Abstract
Background: Isoflavones are naturally occurring plant estrogens that are abundant in soy. Although purported to protect against bone loss, the efficacy of soy isoflavone supplementation in the prevention of osteoporosis in postmenopausal women remains controversial.
Objective: Our aim was to test the effect of soy isoflavone supplementation on bone health.
Design: A multicenter, randomized, double-blind, placebo-controlled 24-mo trial was conducted to assess the effects of daily supplementation with 80 or 120 mg of soy hypocotyl aglycone isoflavones plus calcium and vitamin D on bone changes in 403 postmenopausal women. Study subjects were tested annually and changes in whole-body and regional bone mineral density (BMD), bone mineral content (BMC), and T scores were assessed. Changes in serum biochemical markers of bone metabolism were also assessed.
Results: After study site, soy intake, and pretreatment values were controlled for, subjects receiving a daily supplement with 120 mg soy isoflavones had a statistically significant smaller reduction in whole-body BMD than did the placebo group both at 1 y (P < 0.03) and at 2 y (P < 0.05) of treatment. Smaller decreases in whole-body BMD T score were observed among this group of women at 1 y (P < 0.03) but not at 2 y of treatment. When compared with the placebo, soy isoflavone supplementation had no effect on changes in regional BMD, BMC, T scores, or biochemical markers of bone metabolism.
Conclusion: Daily supplementation with 120 mg soy hypocotyl isoflavones reduces whole-body bone loss but does not slow bone loss at common fracture sites in healthy postmenopausal women. This trial was registered at clinicaltrials.gov as NCT00665860.
INTRODUCTION
Prevention of bone loss during menopause is the primary strategy used to reduce the risk of osteoporotic fractures and the ensuing physical and economic burdens (1). For years, hormone therapy (HT) was the treatment of choice because it prevents bone loss and osteoporotic fractures in postmenopausal women (2, 3). However, women have sought alternative therapies for preventing osteoporosis because of the increased risks of breast cancer (4, 5) and endometrial cancer (6, 7) associated with HT. Soy isoflavones, which are compounds with chemical structures similar to estrogen, have been proposed as alternatives to HT for preventing bone loss; yet the evidence supporting their efficacy is mixed due to variations in isoflavone products, study designs, and statistical analyses. For example, postmenopausal women receiving 110 mg of isoflavone-enriched foods containing 60–75% genistein for 1 y reported no benefit over placebo (8). However, supplementation with 54 mg of genistein alone was effective for slowing bone loss at the lumbar spine (LS) and femoral neck (FN) over 2 y in osteopenic postmenopausal women (9). Additionally, a 1-y study of 203 Chinese postmenopausal women with intakes of 40 or 80 mg/d of combined soy hypocotyl isoflavones showed a bone-sparing effect in total hip (TH) and trochanter bone mineral content (BMC) (10). That study reported benefits with the 80-mg dose only, and neither BMC of the LS nor bone mineral density (BMD) at any skeletal site was affected significantly.
In the current study, Osteoporosis Prevention Using Soy (OPUS), we sought to determine whether over a 2-y interval daily supplementation with 80 or 120 mg of soy hypocotyl aglycone isoflavones could reduce bone loss in healthy postmenopausal women, as assessed by measurements of BMD, BMC, and serum biochemical markers of bone metabolism.
SUBJECTS AND METHODS
Study design
The OPUS study was a multicenter, randomized, double-blind, placebo-controlled, 2-y intervention trial conducted at Baylor College of Medicine, at the University of California at Davis in collaboration with the Kaiser Foundation Research Institute, and at the University of Georgia between March 2002 and June 2006. Study subjects provided written informed consent, and the study’s protocol was approved by the institutional review board for human studies at each institution.
Menopausal women between the ages of 40 and 60 y with a serum follicle-stimulating hormone concentration >30 IU/mL and at least 12 mo of amenorrhea were eligible to participate in the study provided that they were nonvegetarians and nonrunners. Soy food consumption of less than one serving per week was acceptable. Women with bilateral oophorectomy also qualified for the study 6-mo post surgery if other criteria were met.
Exclusion criteria included an abnormal screening mammogram, Papanicolau test, or blood chemistry; a body mass index (in kg/m2) > 30; and a history of osteoporosis, spine, and/or hip fracture, cancer, or active liver, kidney, gallbladder, and heart disease. Smokers were not enrolled in the study because they have accelerated bone loss and lower BMD of the lower spine and other skeletal sites (11–13). Osteopenic women (LS BMD T score <1.5) were excluded from the study. Women were excluded from the study if they were being treated with bisphosphonates, calcitonin, fluoride, corticosteroids, tamoxifen, raloxifene, letrozole, or HT. Women also were excluded from the study if they were taking supplements such as black cohosh, blue cohosh, dong quai, caltrate 600+Soy (Wyeth Consumer Healthcare, Richmond, VA), Estroven (Amerifit Brands, Cromwell, CT), ginseng, Healthy Women (Johnson & Johnson, Langhome, PA), Opti-Soy (Optimum Nutrition Inc, Aurora, IL), PhytoFem (The Nutri Centre, London, United Kingdom), Probalance (Antiaging Systems, Sark, United Kingdom), Promensil (Natrol Inc, Chatsworth, CA), Remifemin (Enzymatic Therapy Inc, Green Bay, WI), Rimostil (Novogen Inc, New Canaan, CT), or Trinovin (Novogen Inc).
