Abstract
Deacetylases of the Sir2 family regulate lifespan and response to stress. We have examined the evolutionary history of Sir2 and Hst1, which arose by gene duplication in budding yeast and which participate in distinct mechanisms of gene repression. In Saccharomyces cerevisiae, Sir2 interacts with the SIR complex to generate long-range silenced chromatin at the cryptic mating-type loci, HMLα and HMR a. Hst1 interacts with the SUM1 complex to repress sporulation genes through a promoter-specific mechanism. We examined the functions of the non-duplicated Sir2 and its partners, Sir4 and Sum1, in the yeast Kluyveromyces lactis, a species that diverged from Saccharomyces prior to the duplication of Sir2 and Hst1. KlSir2 interacts with both KlSir4 and KlSum1 and represses the same sets of target genes as ScSir2 and ScHst1, indicating that Sir2 and Hst1 subfunctionalized after duplication. However, the KlSir4-KlSir2 and KlSum1-KlSir2 complexes do not function as the analogous complexes do in S. cerevisiae. KlSir4 contributes to an extended repressive chromatin only at HMLα and not at HMR a. In contrast, the role of KlSum1 is broader. It employs both long-range and promoter-specific mechanisms to repress cryptic mating-type loci, cell-type–specific genes, and sporulation genes and represents an important regulator of cell identity and the sexual cycle. This study reveals that a single repressive complex can act through two distinct mechanisms to regulate gene expression and illustrates how mechanisms by which regulatory proteins act can change over evolutionary time.
Author Summary
Sir2 deacetylases are found in organisms ranging from bacteria to mammals. Sir2 from the yeast Saccharomyces cerevisiae deacetylates histones and is part of the SIR complex that spreads across chromatin to repress gene expression. A related histone deacetylase, Hst1, interacts with a DNA–binding protein, Sum1, to repress genes in a promoter-specific manner. Hst1 and Sir2 are paralogs, arising from a duplication about 100 million years ago. To understand how Sir2 and Hst1 have diverged, as well as to investigate the evolutionary relationship between spreading and non-spreading mechanisms of gene repression, we have characterized the function of a non-duplicated Sir2 from the yeast Kluyveromyces lactis, a species that diverged from Saccharomyces prior to this duplication. We found that KlSir2 is part of both the SIR and SUM1 complexes, indicating that the ancestral Sir2 had both Sir2- and Hst1-like properties. Interestingly, we found that, in K. lactis, the Sir2-Sum1 complex not only uses a promoter-specific mechanism to repress the same sets of genes as S. cerevisiae, it also forms extended chromatin structures to repress gene transcription. Our results illustrate how mechanisms by which regulatory proteins act can change over evolutionary time.
Introduction
Deacetylases of the Sir2 family are key regulators of lifespan and stress resistance in many organisms ranging from yeast to humans [1]. These enzymes couple deacetylation with hydrolysis of NAD+ and consequently their activity is linked to the metabolic state of the cell [2]. Despite having a well-conserved enzymatic activity, Sir2 family members act on a wide variety of substrates and serve a diverse set of biological functions [3],[4]. To explore the process by which Sir2 deacetylases have diversified, we examined the evolutionary history of two family members from budding yeast, Sir2 and Hst1 [5],[6], which arose in a whole-genome duplication [7],[8],[9], yet have distinct functions.
Gene duplication is an important force in evolution because it allows variation to occur without compromising the original function of the gene. Preservation of duplicate genes, or paralogs, is proposed to occur through at least two mechanisms, neofunctionalization and subfunctionalization. In the neofunctionalization model, one duplicate retains the original function, leaving the other gene free of selective constraint and able to evolve a new function [10]. Alternatively, in the subfunctionalization model, if the ancestral gene had multiple functions, duplicated genes could each lose one of the original functions and together retain the entire set of ancestral functions [11]. Only a few studies have characterized the path by which paralogs have diverged [12],[13],[14],[15]. To investigated how Sir2 and Hst1 diverged, we have characterized the function of a representative non-duplicated Sir2 from Kluyveromyces lactis, a budding yeast species that diverged from S. cerevisiae prior to the whole-genome duplication [16].
The functions of Sir2 and Hst1 in S. cerevisiae are well understood. Sir2 interacts with the histone-binding proteins Sir3 and Sir4, and together these proteins generate an extended silenced domain at the telomeres and cryptic mating-type loci, HMLα and HMR a [17]. The HM loci are flanked by silencers that recruit Sir proteins through DNA binding proteins to initiate the formation of silenced chromatin. The telomere repeats also recruit Sir proteins. Sir2, Sir3, and Sir4 spread from sites of recruitment through a sequential deacetylation mechanism that is independent of DNA sequence [18],[19],[20]. Sir2 deacetylates nearby nucleosomes, creating high affinity binding sites for Sir3 and Sir4, which bind preferentially to deacetylated tails of histones H3 and H4. Sir3 and Sir4 then recruit additional Sir2 to newly deacetylated nucleosomes. As Sir proteins spread, they generate a specialized chromatin structure that is restrictive to transcription.
Unlike Sir2, Hst1 does not spread. It is part of the SUM1 complex that represses over fifty genes that are involved in sporulation, NAD+ biosynthesis, and α-cell identity [21],[22],[23],[24]. Sum1 is a DNA binding protein that associates with a conserved sequence, the middle sporulation element, found in the promoters of target genes [21],[23],[25]. Hst1 deacetylates the tails of histones H3 and H4 [26],[27], and this deacetylation is thought to be important for its repressive function. The third member of the complex, Rfm1, mediates the interaction between Sum1 and Hst1 [22].
Genes regulated by Sir2 and Hst1 are critical to cell identity as well as the sexual cycle, and consequently these deacetylases have the potential to coordinate the timing of the life cycle with NAD+ availability. Hst1 plays a role in cell-type identity by repressing several α-specific genes [24]. Hst1 also represses a number of mid-sporulation genes, and this repression must be relieved for completion of the sexual cycle [23]. The mating-type of haploid yeast cells, which can be a or α, is determined by the MAT locus, which encodes transcription factors that regulate cell-type specific genes [28]. These transcription factors are also encoded at HMLα and HMR a, but are silenced by the SIR complex and serve as repositories for mating-type switching. Sir2 maintains cell identity by preventing the cell from simultaneously expressing both a- and α-specific transcription factors.
Compared to ScSir2 and ScHst1, the biological function of the non-duplicated KlSir2 is less understood. KlSir2 is thought to have properties similar to both Sir2 and Hst1, as it complements both sir2Δ and hst1Δ mutations in S. cerevisiae [26],[29]. In K. lactis, KlSir2 represses the HM loci [30],[31], and a sir2Δ mutation results in reduced mating and sporulation defects [29]. Prior to this study, it was not known whether KlSir2 regulates sporulation genes as ScHst1 does.
Few studies have investigated silencing in K. lactis, yet the mechanism differs substantially from that in S. cerevisiae. KlSir2 and the histone binding protein KlSir4 contribute to the silencing of HMLα [30],[31]. However, there is no distinct Sir3 protein in K. lactis. Additionally, the silencer elements that recruit silencing factors are not conserved between K. lactis and S. cerevisiae [32]. Silencers in S. cerevisiae consist of binding sites for ORC, Rap1, and Abf1, whereas in K. lactis, binding sites for these factors have not been identified at the HM loci. Instead the only defined silencer consists of a KlReb1 binding site and two other uncharacterized DNA sequences [32].
In this study, we examined the functions of the non-duplicated KlSir2 and found that it interacts with both KlSir4 and KlSum1. However, the SIR and SUM1 complexes in K. lactis do not function exactly as the analogous complexes do in S. cerevisiae. The KlSum1-KlSir2 complex contributes to silencing at both HM loci as well as sporulation and cell-type specific genes and achieves repression by both long-range and promoter-specific mechanisms. In contrast, KlSir4 only contributes to silenced chromatin at HMLα, but not at HMR a. This study enhances our understanding of the process by which duplicated genes diverge and provides insights into the connections between promoter-specific and regional silencing.
Results
KlSir2 physically associates with both KlSir4 and KlSum1
To determine whether the non-duplicated KlSir2 has functions analogous to both ScSir2 and ScHst1, we first identified its binding partners in K. lactis (described in Table 1). If KlSir2 functions similarly to ScSir2, it should associate with KlSir4, and if it has a function analogous to ScHst1 it should associate with KlSum1. Trans-species complementation experiments previously demonstrated that KlSir2 associates with both ScSir4 and ScSum1 in S. cerevisiae [26], suggesting that analogous interactions occur in K. lactis. We created a K. lactis strain with alleles of KlSIR2-HA, KlSIR4-Flag and myc-KlSUM1 integrated at their chromosomal locations. All three tagged proteins were detectable by immunoblotting (Figure 1) and maintained wild-type function, as assessed by RT-PCR analysis of genes repressed by these proteins (data not shown).
Table 1. Overview of K. lactis genes described in this study.
| Common Name | K. lactis systematic name | S. cerevisiae homolog | Conservation1 | Biological Function in S. cerevisiae |
| KlSIR2 | KLLA0F14663g | ScSIR2 2 | 56 (78) | Silences HML, HMR, telomeres, and the rDNA locus, in complex with Sir4 and Sir3 |
| ScHST1 2 | 63 (84) | Repressor of middle sporulation-specific genes, in complex with Rfm1 and Sum1 | ||
| KlSUM1 | KLLA0C14696g | ScSUM1 | 33 (59) | Repressor of middle sporulation-specific genes, in complex with Rfm1 and Hst1 |
| KlRFM1 | KLLA0C07062g | ScRFM1 | 36 (63) | Repressor of middle sporulation-specific genes, in complex with Hst1 and Sum1 |
| KlSIR4 3 | KLLA0F14320g | See Figure S1 | Silences HML, HMR and telomeres, in complex with Sir2 and Sir3 | |
| KlASF2 3 | KLLA0F13998g | See Figure S1 | Anti-silencing protein that causes derepression of silent loci when overexpressed |
1 Percent identity (percent similar), calculated from FASTA sequence alignments.
2 SIR2 and HST1 are a duplicate gene pair, duplicated in the whole-genome duplication.
3 SIR4 and ASF2 are a tandem duplicate gene pair, duplicated prior to the whole-genome duplication.
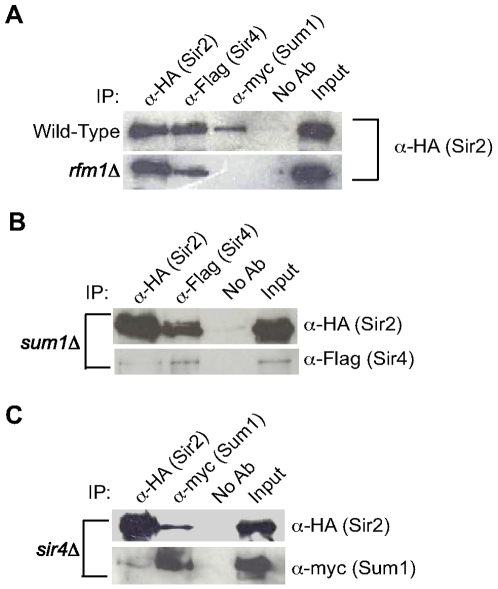
Figure 1. KlSir2 co-precipitates with KlSir4 and KlSum1.
