 3 (n = 21) | P value2 | |
| Child body fat by DXA (%) | 19.7 ± 2.6 | 28.2 ± 2.6 | 39.3 ± 4.3 | 0.00013–5 |
| BMI z score | −0.49 ± 9.57 | 0.14 ± 0.82 | 1.74 ± 0.77 | 0.00013–5 |
| Age (y) | 8.8 ± 1.8 | 10.2 ± 1.3 | 9.5 ± 1.6 | 0.015 |
| Height (cm) | 131.7 ± 13.0 | 142.3 ± 9.3 | 139.1 ± 10.7 | 0.0135 |
| CDC weight percentile | 39.8 ± 27.5 | 66.0 ± 19.1 | 88.0 ± 11.4 | 0.00013–5 |
| Activity (h) | 338 ± 295 | 244 ± 172 | 254 ± 225 | 0.48 |
| Inactivity (h) | 612 ± 250 | 606 ± 229 | 624 ± 465 | 0.99 |
| Total energy (kcal) | 1874 ± 661 | 1938 ± 571 | 1961 ± 564 | 0.90 |
| Protein intake (g) | 59 ± 25 | 67 ± 24 | 68 ± 16 | 0.38 |
| Carbohydrate intake (g) | 263 ± 68 | 253 ± 70 | 250 ± 70 | 0.82 |
| Fat intake (g) | 68 ± 35 | 75 ± 28 | 79 ± 32 | 0.53 |
| Waist circumference (cm) | 55.3 ± 5.0 | 62.0 ± 6.8 | 72.0 ± 8.0 | 0.00013–5 |
| Thigh circumference (cm) | 39.4 ± 4.8 | 46.5 ± 4.5 | 49.7 ± 8.7 | 0.000135 |
| Waist:thigh ratio | 1.41 ± 0.12 | 1.34 ± 0.12 | 1.50 ± 0.37 | 0.094 |
| Sum of 7 skinfold thicknesses (mm) | 50.1 ± 9.8 | 83.2 ± 23.1 | 124.7 ± 30.1 | 0.00013–5 |
| Skinfold thickness ratio | 0.696 ± 0.156 | 0.871 ± 0.265 | 1.198 ± 0.381 | 0.00013–5 |
| Systolic BP (mm Hg) | 105 ± 8 | 109 ± 5 | 114 ± 13 | 0.013 |
| Diastolic BP (mm Hg) | 59 ± 8 | 58 ± 8 | 61 ± 8 | 0.50 |
| Fasting glucose (mmol/L)6 | 4.8 ± 0.3 | 4.9 ± 0.2 | 4.9 ± 0.1 | 0.32 |
| Fasting insulin (pmol/L)7 | 43 ± 13 | 60 ± 30 | 94 ± 46 | 0.00134 |
| HOMA-IR7 | 1.55 ± 0.56 | 2.22 ± 1.15 | 3.42 ± 1.72 | 0.00234 |
| Fasting FFA (g/L)6 | 0.247 ± 0.083 | 0.199 ± 0.059 | 0.204 ± 0.042 | 0.13 |
| Total cholesterol (mmol/L)6 | 4.07 ± 0.78 | 4.01 ± 0.54 | 3.96 ± 0.39 | 0.90 |
| Triglyceride (mmol/L)6 | 0.63 ± 0.29 | 0.72 ± 0.32 | 1.23 ± 0.77 | 0.00934 |
| HDL cholesterol (mmol/L)6 | 1.28 ± 0.28 | 1.20 ± 0.24 | 1.10 ± 0.22 | 0.26 |
| LDL cholesterol (mmol/L)8 | 2.64 ± 0.65 | 2.56 ± 0.49 | 2.44 ± 0.47 | 0.64 |
| Leptin (ng/mL)6 | 2.5 ± 0.6 | 7.6 ± 4.7 | 15.9 ± 7.0 | 0.00013–5 |
| TNF-α (pg/mL)6 | 8.8 ± 6.9 | 5.6 ± 6.9 | 7.8 ± 10.2 | 0.55 |
All values are means ± SDs. CDC, Centers for Disease Control and Prevention; DXA, dual-energy X-ray absorptiometry; BP, blood pressure; TNF-α, tumor necrosis factor-α; HOMA-IR, homeostasis model assessment of insulin resistance; FFA, free fatty acid.
ANOVA, Kruskal-Wallis test, and chi-square test.
Significant difference between tertiles 1 and 3, P < 0.05 (Fisher's protected least-significant difference).
