Abstract
Central sensitization, caused either by tissue inflammation or peripheral nerve injury, plays an important role in persistent pain. An animal model of capsaicin-induced pain has well-defined peripheral and central sensitization components, thus is useful for studying the analgesic effect on two separate components. The focus of this study is to examine the analgesic effects of electroacupuncture (EA) on capsaicin-induced secondary hyperalgesia, which represents central sensitization.
Capsaicin (0.5%, 10 μl) was injected into the plantar side of the left hind paw, and foot withdrawal thresholds in response to von Frey stimuli (mechanical sensitivity) were determined for both primary and secondary hyperalgesia in rats. EA (2 Hz, 3 mA) was applied to various pairs of acupoints, GB30-GB34, BL40-BL60, GV2-GV6, LI3-LI6 and SI3-TE8, for 30 min under isofluraine anesthesia and then the effect of EA on mechanical sensitivity of paw was determined.
EA applied to the ipsilateral SI3-TE8, but none the other acupoints, significantly reduced capsaicin-induced secondary hyperalgesia but not primary hyperalgesia. EA analgesic effect was inhibited by a systemic non-specific opioid receptor (OR) antagonist or an intrathecal μ- or δ-OR antagonist. EA analgesic effect was not affected by an intrathecal κ-OR antagonist or systemic adrenergic receptor antagonist.
This study demonstrates that EA produces a stimulation point specific analgesic effect on capsaicin-induced secondary hyperalgesia (central sensitization), mediated by activating endogenous spinal μ and δ opioid receptors.
Keywords: electroacupuncture, capsaicin, hyperalgesia, opioid
1 Introduction
The use of acupuncture as an alternative approach for the management of persistent pain is receiving increasing recognition in pain clinics because conventional treatments for persistent pain are often unsuccessful and frequently cause side effects [42, 58]. In spite of laboratory evidence of acupuncture analgesia in persistent pain in animals and the widespread use of acupuncture at pain clinics, the results from controlled clinical studies are still highly contradictory [14, 50]. On the other hand, animal experiments have shown that electroacupuncture (EA) is very effective in ankle sprain pain and chronic inflammatory pain [20, 28, 63]. Thus, it is important to illuminate the mechanisms of EA analgesia for better understanding of EA effects on persistent pain and the development of mechanism-based treatment paradigms for persistent pain.
Peripheral and/or central sensitization play important roles in the initiation and maintenance of persistent pain, such as inflammatory pain, neuropathic pain, postherpetic neuralgia, referred pain, and postoperative pain [10]. Peripheral sensitization manifests as hyperalgesia in the injured region (primary hyperalgesia), and is characterized by the sensitized nociceptors responding more vigorously to a suprathreshold stimulus, as well as lowered thresholds for activation. On the other hand, central sensitization is responsible for spreading pain and hyperalgesia to uninjured tissue (secondary hyperalgesia), and is involved in pain-transmission neurons in the central nervous system (CNS), often in the dorsal horn of the spinal cord. Since the degree of contribution of peripheral and central sensitization varies in different pain conditions, any therapeutic interventions, including acupuncture, should examine the effectiveness for each component in any given persistent pain.
The capsaicin-induced hyperalgesia is an attractive animal model for studying the mechanisms of persistent pain. The reason is that pain produced by capsaicin, a pungent agent found in the chili pepper, has well-defined peripheral and central sensitization components that are manifested as primary and secondary hyperalgesia, respectively [11]. Primary hyperalgesia, occurring at the capsaicin-injection site, is explained by peripheral sensitization. The secondary hyperalgesia, which develops in the surrounding region not directly affected by capsaicin, is due to central sensitization of the spinal dorsal horn neurons [4, 46]. Therefore, this model provides an excellent opportunity to study the effect of electroacupuncture (EA) on central and peripheral mechanisms differentially.
The aims of this study are: 1) to see if EA produces an analgesic effect on capsaicin-induced hyperalgesia; 2) to find out which acupoint is most effective in producing analgesia; 3) to test if there is a point-specificity of EA; 4) to investigate neurotransmitters involved in EA analgesia; and 5) to identify the site of action for EA induced analgesia – central vs. peripheral sensitization.
2 Materials and methods
2.1 Experimental animals
Adult male Sprague-Dawley rats (270-330 g, Harlan Sprague-Dawley Co., Houston, TX) were used. The rats were housed in groups of three to four in plastic cages with soft bedding under a reversed 12-hour light/dark cycle (dark cycle: 8:00 a.m.–8:00 p.m.). Rats were housed in the same room at a constant ambient temperature with free access to food and water. All rats were housed for at least 7 days under these conditions prior to any experimental manipulations. All procedures involving the use of animals conformed to the guidelines of the International Association for the Study of Pain [65] and the National Institutes of Health and were approved by the Institutional Animal Care and Use Committee at the University of Texas Medical Branch.
2.2 Capsaicin injection
The procedure for capsaicin injection has been previously described in detail [33]. In brief, each rat was anesthetized with isoflurane, and a 27-gauge needle attached to a Hamilton syringe was inserted at a site near the heel (marked X on foot pad in Fig. 1A) and was advanced to the mid-plantar region (site I in Fig. 1A). Capsaicin (20 μg in 20 μl olive oil) was injected intradermally into the site I. Pressure was applied to the needle insertion site (site X) for 1 minute after removal of the needle to prevent leakage of the solution. Anesthesia was discontinued, and rats were returned to their cages when fully recovered from anesthesia. It usually took about 5 minutes for the rats to wake up and move around freely after anesthesia.
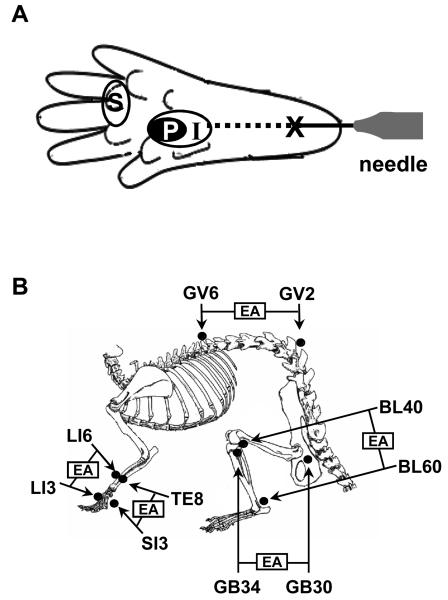
Fig. 1.
Schematic representation of the experimental design. (A) Drawing showing the sites of capsaicin injection and behavioral testing in the hind paw. For capsaicin injection, a 27 gauge hypodermic needle was inserted intradermally at the heel of the foot (X) and advanced to the injection site (I), and capsaicin (20 μg in 20 μl olive oil) was injected. Primary and secondary hyperalgesia were measured at the areas marked ‘P’ and ‘S’, respectively. (B) Anatomical locations of 5 pairs of acupoints used in this study. EA was applied ipsilaterally to the capsacin-injected paw at the following pairs of points; GB30-GB34, BL40-BL60, GV2-GV6, LI3-LI6 and SI3-TE8.
2.3 Behavioral testing for assessment of mechanical thresholds
All behavioral testing, except experiment 1 (exploring effective acupuncture points), was performed in a blinded fashion so that the investigator conducting the tests was not aware of the nature of manipulations done to the animals.
