Abstract
BTBR T+tf/J (BTBR) is an inbred strain of mice that displays prominent social deficits and repetitive behaviors analogous to the defining symptoms of autism, along with a complete congenital agenesis of corpus callosum. BTBR is genetically distant from the widely used C57BL/6J (B6) strain, which exhibits high levels of sociability, low repetitive behaviors, and an intact corpus callosum. Emerging evidence implicates compromised inter-hemispherical connectivity in some cases of autism. We investigated the hypothesis that the disconnection of corpus callosum (CC) fiber tracts contribute to behavioral traits in mice that are relevant to the behavioral symptoms of autism. Surgical lesion of the CC in B6 mice at postnatal day 7 had no effect on juvenile play and adult social approach, and did not elevate repetitive self-grooming. No correlations were detected between rostral-caudal extent of the CC lesion and behavioral scores. In addition, LP/J, the strain that is genetically closest to BTBR but has an intact CC, displayed juvenile play deficits and repetitive self-grooming similar to those seen in BTBR. These corroborative results offer evidence against the hypothesis that the corpus callosum disconnection is a primary cause of low sociability and high repetitive behaviors in inbred mice. Our findings indicate that genes mediating other aspects of neurodevelopment, including those whose mutations underlie more subtle disruptions in white matter pathways and connectivity, are more likely to contribute to the aberrant behavioral phenotypes in the BTBR mouse model of autism.
Keywords: corpus callosum, BTBR T+tf/J mice, autism, mouse models, social interactions, repetitive self-grooming
Introduction
Autism is a neurodevelopmental disorder defined by impaired social interactions, communications deficits, and repetitive behaviors (DiCicco-Bloom et al., 2006; Lord et al., 2006). While the causes are unknown, both genetic and environmental factors have been implicated (Herbert et al., 2006; Abrahams and Geschwind, 2008). Differential concordance rates between monozygotic twins (60%-90%) versus dizygotic twins and siblings (1-10%), along with a 4:1 male:female ratio, lend support to a strong genetic component (Veenstra-Vanderweele et al., 2004; Grice and Buxbaum, 2006; Newschaffer et al., 2007; Abrahams and Geschwind, 2008). Recent evidence indicates a possible association between aberrant patterns of neural connections and autism (Geschwind and Levitt, 2007; Minshew and Williams, 2007; Rippon et al., 2007). Functional imaging studies reported less activation of brain regions and reduced functional connectivity in adults with autism during several social and cognitive tasks, suggesting cortical underconnectivity in autism (Castelli et al., 2002; Just et al., 2004; Just et al., 2007; Kana et al., 2007; Kennedy and Courchesne, 2008; Kleinhans et al., 2008; Koshino et al., 2008). The corpus callosum (CC) is the largest connective structure in the brain and provides the majority of inter-hemispherical cortical connections (Paul et al., 2007). Structural imaging studies reported reduced sizes of sub-regions of CC in some individuals with autism (Egaas et al., 1995; Piven et al., 1997; Manes et al., 1999; Hardan et al., 2000; Brambilla et al., 2003; Boger-Magiddo et al., 2006; Vidal et al., 2006; Geschwind and Levitt, 2007; Kilian et al., 2008). Voxel-based imaging and diffusion tensor imaging indicate abnormal structural integrity of white matter in autism (Barnea-Goraly et al., 2004; Chung et al., 2004; Alexander et al., 2007; Keller et al., 2007; Bonilha et al., 2008; Sundaram et al., 2008). Further, more than one third of children with congenital agenesis of the corpus callosum (AgCC) have social problems, suggesting that reduced CC may contribute to abnormalities in social behaviors in some genetically-based neurodevelopmental disorders (Badaruddin et al., 2007; Paul et al., 2007).
Animal models offer opportunities to test genetic hypotheses and evaluate proposed treatments. Reverse genetic approaches are elucidating phenotypes of mice with mutations in candidate genes for autism (Young, 2001; Winslow and Insel, 2002; Young et al., 2002; Long et al., 2004; Takayanagi et al., 2005; Cheh et al., 2006; Kwon et al., 2006; Mineur et al., 2006; Crawley et al., 2007; Scearce-Levie et al., 2007; Tabuchi et al., 2007; Ehninger et al., 2008; Hung et al., 2008; Jamain et al., 2008; Chadman et al., 2008). Forward genetic approaches identify inbred strains of mice with phenotypes relevant to autism, and explore mechanisms responsible for the phenotypes (Brodkin, 2007; Moy et al., 2007; Panksepp and Lahvis, 2007; McFarlane et al., 2008). Using forward genetics, we discovered an obscure inbred strain, BTBR T+tf/J (BTBR), that exhibits behavioral traits with face validity for the diagnostic symptoms of autism, including aberrant reciprocal social interactions as juveniles and adults, reduced social transmission of food preference, and repetitive self-grooming (Bolivar et al., 2007; Moy et al., 2007; Yang et al., 2007b; McFarlane et al., 2008). A complete congenital agenesis of the corpus callosum is well-documented in BTBR (Wahlsten et al., 2003; MacPherson et al., 2008). BTBR males and females are within the normal range on measures of general health, home cage behaviors, home cage nesting, parental care, neurological reflexes, vision, olfaction, hearing, open field locomotion, anxiety-related behaviors, cognitive abilities on acquisition of spatial navigation in the Morris water maze, appetitive learning in a T-maze task, and odor discrimination learning (Zagreda et al., 1999; Bolivar et al., 2007; Moy et al., 2007; MacPherson et al., 2008; McFarlane et al., 2008), ruling out potential confounds from these domains. To experimentally test the hypothesis that CC disconnection leads to autism-like behavioral traits in mice, we lesioned the CC at postnatal day 7 in C57BL/6J (B6) mice and compared their juvenile and adult social behaviors and repetitive self-grooming with sham and non-operated B6. LP/J, the inbred strain that is genetically closest to BTBR (Witmer et al., 2003; Petkov et al., 2004) but has an intact corpus callosum, was compared to BTBR and B6 on the same behavioral assays. Findings from these two complementary approaches indicate that factors other than, or in addition to, CC absence mediate social deficits and repetitive behaviors in BTBR mice.
Materials and methods
Animals
C57BL/6J (B6), BTBR T+tf/J (BTBR), and LP/J mice breeding pairs were purchased from The Jackson Laboratory (Bar Harbor, ME) and bred at NIMH in Bethesda, Maryland. Breeding, housing, and behavioral testing were conducted in accordance with the NIH Guidelines for the Care and Use of Laboratory Animals and approved by the National Institute of Mental Health Animal Care and Use Committee. Subject mice were weaned at postnatal day 20-22, then group housed by sex in standard mouse cages containing 2-4 mice. Standard rodent chow and tap water were available ad libitum. In addition to standard bedding, a Nestlet square and a cardboard tube were provided in each cage. The colony room was maintained on a 12:12 light/dark cycle with lights on at 6:00 AM, at 20°C and 55% humidity. All experiments were conducted between 10:00 AM and 4:00 PM. Recent experiments from our laboratory found similar behavioral phenotypes of BTBR and B6 tested during the dark and light phases of their circadian cycles (Yang et al., 2007a). Two cohorts of male B6 were used as the control groups, one for comparison with sham operated and CC lesioned male B6, and one for comparison with male LP/J and BTBR inbred strains. Male non-operated B6, sham-operated B6, and CC-lesioned B6 were compared in the first set of experiments. Male B6, LP/J, and BTBR were compared in the second set of experiments. Female B6, LP/J, and BTBR were compared in the third set of experiments. Mice used as the novel targets in the social approach test were 129Sv/ImJ mice of the same sex as the subject, aged 8-14 weeks old, purchased from The Jackson Laboratory. The novel mice had no previous physical contact with subject mice.
Corpus callosum transection and histological verification
Since both sexes of B6 exhibit high levels of social behaviors, we lesioned male B6 only to test whether the presence of an intact CC is critical for the expression of sociability. Female B6 were compared with LP/J and BTBR on juvenile and adult social tasks. Surgical procedures in mouse pups were adapted from previously described methods (Manhaes et al., 2007). Corpus callosum transection was performed on postnatal day 7 (P7), under a stereoscopic microscope using the freehand method. Pups were separated from the dam one at a time, to reduce maternal stress (Manhaes et al., 2007). General anesthesia was induced by cooling the subject at -10°C for 5 min. Hypothermia was maintained throughout the surgery by placing the pup on wet ice contained in a plastic box covered with dry cotton surgical gauze. The scalp was cleaned with Betadine solution and 70% ethanol. An incision was made in the scalp to reveal the skull surface and visualize lambda. A size 5 scalpel was inserted vertically through the sagittal suture, directly behind the coronal suture, and moved rostrocaudally to transect the CC at a vertical depth of 3.0 mm (Paxinos et al., 2006). The incision was then closed with tissue adhesive (Nexaband, Abbott Laboratories, Chicago). Sham controls received the same procedures except that the scalpel was not inserted into the brain. Following the completion of the surgery, typically of 3 minutes duration, each pup was placed in an incubator maintained at 35° C for 10 min or until normal movement was observed. The incubator chamber contained home cage nest bedding placed near the pup to facilitate subsequent acceptance by the mother when the pup was returned to the home cage. Behavioral testing began fourteen days after surgery, beginning with the juvenile play test at postnatal day 21 (P21), the day before weaning. After the full sequence of behavioral tests was completed, adult mice (12 – 16 weeks) were sacrificed by cervical dislocation and decapitated. Brains were removed and stored in 4% formaldehyde solution for at least 48 h before sectioning coronally at 80 μm using a vibratome (Electron Microscopy Sciences, OTS-3000-03, Hatfield, Pennsylvania). Sections were stained with thionin and examined under a light microscope. The percentage of CC lesion in each mouse was calculated by dividing the rostrocaudal length of CC displaying evidence of lesion by the entire callosal length of 3.64 mm, extending from bregma 1.18 to bregma -2.46 (Franklin and Paxinos, 1997). Any associated lesions to the septum, anterior commissure, hippocampus, and hippocampal commissure were noted for each mouse.
Mouse behavioral tests relevant to the behavioral symptoms of autism
Juvenile Play
Juvenile play at P21 was conducted in the Noldus PhenoTyper Observer 3000 chamber (Noldus, Leesburg, Virginia) as previously described (Yang et al., 2007b; McFarlane et al., 2008). The floor of the arena was covered with a 0.5 cm layer of clean bedding. Subjects were individually housed in standard mouse cages for one hour prior to the play session. Two mice of the same age and sex but from different litters were then placed in the arena and their interactions were recorded for 10 min, the period during which the majority of social interactions occur. For the CC lesion study, mice of the same treatment were paired, i.e. two lesioned juvenile males, or two sham juvenile males. For non-operated mice, age-matched juvenile B6 mice were used as the play partners for B6, BTBR and LP/J subjects. B6 was chosen as the partner because this strain is neither unusually high nor unusually low on most behavioral traits, does not exhibit repetitive self-grooming like BTBR, and does not exhibit vertical jumping behaviors like LP/J. Behaviors were subsequently scored from digital videotapes by a highly trained observer, using Noldus Observer 5.0 software. The observer was uninformed of the sham or lesion condition during scoring. However, the identity of the strains was generally obvious from fur color patterns. Behaviors of interest included Follow (one mouse walks straight behind its partner, keeping pace with the one ahead), Nose-to-nose sniffing (sniffing the nose and face of the partner), Push and crawl (push = pushing head/snout underneath the partner's body or squeezing itself between the wall/floor and the partner; Crawl = crawling over or under the partner's body). The last two similar movements were subsequently combined as a single parameter, “push-crawl”, in the present study. These behaviors have been used in previous studies as parameters of play behaviors in juvenile mice (Terranova and Laviola, 2005; Ricceri et al., 2007; McFarlane et al., 2008). Vertical jumping (upward jumping, usually against the wall) and bouts of Arena exploration (walking around the arena, sniffing or digging the bedding) were scored as control measures of motor activity and exploratory tendencies. All behaviors were analyzed for frequency of occurrence, i.e. number of bouts.