(A) KlSir2-HA, KlSir4-Flag or myc-KlSum1 was precipitated from a lysate prepared from wild-type (LRY2285) or rfm1Δ (LRY2528) strains, and the precipitated material was examined by immunoblotting with an antibody against the HA tag to detect KlSir2-HA. The input represents 33% of the IP. (B) KlSir2-HA or KlSir4-Flag was immunoprecipitated from a sum1Δ strain (LRY2158), and the precipitated material was examined with an antibody against the HA tag to detect KlSir2-HA or the Flag tag to detect KlSir4-Flag. (C) KlSir2-HA or myc-KlSum1 was immunoprecipitated from a sir4Δ strain (LRY2282), and the precipitated material was examined with an antibody against the HA tag to detect KlSir2-HA or the myc tag to detect myc-KlSum1.
If KlSir2 associates with both KlSir4 and KlSum1, it should co-precipitate with these proteins, and indeed, KlSir2 did co-precipitate with both KlSir4 and KlSum1 (Figure 1A). In S. cerevisiae, the association of ScSum1 with ScHst1 requires ScRfm1 [22]. To determine if Rfm1 mediates the interaction between Sum1 and Sir2 in K. lactis, we examined whether the co-precipitation between KlSir2 and KlSum1 persisted in the absence of KlRfm1. There was no observable co-precipitation between KlSir2 and KlSum1 in an rfm1Δ strain (Figure 1A), suggesting that the architecture of the SUM1 complex is conserved between S. cerevisiae and K. lactis.
Given the association of KlSir2 with both KlSir4 and KlSum1, all three proteins might be part of a stable complex. However, a co-precipitation between KlSir4 and KlSum1 was not detected (data not shown), although we could not distinguish whether this result reflected the absence of a complex containing KlSir4 and KlSum1 or simply its instability. Nevertheless, if this complex does exist, the components are not mutually dependent on one another for association, as KlSir2 and KlSir4 still co-precipitated in the absence of KlSum1 (Figure 1B) and KlSir2 and KlSum1 co-precipitated in the absence of KlSir4 (Figure 1C). Therefore, KlSir2 forms independent associations with both KlSir4 and KlSum1, a finding consistent with KlSir2 having functions analogous to those of both ScSir2 and ScHst1.
KlSir2, KlSir4, and KlSum1 repress HMLα
We next investigated whether the Sir4-Sir2 and Sum1-Sir2 complexes have the same repressive functions in K. lactis as they do in S. cerevisiae. If these functions are conserved, deletion of KlSIR4 should derepress the HM loci, deletion of KlSUM1 should derepress mid-sporulation genes, and deletion of KlSIR2 should derepress both HM loci and mid-sporulation genes. We first examined silencing at HMLα, which is known to be repressed by KlSir2 and KlSir4 [30],[31]. To extend this previous result and address the role of KlSum1 at HMLα, we isolated RNA from MAT a wild-type, sir2Δ, sir4Δ, sum1Δ, and rfm1Δ strains and examined the expression of HMLα1, HMLα2 and HMLα3 by quantitative RT-PCR. All three genes were significantly derepressed in the absence of KlSir2 and modestly derepressed in the absence of KlSir4 (Figure 2), consistent with previous reports. Surprisingly, deletion of KlSum1 resulted in derepression of HMLα to a similar extent as observed in the sir2Δ strain. In contrast to KlSum1, deletion of KlRfm1 had very little effect on the transcription of HMLα. This result suggests that KlSir2 does not require KlRfm1 to act at HMLα and therefore may act independently of KlSum1. In this case, a sir2Δ sum1Δ double deletion might disrupt silencing to a greater extent than either single deletion. However, there was no difference in transcription of HMLα in a sir2Δ sum1Δ strain compared to a sir2Δ or sum1Δ strain (Figure 2).
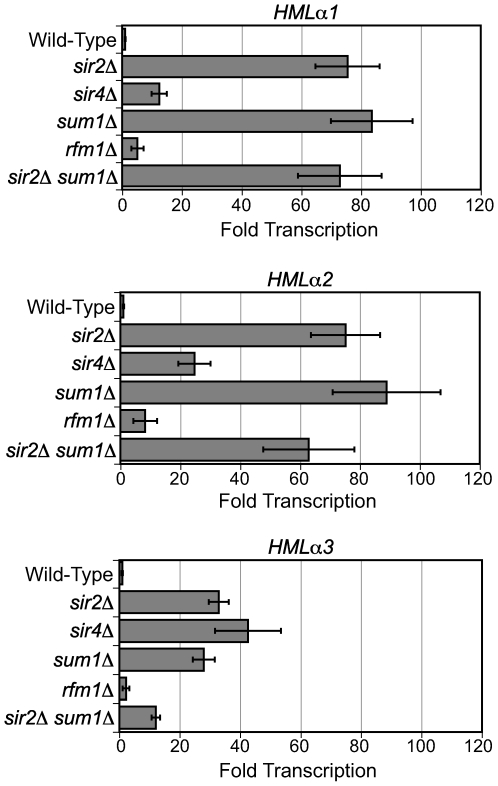
Figure 2. KlSir2, KlSir4, and KlSum1 silence the cryptic mating-type locus HMLα.
Quantitative RT–PCR analysis of HMLα1, HMLα2 and HMLα3 mRNA in wild-type (CK213), sir2Δ (SAY569), sir4Δ (LRY2038), sum1Δ (LRY2035), rfm1Δ (LRY2528), and sir2Δ sum1Δ (LRY2533) strains. The amount of cDNA was first normalized to the control locus ACT1. The values shown here represent the relative amount of cDNA for each deletion strain compared to the wild-type strain. Error bars represent the SEM.
To confirm that these phenotypes resulted from the deletions of the intended genes, plasmids expressing the wild-type KlSIR2, KlSIR4 and KlSUM1 genes were introduced into the corresponding deletion strains. In all cases, repression was restored (data not shown). These results reveal that KlSum1, in addition to KlSir2 and KlSir4, contributes to the silencing of HMLα. Thus, KlSum1 behaves differently than its ortholog in S. cerevisiae, as the deletion of ScSum1 does not alter the expression of ScHMLα [33].
It is interesting to note that in both the sir2Δ and sum1Δ strains the induction of HMLα3 was modest compared to HMLα1 or HMLα2, suggesting that HMLα3 may be regulated differently than the other two genes at HMLα. The α3 gene, which is specific to Kluyveromyces, is proposed to be a MULE family DNA transposase [34] and is required for mating [30].
The modest derepression of the HMLα locus observed in the sir4Δ strain suggested that another protein might compensate for KlSir4 in its absence. The SIR4 gene was duplicated in tandem prior to the whole-genome duplication, and each of the tandem duplicates was retained as a single gene after the whole-genome duplication [7]. This ancient duplicate of Sir4, Asf2 (Anti-Silencing Factor 2), reduces silencing when over-expressed in S. cerevisiae [35]. The SIR4 and ASF2 genes are rapidly evolving, making it difficult to determine which K. lactis gene is orthologous to which S. cerevisiae gene (Figure S1). Gene KLLAOF14320g has been designated KlSIR4 based on functional studies [31], and therefore we refer to the other gene (KLLA0F13398g) as KlASF2. To determine whether its common ancestry with KlSir4 enables KlAsf2 to silence HMLα in the absence of KlSir4, we constructed both asf2Δ and asf2Δ sir4Δ strains and examined expression of the HMLα genes. The lack of KlAsf2 resulted in the further repression of all three genes to less than one-tenth the level of the wild-type strain, and the double deletion of asf2Δ and sir4Δ resembled the single sir4Δ deletion (Figure S2). Therefore, KlASF2 does not have a SIR4-like function. In fact, KlASF2, like ScASF2, is antagonistic to silencing.
KlSir2, KlSir4, and KlSum1 spread across HMLα but not MATα
Given the surprising result that KlSum1 affects the expression of HMLα, it was important to investigate whether KlSum1 acts directly at HMLα to silence transcription. We also examined the association of KlSir2 and KlSir4 with HMLα, as the association of these proteins with HMLα had not been assessed previously. We used chromatin immunoprecipitation to map the distributions of KlSir2, KlSir4, KlSum1 and KlRfm1 across HMLα. We observed a robust enrichment of all four proteins across the entire HMLα locus (Figure 3A), demonstrating that not only KlSir2 and KlSir4, but also the components of the SUM1 complex, KlSum1 and KlRfm1, spread across this locus. Therefore, KlSum1 contributes directly to transcriptional silencing at HMLα.
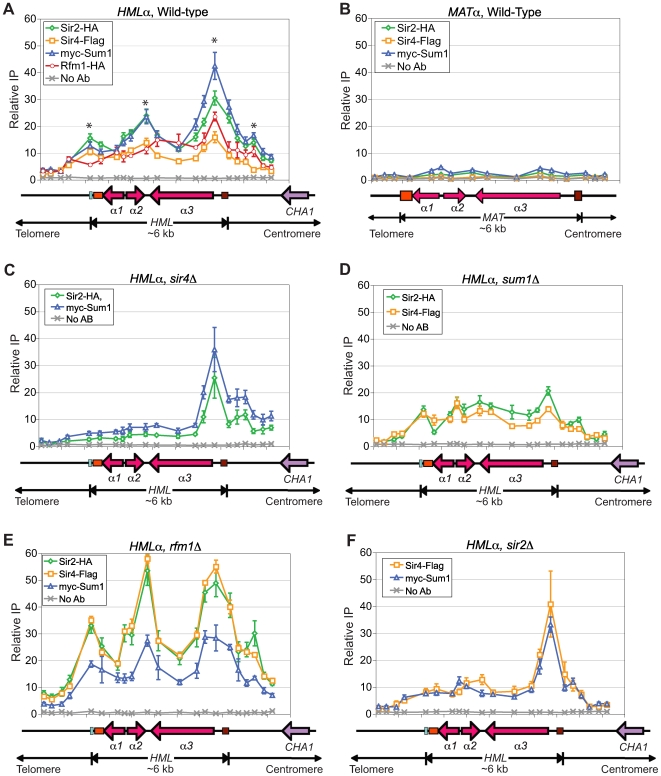
Figure 3. KlSir2, KlSir4, KlSum1, and KlRfma1 spread across HMLα.