The 50% foot withdrawal thresholds, in response to mechanical stimuli applied to the capsaicin-injected hind paw, were measured and used as an indicator for the nociceptive thresholds (and the level of pain). Mechanical thresholds were assessed before and up to 6 hours after capsaicin injection. For each test, the animal was placed in a plastic chamber (8.0 × 8.5 × 20 cm) on top of a mesh screen platform and habituated for at least 10 minutes. Thresholds were determined by the up-down method [6, 13], using a set of von Frey monofilaments (von Frey numbers: 3.65, 3.87, 4.10, 4.31, 4.52, 4.74, 4.92 and 5.16; equivalent to 0.45, 0.74, 1.26, 2.04, 3.31, 5.50, 8.32 and 14.45 g, respectively). The primary and secondary hyperalgesia were measured by applying stimuli to the capsaicin injection site (site P) and the adjacent surrounding region (the base or the proximal part of the third and fourth digits, site S), respectively (Fig. 1A). A von Frey filament was applied perpendicular to the designated site with a sufficient force to bend the filament slightly for 2–3 seconds. An abrupt withdrawal of the foot during stimulation or immediately after stimulus removal was considered a positive response. The first stimulus was always applied with the 4.31 filament. If there was a positive response, the next lower filament was used, and if not, the next higher filament was applied. This testing pattern was continued until 6 responses were recorded from the first change of response (either higher or lower than the first stimulus, depending on whether the first response was negative or positive). The responses were then converted into a 50% threshold value using the formula: 50% threshold = 10(X + kd)/104, where X is the value of the final von Frey hair used in logarithmic units, k is the tabular value for positive/negative responses, and d is the mean difference between stimuli in logarithmic units (0.22) [13]. When positive or negative responses were still observed at the end of a stimulus session, respective values of 3.54 or 5.27 were assigned by assuming a value of ± 0.5 for k in these cases. The behavioral data were plotted using a linear scale in von Frey values.
2.4 Electroacupuncture (EA)
With the rats under gas anesthesia (isoflurane 1.25% in air), EA was applied by stimulating two points with electrical current through two stainless steel acupuncture needles (one needle at each acupoint). The needles (size: 0.10 mm in diameter and 7 mm in length of needle shaft) were inserted into acupuncture points at a depth of 5 mm and electrical stimulation was applied using a Grass S88 stimulator equipped with an SIU5 isolation unit (Grass Medical Instruments, Quincy, MA, USA). Trains of four pulses (1 ms long square wave pulses, 100 Hz of intra-train frequency) repeated at a rate of 2 Hz were delivered at an intensity of 3 mA. This stimulus paradigm was successfully used to produce acupuncture analgesia in a rat ankle-sprain pain model [28, 29]. The current delivered was monitored at all times and the polarity was reversed every 60 s to prevent polarization of the electrodes. The total duration of EA stimulation was 30 min. Immediately after the termination of EA, anesthesia was discontinued and the rats were returned to their cages. Rats resumed their normal activity within 5–10 min.
For the search for effective acupoints that induce analgesia on capsaicin-induced hyperalgesia (experiment 1), we tested several commonly used acupuncture points based on literature [24, 25, 47]. Acupuncture to certain acupoints, such as BL40 (Wei-Chung) [39], BL60 (K'un-Lun) [37], GB30 (Huan-T'iao) and/or GB34 (Yang-Lin-Chuan) [36, 38], GV2 (Yao-Shu) and GV6 (Chi-Chung) [8], SI3 (Hou-Hsi) [21] and TE8 (San-Yang-Lo) [22, 30, 43], has been reported to be effective to either alleviate pain or induce analgesia in animal models of neuropathic or inflammatory pain. Based on these previous studies, the following combinations of acupoints were selected for our investigation of the analgesic effects of EA on capsaicin-induced hyperalgesia in rats. The acupoints tested in this study include the combinations of GB30-GB34, BL40-BL60, GV2-GV6, LI3-LI6 and SI3-TE8. EA was applied to a pair of points located on the ipsilateral side to the capsaicin-injected paw (Fig. 1B). When an effective pair of acupoints was found, the effect of EA on each acupoint was also tested, using an EA needle constructed with a pair of acupuncture needles (2 mm apart). This study adopted the transpositional method, which locates animal acupoints on the surface of their skin corresponding to the human anatomic sites of acupoints [61].
2.5 Drug treatments
To explore possible mechanisms of EA-induced antihyperalgesia, we tested the possible involvement of endogenous opioid and adrenergic analgesic systems by using pharmacological receptor blockers, since these two are known to be involved in acupuncture analgesia [17, 28, 63]. As a systemic treatment, intraperitoneal injection was made for the following drugs: naltrexone hydrochloride (a nonspecific opioid receptor antagonist; 10 mg/ml in saline; 10 mg/kg) [29] and phentolamine hydrochloride (a nonspecific adrenergic receptor antagonist; 5 mg/ml in saline; 5 mg/kg) [3]. As a local spinal cord treatment, intrathecal injection was made for the following drugs: naltrexone hydrochloride (10 μg in 30 μl saline) [28]; D-Phe-Cys-Tyr-D-Trp-Arg-Pen-Thr-NH2 (CTAP; μ-opioid receptor antagonist, 3 μg in 30 μl saline) [7]; nor-binaltorphimine dihydrochloride (nBNI; kappa opioid receptor antagonist, 7.3 μg in 30 μl saline) [16]; and naltrindole hydrochloride (NTI, δ opioid receptor antagonist, 9 μg in 30 μl saline) [62]. All of the above agents used in this study were obtained from Sigma (St. Louis, MO, USA).
For intrathecal injection, we used a modified lumbar puncture technique [33]. Briefly, the spinal process of the sixth lumbar (L6) was palpated with the index finger, and a 27-gauge hypodermic needle (32 mm) connected to a 100-μl Hamilton syringe was inserted from the caudal end, 2-3 mm lateral to the L6 spinous process at a 45° angle to the vertebral column and was pushed slowly toward the cranioventral direction. When a sudden lateral tail movement was observed, a compound or saline was injected slowly for 30 seconds and the syringe was held in place for over 10 seconds to prevent outflow of the drug. The i.t. injection of 30 μl of a drug by the modified lumbar puncture technique [33] shows the average spread of a drug up to T10-T11 levels and the success rate of the lumbar puncture is >95% in our hand.
2.6 Data analysis
Behavioral data are presented as mean ± SEM (standard error of the mean). The data were analyzed by one- or two-way repeated-measurement analysis of variance (ANOVA), followed by post hoc testing using the Holm-Sidak method. P values ≤0.05 were considered statistically significant.
3 Results
The average 50% foot withdrawal threshold of the rat hind paw to von Frey stimuli was the von Frey value of 5.27, equivalent to a bending force of 18.7g, before capsaicin injection. One hour after capsaicin injection, mechanical thresholds were markedly decreased to the von Frey values of 4.25 ± 0.01 (1.79 ± 0.03 g, n=36) at the injection site (primary hyperalgesia; P area in Fig. 1A) and 4.30 ± 0.01 (1.96 ± 0.03 g, n=36) at the base or the proximal part of the third and fourth digits (secondary hyperalgesia; S area in Fig. 1A). The mechanical thresholds remained at significantly reduced levels for up to 4-5 hr following injection, as shown in Fig. 2 and 3.
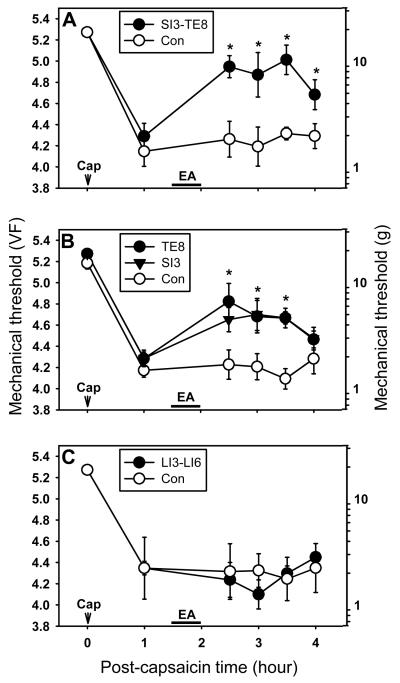
Fig. 2.
