Abstract
Background: Better early childhood nutrition improves schooling, adult health, skills, and wages, but there is little evidence regarding its effect on the next generation.
Objective: We assessed whether nutritional supplementation in children aged <7 to 15 y affected their children's nutritional status 29–38 y later.
Design: We studied 791 children 0–12 y who were offspring of 401 Guatemalan women who had participated as children in a nutritional supplementation trial in which 2 villages were randomly assigned to receive a nutritious supplement (atole) and 2 were assigned to receive a less-nutritious supplement (fresco). We compared anthropometric indicators between the offspring of mothers exposed to atole and the offspring of mothers exposed to fresco.
Results: Compared with the offspring of women exposed to fresco, the offspring of women exposed to atole had a 116-g (95% CI: 17, 215 g) higher birth weight, were 1.3-cm (0.4, 2.2 cm) taller, had a 0.6-cm (0.4, 0.9 cm) greater head circumference, had a 0.26 (0.09, 0.43) greater height-for-age z score, and had a 0.20 (0.02, 0.39) greater weight-for-age z score. The association for height differed by offspring sex. Sons of women exposed to atole were 2.0-cm (95% CI: 1.0, 3.1 cm) taller than the sons of women exposed to fresco. Supplementation was not associated with 6 other offspring anthropometric indicators that reflect measures of adiposity. Supplementation in boys did not affect their children's anthropometric measures.
Conclusion: Nutritional supplementation in girls is associated with substantial increases in their offsprings' (more for sons) birth weight, height, head circumference, height-for-age z score, and weight-for-age z score.
INTRODUCTION
Over 200 million children aged <5 y in developing countries are not reaching their developmental potential (1, 2). Small newborn size and childhood stunting predict short stature, reduced lean body mass, less schooling, diminished intellectual functioning, and reduced wage rates in adulthood (3–6). These same factors may also affect the next generation through multiple pathways, including parental phenotype (particularly the intrauterine environment), pelvic size, health and education of the mothers, and possibly epigenetic channels (7–10).
Little high-quality evidence of nongenetic intergenerational determinants of body size exists. Studies associating birth weights across generations, for example, generally do not control for intergenerationally correlated genetic endowments or family background (11). Likewise, the positive intergenerational association in schooling is attenuated following appropriate control for genetic, family, and community background factors (12–14). Thus, the effect of improvements in child nutrition on next-generation growth and development are not well understood.
We used quasi-experimental data from Guatemala to investigate the effect of early-life nutrition of women on 11 anthropometric indicators of their offspring under 12 y of age: birth weight, height, weight, BMI, head and arm circumferences, triceps and subscapular skinfold thicknesses, and height-for-age, weight-for-age, and BMI-for-age z scores.
SUBJECTS AND METHODS
Study participants and procedures
Between 1969 and 1977, the Institute of Nutrition of Central America and Panama (INCAP) undertook a study of the effect of improved energy and protein intakes on the physical and mental development of children from 4 villages of mixed Spanish-Amerindian ethnic origin in El Progreso, Guatemala (15–17). Two villages, one from each pair matched on population size, were randomly assigned to receive nutritional supplements called atole or fresco. Atole is a gruel-like drink made from Incaparina (a vegetable protein mixture), dry skim milk, and sugar; it provided 6.4 g protein and 380 kJ (91 kcal) energy/100 mL. Fresco contained no protein, and provided 138 kJ (33 kcal)/100 mL from sugar. From October 1971, both supplements were fortified with micronutrients in equal concentrations by volume. The supplements were available to all villagers twice daily throughout the study at central locations in each village, but records of attendance and consumption were kept only for children younger than 7 y. INCAP also established and maintained medical services for each village. For children younger than 7 y, participation (defined as any attendance) was between 65% and 85% and varied little by village or age (18). For children younger than 3 y, daily attendance and the daily average volume of supplement consumed were higher in villages assigned to atole than in those assigned to fresco (18), which resulted in protein, energy, and micronutrient intakes from the supplements being higher in atole villages (19). For children 4–7 y of age, the average volume of fresco ingested was greater than the average volume of atole ingested, with the result that the energy gap from supplementation was much less than for children under 3 y of age, but still favored children in the atole villages, and the micronutrient gap from supplementation was reversed (18). The salient difference in intakes for children 4–7 y of age was in protein, favoring children in the atole villages (18).