The study consisted of 2 periods: a screening phase and a double-blind study period of 24 mo. At baseline, informed consent was obtained, after which a screening mammogram and a physical examination that included a Papanicolau test, a stool guaiac test, clinical blood chemistries, and dual-energy X-ray absorptiometry (DXA) scans were performed. After the screening, 135 women at each study site were randomized within 9 time blocks of 15 so that one-third of the participants were assigned to receive 80 mg soy isoflavones/d, one-third to receive 120 mg soy isoflavones/d, and the remaining one-third to receive a placebo. All the investigators, research staff, and subjects were blinded to the treatment codes. DXA measurements, well-women examinations, and blood draws were scheduled for visits at baseline and at 12 and 24 mo. Each woman was supplemented daily with 1000 mg calcium carbonate (400 mg calcium) and a one-a-day multivitamin that delivered 400 IU vitamin D.
BMD measurements
DXA (Delphi A; Hologic Inc, Bedford, MA) was used to assess changes in whole-body (WB), LS, TH, FN, and trochanter BMD and BMC. To minimize differences in BMD and BMC measurements between DXA instruments (14–16), 3 identical instruments were employed at the 3 study sites. All DXA measurements were made by using a single-scan mode, and each site used the same DXA instrument and software throughout the study. The DXA manager at the Children’s Nutrition Research Center traveled to the study sites to standardize all the DXA procedures. The same spine, femur, and soft-tissue phantoms were used to cross-calibrate the DXA instruments at quarterly intervals (17). To assess the interchangeability of the DXA data among the 3 study sites, an in vivo cross-calibration of the 3 DXA instruments was carried out before the start of the study by measuring the WB and regional BMD and BMC of 10 volunteers with the 3 DXA instruments within 3 wk (18, 19). All DXA scans were analyzed by a single investigator at the Children’s Nutrition Research Center to minimize variation in data interpretation (20). A coefficient of variation of 0.37% was observed for each instrument from scans of the spine phantom.
Biochemical markers of bone metabolism
Serum samples were analyzed for osteocalcin, bone-specific alkaline phosphatase (BAP), and cross-linked N-telopeptides of type 1 collagen (NTx). Serum osteocalcin was measured by using a radioimmunoassay kit (Incstar, Stillwater, MN). BAP was measured with an immunoradiometric assay (Hybritech Inc, San Diego, CA). Serum NTx was measured by using the OsteoMark NTx serum competitive-inhibition enzyme-linked immunosorbent assay (Ostex International Inc, Seattle, WA). The coefficients of variation for the osteocalcin, BAP, and NTx assays were 4.3%, 3.6%, and 3.6%, respectively.
Soy isoflavone and placebo tablets
The soy hypocotyl isoflavones were manufactured by Frutarum Netherlands BV (Veenendaal, Netherlands). The placebo and isoflavone tablets were manufactured and packaged by Pharma Consulting & Industries BV (Eede, Netherlands). Each isoflavone tablet contained 40.51 mg aglycone isoflavones (daidzein: 22.01 mg; glycitein: 13.54 mg; genistein: 4.96 mg) with the majority (>95%) in the form of glycosides and reflected the natural composition of the soy germ. The placebo tablets were filled with cellulose along with common processing aids such as sodium carboxymethylcellulose, silicon dioxide, magnesium stearate, titanium dioxide, and iron oxide. The same filler material and processing aids were used in the isoflavone tablets. Each woman ingested 3 tablets each day—one at breakfast, one at lunch, and one at dinner. The study subjects returned every 6 mo to receive a new 6-mo supply of the tablets and the calcium and multivitamin supplements.
Soy intake
Each woman completed a soy food questionnaire at baseline (21, 22). The questionnaire was analyzed by the Fred Hutchinson Cancer Research Center Nutrition Assessment Shared Resource (Seattle, WA). The staff at the Nutrition Assessment Shared Resource was not involved in the study and was blinded to the treatment codes.
Physical activity recall and compliance measure
Each woman completed a 7-Day Physical Activity Recall Questionnaire (23–25) to estimate her energy expenditure (kcal/d) at baseline. Subjects were asked to maintain their current diet and exercise regimens throughout the study.
