Abstract
With the increasing interest in treatments for neonatal brain injury, bedside methods for detecting and assessing injury status and evolution are needed. We aimed to determine whether cerebral tissue oxygenation (StO2), cerebral blood volume (CBV), and estimates of relative cerebral oxygen consumption (rCMRO2) determined by bedside frequency-domain near-infrared spectroscopy (FD-NIRS) have the potential to distinguish neonates with brain injury from those with non-brain issues and healthy controls. We recruited 43 neonates ≤ 15 days old and > 33 weeks gestational age (GA): 14 with imaging evidence of brain injury, 29 without suspicion of brain injury (4 unstable, 6 stable, and 19 healthy). A multivariate analysis of variance with Newman–Keuls post hoc comparisons confirmed group similarity for GA and age at measurement. StO2 was significantly higher in brain injured compared with unstable neonates, but not statistically different from stable or healthy neonates. Brain-injured neonates were distinguished from all others by significant increases in CBV and rCMRO2. In conclusion, although NIRS measures of StO2 alone may be insensitive to evolving brain injury, increased CBV and rCMRO2 seem to be useful for detecting neonatal brain injury and suggest increased neuronal activity and metabolism occurs acutely in evolving brain injury.
Keywords: NIRS (near infrared spectroscopy), hypoxic ischemic injury, stroke, neonate, CBV (cerebral blood volume), CMRO2 (cerebral metabolic rate of oxygen consumption
Introduction
Perinatal brain injury remains a common cause of morbidity and mortality, placing heavy burdens on families and society. Many types of brain injury, including hypoxic-ischemic insults, focal arterial ischemic strokes, hypoglycemia, and metabolic disorders, may become evident in the first days of life, often with a common clinical manifestation of seizures. Confirming brain injury at bedside and determining the type and severity remain a challenge, as do bedside identification and monitoring of injuries likely involving ongoing processes of oxidative stress, excitotoxicity, inflammation, repair, and cell death, evolving over hours to weeks (Ferriero, 2004). As new treatment strategies for neonatal brain injuries (e.g., cooling, novel-neuro-protection, and anti-seizure agents (Ferriero, 2004; Glass and Ferriero, 2007; Perlman, 2006)) become available, we are faced with significant challenges in selecting appropriate patients and adequate means of monitoring response to therapy as treatment is introduced.
To evaluate the effectiveness and better understand the mechanisms of action of new therapies, better bedside methods for detecting and monitoring the evolution of brain injury are needed. Amplitude-integrated EEG (aEEG) and head ultra-sound (US) remain the most common bedside methods. Although aEEG has an additive role in early assessment (al Naqeeb et al, 1999; Shalak et al, 2003), the sensitivity of aEEG alone to neonatal seizures is in question, and the relationship between aEEG and injury unclear (Shellhaas et al, 2007). Also, US is notoriously insensitive to acute brain injury (Blankenberg et al, 2000; Silverstein et al, 2008).
Near-infrared spectroscopy (NIRS), an inexpensive bedside method for evaluating oxy- and deoxy-hemoglobin levels in the brain, provides information about brain health. However, early attempts at using NIRS to detect and monitor neonatal brain injury were disappointing (Greisen, 2006). Major obstacles to successful clinical application of NIRS have been: (1) lack of quantification and therefore an inability to compare at-risk and normal neonates and (2) wide dispersion of results making it difficult to distinguish normal neonates from those with brain injury.
The past decade has seen significant advances in NIRS technique addressing these failings. First, frequency-domain and time-domain (FD-NIRS and TD-NIRS, respectively) devices allow quantification of brain tissue oxygen (StO2) and total hemoglobin concentration (HbT) (Fantini et al, 1995; Ijichi et al, 2005; Zhao et al, 2005). Second, the use of multiple wavelengths and light source-detector pairs yields redundant data at multiple wavelengths and thus an ability to use objective criteria for distinguishing good from bad quality data. This approach has been used successfully to measure the evolution of cerebral blood volume (CBV), StO2, and the cerebral metabolic rate of oxygen consumption (CMRO2) over the first year of normal brain development (Franceschini et al, 2007).
Here, we assessed the potential role of FD-NIRS in evaluating neonatal brain health. We hypothesized that neonates with recent brain injuries would have: (1) increased CBV, (2) increased StO2, and (3) altered CMRO2 compared with neonates without brain injury.
Materials and methods
Subject Selection
This study was part of a larger prospective study in which any neonate in the hospital (both inpatient and outpatient in physician's office) was eligible for recruitment into our FD-NIRS study. Subjects with clinical suspicion of brain injury were targeted for recruitment and subjects without suspicion of brain injury, including normal controls, were recruited on a random basis. We attempted to recruit infants with suspected brain injury as soon as possible after clinical identification, but parental distress and time required for parental consideration resulted in a wide variation between suspected time of injury and FD-NIRS measurement. Subjects were recruited between October 2004 and September 2007. Our institutional review board approved the study and parents provided informed consent.
Subject Classification
Infants were divided into four clinical groups: (1) ‘brain injury’, (2) no brain injury but cardiovascular or respiratory instability (unstable), (3) no brain injury or instability (stable), and (4) ‘healthy’. Any neonate with intraventricular hemorrhage or parenchymal brain abnormalities on US or magnetic resonance imaging (MRI) were included in the brain-injured category. If a neonate was clinically suspected to have brain injury but the MRI was read by a pediatric neuroradiologist as normal, it was not included in the ‘brain injury’ group. Neonates were assigned to the ‘unstable’ group if there was no clinical suspicion of brain injury, but pressors and/or ECMO and/or intubation and ventilation were a clinical necessity at the time of the FD-NIRS study. Neonates were assigned to the ‘stable’ group if they had adverse perinatal events or surgery but no clinical suspicion of brain injury and no cardiovascular or respiratory support was required. Healthy or premature neonates being monitored because of gestational age (GA) but without known additional medical issues were assigned to the ‘healthy’ group (see Table 1 for details).
Table 1.