Adult social approach test and self-grooming
Social approach behaviors were tested between 8 to 12 weeks of age, in an automated three-chambered apparatus using methods previously described (Moy et al., 2004; Nadler et al., 2004; Crawley et al., 2007; Moy et al., 2007; Yang et al., 2007b; McFarlane et al., 2008). The apparatus was a rectangular, three-chambered box made of clear polycarbonate. Retractable doorways built into the two dividing walls controlled access to the side chambers. Number of entries and time spent in each chamber were automatically detected by photocells embedded in the doorways and tallied by the software. The test session began with a 10 min habituation session in the center chamber only, followed by a 10 min habituation to all three chambers. Lack of innate side preference was confirmed during the second 10 minute habituation. The subject was then briefly confined to the center chamber while the novel object (an inverted stainless steel wire pencil cup, Galaxy, Kitchen Plus, http://www.kitchen-plus.com) was placed in one of the side chambers. A novel mouse, previously habituated to the enclosure, was placed in an identical wire cup located in the other side chamber. A disposable plastic drinking cup containing a lead weight was placed on the top of each inverted wire pencil cup to prevent the subject from climbing on top. The side containing the novel object and the novel mouse alternated between the left and right chambers across subjects. After both stimuli were positioned, the two side doors were simultaneously lifted and the subject was allowed access to all three chambers for 10 min. In addition to the automatically tallied time spent in each chamber and entries into each chamber, an observer with stopwatches scored time spent sniffing the novel object and the novel mouse and the duration of self-grooming exhibited by the subject mouse during the 10 min test session. The apparatus was cleaned with 70% ethanol and water between subjects. 129Sv/ImJ was used as the “novel mouse” because this strain is inactive and passive, rarely displays irritability when confined under the wire cup, and never exhibits aggressive behaviors towards subject mice. Using a minimally active partner is a strategy that allows all approaches to be initiated by the adult subject mouse only.
Elevated plus-maze, light ↔ dark exploration, open field locomotion, and rotarod test
To control for potential confounding factors such as anxiety-like traits and unusual motor phenotypes that could confound the interpretation of the social traits, a sequence of control tests was performed when the subjects were 8 to 12 weeks of age, including: elevated plus-maze (EPM), light ↔ dark exploration, open field locomotion, and rotarod coordination and balance. In the EPM, the mouse was placed in the center, facing a closed arm, and allowed to freely explore the apparatus for 5 min. Time spent and number of entries into the open arms (20 lux) and closed arms were scored by an investigator, using Observer software (Noldus Information Technology, Leesburg, Virginia; Karlsson et al., 2005). In the light↔dark exploration test (Crawley and Goodwin, 1980), the mouse was placed in the light compartment facing away from the partition, and allowed to freely explore the apparatus for 10 min. Time spent and number of full-body transitions between the light (300 lux) and dark (3 lux) compartments were automatically scored, as previously described (Mathis et al., 1995; Holmes et al., 2001). In the open field test, the mouse was allowed to freely explore the apparatus (20 lux) for 10 min. Total distance traveled in the arena, vertical activity, and time spent in the center were automatically scored by software linked to the photocell detectors (Accuscan, Columbus, OH). Motor coordination and balance were evaluated in male mice on three consecutive 5 minute trials on an accelerating rotarod, 4-40 rpm/5 min (Ugo Basile, Stoelting, Wood Dale, IL; Bailey et al., 2007). The inter-trial interval was 60 sec.
General health, neurological reflexes, sensory and motor abilities
To examine physical effects of the CC transection, lesioned and sham B6 were tested for general health, as previously described (Bailey et al., 2007; Crawley et al., 2007). General health assessment included scoring fur and whisker condition, as well as limb and body tone. Neurological reflex tests included forepaw reaching, righting reflex, trunk curl, whisker twitch, pinna twitch, eyeblink response, and toe pinch. The reactivity level of the mice was assessed with tests measuring responsiveness to petting, intensity of a dowel biting response, and level of vocalization during handling. Body weight was measured at 10 weeks of age.
Statistical analysis
For the automated social approach test, time spent in the two side chambers was compared using within-group Repeated Measures ANOVAs, with the factor of chamber side (novel mouse side vs. novel object side). Time spent in the center chamber is included on the graphs for illustrative purposes. Time spent sniffing the novel mouse versus the novel object was also analyzed using within-group Repeated Measures ANOVAs, with the factor of chamber side (novel mouse side vs. novel object side). Entries into all chambers were summed as “total entries” and analyzed using One Way ANOVA across groups, as a measure of general exploratory activity. Rotarod performance (latency to fall) was analyzed with Repeated Measure ANOVAs followed by Scheffe test for post-hoc comparisons. All other behavior assays were analyzed using One Way ANOVA. Scheffe test was used for post-hoc comparisons following a significant overall ANOVA F value. Pearson's r was used to evaluate correlations between CC lesion size and social scores. Correlation analyses included lesioned mice only.
Results
Histology
Figure 1 illustrates representative cross sections of brains of sham operated B6, CC lesioned B6, LP/J, and BTBR male mice. The intact CC in a representative sham operated B6 mouse brain cross section is illustrated in 1A and 1E. A representative CC lesion performed in B6 mice on P7 is illustrated in 1B. CC lesions remained clearly visible at the time of sacrifice, 3-4 months of age. Light microscopic examination of sections from the 35 lesioned mice found that the most rostral part of CC was lesioned in all mice, while variable portions of the caudal CC remained. Complete transections throughout the rostral-caudal dimension were obtained in 7 mice. An average of 70.3±3.5% length of CC was absent, with a range of 33% - 100% completeness of the lesion in the rostral-caudal dimension. The hippocampal commissure was partially severed in 20 mice, and minor damage was noted in the anterior medial hippocampus in 17 mice. The anterior commissure was intact in all mice.
Figure 1.
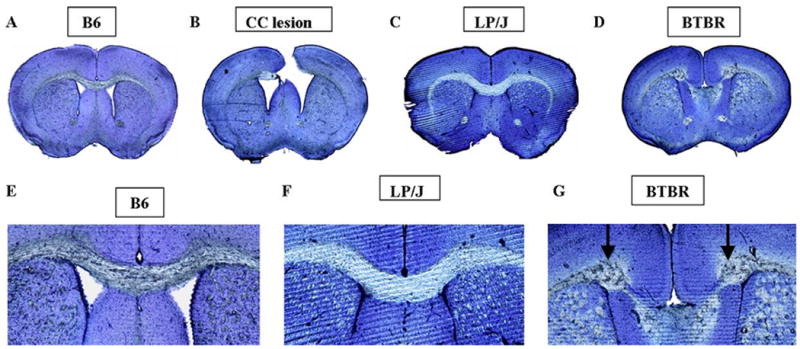
Photomicrographs of thioin-stained representative coronal sections depicting the corpus callosum (CC) region in representative (A) B6 control mice; (B) B6 mice in which the CC was lesioned on postnatal day 7 (tissue obtained from subjects sacrificed at 3 months of age); (C) LP/J mice with intact CC; (D) BTBR mice in which the corpus callosum is congenitally absent. Close-ups of the CC region are shown in (E) B6; (F) LP/J; (G) BTBR. Probst bundles appear in BTBR only (arrows). Scale bar 80=μm.
A representative BTBR brain is shown in cross section in Figs. 1D and 1G. Histological analysis showed that the CC was absent in all 10 BTBR brains. A representative LP/J brain is shown in Figs. 1C and 1F. The CC was present in all 10 LP/J brains that were analyzed. The complete absence of the CC in BTBR and the normal thickness of the CC in LP/J confirm previously published findings (Wahlsten et al., 2003) and the database of The Mouse Brain Library (www.mbl.org).
General health evaluation in CC-lesioned B6 and sham B6 mice
Table 1 shows that CC-lesioned B6 and sham B6 were normal on measures of general health, neurological reflexes, and sensory abilities. No mice exhibited physical abnormalities or aberrant behaviors. Body weights of both groups were within the normal range of adult male B6 mice (http://jaxmice.jax.org).
Table 1.
General health and neurological reflexes in sham and corpus callosum lesioned (CC lesion) adult male B6 mice. Both groups displayed scores in the normal range for untreated B6 mice (data not shown).
| Sham (N=18) | CC lesion (N = 30) | |
|---|---|---|
| Body weight (g) | 28.10±0.52 | 26.93±0.54 |
| Fur condition (3 pt scale) | 3.0 | 3.0 |
| Bald patches (%) | 0 | 0 |
| Missing whiskers (%) | 0 | 0 |
| Piloerection (%) | 0 | 0 |
| Body tone (3 pt scale) | 2.0 | 2.0 |
| Limb tone (3 pt scale) | 2.0 | 2.0 |
| Positional passivity (%) | 27.8 | 33.3 |
| Trunk curl (%) | 100 | 66.7 |
| Forepaw reach (%) | 100 | 100 |
| Righting reflex (%) | 100 | 100 |
| Corneal (%) | 100 | 100 |
| Pinna (%) | 100 | 100 |
| Vibrissae (%) | 100 | 100 |
| Toe pinch (%) | 100 | 100 |
| Petting escape (%) | 88.9 | 80 |
| Struggle/vocalization (%) | 12.5 | 4 |
| Dowel biting (3 pt scale) | 0 | 0 |
Juvenile play behaviors in control, sham, and CC lesioned B6 mice
Figure 2 displays parameters of social interactions in 21 day old B6, sham-operated B6, and CC lesioned B6 mice. Bouts of nose-to-nose sniffing did not differ across groups (F2,58=2.11, NS = not significant). No group differences were detected for number of bouts of following (F2,58=0.29, NS), number of bouts of push-crawl play-soliciting attempts (F2,58=0.66, NS), general exploration of the test arena (F2,58=1.81, NS), or vertical jumping (F2,58=1.50, NS, Table 2).
Figure 2.
Juvenile play behaviors were similar in male mice with early postnatal corpus callosum lesions (CC lesion), sham lesions (sham) and non-operated C57BL/6J controls (B6). No significant group differences were detected with One Way ANOVA for (A) nose-to-nose sniffing (B) following (C) push-crawl play-soliciting attempts, and (D) arena exploration. B6 N=8, sham N=22, CC lesion N=31. For all bar graphs shown in Figures 2, 3, 5-8 data are presented as mean + standard error of the mean.
Table 2.
(A) Corpus callosum lesions did not alter motor functions in male B6 mice; Corpus callosum lesion reduced anxiety-like behaviors in the elevated plus-maze test but not in the light ↔ dark test (B) Motor functions and anxiety-related traits in male B6, LP/J, and BTBR. (C) Motor functions and anxiety-related traits in female B6, LP/J, and BTBR.