Compliance was assessed by pill counts when the participants returned to the study sites to pick up their next 6-mo supply of tablets.
Study outcomes
The primary outcome was the change in WB, LS, TH, FN, and trochanter BMD and BMC from baseline over 2 y. The secondary outcome was the change in biochemical markers of bone metabolism (osteocalcin, BAP, NTx) from baseline over 2 y.
Statistical analysis
A total of 102 women were required in each group to provide a statistical power of 80% to detect a difference of 0.0223 g/cm2 in LS BMD between the treatment groups and placebo at 2 y, assuming a SD of 0.0537 g/cm2 and a 2-sided α of 0.05. We planned to recruit 400 women to allow for a 30% attrition rate during the 2-y period. The attrition rate was based on that reported in a 24-mo clinical trial documenting the effect of transdermal 17 β-estradiol on bone loss in menopausal women (26). The sample size was expected to detect differences in osteocalcin of 1.0 ng/mL and in BAP of 2.0 U/L.
Potential confounding was assessed by comparing treatment groups with respect to baseline demographic and clinical characteristics. Characteristics shown to be different between groups to a clinically important degree were included as covariates in the analyses. Generalized estimating equations were used to assess each outcome with respect to the effects of treatment group, time, and interaction between treatment and time while accounting for clinical site, confounders, and the corresponding baseline value of the outcome variable. A significant treatment-by-time interaction was followed by group comparison at years 1 and 2. If the interaction was not significant, that term was dropped from the model, and the analysis was repeated to assess the main effect of treatment. Because the correlation between a given outcome variable and its baseline value is likely to be stronger at year 1 than at year 2, leading to a more precise assessment at year 1, this analysis was followed by a separate analysis at years 1 and 2 if the treatment effect was suggestive. Analyses were performed with the SPSS software (version 16; SPSS Inc, Chicago, IL) on an intent-to-treat basis, and the sequential Sidak multiple comparison procedure was applied to all pairwise comparisons between the 3 treatment groups.
The manufacturers of the supplements were not involved in the study design or data analysis. The academic authors had full and unrestricted rights to analyze, interpret, and publish the results.
RESULTS
Study subjects
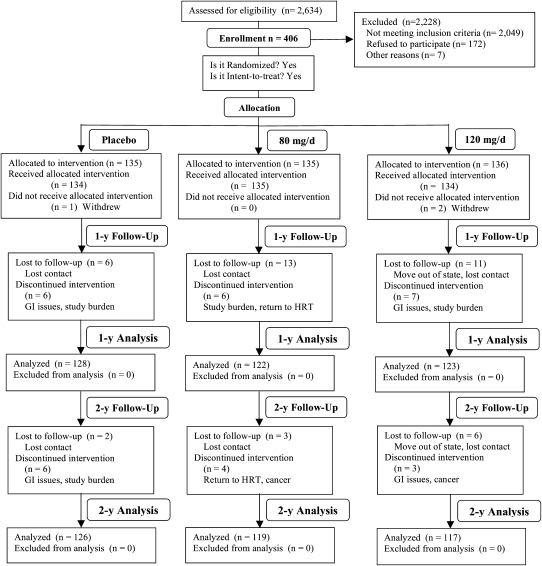
From March 2002 to June 2006, a total of 2634 women underwent screening. As shown in Figure 1, 406 were randomly assigned to the following treatment groups: 135 were assigned to the placebo group, 135 were assigned to the 80-mg/d group, and 136 were assigned to the 120-mg/d group. At 1 y, 30 women dropped out of the study (placebo group: 6; 80-mg/d group: 13; 120-mg/d group: 11). At 2 y, 11 more women dropped out of the study (placebo group: 2; 80-mg/d group: 3; 120-mg/d group: 6). The attrition rates at both time points were not significantly different among the treatment groups. The most common reasons for withdrawal included study burden, gastrointestinal upset, and return to HT.
FIGURE 1.
Flowchart of the study design and subject participation throughout the current study. GI, gastrointestinal; HRT, hormone replacement therapy.
Baseline characteristics of the study subjects are listed in Table 1. Age, age at menarche, age at menopause, years since menopause, body weight, height, body mass index, body composition, and energy expenditure were similar at baseline among the treatment groups. Soy protein intake was significantly different among the treatment groups. Compliance with the study protocol was confirmed by pill counts in that ≈95% of the subjects in all 3 groups consumed >80% of the pills.
TABLE 1.