Subject details
| Group | ID | BW (g) | GA (wk) | APGAR | On day of FD-NIRS |
EEG (age)/imaging (age) | Follow-up (age) | ||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Clinical summary | Intubated | Circ. Sup. | AEM | Age | |||||||
| Brain injury |
9 | 2200 | 35.0 | 3 | HIE, Generalized tonic clonic seizure < 12 h of life |
Y | Phenob., Phenytoin |
2 | EEG: Isoelectric (0 day) MRI: Bilateral thalamus, brainstem low ADC (1 day) |
Withdrawal of support (3 days) | |
| 10 | 2270 | 36.0 | 7 | Mitochondrial disorder, question of seizures < 12 h of life |
Y | Phenob. | 6 | EEG: Bilateral independent sharp waves (6 days) MRI: Bilateral thalamic volume loss (1 day) |
Withdrawal of support (20 days) | ||
| 11 | 2420 | 35.3 | 7 | PHT, mitochondrial disorder, on V-V ECMO, IVH |
Y | Y | Versed, morphine |
3 | EEG: Normal (5 months) HUS: IVH and ventricular dilatation (5 days) MRI: Brainstem, cerebellar, bilateral thalamic and BG volume loss, periventricular WM low ADC (3 months) |
Withdrawal of support (15 months) | |
| 12 | 4260 | 39.0 | 8 | HII, seizures on day 8 of life | Y | Phenob. | 12 | EEG: Numerous electroclinical seizures (10 days) MRI: Bilateral cortical, thalamic, BG and WM low ADC (11 days) |
Withdrawal of support (12 days) | ||
| 17 | 3700 | 40.0 | 9 | Seizure-like movements days 1–2. Off VA ECMO for treatment of PPHN on day 5. Resolving hydrops fetalis |
Y | Y | Phenob. | 6 | EEG: Excessive sharp transients. Diffuse background attenuation. No seizures (6 days) MRI: Cystic WM changes, low ADC in corpus callosum (12 days) |
Delayed speech, increased tone with conus in right leg (19 months) |
|
| 19 | 3700 | 40.9 | 7 | Septic shock, Mec aspiration, on VA ECMO | Y | Y | 3 | EEG: Excessive sharp transients. No seizures (9 days) MRI: Small infarct right centrum semiovale (10 days) |
Developmentally normal (2 years) | ||
| 20 | 4085 | 38.3 | 0 | HIE, seizures within day 1, body cooling for 72 h, Anasarca |
Y | Y | Fentanyl, Versed, Nitric Ox. |
10 | EEG: Bursts of bifronal sharps without focality, attenuations up to 22 secs (8 days) MRI: Diffuse volume loss, bilateral thalamic and BG gliosis (13 days) |
Moderate to Severe Global delay and spasticity (2 years) |
|
| 23 | 3300 | 40.0 | 5 | Respiratory distress at birth. seizures first day of life |
Y | Phenob, Phenytoin |
3 | EEG: Frequent bilateral sharp transients, Frequent left sided seizures (0 day) MRI: Bilateral choroid plexus bleeds, IVH (4 days) |
Developmentally normal (15 months) | ||
| 30 | 3900 | 40.1 | 7 | AVM rupture, pulmonary hypertension, HIE with seizures at birth |
Y | Y | Phenob., Phenytoin |
3 | EEG: Left sided bursts of rhythmic slow waves with intermixed sharp waves. Suppressed background (0 day) MRI: Left frontal hemorrhage, bilateral thalamic and CST low ADC (1 day) |
Withdrawal of Support (3 days) | |
| 31 | 3030 | 40.0 | NA | Hypoglycemia, seizures | Y | Phenob., Phenytoin |
12 | EEG: Alternating attenuated background. (3 days) MRI: Diffuse cortical low ADC (3 days) |
Language and Motor Delay (2 years) | ||
| 35 | 2880 | 40.0 | 9 | Arterial ischemic stroke, seizures first day of life |
Phenob., Phenytoin |
6 | EEG: Status epilepticus followed by attenuated, irregular background on left (1 day) MRI: Left MCA Stroke with low ADC (2 days) |
Motor delay with increased tone on right (7 months) |
|||
| 38 | 2950 | 34.0 | 2 | Venous edema | 1 | MRI: Diffuse edema with high ADC (0 day) | Age appropriate development (17 months) |
||||
| 41 | 3860 | 41.0 | 9 | Partial HII, seizures first day of life | Phenob., Phenytoin |
8 | EEG: Discontinuous background, frequent sharp waves, generalized discharges (2d) MRI: Mutifocal bilateral cortical and WM low ADC (3 days) |
Grossly normal (6 months) | |||
| 43 | 3340 | 40.0 | 3 | Severe perinatal depression, seizures first day of life |
Phenob. | 6 | EEG: Frequent bilateral sharp transients (3 days) MRI: Multifocal cortical low ADC bilateral BG and thalamic low ADC (3 days) |
Increased leg tone (11 wks) | |||
| Unstable | 8 | 2820 | 35.0 | 7 | RDS, hyperbilirubinemia resolved | Y | 4 | HUS: Normal (16 days) | Delayed Motor development (2 years) | ||
| 16 | 2480 | 37.0 | 8 | Gastroshesis, adrenal insuff., sepsis | Y | Y | 1 | HUS: Normal (13 days) | Withdrawal of support (2 months) | ||
| 21 | 3800 | 37.0 | 5 | Perinatal distress, PHT, tricuspid regurgitation |
Y | Y | 4 | HUS: Normal (2 days) | Discharged home at 1 month | ||
| 37 | 2600 | 36.7 | 4 | CDH, respiratory failure. | Y | Y | 3 | HUS: Normal (3 days) | Withdrawal of support (6 wks) | ||
| Stable | 2 | 3800 | 40.0 | 6 | Prior RD and seizure at birth. Perinatal depression. Occasional desats, on nasal canula |
Phenob. | 9 | EEG: Multifocal sharps. No seizures (10 days) MRI: Normal (1 day) |
Age appropriate development (13 months) |
||
| 6 | 3827 | 40.0 | 9 | Stable term, Hirschsprung's disease, 2 days post pull through procedure |
2 | ||||||
| 13 | 1360 | 34.4 | 9 | Premature, 3rd of Triplets, FTT | 8 | Discharged home at 2 wks | |||||
| 18 | 1980 | 33.5 | 8 | RDS, Intubated for 3 days but now on room air, pneumonia |
15 | Mild axial hypotonia, otherwise developmentally normal (6 months) |
|||||
| 36 | 4200 | 40.0 | 9 | No seizures until DOL 3–4. Seizure-like activity days 3–7 of life |
Phenob. | 13 | EEG: Sharp transients. No seizures (14 days) MRI: Normal (10 days) |
||||
| 39 | 2300 | 34.0 | 9 | TEF | Y | 6 | Difficulty swallowing | ||||
| Healthy | 1 | 3500 | 39.5 | 9 | Normal term | 8 | |||||
| 3 | 3659 | 40.0 | 9 | Normal term | 7 | Normal (4 months) | |||||
| 4 | 2150 | 34.0 | 9 | Stable premature | 10 | Normal (8 months) | |||||