* p<.05 versus B6; #p<.05 BTBR versus LP/J (One Way ANOVA followed by Scheffe post-hoc test).
| A. Motor functions and anxiety-like behaviors in male non-operated B6, sham B6, and CC lesion B6, * p<.05 versus B6. | ||||||||
|---|---|---|---|---|---|---|---|---|
| B6 | Sham | CC lesion | ||||||
| Open field activities | Total distance (cm/10 min) | 1610.0 ± 83.9 | 2006.3±116.3 | 2142.2±140.0 | ||||
| Center time (sec/10 min) | 83.3±15.2 | 98.1±6.7 | 78.3±7.4 | |||||
| Vertical activity | 96.1±9.1 | 133.7±16.0 | 111.1±11.6 | |||||
| Vertical jumping in the juvenile play test | 0.75±0.49 | 1.19±0.47 | 2.50±0.85 | |||||
| Rotarod, latency to fall (sec, average of trials) | 208.5±19.61 | 234.18±13.91 | 241.25±9.08 | |||||
| Elevated plus-maze | Open arm time (%) | 31.8±5.3 | 30.5±2.3 | 48.9±2.4 * | ||||
| Open arm entries | 9.6±1.1 | 12.2±1.9 | 16.8±0.8 * | |||||
| Total arm entries | 21.4±0.80 | 26.8±1.3 | 28.3±1.4 | |||||
| Light ↔ dark exploration | Number of transitions | 37.3±3.3 | 47.5±3.6 | 45.8±2.5 | ||||
| Time in the dark chamber (sec) | 313.9±17.5 | 295.0±16.8 | 345.0±16.8 | |||||
| Latency to enter the dark chamber (sec) | 8.9±2.5 | 5.0±1.5 | 4.8±0.7 | |||||
| B. Motor functions and anxiety-like behaviors in male B6, LP/J, and BTBR mice, * p<.05 versus B6; #p<.05 BTBR versus LP/J. | ||||||||
| B6 | LP/J | BTBR | ||||||
| Open field activities | Total distance (cm/10 min) | 1549.9 ± 114.0 | 1025.6±125.3 | 2529.5±223.5 * # | ||||
| Center time (sec/10 min) | 93.9±11.5 | 61.2±37.0 | 94.2±12.7 | |||||
| Vertical activity | 132.3±14.5 | 24.1±6.8 * | 84.0±19.2 # | |||||
| Vertical jumping in the juvenile play test | 0.29±0.13 | 8.33±2.93* | 0±0# | |||||
| Rotarod, latency to fall (sec, average of trials) | 244.901±5.16 | 159.21±23.87* | 95.94±14.54* | |||||
| Elevated plus-maze | Open arm time (%) | 33.4±2.7 | 21.1±3.0* | 38.3±3.1# | ||||
| Open arm entries | 11.4±1.0 | 7.3±0.8* | 17.2±1.5*# | |||||
| Total arm entries | 24.2±1.5 | 18.3±1.3* | 29.2±2.1# | |||||
| Light ↔ dark exploration | Number of transitions | 43.7±2.9 | 18.0±3.4* | 45.2±3.3 # | ||||
| Time in the dark chamber (sec) | 331.5±15.1 | 254.9±44.4* | 335.8±25.2 | |||||
| Latency to enter the dark chamber (sec) | 3.9±1.0 | 150.3±53.3* | 6.9±1.6 # | |||||
| C. Motor functions and anxiety-like behaviors in female B6, LP/J, and BTBR mice, * p<.05 versus B6; #p<.05 BTBR versus LP/J. | ||||||||
| B6 | LP/J | BTBR | ||||||
| Open field activities | Total distance (cm/10 min) | 1740.5±180.0 | 805.4±137.8* | 2446.5±192.2 # | ||||
| Center time (sec/10 min) | 105.5±10.6 | 43.5±10.9 * | 93.0±18.0 # | |||||
| Vertical activity | 121.1±21.8 | 32.1±8.2 * | 77.3±16.1 | |||||
| Vertical jumping in the juvenile play test | 3.4±2.1 | 3.8±2.1 | 0.0±0.0 | |||||
| Elevated plus-maze | Open arm time (%) | 34.1±2.5 | 16.9±3.3* | 38.8±2.7 # | ||||
| Open arm entries | 13.1±0.8 | 5.1±0.8* | 13.8±1.2 # | |||||
| Total arm entries | 28.0±1.4 | 13.1±1.8* | 24.2±1.6 # | |||||
| Light ↔ dark exploration | Number of transitions | 43.7±3.5 | 10.3±2.2 * | 41.2±4.8# | ||||
| Time in the dark chamber (sec) | 386.7±14.0 | 420.0±57.3 | 414.2±26.7 | |||||
| Latency to enter the dark chamber (sec) | 9.9±2.6 | 82.5±49.2 | 6.4±1.1 | |||||
Adult social approach behaviors and self-grooming behavior in control B6, sham, and CC lesioned B6 mice
Figure 3 displays adult social approach (Fig. 3A-C) and repetitive self-grooming (Fig. 3D) results, obtained simultaneously in 8-12 week old male non-operated control B6 (B6), sham B6, and CC lesioned B6 mice. Non-operated B6 spent more time in the chamber containing the novel mouse than in the chamber containing the novel object (F1,10=38.11, p<.0001). Similarly, sham B6 (F1,18=54.38, p<.0001) and CC lesioned B6 (F1,34=150.35, p<.0001) spent more time in the chamber containing the novel mouse than in the chamber containing the novel object. Time spent sniffing the novel mouse showed a similar pattern to time in the side chambers. B6 (F1,10=112.25, p<.0001), sham B6 (F1,18 =70.72, p<.0001) and CC lesioned B6 (F1,34 =181.50, p<.0001) spent more time sniffing the novel mouse than the novel object. A significant main group effect was found for total entries to the side chambers (F2,58=4.35, p<.05). However, Scheffe post-hoc test did not reveal significant differences when sham and lesioned groups were compared to the non-operated B6 group. Cumulative time spent in self-grooming during the social approach test was low in all three groups and not significantly different across groups (F2,58=0.23, NS).
Figure 3.
Adult sociability and levels of self-grooming were similar in male mice with early postnatal corpus callosum lesions (CC lesion), sham lesions (Sham) and non-operated C57BL/6J controls (B6). (A) Sociability in the automated three chambered task is defined as spending significantly more time in the side chamber containing a novel mouse than in the side chamber containing a non-social novel object, an inverted wire pencil cup. Repeated Measure ANOVA revealed significant sociability in B6 (F1,10=38.11, p<.0001), sham (F1,18=54.38, p<.0001), and CC-lesioned B6 (F1,34=150.35, p<.0001); (B) Time spent sniffing the inverted wire cup containing the novel mouse versus sniffing the empty inverted wire cup is a complementary and more sensitive measure of sociability. Repeated Measure ANOVA revealed that B6 (F1,10=112.25, p<.0001), sham (F1,18 =70.72, p<.0001) and CC lesioned B6 (F1,34 =181.50, p<.0001) all spent more time sniffing the novel mouse than the novel object; (C) One Way ANOVA revealed a significant main group effect for total entries (F2,58=4.35, p<.05). However, Scheffe post-hoc test did not reveal significant differences when sham and lesioned groups were compared to the non-operated B6 group. (D) Repetitive self-grooming was simultaneously scored during the social approach test. CC lesion did not alter the amount of self-grooming as compared to sham and B6 mice (F2,58=0.23, NS). B6 N=11, Sham N=19, CC lesion N=35. *p <.01 comparison between novel object and novel mouse.
Lack of correlation between extent of CC lesion and behavioral scores in male B6 mice
Figure 4 shows a scattergram plot of the percentage of corpus callosum reduction in each male CC lesioned mouse and its score on measures of juvenile play, adult social approach and repetitive self-grooming. Only lesioned mice were included in the statistical analysis. Correlation values between percentage of CC lesion and play behaviors were: nose-to-nose sniff, r=0.304, NS; follow, r=0.469, NS; push-crawl, r=0.577, NS, indicating that the exact length of the remaining posterior CC did not correlate with scores on play behaviors. Correlation values for scores in the adult social approach test were: time spent in the chamber containing the novel mouse, r=0.288, NS; time spent sniffing the novel mouse, r=0.216, NS; cumulative time spent in self-grooming, r=0.106, NS, indicating that the exact length of the remaining posterior CC did not correlate with adult sociability or amount of repetitive self-grooming. The 7 mice with complete CC transections scored in the same range as sham controls on all parameters. The 20 mice with partial hippocampal commissure lesions similarly scored in the same range as sham controls on all parameters.
Figure 4.
Lack of correlation between percentage of lesion-induced corpus callosum reduction and scores for juvenile play behaviors (A-C), adult sociability (D, E), and self-grooming (F). Correlation analyses included lesioned mice only, using Pearson's r test. (A) Number bouts of nose-to-nose sniffing (r=0.304, NS); (B) Number of bouts in which one juvenile male mouse followed the other juvenile mouse (r=0.469, NS); (C) Number of bouts of push-crawl interactions (r=0.577, NS); (D) Time spent by the adult male subject mouse in the chamber containing the novel mouse (r=0.288, NS); (E) Time spent sniffing the novel mouse (r=0.216, NS); (F) Time spent self-grooming by the adult male subject mouse in the social approach apparatus (r=0.106, NS). Sham surgery mice (black triangles) were not included in the statistical analyses of r values, but are shown here for illustrative purposes only, as controls with zero for their lesion values. Sham B6: N=22 for juvenile play, N=19 for social approach. Corpus callosum-lesioned B6 (white triangles): N=31 for juvenile play, N=35 for social approach. Seven mice displayed complete 100% lesions, with no connections visible between the left and right cerebral cortex throughout the rostral-caudal dimension. Social scores and self-grooming remained within control ranges in mice with complete and partial lesions.
Control measures of motor functions and anxiety-related behaviors in control B6, sham, and CC lesioned B6 mice
Table 2 shows results for adult male B6, sham, and CC lesioned B6 on open field exploratory locomotor activity and on rotarod coordination and balance. In the open field, no significant differences were detected across the three groups on total distance traversed in the open field (F2,52=2.27, NS), time spent in the center of the open field (F2,52=1.62, NS), or vertical activity (F2,52=1.29, NS). On the rotarod, no significant differences were detected across the three groups on latency to fall (F2,51=1.25, NS).
Table 2 displays results for adult male B6, sham, and CC lesioned B6 on the elevated plus-maze and light ↔ dark exploration tests for anxiety-related behaviors. In the elevated plus-maze, significant group differences were found for the percentage of time spent in the open arm (F2,61=16.62, p<.001) and the number of open arm entries (F2,61=12.92, p<.0001). Post hoc Scheffe tests revealed that CC lesioned B6 had higher levels of % open arm time and made more open arm entries as compared to non-operated B6 and sham B6 (p<.01 for each comparison). Number of total arm entries did not differ across groups (F2,61=3.00, NS). In the light ↔ dark exploration test, no significant differences were detected across groups on the number of transitions between the light and dark compartments (F2,61=1.52, NS), the cumulative time spent in the dark chamber (F2,61=2.35, NS), or the latency to first entry into the dark chamber (F2,61=1.83, NS).