Significant difference between tertiles 2 and 3, P < 0.05 (Fisher's protected least-significant difference).
Significant difference between tertiles 1 and 2, P < 0.05 (Fisher's protected least-significant difference).
For combined tertiles 1–3: 6n = 39, 7n = 37, 8n = 38.
RESULTS
Eighty-nine children participated in the follow-up study: 37 boys and 52 girls. Thirty-seven were children of GDM mothers, and 52 were children of NGT mothers. The mean age at follow-up was 8.8 ± 1.8 y (range: 6.1–11.9 y). Parental demographic characteristics and neonatal body measurements in the NGT and GDM study populations are shown in Table 1.
At follow-up we compared the children of the NGT and GDM women to determine whether there were any differences in CDC weight percentile (Table 2), body composition (Table 3), morphometric measures, dietary data, activity levels, and laboratory results. The children of the GDM women had significantly greater BMI z scores (P = 0.03, Table 2; P = 0.02, Table 3) than did the children of the NGT women. No other significant differences in any of the measured variables were observed between the 2 groups, except for significantly greater fasting insulin and HOMA-IR values in the children of GDM mothers than in the children of NGT mothers when compared in relation to percentage body fat at the time of follow-up (Table 3). There was a trend for cholesterol (P = 0.09) and LDL cholesterol (P = 0.08) to be higher in the children of NGT mothers than in the children of GDM mothers.
We next analyzed the data to determine whether there were any differences in maternal or paternal factors at the time of birth (Table 4) or in neonatal factors (Table 5) after each child had a CDC weight-for-age and sex percentile assigned. The data from all the children were rank ordered and are presented as tertiles.
Parental data at birth are shown in Table 4. Compared with mothers of children in tertiles 1 or 2, mothers of children in the upper tertile had significantly greater pregravid weight and BMI but less weight gain. Paternal weight was significantly greater in fathers of children in tertile 3 than in fathers of children in tertile 1. Neonatal morphometric data are shown in Table 5. Compared with infants in tertile 1, infants in tertile 2 had significantly greater birth weight, DXA-estimated percentage body fat, and fat mass.
Anthropometric and metabolic measures of the young children in relation to the CDC weight percentiles at the time of follow-up are shown in Table 6. Children in tertile 1 weighed less and were shorter than those in tertiles 2 or 3. The children in tertile 3 had significantly greater calorie, protein, and fat intakes than did those in tertiles 1 and 2. Skinfold thicknesses and the central to peripheral skinfold thickness ratio in children in tertile 3 were approximately double those of children in tertile 1. HOMA-IR estimates were significantly different between the groups (P = 0.0005), ie, insulin resistance was more than 2-fold greater in children in tertile 3 than in children in tertile 1. The results shown in Table 6 were similar when adiposity was expressed on the basis of CDC BMI percentiles for age and sex. The mean percentiles for CDC BMI, adjusted for age and sex, were 23%, 69%, and 94% for tertiles 1, 2, and 3, respectively. (See supplementary Tables 1–3 under “Supplementary data” in the online issue.)
We next analyzed the data to determine whether there were any differences in maternal or paternal factors at the time of birth (Table 7) or in neonatal factors (Table 8) after each child had percentage body fat estimated by DXA. The data for all of the children were again rank ordered and presented as tertiles.
Sixty-three children completed the DXA evaluation: 38 children of NGT mothers and 25 children of GDM mothers. Mean percentage body fat values were 20%, 28%, and 39% in tertiles 1, 2, and 3, respectively. Parental data at birth are shown in Table 7. Mothers of children in the upper tertile had significantly greater pregravid BMI than did mothers of children in tertiles 1 or 2. Paternal BMI was not significantly different between the 3 tertiles. Neonatal morphometric data are shown in Table 8. Children in tertile 3 had significantly greater percentage body fat at birth than did those in tertile 1.
Anthropometric and metabolic measures in relation to DXA-estimated percentage body fat at the time of follow-up are shown in Table 9. There were no significant differences in caloric intake or activity or inactivity hours between the groups. Waist circumference and skinfold thickness ratio were greater in children in tertile 3, consistent with increased central obesity. The skinfold thicknesses of children in tertile 3 were 2.5 times those of children in tertile 1. Systolic blood pressure was significantly greater in tertile 3 than in tertiles 1 and 2. HOMA-IR and triglyceride concentrations were 2-fold greater in children in tertile 3 than in children in tertile 1. Circulating leptin was markedly higher in children in tertile 3.