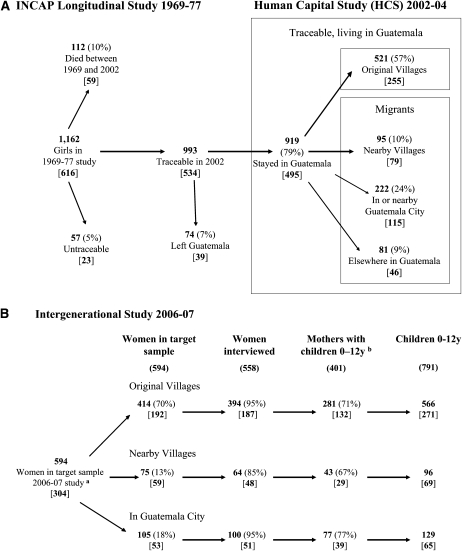
We have been following this cohort of children prospectively (17). Between January 2006 and October 2007 we collected information on the original sample members, their parents, spouses, and children (20). The sampling frame for this survey was developed based on the sample of 1090 living individuals from the 1969–1977 study (hereafter referred to as original sample members, 46% of original sample and 54% of those alive in 2007) who 1) had been interviewed in our previous survey in 2002–2004 (21), 2) were living in or near one of the original study villages or in Guatemala City or its suburbs, and 3) had a biological parent living in the above locations. Of these original sample members, 1009 (92.6%) were interviewed, of whom 824 (436 women, 388 men) reported that they had 1400 living children younger than 12 y of age. We attempted to obtain data on all spouses or partners, all children younger than 12 y of age living in the same household as original sample members, and children of original sample members who lived with a former spouse or partner. We successfully interviewed 558 (93.9%) eligible original sample women, 436 of whom had children aged 0–12 y. The proportion lost to follow-up was similar between mothers from the atole and the fresco villages. The distributions for height-for-age z scores when the mothers were 6-y-old did not differ between those included and those not included (Kolmogorov-Smirnov test for equality of distributions: P = 0.38 overall, 0.96 for those exposed to atole, and 0.24 for those exposed to fresco). For our core analysis we included 791 biological children of 401 mothers who had been exposed to supplementation for whom data on all 10 child anthropometric indicators (excluding weight-for-age z scores for which the standards are available only through 10 y of age) were available. The analysis included 405 children of 200 mothers exposed to atole and 386 children of 201 mothers exposed to fresco. gives The relation between the original cohort of girls and the mothers and children in this study is provided in Figure 1.
FIGURE 1.
A, B: Tracking of the Intergenerational Study 2006–2007 sample (women only). Numbers in brackets reflect number of women from atole villages. INCAP, Institute of Nutrition of Central America and Panama. aOf 992 living original women in 2006–2007, 285 lived outside the study area, 113 were ineligible because they did not complete the HCS 2002–04 and/or did not have an eligible parent, 589 were eligible because they completed the HCS 2002–04 and had an eligible parent, and 5 were ineligible as original members but sampled because the spouse was an eligible sample member. bRefers to number of mothers of children aged 0–12 y with full information.
All adult participants provided informed consent, and parents provided informed consent for their children. The study was approved by the Institutional Review Boards of the International Food Policy Research Institute and Emory University and Latin Ethics, an Institutional Review Board located in Guatemala City.
Characterization of maternal supplement exposure and type
The intervention started in March 1969 in the more populous villages and in May 1969 in the less-populous villages. Supplementation ended in February 1977. We classified the children as born to mothers exposed to atole or fresco. The original study enrolled all children under the age of 7 y at study launch in 1969 and newborns from birth until the study ended in 1977. We defined “exposure to atole” as exposure to this supplement at any age up to 15 y, but including some exposure when <7 y. All of the original sample members in the atole villages were exposed (n = 200 women). We defined “exposure to fresco,” our reference group, in a parallel manner (n = 201 women).
Child anthropometric outcomes
Birth weight was obtained by interviewing the mother. To validate these data, we compared, for 244 children, birth weights obtained from the interview and from an earlier prospective study of birth weights in the 4 study villages (22). The 2 reports of birth weight for the same children had a Pearson correlation coefficient of 0.67. Height was measured to the nearest 0.1 cm, with the subject barefoot and standing with their back to a stadiometer (GPM, Zurich, Switzerland), for children older than 36 mo of age. Length was measured to the nearest 0.1 cm with a wood stadiometer for children younger than 36 mo. Weight (kg) was measured with a digital scale (model 1582; Tanita, Tokyo, Japan) with a precision of 100 g. Head and arm circumferences (cm) were measured to the nearest 0.1 cm with a plastic inextensible measuring tape. Triceps and subscapular skinfold thicknesses (mm) were measured with a Holtain skinfold caliper (Croswell, Wales, United Kingdom). All measures were obtained in duplicate, and the means were used for analysis.
Paternal characteristics
Completed grade of schooling was self-reported. Height was measured to the nearest 0.1 cm with the subjects barefoot and standing with their back to a stadiometer (GPM).
Statistical analysis
We computed means and SDs for each of the key variables, including raw data and z scores (which condition on age and sex), for each of the 2 exposure groups and used t tests to test the equality of the means. We computed intrafamily correlations for internal z scores (which condition on age and sex).
We used linear regression to estimate the relation between offspring anthropometric measures and maternal exposure to the atole supplement using offspring of women exposed to the fresco supplement as the reference. We controlled for offspring sex, child's date of birth (for birth weight estimates), a second-order polynomial in child age (for measures other than birth weight), and a linear term in mother's date of birth to capture cohort and period effects. In our basic specification, we did not control for birth weight, maternal height, maternal schooling, or paternal height because these variables might be pathways through which the nutritional supplements may have affected the child outcomes. We calculated SEs that allow for clustering at the mother level to account for intrafamily correlations and heteroscedasticity of unknown form (23). We report parameter estimates and 95% CIs. Significance was set at P < 0.05. We used Stata version 10.1 (24) for data analysis.
RESULTS
Descriptive statistics for child outcomes and some maternal characteristics disaggregated by maternal exposure are provided in Table 1. This population showed evidence of linear growth retardation, with a mean height-for-age z score [per World Health Organization (25, 26) standards] of −1.20, and 20.1% (n = 159) of the children stunted. The t tests indicate significant differences between the means for offspring of mothers exposed to atole and means for offspring of mothers exposed to fresco for birth weight, head circumference, height-for-age z score, and weight-for-age z score. The significance of the differences in z scores for height and weight but not in the raw data for height and weight may reflect the slightly higher age distribution for those exposed to fresco than for those exposed to atole, for which reason controlling for age is important. The intrafamily correlations are significant for all child anthropometric indicators.