The effect of EA applied to different sites on capsaicin-induced secondary hyperalgesia (behavioral tests done by using the site “S” in Fig. 1A). (A) The effects of EA at SI3-TE8 on capsaicin-induced hyperalgesia. Immediately after measurement of the baseline mechanical threshold, capsaicin was injected intradermally. The mechanical threshold was measured at 1 hr after capsaicin injection and then EA was applied to the SI3-TE8 pair of points ipsilateral to the capsaicin-injected paw for 30 min. Behavior tests were performed at 0.5, 1.0, 1.5 and 2 hr after the termination of acupuncture (up to 4 hr after capsaicin). (B) The effect of single point stimulation at SI3 or TE8 on capsaicin-induced hyperalgesia. (C) The effect of EA at LI3-LI6 pair. Control rats received anesthesia only without EA in all experiments. Data are expressed as means ± SEM. Number of animals - A: 6 in SI3-TE8 and 6 in Con groups; B: 6 in TE8, 6 in SI3, and 5 in Con groups; C: 6 in LI3-LI6 and 6 in Con groups. Data were analyzed by two-way repeated measure Analysis of Variance (ANOVA), followed by the Holm-Sidak post hoc test. *, a value significantly (p<0.05) different from the corresponding value in the control group.
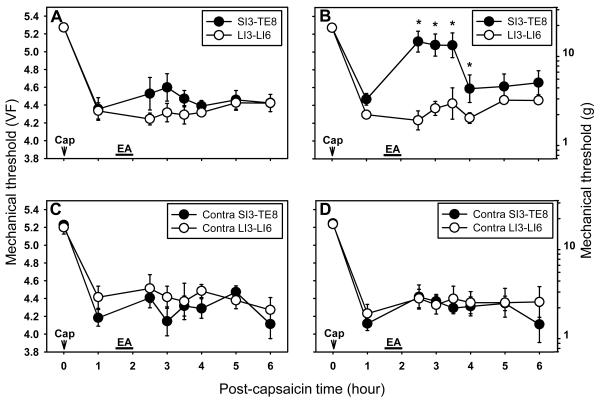
Fig. 3.
The effects of ipsilateral and contralateral EA at SI3-TE8 on capsaicin-induced primary and secondary hyperalgesia. A and B are experiments with ipsilateral EA at SI3-TE8 while C and D are with contralateral EA at the same points. A and C show the threshold measurements for the primary hyperalgesia, while B and D are for the secondary hyperalgesia. Data are expressed as means ± SEM. Number of animals - A and B: 6 in SI3-TE8 and 6 in LI3-LI6 groups; C and D: 6 in Contra SI3-TE8 and 6 in Contra LI3-LI6 groups. Data were analyzed by two-way repeated measure ANOVA, followed by the Holm-Sidak post hoc test. *, a value significantly (p<0.05) different from the corresponding value in the control group (LI3-LI6 EA group).
3.1 Experiment 1 - Are there any acupoints that can produce antihyperalgesic effects on capsaicin-induced secondary hyperalgesia?
As an initial step, the effective analgesic EA points on capsaicin-induced hyperalgesia were identified by applying EA at various points and then measuring the secondary hyperalgesia. A total of 5 pairs of acupoints (GB30-GB34, BL40-BL60, GV2-GV6, LI3-LI6 and SI3-TE8) were tested. Testing of each pair of acupoints was done on 12 rats, randomly divided into 2 groups of control (n=6) and acupuncture (n=6). On the day of the experiment, rats were placed in the behavioral testing chamber and baseline foot withdrawal thresholds to mechanical stimuli were measured. After measuring baseline thresholds, each rat received a capsaicin injection. One hour after capsaicin injection, mechanical thresholds were measured again and then EA was applied (begun 1.5 hr after capsaicin injection) for 30 min at a chosen pair of points ipsilateral to the capsaicin-injected paw. Behavioral tests were performed at 0.5, 1.0, 1.5 and 2 hr after the termination of acupuncture (up to 4 hr after capsaicin). Control rats received anesthesia only without EA stimulation.
EA at BL40-BL60 significantly increased mechanical thresholds only at the time point of 2 hr after termination of EA compared to the control. EA application to GB30-GB34 and GV2-GV6 failed to show any significant changes in mechanical thresholds compared to the non-EA groups (data not shown). On the other hand, EA at SI3-TE8 points greatly reduced the capsaicin-induced secondary hyperalgesia to von Frey value of 4.9 ± 0.10 (8.00 ± 0.18 g, n=6) at 30 min after termination of EA. This von Frey value is significantly different when compared to the pre-EA values of 4.28 ± 0.12 (1.92 ± 0.61 g, P<0.01) or non-treated control of 4.26 ± 0.15 (1.83 ± 0.65 g, P<0.01). These effects lasted longer than 2 hr (Fig. 2A). To examine which one of those 2 points is critical for analgesic effects, EA was applied to a single point, either SI3 or TE8, and the results are shown in Fig. 2B. The magnitude of antihyperalgesic effect of EA at SI3 (n=6) is similar to that at TE8 (n=6) but little less than EA to both of the points.
We also tested EA at a pair of LI3 and LI6 on the forelimb. As shown in Fig. 2C, EA at LI3-LI6 did not produce any analgesia at all. Accordingly, we chose pairs of SI3-TE8 and LI3-LI6 for EA and EA-control groups, respectively, in the following experiment.
3.2 Experiment 2 - Electroacupuncture at ipsilateral SI3-TE8 induces antihyperalgesic effects on capsaicin-induced secondary hyperalgesia
To further evaluate the effects of EA at SI3-TE8 on capsaicin-induced hyperalgesia, we extended the time for behavior testing of secondary hyperalgesia until EA-induced antihyperalgesic effects dissolve. In addition, the primary hyperalgesia was also measured to explore EA effects on peripheral sensitization. Figure 3 shows the effects of EA at ipsilateral SI3-TE8 on capsaicin-induced primary and secondary hyperalgesia. EA at ipsilateral LI3-LI6 was used as a control group. EA at ipsilateral SI3-TE8 markedly increased the withdrawal thresholds on the secondary hyperalgesia site up to 2 hr after EA compared to the control group (Fig. 3B, P<0.05), thus confirming the previous results shown in Fig. 2A. On the other hand, there was no significant change on primary hyperalgesia (Fig. 3A). EA applied to ipsilateral LI3-LI6 did not change either the primary or the secondary hyperalgesia induced by capsaicin (Fig. 3A & 3B).
We also examined the effect of EA applied to the contralateral SI3-TE8 or LI3-LI6 points on capsaicin-induced hyperalgesia. In contrast to the antihyperalgeic effect of EA on ipsilateral SI3-TE8, EA at contralateral SI3-TE8 (n=6) did not produce any effect on capsaicin-induced hyperalgesia (Fig. 3C & 3D). EA at contralateral LI3-LI6 (n=6) had no effect on capsaicin-induced hyperalgesia (Fig. 3C & 3D).
3.3 Experiment 3 - The endogenous opioid system mediates EA-induced antihyperalgesia
To explore the possible involvement of endogenous adrenergic or opioid system in EA-induced antihyperalgesia, we examined the effect of systemically injected phentolamine (PTL, a non-specific adrenergic receptor antagonist; 5 mg/kg in 5 mg/ml saline) or naltrexone hydrochloride (NTX, a non-specific opioid receptor antagonist; 10 mg/kg in 10 mg/ml saline) on EA-induced antihyperalgesia.
