Abstract
Background
Premenstrual dysphoric disorder (PMDD) is a heritable mood disorder that is triggered by gonadal steroids during the luteal phase in susceptible women.
Methods
We performed haplotype analyses of estrogen receptors alpha and beta (ESR1 and ESR2) in 91 women with prospectively confirmed PMDD and 56 controls to investigate possible sources of the genetic susceptibility to affective dysregulation induced by normal levels of gonadal steroids. We also examined associations with the Val158Met SNP of the gene for COMT, an enzyme involved in estrogen metabolism and prefrontal cortical activation.
Results
Four SNPS in intron 4 of ESR1 showed significantly different genotype and allele distributions between patients and controls. Significant case-control differences were seen in sliding-window analyses of two, three, and four marker haplotypes, but only in those haplotypes containing SNPs in intron 4 that were positive in the single-locus analysis. No significant associations were observed with ESR2 or with the COMT Val158Met polymorphism, although the significant associations with ESR1 were observed only in those with the Val/Val genotype.
Conclusion
These are the first positive (albeit preliminary) genetic findings in this reproductive endocrine-related mood disorder and involve the receptor for a hormone that is pathogenically relevant.
Keywords: premenstrual dysphoric disorder, PMDD, ESR1, estrogen receptor, alpha gene
Premenstrual Dysphoric Disorder (PMDD) is a reproductive endocrine-related mood disorder that affects approximately 5%–8% of women and is associated with substantial morbidity. While affective disturbance in this condition has been presumed to be linked to hormonal changes over the course of the menstrual cycle, the exact role of hormones has been elusive. PMDD is not characterized by abnormal gonadal steroid levels or disordered hypothalamic-pituitary-ovarian axis function (Rubinow 1992). Nonetheless, we have demonstrated that exposure to normal levels of gonadal steroids can trigger depressed mood in women with PMDD, but not in those without such history (Schmidt et al 1998). Consequently, any pathophysiological explanation for PMDD must account for both the triggering of mood symptoms by gonadal steroids and the vulnerability to experience affective disturbance in association with levels of gonadal steroids that are without effect on mood in women lacking a history of PMDD.
Genotypic differences are likely to mediate a differential behavioral response to gonadal steroids. In both animals and humans, polymorphic variants in genes encoding gonadal steroid receptors associate with both altered sensitivity and differential response to gonadal steroids. These gonadal steroid receptor polymorphisms have been shown to alter receptor transcriptional efficacy (e.g., CAG repeat in exon 1 of the androgen receptor; progins insertion in intron 7 of the progesterone receptor) and are associated with differential illness risk (i.e., prostate cancer, breast cancer) (Zhang et al 2004; Beilin et al 1999; Giovannucci et al 1997; Wang-Gohrke et al 2000). Additionally, the susceptibility to the disruptive effects of estradiol on reproductive development differs enormously (up to 100-fold) between mouse strains, with genotype controlling more of the variance than the dose of estradiol employed (Spearow et al 1999). There is precedent, then, for inferring that polymorphisms in genes in the gonadal steroid signaling pathway or in gonadal steroid-regulated genes may alter the nature or strength of the steroid signal as well as the clinical and behavioral phenotype. Moreover, a genetic contribution to PMDD is supported by the results of both family and twin studies (Wilson et al 1991; Kendler et al 1992; Condon 1993).
Several genetic polymorphisms have been tested for possible association to PMDD (Melke et al 2003; Damberg et al 2005) without any positive reports thus far [e.g., variants in the serotonin transporter (SLC6A4) including the 5′HTTLPR polymorphism (Heils et al 1995), an intron 2 VNTR (Ogilvie et al 1996), and a G to T single nucleotide polymorphism (SNP) in the 3′ UTR region (Battersby et al 1999); and an intron 2 VNTR in the gene for transcription factor activating protein 2beta (AP-2beta) (Damberg et al 2005)]. In the absence of validated functional polymorphisms in potentially causative genes, genetic association relies on typing marker alleles across samples as potential proxies for causative mutations. The extent of gene sequence diversity, however, has made apparent the limited power of single SNP-based candidate gene studies and consequently raised questions about their validity (Hoehe 2003). Gene-based haplotype analysis employs a set of SNPs (or other markers) that when analyzed together can have substantially greater power to capture the genetic diversity of the sample compared to single SNPs.
In this study, we initiated gene-based haplotype analyses of estrogen receptors (ER) alpha and beta in women with PMDD and controls. We selected these genes for two reasons: 1) our prior demonstration of the triggering of affective dysregulation in women with PMDD when exogenous estradiol was administered in the context of GnRH agonist-induced hypogonadism (Schmidt et al 1998); 2) recent studies identifying the importance of ER beta in animal models of anxiety and depression (Walf et al 2004; Krezel et al 2001; Rocha et al 2005) and of estrogen receptor alpha in arousal (Garey et al 2003). In addition, we examined associations with the Val158Met SNP (rs4680) in the gene for catechol-O-methyltransferase (COMT), an enzyme involved in estradiol metabolism, implicated in sex hormone-mediated cancer (Tanaka et al 2006; Sazci et al 2004), and observed in other studies to regulate activity in the prefrontal cortex, a brain region implicated as dysfunctional in PMDD (Rubinow et al in press).
2. Materials and Methods
2.1. Subjects and recruitment procedures
We studied 91 women with PMDD and 56 women who served as an asymptomatic comparison group. Medication-free Caucasian women with regular menstrual cycles were selected from respondents to newspaper advertisements for volunteers with a history of PMDD or without any history of menstrual cycle-related mood changes. Subjects from both groups had similar demographic and socio-economic characteristics. Before entry into the study, prospective participants were screened with a daily visual analogue scale of self-ratings of affective symptoms (i.e., a 100 mm line bracketed by severity extremes [none to worst ever] for sadness and irritability/anxiety) (Rubinow et al 1984). In at least two of three menstrual cycles, women diagnosed with PMDD (n = 91, age = 39.5. yrs, SD = 5.9) showed a 30% (or greater) higher mean level (adjusted for the range of the scale employed) of sadness and/or irritability/anxiety symptoms in the week before menses than in the week following the end of menses. (Adjustment consisted of dividing 30% by the percent of the scale spanned by the extreme high and low ratings (Smith et al 2003).) This criterion operationalizes the DSM-IV severity and cyclicity criteria for PMDD (Smith et al 2003), and all patient volunteers met DSM-IV criteria. On the daily ratings control women without PMDD (n = 56, age = 40.6 yrs, SD = 9.0) showed no evidence of mood changes related to menstrual cycle phase. All subjects were given a Structured Clinical Interview for DSM-III-R (SCID) (Spitzer et al 1990) and a modified Schedule for Affective Disorders and Schizophrenia-Lifetime (SADS-L) (Spitzer et al 1979); women with PMDD were required to have no current or recent (< 2 years) Axis I condition, and control women without PMDD were required to have no current or past history of an Axis I condition. The two year requirement eliminated subjects with significant current or recent comorbidities, which could serve as confounds, but did not eliminate the majority of subjects with PMDD with more remote histories of Axis I disorders. All subjects provided written informed consent, and the protocol was approved by the National Institute of Mental Health institutional review board. All subjects reported menstrual cycles of regular length, ranging between subjects from 21 to 33 days, and none had any significant medical illness either at intake or at time of testing or within the previous year.