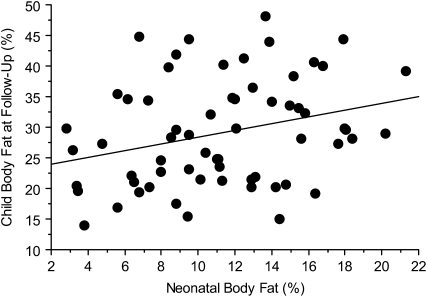
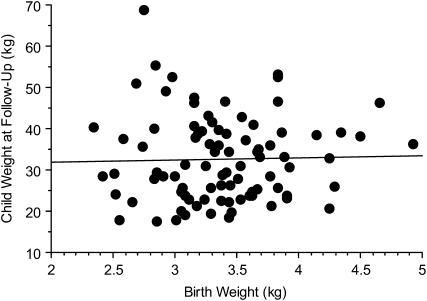
There was a positive correlation between percentage body fat at birth and at follow-up (r = 0.29, P = 0.02; Figure 1), but no significant correlation between birth weight and weight at follow-up (r = 0.03, P = 0.79; Figure 2). We next performed a multivariate logistic regression analysis to determine the predictors of a child being in the upper tertile of the percentile weight for age and sex. The variables that were entered into the model included those with a P value < 0.10 in Tables 4 and 5, ie, maternal pregravid weight, BMI, weight gain during pregnancy, family history of diabetes, and paternal weight and BMI. The neonatal variables included were birth weight, fat mass, and percentage body fat. Maternal pregravid BMI was consistently identified as the best predictor of obesity in the offspring as children. The odds of having a child in the upper tertile for the CDC percentile weight for a mother with a pregravid BMI > 30 was 3.8 times that of a mother with a pregravid BMI < 30 [odds ratio (OR): 3.75; 95% CI: 1.39, 10.10; P = 0.009). This model improved when adjusted for sex and group, ie, NGT or GDM (OR: 4.03; 95% CI: 1.23, 13.22; P = 0.02).
FIGURE 1.
Correlation between percentage body fat in neonates at birth and percentage body fat in children at follow-up. r = 0.29, P = 0.02 (n = 63).
FIGURE 2.
Correlation between birth weight and weight at follow-up in the children. r = 0.03, P = 0.79 (n = 89).
A multivariate logistic regression analysis was used to determine the best predictors of a child's DXA-estimated adiposity at follow-up. The variables entered into the model included those with a P value < 0.10, as noted in Tables 7 and 8. Included in the model were maternal pregravid weight and BMI, family history of diabetes, gestational age at delivery, and percentage body fat at birth. The only variables of significance in the model were maternal pregravid BMI (r = −0.40155, P = 0.001) and family history of diabetes (r = 0.23102, P = 0.04). Mothers with a pregravid BMI of ≥30 were 5.4 times as likely than a mother with a BMI < 30 to have children in the upper tertile for percentage body fat at follow-up (OR: 5.45; 95% CI: 1.62, 18.41; P = 0.006). This relation improved after sex (OR: 6.36; 95% CI: 1.77, 22.88; P = 0.004) or sex and group (OR: 7.75; 95% CI: 1.51, 37.74; P = 0.01) were adjusted for. The strength of this relation did not change when measures of neonatal adiposity were included in the analysis.
The variables entered into the multivariate logistic regression models were also entered into the stepwise regression analyses models. The predictors for a child being in the upper tertile of percentage weight for age and sex were maternal pregravid BMI (P = 0.003) followed by paternal weight (P = 0.02). These 2 factors explained 11.4% of the variance (R2 = 0.114) in the CDC weight percentiles of the children at the time of follow-up. Similarly, for identifying offspring in the upper tertile for percentage body fat, maternal pregravid BMI again was the strongest and only perinatal predictor (P = 0.002). Maternal pregravid BMI accounted for 17.6% of the variance (R2 = 0.176) in the DXA estimates of percentage body fat in the children at the time of follow-up.