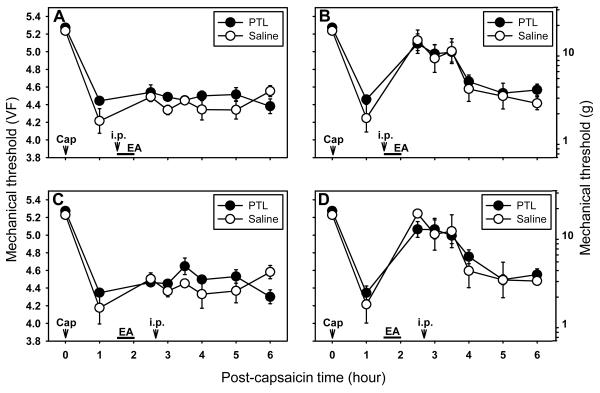
When phentolamine (PTL, 5 mg/kg, i.p., n=5) was administered at 10 min after the beginning of EA to the ipsilateral SI3-TE8 in capsaicin treated rats, there was no significant change in mechanical thresholds as compared to the saline injected control group (n=6) in either primary (Fig. 4A) or secondary (Fig. 4B) hyperalgesia. Thus, the results showed that systemic phentolamine failed to block the development of EA-induced antihyperalgesic effect on the secondary hyperalgesia. In addition, when phentolamine was injected during the maintenance period of EA-induced antihyperalgesia (n=8), it did not change EA-induced antihyperalgesic effects as compared to the saline (n=5) control group in either primary (Fig. 4C) or secondary (Fig. 4D) hyperalgesia.
Fig. 4.
Effect of systemic phentolamine (PTL, a non-specific adrenergic receptor antagonist) on capsaicin-induced primary and secondary hyperalgesia. A and B show the effect of pretreatment of systemic PTL (i.p., 5 mg/kg) on EA-induced antihyperalgesic effect on the primary (A) and secondary (B) hyperalgesia. Phentolamine (or saline) was injected intraperitoneally (i.p.) 10 min after beginning of EA. C and D show the effect of systemic PTL (i.p., 5 mg/kg) injected during the maintenance of EA-induced antihyperalgesia on the primary (C) and secondary (D) hyperalgesia. Phentolamine (or saline) was injected intraperitoneally (i.p.) immediately after behavior measurement at 0.5 hr post EA. Data are expressed as means ± SEM. Number of animals - A and B: 5 in PTL and 6 in Saline groups; C and D: 8 in PTL and 5 in Saline groups. Data were analyzed by two-way repeated measure ANOVA, followed by the Holm-Sidak post hoc test. *, a value significantly (p<0.05) different from the corresponding value in the saline control group.
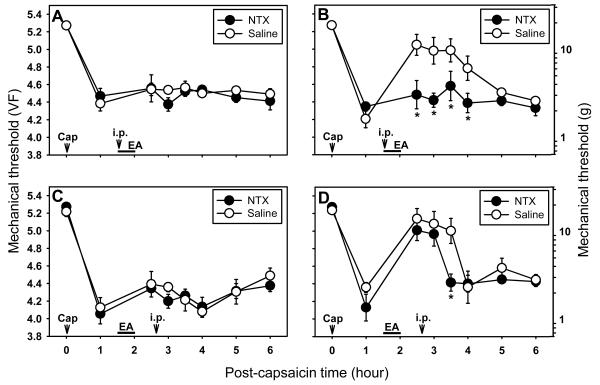
On the other hand, when naltrexone (NTX; a non-specific opioid receptor antagonist) was administered systemically (10 mg/kg, i.p., n=6) 10 min after the initiation of EA, EA-induced antihyperalgesic effect on the secondary hyperalgesia was completely blocked (Fig. 5B). When naltrexone was administered after EA-induced antihyperalgesia was completely developed (0.5 hr after the termination of EA), it showed a slightly delayed inhibition of the EA-induced antihyperalgesic effect (n=6, Fig. 5D). Naltrexone did not show any change in capsaicin-induced primary hyperalgesia (Fig. 5A & 5C). These data suggest that the endogenous opioid system is involved in mediating EA-induced antihyperalgesic effects on capsaicin-induced secondary hyperalgesia.
Fig. 5.
Effect of systemic naltrexone (NTX; a non-specific opioid receptor antagonist) on capsaicin-induced primary and secondary hyperalgesia. A and B show the effect of pretreatment of systemic NTX (i.p., 10 mg/kg) on EA-induced antihyperalgesic effect on the primary (A) and secondary (B) hyperalgesia. Naltrexone (or saline) was injected intraperitoneally (i.p.) 10 min after beginning of EA. C and D show the effect of systemic NTX (i.p., 10 mg/kg) injected during the maintenance of EA-induced antihyperalgesia on the primary (C) and secondary (D) hyperalgesia. Naltrexone (or saline) was injected intraperitoneally (i.p.) immediately after behavior measurement at 0.5 hr post EA. Data are expressed as means ± SEM. Number of animals - A and B: 6 in NTX and 7 in Saline groups; C and D: 6 in NTX and 6 in Saline groups. Data were analyzed by two-way repeated measure ANOVA, followed by the Holm-Sidak post hoc test. *, a value significantly (p<0.05) different from the corresponding value in the saline control group.
3.4 Experiment 4 - The spinal opioid system is critical for EA-induced antihyperalgesia
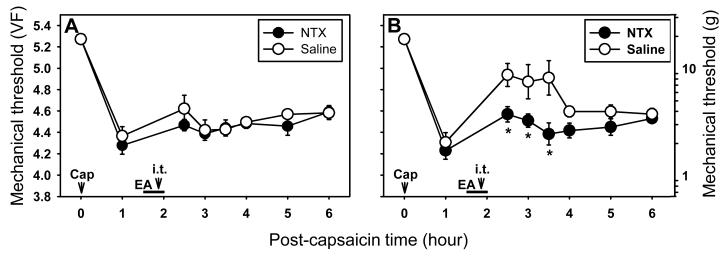
Since it was determined that the endogenous opioid system is involved in the EA-induced antihyperalgesic effect, the possible involvement of the spinal opioid system was tested by injecting naltrexone intrathecally. In capsaicin-treated rats, EA was applied at ipsilateral SI3-TE8 (n=15) and then NTX (10 μg in 30 μl saline, n=8) was injected intrathecally 10 min before the termination of EA. The remaining 7 rats received saline intrathecally instead of NTX. Mechanical thresholds were measured for up to 4 hr after termination of EA. EA at SI3-TE8 did not affect primary hyperalgesia in either the NTX or saline groups (Fig. 6A). Intrathecal NTX almost completely inhibited EA-induced antihyperalgesic effects on secondary hyperalgesia (Fig. 6B). The data suggest that antihyperalgesic effects of EA on capsaicin-induced secondary hyperalgesia are mediated mainly by the endogenous spinal opioid system.
Fig. 6.
Effects of intrathecal naltrexone on capsaicin-induced primary and secondary hyperalgesia. A and B show the effect of intrathecal NTX (10 μg in 30 μl saline) on EA-induced antihyperalgesic effect on the primary (A) and secondary (B) hyperalgesia. Naltrexone (or saline) was injected inthecally (i.t.) 10 min before termination of EA (20 min after start of EA). Data are expressed as means ± SEM. Number of animals - A and B: 8 in NTX and 7 in Saline groups. Data were analyzed by two-way repeated measure ANOVA, followed by the Holm-Sidak post hoc test. *, a value significantly (p<0.05) different from the corresponding value in the saline control group.