(A) The association of KlSir2-HA, KlSir4-Flag, myc-KlSum1 (LRY2239) and KlRfm1-HA (LRY2327) with HMLα as assessed by chromatin IP followed by quantitative PCR. The y-axis represents the relative enrichment normalized to a control locus, RRP7, which is not detectably associated with KlSir2, KlSir4 or KlSum1. A diagram of the HMLα locus is shown under the x-axis. The aqua bar represents the characterized silencer and the orange and brown boxes represent sequences found at HMLα, MAT, and HMR a loci. Asterisks indicate the peaks of enrichment. (B) The association of KlSir2-HA, KlSir4-Flag and myc-KlSum1 with the MATα locus in a strain in which the α-cassette is only found at MAT (LRY2398). (C) The association of KlSir2-HA and myc-KlSum1 with HMLα in a sir4Δ strain (LRY2281). (D) The association of KlSir2-HA and KlSir4-Flag with HMLα in a sum1Δ strain (LRY2158). (E) The association of KlSir2-HA, KlSir4-Flag and myc-KlSum1 with HMLα in a rfm1Δ strain (LRY2528). (F) The association of KlSir4-Flag and myc-KlSum1 with HMLα in a sir2Δ strain (LRY2388). All y-axes are set to the same scale to facilitate the comparison of protein associations in different experiments. Error bars represent the SEM.
The enrichment of KlSir2, KlSir4 and KlSum1 peaked at a previously identified silencer ([32], represented as an aqua bar in Figure 3), suggesting that this sequence may stabilize the association of silencing proteins with chromatin. Three other peaks were also observed (indicated by asterisks in Figure 3A): one in the intergenic region in which the α2 and α3 genes converge, one in the α3 promoter, and a smaller peak on the centromere proximal side of HMLα. These peaks could represent additional silencers or proto-silencers. Curiously, two of the peaks coincided with sequences that are conserved between the transcriptionally silent HMLα locus and the transcriptionally active MATα locus. If these peaks represent binding sites for silencing factors, then these factors might be recruited to MATα. To examine this possibility, we constructed a strain in which the α-cassette at HML was replaced with an a-cassette, so that the only α-cassette in the genome was at the MAT locus. Using this strain, we investigated whether KlSir2, KlSir4 or KlSum1 associated with the MAT locus. All three proteins associated with control loci (data not shown). However, we observed no significant enrichment of KlSum1, KlSir2 or KlSir4 anywhere along the MATα locus (Figure 3B). Therefore, the peaks of silencing proteins at the α3 promoter and the α2–α3 intergenic regions are specific to the HMLα locus, and these sequences cannot recruit silencing proteins independently.
Both KlSir4 and KlSum1 recruit KlSir2 to HMLα
Sir2 deacetylases lack DNA-binding and histone-binding domains and consequently are recruited to chromatin through adaptor proteins such as Sum1, a DNA binding protein, or Sir4, a histone binding protein. To determine whether KlSir4 and/or KlSum1 recruit KlSir2 to HMLα, we examined the association of KlSir2 with HMLα in strains lacking these proteins. In a sir4Δ strain, the enrichments of KlSir2 and KlSum1 were significantly reduced over the silencer and across the open reading frames of α1, α2, and α3 (Figure 3C). However, the associations of KlSir2 and KlSum1 with the promoter of α3 and centromere-proximal side of HMLα were unchanged. Thus, there may be different requirements for the assembly of silenced chromatin on the two sides of the HMLα locus. On the telomere-proximal side, containing the known silencer, KlSir4 is important for the recruitment and spreading of silencing proteins. However, on the centromere-proximal side, the recruitment of KlSum1 and KlSir2 is independent of KlSir4.
The ability of KlSir2 to associate with the centromere-proximal side HMLα in the absence of KlSir4 suggests that another protein is recruiting KlSir2 to this region. To determine whether KlSum1 is required for the recruitment or spreading of KlSir2 and KlSir4, we examined the associations of these proteins with HMLα in a sum1Δ strain. The deletion of KlSum1 caused a reduction in the association of KlSir2 at the α2–α3 intergenic region, the α3 promoter and on the centromere-proximal side of the HMLα locus. There was no observable difference in the association of KlSir4 with HMLα (Figure 3D). These results suggest that KlSum1 is important for stabilizing the association of KlSir2 with the HMLα locus, particularly at the α3 promoter and centromere-proximal regions, but that it is not absolutely required for the recruitment or spreading of either KlSir2 or KlSir4. Together, these results indicate that neither KlSir4 nor KlSum1 is solely responsible for the recruitment of KlSir2 to HMLα. This finding is consistent with the independent interactions of KlSir2 with KlSir4 and KlSum1 (Figure 1).
The greater level of transcription of HMLα in a sir2Δ strain compared to an rfm1Δ strain (Figure 2) suggests that KlRfm1 is not critical for the recruitment of KlSir2 or other silencing proteins. In fact, in the absence of KlRfm1, all three silencing proteins, KlSir2, KlSir4 and KlSum1, still associated with the entire HMLα locus (Figure 3E). The enrichment of KlSum1 was indistinguishable between the wild-type and rfm1Δ strains, indicating that its association with HMLα does not require KlRfm1 and may be an inherent property of the Sum1 protein. Interestingly, the enrichments of both KlSir2 and KlSir4 were significantly enhanced in the rfm1Δ strain compared to the wild-type strain, although the overall pattern, with peaks of association at the silencer, α2–α3 intergenic region, α3 promoter and centromere-proximal side of HMLα, was maintained. Perhaps in the absence of KlRfm1, KlSir2 is better able to associate with KlSir4.
In S. cerevisiae, the deacetylase activity of Sir2 is required for the spreading of Sir3 and Sir4 [18],[19],[20]. To determine whether a similar requirement exists in K. lactis, we examined the associations of KlSir4 and KlSum1 with HMLα in a sir2Δ strain. KlSir4 and KlSum1 were reduced over the silencer and the three open reading frames (Figure 3F). However, both silencing proteins remained strongly associated with the α3 promoter, and KlSir4 displayed a more robust enrichment with this region in the absence of KlSir2. This pattern of association is similar to the distribution of KlSum1 and KlSir2 in the sir4Δ strain (Figure 3C). Therefore, KlSir2 may contribute to the assembly of silenced chromatin on the telomere-proximal side of HMLα, but it is not required to assemble these factors at the α3 promoter.
KlSum1 associates with HMLα independently of KlSir2 and KlSir4
Given that KlSum1 is a DNA-binding protein, we were curious whether it binds directly to a sequence at HMLα. The mid-sporulation element (MSE) consensus sequence, to which Sum1 binds in S. cerevisiae, appears to be conserved in K. lactis, as it occurs at the promoters of a number of sporulation genes (data not shown). However, a match to the MSE consensus sequence was not found in the known telomere-proximal silencer (aqua box) or the rest of the HMLα locus. Moreover, the observation that the enrichment of KlSum1 was significantly reduced on the telomere-proximal side of HMLα in the absence of KlSir4 or KlSir2 (Figure 3C and 3F) makes it unlikely that KlSum1 binds directly to this side of the locus. Furthermore, KlSum1 did not associate with the MATα locus (Figure 3B), indicating that the sequences conserved between MATα and HMLα are unable to recruit KlSum1 directly. It remains possible that KlSum1 binds directly to a non-MSE sequence on the centromere-proximal side of the HMLα, and KlSum1 did associate with this region of HMLα in the absence of both KlSir2 and KlSir4 (Figure S3), indicating that the recruitment of KlSum1 to HMLα is independent of KlSir2 and KlSir4. However, it is also possible that another, unidentified protein recruits KlSum1 to this region.
KlSir2 and KlSum1, but not KlSir4, repress HMRa
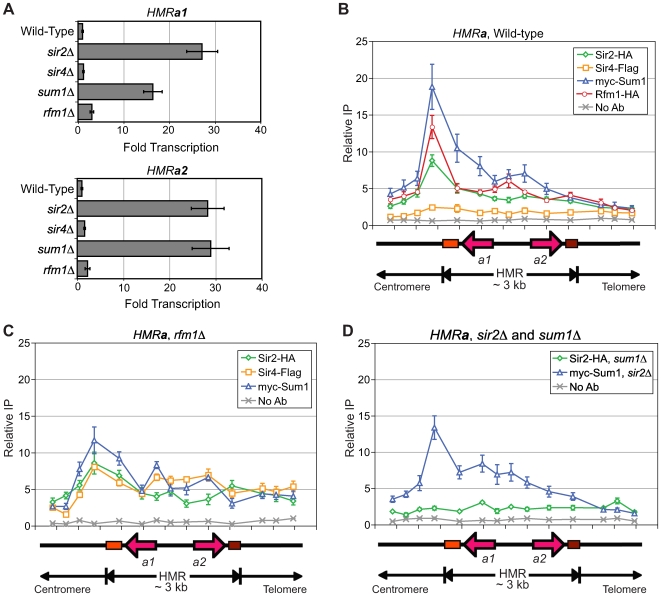
We next investigated the roles KlSir2, KlSir4, KlSum1 and KlRfm1 have in regulating the other cryptic mating-type locus, HMR a. In S. cerevisiae, both HM loci are silenced by the same set of Sir proteins. However, in K. lactis, deletion of KlSir4 had little effect on the expression of the a1 or a2 genes found at HMR a (Figure 4A). Furthermore, deletion of KlAsf2, the paralog of KlSir4, either singly or in conjunction with KlSir4 did not result in derepression of HMR a (Figure S4). In contrast, deletion of KlSir2 or KlSum1 resulted in a substantial derepression of HMR a1 and HMR a2, whereas deletion of KlRfm1 resulted in very little change in HMR a1 or HMR a2 expression (Figure 4A). These results suggest that only a subset of the proteins that contribute to the silencing of HMLα also repress HMR a.
Figure 4. KlSir2 and KlSum1, but not KlSir4, silence and spread across HMRa.
(A) Quantitative RT-PCR analysis of HMR a 1 and HMR a 2 in wild-type (SAY538), sir2Δ (SAY544), sir4Δ (LRY1946), sum1Δ (LRY1947), and rfm1Δ (LRY2529) strains. The fold induction was determined as for Figure 2. (B) The association of KlSir2-HA, KlSir4-Flag, myc-KlSum1 (LRY2285) and KlRfm1-HA (LRY2328) with HMRa as assessed by chromatin IP followed by quantitative PCR. (C) The association of KlSir2-HA (LRY2528), myc-KlSum1 and KlSir4-Flag (LRY2529) with HMR a in a rfm1Δ strain. (D) The association of KlSir2-HA with HMR a in a sum1Δ strain (LRY2126) and the association of myc-KlSum1 with HMR a in a sir2Δ strain (LRY2390). All y-axes are set to the same scale to facilitate the comparison of protein associations in different experiments. Error bars represent the SEM.
To determine whether KlSir2 and KlSum1 act directly at HMR a, we examined their association by chromatin immunoprecipitation. We observed an asymmetric distribution of KlSir2 and KlSum1, as well as KlRfm1, with the HMR a locus. A substantial peak of enrichment was observed on the centromere-proximal side of HMR a, and a shoulder extended across the open reading frames (Figure 4B). The peak likely indicates the location of a silencer element. In contrast to KlSir2 and KlSum1, there was no significant association of KlSir4 with any part of HMR a, consistent with the deletion of SIR4 resulting in no change in the transcription of HMR a1 and HMR a2. These results indicate that KlSum1 and KlSir2, but not KlSir4, are responsible for repressing HMR a. Thus, the mechanisms of silencing at HMR a and HMLα are distinct.