| 5 | 1970 | 33.6 | 9 | Stable premature | 11 | Normal (3 years) | |||||
| 7 | 1880 | 34.1 | 9 | Stable premature | 8 | Normal (2 years) | |||||
| 14 | 2115 | 34.4 | 9 | Stable premature, 2nd of triplets | 8 | ||||||
| 15 | 2315 | 34.4 | 9 | Stable premature, 1st of triplets | 8 | ||||||
| 22 | 2470 | 35.1 | 9 | Stable premature | 2 | ||||||
| 24 | 3632 | 38.0 | NA | Normal term | 1 | ||||||
| 25 | 3541 | 41.0 | NA | Normal term | 1 | ||||||
| 26 | 3600 | 41.5 | NA | Normal term | 2 | ||||||
| 27 | 3720 | 40.0 | NA | Normal Term | 0 | ||||||
| 28 | 3690 | 40.1 | NA | Normal term | 2 | Normal (2 years) | |||||
| 29 | 4500 | 41.0 | NA | Normal term | 1 | ||||||
| 32 | 2110 | 33.9 | 9 | Stable premature | 8 | ||||||
| 33 | 2715 | 34.0 | 9 | Stable premature | 7 | ||||||
| 34 | 2420 | 34.0 | 9 | Stable premature | 7 | ||||||
| 40 | 2645 | 36.7 | NA | Stable premature | 6 | Normal (18 months) | |||||
| 42 | 3180 | 42.0 | 8 | Normal term | 2 | Normal (18 months) | |||||
ADC=apparent diffusion coefficient; AEM=antiepileptic medication; AVM=arterio-venous malformation; BG=basal ganglia; BW=birth weight; circ. sup.=circulatory support; CDH=congenital diaphragmatic hernia; CST=corticospinal tract; DOL=day of life; FTT=failure to thrive; HIE=hypoxic ischemic encephalopathy; HII=hypoxic ischemic injury; HUS=head ultrasound; IVH=intraventricular hemorrhage; MCA=middle cerebral artery; phenob.=phenobarbital; PHT=pulmonary hypertension; PPHN=persistent pulmonary hypertension of the newborn; RD(S)=respiratory distress (syndrome); TEF=tracheoesophageal fistula; VA ECMO=venoarterial extracorporeal membrane oxygenation; V-V ECMO=veno-venous ECMO; WM=white matter.
Frequency-Domain Near-Infrared Spectroscopy Protocol
Measurements were obtained from up to four different regions of the head: frontal (FpZ, Fp1, Fp2), central (C2, C3, C4, C5), temporal (T3, T4), and occipital (O1, O2). We acquired data for 8 sec in each location, with up to three repeated measurements. The total number of positions and repetitions depended on the subject's cooperation and presence of monitoring devices. Total examination time was < 20 mins.
Frequency-Domain Near-Infrared Spectroscopy Instrument
We used a customized commercial FD oximeter (Imagent, ISS Inc) with 32 laser sources and 4 photomultiplier tube detectors (Fantini et al, 1995). For these measurements, we used seven lasers (emitting at 670, 690, 750, 760, 780, 810, and 830 nm) combined in a fiber bundle, and four detectors coupled to four fiber bundles. Fiber bundles (2.5 mm diameter) were arranged in a row on a black rubber probe (5×2×0.5 cm3) with source-detector distances of 1, 1.5, 2, and 2.5 cm. Multiple distances are necessary to quantify absorption and scattering coefficients with this system (Fantini et al, 1995). As we separate absorption and scattering, there is no need to assume a pathlength factor at each wavelength as with continuous-wave systems. The source-detector separations we chose (1 to 2.5 cm) are adequate for a depth penetration of about 1 cm, which includes the cerebral cortex in neonates. The laser fluence at the skin is < 0.64 W/cm2, well below the ANSI standard limits, allowing for safe measurements. The FD-NIRS instrument is quite compact and can be moved on a small cart to the infant's bedside in the hospital.
Quantification of StO2, Cerebral Blood Volume, and Estimation of CMRO2 Changes
The amplitude and phase data collected at each wave-length are used to determine average absorption and scattering coefficients using the multi-distance FD method (Fantini et al, 1995). We discard all data points with R2 < 0.97 for the linear fit of the raw optical data versus source-detector distance. Furthermore, data are discarded if the P > 0.05 for the fit of the seven absorption coefficients with the hemoglobin spectra or for the linear fit of the reduced scattering coefficient versus wavelength. Using these objective rejection criteria, only the highest quality data are retained for further analysis.
Hemoglobin concentration and oxygenation are derived by fitting the absorption coefficients at seven wavelengths with the hemoglobin spectra using extinction coefficients reported in the literature (Wray et al, 1988) and a 75% concentration of water (Wolthuis et al, 2001). For these calculations, we developed a graphical interface program implemented in MATLAB (The Mathworks, Inc). Multiple measurements in the same location are averaged together, and for this paper results are averaged over all positions.
CBV was calculated using the following equation (Ijichi et al, 2005):
where CBV is in mL/100 g, HbT [μMol] is the total hemoglobin concentration measured with FD-NIRS, MWHb = 64,500 [g/Mol] is the molecular weight of hemoglobin, and Dbt is the brain tissue density (1.05 g/mL) (Kretschmann et al, 1986). When hemoglobin concentration in the blood (HGB, g/dL) was not available in clinical charts, standard normal values of HGB for age were used (Oski and Naiman, 1982). A discussion of the uncertainties in the above physiologic parameters has been reported earlier (Franceschini et al, 2007).
Relative CMRO2 (rCMRO2) was calculated using the following equation (Ijichi et al, 2005):
where SaO2 = arterial oxygenation obtained from the pulse oximeter, the subscript ‘o’ indicating the reference values, and under the assumption of a constant power law relation between changes in blood flow (CBF) and blood volume with 2≤β≤5 (Brown et al, 2003; Grubb et al, 1974). rCMRO2 for each baby was calculated as the ratio between the subject and the average of healthy controls.