Juvenile play behaviors in male B6, LP/J, and BTBR inbred mice
Figure 5 displays parameters of social interactions between 21 day old male B6, LP/J, and BTBR mice. Significant strain differences were found for nose-to-nose sniffing (F2,37=18.36, p<.0001), following the partner mouse (F2,37=8.27, p<.001), and push-crawl play-soliciting attempts (F2,37=38.10, p<.0001). Scheffe post hoc tests revealed that LP/J and BTBR exhibited fewer bouts of nose-to-nose sniffing, fewer bouts of following, sand fewer bouts of push-crawl as compared to B6 (p<.01 for each comparison). Strain difference for general exploration of the test arena was significant (F2,37=20.41, p<.0001). BTBR showed more bouts of arena exploration than B6 and LP/J (p<.01 for each comparison). The difference between LP/J and B6 was not significant (p>.05), ruling out an inactivity explanation for the lower social interactions in juvenile BTBR and LP/J. Vertical jumping differed significantly among the strains, (F2,37=6.42, p<.01, Table 2), with LP/J exhibiting more vertical jumps than B6 and BTBR (p<.05 for each comparison).
Figure 5.
Deficits in juvenile play behaviors in 21 day old male LP/J and BTBR T+tf/J (BTBR) as compared to C57BL/6J (B6). One Way NOVA revealed significant strain differences in (A) nose-to-nose sniffs (F2,37=18.36, p<.0001) (B) following the partner mouse (F2,37=8.27, p<.001), (C) push-crawl play-soliciting attempts (F2,37=38.10, p<.0001), and (D) arena exploration (F2,37=20.41, p<.0001). Scheffe tests revealed that LP/J and BTBR exhibited fewer bouts of nose-to-nose sniffs, fewer bouts of following and fewer bouts of push-crawl as compared to B6 (p<.01 for each comparison) and that BTBR showed more bouts of arena exploration than B6 and LP/J (p<.01 for each comparison) B6 N=14, LP/J N=15, BTBR N=11. *p<.01 versus B6; # p<.01 BTBR versus LP/J
Adult social approach behaviors and self-grooming behavior in male B6, LP/J, and BTBR inbred mice
Figure 6 displays social approach and repetitive self-grooming results, obtained simultaneously in 8-12 week old male B6, LP/J, and BTBR mice. B6 spent more time in the chamber containing the novel mouse than in the chamber containing the novel object (F1,9 =63.01, p<.0001). BTBR did not spend more time in the chamber containing the novel mouse than in the chamber containing the novel object (F1,11=3.26, NS). Some of the LP/J mice exhibited very low locomotor activity that confounded the interpretation of scores on the social approach task, and were therefore removed from the statistical analysis. Eighteen LP/J with normal locomotion (total entries > 12) spent more time in the chamber containing the novel mouse than in the chamber containing the novel object (F1,17 =18.83, p<.001). More time sniffing the novel mouse than the novel object was seen in B6 (F1,9 =86.63 p<.0001) and LP/J (F1,17=28.44 p<.0001), but not in BTBR (F1,11=3.26, NS). Total entries into the side chambers showed a significant main group effect (F2,37 =4.85, p<.01). Post hoc Scheffe test revealed that BTBR made more entries than LP/J (p<.05).
Figure 6.
Deficits in sociability in adult male BTBR T+tf/J (BTBR) and repetitive self-grooming in BTBR and LP/J as compared to C57BL/6J (B6). Sociability in the automated three-chambered apparatus is defined as spending significantly more time in the side chamber containing a novel mouse than in the side chamber containing a non-social novel object. (A) Repeated Measure ANOVA revealed significant sociability in B6 (F1,9 =63.01, p<.0001) and LP/J (F1,17 =18.83, p<.001), but not in BTBR(F1,11=3.26, NS); (B) Time spent sniffing the novel mouse versus the novel object provides a complementary measure of sociability. Repeated Measure ANOVA revealed that B6 (F1,9 =86.63 p<.0001) and LP/J (F1,17=28.44 p<.0001) spent more time sniffing the novel mouse than the novel object, while BTBR (F1,11=3.26, NS) did not; (C) Total entries into the side chambers showed a significant main group effect (One Way ANOVA, F2,37 =4.85, p<.01). Scheffe tests revealed that BTBR made more entries than LP/J (p<.05); (D) Repetitive self-grooming was simultaneously measured during the social approach test. One Way ANOVA indicated that LP/J and BTBR spent more time engaged in self-grooming as compared to B6 (p<.0001 for each comparison). B6 N=10, LP/J N=18, BTBR N=12. Panels A and B, *p<.01 comparison between novel mouse and novel object sides; Panel C: #, p<.05 BTBR versus LP/J; Panel D, * p<.0001 versus B6.
Total cumulative time spent in self-grooming during the social approach test was significant across groups (F2,37=23.47, p<.0001). As compared to B6, both LP/J and BTBR exhibited higher levels of self-grooming (p<.0001 for each comparison). No significant strain differences were detected between LP/J and BTBR.
The number of entries in the automated 3-chambered social approach apparatus was very low in approximately one third of LP/J males. Total chamber entries were fewer than 12 in these individuals during the adult social approach test. Instead of exploring the apparatus as B6 and BTBR strains did, this subset of LP/J tended to sit still in one place for a very long time during the test session. Their data were therefore analyzed separately. The 9 inactive male LP/J did not display sociability, i.e. did not spend more time in the chamber containing a novel mouse than in the chamber containing a novel object (time in the chamber with the novel mouse: 275.1±72.8; time in the chamber with the novel object: 174.3±68.1; F1,8=0.60, NS), but did spend significantly more time sniffing the novel mouse than the novel object (time spent sniffing the novel mouse: 79.2±11.9; time spent sniffing the novel object: 22.9±7.1; F1,8=17.34, p<.01). This subset, whose total entries were 7.56±1.46, was not included in the data shown in Figure 3.
Control measures of motor functions and anxiety-related behaviors in male B6, LP/J, and BTBR inbred mice
Table 2 displays results for adult male B6, LP/J and BTBR mice on open field exploratory locomotor activity and on rotarod coordination and balance. In the open field test, a significant strain difference was found for total distance (F2,41=18.80, p<.001), with BTBR traversing a greater total distance than B6 and LP/J (p<.01 for each comparison). Time spent in the center of the open field did not differ across groups (F2,41=0.77, NS). The strain difference for vertical activity was significant (F2,41=15.87, p<.001). LP/J exhibited fewer vertical movements than B6 (p<.01) and BTBR (p<.05). On the rotarod, Repeated Measure ANOVA revealed a significant strain difference was found for latency to fall (F2,33=18.27, p<.0001), with both BTBR (p<.0001) and LP/J (p<.0001) falling sooner than B6.
Table 2 displays results for adult male B6, LP/J and BTBR male mice on the elevated plus-maze and light ↔ dark exploration tests for anxiety-related behaviors. In the elevated plus-maze test, significant strain differences were found for the percentage of time spent in the open arm (F2,47=8.19, p<.0001), number of open arm entries (F2,47=17.01, p<.001), and number of total arm entries (F2,47=9.45, p<.001). As compared to B6 and BTBR, LP/J scored lower on all three measures (p<.05 or less for each comparison). BTBR made more open arm entries than B6 (p<.05). In the light ↔ dark exploration test, significant strain differences were found for the number of transitions (F2,60=24.66, p<.0001), cumulative time spent in the dark chamber (F2,60=3.46, p<.05), and the latency to enter the dark chamber (F2,60=7.99, p<.0001). As compared to B6 and BTBR, LP/J made fewer transitions between the light and dark compartments and exhibited longer latency to enter the dark chamber (p<.01 for each comparison). LP/J also spent less time in the dark chamber than B6 (p<.05). BTBR and B6 did not differ significantly on any of the measures in the light ↔ dark test. Fewer total arm entries in the elevated plus-maze and longer latency to enter the dark chamber in the light ↔ dark exploration test indicated that lower general exploratory activity of LP/J may explain its anxiety-like behaviors.
Juvenile play behaviors in female B6, LP/J, and BTBR inbred mice
Figure 7 displays parameters of juvenile social interaction in 21 day old female B6, LP/J, and BTBR mice. Significant strain differences were found for nose-to-nose sniffs (F2, 30=18.74, p<.0001), following the play partner (F2.30=7.83, p<.01), push-crawl play-soliciting attempts (F2,30=14.95, p<.0001), and arena exploration (F2,30 =20.45, p<.0001). LP/J females scored lower than B6 on all four measures (p<.01 for each) and lower than BTBR females on push-crawl and arena exploration (p<.01 for each). BTBR females made fewer nose-to-nose sniffs than B6 (p<.01) and showed a trend toward reduced following. Arena exploration did not differ significantly between BTBR and B6 females.
Figure 7.
Juvenile play behaviors in 21 day old female B6, LP/J, and BTBR inbred mice. One Way ANOVA revealed significant strains differences in (A) nose-to-nose sniffs (F2, 30=18.74, p<.0001), (B) following the play partner (F2.30=7.83, p<.01), (C) push-crawl play-soliciting attempts (F2,30=14.95, p<.0001), and (D) arena exploration (F2,30 =20.45, p<.0001). Scheffe post hoc tests revealed that LP/J scored lower than B6 on all four measures (p<.01 for each comparison) and lower than BTBR females on push-crawl and arena exploration (p<.01 for each comparison). BTBR females made fewer nose-to-nose sniffs than B6 (p<.01) and showed a trend toward reduced following. B6 N=12, LP/J N=15, BTBR N=9. * p<.01 versus B6; # p<.01 BTBR versus LP/J.
Adult social approach behaviors and self-grooming behavior in female B6, LP/J, and BTBR inbred mice
Figure 8 displays social approach behaviors and self-grooming behavior in adult female mice of the inbred strains of B6, LP/J, and BTBR. B6 and LP/J females spent more time in the chamber containing a novel mouse than in the chamber containing a novel object (B6: F1, 12=94.93, p<.0001; LP/J: F1,20=6.15, p<.05); BTBR females did not spend more time in the chamber containing a novel mouse than the chamber containing a novel object (F1,11=3.26, NS). B6 and LP/J spent more time sniffing the novel mouse than the novel object (B6: F1, 12 =91.5, p<.0001; LP/J: F1,20=24.94, p<.0001); BTBR did not spend more time sniffing the novel mouse than the novel object (F1,12 =3.26, NS). Total entries into the side chambers showed a significant main group effect (F2,36 =10.47, p<.001). Post hoc analysis revealed that LP/J made fewer entries than B6 and BTBR (p<.01 for each comparison). Strain difference for repetitive self-grooming was significant (F2, 36=23.27, p<.001). BTBR and LP/J spent more time in self-grooming as compared to B6 (p<.01 for each comparison)
Figure 8.
Deficits in sociability in female BTBR and repetitive self-grooming in female BTBR and LP/J as compared to B6. Repeated Measure ANOVA revealed significant sociability in B6 (F1, 12=94.93, p<.0001) and LP/J females (F1,20=6.15, p<.05), but not in BTBR females (F1,11=3.26, NS); (B) Repeated Measure ANOVA revealed that B6 (F1, 12 =91.5, p<.0001) and LP/J females (F1,20=24.94, p<.0001) spent more time sniffing the novel mouse than the novel object, while BTBR females did not (F1,12 =3.26, NS); (C) Total entries into the side chambers showed a significant main group effect (One Way ANOVA, F2,36 =10.47, p<.001). Scheffe tests revealed that LP/J made fewer entries than B6 and BTBR (p<.01 for each comparison). (D) Repetitive self-grooming was simultaneously measured during the social approach test. Strain difference for self-grooming was significant (F2, 36=23.27, p<.001). BTBR and LP/J spent more time in self-grooming as compared to B6 (p<.01 for each comparison). B6 N=12, LP/J N=21, BTBR N= 13. Panels A and B, * p<.01 comparison between novel mouse and the novel objects side; Panel C and D, *p<.01 versus B6; # p<.01 BTBR versus LP/J.