Curiously, KlRfm1 associated with HMR a (Figure 4B), yet was not required for repression of the HMR a1 and HMR a2 genes (Figure 4A). We examined the association of KlSum1 and KlSir2 with HMR a in a rfm1Δ strain and found that KlSum1 was only slightly reduced at the proposed silencer (Figure 4C). Intriguingly, KlSir2 was still able to associate with HMR a in the absence of KlRfm1, despite the fact that it no longer co-precipitated with KlSum1 (Figure 1A). We propose that the absence of KlRfm1 may enable KlSir4 to interact with KlSir2 and KlSum1, thereby stabilizing the association of KlSir2 with HMR a. To test this hypothesis, we assessed whether KlSir4 associated with HMR a in an rfm1Δ strain, and indeed, KlSir4 associated with HMR a (Figure 4C). This result is reminiscent of the increase in KlSir4 at HMLα in the absence of KlRfm1 (Figure 3E).
To determine whether KlSum1 and KlSir2 depended on one another for association with HMR a, we performed chromatin immunoprecipitation experiments in the absence of KlSum1 or KlSir2. In the absence of KlSum1, KlSir2 no longer associated with any region of the HMR a locus (Figure 4D), and therefore KlSum1 was required for recruitment of KlSir2 to HMR a. This result contrasts with what was observed in the absence of KlRfm1 (Figure 4C). Deletion of KlSir2, like deletion of KlRfm1, resulted in a reduced association of KlSum1 with the proposed silencer at HMR a. Despite this reduction, KlSum1 still spread across HMR a (Figure 4D). Thus, the association and spreading of KlSum1 does not require KlSir2 or KlRfm1.
KlSir2 and KlSum1 repress mid-sporulation genes in a promoter-specific manner
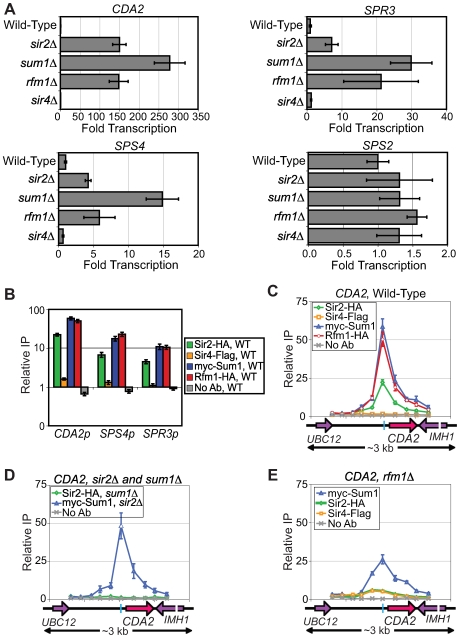
In S. cerevisiae, the Sum1-Hst1 complex represses mid-sporulation genes. To assess whether KlSir2 regulates mid-sporulation genes in a manner similar to ScHst1, we isolated RNA from wild-type, sir2Δ, sum1Δ and rfm1Δ strains and examined expression of the K. lactis orthologs of the mid-sporulation genes CDA2, SPR3, SPS4, and SPS2 that are repressed by ScHst1 in S. cerevisiae [23]. Deletion of KlSir2, KlSum1 and KlRfm1 all resulted in derepression of CDA2, SPS4, and SPR3, but not SPS2 (Figure 5A, note the different scales of the x-axes). We also examined whether KlSir4 has a role in regulating transcription of these genes, as KlSir2 and KlSum1 functioned with KlSir4 to regulate HMLα. However, the sir4Δ strain had no effect on the expression of CDA2, SPS4, SPR3 or SPS2 (Figure 5A). Therefore, KlSum1, KlSir2 and KlRfm1, repress sporulation genes independently of KlSir4. In addition, many (CDA2, SPS4 and SPR3), but not all (SPS2) of the targets of the Sum1-Hst1 complex in S. cerevisiae are also targets in K. lactis.
Figure 5. KlSir2, KlSum1, and KlRfm1 repress sporulation genes in a promoter-specific manner.
(A) Quantitative RT-PCR analysis of CDA2 (KLLA0C17226g), SPS4 (KLLA0F08679g), SPR3 (KLLA0B08129g) and SPS2 (KLLA0C01001g) mRNA in wild-type (SAY538), sir2Δ (SAY544), sum1Δ (LRY1947), rfm1Δ (LRY2529) and sir4Δ (LRY1946) strains. The fold induction was determined as for Figure 2. (B) The association of KlSir2-HA, KlSir4-Flag, myc-KlSum1 (LRY2285) and KlRfm1-HA (LRY2328) with the promoters of CDA2, SPS4 and SPR3 was assessed by chromatin IP followed by quantitative PCR. The y-axis is a log-scale. (C) Distribution of KlSir2-HA, KlSir4-Flag, myc-KlSum1 (LRY2285) and KlRfm1-HA (LRY2328) across the CDA2 locus. The blue bar in the schematic represents the conserved MSE sequence. (D) The association of KlSir2-HA with CDA2 in a sum1Δ strain (LRY2126) and association of myc-KlSum1 with CDA2 in a sir2Δ strain (LRY2390). (E) The association of KlSir2-HA and myc-KlSum1 with CDA2 in a rfm1Δ strain (LRY2529). All y-axes are set to the same scale to compare changes in protein association across experiments. Error bars represent the SEM.
To determine if KlSir2, KlSum1, and KlRfm1 repress mid-sporulation genes directly, we used chromatin immunoprecipitation to assess the association of KlSir2, KlSum1, KlRfm1 and KlSir4 with the promoters of these genes. KlSir2, KlSum1 and KlRfm1 were enriched at the promoters of CDA2, SPS4 and SPR3 (Figure 5B), suggesting that these proteins repress these genes directly, presumably as a complex. In contrast, KlSir4 did not associate with mid-sporulation genes, consistent with the sir4Δ strain having no effect on transcription. To address whether KlSir2, KlSum1 and KlRfm1 spread at sporulation genes, as they do at HMLα and HMR a, we examined a 3-kb region around the CDA2 promoter and open reading frame. A relatively narrow peak of KlSum1, KlRfm1 and KlSir2 coincided with an MSE consensus sequence at the promoter of CDA2 (indicated by the blue bar in the schematic), and the association of these proteins diminished significantly in both directions (Figure 5C), suggesting that these proteins do not spread at the CDA2 locus. Therefore, the ability of the SUM1 complex to spread differs between the HM loci and mid-sporulation genes.
We had observed at HMLα that KlAsf2 was antagonistic to silencing (Figure S2), and it was possible that KlAsf2 restricts the spreading of the Sum1-Sir2 complex at sporulation genes and therefore accounts for the difference in spreading at HMR a compared to sporulation genes. To test this hypothesis, we assessed the distribution of KlSum1 and KlSir2 at the sporulation gene CDA2 in an asf2Δ strain. We observed no changes in the distribution of KlSir2 and KlSum1 across the CDA2 locus (Figure S5A). Furthermore, the transcription of several mid-sporulation genes was not altered (Figure S5B). Therefore, KlAsf2 only antagonized silencing at HMLα.
We discovered that KlSir2 was more dependent on KlRfm1 for recruitment to CDA2 as compared to HMR a. At HMR a, KlSir2 required KlSum1 but not KlRfm1 for recruitment (Figure 4C and 4D). In contrast, the association of KlSir2 with CDA2 was greatly reduced in both sum1Δ and rfm1Δ strains (Figure 5D and 5E). This dependence was similar to what has been observed for the S. cerevisiae SUM1 complex at mid-sporulation genes. One potential explanation for the reduced role of KlRfm1 at the HM loci is the ability of KlSir4 to compensate for the loss of KlRfm1. For example, at both HMLα and HMR a, the association of KlSir4 increased in the absence of KlRfm1 (Figure 3E and Figure 4C). In keeping with the greater role of KlRfm1 at CDA2, we observed only a modest increase in the association of KlSir4 (Figure 5D) in the absence of KlRfm1. We also found that the ability of KlSum1 to associate with the promoter of CDA2 was unaltered in the absence of KlSir2 (Figure 5D), and was reduced, but not abolished, in the absence of KlRfm1 (Figure 5E). Thus, KlRfm1 contributes to the ability of the SUM1 complex to associate with DNA. We conclude that the promoter-specific mechanism by which the SUM1 complex represses mid-sporulation genes is conserved between K. lactis and S. cerevisiae.
KlSum1 and KlSir2 also repress cell-type–specific genes
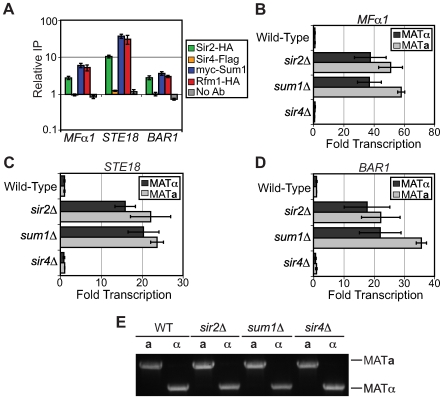
The KlSum1-KlSir2 complex is clearly critical to the regulation of sexual identity and the sexual cycle as it represses both the HM loci and sporulation genes. However, the Sum1-Sir2 complex may have an even broader role in controlling sexual identity. It has recently been shown in both Saccharomyces bayanus and S. cerevisiae that Sum1 represses α-specific genes [24]. To investigate whether the Sum1-Sir2 complex in K. lactis also represses α-specific genes or other cell-type specific genes, we examined whether promoters of cell-type specific genes were associated with KlSir2. Remarkably some, but not all, α-specific, a-specific and haploid-specific genes were associated with KlSir2 (Figure 6A and data not shown). For example, the α-specific gene MFα1, the a-specific gene BAR1, and the haploid-specific gene STE18 were associated with KlSir2, KlSum1, and KlRfm1, but not KlSir4 (Figure 6A).
Figure 6. KlSir2 and KlSum1 repress cell-type–specific genes.
(A) Association of KlSir2-HA, KlSir4-Flag, myc-KlSum1 (LRY2285) and KlRfm1-HA (LRY2328) at the MFα1 (KLLA0E19173g), STE18 (KLLA0E06138g) and BAR1 (KLLA0D15917g) promoters in a MATα strain as assessed by chromatin IP followed by quantitative PCR. The y-axis is a log-scale. (B) Quantitative RT–PCR analysis of MFα1 mRNA in MATα wild-type (SAY538), sir2Δ (SAY544), sum1Δ (LRY1947), and sir4Δ (LRY1946) strains and MAT a wild-type (CK213), sir2Δ (SAY569), sum1Δ (LRY2035) and sir4Δ (LRY2038) strains. (C) Quantitative RT-PCR analysis of STE18 mRNA in the same strains analyzed in (B). (D) Quantitative RT-PCR analysis of BAR1 mRNA in the same strains analyzed in panel B. Error bars represent the SEM. (E) PCR amplification of MAT loci in strains analyzed in (B–D) using mating-type specific primers.