The calculation of rCMRO2 depends on SaO2 and the exponent β as well as StO2, HGB, and CBV discussed above. Uncertainty in SaO2 is < 3%. Both value and uncertainty in β are unknown for neonates. The minimum value of β = 2 is set by the Poiseuille's Law for laminar flow at fixed pressure. As the arterial, capillary, and venous vascular compartments are compliant, larger values of β are expected (Mandeville et al, 1999). In adult monkey brains, β has been measured at 2.6 when assessing the normal physiological response to changes in CO2 (Grubb et al, 1974). In human functional MRI studies, β varies between 2.8 for stimuli duration > 30 secs and five for rapid stimuli (Mandeville et al, 1999). As the value of β in the neonate is unknown, we estimated rCMRO2 with β = 2.6 but also examined the results with β = 2, 3, 4, and 5 to get a bound on our error.
Statistical Analysis
First, a multivariate analysis of variance (ANOVA) was performed to determine whether GA at birth and at measurement were homogeneous between the four clinical groups. Second, a multivariate ANOVA was performed to determine whether clinical group, GA at birth or age at measurement had a significant effect on CBV, StO2, or rCMRO2. GA at birth was defined as preterm ( < 38 weeks) or term (38 to 42 weeks) and age at measurement as < 3 days or > 3 days. Newman–Keuls post hoc comparisons were performed to determine whether GA at birth, age at measurement or clinical group had significant effects on each hemodynamic variable. Five β values between 2 and 5 were explored when performing post hoc comparisons for rCMRO2. For all statistical tests, P-values < 0.05 were considered significant. Sensitivity and specificity for diagnosis of brain injury based on the scatterplot of rCMRO2 (with β = 2.6) against CBV were calculated.
Results
With a success rate of approximately 30%, 80 neonates were recruited into the study: 19 were excluded due to GA < 33 weeks, 16 because measurements started after two weeks of life, 1 due to poor data quality, and 1 due to equipment malfunction. Therefore, 43 neonates were included in this study (Table 1), 14 (33%) were in the brain injury group, 4 (9%) in the unstable group, 6 (14%) in the stable group, and 19 (44%) in the healthy group. Mean GA at birth was 37.5 (range, 33.6 to 41.5) weeks. Mean age at the time of FD-NIRS was 5.7 days (range, 0 to 15). Twenty-one (49%) were born prematurely ( < 38 weeks GA at birth). The first ANOVA did not show any significant effect of the group on GA at birth and age at measurement, which means the groups were homogeneous relative to these parameters.
HGB values obtained the same day were available in 34 neonates, within 1 to 3 days in 22 and within 4 to 8 days in 12. In nine, HGB values were not available or were > 8 days far from the day of our measurement (Supplementary Table 2). When available, pCO2 values were documented (Supplementary Table 2). There was no significant correlation between CBV and pCO2 (Supplementary Figure 4, online), suggesting that changes in pCO2 were not a major contributing factor to differences in CBV.
For FD-NIRS data analysis, the average number of positions used across all babies was on average 5±3 locations. In 90% of neonates we kept forehead measurements and in 60 to 65% of the babies we kept temporal and parietal (left and right) measurements. The inter-location coefficient of variation (COV, standard deviation/mean ratio) of CBV in our subjects is 18±8%. The intra-location COV is 11±1% in CBV and 4±1% in StO2.
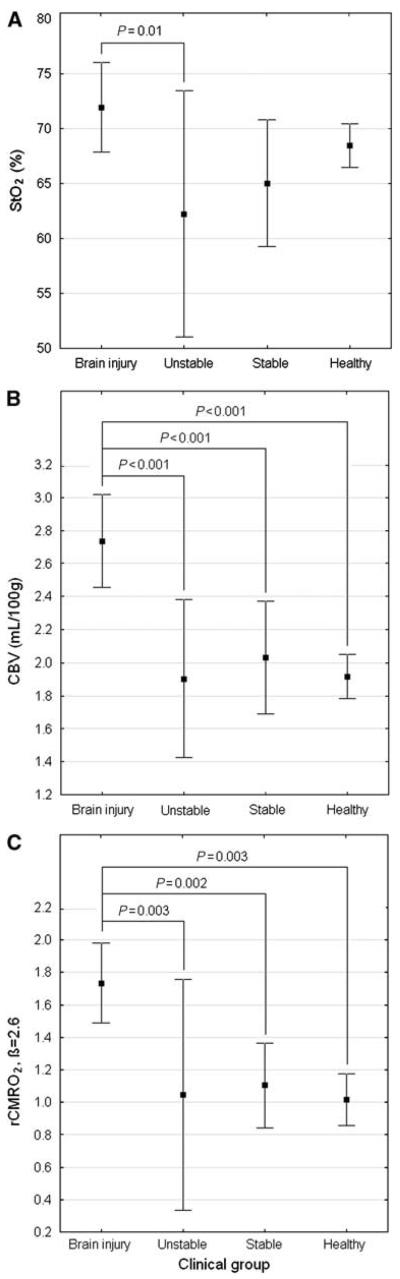
The ANOVA identified a significant effect of the clinical group on CBV, StO2, and rCMRO2 (F = 6.2, P < 0.001). Although StO2 was significantly higher in the brain-injured group compared with the unstable group, no other statistical differences in StO2 were detected between groups by post hoc Newman–Keuls test. In particular, brain-injured and normal groups were not significantly different (Figure 1A). In contrast, CBV was significantly higher in the brain-injured group compared with every other group. Furthermore, no significant differences in CBV were found among the three groups without brain injury (Figure 1B). For β = 2.6, rCMRO2 was significantly higher in the brain-injured group compared with every other group, and no significant differences were found among the three groups without brain injury (Figure 1C; Supplementary Figure 3, online).
Figure 1.
(A) Average (±95% confidence interval) of StO2 for each clinical group, with significant P-value. (B) Average (±95% confidence interval) of CBV for each clinical group, with significant P-values. (C) Average (±95% confidence interval) of rCMRO2 for each clinical group, for β = 2.6 and with significant P-values.