As found in the males, approximately one third of LP/J females exhibited low levels of activity, i.e. total chamber entries were fewer than 12, in the adult social approach test. Their data were therefore analyzed separately. The 11 inactive female LP/J did not spend more time in the chamber containing a novel mouse than in the chamber containing a novel object (time in the chamber with the novel mouse: 157.5±71.7; time in the chamber with the novel object: 127.0±60.7; F1,10 =0.08, NS) and did not spend more time sniffing the novel mouse than the novel object (time spent sniffing the novel mouse: 62.5±23.0; time spent sniffing the novel object:17.3±10.0; F1,10=3.55, NS). This subset, whose total entries were 3.36±1.13, was not included in the data shown in Figure 8.
Control measures of motor functions and anxiety-related behaviors in female B6, LP/J, and BTBR inbred mice
Table 2 displays adult open field activities and bouts of jumping during the juvenile play test in female B6, BTBR, and LP/J mice. Significant strain differences were found for total distance (F2, 46=25.01, p<.001), center time (F2, 46=7.55, p<.01), and vertical activity (F2, 46=7.75, p<.01). As compared to B6 and BTBR, LP/J females traversed shorter total distances (p<.01 for each) and spent less time in the center (p<.05 for each). BTBR females traveled longer total distances than B6 (p<.01). LP/J made fewer vertical movements as compared to B6 (p<.01). Vertical jumping during the juvenile play test did not differ across strains (F2, 30=1.10, NS).
Table 3 displays anxiety-related tests in adult female mice of B6, LP/J, and BTBR. In the elevated plus-maze test, significant strain differences were found for the percentage of time spent in the open arm (F2,43=16.10, p<.0001), number of open arm entries (F2,43=10.08, p<.001), and number of total arm entries (F2,43=21.049, p<.001). As compared to B6 and BTBR females, LP/J females had lower % open arm time, made fewer open arm entries, and fewer total entries (p<.001 for each comparison). BTBR and B6 did not differ significantly on any of these measures. In the light ↔ dark exploration test, a significant strain difference was found for the number of transitions (F2, 43=20.26, p<.001). LP/J made fewer transitions than B6 and BTBR (p<.01 for each comparison). No significant strain difference was found for time spent in the dark chamber (F2, 43=0.72, NS). Strain difference was significant for the latency to enter the dark chamber (F2, 43=3.27, p<.05), but Scheffe post hoc test did not detect significant differences among the three strains.
Table 3.
Summary of corpus callosum lesioned, sham surgery, and inbred strains of mice on behaviors relevant to the first diagnostic symptom of autism, qualitative impairment in social interaction, and to the third diagnostic symptom of autism, stereotyped and repetitive patterns of behavior with restricted interests. Exploratory locomotion and motor coordination are summarized as procedural controls for the social tasks.
| C57BL/6J | C57BL/6J with corpus callosum lesion | LP/J | BTBR T+tf/J | |
|---|---|---|---|---|
| Corpus callosum | Present | Partially or completely disconnected | Present | Congenital absence |
| Juvenile play behaviors | High | High | Low | Low |
| Adult social approach | High | High | High | Low |
| Repetitive self-grooming | Low | Low | High | High |
| Open field exploratory behaviors | Medium | Moderately elevated | Low | High |
| Elevated plus-maze anxiety-like behaviors | Normal | Anxiolytic-like | Anxiety-like | Normal |
| Light ↔ dark exploration anxiety-like behaviors | Normal | Normal | Anxiety-like | Normal |
| Motor coordination | Good | Good | Poor | Poor |
Discussion
The BTBR inbred strain of mice displays impaired reciprocal social interactions as juveniles, reduced social approach and reduced reciprocal social interactions as adults, less social transmission of food preference, qualitatively and quantitatively unusual vocalizations (Scattoni et al., 2008), and high levels of repetitive self-grooming across developmental ages, as compared to the standard B6 inbred strain (Bolivar et al., 2007; Moy et al., 2007; McFarlane et al., 2008), representing traits relevant to the first, second, and third diagnostic symptoms of autism. The currently known major neuroanatomical abnormalities in BTBR are a complete congenital agenesis of the corpus callosum and a reduced hippocampal commissure, along with a normal anterior commissure (Wahlsten et al., 2003; Kusek et al., 2007). One possible explanation for the behavioral abnormalities in BTBR is its acallosal condition and/or other white matter connectivity. We addressed the corpus callosum hypothesis using two complementary approaches: 1) experimental lesions of the corpus callosum in a standard inbred strain, B6, at postnatal day 7; and 2) comparison of BTBR with the inbred strain LP/J, which is adjacent to BTBR on the mouse family tree but has an intact corpus callosum. The acallosal BTBR displayed lack of adult sociability, low scores on some measures of juvenile play, and high levels of repetitive self-grooming in the present study, consistent with previous reports from multiple cohorts tested in three different laboratories (Moy et al., 2007; Yang et al., 2007; McFarlane et al., 2008). However, postnatal surgical disconnection of CC did not impair behaviors in either the juvenile social interaction test or the adult social approach test, and did not elevate self-grooming, providing evidence against the hypothesis that CC disconnection is a causal factor for social deficits and/or repetitive behaviors in mice.
Our findings are consistent with human studies which report that children with congenital CC agenesis (AgCC) do not uniformly exhibit autism-like symptoms. While a high percentage (∼40%) of children with AgCC are found to have unusual social interactions (Badaruddin et al., 2007), the forms of social abnormalities most frequently cited in AgCC patients are “emotional immaturity, lack of introspection, impaired social competence, general deficits in social judgment and planning, and poor communication of emotions” (Paul et al., 2006; 2007). Core symptoms of autism, such as aloofness and repetitive/restricted behaviors, are not major characteristics of AgCC children (Badaruddin et al., 2007). The social problems of AgCC individuals may be more closely related to their deficits in cognitive integration, for which the CC might be important. Further, social problems typically do not become obvious in AgCC children until 6 years of age, whereas symptoms of autism are usually detected before age 2 and the diagnosis can be reliably made by age 3 (Volkmar et al., 2005; Geschwind and Levitt, 2007). In addition, human epilepsy patients who received surgical lesions of the corpus callosum displayed no adverse consequences on neurophysiological measures, physical health, motor functions, and remain largely normal in terms of personality and social behaviors (Gazzaniga et al., 1975; Wilson et al., 1978; Sass et al., 1988; Mamelak et al., 1993; Lassonde and Sauerwein, 1997; Rougier et al., 1997; Devinsky and Laff, 2003). These behavioral differences between callostomy patients and AgCC children versus autistic individuals support our interpretation that CC disconnection is not the causal factor for autism-like behavioral symptoms in mice.
Although CC lesioned B6 did not resemble BTBR in social and repetitive behaviors, they showed phenotypes similar to BTBR in two non-social tests, namely the elevated plus-maze anxiety test and the open field exploration test. Both CC lesioned and BTBR mice displayed high exploration and low anxiety-like scores on some components of these tasks. It is thus instructive to compare the elevated open field exploratory activity and anxiolytic-like scores on the elevated plus-maze in intact B6 mice, CC lesioned mice, and in the acallosal BTBR strain. One interpretation of the similarities between BTBR and CC lesioned B6 in these tests is that different genes mediate exploratory, anxiety-related, and social traits. It is also possible that the CC plays a role in regulating exploratory and anxiety-like traits that are dissociable from sociability. A third interpretation for the anxiolytic-like profile of CC lesioned mice is that CC lesioned pups received additional maternal care when returned to the nest, e.g. extra licking and grooming by their dams, an environmental factor that reduces anxiety-like scores in rodents (Caldji et al., 1998; Meaney, 2001). Further, the required checking of post-lesion mice by the investigators and animal caretakers in our vivarium involved extra handling, a factor that could reduce anxiety-like scores (Vallee et al., 1997).
It is important to recognize that experimental lesions at postnatal day 7 do not replicate congenital CC agenesis, which begins at an embryonic stage. Therefore, the present neonatal CC lesion is not expected to completely recapitulate the phenotype of the acallosal BTBR strain, in which the CC is absent from the earliest stages of brain development, and which may incorporate other intrahemispheric and long pathway connectivity abnormalities that have not yet been discovered. Further, unidentified genes in the B6 background could conceivably protect B6 mice from the behavioral consequences of CC lesion at postnatal day 7, thus complicating a direct comparison of behavioral phenotypes in BTBR and CC lesioned B6.
Another explanation, which will be important for future research, is the possibility that the lack of corpus callosum and partial reduction in hippocampal commissure size in BTBR (Wahlsten et al., 2003) may represent more pervasive abnormalities in commissural development, white matter pathways, and connectivity. Preliminary examination of the brains of BTBR revealed unusual characteristics in several structures other than CC, e.g. Probst bundles. It will be interesting to pursue in-depth imaging of white matter pathways in BTBR, to understand the scope of connectivity dysfunctions in this mouse strain. Human imaging studies of individuals with autism have reported white matter structural deficits and connectivity abnormalities (Castelli et al., 2002; Casanova, 2004; Chung et al., 2004; Just et al., 2004, 2007; Waiter et al., 2005; Boger-Megiddo et al., 2006; Cherkassky et al., 2006; Geschwind and Levitt, 2007; Kana et al., 2007; Keller et al., 2007; Koshino et al., 2008; Sundaram et al., 2008), offering a compelling hypothesis for the cause of autism and other neurodevelopmental disorders. Structural and functional imaging of the BTBR mouse brain across developmental ages will be needed to fully understand the neuroanatomical irregularities in fiber tracts that may underlie its autistic-like behavioral abnormalities.
LP/J is the closest genetic relative to BTBR (Witmer et al., 2003; Petkov et al., 2004). LP/J offers an interesting comparison to BTBR because the CC is intact in LP/J while absent in BTBR. Results from the present experiments revealed that LP/J mice are similar to BTBR on some traits but differ on others (Table 3). At early juvenile ages, LP/J displayed less reciprocal social interactions than B6, consistent with the BTBR phenotype. At adult ages, LP/J displayed high levels of repetitive self-grooming, consistent with the BTBR phenotype. In contrast, adult sociability was significant in LP/J and B6 but not in BTBR. One interpretation is that social deficits are present at young ages in LP/J, but are resolved over the course of development by adult ages, while repetitive behaviors persist into adult ages. Another interpretation is that unusual phenotypes in other aspects of LP/J behaviors confound the direct comparisons with BTBR. Some of the LP/J mice displayed unusual behaviors not seen in BTBR, including sporadic vertical jumping during the juvenile play test, locomotor inactivity in the open field, and anxiety-like traits.
Low exploratory activity, high anxiety-like scores, and high adult sociability in LP/J are opposite in direction to the BTBR and CC lesioned phenotypes. However, physical differences in LP/J could be the source of anxiety-related behavioral traits, including their smaller body size (Mouse Phenome Database, http://phenome.jax.org/pub-cgi/phenome/mpdcgi?rtn=docs/home) and lower reproduction. In our breeding facility, typical litter sizes were 1-4 pups/litter for LP/J and 8-12 pups/litter for BTBR (unpublished observations). Since the strains were tested as groups, rather than identified as individuals, it was not possible to conduct longitudinal comparisons to determine whether the same individuals displayed low open field activity and low entries during adult social approach, and/or were the same individuals with unusual scores on other tasks and physical measures. Comparisons across tests for LP/J individuals will be useful for future studies.