2.2. Genotyping
Genomic DNA was extracted from peripheral lymphocytes from 20 ml of whole blood with the Puregene DNA isolation kit (Gentra Systems, Minneapolis, MN), used according to the manufacturer’s instructions.
A total of 24 SNPs were selected from the Celera and dbSNP (http://www.ncbi.nlm.nih.gov/SNP/) databases to test for association of PMDD with estrogen receptor alpha (ESR1) and estrogen receptor beta (ESR2). Initially we examined 10 SNPs that spanned a 295.7 kilobase (kb) genomic DNA interval encompassing the ESR1 transcript (GenBank NM_000125) and attempted 8 SNPs that spanned the ESR2 transcript (GenBank NM_001437). One ESR2 SNP failed, and primers and probes for another could not be successfully produced; therefore, 6 ESR2 SNPs were genotyped. We selected SNPs that are at roughly 30 kb intervals for ESR1 or 10 kb intervals for ESR2, spanned the whole gene coding region, and, with two exceptions (low frequency SNPs previously validated in the literature), had a minor allele frequency > 10%. Regions identified as showing positive associations with diagnosis (PMDD vs. controls) were further interrogated with additional SNPs. This resulted in the genotyping of six additional SNPs in ESR1. The COMT Val158Met polymorphism was also genotyped; primer and probe were designed by Applied Biosystems, Assay-by-Design as previously described (Chen et al 2004). The map positions and polymorphism information for the SNPs tested are shown in Tables 1 and 2. The Haploview 3.2 program was used to determine which of these SNPs were also ht-SNPs (Barrett et al 2005).
Table 1.
ESR1 marker and map information
| NCBI dbSNP |
Coding Strand |
Chromosomal | Intermarker | Distance from M014 |
|||
|---|---|---|---|---|---|---|---|
| SNP | rs# | Alleles1 | MAF2 | Location | Position3 | Distance4 | |
| L0017 | rs6920483 | G/A | 0.487 | intron 1 | 152223435 | 0 | 0 |
| L0018 | rs827421 | T/C | 0.447 | intron 1 | 152249236 | 25801 | 25801 |
| L0019 | rs7774230 | C/T | 0.447 | intron 2 | 152256353 | 7117 | 32918 |
| L0020 | rs11155818 | A/G | 0.041 | intron 2 | 152276244 | 19891 | 52809 |
| L0024 | rs7761846 | T/C | 0.117 | intron 3 | 152304622 | 28378 | 81187 |
| L0055 | rs4870062 | T/G | 0.378 | intron 3 | 152329732 | 25110 | 106297 |
| L0058 | rs1801132 | C/G | 0.229 | intron 4 | 152357636 | 27904 | 134201 |
| L0025 | rs3003917 | A/G | 0.220 | intron 4 | 152358582 | 946 | 135147 |
| L0060 | rs3020314 | C/T | 0.423 | intron 4 | 152362786 | 4204 | 139351 |
| L0061 | rs3020377 | G/A | 0.416 | intron 4 | 152364512 | 1726 | 141077 |
| L0057 | rs3020317 | T/C | 0.209 | intron 4 | 152370855 | 6343 | 147420 |
| L0026 | rs1884051 | A/G | 0.373 | intron 4 | 152375393 | 4538 | 151958 |
| L0056 | rs1884054 | A/C | 0.423 | intron 4 | 152383680 | 8287 | 160245 |
| L0021 | rs932477 | G/A | 0.095 | intron 4 | 152396710 | 13030 | 173275 |
| L0023 | rs926779 | G/A | 0.366 | intron 5 | 152448034 | 51324 | 224599 |
| L0022 | rs2813543 | G/A | 0.156 | 3′ UTR | 152516592 | 68558 | 293157 |
major/minor
minor allele frequency
from the UCSC May 2004 freeze
in base pairs
Table 2.
ESR2 marker and map information
| NCBI dbSNP | Coding Strand | Chromosomal | Intermarker | Distance from M014 | |||
|---|---|---|---|---|---|---|---|
| SNP | rs# | Alleles1 | MAF2 | Location | Position3 | Distance4 | |
| L0032 | rs1952586 | A/G | 0.139 | 5′ UTR | 63829170 | 0 | 0 |
| L0034 | rs7159462 | C/T | 0.043 | 5′ UTR | 63828629 | 541 | 541 |
| L0031 | rs6573553 | G/T | 0.441 | 5′ UTR | 63824114 | 4515 | 5056 |
| L0030 | rs1256030 | C/T | 0.361 | intron 1 | 63816915 | 7199 | 11714 |
| L0033 | rs1256048 | G/T | 0.281 | intron 3 | 63798033 | 18882 | 26081 |
| L0029 | rs6573549 | C/T | 0.337 | intron 5 | 63791402 | 6631 | 25513 |
| L0028 | rs8017441 | A/G | 0.21 | intron 6 | 63785547 | 5855 | 12486 |
| L0027 | rs1256061 | A/C | 0.421 | intron 6 | 63773346 | 12201 | 18056 |
major/minor
minor allele frequency
from the UCSC May 2004 freeze
in base pairs
Amplification reactions were performed in a 384-well format in a total reaction volume of 10 μl with 7.5 ng of dried genomic DNA, 5 μl of 2 × AmpliTag Gold® PCR master mix (Applied Biosystems), 0.1 μl (1000 nM) of each primer, 0.02 μl (100 nM) of each probe, and 3.76 μl of 1 × TE buffer. The plates were then placed in a thermal cycler (PE 9700; Applied Biosystems) and were heated at 50°C for two minutes and 95°C for 10 minutes, followed by 40 cycles of 95°C for 30s and 60°C for one minute. Plates were then transferred to the Prism 7900HT (Applied Biosystems), in which the fluorescence intensity in each well of the plate was read. Water was used as a negative control. Greater than 96% of genotypes were called. In addition, consistency of genotyping was determined by loading four case and four control samples in duplicate in each genotyping plate; reliability of genotyping was 99.6%. Interplate and intraplate duplicate testing of known DNAs was performed.