TABLE 1.
Children's and mothers' characteristics in the Intergenerational Transfers Study (Guatemala, 2006–2007), by type of supplementation received1
| Mother exposed to atole (n = 405) | Mother exposed to fresco (n = 386) | P for equality of means (atole − fresco)2 | Intrafamily correlation3 | |
| Offspring characteristics (n = 791) | ||||
| Birth weight (g) | 3311 ± 529.64 | 3200 ± 587.8 | <0.01 | 0.465 |
| Height (cm) | 114.0 ± 20.3 | 113.5 ± 21.3 | 0.75 | 0.425 |
| Weight (kg) | 22.8 ± 9.8 | 22.7 ± 9.7 | 0.94 | 0.405 |
| BMI (kg/m2) | 16.8 ± 2.6 | 16.8 ± 2.5 | 0.98 | 0.325 |
| Head circumference (cm) | 49.8 ± 2.7 | 49.2 ± 2.9 | <0.01 | 0.455 |
| Arm circumference (cm) | 17.9 ± 2.8 | 18.0 ± 3.0 | 0.84 | 0.365 |
| Triceps skinfold thickness (mm) | 9.8 ± 3.4 | 9.7 ± 3.7 | 0.63 | 0.385 |
| Subscapular skinfold thickness (mm) | 6.5 ± 2.9 | 6.8 ± 3.1 | 0.23 | 0.365 |
| Height-for-age z score | −1.1 ± 1.0 | −1.3 ± 1.0 | <0.01 | 0.425 |
| Weight-for-age z score6 | −0.4 ± 1.0 | −0.6 ± 1.0 | 0.02 | 0.435 |
| BMI-for-age z score | 0.3 ± 1.0 | 0.3 ± 1.0 | 0.70 | 0.315 |
| Male (%) | 48.6 | 52.6 | 0.27 | |
| Age (mo) | 84.1 ± 37.8 | 86.4 ± 39.8 | 0.41 | |
| Mothers' characteristics (n = 401) | ||||
| Current age (y) | 35.5 ± 3.7 | 35.7 ± 4.5 | 0.71 | |
| Height (cm) | 151.3 ± 5.1 | 149.7 ± 4.9 | <0.01 | |
| Completed grades of schooling | 4.6 ± 3.2 | 5.3 ± 2.9 | 0.02 | |
Mothers were supplemented as children in the 1969–1977 study; the offspring were aged 0–12 y in the 2006–2007 study.
Two-sample t test: null hypothesis is difference in means equals zero; alternative hypothesis is difference in means is different from zero.
One-factor ANOVA: offspring anthropometric measures in 2006–2007. Intrafamily correlation was calculated by using internal z scores (calculated from the sample) for height, weight, BMI, head and arm circumferences, and triceps and subscapular skinfold thicknesses.
Mean ± SD (all such values).
Significantly different from 0, P < 0.01.
Weight-for-age z score covers up to age 120 completed months; thus, numbers of children in the atole and fresco groups are 328 and 293, respectively.
Maternal childhood exposure to atole in comparison with exposure to fresco was associated with a 116-g (95% CI: 17, 215 g) higher birth weight, 1.3-cm (0.4, 2.2 cm) greater height, 0.6-cm (0.4, 0.9 cm) greater head circumference, 0.26 (0.09, 0.43) greater height-for-age z score, and 0.20 (0.02, 0.39) greater weight-for-age z score (Table 2).
TABLE 2.
Association between maternal exposure to atole in comparison with fresco for mothers born between 1962 and 1977 in Guatemala and offspring anthropometric measures in 2006–20071
| Birth weight | Height | Weight | BMI | Head circumference | Arm circumference | Triceps skinfold thickness | Subscapular skinfold thickness | Height-for-age z score | Weight-for-age z score2 | BMI-for-age z score | |
| g | cm | kg | 2 | cm | cm | mm | mm | ||||
| Maternal exposure to atole | 116 | 1.34 | 0.68 | 0.13 | 0.63 | 0.10 | 0.19 | −0.15 | 0.26 | 0.20 | 0.03 |
| 95% CI | 17, 215 | 0.43, 2.24 | −0.10, 1.46 | −0.22, 0.49 | 0.37, 0.89 | −0.21, 0.41 | −0.35, 0.73 | −0.60, 0.29 | 0.09, 0.43 | 0.02, 0.39 | −0.13, 0.19 |
| P | 0.02 | 0.01 | 0.09 | 0.46 | 0.01 | 0.51 | 0.49 | 0.49 | 0.01 | 0.03 | 0.67 |
n = 791 children and 401 mothers. Mothers were supplemented as children in the 1969–1977 study; the offspring were aged 0–12 y in the 2006–2007 study. Exposure to atole, the more-nutritious supplement, is a dummy variable that equals 1 for children born to mothers exposed to atole when aged <15 y. Offspring of mothers exposed to fresco, the less-nutritious supplement, constitutes the reference group. P values and 95% CIs were calculated allowing for clustering at the mother level. Additional variables included but not reported are offspring sex, child's date of birth (for birth weight estimates), a second-order polynomial in child age (for measures other than birth weight), and a variable for mother's date of birth.