Although BTBR and LP/J are immediately adjacent on the mouse family tree (Witmer et al., 2003; Petkov et al., 2004), there is still a substantial percentage (∼24%) of SNP differences between these two strains (Petko M. Petkov, The Jackson Laboratory, personal communication). In addition, the proportion of microsatellite marker alleles that are identical between BTBR and LP/J is 54.7%, indicating substantive genetic differences (Wahlsten et al., 2003). Separate alleles may regulate functions relevant to the corpus callosum and other white matter pathways, and relevant to the adult social behaviors, fertility, locomotor activity, and anxiety-like behaviors that were found to differ between LP/J and BTBR in the present study. Thus, genes that regulate brain development, connectivity, social behaviors, locomotion, anxiety-related traits, and reproductive functions could interact with genes responsible for CC development, complicating the unambiguous interpretation of the cause of behavioral similarities and differences between LP/J and BTBR.
Corpus callosum reductions in cross sectional area have been reported by Wahlsten and co-workers for several other inbred strains of mice, including 129S1/SvImJ, A/J, BALB/c, C3H/HeJ, NZB/B1NJ, SJL/J, and SWR/J (Wahlsten et al., 2003). BALB/cJ displayed low sociability at age 30 days (Brodkin, 2007; Fairless et al., 2008), interacted at low levels with former cagemates at 4-6 days postweaning (Panksepp et al., 2007), and showed low scores on socially conditioned place preference (Panksepp and Lahvis, 2007). BALB/cBy/J males at age 6-7 weeks displayed low social approach (Moy et al., 2007). Similarly, 129S1/SvImJ and A/J showed low levels of sociability in several tasks (Brodkin et al., 2004; Bolivar et al., 2007; Moy et al., 2007). However, it must be noted that concomitant low levels of exploratory activity and comparatively high levels of anxiety-like traits have been extensively described for BALB, A/J, and 129S1/SvImJ (Mathis et al., 1995; Griebel et al., 2000; van Gaalen and Steckler, 2000; Bouwknecht and Paylor, 2002; Cook et al., 2002; Moy et al., 2007), raising potential artifactual confounds for interpreting social approach and social interaction deficits in these strains. Further, some strains with modest CC sizes, including C3H and SWR/J, showed high social approach scores, whereas NOD/LtJ, the strain with the a large CC, exhibited low sociability (Wahlsten et al., 2003; Moy et al., 2007, 2008), indicating that reduced corpus callosum size is unlikely to be a definitive predictor of social scores in mice. Interactions of multiple genes and other biological, neuroanatomical, and behavioral factors are likely to contribute to social traits in mice and other species.
No major sex differences were detected on social scores and repetitive behaviors in B6, BTBR, and LP/J. BTBR females showed low scores on nose-to-nose sniffing and following during juvenile play, lack of adult sociability in the three-chambered task on chamber time and sniffing time, and high self-grooming, similar to BTBR males. Both sexes of LP/J showed low scores on all measures of juvenile play, and exhibited high levels of repetitive grooming. The general lack of sex differences in BTBR and LP/J mice suggests that X-linked genes and sexually dimorphic factors are unlikely to be primary substrates for the social deficits and repetitive behaviors in these two strains. Other hypotheses that have recently been ruled out include the effects of early maternal care (Yang et al., 2007b) and circadian factors (Yang et al., 2007a) on the BTBR behavioral phenotype.
In conclusion, our findings suggest that early postnatal surgical disconnection of the corpus callosal fiber tract in B6 mice does not result in the social deficits or repetitive self-grooming that characterize BTBR mice, which have a congenital agenesis of the CC. In addition, LP/J, the inbred strain that is genetically closest to BTBR but has an intact corpus callosum, shares some autism-relevant behavioral traits with BTBR, including juvenile social deficits and repetitive self-grooming. Juvenile reciprocal social interactions, adult social approach, general exploratory activity, anxiety-related behaviors, and corpus callosum integrity appear to represent discrete traits with separable genetic determinants. Taken together, these several findings suggest that genes regulating other aspects of connectivity and brain development, rather than those regulating CC development specifically, are more likely to be responsible for the social and repetitive abnormalities in BTBR. Unusual single nucleotide polymorphisms in BTBR (McFarlane et al., 2008) and genetic loci on the X chromosome linked to corpus callosum absence in BTBR (Kusek et al., 2007) have been reported. It will be important to conduct a comprehensive investigation of unusual alleles, epigenetic factors, and copy number variants in the BTBR background, to increase our understanding of the genetic basis of autism-like phenotypes in the BTBR mouse model of autism.
Acknowledgments
We thank Professor Douglas Wahlsten for his excellent suggestion to compare LP/J and BTBR T+tf/J, Dr. Petko Petkov for information on the LP/J genetic background, and Drs. Elliorr Sherr, Lynn Paul, and Ralph Adolphs for helpful discussion of human acallosal syndrome. Mr. Timothy Sullivan provided assistance with histological analysis. Ms. Janet Stephens, NIH Medical Arts, contributed macrophotography expertise for Figure 1. Supported by the National Institute of Mental Health Intramural Research Program.
Abbreviations
- BTBR
BTBR T+tf/J
- B6
C57BL/6J
- CC
corpus callosum
References
- Abrahams BS, Geschwind DH. Advances in autism genetics: on the threshold of a new neurobiology. Nat Rev Genet. 2008;9:341–355. doi: 10.1038/nrg2346. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alexander AL, Lee JE, Lazar M, Boudos R, DuBray MB, Oakes TR, Miller JN, Lu J, Jeong EK, McMahon WM, Bigler ED, Lainhart JE. Diffusion tensor imaging of the corpus callosum in Autism. Neuroimage. 2007;34:61–73. doi: 10.1016/j.neuroimage.2006.08.032. [DOI] [PubMed] [Google Scholar]
- Badaruddin DH, Andrews GL, Bolte S, Schilmoeller KJ, Schilmoeller G, Paul LK, Brown WS. Social and behavioral problems of children with agenesis of the corpus callosum. Child Psychiatry Hum Dev. 2007;38:287–302. doi: 10.1007/s10578-007-0065-6. [DOI] [PubMed] [Google Scholar]
- Bailey KR, Pavlova MN, Rohde AD, Hohmann JG, Crawley JN. Galanin receptor subtype 2 (GalR2) null mutant mice display an anxiogenic-like phenotype specific to the elevated plus-maze. Pharmacol Biochem Behav. 2007;86:8–20. doi: 10.1016/j.pbb.2006.11.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barnea-Goraly N, Kwon H, Menon V, Eliez S, Lotspeich L, Reiss AL. White matter structure in autism: preliminary evidence from diffusion tensor imaging. Biol Psychiatry. 2004;55:323–326. doi: 10.1016/j.biopsych.2003.10.022. [DOI] [PubMed] [Google Scholar]
- Boger-Megiddo I, Shaw DW, Friedman SD, Sparks BF, Artru AA, Giedd JN, Dawson G, Dager SR. Corpus callosum morphometrics in young children with autism spectrum disorder. J Autism Dev Disord. 2006;36:733–739. doi: 10.1007/s10803-006-0121-2. [DOI] [PubMed] [Google Scholar]
- Bolivar VJ, Walters SR, Phoenix JL. Assessing autism-like behavior in mice: variations in social interactions among inbred strains. Behav Brain Res. 2007;176:21–26. doi: 10.1016/j.bbr.2006.09.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bonilha L, Cendes F, Rorden C, Eckert M, Dalgalarrondo P, Li LM, Steiner CE. Gray and white matter imbalance--typical structural abnormality underlying classic autism? Brain Dev. 2008;30:396–401. doi: 10.1016/j.braindev.2007.11.006. [DOI] [PubMed] [Google Scholar]
- Bouwknecht JA, Paylor R. Behavioral and physiological mouse assays for anxiety: a survey in nine mouse strains. Behav Brain Res. 2002;136:489–501. doi: 10.1016/s0166-4328(02)00200-0. [DOI] [PubMed] [Google Scholar]
- Brambilla P, Hardan A, di Nemi SU, Perez J, Soares JC, Barale F. Brain anatomy and development in autism: review of structural MRI studies. Brain Res Bull. 2003;61:557–569. doi: 10.1016/j.brainresbull.2003.06.001. [DOI] [PubMed] [Google Scholar]
- Brodkin ES. BALB/c mice: low sociability and other phenotypes that may be relevant to autism. Behav Brain Res. 2007;176:53–65. doi: 10.1016/j.bbr.2006.06.025. [DOI] [PubMed] [Google Scholar]
- Brodkin ES, Hagemann A, Nemetski SM, Silver LM. Social approach-avoidance behavior of inbred mouse strains towards DBA/2 mice. Brain research. 2004;1002:151–157. doi: 10.1016/j.brainres.2003.12.013. [DOI] [PubMed] [Google Scholar]
- Caldji C, Tannenbaum B, Sharma S, Francis D, Plotsky PM, Meaney MJ. Maternal care during infancy regulates the development of neural systems mediating the expression of fearfulness in the rat. Proceedings of the National Academy of Sciences of the United States of America. 1998;95:5335–5340. doi: 10.1073/pnas.95.9.5335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Casanova MF. White matter volume increase and minicolumns in autism. Ann Neurol. 2004;56:453. doi: 10.1002/ana.20196. author reply 454. [DOI] [PubMed] [Google Scholar]
- Castelli F, Frith C, Happe F, Frith U. Autism, Asperger syndrome and brain mechanisms for the attribution of mental states to animated shapes. Brain. 2002;125:1839–1849. doi: 10.1093/brain/awf189. [DOI] [PubMed] [Google Scholar]
- Chadman KK, Gong S, Scattoni ML, Boltuck SE, Gandhy SU, Heintz N, Crawley JN. Minimal aberrant behavioral phenotypes of neuoligin-3 R451C knockin mice. Autism Research. 2008;1:147–158. doi: 10.1002/aur.22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cheh MA, Millonig JH, Roselli LM, Ming X, Jacobsen E, Kamdar S, Wagner GC. En2 knockout mice display neurobehavioral and neurochemical alterations relevant to autism spectrum disorder. Brain research. 2006;1116:166–176. doi: 10.1016/j.brainres.2006.07.086. [DOI] [PubMed] [Google Scholar]