2.3. Statistical analysis
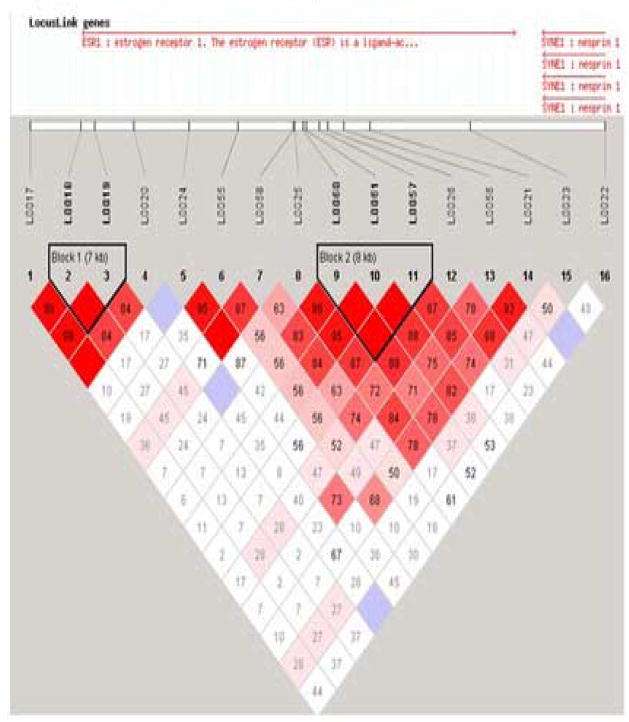
The 25 SNPs were tested for Hardy-Weinberg equilibrium in the Caucasian individuals. Differences in genotype distributions between cases and controls were tested for significance using a χ2 test (unadjusted for multiple comparisons). The standardized measure of linkage disequilibrium (LD), denoted as D′, was estimated with software (Haploview 3.1) from haplotype frequencies based on alleles at all possible pairs of SNP loci. A graphical overview of the LD plot was constructed using Haploview software (see Figure 1).
Figure 1.
Marker to Marker Linkage Disquillibrium - HAPLOVIEW D′
COCAPHASE, a method of standard unconditional logistic regression, was used to test for single-locus association with our case-control data (http://www.hgmp.mrs.ac.uk/~fdudbrid/software/unphased/). The SNPHAP program (version 1.0, http://www.gene.cimr.cam.ac.uk/clayton/software/) was used to infer ESR1 haplotype frequencies in case and control samples. Haplotype frequencies were compared more rigorously using the GENECOUNTING program (version 2.0, 1/04; (Zhao et al 2002)). This program, which in our sample can be used for up to 10 SNP haplotypes, assesses the significance of each haplotype individually by permutation (here, n = 1000), producing empirical p-values, and also performs a global measure of association. We used this program to estimate multi-marker haplotype frequencies and to test each haplotype for differences in frequency between our subject groups. In addition to the single-locus analysis, then, haplotype analysis was performed using two, three, and four marker moving-windows. The association between individual SNPs and diagnosis was compared across COMT genotypes with the Cochran-Mantel-Haenszel odds ratio test (SPSS version 14.0.2, SPSS, Inc, Chicago, IL). Additionally, to rule out the possible contribution to the associations observed of a mood disorder in general (vs PMDD), single-locus associations were recalculated after the 29 PMD subjects with a past history of MDD were deleted.
3. Results
3.1. Genotype distribution
The marker to marker linkage disequilibrium map constructed by the program HAPLOVIEW is shown in Figure 1. All 16 SNPs tested in ESR1 (see Table 1 and Figure 2) were in Hardy–Weinberg equilibrium in both PMDD and control groups. Four SNPs, all located in intron 4 showed significantly different allele and genotype frequencies between patients and controls (Table 3A), and for each SNP the 1 was the risk allele. Reanalysis of the data after excluding the 29 PMD subjects with a past history of MDD produced identical genotypic results (despite the decreased sample size) and nearly identical allelic results, with the exception that the contiguous intron 4 SNPs L0058 + L0057 also demonstrated significant case control associations. No significant association was observed with any of the eight SNPs (see Table 3B) in ESR2 (estrogen receptor beta) or with the COMT Val158Met polymorphism (data not shown).
Figure 2.
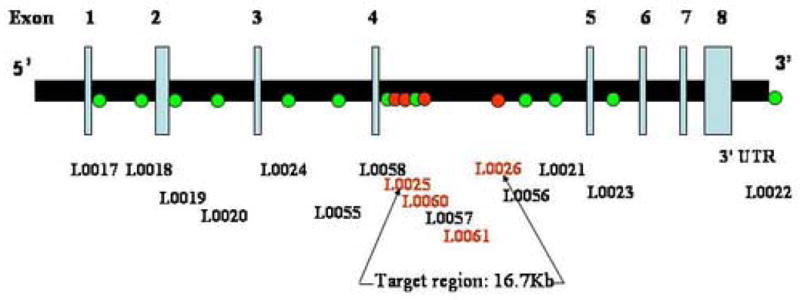
SNPs genotyped in ESR1 (6q25.1)
Table 3.