Baseline descriptive statistics of study subjects
| Soy isoflavone–treated groups |
||||
| Placebo group | 80 mg/d | 120 mg/d | P value1 | |
| n | 134 | 135 | 134 | |
| Age (y) | 55.0 ± 3.72 | 54.9 ± 4.0 | 54.5 ± 4.1 | 0.55 |
| Age at menarche (y) | 12.7 ± 1.5 | 12.9 ± 1.5 | 12.8 ± 1.6 | 0.46 |
| Age at menopause (y) | 48.2 ± 5.1 | 48.5 ± 5.5 | 47.6 ± 6.3 | 0.42 |
| Years since menopause (y) | 6.7 ± 5.3 | 6.4 ± 5.2 | 6.9 ± 6.5 | 0.64 |
| Weight (kg) | 68.0 ± 9.8 | 68.8 ± 13.2 | 67.9 ± 10.2 | 0.77 |
| Height (cm) | 163.8 ± 6.7 | 164.8 ± 5.9 | 165.0 ± 6.0 | 0.24 |
| BMI (kg/m2) | 25.4 ± 3.4 | 25.3 ± 4.5 | 24.9 ± 3.2 | 0.59 |
| Lean body mass (kg) | 41.4 ± 4.3 | 42.4 ± 4.8 | 42.2 ± 4.8 | 0.17 |
| Body fat (kg) | 24.9 ± 6.0 | 25.0 ± 6.4 | 24.4 ± 6.9 | 0.73 |
| Soy protein intake (g/wk) | 168 ± 332 | 416 ± 863 | 278 ± 582 | 0.0073 |
| Energy expenditure (kcal/d) | 2685 ± 592 | 2668 ± 552 | 2675 ± 672 | 0.97 |
| Bone mineral density (g/cm2) | ||||
| Whole body | 1.100 ± 0.084 | 1.115 ± 0.090 | 1.121 ± 0.093 | 0.156 |
| Lumbar spine | 1.013 ± 0.094 | 1.029 ± 0.114 | 1.030 ± 0.105 | 0.312 |
| Total hip | 0.896 ± 0.106 | 0.921 ± 0.106 | 0.916 ± 0.098 | 0.109 |
| Femoral neck | 0.759 ± 0.100 | 0.777 ± 0.106 | 0.767 ± 0.097 | 0.366 |
| Trochanter | 0.672 ± 0.089 | 0.694 ± 0.091 | 0.688 ± 0.089 | 0.114 |
values by one-factor analysis of variance among the 3 treatment groups.
Mean ± SD (all such values).
Due to its skewed distribution, soy protein intake was compared by using the Kruskal-Wallis test.
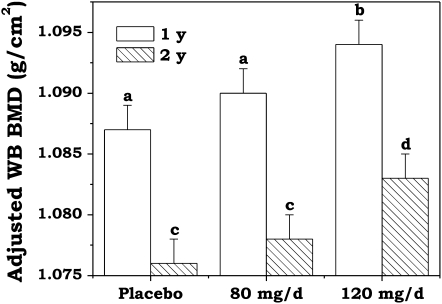
Treatment group differences in WB BMD after 1 and 2 y of soy isoflavone supplementation with adjustment for study site, soy protein intake, and pretreatment values are shown in Figure 2. The group-by-time interaction was not significant (P = 0.62), which indicates that differences between treatment groups were similar at years 1 and 2. The adjusted WB BMD was significantly higher among the menopausal women receiving 120 mg soy isoflavones/d after both 1 and 2 y of treatment than that in those receiving the placebo (P = 0.04 by using sequential Sidak adjustment for multiple comparisons). The adjusted WB BMD values among the women receiving the lower amount of supplementation (80 mg/d) fell between the values reported for the women receiving the placebo and the women receiving the higher dosage but did not reach significance when compared with those receiving the placebo (P = 0.13). Differences between year 1 and year 2 means were significant across treatment groups (P < 0.001).
FIGURE 2.
Mean (±SEM) whole-body (WB) bone mineral density (BMD) values by supplementation group, with 1- and 2-y outcomes as the dependent variable, according to a general linear model with adjustment for study site, soy protein intake, and pretreatment values. Letters above the columns at 1 y (placebo: n = 128; 80 mg soy isoflavones/d: n = 122; 120 mg soy isoflavones/d: n = 123) and at 2 y (placebo: n = 126; 80 mg soy isoflavones/d: n = 119; 120 mg soy isoflavones/d: n = 117) denote differences between the treatment groups where a and c = placebo compared with 80 mg/d (P > 0.05) and b and d = placebo compared with 120 mg/d (P < 0.05).
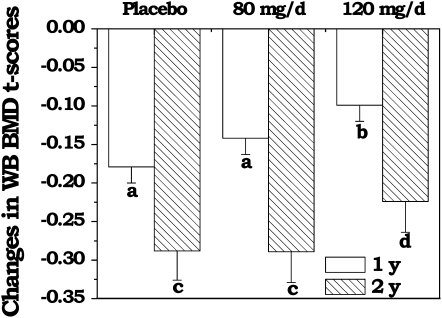
The changes in WB BMD T scores among the 3 treatment groups after 1 and 2 y of soy isoflavone supplementation with adjustment for study site, soy protein intake, and pretreatment values are shown in Figure 3. There was no evidence of an interaction between group and time (P = 0.53). However, the reduction in the adjusted WB BMD T score was significantly less among the menopausal women receiving 120 mg soy isoflavones/d after 1 y (P < 0.03) of treatment when compared with the placebo. The benefit, however, did not reach significance after 2 y of treatment (P > 0.49). The change in adjusted WB BMD T scores among women receiving the lower amount of supplementation (80 mg/d) did not reach significance compared with that in those receiving the placebo.