- Cherkassky VL, Kana RK, Keller TA, Just MA. Functional connectivity in a baseline resting-state network in autism. Neuroreport. 2006;17:1687–1690. doi: 10.1097/01.wnr.0000239956.45448.4c. [DOI] [PubMed] [Google Scholar]
- Chung MK, Dalton KM, Alexander AL, Davidson RJ. Less white matter concentration in autism: 2D voxel-based morphometry. Neuroimage. 2004;23:242–251. doi: 10.1016/j.neuroimage.2004.04.037. [DOI] [PubMed] [Google Scholar]
- Cook MN, Bolivar VJ, McFadyen MP, Flaherty L. Behavioral differences among 129 substrains: implications for knockout and transgenic mice. Behav Neurosci. 2002;116:600–611. [PubMed] [Google Scholar]
- Crawley J, Goodwin FK. Preliminary report of a simple animal behavior model for the anxiolytic effects of benzodiazepines. Pharmacol Biochem Behav. 1980;13:167–170. doi: 10.1016/0091-3057(80)90067-2. [DOI] [PubMed] [Google Scholar]
- Crawley JN, Chen T, Puri A, Washburn R, Sullivan TL, Hill JM, Young NB, Nadler JJ, Moy SS, Young LJ, Caldwell HK, Young WS. Social approach behaviors in oxytocin knockout mice: comparison of two independent lines tested in different laboratory environments. Neuropeptides. 2007;41:145–163. doi: 10.1016/j.npep.2007.02.002. [DOI] [PubMed] [Google Scholar]
- Devinsky O, Laff R. Callosal lesions and behavior: history and modern concepts. Epilepsy Behav. 2003;4:607–617. doi: 10.1016/j.yebeh.2003.08.029. [DOI] [PubMed] [Google Scholar]
- DiCicco-Bloom E, Lord C, Zwaigenbaum L, Courchesne E, Dager SR, Schmitz C, Schultz RT, Crawley J, Young LJ. The developmental neurobiology of autism spectrum disorder. J Neurosci. 2006;26:6897–6906. doi: 10.1523/JNEUROSCI.1712-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Egaas B, Courchesne E, Saitoh O. Reduced size of corpus callosum in autism. Arch Neurol. 1995;52:794–801. doi: 10.1001/archneur.1995.00540320070014. [DOI] [PubMed] [Google Scholar]
- Ehninger D, Han S, Shilyansky C, Zhou Y, Li W, Kwiatkowski DJ, Ramesh V, Silva AJ. Reversal of learning deficits in a Tsc2+/- mouse model of tuberous sclerosis. Nat Med. 2008;14:843–848. doi: 10.1038/nm1788. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fairless AH, Dow HC, Toledo MM, Malkus KA, Edelmann M, Li H, Talbot K, Arnold SE, Abel T, Brodkin ES. Low sociability is associated with reduced size of the corpus callosum in the BALB/cJ inbred mouse strain. Brain research. 2008 doi: 10.1016/j.brainres.2008.07.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Franklin KBJ, Paxinos G. The Mouse Brain: in Stereotaxic Coordinates. Academic Press; San Diego, CA: 1997. [Google Scholar]
- Gazzaniga MS, Risse GL, Springer SP, Clark DE, Wilson DH. Psychologic and neurologic consequences of partial and complete cerebral commissurotomy. Neurology. 1975;25:10–15. doi: 10.1212/wnl.25.1.10. [DOI] [PubMed] [Google Scholar]
- Geschwind DH, Levitt P. Autism spectrum disorders: developmental disconnection syndromes. Curr Opin Neurobiol. 2007;17:103–111. doi: 10.1016/j.conb.2007.01.009. [DOI] [PubMed] [Google Scholar]
- Griebel G, Belzung C, Perrault G, Sanger DJ. Differences in anxiety-related behaviours and in sensitivity to diazepam in inbred and outbred strains of mice. Psychopharmacology. 2000;148:164–70. doi: 10.1007/s002130050038. [DOI] [PubMed] [Google Scholar]
- Grice DE, Buxbaum JD. The genetics of autism spectrum disorders. Neuromolecular Med. 2006;8:451–460. doi: 10.1385/NMM:8:4:451. [DOI] [PubMed] [Google Scholar]
- Hardan AY, Minshew NJ, Keshavan MS. Corpus callosum size in autism. Neurology. 2000;55:1033–1036. doi: 10.1212/wnl.55.7.1033. [DOI] [PubMed] [Google Scholar]
- Herbert MR, Russo JP, Yang S, Roohi J, Blaxill M, Kahler SG, Cremer L, Hatchwell E. Autism and environmental genomics. Neurotoxicology. 2006;27:671–684. doi: 10.1016/j.neuro.2006.03.017. [DOI] [PubMed] [Google Scholar]
- Holmes A, Hollon TR, Gleason TC, Liu Z, Dreiling J, Sibley DR, Crawley JN. Behavioral characterization of dopamine D5 receptor null mutant mice. Behav Neurosci. 2001;115:1129–1144. [PubMed] [Google Scholar]
- Hung AY, Futai K, Sala C, Valtschanoff JG, Ryu J, Woodworth MA, Kidd FL, Sung CC, Miyakawa T, Bear MF, Weinberg RJ, Sheng M. Smaller dendritic spines, weaker synaptic transmission, but enhanced spatial learning in mice lacking Shank1. J Neurosci. 2008;28:1697–1708. doi: 10.1523/JNEUROSCI.3032-07.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jamain S, Radyushkin K, Hammerschmidt K, Granon S, Boretius S, Varoqueaux F, Ramanantsoa N, Gallego J, Ronnenberg A, Winter D, Frahm J, Fischer J, Bourgeron T, Ehrenreich H, Brose N. Reduced social interaction and ultrasonic communication in a mouse model of monogenic heritable autism. Proc Natl Acad Sci U S A. 2008;105:1710–1715. doi: 10.1073/pnas.0711555105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Just MA, Cherkassky VL, Keller TA, Kana RK, Minshew NJ. Functional and anatomical cortical underconnectivity in autism: evidence from an FMRI study of an executive function task and corpus callosum morphometry. Cereb Cortex. 2007;17:951–961. doi: 10.1093/cercor/bhl006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Just MA, Cherkassky VL, Keller TA, Minshew NJ. Cortical activation and synchronization during sentence comprehension in high-functioning autism: evidence of underconnectivity. Brain. 2004;127:1811–1821. doi: 10.1093/brain/awh199. [DOI] [PubMed] [Google Scholar]
- Kana RK, Keller TA, Minshew NJ, Just MA. Inhibitory control in high-functioning autism: decreased activation and underconnectivity in inhibition networks. Biol Psychiatry. 2007;62:198–206. doi: 10.1016/j.biopsych.2006.08.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Karlsson RM, Choe JS, Cameron HA, Thorsell A, Crawley JN, Holmes A, Heilig M. The neuropeptide Y Y1 receptor subtype is necessary for the anxiolytic-like effects of neuropeptide Y, but not the antidepressant-like effects of fluoxetine, in mice. Psychopharmacology (Berl) 2008;195:547–557. doi: 10.1007/s00213-007-0945-2. [DOI] [PubMed] [Google Scholar]
- Keller TA, Kana RK, Just MA. A developmental study of the structural integrity of white matter in autism. Neuroreport. 2007;18:23–27. doi: 10.1097/01.wnr.0000239965.21685.99. [DOI] [PubMed] [Google Scholar]
- Kennedy DP, Courchesne E. The intrinsic functional organization of the brain is altered in autism. Neuroimage. 2008;39:1877–1885. doi: 10.1016/j.neuroimage.2007.10.052. [DOI] [PubMed] [Google Scholar]
- Kilian S, Brown WS, Hallam BJ, McMahon W, Lu J, Johnson M, Bigler ED, Lainhart J. Regional callosal morphology in autism and macrocephaly. Dev Neuropsychol. 2008;33:74–99. doi: 10.1080/87565640701729821. [DOI] [PubMed] [Google Scholar]
- Kleinhans NM, Richards T, Sterling L, Stegbauer KC, Mahurin R, Johnson LC, Greenson J, Dawson G, Aylward E. Abnormal functional connectivity in autism spectrum disorders during face processing. Brain. 2008;131:1000–1012. doi: 10.1093/brain/awm334. [DOI] [PubMed] [Google Scholar]
- Koshino H, Kana RK, Keller TA, Cherkassky VL, Minshew NJ, Just MA. fMRI investigation of working memory for faces in autism: visual coding and underconnectivity with frontal areas. Cereb Cortex. 2008;18:289–300. doi: 10.1093/cercor/bhm054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kusek GK, Wahlsten D, Herron BJ, Bolivar VJ, Flaherty L. Localization of two new X-linked quantitative trait loci controlling corpus callosum size in the mouse. Genes Brain Behav. 2007;6:359–363. doi: 10.1111/j.1601-183X.2006.00264.x. [DOI] [PubMed] [Google Scholar]
- Kwon CH, Luikart BW, Powell CM, Zhou J, Matheny SA, Zhang W, Li Y, Baker SJ, Parada LF. Pten regulates neuronal arborization and social interaction in mice. Neuron. 2006;50:377–388. doi: 10.1016/j.neuron.2006.03.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lassonde M, Sauerwein C. Neuropsychological outcome of corpus callosotomy in children and adolescents. J Neurosurg Sci. 1997;41:67–73. [PubMed] [Google Scholar]
- Long JM, LaPorte P, Paylor R, Wynshaw-Boris A. Expanded characterization of the social interaction abnormalities in mice lacking Dvl1. Genes Brain Behav. 2004;3:51–62. doi: 10.1046/j.1601-183x.2003.00045.x. [DOI] [PubMed] [Google Scholar]
- Lord C, Risi S, DiLavore PS, Shulman C, Thurm A, Pickles A. Autism from 2 to 9 years of age. Arch Gen Psychiatry. 2006;63:694–701. doi: 10.1001/archpsyc.63.6.694. [DOI] [PubMed] [Google Scholar]
- MacPherson P, McGaffigan R, Wahlsten D, Nguyen PV. Impaired fear memory, altered object memory and modified hippocampal synaptic plasticity in split-brain mice. Brain research. 2008;1210:179–188. doi: 10.1016/j.brainres.2008.03.008. [DOI] [PubMed] [Google Scholar]
- Mamelak AN, Barbaro NM, Walker JA, Laxer KD. Corpus callosotomy: a quantitative study of the extent of resection, seizure control, and neuropsychological outcome. J Neurosurg. 1993;79:688–695. doi: 10.3171/jns.1993.79.5.0688. [DOI] [PubMed] [Google Scholar]
- Manes F, Piven J, Vrancic D, Nanclares V, Plebst C, Starkstein SE. An MRI study of the corpus callosum and cerebellum in mentally retarded autistic individuals. J Neuropsychiatry Clin Neurosci. 1999;11:470–474. doi: 10.1176/jnp.11.4.470. [DOI] [PubMed] [Google Scholar]
- Manhaes AC, Abreu-Villaca Y, Schmidt SL, Filgueiras CC. Neonatal transection of the corpus callosum affects rotational side preference in adult Swiss mice. Neurosci Lett. 2007;415:159–163. doi: 10.1016/j.neulet.2007.01.012. [DOI] [PubMed] [Google Scholar]
- Mathis C, Neumann PE, Gershenfeld H, Paul SM, Crawley JN. Genetic analysis of anxiety-related behaviors and responses to benzodiazepine-related drugs in AXB and BXA recombinant inbred mouse strains. Behav Genet. 1995;25:557–568. doi: 10.1007/BF02327579. [DOI] [PubMed] [Google Scholar]
- Meaney MJ. Maternal care, gene expression, and the transmission of individual differences in stress reactivity across generations. Ann Rev Neurosci. 2001;24:1161–1192. doi: 10.1146/annurev.neuro.24.1.1161. [DOI] [PubMed] [Google Scholar]
- McFarlane HG, Kusek GK, Yang M, Phoenix JL, Bolivar VJ, Crawley JN. Autism-like behavioral phenotypes in BTBR T+tf/J mice. Genes Brain Behav. 2008;7:152–163. doi: 10.1111/j.1601-183X.2007.00330.x. [DOI] [PubMed] [Google Scholar]