The ANOVA also identified a significant effect of GA at birth on the hemodynamic variables (F = 3.5, P = 0.03). Post hoc Newman–Keuls test detected that rCMRO2 was significantly higher for GA at birth > 38 weeks (P = 0.006), but GA at birth did not significantly affect CBV or StO2. Age at measurement ( < 3 days versus > 3 days) did not significantly affect any of the hemodynamic variables.
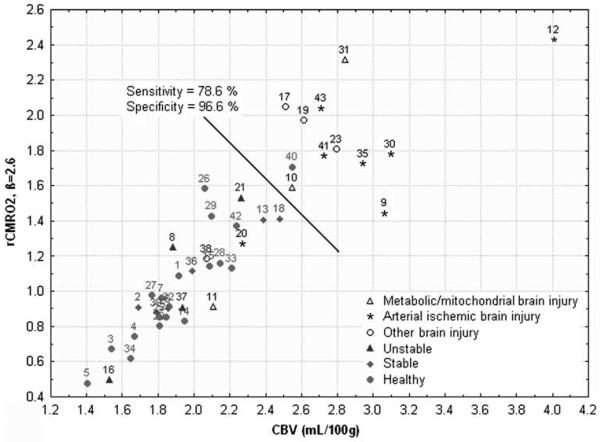
Using the scatterplot of rCMRO2 (β = 2.6) against CBV, the sensitivity of our method for detecting brain injury was 78.6% at a specificity of 96.6% (Figure 2). In brain injuries without increased rCMRO2 and CBV (subjects 11, 38, 20), the first two had no seizures and last one was 10 days post-body cooling for HII.
Figure 2.
Scatterplot of rCMRO2 against CBV for each clinical group, for β = 2.6. The black line represents the cutoff for sensitivity and specificity calculation.
Discussion
In this pilot study, there was no significant difference in StO2 between brain-injured and normal neonates, suggesting that StO2 alone may be insensitive to evolving brain injury. However, CBV and estimates of rCMRO2 were significantly increased in the brain-injured group compared with all other clinical groups despite the diversity of brain injury types. In this small study, combining CBV and rCMRO2 resulted in encouraging sensitivity and specificity values for FD-NIRS for detecting brain injury within the first two weeks of life.
Many neonatal NIRS studies have focused on StO2 (Austin et al, 2006; Benaron et al, 1992; Isobe et al, 2000; Toet et al, 2006; Tsuji et al, 2000; Wong et al, 2008; Wyatt et al, 1986), following the assumption that tissue oxygenation is a good marker of brain health. In a study of severe hypoxic-ischemic injury, increased cerebral tissue oxygenation in the first 48 h of life was correlated with poor outcomes (Toet et al, 2006). We also observed an increase in StO2 in the brain-injured group, but this was not significant. Although the lack of significance in our study may be due to the later time periods and more heterogeneous nature of the brain insults, it is also possible that StO2 is not a sensitive marker of evolving injury. We have observed earlier that StO2 is not a sensitive marker of brain development in the first year of life, showing little change as CBV increases with age (Franceschini et al, 2007). In this case, we hypothesized that CBV increases to meet increasing neuronal demands in such a way that StO2 remains relatively stable. Here, we hypothesize that StO2 is useful for monitoring the severity of the primary insult. However, after the insult and during the injury evolution, cerebral perfusion increases in an effort to stabilize StO2, as during development. Future studies with more patients and multiple time points starting closer to birth are needed to monitor the temporal evolution of StO2 and test this hypothesis in humans.
Increased CBV after neonatal brain injury in the first week of life has been reported in prior NIRS studies (Meek et al, 1999; Wyatt et al, 1990). Here, we extend the time window to 2 weeks and show that the increase in CBV is associated with relative increases in CMRO2. These increases in CBV and CMRO2 suggest increased neuronal activity (Huppert et al, 2006; Uludag et al, 2004). In fact, the trend of an increase in StO2 in the brain group is in keeping with increased neuronal activity. Functional MRI, positron emission tomography, and NIRS studies have shown us that normal brain function is associated with a local increase in oxy-hemoglobin and a decrease in deoxy-hemoglobin concentrations, and an overall increase in local CBV and CBF (Fox et al, 1988; Malonek and Grinvald, 1996).
Increased neuronal activity in neonatal brain injury has two important implications: (1) increased cerebral perfusion after cerebral injury may be due to increased neuronal activity and not loss of vascular control; (2) increased neuronal activity may persist for days after injury despite standard anti-seizure medication and a lack of EEG and clinical signs of seizures. A seizure is defined as a transient symptom of excessive or synchronous neuronal activity in the brain (Fisher et al, 2005). This abnormal synchronous neuronal activity can be detected with EEG and is associated with increased neuronal activity. Unlike many epilepsy syndromes, seizure activity in neonatal brain injury occurs secondary to injury and excitotoxic cascades. Therefore, we hypothesize that neuronal synchrony may not occur and EEG results may be misleading. Thus, we hypothesize that increases in CBV and rCMRO2 can be seen without frank clinical or electrographic seizure activity due to incoherent increased neuronal activity resulting from excitotoxic injury. Another possibility for elevated CBV and rCMRO2 in the absence of EEG seizure activity is that the neurons are unable to generate an action potential due to injury but are continuing to consume large amounts of oxygen due to excitotoxic stress. Further studies correlating FD-NIRS directly with EEG at the time of FD-NIRS measurement are needed to explore this relationship further.
Although there have been previous reports of CMRO2 in neonates, these studies required radioactive tracers, invasive procedures or ventilated subjects (Altman and Volpe, 1991; Elwell et al, 2005; Skov et al, 1993; Yoxall and Weindling, 1998). Therefore, the numbers in these studies are small and no normal neonates are included. Higher CMRO2 rates were reported in term and near-term neonates compared with premature neonates. However, the term and near-term neonates often suffered from hypoxic-ischemic insults, whereas the premature neonates had other types of injuries, including hyaline membrane disease, respiratory distress syndrome, or intraventricular hemorrhage. It is, therefore, impossible to determine whether the term neonates had higher CMRO2 due to increased maturity or to evolving cerebral injury.