3.5 Experiment 5 - μ- and δ-opioid receptors are involved to mediate EA-induced antihyperalgesia
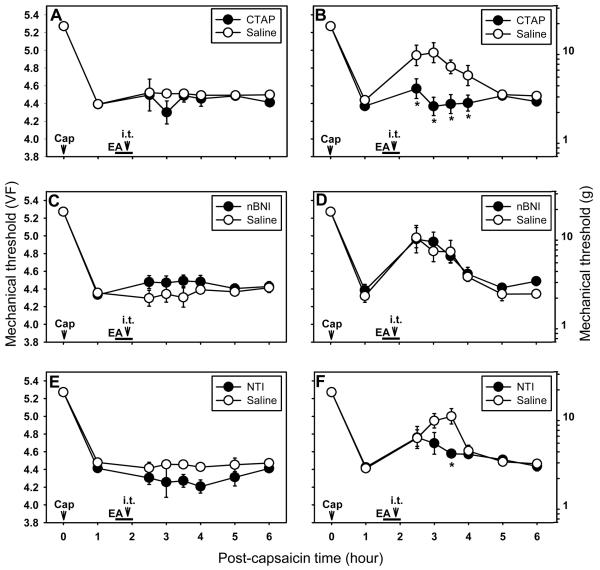
To explore which subtypes of opioid receptors are involved in mediating EA-induced antihyperalgesic effects in the spinal cord, we examined the effects of intrathecally injected specific μ-, δ- and κ-opioid receptor antagonists on EA-induced antihyperalgesia. The drugs used include a μ-opioid receptor antagonist, D-Phe-Cys-Tyr-D-Trp-Arg-Pen-Thr-NH2 (CTAP; 3 μg), a κ-opioid receptor antagonist, nor-binaltorphimine dihydrochloride (nBNI; 7.3 μg) and a δ-opioid receptor antagonist, naltrindole hydrochloride (NTI; 9 μg). The test set for each drug consisted of 12 rats randomly divided into 2 groups of drug treatment (n=6) and saline control (n=6). Each drug was dissolved in saline and administered in a volume of 30 μl and injected intrathecally 10 min before termination of EA at SI3-TE8 in capsaicin-injected rats. The same volume of saline was injected in control groups. The results are shown in Fig. 7. The intrathecal injection of CTAP almost completely blocked the development of antihyperalgesic effect by EA on secondary hyperalgesia (Fig. 7B). The κ opioid receptor antagonist, nBNI, did not influence the EA-induced antihyperalgesic effects (Fig. 7D) but a δ opioid receptor antagonist, NTI, reduced EA-induced antihyperalgesic effects moderately for about one hour as compared to the saline control group (P<0.01, Fig. 7F). EA at SI3-TE8 did not change primary hyperalgesia in either compounds or saline groups (Fig. 7A, 7C, and 7E). These data suggest that EA analgesia on capsaicin-induced secondary hyperalgesia is mediated by spinal μ- and δ-opioid receptors, but not by κ-opioid receptors.
Fig. 7.
Effects of intrathecal injection of subtypes of opioid antagonists on capsaicin-induced primary and secondary hyperalgesia. A and B show the effect of intrathecal D-Phe-Cys-Tyr-DTrp-Arg-Pen-Thr-NH2 (CTAP; μ-opioid receptor antagonist, 3 μg), C and D show the effect of nor-binaltorphimine dihydrochloride (nBNI; κ-opioid receptor antagonist, 7.3 μg), and E and F show the effect of intrathecal naltrindole hydrochloride (NTI; δ-opioid receptor antagonist, 9 μg). A, C, and E are for the primary hyperalgesia while B, D, and E are for the secondary hyperalgesia. All intrathecal injections were made at 10 min before termination of EA (20 min after start of EA). Data are expressed as means ± SEM. Number of animals - A and B: 6 in CTAP and 6 in Saline groups; C and D: 6 in nBNI and 6 in Saline groups; E and F: 6 in NTI and 6 in Saline groups. Data were analyzed by two-way repeated measure ANOVA, followed by the Holm-Sidak post hoc test. *, a value significantly (p<0.05) different from the corresponding value in the saline control group.
4 Discussion
The present study explored the effects of EA on capsaicin-induced hyperalgesia in rats. EA applied to ipsilateral SI3-TE8 suppressed capsaicin-induced secondary hyperalgesia, but not primary hyperalgesia. Single-point stimulation at either SI3 or TE8 also produced similar effects with a lesser magnitude, thus suggesting that both sites have synergistic and overlapping effect. This antihyperalgesic effect by EA was inhibited by a spinal injection of non-specific-, μ- or δ-opioid receptor antagonist but not by an antagonist for adrenergic receptor or κ-opioid receptor. The data suggest that the antihyperalgesic effects of EA at ipsilateral SI3-TE8 are mediated by spinal μ- and δ-opioid receptors, but not by adrenergic receptors or κ-opioid receptors. Since the majority of acupuncture studies used animal models in which both peripheral and central pain components are combined, it has been argued whether EA functions mainly via a central or peripheral mechanism or both. This study demonstrates that EA produces antihyperalgesic effects mainly via a central mechanism, because this study utilized the rat capsaicin pain model in which central and peripheral pain components were tested separately.
4.1 EA at SI3-TE8 produces analgesia on capsaicin-induced secondary hyperalgesia
Intradermal capsaicin injection produces primary and secondary hyperalgesia, due to peripheral and central sensitization, respectively [52]. Therefore, capsaicin-induced hyperalgesia is a good model to study the effect of electroacupuncture (EA) on central and peripheral sensitization independently. Among 5 different combinations of acupoints (GB30-GB34, BL40-BL60, GV2-GV6, LI3-LI6 and SI3-TE8) tested, only EA at SI3-TE8 on the ipsilateral to the capsaicin injection side alleviates capsaicin-induced secondary hyperalgesia. EA at the single point of SI3 or TE8 also significantly suppresses secondary hyperalgesia. The acupoint SI3 has been used empirically for the treatment of pain in the neck, chest, and lumbar region, as well as headache and fever [24, 25, 31]. The acupoint TE8 has also been used clinically for treatment of pain in the scapula and forelimb and headache [24, 25]. The present studies provided evidence that EA at SI3 and TE8 (these acupoints have been used empirically for pain management) has analgesic effects on capsaicin-induced secondary hyperalgesia
4.2 Point-and disease-specificity of SI3-TE8
EA-induced analgesic effects in capsaicin-induced hyperalgesia is produced only by stimulation at SI3 and/or TE8 of the forelimb, but not at nearby points (LI3-LI6) or several other points (GB30-GB34, BL40-BL60 and GV2-GV6), thus indicating point-specificity of EA effects. Acupuncture is believed to have ‘point- and disease-specificity’ meaning that specific acupoints have specific functions and may treat certain types of diseases. However, there has been a certain degree of skepticism about ‘point- and disease-specificity.’ Our previous studies showed that ankle sprain pain was relieved by EA at SI6, but not at nearby LI4 [29], thus suggesting the ‘point-specificity’ of acupuncture. Kim and his colleagues [26] also showed that acupuncture at HT7, but not at nearby point TE8, suppresses dopamine release in the nucleus accumbens and behavioral hyperactivity in the morphine addiction model. In contrast, Cho and his colleagues [9] reported that there was no statistical difference between acupoint and nonacupoint acupuncture in a functional MRI study using an experimental human pain model, thus suggesting no point specificity. Therefore, the point specificity of acupuncture is still a controversial issue and is a subject of further study.
It is worth note that our results showed that EA only at the ipsilateral SI3-TE8, but not the same sites on the contralateral side, has a significant analgesic effect on the capsaicin-induced secondary hyperalgesia. Previous studies have shown that unilateral acupuncture can produce effects on the ipsilateral, contralateral or bilateral loci, depending on their pathological conditions [34, 37, 38, 41, 56, 64]. The ipsilateral efficacy of EA in our study suggests that the EA effect is conveyed through the ipsilateral descending inhibitory system. This is reasonable since it is well known that many descending inhibitory systems from the amygdala, periaqueductal gray (PAG) and rostroventral medulla (RVM) influence nociceptive signals entering primarily the ipsilateral spinal cord [35, 40, 53, 54] and are affected by ipsilateral acupuncture [2, 44, 45].