Weight-for-age z score covers up to age 120 completed months; thus, n = 621 children and 360 mothers.
We tested for significant differences between associations for male and female offspring (see Supplementary Table T2 B under “Supplemental data” in the online issue). Only for height was there a difference, with a larger association for sons (P = 0.04). We estimated the relations for the child anthropometric indicators that were significantly associated with maternal childhood exposure to atole in Table 2, separately by offspring sex (Table 3). Maternal childhood exposure to atole in comparison with exposure to fresco was associated for sons with a 123-g (95% CI: −1.8, 248 g) greater birth weight, 2.0-cm (1.0, 3.1 cm) greater height, 0.6-cm (0.2, 0.9 cm) greater head circumference, and 0.38 (0.16, 0.59) greater height-for-age z score. Maternal childhood exposure to atole in comparison with exposure to fresco was associated for daughters with a 0.7-cm (0.4, 1.0 cm) greater head circumference.
TABLE 3.
Association between maternal exposure to atole in comparison with fresco for mothers born between 1962 and 1977 in Guatemala and anthropometric measures of male and female offspring in 2006–20071
| Birth weight | Height | Head circumference | Height-for-age z score | Weight-for-age z score2 | |
| g | cm | cm | |||
| Boys (n = 400) | |||||
| Maternal exposure to atole | 123 | 2.04 | 0.56 | 0.38 | 0.21 |
| 95% CI | −1.8, 248.3 | 0.97, 3.10 | 0.22, 0.90 | 0.16, 0.59 | −0.04, 0.45 |
| P | 0.05 | 0.01 | 0.01 | 0.01 | 0.10 |
| Girls (n = 391) | |||||
| Maternal exposure to atole | 106 | 0.60 | 0.69 | 0.14 | 0.20 |
| 95% CI | −23, 234.6 | −0.63, 1.84 | 0.35, 1.03 | −0.08, 0.37 | −0.05, 0.44 |
| P | 0.11 | 0.34 | 0.01 | 0.21 | 0.11 |
Mothers were supplemented as children in the 1969–1977 study; the offspring were aged 0–12 y in the 2006–2007 study. Exposure to atole, the more-nutritious supplement, is a dummy variable that equals 1 for children born to mothers exposed to atole when aged <15 y. Offspring of mothers exposed to fresco, the less-nutritious supplement, constitutes the reference group. P values and 95% CIs were calculated allowing for clustering at the mother level. Additional variables included but not reported are offspring sex, a second-order polynomial in child age, and a variable for mother's date of birth.
Weight-for-age z score covers up to age 120 completed months; thus, n = 306 boys and 315 girls.
We assessed the robustness of our basic results in Table 2. We found the following:
1) Paternal, instead of maternal, exposure to atole was not associated with any of the 11 offspring measures (see Supplementary Table T2a under “Supplemental data” in the online issue).
2) The use of the Huber-White (27, 28) approach to estimate SEs, which allows for heteroscedasticity of unknown form but not for clustering, resulted in greater precision; therefore, the precision of our basic estimates in Table 2 with clustering on mothers appears conservative (see Supplementary Table T2b under “Supplemental data” in the online issue).
3) Adjustment for log maternal height and maternal schooling attainment reduced the magnitude and precision of the estimate for birth weight, but did not change the other estimated exposure associations substantially, although one or both of these controls had significant coefficient estimates for all of the child outcomes except for BMI and BMI-for-age z score (see Supplementary Table T2c under “Supplemental data” in the online issue).
4) Adjustment for log paternal height (because mothers who were better nourished as children may have attracted taller mates) reduced the precision somewhat particularly for weight-for-age z score (P = 0.06) but did not change the estimated exposure associations substantially even though log paternal height had significant associations with child height, head circumference, and height-for-age z score (see Supplementary Table T2d under “Supplemental data” in the online issue).
5) Adjustment for birth weight for the other 10 outcomes did not change the exposure coefficients substantially, even though birth weight had significant positive coefficient estimates for 7 of the other child anthropometric outcomes: height, weight, head circumference, arm circumference, height-for-age z score, weight-for-age z score, and BMI-for-age z score (see Supplementary Table T2e under “Supplemental data” in the online issue).
6) The estimated associations declined with birth order for height, height-for-age z score, and weight-for-age z score but not for birth weight or head circumference (see Supplementary Table T2f under “Supplemental data” in the online issue).
7) Control for fixed effects by including a dichotomous variable for the less-populous atole and fresco villages, consistent with the original design emphasis on less- compared with more-populous villages, did not change the pattern of significant associations (see Supplementary Table T2g under “Supplemental data” in the online issue).
8) Adjustment for observed village characteristics related to health (whether a doctor was present when the mother was 2, 7, 12, and 15 y of age) and schooling (students/teacher in primary school when mother was 7, 12, and 15 y of age) did not change the pattern of significant associations substantially, although the coefficient of maternal exposure to atole in the child-weight relation became significant (see Supplementary Table T2h under “Supplemental data” in the online issue).
9) Use of the Donald-Lang (29) differences-in-difference estimator based on the mother's village birth-year means (after conditioning out variables that vary at the individual level), which is robust with a small number of villages, did not change the magnitudes of the estimates substantially, although only coefficients for height and head circumference remained significant (see Supplementary Table T2i under “Supplemental data” in the online issue).