We averaged results from all locations as only three subjects (19, 30, and 35) had marked asymmetry to the MR imaging findings and 12 out of the 43 did not have high quality FD-NIRS results bilaterally to allow accurate lateralization. We could not assess laterality of injury in subject 19, as only middle forehead measurements were acceptable for analysis. For the remaining 28 subjects for whom high quality bilateral data were available and markedly asymmetric injury was not present on MRI, the average difference between left and right temporal/parietal regions was 17±11% for CBV and 3±2% for StO2. These left and right asymmetry values are within our reproducibility errors (11±1% for CBV and 4±1% for StO2) and consistent with the mean COV calculated across all head locations (18±8% for CBV and 4±2% for StO2). For patient 30 with the large left frontal hematoma due to an arterio-venous malformation (AVM) rupture, differences in left and right temporal/parietal hemispheres were 1% for CBV and 0% for StO2. However, the diffusion weighted images (DWIs) in this patient showed evidence of a bilaterally symmetric profound hypoxic-ischemic injury that was confirmed on pathology. Therefore, this subject had both an AVM that ruptured and profound global hypoxic-ischemic injury. The large left frontal hematoma was over 2 cm below the cortex within the deep white matter of the left frontal lobe. As the depth penetration of FD-NIRS is only 1 cm, it is likely that the FD-NIRS measurements were dominated by the global symmetric HII affecting cortical neuronal health. In subject 35, the asymmetries were higher than those in any other subject, with left-sided CBV 46% larger than right and left-sided StO2 24% larger than right. This correlated with the large left MCA arterial ischemic stroke identified on MRI. The next subject with CBV asymmetry larger than the average was subject 41 with 38% left/right difference (StO2 asymmetry only 3% in this subject). This subject had asymmetric partial hypoxic-ischemic injury on DWI with the left hemisphere more severely affected than the right. The next subject with StO2 asymmetry larger than the average was subject 43 with 10% left/right difference (CBV asymmetry only 7%). This subject also had asymmetric HII on DWI with more cortical low apparent diffusion coefficient (ADC) on the left. Therefore, as these few cases suggest, FD-NIRS has the potential to lateralize injury, although better determination of normal laterality and confirmation with more cases of asymmetric injury is needed.
This study encompassed a large range of brain injuries, including hypoglycemic, metabolic, hypoxic ischemic, and focal arterial ischemic injuries (Table 1). We allowed this diversity to determine whether FD-NIRS measures of StO2, CBV, and/or rCMRO2 could detect a unique response to primary brain injuries distinguishing them from normal neonates and neonates with non-brain issues. Using the sensitivity and specificity values in Supplementary Figure 3, 12 neonates, had increased CBV and rCMRO2 with 11 out of the 12 from the brain injury group. All nine patients who had an MRI within 10 days of the NIRS study and at least one site with low ADC (subjects 9, 12, 17, 19, 30, 31, 35, 41, and 43) were in this group with elevated CBV and rCMRO2. Arterial patterns of hypoxic-ischemic injury (HIE, HII, and arterial stroke) were present on MRI in 6 out of the 9 of these patients with low ADC associated with high CBV and rCMRO2 (subjects 9, 12, 30, 35, 41, and 43). However, elevated CBV and rCMRO2 were observed in subject 10 and 23 even though no ADC abnormalities were present on recent DWI. Subject 10 had a mitochondrial disorder and subject 23 had bilateral choroids plexus bleeds with IVH on MRI after respiratory distress and seizures at birth. We hypothesize that all these disorders share the common mechanism of hyperperfusion due to increased neuronal activity, with the increased neuronal activity possibly due to excitotoxic cascades. Although brain injuries with high CBV and rCMRO2 are most commonly associated with low ADC, the absence of low ADC in two subjects suggests that neuronal dysfunction can occur in the absence of low ADC. In addition, in patients 9, 12, 17, 19, and 30, the regions of low ADC were deep and, therefore, unlikely to be directly sampled by FD-NIRS. These findings suggest that if the brain injury is severe enough to cause ADC to decrease in any region of the brain, it may be severe enough to result in global changes in cortical CBV and rCMRO2. We hypothesize that CBV and rCMRO2 increases may be due to neurons that are functioning abnormally but are not necrotic. Neuronal dysfunction could be secondary to primary insults, disrupted connections to deep gray nuclei or deep gray nuclei injury.
Not all brain injury subjects exhibited increases in CBV and rCMRO2. For example, one neonate 10 days after hypoxic-ischemic injury (20), one with vasogenic edema that resolved (38), and one with a mitochondrial disorder (11) had CBV and rCMRO2 results within the normal rage. As other subjects with more acute HII showed increased CBV and rCMRO2, we expect that hypoxic-ischemic injuries evolve over time, eventually normalizing and probably going on to have lower rCMRO2 than normal. The lack of increased CBV and rCMRO2 in neonates with vasogenic edema and mitochondrial disorder suggests that FD-NIRS may help distinguish different types and severity of brain injury. However, larger studies are needed to determine the role of FD-NIRS in distinguishing different types of neonatal brain injury and the role of FD-NIRS in monitoring the evolution of neonatal brain injury.
Variations in HGB may have contributed to errors in CBV calculations as we did not have HGB values for all infants. Values were available for all unstable neonates and most with brain injury. We did not have IRB approval to do blood work if no values were available in the clinical chart. In these cases we used standard normal values for age. To estimate the magnitude of the error and bias on CBV and rCMRO2 values due to use of table values, we repeated the statistical analysis using table values for all neonates and with a value of 15 g/dL for all neonates. In both cases increased CBV and CMRO2 in the brain injury group compared with all others maintained significant ANOVA but with higher P-values. In summary, the errors introduced by using table values for HGB are not likely large enough to alter the findings in this study.
Also, the error in rCMRO2 is unknown because the value of β is unknown. We show, however, that over a large physiological range of β, rCMRO2 remains significantly elevated in brain injured compared with all other groups (Supplementary Figure 3, online). Direct measurements of CBF in the future will improve the accuracy of rCMRO2 estimates and better understanding of neurovascular coupling in injury and development.
In summary, this pilot study suggests that NIRS measures of StO2 alone may be insensitive to evolving neonatal brain injury. However, increased CBV and increased relative CMRO2 on bedside FD-NIRS may be useful for detecting or monitoring evolving neonatal brain injury as these increases seem to distinguish brain injured from all other neonates. The observed increase in rCMRO2 suggests that the increased perfusion seen in neonatal brain injury may be related to increased neuronal activity, despite the lack of EEG or clinical signs of seizures. Larger prospective longitudinal studies with age-matched controls and neonates with each subtype of brain injury are required to confirm these findings and better determine the sensitivity and specificity of FD-NIRS measures of CBV and rCMRO2. In particular, the sensitivity and specificity of these FD-NIRS measures in the critical first few hours of life is still unclear.