FIGURE 3.
Mean (±SEM) changes in whole-body (WB) bone mineral density (BMD) T scores by supplementation group, with 1- and 2-y outcomes as the dependent variable, according to a general linear model with adjustment for study site, soy protein intake, and pretreatment values. Letters below the columns at 1 y (placebo: n = 128; 80 mg soy isoflavones/d: n = 122; 120 mg soy isoflavones/d: n = 123) and at 2 y (placebo: n = 126; 80 mg soy isoflavones/d: n = 119; 120 mg soy isoflavones/d: n = 117) denote differences between the treatment groups where a and c = placebo compared with 80 mg/d (P > 0.05), b = placebo compared with 120 mg/d (P < 0.05), and d = placebo compared with 120 mg/d (P > 0.05).
The benefit of soy isoflavone supplementation at 120 mg/d in reducing the loss of WB BMD, however, was not reflected in BMC values among the regional sites or among the serum biochemical markers of bone metabolism.
Adverse events
Serious adverse events included one woman receiving the 120 mg soy isoflavones/d treatment being diagnosed with breast cancer at 14 mo and one woman receiving the 80 mg soy isoflavones/d treatment being diagnosed with stage 1B, grade III, squamous carcinoma of the endometrium at 21 mo. Pathologic examination of the affected endometrium tissue revealed the lack of estrogen receptors. In summary, the major adverse events are the same or fewer than would be predicted in a comparable study population (27).
DISCUSSION
On the basis of evidence from in vitro studies of cultured bone cells, in vivo studies of animal models for postmenopausal osteoporosis, human observational/epidemiologic studies, and short-term dietary intervention studies, isoflavones are believed to protect against bone loss (28). Contrary to these reports, we showed that supplementation with 2 doses of soy isoflavones for 2 y in healthy postmenopausal women was not effective in slowing bone loss at key fracture sites (LS and TH). Soy isoflavones did, however, slow WB bone loss.
The declines in WB BMD and in WB BMD T scores as shown in Figures 2 and 3 represent a normal physiologic progression for postmenopausal women over time, particularly in the initial years after menopause. Among our healthy postmenopausal women who were assigned to the placebo group, the unadjusted WB BMD dropped 2.3% in year 1 and 0.9% between year 1 and year 2. In 2 studies that reported changes in WB BMD (29, 30), the annual drop in WB BMD was reported to be ≈0.6%. However, these 2 studies involved participants who were much older (average age of 68 y compared with 55 y among our study participants). One of the studies (29) involved older Chinese women with low WB, LS, TH, FN, and trochanter BMD. Therefore, a comparison of changes in WB BMD between our younger menopausal women with healthy bone status and those reported in these 2 studies is not ideal. However, in a cohort of 1901 American women who participated in the Study of Women’s Health Across the Nation (31), the rates of BMD loss from the LS and TH were 0.022 g · cm−2 · y−1 (2.0%) and 0.013 g · cm−2 · y−1 (1.4%), respectively, among the postmenopausal women. Among our study participants who were assigned to the placebo group, the rates of BMD loss from the LS and TH were 0.022 g · cm−2 · y−1 (2.0%) and 0.012 g · cm−2 · y−1 (1.3%), respectively. Therefore, the rates of BMD loss or change in T scores of our study participants are expected to be similar to those observed in healthy postmenopausal American women.
The fact that the OPUS trial did not corroborate the positive findings from other soy isoflavone trials may be related to differences in the bone health of the study participants, the soy products employed, and variations in methodologic approaches. Participants in the OPUS trial were healthy compared with participants from the genistein supplementation trial who were osteopenic or had 3 risk factors for osteoporosis (32). Furthermore, improvements in TH and trochanter BMD were reported among menopausal Chinese women with low initial BMC or BMD, low body weights, and low intakes of dietary calcium after 1 y of treatment with a soy-germ formulation (80 mg aglycone isoflavones/d) similar to that used in the current project (10). In comparison, the women in the OPUS study had normal baseline BMD values as part of the study inclusion criteria; soy isoflavone supplementation may therefore be more effective in menopausal women with low bone quality and or risk factors for osteoporosis.