DISCUSSION
The purpose of this study was to determine which perinatal factors had the strongest correlation with childhood adiposity in a cohort of children whose mothers had NGT or GDM during their pregnancy. The results strongly support the contention that maternal pregravid obesity is a significant risk factor for childhood obesity and subsequent metabolic dysregulation. On the basis of our results, children in the upper tertile not only had a greater percentage body fat, but also had evidence of central obesity on the basis of anthropometric measurements. Children in the upper tertiles also had evidence of metabolic dysfunction, including significantly higher systolic blood pressure, greater insulin resistance, higher fasting serum triglycerides, and lower HDL concentrations compared with the children in the lower tertiles. These results are consistent with the findings of Boney et al (19), who reported that, in an 11-y follow-up study, children of women with NGT but a pregravid BMI > 27.3 were at risk of developing the metabolic syndrome, including ≥2 of the following: obesity, hypertension, dyslipidemia, and glucose intolerance. The risk of the metabolic syndrome was greater (29%) in infants born large for gestational age to NGT women than in infants of GDM women born of average weight for gestational age (21%). These results are consistent with our findings that maternal pregravid obesity is a risk factor for obesity and metabolic dysregulation in the offspring.
In a review of the literature, Oken and Gillman (20) reported that most studies showed a positive correlation between birth weight and childhood obesity. Many of these reports included epidemiologic studies with large numbers of subjects. These studies, however, are limited because of incomplete data regarding gestational age, parental body size, use of tobacco, and socioeconomic factors (20). For each 1-kg increase in birth weight, there was an increase in BMI ranging from 0.5 to 0.7 (21, 22). Although in our study there was no correlation between birth weight and weight of the children at follow-up (Figure 2), there was a significant correlation between percentage body fat at birth and at follow-up (Figure 1). These data suggest that estimates of body composition rather birth weight may be the important risk factor. Only with a larger number of study subjects in diverse populations will we able to better test this hypothesis.
One of the most important results from this study was the finding that maternal pregravid obesity, as estimated by BMI, was the strongest perinatal predictor of childhood obesity in contrast with either maternal glucose homeostasis or weight gain during pregnancy. Long-term follow-up studies of offspring of diabetic Pima Indian women by Dabelea et al (23) reported that fetal exposure to maternal diabetes in utero increases the risk of both obesity and type 2 diabetes 5–20 y later. Furthermore, Hillier et al (24) recently reported in a large multiethnic population that there was a positive trend for increasing childhood obesity at age 5–7 y across the range of glucose screening values during pregnancy. Increasing fasting maternal glucose was particularly associated with an increased risk of childhood obesity, as estimated by CDC weight criteria. These relations remained significant after maternal age, parity, pregnancy weight gain, ethnicity, macrosomia at birth, and infant sex were adjusted for. However, in neither of these studies was maternal pregravid obesity evaluated as a risk factor for childhood obesity.
Similar to what was observed in infants of women with GDM, we previously showed that maternal pregravid obesity alone is a significant risk factor for fetal obesity (25). Neonates of obese women with NGT have a higher weight at birth than do neonates of average-weight women, because of a higher amount of fat, not fat-free mass (9). Our data are consistent with those of a study of 8400 children born to obese women. The offspring of obese women had a 2.5-fold increased risk of BMI >95th percentile at 2–4 y of age compared with children of normal-weight mothers (BMI ≥18.5 and <25) in early pregnancy (11). Thus, on the basis of our results, maternal pregravid obesity rather than dysregulation of glucose homeostasis (GDM) appears to be a stronger risk factor for the development of childhood obesity.
How then does maternal pregravid obesity affect fetal and childhood growth? Recently, evidence has accumulated that obesity is an inflammatory condition that increases the risk of insulin resistance (26, 27). There is also evidence that pregnancy is an inflammatory condition (28). The decreases in insulin sensitivity are strongly correlated with the changes in circulating cytokines (29), many of which are produced by the placenta (30). Because of increased inflammation and an associated decrease in insulin sensitivity, pregnant obese women, even those with normal glucose tolerance, are more insulin resistant than are lean or average-weight women (25). These alterations in insulin sensitivity in obese pregnant women result in a greater availability of nutrients, such as glucose and lipids, which are preferential energy sources and substrates for feto-placental growth (31).
The strength of this study was that it was conducted in a longitudinal cohort of a diverse group of women (NGT and GDM) and their offspring studied prospectively over 18 y. The estimates of adiposity were made by using methods specifically designed to estimate neonatal and childhood body composition. The same investigators were involved in the study from the inception of the study design through the obstetrical and neonatal measures and follow-up during childhood. Our study also had some limitations. The number of study subjects was relatively small. Additionally, not all of the children allowed us to obtain DXA measurements or to perform fasting laboratory studies. Although maternal pregravid obesity was the strongest predictor of childhood obesity, it explained only 18% of the variance in childhood adiposity. Genetic, epigenetic, and the postpregnancy environment are significant factors that we have not addressed but that play an obvious role. Last, because there was limited equipment available to estimate body composition in neonates and in children, we estimated body composition in neonates using TOBEC and in children using DXA. Although there was a strong correlation between TOBEC and DXA measures in neonates (r = 0.87), DXA is known to overestimate fat mass and underestimate FFM (32, 33). We were unable to perform cross-calibration estimates secondary to differences in the DXA software for neonates and children.