Supplementary Material
Acknowledgments
Funding sources were National Institute of Health and Massachusetts General Hospital: grant numbers K23NS42758 and Claflin Award (PE Grant); grant numbers, RO1-HD42908, Claflin Award, and MGH interim support (MA Franceschini). The authors sincerely thank the following for their assistance: Kara Arvin, Robert Insoft, Joe Mandeville, Sol Diamond, Sara Barnett, Sonal Thaker, Eleni Themelis, Tina Chaves, Shalini Nadgir, Weichen Wu, as well as all the nurses and staff at MGH involved with neonatal care.
Footnotes
Disclosure/conflict of interest
The authors declare no conflict of interest.
Supplementary Information accompanies the paper on the Journal of Cerebral Blood Flow & Metabolism website (http://www.nature.com/jcbfm)
References
- al Naqeeb N, Edwards AD, Cowan FM, Azzopardi D. Assessment of neonatal encephalopathy by amplitude-integrated electroencephalography. Pediatrics. 1999;103:1263–71. doi: 10.1542/peds.103.6.1263. [DOI] [PubMed] [Google Scholar]
- Altman DI, Volpe JJ. Positron emission tomography in newborn infants. Clin Perinatol. 1991;18:549–62. [PubMed] [Google Scholar]
- Austin T, Gibson AP, Branco G, Yusof RM, Arridge SR, Meek JH, Wyatt JS, Delpy DT, Hebden JC. Three dimensional optical imaging of blood volume and oxygenation in the neonatal brain. Neuroimage. 2006;31:1426–33. doi: 10.1016/j.neuroimage.2006.02.038. [DOI] [PubMed] [Google Scholar]
- Benaron DA, Benitz WE, Ariagno RA, Stevenson DK. Noninvasive methods for estimating in vivo oxigenation. Clin Pediatr. 1992;31:258–73. doi: 10.1177/000992289203100501. [DOI] [PubMed] [Google Scholar]
- Blankenberg FG, Loh NN, Bracci P, D'Arceuil HE, Rhine WD, Norbash AM, Lane B, Berg A, Person B, Coutant M, Enzmann DR. Sonography, CT, and MR imaging: a prospective comparison of neonates with suspected intracranial ischemia and hemorrhage. AJNR Am J Neuroradiol. 2000;21:213–8. [PMC free article] [PubMed] [Google Scholar]
- Brown DW, Hadway J, Lee TY. Near-infrared spectroscopy measurement of oxygen extraction fraction and cerebral metabolic rate of oxygen in newborn piglets. Pediatr Res. 2003;54:861–7. doi: 10.1203/01.PDR.0000090928.93045.BE. [DOI] [PubMed] [Google Scholar]
- Elwell CE, Henty JR, Leung TS, Austin T, Meek JH, Delpy DT, Wyatt JS. Measurement of CMRO2 in neonates undergoing intensive care using near infrared spectroscopy. Adv Exp Med Biol. 2005;566:263–8. doi: 10.1007/0-387-26206-7_35. [DOI] [PubMed] [Google Scholar]
- Fantini S, Franceschini MA, Maier JS, Walker SA, Barbieri B, Gratton E. Frequency-domain multichannel optical detector for non-invasive tissue spectroscopy and oximetry. Opt Eng. 1995;34:32–42. [Google Scholar]
- Ferriero DM. Neonatal brain injury. N Engl J Med. 2004;351:1985–95. doi: 10.1056/NEJMra041996. [DOI] [PubMed] [Google Scholar]
- Fisher RS, van Emde Boas W, Blume W, Elger C, Genton P, Lee P, Engel J., Jr Epileptic seizures and epilepsy: definitions proposed by the International League Against Epilepsy (ILAE) and the International Bureau for Epilepsy (IBE) Epilepsia. 2005;46:470–2. doi: 10.1111/j.0013-9580.2005.66104.x. [DOI] [PubMed] [Google Scholar]
- Fox PT, Raichle ME, Mintun MA, Dence C. Nonoxidative glucose consumption during focal physiologic neural activity. Science. 1988;241:462–4. doi: 10.1126/science.3260686. [DOI] [PubMed] [Google Scholar]
- Franceschini MA, Thaker S, Themelis G, Krishnamoorthy KK, Bortfeld H, Diamond SG, Boas DA, Arvin K, Grant PE. Assessment of infant brain development with frequency-domain near-infrared spectroscopy. Pediatr Res. 2007;61:546–51. doi: 10.1203/pdr.0b013e318045be99. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Glass HC, Ferriero DM. Treatment of hypoxic-ischemic encephalopathy in newborns. Curr Treat Options Neurol. 2007;9:414–23. doi: 10.1007/s11940-007-0043-0. [DOI] [PubMed] [Google Scholar]
- Greisen G. Is near-infrared spectroscopy living up to its promises? Semin Fetal Neonatal Med. 2006;11:498–502. doi: 10.1016/j.siny.2006.07.010. [DOI] [PubMed] [Google Scholar]
- Grubb RL, Jr, Raichle ME, Eichling JO, Ter-Pogossian MM. The effects of changes in PaCO2 on cerebral blood volume, blood flow, and vascular mean transit time. Stroke. 1974;5:630–9. doi: 10.1161/01.str.5.5.630. [DOI] [PubMed] [Google Scholar]
- Huppert TJ, Hoge RD, Diamond SG, Franceschini MA, Boas DA. A temporal comparison of BOLD, ASL, and NIRS hemodynamic responses to motor stimuli in adult humans. Neuroimage. 2006;29:368–82. doi: 10.1016/j.neuroimage.2005.08.065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ijichi S, Kusaka T, Isobe K, Okubo K, Kawada K, Namba M, Okada H, Nishida T, Imai T, Itoh S. Developmental changes of optical properties in neonates determined by near-infrared time-resolved spectroscopy. Pediatr Res. 2005;58:568–73. doi: 10.1203/01.PDR.0000175638.98041.0E. [DOI] [PubMed] [Google Scholar]
- Isobe K, Kusaka T, Fujikawa Y, Kondo M, Kawada K, Yasuda S, Itoh S, Hirao K, Onishi S. Changes in cerebral hemoglobin concentration and oxygen saturation immediately after birth in the human neonate using full-spectrum near infrared spectroscopy. J Biomed Opt. 2000;5:283–6. doi: 10.1117/1.429997. [DOI] [PubMed] [Google Scholar]