| Table 3A. ESR1 - Single SNP analysis of association with PMDD | ||||
|---|---|---|---|---|
| PMDD (n=91) | Controls (n=56) | P values |
||
| SNP | MAF | MAF | Alleles | Genotypes |
| L0017 | 0.443 | 0.473 | 0.626 | 0.8659 |
| L0018 | 0.411 | 0.429 | 0.769 | 0.7016 |
| L0019 | 0.411 | 0.429 | 0.769 | 0.7016 |
| L0020 | 0.017 | 0.036 | 0.316 | 1.000 |
| L0024 | 0.094 | 0.063 | 0.326 | 0.5672 |
| L0055 | 0.317 | 0.352 | 0.552 | 0.7712 |
| L0058 | 0.200 | 0.295 | 0.070 | 0.1814 |
| L0025 | 0.125 | 0.246 | 0.009 | 0.0169 |
| L0060 | 0.279 | 0.434 | 0.008 | 0.0223 |
| L0061 | 0.276 | 0.425 | 0.011 | 0.0357 |
| L0057 | 0.136 | 0.226 | 0.057 | 0.1212 |
| L0026 | 0.287 | 0.420 | 0.020 | 0.0437 |
| L0056 | 0.324 | 0.380 | 0.339 | 0.5855 |
| L0021 | 0.062 | 0.130 | 0.053 | 0.1203 |
| L0023 | 0.285 | 0.348 | 0.261 | 0.4103 |
| L0022 | 0.182 | 0.173 | 0.845 | 0.5149 |
| Table 3B. SNPs in ESR2 are not associated with PMDD: pairwise anaylsis | ||||
|---|---|---|---|---|
| PMS (n=91) | Controls (n=56) | P values | ||
| SNP | Minor allele frequency | Minor allele frequency | Alleles | Genotypes |
| L0027 | 0.448 | 0.519 | 0.252 | 0.4627 |
| L0028 | 0.138 | 0.098 | 0.338 | 0.442 |
| L0030 | 0.461 | 0.391 | 0.241 | 0.493 |
| L0031 | 0.476 | 0.422 | 0.382 | 0.245 |
| L0032 | 0.135 | 0.147 | 0.779 | 0.224 |
| L0034 | 0.074 | 0.127 | 0.138 | 0.283 |
note: p values were caculated using the χ2 test.
3.2. Haplotype analysis
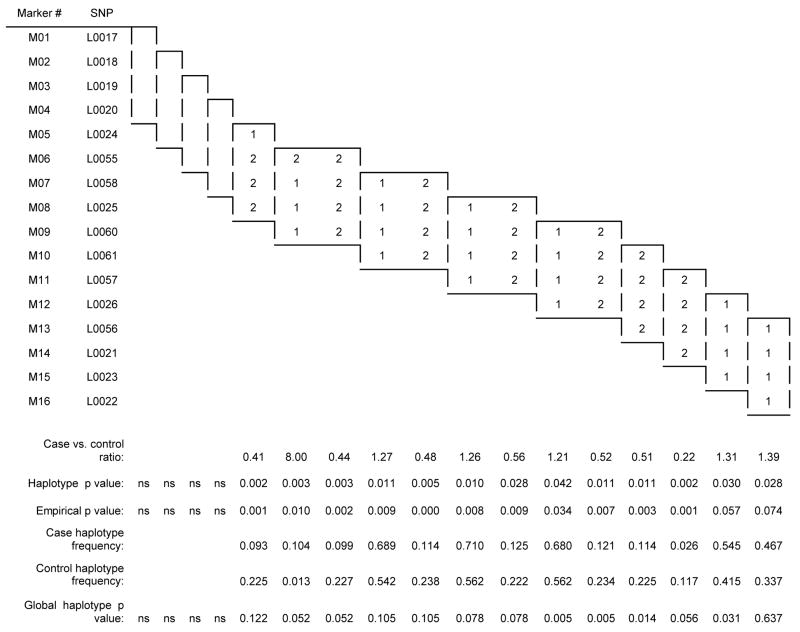
Significant case-control differences were seen in the two-, three-, and four-marker haplotypes analyses (Tables 4A, 4B, and 4C, respectively) in those haplotypes containing the SNPs in intron 4 that were positive in the single-locus analysis. In all three analyses, the first significant haplotypes (global and empirical p values) contained SNPs L0025 or L0060 or both, and the last significant two and three marker haplotypes began with L0026. Almost every significant association consisted of the common 11, 111 and 1111 haplotypes as risk and the 22, 222, and 2222 haplotype as protective. No significant associations were observed upstream of the L0025 locus or downstream of the L0026 locus. L0026 (rs1884051) and L0060 (rs3020314) are ht-SNPS in block 6, and the only other ht-SNP that we genotyped in ESR1 was the slightly upstream L0058 (rs1801132).
Table 4.
| Table 4A ESR1 two-marker haplotype results |
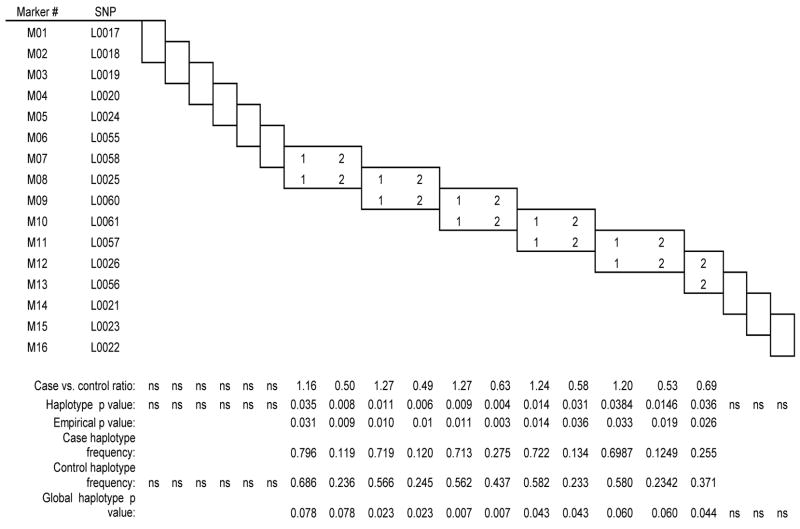 |
| Table 4B ESR1 three-marker haplotype results |
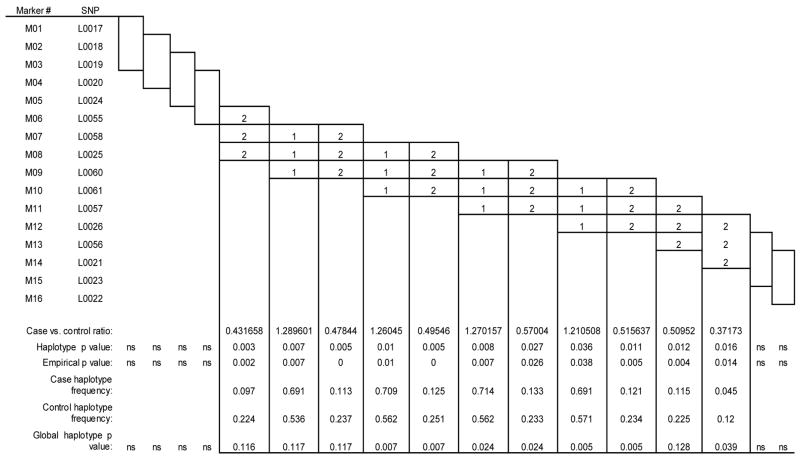 |
| Table 4C ESR1 four-marker haplotype results |
 |
3.3 Effect of COMT genotype on ESR1 association results
The significant differences in the allele frequencies between patients and controls of four SNPs in intron 4 of ESR1 were observed only in subjects with the COMT Val/Val homozygote genotype (Table 5). No associations were observed in Met/Met homozygotes or in Val/Met heterozygotes, and the Cochran-Mantel-Haenszel test showed the single SNP associations to be significantly different in the Val/Val compared with the combined Val/Met and Met/Met groups for markers 25, 26, 60, 61, as well as for 57 and 21 (odds ratios varied between 1.7 and 3).