4.3 Suppression of central sensitization by EA
The present data show that EA at SI3-TE8 has analgesic effects on secondary hyperalgesia, but not primary, in the rat intradermal capsaicin model, suggesting the inhibition of central sensitization. Although acupuncture has been used clinically for alleviation of various types of pain [1, 50, 58], there is not enough scientific validation for the use of acupuncture in persistent pain. Our study provides evidence that acupuncture inhibits central sensitization, the critical mechanism of persistent pain.
The processing of pain information occurs at central (spinal and supraspinal) and peripheral sites, and thus modification (attenuation or exacerbation) of pain levels can be achieved through interventions at multiple sites [12]. Although the exact locations where EA modifies pain are not clearly identified, acupuncture is shown to activate the ascending sensory pathways such as spinal dorsal horn and thalamus [32, 48, 60], or the descending pain inhibitory mechanisms, such as opioid, adrenergic and serotonergic pathways [17, 28, 36, 51]. The above evidence suggests the central mechanism of EA analgesia. On the other hand, NMDA receptor expression in primary sensory neurons is inhibited by EA [56], suggesting the peripheral effect of EA analgesia. The present data show that EA reduces secondary hyperalgesia, but not primary hyperalgesia, through activation of the central opioid system, thus confirming EA modulation of pain through a central, but not peripheral, mechanism.
4.4 Mediation of spinal μ- and δ-opioid receptors, but not κ-receptors in EA-induced antihyperalgesia
There are three distinct opioid receptor (OR) types in the central nervous system; μ, δ and κ receptors [5]. Since the EA-induced antihyperalgesia was inhibited by intrathecal injection of a μ-OR antagonist, or a δ-OR antagonist, but not by a κ-OR antagonist, these data suggest that EA analgesia is mediated by the μ- and δ ORs in the spinal cord. Consistent with our study, previous studies have reported that EA is mediated by the μ and δ, but not κ, ORs in hyperalgesia induced by complete Freund's adjuvant (CFA) inflammation [63].
EA induces release of various endogenous opioids, including endomorphins, endorphin, enkephalin and dynorphin [18, 57]. Endomorphins, enkephalin and dynorphin possess a selective high affinity for μ, δ and κ ORs, respectively. Endorphin is known to have a high affinity for both μ and δ ORs in the spinal cord [59]. The terminals and fibers containing these opioids are concentrated in the superficial dorsal horn in the spinal cord and exert inhibitory effects on nociceptive transmission [5]. Previous studies have demonstrated that stimulation frequencies of EA or transcutaneous electrical nerve stimulation (TENS) determine the types of opioid peptides released in the central nervous system and thus the types of pain controlled. It has been shown that low frequency (2 Hz) stimulation releases endomorphins, enkephalin and/or endorphin and activates μ/δ-ORs, while high frequency (100 Hz) stimulation releases dynorphin to act on κ-ORs in the spinal cord [17-19, 55]. In addition, low frequency stimulation reduces mainly secondary hyperalgesia but high frequency stimulation reduces primary hyperalgesia [15, 27]. The EA parameters used in the present study likely activated the descending pain inhibitory system that releases opioid peptides in the spinal cord [23, 48, 49].
4.5 Conclusion
This study demonstrates that EA at ipsilateral SI3-TE8 suppresses capsaicin-induced secondary hyperalgesia (central sensitization) in a point-specific manner. This EA analgesia is mediated, at least in part, by spinal μ and δ opioid receptors. An important finding of this study is that EA selectively inhibits centrally mediated pain via suppressing central sensitization.
Acknowledgements
This work was supported by NIH/NCCAM grant 5 R01 AT001474.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
There are no conflicts of interest.
References
- 1.NIH Consensus Conference Acupuncture. JAMA. 1998;280:1518–24. [PubMed] [Google Scholar]
- 2.Bai L, Qin W, Tian J, Liu P, Li L, Chen P, Dai J, Craggs JG, von Deneen KM, Liu Y. Time-varied characteristics of acupuncture effects in fMRI studies. Hum Brain Mapp. 2009 doi: 10.1002/hbm.20769. doi:10.1002/hbm.20769. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Barres C, de Souza Neto EP, Julien C. Effect of alpha-adrenoceptor blockade on the 0.4 Hz sympathetic rhythm in conscious rats. Clin Exp Pharmacol Physiol. 2001;28:983–5. doi: 10.1046/j.1440-1681.2001.03561.x. [DOI] [PubMed] [Google Scholar]
- 4.Baumann TK, Simone DA, Shain CN, LaMotte RH. Neurogenic hyperalgesia: the search for the primary cutaneous afferent fibers that contribute to capsaicin-induced pain and hyperalgesia. J Neurophysiol. 1991;66:212–27. doi: 10.1152/jn.1991.66.1.212. [DOI] [PubMed] [Google Scholar]
- 5.Bodnar RJ, Klein GE. Endogenous opiates and behavior: 2005. Peptides. 2006;27:3391–478. doi: 10.1016/j.peptides.2006.07.011. [DOI] [PubMed] [Google Scholar]
- 6.Chaplan SR, Bach FW, Pogrel JW, Chung JM, Yaksh TL. Quantitative assessment of tactile allodynia in the rat paw. J Neurosci Methods. 1994;53:55–63. doi: 10.1016/0165-0270(94)90144-9. [DOI] [PubMed] [Google Scholar]
- 7.Chen SR, Pan HL. Blocking mu opioid receptors in the spinal cord prevents the analgesic action by subsequent systemic opioids. Brain Res. 2006;1081:119–25. doi: 10.1016/j.brainres.2006.01.053. [DOI] [PubMed] [Google Scholar]
- 8.Chen YY, Zhang W, Chen YL, Chen SJ, Dong H, Zeng YS. Electro-acupuncture improves survival and migration of transplanted neural stem cells in injured spinal cord in rats. Acupunct Electrother Res. 2008;33:19–31. doi: 10.3727/036012908803861212. [DOI] [PubMed] [Google Scholar]
- 9.Cho ZH, Son YD, Han JY, Wong EK, Kang CK, Kim KY, Kim HK, Lee BY, Yim YK, Kim KH. fMRI neurophysiological evidence of acupuncture mechanisms. Med Acupuncture. 2003;14:16–22. [Google Scholar]
- 10.Coderre TJ, Katz J. Peripheral and central hyperexcitability: differential signs and symptoms in persistent pain. Behav Brain Sci. 1997;20:404–19. doi: 10.1017/s0140525x97251484. discussion 435-513. [DOI] [PubMed] [Google Scholar]
- 11.Coderre TJ, Melzack R. Cutaneous hyperalgesia: contributions of the peripheral and central nervous systems to the increase in pain sensitivity after injury. Brain Res. 1987;404:95–106. doi: 10.1016/0006-8993(87)91359-x. [DOI] [PubMed] [Google Scholar]
- 12.DeLeo JA. Basic science of pain. J Bone Joint Surg Am. 2006;88(Suppl 2):58–62. doi: 10.2106/JBJS.E.01286. [DOI] [PubMed] [Google Scholar]