These data provide strong evidence that obesity may be programmed in utero through the adverse metabolic status of obese mothers. From a public health perspective, because ≈60% of reproductive age women are overweight or obese (3), the issue of maternal obesity during pregnancy needs to be strongly considered relative to both the short- and long-term morbidity in the mother and her offspring. If prevention of obesity is the goal, as opposed to treatment, then addressing maternal pregravid obesity assumes importance not only for the women but for their offspring as well.
Supplementary Material
Acknowledgments
We thank the staff of the Clinical Research Unit NCRR CTSA at MetroHealth Medical Center for their support of this project.
The authors' responsibilities were as follows—PMC: had full access to all the data in the study and takes responsibility for the integrity of the data and the accuracy of the analyses; PMC and SHd-M: study concept and design, drafting of the manuscript, funding, and critical revision of the manuscript for important intellectual content; KF, AT, LH-P, and PM: acquisition of data; and SBA, PMC, and LH-P: statistical analysis. None of the authors declared any financial conflict of interest.
REFERENCES
- 1.World Health Organization Obesity: preventing and managing a global epidemic. World Health Organ Tech Rep Ser 2000;899;1–4 [PubMed] [Google Scholar]
- 2.Flegal KM, Carroll MD, Ogden CL, Johnson CL. Prevalence and trends in obesity among U.S. adults, 1999-2000. JAMA 2002;288:1723–7 [DOI] [PubMed] [Google Scholar]
- 3.Ogden CLC, Carroll MD, Curtin LR, McDowell MA, Tabac CJ, Flegal KM. Prevalence of overweight and obesity in the United States, 1999-2004. JAMA 2006;295:1549–55 [DOI] [PubMed] [Google Scholar]
- 4.Barker DJP, Osmond C, Simmonds SJ, Wield GA. The relation of small head circumference and thinness at birth to death from cardiovascular disease in adult life. BMJ 1993;306:422–6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Gluckman PD, Hanson MA, Pina LC. The developmental origins of adult disease. Matern Child Nutr 2005;1:130–41 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Fewtrell MS, Morley R, Abbott RA, et al. Catch-up growth in small-for-gestational-age infants: a randomized trial. Am J Clin Nutr 2001;74:516–23 [DOI] [PubMed] [Google Scholar]
- 7.Ananth CV, Wen SW. Trends in fetal growth among singleton gestations in the United States and Canada, 1985 through 1998. Semin Perinatol 2002;26:260–7 [DOI] [PubMed] [Google Scholar]
- 8.Catalano PM. Management of obesity in pregnancy. Obstet Gynecol 2007;109:419–33 [DOI] [PubMed] [Google Scholar]
- 9.Sewell MF, Huston-Presley L, Super DM, Catalano PM. Increased neonatal fat mass, and not lean body mass is associated with maternal obesity. Am J Obstet Gynecol 2006;195:1100–3 [DOI] [PubMed] [Google Scholar]
- 10.Catalano PM, Thomas A, Huston-Presley L, Amini SB. Increased fetal adiposity: a very sensitive marker of abnormal in utero development. Am J Obstet Gynecol 2003;189:1698–704 [DOI] [PubMed] [Google Scholar]
- 11.Whitaker RC. Predicting preschooler obesity at birth: the role of maternal obesity in early pregnancy. Pediatrics 2004;114:e29–36 [DOI] [PubMed] [Google Scholar]
- 12.Dabelea D, Hanson RL, Lindsay RS, et al. Intrauterine exposure to obesity conveys risks for type 2 diabetes and obesity: a study of discordant sibships. Diabetes 2000;49:2208–11 [DOI] [PubMed] [Google Scholar]
- 13.Amini SB, Catalano PM, Hirsch V, Mann LI. Analysis of birthweight by gestational age: using a computerized perinatal database 1975-1992. Obstet Gynecol 1994;83:342–52 [PubMed] [Google Scholar]
- 14.National Diabetes Data Group Classification and diagnosis of diabetes mellitus and other categories of glucose intolerance. Diabetes 1979;28:1039–57 [DOI] [PubMed] [Google Scholar]