10) Conditioning on grandparental socioeconomic status in 1975 did not change the estimates substantively (see Supplementary Table T2j under “Supplemental data” in the online issue).
11) Conditioning on grandmother height did not change the estimates substantially (see Supplementary Table T2k under “Supplemental data” in the online issue).
12) A test of whether the associations for exposure differed between those mothers exposed only when <3 y of age, those exposed completely from 0 to 3 y of age, those exposed when <3 y of age but not starting at birth, and those exposed only when >3 y of age did not indicate significant differences (see Supplementary Table T2l under “Supplemental data” in the online issue).
13) Use of the maximum number of observations for each offspring anthropometric indicator did not change the estimated associations substantively (see Supplementary Table T2m under “Supplemental data” in the online issue).
14) Use of internal z scores (calculated from the sample) for height, weight, BMI, head circumference, arm circumference, and triceps and subscapular skinfold thicknesses resulted in significant associations with height, weight, and head circumference (see Supplementary Table T2n under “Supplemental data” in the online issue).
15) Addition of offspring of women who received neither supplement because they did not live in the 4 villages during the supplementation period (but married men who were exposed to the supplement) to the offspring of women who received the supplements did not alter the estimates of receiving atole or fresco substantively (see Supplementary Table T2o under “Supplemental data” in the online issue).
16) Control for attrition with the use of Fitzgerald, Gottschalk, and Moffitt (30, 31) methodology did not change the estimates substantially, although there was somewhat more imprecision so that P > 0.05 for birth weight and weight-for-age z score (see Supplementary Tables T2p and T2q under “Supplemental data” in the online issue).
17) Exclusion of the 12-y-olds, who were most likely to be peripubertal in our sample, to investigate whether the differences in the height estimates by sex might be related to sex differences in entering puberty did not change the estimates substantially (see Supplementary Table T3a under “Supplemental data” in the online issue).
DISCUSSION
We report intergenerational associations of a nutritional intervention in early childhood with mothers' offspring's anthropometric indicators. We found that maternal exposure to a high-calorie and high-protein nutritional supplement (atole) in comparison with maternal exposure to a low-calorie and no-protein nutritional supplement (fresco) in the first 15 y of life, but including some exposure in the first 7 y of life, had significant and substantial associations with offspring birth weight, height, head circumference, and height-for-age and weight-for-age z scores. Our results are robust to a wide range of sensitivity tests, including alternative approaches to computation of SEs; possible pathways for effects such as mother's height and schooling attainment, paternal height, children's birth weight, and children's birth order; parental family background, including socioeconomic status and grandmaternal height; use of internal z scores; adjustment for village fixed effects for the 2 less-populous villages; adjustment for attrition; use of the maximum number of available observations for each offspring anthropometric indicator; and consideration of children born to mothers of the same ages who were not exposed to the supplement because they were not born or raised in the 4 villages. These results suggest strong intergenerational associations with birth weight, height, head circumference, height-for-age z score, and weight-for-age z score for male and female offspring combined. The results suggest significantly larger associations for sons than for daughters for height. Separate estimates for sons suggest significant associations for birth weight, height, head circumference, and height-for-age z score. Separate estimates for daughters suggest significant associations only for head circumference.
One previous examination of aspects of associations between maternal exposure to supplements and child size has been conducted, which used data collected between 1996 and 1999 in this same longitudinal study population (32). That study found that children born to women who received atole were taller (age-adjusted difference: 0.8 cm; 95% CI: 0.2, 1.4 cm) than were children whose mothers received fresco (32). Several differences in the model specifications and samples used across these 2 analyses were observed. The present study, in comparison with the earlier study, has a larger sample of children (791 compared with 263), larger sample of mothers (401 compared with 231), a wider age range for children (0–12 y compared with 0–3 y), a larger number of anthropometric offspring outcomes (11 compared with 1, height), and a wider set of robustness tests (eg, alternative treatment of standard errors, adjustment for attrition, inclusion of fixed effects for less-populous villages, aggregation to birth-year/village cohorts, use of internal z scores, adjustment for paternal height and child birth order, and alternative estimates that include mothers not exposed to the supplements) but fewer observations per child (one compared with multiple measurements). Our results reinforced the basic result of that previous study that, based on the original experimental design, maternal supplementation with atole relative to fresco was associated with offspring linear growth. Our estimates differed with regard to linear growth in that our estimates covered a wider age range (0–12 y compared with 0–3 y), and our estimates more strongly indicated that male offspring alone were the beneficiaries of these intergenerational associations (P = 0.04 in our case for heterogeneity by sex for child height compared with P = 0.08 in the previous study). Our results also suggest a wider range of associations, including with birth weight, offspring head circumference, and weight-for-age z score. Our birth weight results are noteworthy because of the growing literature describing strong effects of birth weight over the life cycle (3, 11, 33, 34). Our results further suggest that for birth weight, height, and height-for-age z scores, the associations were significant only for sons when offspring was considered separately by sex, although for head circumference they were significant for both sons and daughters.