To determine whether the Sum1-Sir2 complex represses these genes, RNA was isolated from both MAT a and MATα cells and expression of MFα1, STE18, and BAR1 was examined by quantitative RT-PCR. MFα1 encodes α-pheromone and in S. cerevisiae is expressed in MATα cells but not in MAT a cells. However in K. lactis, deletion of KlSum1 or KlSir2 resulted in the derepression of MFα1 in both cell types to a comparable extent (Figure 6B). Quantification of cDNA from wild-type cells revealed that MFα1 was repressed to a similar degree in both MAT a and MATα cells (Figure S6). These findings suggest that during vegetative growth, haploid K. lactis cells are not transcribing or producing α-pheromone, regardless of their mating-type identity, and that the Sum1-Sir2 complex contributes to the repression of this gene.
STE18 encodes the G protein gamma subunit in the mating signaling pathway and in S. cerevisiae is expressed in both MATα and MAT a haploid cells. In K. lactis, STE18, like MFα1, was repressed in both MATα and MAT a cells (Figure S6), and deletion of either KlSir2 or KlSum1 resulted in derepression of STE18 in both cell types (Figure 6C). BAR1 encodes an α-pheromone protease that in S. cerevisiae is expressed to a greater extent in MAT a than MATα cells. This pattern of gene expression was also found in K. lactis (Figure S6). However, as for MFα1 and STE18, deletion of KlSum1 or KlSir2 resulted in the derepression of BAR1 in both MAT a and MATα cells (Figure 6D). To verify that we had correctly identified the mating-type of the strains used for these experiments, we analyzed a segment of the MAT locus using mating-type specific PCR primers that yield different sized products in MAT a and MATα strains. All strains had the expected genotypes (Figure 6E). Together, these results suggest that the KlSum1-KlSir2 complex represses a variety of cell-type specific genes as well as mid-sporulation genes and the HM loci. Therefore, this complex represents an important regulator of yeast sexual identity and activity.
Discussion
The Sum1-Sir2 complex employs multiple mechanisms to repress transcription
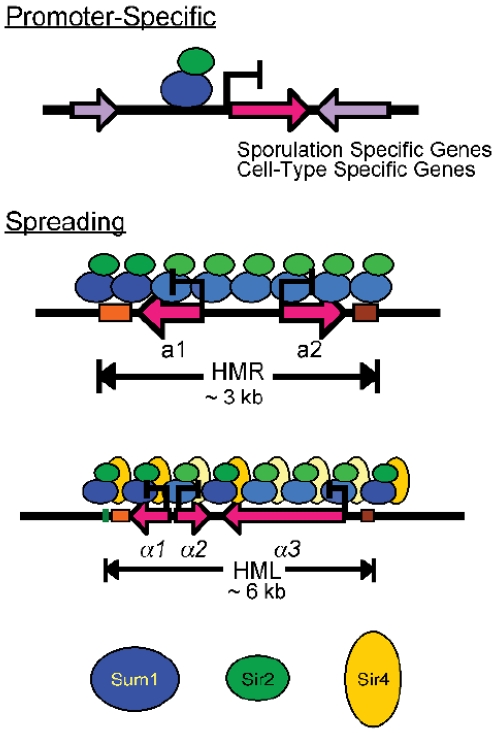
This study has made the striking discovery that the Sum1-Sir2 complex in K. lactis achieves repression through several distinct mechanisms (Figure 7). In S. cerevisiae, the Sum1-Hst1 complex functions primarily as a promoter-specific repressor of mid-sporulation, α-specific, and NAD+-biosynthetic genes, and loss of ScSum1 or ScHst1 do not alter the expression of the HM loci [6],[33]. In contrast, in K. lactis, the Sum1-Sir2 complex not only uses a promoter-specific mechanism to repress the same sets of genes as in S. cerevisiae (Figure 7, top panel), it also has a major role in silencing the HM loci by forming extended chromatin structures (Figure 7, middle and lower panels).
Figure 7. Mechanisms of repression mediated by KlSum1-KlSir2.
The KlSum1-KlSir2 complex participates in multiple mechanisms of repression: a promoter-specific mechanism that represses mid-sporulation and cell-type specific genes (top) as well as a long-range spreading mechanism that silences the cryptic mating-type loci either with Sir4 (HMLα; bottom) or without Sir4 (HMR a; middle). The darker shaded proteins represent stronger association than the lighter colored proteins.
Interestingly, the KlSum1-KlSir2 complex acts differently at HMLα (lower panel), where it works in conjunction with KlSir4, compared to HMR a (middle panel), where KlSir4 is not normally present. Thus, the mechanism by which HMR a is silenced is unlike the mechanism employed at HMLα. The absence of KlSir4 at HMR a is surprising, as the spreading of silencing proteins is thought to require a histone-binding protein, such as KlSir4, and neither KlSum1 nor KlSir2 is known to have this capacity. An important subject for future studies will be to determine how the spreading capacity of the KlSum1-KlSir2 complex is modulated at different genomic locations. It is possible that factors associated with the HM loci promote the spreading of KlSum1-KlSir2. For example, silencers may recruit additional proteins that facilitate the spreading process. We have recently found that the HMR-E silencer in S. cerevisiae can promote the assembly of silenced chromatin through a mechanism that is independent of recruitment [36], and it is possible that silencers in K. lactis have similar properties. Alternatively, factors associated with the promoters of mid-sporulation genes may limit or disable the spreading of KlSum1-KlSir2.
This study also revealed that, although the KlSum1-KlSir2 and KlSir4-KlSir2 complexes cooperate at HMLα, they have distinct contributions to chromatin assembly and transcriptional repression. For example, the KlSir4-KlSir2 complex was critical for assembly of silencing proteins on the telomere proximal side of HMLα. However, silenced chromatin on the centromere-proximal side did not depend on KlSir2 or KlSir4, but was affected by the loss of KlSum1. These results suggest that the chromatin structure differs on the two sides of HMLα, perhaps due to different types of silencer elements. Another indication that the KlSum1-KlSir2 and KlSir4-KlSir2 complexes have independent properties is the observation that the associations of KlSir4 and KlSir2 increased at HMLα and HMR a in the absence of KlRfm1. This result suggests that KlSir4 and KlRfm1 may compete for association with KlSir2.
Association of silencing factors does not correlate with transcriptional activity at HMLα
One puzzling observation was that the absence of KlSir4 resulted in a relatively modest induction of the HMLα1 and HMLα2 genes despite a significant decrease in the associations of both KlSir2 and KlSum1 with the α1–α2 promoter. Conversely, the absence of KlSum1 resulted in a large increase of transcriptional activity yet had seemingly little effect on the associations of KlSir2 and KlSir4 with HMLα. These results are reminiscent of observations that, in some situations, Sir proteins in S. cerevisiae associate with HM loci but do not achieve repression [37],[38]. We speculate that the presence of the KlSum1-KlSir2 complex at HMLα is more critical for repression than is the presence of KlSir4. Moreover, KlSum1 and KlSir2 must be able to achieve repression over a distance, because their presence at the HMLα3 promoter is sufficient to repress the HMLα1 and HMLα2 genes. Similarly, KlSum1 and KlSir2 may act at distance at HMR a, as their greatest enrichment is some distance from the promoter. In contrast, the KlSir4-KlSir2 complex appears to be somewhat permissive to transcription in the absence of KlSum1. Perhaps this chromatin structure serves another biological function, such as preventing illegitimate mating-type switching. While K. lactis is considered to be a homothallic yeast species [39], an ortholog of the HO endonuclease, which initiates switching in S. cerevisiae, has not been identified [40], and mating-type switching presumably occurs through spontaneous homologous recombination. These switching events are relatively rare had have not been studied recently [39].
SIR2 and HST1 subfunctionalized after duplication
This study was initiated to investigate how the deacetylases SIR2 and HST1 diverged after duplication. Two models, subfunctionalization and neofunctionalization, have been proposed to explain how duplicated genes diverge. We used the non-duplicated KlSir2 as a proxy for the ancestral protein and found that it interacted with both KlSir4 and KlSum1 (Figure 1), the partners of ScSir2 and ScHst1, respectively. Furthermore, KlSir2 functioned as a promoter-specific repressor of sporulation genes (similar to ScHst1; Figure 5) and also as a silencing factor that spreads across the HM loci (similar to ScSir2; Figure 2, Figure 3, Figure 4). Therefore, KlSir2 has both Hst1- and Sir2-like functions. The most parsimonious interpretation of these results is that the ancestral deacetylase also had both functions and that subfunctionalization occurred after duplication. This conclusion is supported by the observation that ScSir2 has retained the ability to substitute for ScHst1 in its absence [26]. This is an important contribution to the understanding of the evolution of duplicated genes, as it provides an example of subfunctionalization of protein-protein interactions as opposed to partitioning of expression patterns, which have previously been documented [41].
Previous work provides insight into how the subfunctionalization of SIR2 and HST1 occurred. A chimeric protein consisting of the N-terminus of ScSir2 and the C-terminus of ScHst1 has both Sir2- and Hst1-like functions in S. cerevisiae [26],[42]. This observation suggests that different regions of the deacetylases are important for specifying interactions with the SIR and SUM1 complexes. It is likely that the ancestral deacetylase used these same domains to interact with the SIR and SUM1 complexes. After SIR2 was duplicated, the two copies likely acquired mutations that reduced their affinities for either the SIR or SUM1 complexes, leading to subfunctionalization.
Over the course of evolution it was not simply the deacetylase that subfunctionalized. The proteins associated with Sir2 and Hst1 are used in different ways to achieve repression of essentially the same sets of genes in S. cerevisiae and K. lactis. Other studies have revealed changes in the transcriptional regulatory circuits of yeasts [13],[43],[44]. However in previous examples, evidence suggested that promoter elements have changed to bring genes under the control of different regulators or alter their expression patterns. This study expands the scope of adaptations that can lead to modifications in transcriptional networks, as it reveals that the molecular mechanisms by which regulatory proteins act can also change over evolutionary time.
Evolution of SIR4 and ASF2 genes
In addition to the paralogs SIR2 and HST1, we investigated a second duplicated gene pair, SIR4 and ASF2. SIR4 and ASF2 were tandemly duplicated prior to the whole genome duplication and to the divergence of Kluyveromyces and Saccharomyces species. Due to their tandem arrangement and rapid rate of sequence change, it has been difficult to determine which gene is the ortholog of ScSIR4 or ScASF2. Functional analysis shows that KLLA0F14320g silences HMLα (Figure 2, Figure 3, and [31]) as thus has a Sir4-like function , whereas KLLA0F13998g antagonizes silencing at HMLα (Figure S2) and thus has Asf2-like function. This experimental evidence seems to contradict phylogenetic analyses implying that KLLA0F13998g is the ortholog of ScSIR4, as it clusters with SIR4 genes from other yeast species, and that KLLA0F13420g is an ortholog of ScASF2, as it clusters with ASF2 genes as well as SIR4 genes from Candida glabrata, S. castellii, S. kluyveri and Ashbya gossypii (Figure S1 and [7]). However, this gene tree does not match the species phylogeny, perhaps due to the rapid rate of sequence change and consequently may not accurately reflect the evolutionary relationships among these genes.