- 15.Robinson TN. Reducing children's television viewing to prevent obesity. A randomized trial. JAMA 1999;282:1561–7 [DOI] [PubMed] [Google Scholar]
- 16.Ainsworth BE, Haskell WL, Leon AS, et al. Compendium of physical activities: classification of energy costs of human physical activities. Med Sci Sports Exerc 1993;25:71–80 [DOI] [PubMed] [Google Scholar]
- 17.Kuczmarski RJ, Ogden CL, Guo SS, et al. 2000 CDC growth charts for the United States: methods and development. Vital Health Stat 11 2002;246:1–190 [PubMed] [Google Scholar]
- 18.Matthews DR, Hosker JP, Rudenski AS, Naylor BA, Treacher DF, Turner RC. Homeostasis model assessment: insulin resistance and β-cell function from fasting plasma glucose and insulin concentrations in man. Diabetologia 1985;28:412–9 [DOI] [PubMed] [Google Scholar]
- 19.Boney CM, Verma A, Tucker R, Vohr BR. Metabolic syndrome in childhood: association with birth weight, maternal obesity and gestational diabetes mellitus. Pediatrics 2005;115:e290–6 [DOI] [PubMed] [Google Scholar]
- 20.Oken E, Gillman MW. Fetal origins of obesity. Obes Res 2003;11:496–506 [DOI] [PubMed] [Google Scholar]
- 21.Sorensen HT, Sabroe S, Rothman KJ, Gillman M, Fischer P, Sorensen TI. Relation between weight and length at birth and body mass index in young adulthood: cohort study. BMJ 1997;315:1137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Loos RJ, Beunen G, Fagard R, Derom C, Vlietinck R. Birth weight and body composition in young adult men—a prospective twin study. Int J Obes Relat Metab Disord 2001;25:1537–45 [DOI] [PubMed] [Google Scholar]
- 23.Dabelea D, Hanson RL, Bennett PH, Roumain J, Knowler WC, Pettitt DJ. Increasing prevalence of type II diabetes in American Indian children. Diabetologia 1998;41:904–10 [DOI] [PubMed] [Google Scholar]
- 24.Hillier TA, Pedaler KL, Schmidt MM, Muller JA, Charles MA, Pettitt DJ. Childhood obesity and metabolic imprinting the ongoing effects of maternal hyperglycemia. Diabetes Care 2007;30:2287–92 [DOI] [PubMed] [Google Scholar]
- 25.Catalano PM, Ehrenberg HM. The short- and long-term implications of maternal obesity on the mother and her offspring. BJOG 2006;113:1126–33 [DOI] [PubMed] [Google Scholar]
- 26.Xu H, Barnes GT, Yans Q, et al. Chronic inflammation in fat plays a crucial role in the development of obesity-related insulin resistance. J Clin Invest 2003;112:1821–30 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Shoelson SE, Herrero L, Naaz A. Obesity, inflammation, and insulin resistance. Gastroenterology 2007;132:2169–80 [DOI] [PubMed] [Google Scholar]
- 28.Ramsay JE, Ferrell WR, Crawford L, Wallace AM, Greer IA, Sattar N. Maternal obesity is associated with dysregulation of metabolic, vascular and inflammatory pathways. J Clin Endocrinol Metab 2002;87:4231–7 [DOI] [PubMed] [Google Scholar]
- 29.Catalano PM, Hoegh M, Minium J, et al. Adiponectin in human pregnancy: implications for regulation of glucose and lipid metabolism. Diabetologia 2006;49:1677–85 [DOI] [PubMed] [Google Scholar]
- 30.Radaelli T, Varastehpour A, Catalano PM, Hauguel-de Mouzon S. Gestational diabetes induces placental genes for chronic stress and inflammatory pathways. Diabetes 2003;52:2951–8 [DOI] [PubMed] [Google Scholar]
- 31.Radaelli T, Lepercq J, Varastehpour A, Basu S, Catalano PM, Hauguel-de Mouzon S. Differential regulation of genes for fetoplacental lipid pathways in pregnancy with gestational and type 1 diabetes mellitus. Am J Obstet Gynecol 2009;201:209.e1–10 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Groh-Wargo S, Moore JJ, Thomas A, Catalano P, O'Connor DL, Lerner E. Dexa vs. TOBEC measurement of body composition in preterm infants. Pediatr Res 2002;51:381A.(abstr) [Google Scholar]
- 33.Little KD, Schubeck D. Comparison of body composition assessment methods in infants. Med Sci Exerc 2001;33:S244.(abstr) [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.