The suggestion of stronger associations with anthropometric indicators for sons than for daughters is intriguing. Sex-specific associations of maternal nutrition with child growth have been reported in the context of fetal programming, with boys being more sensitive to nutritional insults (35, 36). On the other hand, a review of intergenerational height relations finds that mother's height is no more closely associated with son's height than with daughter's height (37). The relatively strong associations between mothers' childhood nutrition and sons' anthropometric indicators may reflect that sons tend to have higher birth weights than daughters (38, 39), so that maternal nutritional status is more constraining for sons than for daughters. The data used in this study indicate that the distribution of birth weights for sons was significantly higher than that for daughters (2-sample Kolmogorov-Smirnov test for equality of distributions: P = 0.01).
Our study had some limitations. In the original INCAP longitudinal study, the 4 villages, and not the individuals in the original sample within them, were randomly assigned to either atole or fresco supplementation. The small number of villages does not provide enough power to estimate the effect of exposure to atole or fresco at the village level. Thus, we used mother-child pairs as the unit of analysis, although in alternative estimates in which the results remained very similar we used village-annual birth cohort means, village fixed effects for the 2 less-populous villages, and observed village characteristics related to health and schooling when the mothers were of different ages. Thus, whereas it is possible that there may be other village characteristics that are correlated with exposure to atole or fresco for which we do not control for in our basic estimates, we perceived that the probability of significant bias was small.
In our study, women were eligible to participate in the intervention from the time their pregnancy was detected through lactation. Other studies of maternal nutrition in pregnancy and outcomes of the third generation (the children born to births of the affected pregnancy) are limited. Data from the Dutch Famine suggest inconsistent associations with birth weight (40–42), and one recent report suggests that the third generation have increased adiposity at birth (43), which suggests that maternal influences in gestation may affect adiposity, unlike the postnatal supplementation we considered here. Our data do not permit differentiation for the association between women who received postnatal supplementation and those who received only prenatal supplementation.
The strengths of our study included the nutritional intervention, which was proven to have increased nutrient intakes and physical growth in children, the extended period of follow-up, the investigation of many anthropometric outcomes for the next generation, and the use of appropriate and robust statistical methods with a range of alternative estimates.
Our results suggest that exposure to the nutritional supplements for females, but not for males, had significant intergenerational associations beyond previously documented associations in their own lives. Our results also suggest that exposure to the nutritional supplement had such associations for exposure at ages >3 y and indeed up to 15 y, although including some exposure when <7 y of age, in contrast with studies finding effects on own growth, education, and wage rates with the same longitudinal study data that report associations only for exposure <3 y (4–6, 16, 19).
The difference between the results for maternal and paternal exposure suggests some insights about the pathways through which the effects occurred, which may have resulted because of the greater reproductive fitness of women if additional energy is directed toward improving the reproductive fitness of females but the productive fitness of males (44–48). The differences may have been due to better own-health care and better mothering because of more-educated or more-healthy mothers and the sex specialization of females in child care (49, 50). However, the pathway probably was not through greater income generated by more education and better health, although income has been emphasized as an important determinant of nutrition (51, 52) because this pathway implies similar effects for females and males. The differences probably did not result because of adding shared knowledge that is pooled between mothers and fathers, as has been suggested to be important in some contexts (53), because this pathway also implies similar effects for females and males. The differences probably did not result through strengthening the bargaining position in households of mothers who have more interest in the nutritional development of their children, although this possibility has been emphasized as being important for investments in children (54, 55) because this pathway implies opposite effects for females and males.
It will be of great interest in future research to better identify the pathways through which nutritional supplementation in mothers when young affects their offspring and to investigate whether there are effects on other child outcomes, including other anthropometric indicators, and why the associations were stronger for male than for female offspring. Finally, our findings underscore the importance of further investigations of the long-term intergenerational effects of improving childhood nutrition on their offspring in other settings.
Acknowledgments
We have benefited from our interactions with the following other members of the study team for the larger project of which this article is a part: Ann DiGirolamo, Ruben Grajeda, Paúl Melgar, Humberto Méndez, Agnes Quisumbing, Usha Ramakrishnan, Luis Fernando Ramírez, Manuel Ramírez-Zea, Kathryn Yount, Alexis Murphy, Scott McNiven, and Meng Wang (the last 3 of whom we especially thank for their excellent research assistance in the preparation of the data for this article). We also have benefited significantly from the comments of the referees on an earlier version of this article.
The authors' responsibilities were as follows—JRB: had full access to all of the data in the study, had final responsibility for the decision to submit for publication, and prepared the initial and final drafts; MCC: undertook the statistical analysis; JRB, MCC, SHP, and ADS: responsible for the statistical modeling of the intervention; RM: researcher in the original longitudinal study; and JRB, JH, RM, and ADS: participated in the development of applications for National Institutes of Health and National Science Foundation grants, and each is the Principal Investigator on at least one such grant that supported the 2002–2004 and 2006–2007 data collection and related analysis including the present article. All authors contributed to the study design, development of standard operating procedures and analytical protocols, critical review and approval of this manuscript, and the writing of the manuscript. All authors declared that they had no conflicts of interest.