Table 5.
Significant ESR1 associations with PMDD are preserved in those with a COMT Val/Val genotype
| Marker | p value in full sample (n=147) | p value in subjects with COMT Val/Val (n=38) | p value in subjects with COMT Val/Met (n=61) | p value in subjects with COMT Met/Met (n=43) | P value by Cochran - Mantel-Haenszel statistic (Val/Val vs grouped Val/Met and MetMet) |
|---|---|---|---|---|---|
| L0017 | 0.626 | 0.135 | 0.489 | 0.631 | 0.593 |
| L0018 | 0.769 | 0.392 | 0.812 | 0.992 | 0.817 |
| L0019 | 0.769 | 0.392 | 0.812 | 0.992 | 0.817 |
| L0020 | 0.316 | 0.242 | 0.345 | 0.271 | 0.325 |
| L0024 | 0.326 | 0.618 | 0.730 | 0.147 | 0.456 |
| L0055 | 0.552 | 0.234 | 0.461 | 0.715 | 0.703 |
| L0058 | 0.070 | 0.288 | 0.576 | 0.129 | 0.073 |
| 0.009 | 0.026 | 0.397 | 0.092 | 0.010 | |
| L0060 | 0.008 | 0.019 | 0.309 | 0.202 | 0.011 |
| L0061 | 0.011 | 0.022 | 0.310 | 0.137 | 0.009 |
| L0057 | 0.057 | 0.003 | 0.873 | 0.369 | 0.033 |
| L0026 | 0.020 | 0.059 | 0.512 | 0.199 | 0.033 |
| L0056 | 0.339 | 0.101 | 0.777 | 0.946 | 0.288 |
| L0021 | 0.053 | 0.072 | 0.161 | 0.250 | 0.017 |
| L0023 | 0.261 | 0.166 | 0.528 | 0.204 | 0.318 |
| L0022 | 0.845 | 0.4162 | 0.5947 | 0.5915 | 0.731 |
p values for the first four columns were calculated with the χ2 test.
Discussion
Our demonstration of an association between allelic variants in the estrogen receptor alpha gene and PMDD is the first positive genetic finding in this disorder, a condition estimated to affect 5–8% of reproductive age women and to be responsible for 14.5 million disability adjusted life-years in the United States (Halbreich et al 2003). The gene for ER alpha (ESR1), a 595 amino acid protein, contains 8 exons, which code for two activation domains, a DNA binding domain, and a ligand binding domain. ESR1 was cloned in 1985 (Walter et al 1985), and a little over a decade later a second ER, ER beta (ESR2), was cloned (Kuiper et al 1996). ER beta is a 530 amino acid protein that is 95% homologous with ER alpha in the DNA binding domain and 53% homologous in the E/F transactivation/ligand binding domain, but is otherwise poorly conserved (Ogawa et al 1998). As ligand-dependent transcription factors, ER alpha and beta regulate the synthesis and metabolism of multiple neurotransmitters and neuropeptides, their receptors, and transporters (McEwen et al 1999). Additionally, both receptors have been shown to play a role in acute, non-genomic cell signaling (Razandi et al 1999; Razandi et al 2002; Kim et al 1999). Through these manifold actions, ER alpha and beta impact on virtually all elements of CNS development and function – neuronal and glial proliferation, migration, differentiation, activation, survival, and death.
The results of twin and family studies suggest that PMDD is a heritable disorder (Kendler et al 1992; Condon 1993; Wilson et al 1991). Kendler et al, for example, observed that premenstrual, menstrual and neurotic symptoms had different genetic and environmental determinants (Kendler et al 1992) and that the heritability of PMS was about 56% (Kendler et al 1998). These studies provide a rational basis for the search for genetic contributions to PMDD. As with all complex genetic disorders, the contribution of any single gene is likely to be quite small. Nonetheless, demonstration of a relationship between genetic variations in ESR1 and PMDD is very promising for several reasons. First, ER alpha plays a major role in arousal (Garey et al 2003), dysfunction of which could underlie somatic, cognitive and affective symptoms of PMDD. Second, ER alpha regulates the signaling of neurotransmitter systems implicated in both the etiopathogenesis and treatment of PMDD. For example, extensive links exist between estrogen and serotonin function, with the latter involved in mood regulation and the selective therapeutic effects of SSRIs in PMDD (Rubinow et al 1998). At least some of the effects of estradiol are mediated through serotonin 1A receptors, which are upregulated through nuclear factor-kappa B (NF-kB) by ER alpha but not ER beta (Wissink et al 2001). Third, the estrogen receptor has clear physiologic relevance in PMDD as the receptor for a hormone that can trigger the onset of symptoms of the disorder (Schmidt et al 1998). Fourth, and perhaps most important, PMDD has been shown to be a disorder of hormone sensitivity; i.e., individuals with the disorder display an abnormal behavioral response to normal levels of estradiol and progesterone (Schmidt et al 1998). Examples of differential sensitivity to the actions of hormones exist in both the human and animal literatures, with both environmental (Meaney et al 2005; Meaney et al 1991; Ladd et al 1996), and genetic sources identified (Bailey et al 2002; Svare 1998; Spearow et al 1999). Of particular note are demonstrations of polymorphic variants in genes encoding reproductive steroid hormone receptors that are associated with both altered transcriptional activity and phenotypic variation. VNTRs in the androgen receptor influence the transcriptional efficiency of the activated receptor (Beilin et al 1999) and are associated in some (Giovannucci et al 1997) but not all (Zeegers et al 2004) studies with increased susceptibility to the development of prostate cancer. Similarly, the progins insertion in the progesterone receptor leads to increased transcriptional effects and diminished susceptibility to the development of breast cancer (Wang-Gohrke et al 2000).