- 13.Dixon WJ. Efficient analysis of experimental observations. Annu Rev Pharmacol Toxicol. 1980;20:441–62. doi: 10.1146/annurev.pa.20.040180.002301. [DOI] [PubMed] [Google Scholar]
- 14.Ezzo J, Berman B, Hadhazy VA, Jadad AR, Lao L, Singh BB. Is acupuncture effective for the treatment of chronic pain? A systematic review. Pain. 2000;86:217–25. doi: 10.1016/S0304-3959(99)00304-8. [DOI] [PubMed] [Google Scholar]
- 15.Gopalkrishnan P, Sluka KA. Effect of varying frequency, intensity, and pulse duration of transcutaneous electrical nerve stimulation on primary hyperalgesia in inflamed rats. Arch Phys Med Rehabil. 2000;81:984–90. doi: 10.1053/apmr.2000.5576. [DOI] [PubMed] [Google Scholar]
- 16.Guirimand F, Strimbu-Gozariu M, Willer JC, Le Bars D. Effects of mu, delta and kappa opioid antagonists on the depression of a C-fiber reflex by intrathecal morphine and DAGO in the rat. J Pharmacol Exp Ther. 1994;269:1007–20. [PubMed] [Google Scholar]
- 17.Han JS. Acupuncture and endorphins. Neurosci Lett. 2004;361:258–61. doi: 10.1016/j.neulet.2003.12.019. [DOI] [PubMed] [Google Scholar]
- 18.Han JS. Acupuncture: neuropeptide release produced by electrical stimulation of different frequencies. Trends Neurosci. 2003;26:17–22. doi: 10.1016/s0166-2236(02)00006-1. [DOI] [PubMed] [Google Scholar]
- 19.Han JS, Chen XH, Sun SL, Xu XJ, Yuan Y, Yan SC, Hao JX, Terenius L. Effect of lowand high-frequency TENS on Met-enkephalin-Arg-Phe and dynorphin A immunoreactivity in human lumbar CSF. Pain. 1991;47:295–8. doi: 10.1016/0304-3959(91)90218-M. [DOI] [PubMed] [Google Scholar]
- 20.Huang C, Hu ZP, Long H, Shi YS, Han JS, Wan Y. Attenuation of mechanical but not thermal hyperalgesia by electroacupuncture with the involvement of opioids in rat model of chronic inflammatory pain. Brain Res Bull. 2004;63:99–103. doi: 10.1016/j.brainresbull.2004.01.006. [DOI] [PubMed] [Google Scholar]
- 21.Jeong J, Yun D, Na C, Yu C, Yun Y, Jo M. Effects of acupuncture at SI3, BL40, SI3-BL40 on neuropathic pain control and c-Fos protein expression in rats. J Kor Soc Acu Mox. 2004;21:240–251. [Google Scholar]
- 22.Jeong SM. Effects of electroacupuncture on minimum alveolar concentration of isoflurane and cardiovascular system in isoflurane anesthetized dogs. J Vet Sci. 2002;3:193–201. [PubMed] [Google Scholar]
- 23.Kalra A, Urban MO, Sluka KA. Blockade of opioid receptors in rostral ventral medulla prevents antihyperalgesia produced by transcutaneous electrical nerve stimulation (TENS) J Pharmacol Exp Ther. 2001;298:257–63. [PubMed] [Google Scholar]
- 24.Kang SB. Acupuncture and Moxibustion based on ancient books. Vol. 1. Iljung co.; Seoul: 2000. [Google Scholar]
- 25.Kim HY, Shim I, Hahm DH, Seo K, Nam TC, Lee HJ. Canine Acupuncture. Korvet Co.; Seoul: 2004. [Google Scholar]
- 26.Kim MR, Kim SJ, Lyu YS, Kim SH, Lee Y, Kim TH, Shim I, Zhao R, Golden GT, Yang CH. Effect of acupuncture on behavioral hyperactivity and dopamine release in the nucleus accumbens in rats sensitized to morphine. Neurosci Lett. 2005;387:17–21. doi: 10.1016/j.neulet.2005.07.007. [DOI] [PubMed] [Google Scholar]
- 27.King EW, Sluka KA. The effect of varying frequency and intensity of transcutaneous electrical nerve stimulation on secondary mechanical hyperalgesia in an animal model of inflammation. J Pain. 2001;2:128–33. doi: 10.1054/jpai.2001.19963. [DOI] [PubMed] [Google Scholar]
- 28.Koo ST, Lim KS, Chung K, Ju H, Chung JM. Electroacupuncture-induced analgesia in a rat model of ankle sprain pain is mediated by spinal alpha-adrenoceptors. Pain. 2008;135:11–9. doi: 10.1016/j.pain.2007.04.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Koo ST, Park YI, Lim KS, Chung K, Chung JM. Acupuncture analgesia in a new rat model of ankle sprain pain. Pain. 2002;99:423–31. doi: 10.1016/S0304-3959(02)00164-1. [DOI] [PubMed] [Google Scholar]
- 30.Kuang X, Su Y, Guo H, Liu W, Geng W, Li S. Study on combined acupunture with general anesthesia in pneumonectomy. Chinese Journal of Integrative Medicine. 1996;2:255–257. [PubMed] [Google Scholar]
- 31.Kvorning N, Holmberg C, Grennert L, Aberg A, Akeson J. Acupuncture relieves pelvic and low-back pain in late pregnancy. Acta Obstet Gynecol Scand. 2004;83:246–50. doi: 10.1111/j.0001-6349.2004.0215.x. [DOI] [PubMed] [Google Scholar]
- 32.Lao L, Zhang RX, Zhang G, Wang X, Berman BM, Ren K. A parametric study of electroacupuncture on persistent hyperalgesia and Fos protein expression in rats. Brain Res. 2004;1020:18–29. doi: 10.1016/j.brainres.2004.01.092. [DOI] [PubMed] [Google Scholar]
- 33.Lee I, Kim HK, Kim JH, Chung K, Chung JM. The role of reactive oxygen species in capsaicin-induced mechanical hyperalgesia and in the activities of dorsal horn neurons. Pain. 2007;133:9–17. doi: 10.1016/j.pain.2007.01.035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Leung A, Khadivi B, Duann JR, Cho ZH, Yaksh T. The effect of Ting point (tendinomuscular meridians) electroacupuncture on thermal pain: a model for studying the neuronal mechanism of acupuncture analgesia. J Altern Complement Med. 2005;11:653–61. doi: 10.1089/acm.2005.11.653. [DOI] [PubMed] [Google Scholar]
- 35.Levine R, Morgan MM, Cannon JT, Liebeskind JC. Stimulation of the periaqueductal gray matter of the rat produces a preferential ipsilateral antinociception. Brain Res. 1991;567:140–4. doi: 10.1016/0006-8993(91)91446-8. [DOI] [PubMed] [Google Scholar]
- 36.Li A, Wang Y, Xin J, Lao L, Ren K, Berman BM, Zhang RX. Electroacupuncture suppresses hyperalgesia and spinal Fos expression by activating the descending inhibitory system. Brain Res. 2007;1186:171–9. doi: 10.1016/j.brainres.2007.10.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Li WM, Cui KM, Li N, Gu QB, Schwarz W, Ding GH, Wu GC. Analgesic effect of electroacupuncture on complete Freund's adjuvant-induced inflammatory pain in mice: a model of antipain treatment by acupuncture in mice. Jpn J Physiol. 2005;55:339–44. doi: 10.2170/jjphysiol.RP001505. [DOI] [PubMed] [Google Scholar]
- 38.Liu F, Li J, Han Y, Yang ZD, Xiao Y, Liu J, Li M, Peng B, Zhang J, Li LL, Shi J. Effects of electroacupuncture on GDNF positive cell immunoreactivity in local dermal tissue of the inflammatory pain focus in the rat of adjuvant arthritis. Zhongguo Zhen Jiu. 2006;26:436–40. [PubMed] [Google Scholar]
- 39.Ma C, Li CX, Yi JL, Yan LP. Effects of electroacupuncture on glutamate and aspartic acid contents in the dorsal root ganglion and spinal cord in rats with neuropathic pain. Zhen Ci Yan Jiu. 2008;33:250–4. [PubMed] [Google Scholar]