REFERENCES
- 1.Grantham-McGregor S, Cheung YB, Cueto S, et al. Developmental potential in the first 5 years for children in developing countries. Lancet 2007;369:60–70 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Walker SP, Wachs TD, Meeks Gardner J, et al. Child development: risk factors for adverse outcomes in developing countries. Lancet 2007;369:145–57 [DOI] [PubMed] [Google Scholar]
- 3.Alderman H, Behrman JR. Reducing the incidence of low birth weight in low-income countries has substantial economic benefits. World Bank Res Obs 2006;21:25–48 [Google Scholar]
- 4.Hoddinott J, Maluccio JA, Behrman JR, Flores R, Martorell R. The impact of nutrition during early childhood on income, hours worked, and wages of Guatemalan adults. Lancet 2008;371:411–6 [DOI] [PubMed] [Google Scholar]
- 5.Maluccio JA, Hoddinott J, Behrman JB, Quisumbing AR, Martorell R, Stein AD. The impact of improving nutrition during early childhood on education among Guatemalan adults. Econ J 2009;119:734–63 [Google Scholar]
- 6.Victora CG, Adair L, Fall C, et al. for the Maternal and Child Undernutrition Study Group Undernutrition 2: maternal and child undernutrition: Consequences for adult health and human capital. Lancet 2008;371:340–57 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Strauss J, Thomas D. Health, nutrition, and economic development. J Econ Lit 1998;36:766–817 [Google Scholar]
- 8.Whitelaw N, Whitelaw E. How lifetimes shape epigenotype within and across generations. Hum Mol Gen 2006;15:R131–7. [DOI] [PubMed] [Google Scholar]
- 9.Pembrey ME, Bygren LO, Kaati G, et al. ALSPAC Study Team Sex-specific, male-line transgenerational responses in humans. Eur J Hum Genet 2006;14:159–66 [DOI] [PubMed] [Google Scholar]
- 10.Gluckman PD, Hanson MA, Cooper C, Thornburg KL. Effect of in utero and early-life conditions on adult health and disease. N Engl J Med 2008;359:61–73 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Behrman JR, Rosenzweig MR. Returns to birthweight. Rev Econ Stat 2004;86:586–601 [Google Scholar]
- 12.Behrman JR, Rosenzweig MR. Does increasing women's schooling raise the schooling of the next generation? Am Econ Rev 2002;92:323–34 [Google Scholar]
- 13.Black SE, Devereux PJ, Salvanes KG. Why the apple doesn't fall far: understanding intergenerational transmission of human capital. Am Econ Rev 2005;95:437–49 [Google Scholar]
- 14.Plug E. Estimating the effect of mother's schooling on children's schooling using a sample of adoptees. Am Econ Rev 2004;94:358–68 [Google Scholar]
- 15.Habicht J-P, Martorell R, Rivera JA. Nutritional impact of supplementation in the INCAP Longitudinal Study: analytic strategies and inferences. J Nutr 1995;125:1042S–50S [DOI] [PubMed] [Google Scholar]
- 16.Martorell R, Habicht J-P, Rivera JA. History and design of the INCAP longitudinal study (1969-77) and its follow up (1988-89). J Nutr 1995;125(4S):1027S–41S [DOI] [PubMed] [Google Scholar]
- 17.Stein AD, Melgar P, Hoddinott J, Martorell R. Cohort profile: the Institute of Nutrition of Central America and Panama (INCAP) nutrition trial cohort study. Int J Epidemiol (Epub ahead of print 19 February 2008) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Schroeder DG, Kaplowitz H, Martorell R. Patterns and predictors of participation and consumption of supplement in an intervention study in rural Guatemala. Food Nutr Bull 1992;14:191–200 [Google Scholar]
- 19.Martorell R. Overview of long-term nutrition intervention studies in Guatemala, 1968–1989. Food Nutr Bull 1992;14:270–7 [Google Scholar]
- 20.Melgar P, Ramírez LF, McNiven S, et al. Resource flows among three generations in Guatemala study (2007-08): definitions, tracking, data collection, coverage, and attrition. Washington, DC: IFPRI, 2008 [Google Scholar]
- 21.Martorell R, Behrman JR, Flores R, Stein AD. Rationale for a follow-up focusing on economic productivity. Food Nutr Bull 2005;26(suppl):S5–14 [DOI] [PubMed] [Google Scholar]
- 22.Ramakrishnan U, Martorell R, Schroeder DG, Flores R. Role of intergenerational effects on linear growth. J Nutr 1999;129(suppl):544S–9S [DOI] [PubMed] [Google Scholar]
- 23.Wooldridge JM. Cluster-sample methods in applied econometrics. Am Econ Rev 2003;93:133–8 [Google Scholar]
- 24.StataCorp Stata statistical software: release 10.1. College Station, TX: Stata Corporation, 2007 [Google Scholar]
- 25.World Health Organization WHO child growth standards: length/height-for-age, weight-for-age, weight-for-length, weight-for-height and body mass index-for-age: methods and development. Geneva, Switzerland: World Health Organization, 2006 [Google Scholar]
- 26.World Health Organization WHO child growth standards: head circumference-for-age, arm circumference-for-age, triceps skinfold-for-age and subscapular skinfold-for-age: methods and development. Geneva, Switzerland: World Health Organization, 2007 [Google Scholar]