The SNPs in ESR1 that are positively associated with PMDD are located within a 16 kb region of intron 4. While this corresponds to a non-coding region of the gene, the findings nonetheless may be of physiological significance: 1) the SNPs may be in linkage disequilibrium with a nearby “causative” polymorphism; 2) intronic SNPs may be functional, involving regulatory sequences (e.g., enhancers) that can regulate expression levels (Cai et al 2003); 3) intronic SNPs have been shown to alter mRNA folding and hence a range of mRNA processing events, including splicing (Shen et al 1999); 4) intronic SNPs in the GR are associated with altered glucocorticoid sensitivity (Stevens et al 2004; Wust et al 2004; Stevens et al 2004).
Results of the single-locus analyses were strengthened in the haplotype analyses. In the haplotypes generated from the moving window analyses, the 2-allele containing haplotype was strongly over-represented in the controls (e.g., case-control ratio of .39, empirical p = 0.0000 for L0057/L0026). All haplotypes containing intron 4 loci L0025, L0060, L0061, or L0026 significantly distinguished patients from controls, in marked contrast to intron 4 haplotypes containing none of the four significant SNPs. Given that the SNPs tested spanned from intron 1 to the 3′ UTR of ESR1, these data suggest that the 5′ region of intron 4 is particularly and perhaps selectively associated with the susceptibility to experience PMDD.
As mentioned above, psychiatric disorders are not inherited in simple Mendelian pattern and instead probably represent low penetrance effects of common variations of multiple interacting genes that confer increased susceptibility (Weinberger et al 2006). As such, we were interested in examining the significantly associated variants in ESR1 (multi-SNP association) against the background of gene polymorphisms that do not appear associated with the diagnosis of PMDD but may nevertheless be involved. The Val158Met polymorphism in the COMT gene was selected for the following reasons: 1) COMT is involved in estrogen metabolism, performing O-methylation of 2- and 4-hydroxy estrogen metabolites (catecholestrogens); 2) the COMT gene contains estrogen response elements (Xie et al 1999), consistent with its regulation (decrease) by estradiol in vitro (Jiang et al 2003), an effect that is presumed to be mediated by ER alpha (Jiang et al 2003); 3) COMT is implicated in sex steroid associated cancers (Tanaka et al 2006; Sazci et al 2004); 4) COMT is responsible for regulating dopamine levels in the PFC, which are critical for modulation of cognitive function and “tuning” of the PFC (the ratio of task-related to task-unrelated neuronal firing), and the PFC is a brain region in which estradiol has been shown to regulate cerebral blood flow and function in humans (Berman et al 1997; Keenan et al 2001); 5) the COMT Val/Met polymorphism has been linked to the predisposition to several psychiatric disorders, including schizophrenia and OCD (Tunbridge et al in press; Karayiorgou et al 1997) and has been shown to moderate the effects of environmental factors in determining disease expression (Caspi et al 2005). The manifold interactions of COMT and estradiol and their convergence in areas of the brain critical in regulating mood and hypothesized as dysfunctional in PMDD (Rubinow et al in press) provided compelling justification for investigation of possible epistasis (gene-gene interactions). We found that the intron 4 SNPs in ESR1 were significantly associated with PMDD only in those individuals with a Val/Val genotype; patient groups with Val/Met or Met/Met genotypes were not associated, either individually or when combined.
The Val/Val genotype has been tentatively associated with decreased PFC dopamine and decreased tuning efficiency and signal to noise of prefrontal related circuitry. Estradiol regulates PFC blood flow in humans (Berman et al 1997), and Korol (2002; 2004) has demonstrated that E2 biases toward or against the activation of circuits mediating different forms of cognition: high estrogen favoring place-activated learning and low estrogen levels response-dependent learning. It is tempting to speculate, therefore, that disturbed ER alpha signaling in the luteal phase may interact with decreased PFC efficiency in those with the Val/Val genotype so as to permit the expression of a dysphoric state suggestive of disinhibited subcortical (e.g., amygdala) activity. Indirect support for this speculation is derived from unpublished data (Dancer et al., unpublished) showing luteal phase-related compromise of PFC function in women with PMDD but not in control women.
A strength of our study is the prospective demonstration of the presence or absence of luteal phase-specific symptoms in patients and controls, respectively. Nonetheless, several caveats should be noted. First, ascertainment of large samples of such carefully screened and evaluated individuals is arduous, and, consequently, our sample sizes are not large. Further, the strength of the single-locus associations is such that they would not survive correction for multiple testing. Replication, therefore, is clearly required, and our results must be viewed as preliminary until such replication occurs. Second, given the relative uniqueness of our controls in having neither a past psychiatric history nor any menstrual cycle-related symptoms, our findings may reflect the existence of protective alleles in the controls rather than susceptibility alleles in the patients. Indeed, our controls may be “supernormal” and unrepresentative of the overall population without PMDD. Third, the SNPs that we selected did not comprehensively interrogate the genes studied, perhaps resulting in our failure to find associations with SNPs in ESR2. One can certainly not conclude the lack of involvement of this gene on the basis of our data, and replication studies are underway employing ht-SNPs that better assay the haplotypic complexity in each gene. Fourth, as befits a complex genetic disorder, the contribution of the ESR polymorphisms to the expression of PMDD is not large. These caveats notwithstanding, identification of a significant association between PMDD and polymorphic variants of the gene for a physiologically relevant steroid receptor is a significant step forward in the effort to discover the genetic underpinnings of PMDD.
Acknowledgments
The authors gratefully acknowledge the statistical consultation provided by Dr. Patrick Sullivan.