The SUM1-1 mutation in S. cerevisiae
The observation that KlSum1 spreads at the HM loci provides a new perspective on the perplexing SUM1-1 mutation identified in S. cerevisiae. This mutation was originally isolated as a suppressor of a sir2Δ mutation [45] and results from a single point mutation, T988I. It causes Sum1 to re-localize from mid-sporulation promoters to the HM loci and form an extended chromatin structure [46],[47]. It had been thought that the SUM1-1 mutation is a gain-of-function mutation that creates the ability to spread de novo, and it was surprising that a single amino acid change could have such a profound effect. However, this study suggests a new interpretation. The ability of both KlSum1 and ScSum1-1 to spread at HM loci suggests that the ancestral Sum1 also had this ability, which was subsequently lost in the Saccharomyces lineage. Consequently, wild-type ScSum1 probably retains most of the properties necessary to spread, and the T988I mutation unmasks this hidden potential.
Our knowledge of the mechanism of the SUM1-1 mutation may provide insights into how the spreading of KlSum1 is controlled. Residue T988 of ScSum1 is conserved in KlSum1, as well as in many other budding yeasts, and is located in the DNA-binding domain. Mutating this residue reduces the affinity of Sum1 for DNA [48] and replacing threonine 988 with isoleucine enables the protein to associate with new partners - ORC (the Origin Recognition Complex) and itself [47],[48],[49]. These observations led to the hypothesis that the SUM1-1 mutation occurs in an interaction domain, and the switch between threonine and isoleucine causes the protein to interact with different partners [48]. Perhaps this domain of KlSum1 also has the capacity to interact with multiple partners, and the genomic context dictates whether this surface functions as a DNA-binding domain to recruit the Sum1-Sir2 complex to mid-sporulation genes or as a self-associating surface to enable KlSum1 to propagate along the chromatin at the HM loci.
The Sum1-Sir complex as a master regulator of the yeast sexual cycle
The K. lactis Sum1-Sir2 complex plays a critical role as a regulator of sexual identity because it regulates some cell-type specific genes (Figure 6). Within budding yeasts there has been a transition from positive to negative regulation of a-specific genes. Candida albicans requires an activator to turn on a-specific genes in MAT a cells, whereas in S. cerevisiae, a-specific genes are on by default and must be turned off in MATα cells [50]. K. lactis has been proposed to have an intermediate circuitry in regulating cell-type identity [43], as a-specific gene promoters share features of both C. albicans and S. cerevisiae promoters. In this study we have demonstrated that many cell-type specific genes, including a- and α-specific genes are repressed by the KlSum1-KlSir2 complex in both haploid cell types providing an additional level of regulation to sexual identity.
Differences between the life cycles of K. lactis and S. cerevisiae may heighten the importance of the Sum1-Sir2 complex in K. lactis. Vegetative growth of K. lactis occurs predominantly in the haploid phase, and mating occurs in response to nutrient deprivation, leading almost immediately to sporulation [39],[51],[52]. In contrast, S. cerevisiae propagates primarily in the diploid phase. Mating occurs shortly after germination in rich nutrient conditions, but sporulation of the resulting diploid cells is delayed until nutrients become scarce. Thus, unlike S. cerevisiae, K. lactis requires a mechanism to suppress mating of haploid cells under nutrient-rich conditions, and perhaps the Sum1-Sir2 complex contributes to this regulation by repressing some of the α-specific, a-specific, and haploid-specific genes required for mating. The use of a repressive complex containing an NAD+-dependent deacetylase may help connect the sexual cycle of K. lactis with nutrient availability.
Materials and Methods
Yeast media and methods
All K. lactis strains used in this study were grown at 30° in YPD medium containing 1% yeast extract, 2% peptone and 2% glucose. Antibiotic supplements were added to YPD medium at 50 µg/ml of clonNAT and 200 µg/ml of geneticin. Electroporation conditions were as described [53] with the following changes. Cells were washed with LiAc buffer (10 mM Tris pH 7.5, 270 mM sucrose, 1 mM lithium acetate) after initial centrifugation. After treatment with the pre-treating buffer (YPD, 20 mM HEPES pH 8.0, 25 mM DTT), cells were resuspended in LiAc buffer to a final concentration of 2×109 cells/ml and electroporation was performed in a 0.2 cm cuvette, with a final at volume between 50 and 55 µl. The settings for electroporation were 1,000 V, 25 µF and 300 Ω. Cells transformed with antibiotic resistance markers were grown at 30° in YPD for 3–5 hours before being plated on selective medium.
Mating was carried out by mixing equal volumes of overnight cultures of the two parental strains, plating 4–10 µl on malt extract (ME) medium (2% malt extract, 2% agar) and incubating at 30° for 2–3 days. Cells were then streaked on media to select for diploids and subsequently transferred to ME plates for sporulation. After 3–4 days, the sporulated culture was suspended in 500 µl water, incubated at 56° for 15 minutes, and plated on media to select for alleles of interest. Genotypes were confirmed by PCR.
Yeast strains
Strains used in this study were derived from SAY538 (Table S1). The sir2Δ::KanMX allele was obtained from S. Astrom. The sir2Δ::NatMX, sir4Δ::URA3, asf2Δ::NatMX, sir4Δ asf2Δ::URA3, sum1Δ::NatMX and rfm1Δ::URA3 alleles were complete deletions of the open reading frames generated by one-step gene replacement. The replacement markers NatMX and URA3 were derived from pAGT100 [54] and pRS316 [55], respectively. The HMLa allele was a fortuitous gene conversion event that occurred during the course of crossing a sir2Δ strain. The SIR2-HA, RFM1-HA and SIR4-Flag alleles were constructed by integrating the tag plus a selectable marker at the end of the open reading frame. Tagging cassettes were generated from pAGT105 [54] containing the HA-epitope tag along with the entire open reading frame of NatMX or p3FLAG-KanMX, [56] containing the Flag tag plus KanMX. The myc-SUM1 allele was generated in two steps. First, a myc-URA3-myc-SUM1 construct, derived from p3MPY-3xMyc, [57] was integrated into the K. lactis genome. After correct integration was confirmed by PCR, cells were grown in non-selective media to allow for recombination between the identical myc-tags and cells were plated on 5-FOA to select for the loss of the URA3 marker. In all cases, the correct integration was confirmed by PCR using primers flanking the sites of recombination. To confirm that the tagged proteins were functional, expression of genes regulated by these factors was examined by quantitative RT-PCR. Alleles were moved into various genetic backgrounds (as described in Table S1) through genetic crosses.
Gene expression analysis
RNA was isolated from logarithmically growing cultures of each strain using a hot phenol method [58]. Removal of DNA was as previously described [26]. To verify that there was no contaminating DNA, 1 µl of DNAse-treated material was used in a PCR reaction containing primers to amplify the KlACT1 transcript. 1 µg of DNA-free RNA was used for cDNA synthesis as previously described [26]. To quantify the relative amounts of mRNA transcripts, approximately 0.025 µg of cDNA was analyzed by real-time PCR in the presence of SYBR Green using a Bio-Rad iCycler. The standard curve was generated with genomic DNA isolated from the wild-type strain (SAY538). Oligonucleotide sequences are provided in Table S2. Data were analyzed with iCycler iQ Optical System Software. Transcript levels of queried genes were first normalized to the KlACT1 mRNA for each genetic background. The fold-induction was calculated by normalizing to the wild-type strain. Results represent the average fold induction (relative to wild-type) of at least two independent cultures of each strain background. The standard error measurement (SEM) was calculated from the differences in fold induction of two or more independent cultures from the mean.
Chromatin immunoprecipitation
Chromatin immunoprecipitation was performed by harvesting approximately 50 OD (7×108) of logarithmically growing cells, collected at an OD600 = 1.4. Cells were collected, washed twice in PBS, re-suspended in DMA (10 mM dimethyl adipimidate, 0.1% DMSO, 1× PBS) and rocked at room temperature for 60 minutes to crosslink. Subsequent to crosslinking, cells were washed twice with PBS, re-suspended in 36 ml PBS and rocked with 1% formaldehyde at room temperature for 60 minutes. The preparation of soluble chromatin and immunoprecipitation was performed as previously described [26]. Chromatin IP samples were analyzed by qPCR using a standard curve prepared from input DNA. The amounts of the immunoprecipitated DNA at experimental loci and a control locus, KlRRP7, were determined relative to the input DNA, and the relative enrichment of the experimental loci compared to the control locus was calculated. Oligonucleotide sequences are provided in Figure S7 and Table S3. Results represent the relative immunoprecipitation of two or more independent cultures of each strain background, and the SEM was calculated from differences in the relative enrichment from the mean. No antibody control data represent the average values from multiple chromatin IP experiments using different strains.
Co-immunoprecipitations
Co-immunoprecipitations were performed by harvesting approximately 30 OD (4.2×108) of logarithmically growing cells. The preparation of whole-cell lysates was performed as previously described [26]. Whole-cell lysates were incubated overnight at 4°C with 5 µl of α-HA (Sigma H-6908), α-Flag (Sigma F-7425) or α-myc (Millipore 06-549) antibody. Subsequently, 60 µl of Protein A agarose beads were added and samples were rotated at 4° overnight and protein was eluted in 75 µl 3× protein sample buffer (30% glycerol, 15% β-mercaptoethanol, 0.006% bromophenol blue, 0.1875 M Tris pH 6.8) for 3 minutes at 95°. 20 µl of IP samples and 7.5 µl of whole-cell extracts were electrophoretically fractionated on 7.5% polyacrylamide-SDS gels, transferred to nitro-cellulose membranes, and probed with either mouse polyclonal α-HA antibody (Sigma H-3663), mouse polyclonal α-myc antibody (Calbiochem OP10), rabbit (Sigma F-7425) or mouse (Sigma F-3165) α-Flag antibodies and detected by chemiluminescence (GE RPN2135).
Supporting Information
Phylogenetic gene tree of SIR4 and ASF2 orthologs from several hemiascomycete species. Sequences and nomenclature were obtained from the Yeast Gene Order Browser (YGOB) (Byrne and Wolfe 2005) and analyzed using MEGA (Tamura et al 2007) to construct the neighbor joining gene tree. Bold, red font indicate species that underwent the whole genome duplication. Sc = S. cerevisiae, Sb = S. bayanus, Cg = C. glabrata, Scas = S. castelli, Kp = K. polysporus, Zr = Z. rouxii, Ag = A. gossypii, Sk = S. kluyveri, Kt = K. thermotolerans, Kw = Kwaltii. Common names, as notated in the YGOB, are given along with the systematic names in parantheses. K.lactis common gene names are not given to illustrate how KLLA0F1430g and KLLA0F13998g cluster.