- Mineur YS, Huynh LX, Crusio WE. Social behavior deficits in the Fmr1 mutant mouse. Behav Brain Res. 2006;168:172–175. doi: 10.1016/j.bbr.2005.11.004. [DOI] [PubMed] [Google Scholar]
- Minshew NJ, Williams DL. The new neurobiology of autism: cortex, connectivity, and neuronal organization. Arch Neurol. 2007;64:945–950. doi: 10.1001/archneur.64.7.945. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moy SS, Nadler JJ, Perez A, Barbaro RP, Johns JM, Magnuson TR, Piven J, Crawley JN. Sociability and preference for social novelty in five inbred strains: an approach to assess autistic-like behavior in mice. Genes Brain Behav. 2004;3:287–302. doi: 10.1111/j.1601-1848.2004.00076.x. [DOI] [PubMed] [Google Scholar]
- Moy SS, Nadler JJ, Young NB, Nonneman RJ, Segall SK, Andrade GM, Crawley JN, Magnuson TR. Social approach and repetitive behavior in eleven inbred mouse strains. Behav Brain Res. 2008 doi: 10.1016/j.bbr.2008.03.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moy SS, Nadler JJ, Young NB, Perez A, Holloway LP, Barbaro RP, Barbaro JR, Wilson LM, Threadgill DW, Lauder JM, Magnuson TR, Crawley JN. Mouse behavioral tasks relevant to autism: phenotypes of 10 inbred strains. Behav Brain Res. 2007;176:4–20. doi: 10.1016/j.bbr.2006.07.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nadler JJ, Moy SS, Dold G, Trang D, Simmons N, Perez A, Young NB, Barbaro RP, Piven J, Magnuson TR, Crawley JN. Automated apparatus for quantitation of social approach behaviors in mice. Genes Brain Behav. 2004;3:303–314. doi: 10.1111/j.1601-183X.2004.00071.x. [DOI] [PubMed] [Google Scholar]
- Newschaffer CJ, Croen LA, Daniels J, Giarelli E, Grether JK, Levy SE, Mandell DS, Miller LA, Pinto-Martin J. The Epidemiology of Autism Spectrum Disorders. Annu Rev Public Health. 2007 doi: 10.1146/annurev.publhealth.28.021406.144007. [DOI] [PubMed] [Google Scholar]
- Panksepp JB, Jochman KA, Kim JU, Koy JJ, Wilson ED, Chen Q, Wilson CR, Lahvis GP. Affiliative behavior, ultrasonic communication and social reward are influenced by genetic variation in adolescent mice. PLoS ONE. 2007;2:e351. doi: 10.1371/journal.pone.0000351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Panksepp JB, Lahvis GP. Social reward among juvenile mice. Genes Brain Behav. 2007;6:661–671. doi: 10.1111/j.1601-183X.2006.00295.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paul LK, Brown WS, Adolphs R, Tyszka JM, Richards LJ, Mukherjee P, Sherr EH. Agenesis of the corpus callosum: genetic, developmental and functional aspects of connectivity. Nat Rev Neurosci. 2007;8:287–299. doi: 10.1038/nrn2107. [DOI] [PubMed] [Google Scholar]
- Paul LK, Lautzenhiser A, Brown WS, Hart A, Neumann D, Spezio M, Adolphs R. Emotional arousal in agenesis of the corpus callosum. Int J Psychophysiol. 2006;61:47–56. doi: 10.1016/j.ijpsycho.2005.10.017. [DOI] [PubMed] [Google Scholar]
- Paxinos G, Halliday GM, Watson C, Koutcherov Y, Wang H. Atlas of the Developing Mouse Brain at E17.5, P0 and P6. Academic Press; Burlington: 2006. [Google Scholar]
- Petkov PM, Ding Y, Cassell MA, Zhang W, Wagner G, Sargent EE, Asquith S, Crew V, Johnson KA, Robinson P, Scott VE, Wiles MV. An efficient SNP system for mouse genome scanning and elucidating strain relationships. Genome Res. 2004;14:1806–1811. doi: 10.1101/gr.2825804. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Piven J, Bailey J, Ranson BJ, Arndt S. An MRI study of the corpus callosum in autism. Am J Psychiatry. 1997;154:1051–1056. doi: 10.1176/ajp.154.8.1051. [DOI] [PubMed] [Google Scholar]
- Ricceri L, Moles A, Crawley J. Behavioral phenotyping of mouse models of neurodevelopmental disorders: relevant social behavior patterns across the life span. Behav Brain Res. 2007;176:40–52. doi: 10.1016/j.bbr.2006.08.024. [DOI] [PubMed] [Google Scholar]
- Rippon G, Brock J, Brown C, Boucher J. Disordered connectivity in the autistic brain: challenges for the “new psychophysiology”. Int J Psychophysiol. 2007;63:164–172. doi: 10.1016/j.ijpsycho.2006.03.012. [DOI] [PubMed] [Google Scholar]
- Rougier A, Claverie B, Pedespan JM, Marchal C, Loiseau P. Callosotomy for intractable epilepsy: overall outcome. J Neurosurg Sci. 1997;41:51–57. [PubMed] [Google Scholar]
- Sass KJ, Spencer DD, Spencer SS, Novelly RA, Williamson PD, Mattson RH. Corpus callosotomy for epilepsy. II. Neurologic and neuropsychological outcome. Neurology. 1988;38:24–28. doi: 10.1212/wnl.38.1.24. [DOI] [PubMed] [Google Scholar]
- Scattoni ML, Gandhy SU, Ricceri L, Crawley JN. Unusual repertoire of vocalizations in the BTBR T+tf/J mouse model of autism. PLoS ONE. 2008;3:e3067. doi: 10.1371/journal.pone.0003067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scearce-Levie K, Roberson ED, Gerstein H, Cholfin JA, Mandiyan VS, Shah NM, Rubenstein JL, Mucke L. Abnormal social behaviors in mice lacking Fgf17. Genes Brain Behav. 2008;7:344–354. doi: 10.1111/j.1601-183X.2007.00357.x. [DOI] [PubMed] [Google Scholar]
- Sundaram SK, Kumar A, Makki MI, Behen ME, Chugani HT, Chugani DC. Diffusion Tensor Imaging of Frontal Lobe in Autism Spectrum Disorder. Cereb Cortex. 2008 doi: 10.1093/cercor/bhn031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tabuchi K, Blundell J, Etherton MR, Hammer RE, Liu X, Powell CM, Sudhof TC. A neuroligin-3 mutation implicated in autism increases inhibitory synaptic transmission in mice. Science. 2007;318:71–76. doi: 10.1126/science.1146221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takayanagi Y, Yoshida M, Bielsky IF, Ross HE, Kawamata M, Onaka T, Yanagisawa T, Kimura T, Matzuk MM, Young LJ, Nishimori K. Pervasive social deficits, but normal parturition, in oxytocin receptor-deficient mice. Proc Natl Acad Sci U S A. 2005;102:16096–16101. doi: 10.1073/pnas.0505312102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Terranova ML, Laviola G. Scoring of social interactions and play in mice during adolescence. Curr Protocols Toxicol. 2005:10.11–10.11. doi: 10.1002/0471140856.tx1310s26. [DOI] [PubMed] [Google Scholar]
- van Gaalen MM, Stenzel-Poore MP, Holsboer F, Steckler T. Effects of transgenic overproduction of CRH on anxiety-like behaviour. Eur J Neurosci. 2002;15:2007–2015. doi: 10.1046/j.1460-9568.2002.02040.x. [DOI] [PubMed] [Google Scholar]
- Vallée M, Mayo W, Dellu F, Le Moal M, Simon H, Maccari S. Prenatal stress induces high anxiety and postnatal handling induces low anxiety in adult offspring: correlation with stress-induced corticosterone secretion. J Neurosci. 1997;17:2626–36. doi: 10.1523/JNEUROSCI.17-07-02626.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Veenstra-Vanderweele J, Christian SL, Cook EH., Jr Autism as a paradigmatic complex genetic disorder. Annu Rev Genomics Hum Genet. 2004;5:379–405. doi: 10.1146/annurev.genom.5.061903.180050. [DOI] [PubMed] [Google Scholar]
- Vidal CN, Nicolson R, DeVito TJ, Hayashi KM, Geaga JA, Drost DJ, Williamson PC, Rajakumar N, Sui Y, Dutton RA, Toga AW, Thompson PM. Mapping corpus callosum deficits in autism: an index of aberrant cortical connectivity. Biol Psychiatry. 2006;60:218–225. doi: 10.1016/j.biopsych.2005.11.011. [DOI] [PubMed] [Google Scholar]
- Volkmar F, Chawarska K, Klin A. Autism in infancy and early childhood. Annu Rev Psychol. 2005;56:315–336. doi: 10.1146/annurev.psych.56.091103.070159. [DOI] [PubMed] [Google Scholar]
- Wahlsten D, Metten P, Crabbe JC. Survey of 21 inbred mouse strains in two laboratories reveals that BTBR T/+ tf/tf has severely reduced hippocampal commissure and absent corpus callosum. Brain research. 2003;971:47–54. doi: 10.1016/s0006-8993(03)02354-0. [DOI] [PubMed] [Google Scholar]
- Waiter GD, Williams JH, Murray AD, Gilchrist A, Perrett DI, Whiten A. Structural white matter deficits in high-functioning individuals with autistic spectrum disorder: a voxel-based investigation. Neuroimage. 2005;24:455–461. doi: 10.1016/j.neuroimage.2004.08.049. [DOI] [PubMed] [Google Scholar]
- Wilson DH, Reeves A, Gazzaniga M. Division of the corpus callosum for uncontrollable epilepsy. Neurology. 1978;28:649–653. doi: 10.1212/wnl.28.7.649. [DOI] [PubMed] [Google Scholar]
- Winslow JT, Insel TR. The social deficits of the oxytocin knockout mouse. Neuropeptides. 2002;36:221–229. doi: 10.1054/npep.2002.0909. [DOI] [PubMed] [Google Scholar]
- Witmer PD, Doheny KF, Adams MK, Boehm CD, Dizon JS, Goldstein JL, Templeton TM, Wheaton AM, Dong PN, Pugh EW, Nussbaum RL, Hunter K, Kelmenson JA, Rowe LB, Brownstein MJ. The development of a highly informative mouse Simple Sequence Length Polymorphism (SSLP) marker set and construction of a mouse family tree using parsimony analysis. Genome Res. 2003;13:485–491. doi: 10.1101/gr.717903. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang M, Scattoni ML, Zhodzishsky V, Chen T, Caldwell HK, Young WS, McFarlane HG, Crawley JN. Similar social approach behaviors in BTBR T+ tf/J, C57BL/6J, and vasopressin receptor 1B knockout mice tested on conventional versus reverse light cycles, and in replications across cohorts. Frontiers Behav Neurosci. 2007a;1:9. doi: 10.3389/neuro.08/001.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang M, Zhodzishsky V, Crawley JN. Social deficits in BTBR T+tf/J mice are unchanged by cross-fostering with C57BL/6J mothers. Int J Dev Neurosci. 2007b doi: 10.1016/j.ijdevneu.2007.09.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Young LJ. Oxytocin and vasopressin as candidate genes for psychiatric disorders: lessons from animal models. Am J Med Genet. 2001;105:53–54. [PubMed] [Google Scholar]
- Young LJ, Pitkow LJ, Ferguson JN. Neuropeptides and social behavior: animal models relevant to autism. Mol Psychiatry. 2002;72 2:S38–39. doi: 10.1038/sj.mp.4001175. [DOI] [PubMed] [Google Scholar]
- Zagreda L, Goodman J, Druin DP, McDonald D, Diamond A. Cognitive deficits in a genetic mouse model of the most common biochemical cause of human mental retardation. J Neurosci. 1999;19:6175–6182. doi: 10.1523/JNEUROSCI.19-14-06175.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]