The research was supported by the Intramural Research Program of the NIMH
This work was supported by NIMH Project#1 ZO1 MH00276509. None of the authors has a conflict of interest related directly or indirectly to this work.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Bailey JA, Nephew KP. Strain differences in tamoxifen sensitivity of Sprague-Dawley and Fischer 344 rats. Anti-Cancer Drugs. 2002;13:939–948. doi: 10.1097/00001813-200210000-00006. [DOI] [PubMed] [Google Scholar]
- Barrett JC, Fry B, Maller J, Daly MJ. Haploview: analysis and visualization of LD and haplotype maps. Bioinformatics. 2005;21:263–265. doi: 10.1093/bioinformatics/bth457. [DOI] [PubMed] [Google Scholar]
- Battersby S, Ogilvie AD, Blackwood DHR, Shen S, Muqit MMK, Muir WJ, et al. Presence of multiple functional polyadenylation signals and a single nucleotide polymorphism in the 3′ untranslated region of the human serotonin transporter gene. J Neurochem. 1999;72:1384–1388. doi: 10.1046/j.1471-4159.1999.721384.x. [DOI] [PubMed] [Google Scholar]
- Beilin J, Zajac JD. Function of the human androgen receptor varies according to CAG repeat number within the normal range. Abstr 81st Annu Meeting Endocr Soc. 1999:500. [Google Scholar]
- Berman KF, Schmidt PJ, Rubinow DR, Danaceau MA, Van Horn JD, Esposito G, et al. Modulation of cognition-specific cortical activity by gonadal steroids: a positron-emission tomography study in women. Proc Natl Acad Sci U S A. 1997;94:8836–8841. doi: 10.1073/pnas.94.16.8836. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cai Q, Shu X-O, Jin F, Dai Q, Wen W, Cheng J-R, et al. Genetic polymorphisms in the estrogen receptor α gene and risk of breast cancer: results from the Shanghai Breast Cancer Study. Cancer Epidemiol Biomarkers Prev. 2003;12:853–859. [PubMed] [Google Scholar]
- Caspi A, Moffitt TE, Cannon M, McClay J, Murray R, Harrington H, et al. Moderation of the effect of adolescent-onset cannabis use on adult psychosis by a functional polymorphism in the catechol-o-methyltransferase gene: longitudinal evidence of a gene X environment interaction. Biol Psychiatry. 2005;57:1117–1127. doi: 10.1016/j.biopsych.2005.01.026. [DOI] [PubMed] [Google Scholar]
- Chen J, Lipska BK, Halim N, Ma QD, Matsumoto M, Melhem S, et al. Functional analysis of genetic variation in catechol-O-methyltransferase (COMT): effects on mRNA, protein, and enzyme activity in postmortem human brain. Am J Hum Genet. 2004;75:807–821. doi: 10.1086/425589. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Condon JT. The premenstrual syndrome: a twin study. Br J Psychiatry. 1993;162:481–486. doi: 10.1192/bjp.162.4.481. [DOI] [PubMed] [Google Scholar]
- Damberg M, Westberg L, Berggard C, Landen M, Sundblad C, Eriksson O, et al. Investigation of transcription factor AP-2beta genotype in women with premenstrual dysphoric disorder. Neurosci Lett. 2005;377:49–52. doi: 10.1016/j.neulet.2004.11.068. [DOI] [PubMed] [Google Scholar]
- Garey J, Goodwillie A, Frohlich J, Morgan M, Gustafsson JA, Smithies O, et al. Genetic contributions to generalized arousal of brain and behavior. Proc Natl Acad Sci U S A. 2003;100:11019–11022. doi: 10.1073/pnas.1633773100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Giovannucci E, Stampfer MJ, Krithivas K, Brown M, Brufsky A, Talcott J, et al. The CAG repeat within the androgen receptor gene and its relationship to prostate cancer. Proc Natl Acad Sci U S A. 1997;94:3320–3323. doi: 10.1073/pnas.94.7.3320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Halbreich U, Borenstein J, Pearlstein T, Kahn LS. The prevalence, impairment, impact, and burden of premenstrual dysphoric disorder (PMS/PMDD) Psychoneuroendocrinology. 2003;28:1–23. doi: 10.1016/s0306-4530(03)00098-2. [DOI] [PubMed] [Google Scholar]
- Heils A, Teufel A, Petri S, Seemann M, Bengel D, Balling U, et al. Functional promoter and polyadenylation site mapping of the human serotonin (5-HT) transporter gene. J Neural Transm [Gen Sect ] 1995;102:247–254. doi: 10.1007/BF01281159. [DOI] [PubMed] [Google Scholar]
- Hoehe MR. Haplotypes and the systematic analysis of genetic variation in genes and genomes. Pharmacogenomics. 2003;4:547–570. doi: 10.2217/14622416.4.5.547. [DOI] [PubMed] [Google Scholar]
- Jiang H, Xie T, Ramsden DB, Ho SL. Human catechol-O-methyltransferase down-regulation by estradiol. Neuropharmacology. 2003;45:1011–1018. doi: 10.1016/s0028-3908(03)00286-7. [DOI] [PubMed] [Google Scholar]
- Karayiorgou M, Altemus M, Galke BL, Goldman D, Murphy DL, Ott J, et al. Genotype determining low catechol-O-methyltransferase activity as a risk factor for obsessive-compulsive disorder. Proc Natl Acad Sci U S A. 1997;94:4572–4575. doi: 10.1073/pnas.94.9.4572. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Keenan PA, Ezzat WH, Ginsburg K, Moore GJ. Prefrontal cortex as the site of estrogen’s effect on cognition. Psychoneuroendocrinology. 2001;26:577–590. doi: 10.1016/s0306-4530(01)00013-0. [DOI] [PubMed] [Google Scholar]
- Kendler KS, Karkowski LM, Corey LA, Neale MC. Longitudinal population-based twin study of retrospectively reported premenstrual symptoms and lifetime major depression. Am J Psychiatry. 1998;155:1234–1240. doi: 10.1176/ajp.155.9.1234. [DOI] [PubMed] [Google Scholar]
- Kendler KS, Silberg JL, Neale MC, Kessler RC, Heath AC, Eaves LJ. Genetic and environmental factors in the aetiology of menstrual, premenstrual and neurotic symptoms: a population-based twin study. Psychol Med. 1992;22:85–100. doi: 10.1017/s0033291700032761. [DOI] [PubMed] [Google Scholar]