(0.89 MB EPS)
Quantitative RT–PCR analysis of HMLα1, HMLα2, and HMLα3 mRNA in wild-type (CK213), sir2Δ (SAY569), sir4Δ (LRY2038), asf2Δ (LRY2377), asf2Δ sir4Δ (LRY2374), and sir2Δ asf2Δ (LRY2523) strains. The amount of cDNA was first normalized to the control locus ACT1. The values shown here represent the relative amount of cDNA for each deletion strain compared to the wild-type strain. The data for wild-type sir2Δ and sir4Δ strains is the same as Figure 2. Error bars represent the SEM.
(0.65 MB EPS)
The association of myc-KlSum1 with HMLα as assessed by chromatin IP followed by quantitative PCR in a sir2Δ sir4Δ strain (LRY2530). The y-axis represents the relative enrichment normalized to a control locus, RRP7, which is not detectably associated with KlSir2, KlSir4 or KlSum1. Error bars represent the SEM. A schematic of the HMLα locus is shown under the x-axis.
(0.97 MB EPS)
Quantitative RT–PCR analysis of HMRa1 and HMRa2 in wild-type (SAY538), sir4Δ (LRY1946), asf2Δ (LRY1856), and asf2Δ sir4Δ (LRY1948) strains. The amount of cDNA was first normalized to the control locus ACT1. The values shown here represent the relative amount of cDNA for each deletion strain compared to the wild-type strain. The data for wild-type and sir4Δ strains is the same as Figure 4. Error bars represent the SEM.
(0.61 MB EPS)
(A) Quantitative RT–PCR analysis of CDA2, SPS4, SPR3, and SPS2 mRNA in wild-type (SAY538) and asf2Δ (LRY1856) strains. The amount of cDNA was first normalized to the control locus ACT1. The values shown here represent the relative amount of cDNA for each deletion strain compared to the wild-type strain. The data for the wild-type strain is the same as Figure 5. (B) Association of KlSir2-HA and myc-KlSum1 with CDA2 as assess by chromatin IP followed by quantitative PCR in an asf2Δ strain (LRY2525). Error bars represent the SEM.
(1.00 MB EPS)
Quantitative RT–PCR analysis of the cryptic mating-type loci, mid-sporulation genes and cell-type–specific genes in wild-type strains of MATa and MATα cells. The amount of cDNA was normalized to ACT1.
(0.61 MB EPS)
Schematics of HMLα, MATα, HMRa, and CDA2 with primer sets shown for chromatin IP quantitative PCR.
(2.82 MB EPS)
K. lactis strains used in this study.
(0.03 MB DOC)
Oligonucleotides used for quantitative RT–PCR.
(0.02 MB XLS)
Oligonucleotides used for quantitative chromatin IP.
(0.05 MB XLS)
Acknowledgments
We thank P. Lynch, K. Scott, B. Sullivan, D. Des Marias, K. Wolfe, O. Zill, and an anonymous reviewer for critical reading of this manuscript; D. MacAlpine for technical assistance; and S. Astrom for K. lactis strains and plasmids.
Footnotes
The authors have declared that no competing interests exist.
This research was supported by a grant by the National Institute of Health (GM073991) to LNR. The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
References
- 1.Longo VD, Kennedy BK. Sirtuins in aging and age-related disease. Cell. 2006;126:257–268. doi: 10.1016/j.cell.2006.07.002. [DOI] [PubMed] [Google Scholar]
- 2.Denu JM. The Sir 2 family of protein deacetylases. Curr Opin Chem Biol. 2005;9:431–440. doi: 10.1016/j.cbpa.2005.08.010. [DOI] [PubMed] [Google Scholar]
- 3.Buck SW, Gallo CM, Smith JS. Diversity in the Sir2 family of protein deacetylases. J Leukoc Biol. 2004;75:939–950. doi: 10.1189/jlb.0903424. [DOI] [PubMed] [Google Scholar]
- 4.Verdin E, Dequiedt F, Fischle W, Frye R, Marshall B, et al. Measurement of mammalian histone deacetylase activity. Methods Enzymol. 2004;377:180–196. doi: 10.1016/S0076-6879(03)77010-4. [DOI] [PubMed] [Google Scholar]
- 5.Brachmann CB, Sherman JM, Devine SE, Cameron EE, Pillus L, et al. The SIR2 gene family, conserved from bacteria to humans, functions in silencing, cell cycle progression, and chromosome stability. Genes Dev. 1995;9:2888–2902. doi: 10.1101/gad.9.23.2888. [DOI] [PubMed] [Google Scholar]
- 6.Derbyshire MK, Weinstock KG, Strathern JN. HST1, a new member of the SIR2 family of genes. Yeast. 1996;12:631–640. doi: 10.1002/(SICI)1097-0061(19960615)12:7%3C631::AID-YEA960%3E3.0.CO;2-8. [DOI] [PubMed] [Google Scholar]
- 7.Byrne KP, Wolfe KH. The Yeast Gene Order Browser: combining curated homology and syntenic context reveals gene fate in polyploid species. Genome Res. 2005;15:1456–1461. doi: 10.1101/gr.3672305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Dietrich FS, Voegeli S, Brachat S, Lerch A, Gates K, et al. The Ashbya gossypii genome as a tool for mapping the ancient Saccharomyces cerevisiae genome. Science. 2004;304:304–307. doi: 10.1126/science.1095781. [DOI] [PubMed] [Google Scholar]
- 9.Kellis M, Birren BW, Lander ES. Proof and evolutionary analysis of ancient genome duplication in the yeast Saccharomyces cerevisiae. Nature. 2004;428:617–624. doi: 10.1038/nature02424. [DOI] [PubMed] [Google Scholar]
- 10.Ohno S. Evolution by Gene Duplication. New York: Springer-Verlag; 1970. 160 [Google Scholar]
- 11.Force A, Lynch M, Pickett FB, Amores A, Yan YL, et al. Preservation of duplicate genes by complementary, degenerative mutations. Genetics. 1999;151:1531–1545. doi: 10.1093/genetics/151.4.1531. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.van Hoof A. Conserved functions of yeast genes support the duplication, degeneration and complementation model for gene duplication. Genetics. 2005;171:1455–1461. doi: 10.1534/genetics.105.044057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Hittinger CT, Carroll SB. Gene duplication and the adaptive evolution of a classic genetic switch. Nature. 2007;449:677–681. doi: 10.1038/nature06151. [DOI] [PubMed] [Google Scholar]
- 14.Des Marais DL, Rausher MD. Escape from adaptive conflict after duplication in an anthocyanin pathway gene. Nature. 2008;454:762–765. doi: 10.1038/nature07092. [DOI] [PubMed] [Google Scholar]
- 15.Bridgham JT, Carroll SM, Thornton JW. Evolution of hormone-receptor complexity by molecular exploitation. Science. 2006;312:97–101. doi: 10.1681/01.asn.0000926836.46869.e5. [DOI] [PubMed] [Google Scholar]
- 16.Wolfe KH, Shields DC. Molecular evidence for an ancient duplication of the entire yeast genome. Nature. 1997;387:708–713. doi: 10.1038/42711. [DOI] [PubMed] [Google Scholar]
- 17.Rusche LN, Kirchmaier AL, Rine J. The establishment, inheritance, and function of silenced chromatin in Saccharomyces cerevisiae. Annu Rev Biochem. 2003;72:481–516. doi: 10.1146/annurev.biochem.72.121801.161547. [DOI] [PubMed] [Google Scholar]
- 18.Hoppe GJ, Tanny JC, Rudner AD, Gerber SA, Danaie S, et al. Steps in assembly of silent chromatin in yeast: Sir3-independent binding of a Sir2/Sir4 complex to silencers and role for Sir2-dependent deacetylation. Mol Cell Biol. 2002;22:4167–4180. doi: 10.1128/MCB.22.12.4167-4180.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Luo K, Vega-Palas MA, Grunstein M. Rap1-Sir4 binding independent of other Sir, yKu, or histone interactions initiates the assembly of telomeric heterochromatin in yeast. Genes Dev. 2002;16:1528–1539. doi: 10.1101/gad.988802. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Rusche LN, Kirchmaier AL, Rine J. Ordered nucleation and spreading of silenced chromatin in Saccharomyces cerevisiae. Mol Biol Cell. 2002;13:2207–2222. doi: 10.1091/mbc.E02-03-0175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Bedalov A, Hirao M, Posakony J, Nelson M, Simon JA. NAD+-dependent deacetylase Hst1p controls biosynthesis and cellular NAD+ levels in Saccharomyces cerevisiae. Mol Cell Biol. 2003;23:7044–7054. doi: 10.1128/MCB.23.19.7044-7054.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.McCord R, Pierce M, Xie J, Wonkatal S, Mickel C, et al. Rfm1, a novel tethering factor required to recruit the Hst1 histone deacetylase for repression of middle sporulation genes. Mol Cell Biol. 2003;23:2009–2016. doi: 10.1128/MCB.23.6.2009-2016.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Xie J, Pierce M, Gailus-Durner V, Wagner M, Winter E, et al. Sum1 and Hst1 repress middle sporulation-specific gene expression during mitosis in Saccharomyces cerevisiae. EMBO J. 1999;18:6448–6454. doi: 10.1093/emboj/18.22.6448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Zill OA, Rine J. Interspecies variation reveals a conserved repressor of alpha-specific genes in Saccharomyces yeasts. Genes Dev. 2008;22:1704–1716. doi: 10.1101/gad.1640008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Pierce M, Benjamin KR, Montano SP, Georgiadis MM, Winter E, et al. Sum1 and Ndt80 proteins compete for binding to middle sporulation element sequences that control meiotic gene expression. Mol Cell Biol. 2003;23:4814–4825. doi: 10.1128/MCB.23.14.4814-4825.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Hickman MA, Rusche LN. Substitution as a mechanism for genetic robustness: the duplicated deacetylases Hst1p and Sir2p in Saccharomyces cerevisiae. PLoS Genet. 2007;3:e126. doi: 10.1371/journal.pgen.0030126. doi: 10.1371/journal.pgen.0030126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Robert F, Pokholok DK, Hannett NM, Rinaldi NJ, Chandy M, et al. Global position and recruitment of HATs and HDACs in the yeast genome. Mol Cell. 2004;16:199–209. doi: 10.1016/j.molcel.2004.09.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Fraser JA, Heitman J. Fungal mating-type loci. Curr Biol. 2003;13:R792–795. doi: 10.1016/j.cub.2003.09.046. [DOI] [PubMed] [Google Scholar]