- 27.Huber P. The behavior of maximum likelihood estimates under non-standard conditions. Proceedings of the Fifth Berkeley Symposium in Mathematical Statistics and Probability. Berkeley and Los Angeles, CA: University of California Press, 1967:221–33 [Google Scholar]
- 28.White H. A heteroskedasticity-consistent covariance matrix and a direct test for heteroskedasticity. Econometrica 1980;48:817–38 [Google Scholar]
- 29.Fitzgerald JM, Gottschalk P, Moffitt RA. An analysis of sample attrition in panel data: the Michigan Panel Study of Income Dynamics. J Hum Resour 1998;33:251–99 [Google Scholar]
- 30.Fitzgerald JM, Gottschalk P, Moffitt RA. An analysis of the impact of sample attrition on the second generation of respondents in the Michigan Panel Study of Income Dynamics. J Hum Resour 1998;33:300–44 [Google Scholar]
- 31.Donald SG, Lang K. Inference with difference-in-differences and other panel data. Rev Econ Stat 2007;89:221–33 [Google Scholar]
- 32.Stein AD, Barnhart HX, Hickey M, Ramakrishnan U, Schroeder DG, Martorell R. Prospective study of protein-energy supplementation early in life and of growth in the subsequent generation in Guatemala. Am J Clin Nutr 2003;78:162–7 [DOI] [PubMed] [Google Scholar]
- 33.Conley D, Bennett N. Is biology destiny? birth weight and life chances. Am Sociol Rev 2000;65:458–67 [Google Scholar]
- 34.Almond D, Chay KY, Lee DS. The costs of low birth weight. Q J Econ 2005;120:1031–1083 [Google Scholar]
- 35.Barker DJP. Mothers, babies and health in later life. 2nd ed Edinburgh, United Kingdom: Churchill Livingstone, 1998 [Google Scholar]
- 36.Falkner F, Tanner JM, Human growth: a comprehensive treatise. 2nd ed New York, NY: Plennum Press, 1986 [Google Scholar]
- 37.Mueller WH. Parent-child correlations for stature and weight among school aged children: a review of 24 studies. Hum Biol 1976;48:379–97 [PubMed] [Google Scholar]
- 38.Janssen PA, Thiessen P, Klein M, Whitfield M, Macnab Y, Cullis-Kuhl S. Standards for the measurement of birth weight, length and head circumference at term in neonates of European, Chinese, and South Asian ancestry. Open Med [serial online] 2007;1 Available from: http://www.openmedicine.ca/article/viewArticle/47/45 (accessed 6 June 2009) [PMC free article] [PubMed]
- 39.Hemming K, Hutton JL, Glinianaia S, Jarvis S, Platt M. Differences between European birthweight standards: impact on classification of 'small for gestational age'. Dev Med Child Neurol 2006;48:906–12 [DOI] [PubMed] [Google Scholar]
- 40.Lumey LH, Stein AD, Ravelli ACJ. Timing of prenatal starvation in women and birth weight in their offspring: the Dutch famine birth cohort study. Eur J Obstet Gynecol Reprod Biol 1995;61:23–30 [DOI] [PubMed] [Google Scholar]
- 41.Lumey LH, Stein AD. Offspring birth weights after maternal intrauterine undernutrition: a comparison within sibships. Am J of Epidemiol 1997;146:810–9 [DOI] [PubMed] [Google Scholar]
- 42.Stein AD, Lumey LH. The relationship between maternal and offspring birth weights after maternal prenatal famine exposure: the Dutch Famine Birth Cohort Study. Hum Biol 2000;72:641–54 [PubMed] [Google Scholar]
- 43.Painter RC, Osmond C, Gluckman P, Hanson M, Phillips D, Roseboom T. Transgenerational effects of prenatal exposure to the Dutch famine on neonatal adiposity and health in later life. BJOG 2008;115:1243–9 [DOI] [PubMed] [Google Scholar]
- 44.Bateson P, Barker D, Clutton-Brock T, et al. Development plasticity and human health. Nature 2004;430:419–21 [DOI] [PubMed] [Google Scholar]
- 45.Ellison PT. Evolutionary perspectives on the fetal origins hypothesis. Am J Hum Biol 2005;17:113–8 [DOI] [PubMed] [Google Scholar]
- 46.Bribiescas R. Men: evolutionary and life history. Cambridge, MA: Harvard University Press, 2008 [Google Scholar]
- 47.Ellison PT. On fertile ground: a natural history of human reproduction. Cambridge, MA: Harvard University Press, 2001 [Google Scholar]
- 48.Trivers RL. Parental investment and sexual selection. Campbell B, Sexual selection and the descent of man, 1871-1971. Chicago, IL: Aldine, 1972:136–79 [Google Scholar]
- 49.Behrman JR, Wolfe BL. More evidence on nutrition demand: income seems overrated and women's schooling underemphasized. J Dev Econ 1984;14:105–28 [Google Scholar]
- 50.Becker GS. A treatise on the family. 2nd ed Cambridge, MA: Harvard University Press, 1991 [Google Scholar]
- 51.Bouis HE, Haddad LJ. Are estimates of calorie-income elasticities too high?: a recalibration of the plausible range. J Dev Econ 1992;39:333–64 [Google Scholar]
- 52.World Bank World development report. Washington, DC: World Bank, 1981 [Google Scholar]
- 53.Basu K, Foster JE. On measuring literacy. Econ J 1998;108:1733–49 [Google Scholar]
- 54.Thomas D. Intrahousehold resource allocation: an inferential approach. J Hum Resour 1990;25:635–64 [Google Scholar]
- 55.Haddad LJ, Hoddinott J, Alderman H, Intra-household resource allocation: an inferential approach. Baltimore, MD: The Johns Hopkins University Press for the International Food Policy Research Institute, 1996 [Google Scholar]