- 40.Manning BH. A lateralized deficit in morphine antinociception after unilateral inactivation of the central amygdala. J Neurosci. 1998;18:9453–70. doi: 10.1523/JNEUROSCI.18-22-09453.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Miura K, Ohara T, Zeredo JL, Okada Y, Toda K, Sumikawa K. Effects of traditional “Juci” (contralateral acupuncture) on orofacial nociceptive behavior in the rat. J Anesth. 2007;21:31–6. doi: 10.1007/s00540-006-0443-4. [DOI] [PubMed] [Google Scholar]
- 42.Moulin DE, Clark AJ, Gilron I, Ware MA, Watson CP, Sessle BJ, Coderre T, Morley-Forster PK, Stinson J, Boulanger A, Peng P, Finley GA, Taenzer P, Squire P, Dion D, Cholkan A, Gilani A, Gordon A, Henry J, Jovey R, Lynch M, Mailis-Gagnon A, Panju A, Rollman GB, Velly A. Pharmacological management of chronic neuropathic pain - consensus statement and guidelines from the Canadian Pain Society. Pain Res Manag. 2007;12:13–21. doi: 10.1155/2007/730785. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Nam T, Seo K. Induction of Local and General Analgesia by Electroacupuncture in Dogs. J Vet Clin. 1997;14:244–253. [Google Scholar]
- 44.Napadow V, Kettner N, Liu J, Li M, Kwong KK, Vangel M, Makris N, Audette J, Hui KK. Hypothalamus and amygdala response to acupuncture stimuli in Carpal Tunnel Syndrome. Pain. 2007;130:254–66. doi: 10.1016/j.pain.2006.12.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Napadow V, Makris N, Liu J, Kettner NW, Kwong KK, Hui KK. Effects of electroacupuncture versus manual acupuncture on the human brain as measured by fMRI. Hum Brain Mapp. 2005;24:193–205. doi: 10.1002/hbm.20081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Simone DA, Baumann TK, LaMotte RH. Dose-dependent pain and mechanical hyperalgesia in humans after intradermal injection of capsaicin. Pain. 1989;38:99–107. doi: 10.1016/0304-3959(89)90079-1. [DOI] [PubMed] [Google Scholar]
- 47.Sun S, Chen WL, Wang PF, Zhao ZQ, Zhang YQ. Disruption of glial function enhances electroacupuncture analgesia in arthritic rats. Exp Neurol. 2006;198:294–302. doi: 10.1016/j.expneurol.2005.11.011. [DOI] [PubMed] [Google Scholar]
- 48.Takeshige C, Oka K, Mizuno T, Hisamitsu T, Luo CP, Kobori M, Mera H, Fang TQ. The acupuncture point and its connecting central pathway for producing acupuncture analgesia. Brain Res Bull. 1993;30:53–67. doi: 10.1016/0361-9230(93)90039-e. [DOI] [PubMed] [Google Scholar]
- 49.Takeshige C, Sato T, Mera T, Hisamitsu T, Fang J. Descending pain inhibitory system involved in acupuncture analgesia. Brain Res Bull. 1992;29:617–34. doi: 10.1016/0361-9230(92)90131-g. [DOI] [PubMed] [Google Scholar]
- 50.ter Riet G, Kleijnen J, Knipschild P. Acupuncture and chronic pain: a criteria-based meta-analysis. J Clin Epidemiol. 1990;43:1191–9. doi: 10.1016/0895-4356(90)90020-p. [DOI] [PubMed] [Google Scholar]
- 51.Tian XY, Bian ZX, Hu XG, Zhang XJ, Liu L, Zhang H. Electro-acupuncture attenuates stress-induced defecation in rats with chronic visceral hypersensitivity via serotonergic pathway. Brain Res. 2006;1088:101–8. doi: 10.1016/j.brainres.2006.03.014. [DOI] [PubMed] [Google Scholar]
- 52.Treede RD, Meyer RA, Raja SN, Campbell JN. Peripheral and central mechanisms of cutaneous hyperalgesia. Prog Neurobiol. 1992;38:397–421. doi: 10.1016/0301-0082(92)90027-c. [DOI] [PubMed] [Google Scholar]
- 53.Urban MO, Zahn PK, Gebhart GF. Descending facilitatory influences from the rostral medial medulla mediate secondary, but not primary hyperalgesia in the rat. Neuroscience. 1999;90:349–52. doi: 10.1016/s0306-4522(99)00002-0. [DOI] [PubMed] [Google Scholar]
- 54.Van Bockstaele EJ, Aston-Jones G, Pieribone VA, Ennis M, Shipley MT. Subregions of the periaqueductal gray topographically innervate the rostral ventral medulla in the rat. J Comp Neurol. 1991;309:305–27. doi: 10.1002/cne.903090303. [DOI] [PubMed] [Google Scholar]
- 55.Wang JQ, Mao L, Han JS. Comparison of the antinociceptive effects induced by electroacupuncture and transcutaneous electrical nerve stimulation in the rat. Int J Neurosci. 1992;65:117–29. doi: 10.3109/00207459209003283. [DOI] [PubMed] [Google Scholar]
- 56.Wang L, Zhang Y, Dai J, Yang J, Gang S. Electroacupuncture (EA) modulates the expression of NMDA receptors in primary sensory neurons in relation to hyperalgesia in rats. Brain Res. 2006;1120:46–53. doi: 10.1016/j.brainres.2006.08.077. [DOI] [PubMed] [Google Scholar]
- 57.Wang Y, Zhang Y, Wang W, Cao Y, Han JS. Effects of synchronous or asynchronous electroacupuncture stimulation with low versus high frequency on spinal opioid release and tail flick nociception. Exp Neurol. 2005;192:156–62. doi: 10.1016/j.expneurol.2004.11.003. [DOI] [PubMed] [Google Scholar]
- 58.White A, Tough E, Cummings M. A review of acupuncture clinical trials indexed during 2005. Acupunct Med. 2006;24:39–49. doi: 10.1136/aim.24.1.39. [DOI] [PubMed] [Google Scholar]
- 59.Willis WD, Coggeshall RE. Sensory mechanisms of the spinal cord. Kluwer Academic/Plenum Publishers; New York: 2004. [Google Scholar]
- 60.Wu MT, Hsieh JC, Xiong J, Yang CF, Pan HB, Chen YC, Tsai G, Rosen BR, Kwong KK. Central nervous pathway for acupuncture stimulation: localization of processing with functional MR imaging of the brain--preliminary experience. Radiology. 1999;212:133–41. doi: 10.1148/radiology.212.1.r99jl04133. [DOI] [PubMed] [Google Scholar]
- 61.Yin CS, Jeong HS, Park HJ, Baik Y, Yoon MH, Choi CB, Koh HG. A proposed transpositional acupoint system in a mouse and rat model. Res Vet Sci. 2008;84:159–65. doi: 10.1016/j.rvsc.2007.04.004. [DOI] [PubMed] [Google Scholar]
- 62.Yu LC, Lu JT, Huang YH, Meuser T, Pietruck C, Gabriel A, Grond S, Pierce Palmer P. Involvement of endogenous opioid systems in nociceptin-induced spinal antinociception in rats. Brain Res. 2002;945:88–96. doi: 10.1016/s0006-8993(02)02743-9. [DOI] [PubMed] [Google Scholar]
- 63.Zhang RX, Lao L, Wang L, Liu B, Wang X, Ren K, Berman BM. Involvement of opioid receptors in electroacupuncture-produced anti-hyperalgesia in rats with peripheral inflammation. Brain Res. 2004;1020:12–7. doi: 10.1016/j.brainres.2004.05.067. [DOI] [PubMed] [Google Scholar]
- 64.Zhu B, Xu WD, Rong PJ, Ben H, Gao XY. A C-fiber reflex inhibition induced by electroacupuncture with different intensities applied at homotopic and heterotopic acupoints in rats selectively destructive effects on myelinated and unmyelinated afferent fibers. Brain Res. 2004;1011:228–37. doi: 10.1016/j.brainres.2004.03.034. [DOI] [PubMed] [Google Scholar]
- 65.Zimmermann M. Ethical considerations in relation to pain in animal experimentation. Acta Physiol Scand Suppl. 1986;554:221–33. [PubMed] [Google Scholar]