Although calcium and vitamin D supplementation have been shown to slow bone loss in postmenopausal women and to prevent fractures (33, 34), it is unlikely that calcium and vitamin D supplementation attenuated the isoflavone effects on bone. The majority of calcium and vitamin D trials showed a positive effect on bone in frail or institutional elderly individuals and used higher doses of vitamin D (35). The 400-IU vitamin D dose used in the current study is much less than the doses used in the trials showing vitamin D benefits. Furthermore, the OPUS participants were active, healthy, community-dwelling individuals.
Differences in the composition of the soy supplementation used may also have contributed to differences in the study findings. Daily supplementation with 54 mg genistein for 24 mo has been reported to increase LS and FN BMD and markers of bone formation in osteopenic postmenopausal women (9). The formulation used in the OPUS trial delivered only 15 mg/d of genistein at the highest dosage, which is much less than the 54 mg/d used in the study by Marini et al (9). Therefore, if genistein is the critical isoflavone in protecting bone loss in the LS, the amount of genistein in the OPUS formulation was insufficient to protect against LS bone loss in our healthy study participants.
In the most recent randomized, double-blind, placebo-controlled, parallel, multicenter trial conducted in 237 menopausal women in the Netherlands, Italy, and France (8), treatment with 110 mg aglycone isoflavones/d for 1 y did not benefit BMD or markers of bone metabolism. The use of different DXA instruments that employed different scanning software for the BMD measurements together with the heterogeneity of the population studied could have easily masked the small beneficial effects of soy isoflavones on BMD. Strengths of the OPUS trial included the quality-control procedures used with respect to the DXA scans and bone measurements between the 3 different study sites.
A meta-analysis on the basis of 10 studies with durations ranging from 3 to 24 mo and representing a total of 608 subjects concluded that isoflavone intake >90 mg/d is related to increases in LS BMD (36). However, the majority of the intervention studies had short durations, and because of the length of the adult bone-remodeling cycle (≈12 mo), changes in BMD may represent short-term remodeling rather than sustained long-term effects. A more recent meta-analysis of 10 studies with durations of ≥1 y and representing a total of 896 women showed a tendency toward a weak beneficial effect of isoflavone doses of >80 mg/d on LS BMD (37). However, the overall conclusion was that soy isoflavone supplementation was not likely to result in significantly favorable changes in BMD of the TH and LS. The OPUS trial more definitively addressed the effect of soy isoflavone supplementation on bone health by using a dose response design, which included a higher concentration of isoflavones (120 mg) and a 2-y intervention period. It should be noted that both meta-analyses looked only at regional sites and did not examine WB BMD.
Despite the negative finding and the inconsistent benefits at bone sites reported in many of these human clinical trials, a large prospective cohort study of 24,403 menopausal women in the Shanghai Women’s Health Study (38) showed that the incidence of bone fracture over 4.5 y was inversely related to quintiles of soy protein intake (P < 0.01 for trend). Two other smaller observational studies have noted a positive association of dietary soy isoflavone intake with BMD in postmenopausal women (39, 40). It is possible that lifetime intake of soy is needed to see a significant protective association on bone mass conservation.
In conclusion, the OPUS study results indicated that treatment with 120 mg soy hypocotyl isoflavones/d plus calcium and vitamin D was not effective in slowing bone loss at regional bone sites or in favorably altering biochemical markers of bone turnover. The attenuated WB BMD in healthy postmenopausal women for 2 y probably translates to minimal clinical benefits; yet the long-term significance, particularly with regard to fracture prevention, remains to be determined.
Acknowledgments
The authors’responsibilities were as follows—All authors: study design, execution of the clinical trial, and the preparation of the manuscript; JKF and EOS: managed the database and performed the statistical analyses; MJM, MAC, RLY, and PA: performed the annual well-woman examinations; and KJE and RJS: prepared the DXA Manual of Procedures and analyzed all the DXA data. None of the authors had any conflicts of interest to declare.