- Kretschmann HJ, Kammradt G, Krauthausen I, Sauer B, Wingert F. Brain growth in man. Bibl Anat. 1986;28:1–26. [PubMed] [Google Scholar]
- Malonek D, Grinvald A. Interactions between electrical activity and cortical microcirculation revealed by imaging spectroscopy: implications for functional brain mapping. Science. 1996;272:551–4. doi: 10.1126/science.272.5261.551. [DOI] [PubMed] [Google Scholar]
- Mandeville JB, Marota JJA, Ayata C, Zaharchuk G, Moskowitz MA, Rosen BR, Weisskoff RM. Evidence of a cerebrovascular post-arteriole windkessel with delayed compliance. J Cereb Blood Flow Metab. 1999;19:679–89. doi: 10.1097/00004647-199906000-00012. [DOI] [PubMed] [Google Scholar]
- Meek JH, Elwell CE, McCormick DC, Edwards AD, Townsend JP, Stewart AL, Wyatt JS. Abnormal cerebral haemodynamics in perinatally asphyxiated neonates related to outcome. Arch Dis Child Fetal Neonatal Ed. 1999;81:F110–5. doi: 10.1136/fn.81.2.f110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oski FA, Naiman JL. Hematologic problems in the newborn. Third edition. Major Probl Clin Pediatr. 1982;4:1–360. [PubMed] [Google Scholar]
- Perlman JM. Summary proceedings from the neurology group on hypoxic-ischemic encephalopathy. Pediatrics. 2006;117:S28–33. doi: 10.1542/peds.2005-0620E. [DOI] [PubMed] [Google Scholar]
- Shalak LF, Laptook AR, Velaphi SC, Perlman JM. Amplitude-integrated electroencephalography coupled with an early neurologic examination enhances prediction of term infants at risk for persistent encephalopathy. Pediatrics. 2003;111:351–7. doi: 10.1542/peds.111.2.351. [DOI] [PubMed] [Google Scholar]
- Shellhaas RA, Soaita AI, Clancy RR. Sensitivity of amplitude-integrated electroencephalography for neonatal seizure detection. Pediatrics. 2007;120:770–7. doi: 10.1542/peds.2007-0514. [DOI] [PubMed] [Google Scholar]
- Silverstein FS, Jensen FE, Inder T, Hellstrom-Westas L, Hirtz D, Ferriero DM. Improving the treatment of neonatal seizures: National Institute of Neurological Disorders and Stroke workshop. report. J Pediatr. 2008;153:12–5. doi: 10.1016/j.jpeds.2008.01.041. [DOI] [PubMed] [Google Scholar]
- Skov L, Pryds O, Greisen G, Lou H. Estimation of cerebral venous saturation in newborn infants by near infrared spectroscopy. Pediatr Res. 1993;33:52–5. doi: 10.1203/00006450-199301000-00011. [DOI] [PubMed] [Google Scholar]
- Toet MC, Lemmers PM, van Schelven LJ, van Bel F. Cerebral oxygenation and electrical activity after birth asphyxia: their relation to outcome. Pediatrics. 2006;117:333–9. doi: 10.1542/peds.2005-0987. [DOI] [PubMed] [Google Scholar]
- Tsuji M, Saul JP, du Plessis AJ, Eichenwald E, Sobh J, Crocker R, Volpe JJ. Cerebral intravascular oxygenation correlates with mean arterial pressure in critically ill premature infants. Pediatrics. 2000;106:625–32. doi: 10.1542/peds.106.4.625. [DOI] [PubMed] [Google Scholar]
- Uludag K, Dubowitz DJ, Yoder EJ, Restom K, Liu TT, Buxton RB. Coupling of cerebral blood flow and oxygen consumption during physiological activation and deactivation measured with fMRI. NeuroImage. 2004;23:148–55. doi: 10.1016/j.neuroimage.2004.05.013. [DOI] [PubMed] [Google Scholar]
- Wolthuis R, van Aken M, Fountas K, Robinson JS, Jr, Bruining HA, Puppels GJ. Determination of water concentration in brain tissue by Raman spectroscopy. Anal Chem. 2001;73:3915–20. doi: 10.1021/ac0101306. [DOI] [PubMed] [Google Scholar]
- Wong FY, Leung TS, Austin T, Wilkinson M, Meek JH, Wyatt JS, Walker AM. Impaired autoregulation in preterm infants identified by using spatially resolved spectroscopy. Pediatrics. 2008;121:e604–11. doi: 10.1542/peds.2007-1487. [DOI] [PubMed] [Google Scholar]
- Wray S, Cope M, Delpy DT. Characteristics of the near infrared absorption spectra of cytochrome aa3 and hemoglobin for the noninvasive monitoring of cerebral oxygenation. Biochim Biophys Acta. 1988;933:184–92. doi: 10.1016/0005-2728(88)90069-2. [DOI] [PubMed] [Google Scholar]
- Wyatt JS, Cope M, Delpy DT, Richardson CE, Edwards AD, Wray S, Reynolds EO. Quantitation of cerebral blood volume in human infants by near-infrared spectroscopy. J Appl Physiol. 1990;68:1086–91. doi: 10.1152/jappl.1990.68.3.1086. [DOI] [PubMed] [Google Scholar]
- Wyatt JS, Cope M, Delpy DT, Wray S, Reynolds EOR. Quantification of cerebral oxygenation and haemodynamics in sick newborn infants by near infrared spectrophotometry. Lancet. 1986;2:1063–6. doi: 10.1016/s0140-6736(86)90467-8. [DOI] [PubMed] [Google Scholar]
- Yoxall CW, Weindling AM. Measurement of cerebral oxygen consumption in the human neonate using near infrared spectroscopy: cerebral oxygen consumption increases with advancing gestational age. Pediatr Res. 1998;44:283–90. doi: 10.1203/00006450-199809000-00004. [DOI] [PubMed] [Google Scholar]
- Zhao F, Wang P, Hendrich K, Kim SG. Spatial specificity of cerebral blood volume-weighted fMRI responses at columnar resolution. NeuroImage. 2005;27:416–24. doi: 10.1016/j.neuroimage.2005.04.011. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.