- 29.Chen XJ, Clark-Walker GD. sir2 mutants of Kluyveromyces lactis are hypersensitive to DNA-targeting drugs. Mol Cell Biol. 1994;14:4501–4508. doi: 10.1128/mcb.14.7.4501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Astrom SU, Kegel A, Sjostrand JO, Rine J. Kluyveromyces lactis Sir2p regulates cation sensitivity and maintains a specialized chromatin structure at the cryptic alpha-locus. Genetics. 2000;156:81–91. doi: 10.1093/genetics/156.1.81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Astrom SU, Rine J. Theme and variation among silencing proteins in Saccharomyces cerevisiae and Kluyveromyces lactis. Genetics. 1998;148:1021–1029. doi: 10.1093/genetics/148.3.1021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Sjostrand JO, Kegel A, Astrom SU. Functional diversity of silencers in budding yeasts. Eukaryot Cell. 2002;1:548–557. doi: 10.1128/EC.1.4.548-557.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Chi MH, Shore D. SUM1-1, a dominant suppressor of SIR mutations in Saccharomyces cerevisiae, increases transcriptional silencing at telomeres and HM mating-type loci and decreases chromosome stability. Mol Cell Biol. 1996;16:4281–4294. doi: 10.1128/mcb.16.8.4281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Babu MM, Iyer LM, Balaji S, Aravind L. The natural history of the WRKY-GCM1 zinc fingers and the relationship between transcription factors and transposons. Nucleic Acids Res. 2006;34:6505–6520. doi: 10.1093/nar/gkl888. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Le S, Davis C, Konopka JB, Sternglanz R. Two new S-phase-specific genes from Saccharomyces cerevisiae. Yeast. 1997;13:1029–1042. doi: 10.1002/(SICI)1097-0061(19970915)13:11<1029::AID-YEA160>3.0.CO;2-1. [DOI] [PubMed] [Google Scholar]
- 36.Lynch PJ, Rusche LN. A silencer promotes the assembly of silenced chromatin independently of recruitment. Mol Cell Biol. 2009;29:43–56. doi: 10.1128/MCB.00983-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Kirchmaier AL, Rine J. Cell cycle requirements in assembling silent chromatin in Saccharomyces cerevisiae. Mol Cell Biol. 2006;26:852–862. doi: 10.1128/MCB.26.3.852-862.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Lau A, Blitzblau H, Bell SP. Cell-cycle control of the establishment of mating-type silencing in S. cerevisiae. Genes Dev. 2002;16:2935–2945. doi: 10.1101/gad.764102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Herman A, Roman H. Allele specific determinants of homothallism in Saccharomyces lactis. Genetics. 1966;53:727–740. doi: 10.1093/genetics/53.4.727. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Butler G, Kenny C, Fagan A, Kurischko C, Gaillardin C, et al. Evolution of the MAT locus and its Ho endonuclease in yeast species. Proc Natl Acad Sci U S A. 2004;101:1632–1637. doi: 10.1073/pnas.0304170101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Lynch M, Force A. The probability of duplicate gene preservation by subfunctionalization. Genetics. 2000;154:459–473. doi: 10.1093/genetics/154.1.459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Mead J, McCord R, Youngster L, Sharma M, Gartenberg MR, et al. Swapping the gene-specific and regional silencing specificities of the Hst1 and Sir2 histone deacetylases. Mol Cell Biol. 2007;27:2466–2475. doi: 10.1128/MCB.01641-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Tsong AE, Tuch BB, Li H, Johnson AD. Evolution of alternative transcriptional circuits with identical logic. Nature. 2006;443:415–420. doi: 10.1038/nature05099. [DOI] [PubMed] [Google Scholar]
- 44.Wong S, Wolfe KH. Birth of a metabolic gene cluster in yeast by adaptive gene relocation. Nat Genet. 2005;37:777–782. doi: 10.1038/ng1584. [DOI] [PubMed] [Google Scholar]
- 45.Klar AJ, Kakar SN, Ivy JM, Hicks JB, Livi GP, et al. SUM1, an apparent positive regulator of the cryptic mating-type loci in Saccharomyces cerevisiae. Genetics. 1985;111:745–758. doi: 10.1093/genetics/111.4.745. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Rusche LN, Rine J. Conversion of a gene-specific repressor to a regional silencer. Genes Dev. 2001;15:955–967. doi: 10.1101/gad.873601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Sutton A, Heller RC, Landry J, Choy JS, Sirko A, et al. A novel form of transcriptional silencing by Sum1-1 requires Hst1 and the origin recognition complex. Mol Cell Biol. 2001;21:3514–3522. doi: 10.1128/MCB.21.10.3514-3522.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Safi A, Wallace KA, Rusche LN. Evolution of new function through a single amino acid change in the yeast repressor Sum1p. Mol Cell Biol. 2008;28:2567–2578. doi: 10.1128/MCB.01785-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Lynch PJ, Fraser HB, Sevastopoulos E, Rine J, Rusche LN. Sum1p, the origin recognition complex, and the spreading of a promoter-specific repressor in Saccharomyces cerevisiae. Mol Cell Biol. 2005;25:5920–5932. doi: 10.1128/MCB.25.14.5920-5932.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Tsong AE, Miller MG, Raisner RM, Johnson AD. Evolution of a combinatorial transcriptional circuit: a case study in yeasts. Cell. 2003;115:389–399. doi: 10.1016/s0092-8674(03)00885-7. [DOI] [PubMed] [Google Scholar]
- 51.Herman A, Halvorson H. Genetic Control of Beta-Glucosidase Synthesis in Saccharomyces Lactis. J Bacteriol. 1963;85:901–910. doi: 10.1128/jb.85.4.901-910.1963. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Wesolowski-Louvel M, Beunig, Karin D, Fukuhara, Hiroshi . Kluyveromyces lactis. In: Wolf K, editor. Nonconventional yeasts in Biotechnology: A Handbook. Berlin, Heidelberg, New York: Springer-Verlag; 1996. pp. 139–201. [Google Scholar]
- 53.Sanchez M, Iglesias FJ, Santamaria C, Dominguez A. Transformation of Kluyveromyces lactis by Electroporation. Appl Environ Microbiol. 1993;59:2087–2092. doi: 10.1128/aem.59.7.2087-2092.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Kaufmann A, Philippsen P. Of bars and rings: Hof1-dependent cytokinesis in multiseptated hyphae of Ashbya gossypii. Mol Cell Biol. 2009;29:771–783. doi: 10.1128/MCB.01150-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Sikorski RS, Hieter P. A system of shuttle vectors and yeast host strains designed for efficient manipulation of DNA in Saccharomyces cerevisiae. Genetics. 1989;122:19–27. doi: 10.1093/genetics/122.1.19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Gelbart ME, Rechsteiner T, Richmond TJ, Tsukiyama T. Interactions of Isw2 chromatin remodeling complex with nucleosomal arrays: analyses using recombinant yeast histones and immobilized templates. Mol Cell Biol. 2001;21:2098–2106. doi: 10.1128/MCB.21.6.2098-2106.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Schneider BL, Seufert W, Steiner B, Yang QH, Futcher AB. Use of polymerase chain reaction epitope tagging for protein tagging in Saccharomyces cerevisiae. Yeast. 1995;11:1265–1274. doi: 10.1002/yea.320111306. [DOI] [PubMed] [Google Scholar]
- 58.Schmitt ME, Brown TA, Trumpower BL. A rapid and simple method for preparation of RNA from Saccharomyces cerevisiae. Nucleic Acids Res. 1990;18:3091–3092. doi: 10.1093/nar/18.10.3091. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Phylogenetic gene tree of SIR4 and ASF2 orthologs from several hemiascomycete species. Sequences and nomenclature were obtained from the Yeast Gene Order Browser (YGOB) (Byrne and Wolfe 2005) and analyzed using MEGA (Tamura et al 2007) to construct the neighbor joining gene tree. Bold, red font indicate species that underwent the whole genome duplication. Sc = S. cerevisiae, Sb = S. bayanus, Cg = C. glabrata, Scas = S. castelli, Kp = K. polysporus, Zr = Z. rouxii, Ag = A. gossypii, Sk = S. kluyveri, Kt = K. thermotolerans, Kw = Kwaltii. Common names, as notated in the YGOB, are given along with the systematic names in parantheses. K.lactis common gene names are not given to illustrate how KLLA0F1430g and KLLA0F13998g cluster.
(0.89 MB EPS)
Quantitative RT–PCR analysis of HMLα1, HMLα2, and HMLα3 mRNA in wild-type (CK213), sir2Δ (SAY569), sir4Δ (LRY2038), asf2Δ (LRY2377), asf2Δ sir4Δ (LRY2374), and sir2Δ asf2Δ (LRY2523) strains. The amount of cDNA was first normalized to the control locus ACT1. The values shown here represent the relative amount of cDNA for each deletion strain compared to the wild-type strain. The data for wild-type sir2Δ and sir4Δ strains is the same as Figure 2. Error bars represent the SEM.
(0.65 MB EPS)
The association of myc-KlSum1 with HMLα as assessed by chromatin IP followed by quantitative PCR in a sir2Δ sir4Δ strain (LRY2530). The y-axis represents the relative enrichment normalized to a control locus, RRP7, which is not detectably associated with KlSir2, KlSir4 or KlSum1. Error bars represent the SEM. A schematic of the HMLα locus is shown under the x-axis.
(0.97 MB EPS)
Quantitative RT–PCR analysis of HMRa1 and HMRa2 in wild-type (SAY538), sir4Δ (LRY1946), asf2Δ (LRY1856), and asf2Δ sir4Δ (LRY1948) strains. The amount of cDNA was first normalized to the control locus ACT1. The values shown here represent the relative amount of cDNA for each deletion strain compared to the wild-type strain. The data for wild-type and sir4Δ strains is the same as Figure 4. Error bars represent the SEM.
(0.61 MB EPS)
(A) Quantitative RT–PCR analysis of CDA2, SPS4, SPR3, and SPS2 mRNA in wild-type (SAY538) and asf2Δ (LRY1856) strains. The amount of cDNA was first normalized to the control locus ACT1. The values shown here represent the relative amount of cDNA for each deletion strain compared to the wild-type strain. The data for the wild-type strain is the same as Figure 5. (B) Association of KlSir2-HA and myc-KlSum1 with CDA2 as assess by chromatin IP followed by quantitative PCR in an asf2Δ strain (LRY2525). Error bars represent the SEM.
(1.00 MB EPS)
Quantitative RT–PCR analysis of the cryptic mating-type loci, mid-sporulation genes and cell-type–specific genes in wild-type strains of MATa and MATα cells. The amount of cDNA was normalized to ACT1.
(0.61 MB EPS)
Schematics of HMLα, MATα, HMRa, and CDA2 with primer sets shown for chromatin IP quantitative PCR.
(2.82 MB EPS)
K. lactis strains used in this study.
(0.03 MB DOC)
Oligonucleotides used for quantitative RT–PCR.
(0.02 MB XLS)
Oligonucleotides used for quantitative chromatin IP.
(0.05 MB XLS)