REFERENCES
- 1.NIH Consensus Development Panel on Osteoporosis Prevention, Diagnosis, and Therapy Osteoporosis prevention, diagnosis, and therapy. JAMA 2001;285:785–95 [DOI] [PubMed] [Google Scholar]
- 2.Lafferty FW, Fiske ME. Postmenopausal estrogen replacement: a long-term cohort study. Am J Med 1994;97:66–77 [DOI] [PubMed] [Google Scholar]
- 3.Cauley JA, Seeley DG, Ensrud K, Ettinger B, Black D, Cummings SR. Estrogen replacement therapy and fractures in older women. Ann Intern Med 1995;122:9–16 [DOI] [PubMed] [Google Scholar]
- 4.Colditz GA, Hankinson SE, Hunter DJ, et al. The use of estrogen and progestins and the risk of breast cancer in postmenopausal women. N Engl J Med 1995;332:1589–93 [DOI] [PubMed] [Google Scholar]
- 5.Risch HA, Howe GR. Menopausal hormone usage and breast cancer in Saskatchewan: a record-linkage cohort study. Am J Epidemiol 1994;139:670–83 [DOI] [PubMed] [Google Scholar]
- 6.Woodruff JD, Pickar JH. Incidence of endometrial hyperplasia in postmenopausal women taking conjugated estrogens (Premarin) with medroxyprogesterone acetate or conjugated estrogens alone. Am J Obstet Gynecol 1994;170:1213–23 [DOI] [PubMed] [Google Scholar]
- 7.The Writing Group for the PEPI Trial Effects of estrogen or estrogen/progestin regimens on heart disease risk factors in postmenopausal women. The Postmenopausal Estrogen/Progestin Interventions (PEPI) Trial. JAMA 1995;273:199–208 [PubMed] [Google Scholar]
- 8.Brink E, Coxam V, Robins S, Wahala K, Cassidy A, Branca F. Long-term consumption of isoflavone-enriched foods does not affect bone mineral density, bone metabolism, or hormonal status in early postmenopausal women: a randomized, double-blind, placebo controlled study. Am J Clin Nutr 2008;87:761–70 [DOI] [PubMed] [Google Scholar]
- 9.Marini H, Minutoli L, Polito F, et al. Effects of the phytoestrogen genistein on bone metabolism in osteopenic postmenopausal women: a randomized trial. Ann Intern Med 2007;146:839–47 [DOI] [PubMed] [Google Scholar]
- 10.Chen YM, Ho SC, Lam SS, Ho SS, Woo JL. Beneficial effect of soy isoflavones on bone mineral content was modified by years since menopause, body weight, and calcium intake: a double-blind, randomized, controlled trial. Menopause 2004;11:246–54 [DOI] [PubMed] [Google Scholar]
- 11.Mazess RB, Barden HS. Bone density in premenopausal women: effects of age, dietary intake, physical activity, smoking, and birth-control pills. Am J Clin Nutr 1991;53:132–42 [DOI] [PubMed] [Google Scholar]
- 12.Nguyen TV, Kelly PJ, Sambrook PN, Gilbert C, Pocock NA, Eisman JA. Lifestyle factors and bone density in the elderly: implications for osteoporosis prevention. J Bone Miner Res 1994;9:1339–46 [DOI] [PubMed] [Google Scholar]
- 13.Egger P, Duggleby S, Hobbs R, Fall C, Cooper C. Cigarette smoking and bone mineral density in the elderly. J Epidemiol Community Health 1996;50:47–50 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Modlesky CM, Lewis RD, Yetman KA, et al. Comparison of body composition and bone mineral measurements from two DXA instruments in young men. Am J Clin Nutr 1996;64:669–76 [DOI] [PubMed] [Google Scholar]
- 15.Ellis KJ, Shypailo RJ. Bone mineral and body composition measurements: cross-calibration of pencil-beam and fan-beam dual-energy x-ray absorptiometers. J Bone Miner Res 1998;13:1613–8 [DOI] [PubMed] [Google Scholar]
- 16.Clasey JL, Hartman ML, Kanaley J, et al. Body composition by DEXA in older adults: accuracy and influence of scan mode. Med Sci Sports Exerc 1997;29:560–7 [DOI] [PubMed] [Google Scholar]
- 17.Genant HK, Grampp S, Gluer CC, et al. Universal standardization for dual x-ray absorptiometry: patient and phantom cross-calibration results. J Bone Miner Res 1994;9:1503–14 [DOI] [PubMed] [Google Scholar]
- 18.Ellis KJ, Shypailo RS, Steinberg FM, Lewis RD, Young RL, Wong WW. Reproducibility of fan-beam DXA measurements in adults and phantoms. J Clin Densitom 2004;7:413–8 [DOI] [PubMed] [Google Scholar]
- 19.Finkelstein JS, Butler JP, Cleary RL, Neer RM. Comparison of four methods for cross-calibrating dual-energy x-ray absorptiometers to eliminate systematic errors when upgrading equipment. J Bone Miner Res 1994;9:1945–52 [DOI] [PubMed] [Google Scholar]
- 20.Wahner HW, Looker A, Dunn WL, Walters LC, Hauser MF, Novak C. Quality control of bone densitometry in a national health survey (NHANES III) using three mobile examination centers. J Bone Miner Res 1994;9:951–60 [DOI] [PubMed] [Google Scholar]
- 21.Kirk P, Patterson RE, Lampe J. Development of a soy food frequency questionnaire to estimate isoflavone consumption in US adults. J Am Diet Assoc 1999;99:558–63 [DOI] [PubMed] [Google Scholar]
- 22.Frankenfeld CL, Patterson RE, Horner NK, et al. Validation of a soy food-frequency questionnaire and evaluation of correlates of plasma isoflavone concentrations in postmenopausal women. Am J Clin Nutr 2003;77:674–80 [DOI] [PubMed] [Google Scholar]
- 23.Blair SN. How to assess exercise habits and physical fitness. : Matarazzo JD, Miller NE, Weiss SM, Behavioral health: a handbook of health enhancement and disease prevention. New York: John Wiley & Sons, 1984:424–47 [Google Scholar]
- 24.Blair SN, Haskell WL, Ho P, et al. Assessment of habitual physical activity by a seven-day recall in a community survey and controlled experiments. Am J Epidemiol 1985;122:794–804 [DOI] [PubMed] [Google Scholar]
- 25.Sallis JF, Haskell WL, Wood PD, et al. Physical activity assessment methodology in the Five-City Project. Am J Epidemiol 1985;121:91–106 [DOI] [PubMed] [Google Scholar]
- 26.Field CS, Ory SJ, Wahner HW, Herrmann RR, Judd HL, Riggs BL. Preventive effects of transdermal 17 beta-estradiol on osteoporotic changes after surgerical menopause: a two-year placebo-controlled trial. Am J Obstet Gynecol 1993;168:114–21 [DOI] [PubMed] [Google Scholar]
- 27.American Cancer Society Cancer facts & figures, 2008. Atlanta, GA: American Cancer Society, 2008 [Google Scholar]
- 28.Setchell KD, Lydeking-Olsen E. Dietary phytoestrogens and their effect on bone: evidence from in vitro and in vivo, human observational, and dietary intervention studies. Am J Clin Nutr 2003;78:593S–609S [DOI] [PubMed] [Google Scholar]
- 29.Chen YM, Ho SC, Lam SS, Ho SS, Woo JL. Soy isoflavones have a favorable effect on bone loss in Chinese postmenopausal women with lower bone mass: a double-blind, randomized, controlled trial. J Clin Endocrinol Metab 2003;88:4740–7 [DOI] [PubMed] [Google Scholar]
- 30.Booth SL, Dallal G, Shea MK, Gundberg C, Peterson JW, Dawson-Hughes B. Effect of vitamin K supplementation on bone loss in elderly men and women. J Clin Endocrinol Metab 2008;93:1217–23 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Finkelstein JS, Brockwell SE, Mehta V, et al. Bone mineral density changes during the menopause transition in a multiethnic cohort of women. J Clin Endocrinol Metab 2008;93:861–8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Lydeking-Olsen E, Beck-Jensen JE, Setchell KD, Holm-Jensen T. Soymilk or progesterone for prevention of bone loss: a 2 year randomized, placebo-controlled trial. Eur J Nutr 2004;43:246–57 [DOI] [PubMed] [Google Scholar]
- 33.Dawson-Hughes B, Harris SS, Krall EA, Dallal GE, Falconer G, Green CL. Rates of bone loss in postmenopausal women randomly assigned to one of two dosages of vitamin D. Am J Clin Nutr 1995;61:1140–5 [DOI] [PubMed] [Google Scholar]
- 34.Cranney A, Horsley T, O’Donnell S, et al. Effectiveness and safety of vitamin D in relation to bone health. Evid Rep Technol Assess (Full Rep) 2007:1–235 [PMC free article] [PubMed] [Google Scholar]
- 35.Dawson-Hughes B, Bischoff-Ferrari HA. Therapy of osteoporosis with calcium and vitamin D. J Bone Miner Res 2007;22(suppl 2):V59–63 [DOI] [PubMed] [Google Scholar]
- 36.Ma DF, Qin LQ, Wang PY, Katoh R. Soy isoflavone intake increases bone mineral density in the spine of menopausal women: meta-analysis of randomized controlled trials. Clin Nutr 2008;27:57–64 [DOI] [PubMed] [Google Scholar]
- 37.Liu J, Ho SC, Su YX, Chen WQ, Zhang CX, Chen YM. Effect of long-term intervention of soy isoflavones on bone mineral density in women: a meta-analysis of randomized controlled trials. Bone 2009;44:948–53 [DOI] [PubMed] [Google Scholar]
- 38.Zhang X, Shu XO, Li H, et al. Prospective cohort study of soy food consumption and risk of bone fracture among postmenopausal women. Arch Intern Med 2005;165:1890–5 [DOI] [PubMed] [Google Scholar]
- 39.Mei J, Yeung SS, Kung AW. High dietary phytoestrogen intake is associated with higher bone mineral density in postmenopausal but not premenopausal women. J Clin Endocrinol Metab 2001;86:5217–21 [DOI] [PubMed] [Google Scholar]
- 40.Somekawa Y, Chiguchi M, Ishibashi T, Aso T. Soy intake related to menopausal symptoms, serum lipids, and bone mineral density in postmenopausal Japanese women. Obstet Gynecol 2001;97:109–15 [DOI] [PubMed] [Google Scholar]