- Kim HP, Lee JY, Jeong JK, Bae SW, Lee HK, Jo I. Nongenomic stimulation of nitric oxide release by estrogen is mediated by estrogen receptor alpha localized in caveolae. Biochem Biophys Res Commun. 1999;263:257–262. doi: 10.1006/bbrc.1999.1348. [DOI] [PubMed] [Google Scholar]
- Korol DL. Role of estrogen in balancing contributions from multiple memory systems. Neurobiol Learn Mem. 2004;82:309–323. doi: 10.1016/j.nlm.2004.07.006. [DOI] [PubMed] [Google Scholar]
- Korol DL, Kolo LL. Estrogen-induced changes in place and response learning in young adult female rats. Behav Neurosci. 2002;116:411–420. doi: 10.1037//0735-7044.116.3.411. [DOI] [PubMed] [Google Scholar]
- Krezel W, Dupont S, Krust A, Chambon P, Chapman PF. Increased anxiety and synaptic plasticity in estrogen receptor beta-deficient mice. Proc Natl Acad Sci U S A. 2001;98:12278–12282. doi: 10.1073/pnas.221451898. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuiper GGJM, Enmark E, Pelto-Huikko M, Nilsson S, Gustafsson J-A. Cloning of a novel estrogen receptor expressed in rat prostate and ovary. Proc Natl Acad Sci U S A. 1996;93:5925–5930. doi: 10.1073/pnas.93.12.5925. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ladd CO, Owens MJ, Nemeroff CB. Persistent changes in corticotropin-releasing factor neuronal systems induced by maternal deprivation. Endocrinology. 1996;137:1212–1218. doi: 10.1210/endo.137.4.8625891. [DOI] [PubMed] [Google Scholar]
- McEwen BS, Alves SE. Estrogen actions in the central nervous system. Endocr Rev. 1999;20:279–307. doi: 10.1210/edrv.20.3.0365. [DOI] [PubMed] [Google Scholar]
- Meaney MJ, Mitchell JB, Aitken DH, Bhatnagar S, Bodnoff SR, Iny LJ, et al. The effects of neonatal handling on the development of the adrenocortical response to stress: implications for neuropathology and cognitive deficits in later life. Psychoneuroendocrinology. 1991;16:85–103. doi: 10.1016/0306-4530(91)90072-2. [DOI] [PubMed] [Google Scholar]
- Meaney MJ, Szyf M. Environmental programming of stress responses through DNA methylation: life at the interface between a dynamic environment and a fixed genome. Dial Clin Neurosci. 2005;7:103–123. doi: 10.31887/DCNS.2005.7.2/mmeaney. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Melke J, Westberg L, Landen M, Sundblad C, Eriksson O, Baghei F, et al. Serotonin transporter gene polymorphisms and platelet [3H]paroxetine binding in premenstrual dysphoria. Psychoneuroendocrinology. 2003;28:446–458. doi: 10.1016/s0306-4530(02)00033-1. [DOI] [PubMed] [Google Scholar]
- Ogawa S, Inoue S, Watanabe T, Hiroi H, Orimo A, Hosoi T, et al. The complete primary structure of human estrogen receptor beta (hER beta) and its heterodimerization with ER alpha in vivo and in vitro. Biochem Biophys Res Commun. 1998;243:122–126. doi: 10.1006/bbrc.1997.7893. [DOI] [PubMed] [Google Scholar]
- Ogilvie AD, Battersby S, Bubb VJ, Fink G, Harmar AJ, Goodwin GM, et al. Polymorphism in serotonin transporter gene associated with susceptibility to major depression. Lancet. 1996;347:731–733. doi: 10.1016/s0140-6736(96)90079-3. [DOI] [PubMed] [Google Scholar]
- Razandi M, Oh P, Pedram A, Schnitzer J, Levin ER. ERs associate with and regulate the production of caveolin: implications for signaling and cellular actions. Mol Endocrinol. 2002;16:100–115. doi: 10.1210/mend.16.1.0757. [DOI] [PubMed] [Google Scholar]
- Razandi M, Pedram A, Greene GL, Levin ER. Cell membrane and nuclear estrogen receptors (ERs) originate from a single transcript: studies of ER alpha and ER beta expressed in Chinese hamster ovary cells. Mol Endocrinol. 1999;13:307–319. doi: 10.1210/mend.13.2.0239. [DOI] [PubMed] [Google Scholar]
- Rocha BA, Fleischer R, Schaeffer JM, Rohrer SP, Hickey GJ. 17 β-estradiol-induced antidepressant-like effect in the forced swim test is absent in estrogen receptor-β knockout (BERKO) mice. Psychopharmacology. 2005;179:637–643. doi: 10.1007/s00213-004-2078-1. [DOI] [PubMed] [Google Scholar]
- Rubinow DR. The premenstrual syndrome: new views. J A M A. 1992;268:1908–1912. [PubMed] [Google Scholar]
- Rubinow DR, Roy-Byrne PP, Hoban MC, Gold PW, Post RM. Prospective assessment of menstrually related mood disorders. Am J Psychiatry. 1984;141:684–686. doi: 10.1176/ajp.141.5.684. [DOI] [PubMed] [Google Scholar]
- Rubinow DR, Schmidt PJ. Gonadal steroid regulation of mood: the lessons of premenstrual syndrome. Front Neuroendocrinol. 2005 doi: 10.1016/j.yfrne.2006.02.003. in press. [DOI] [PubMed] [Google Scholar]
- Rubinow DR, Schmidt PJ, Roca CA. Estrogen-serotonin interactions: implications for affective regulation. Biol Psychiatry. 1998;44:839–850. doi: 10.1016/s0006-3223(98)00162-0. [DOI] [PubMed] [Google Scholar]
- Sazci A, Ergul E, Utkan NZ, Canturk NZ, Kaya G. Catechol-O-methyltransferase Val 108/158 Met polymorphism in premenopausal breast cancer patients. Toxicology. 2004;204:197–202. doi: 10.1016/j.tox.2004.06.026. [DOI] [PubMed] [Google Scholar]
- Schmidt PJ, Nieman LK, Danaceau MA, Adams LF, Rubinow DR. Differential behavioral effects of gonadal steroids in women with and in those without premenstrual syndrome. N Engl J Med. 1998;338:209–216. doi: 10.1056/NEJM199801223380401. [DOI] [PubMed] [Google Scholar]
- Shen LX, Basilion JP, Stanton VP., Jr Single-nucleotide polymorphisms can cause different structural folds of mRNA. Proc Natl Acad Sci U S A. 1999;99:7871–7876. doi: 10.1073/pnas.96.14.7871. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith MJ, Schmidt PJ, Rubinow DR. Operationalizing DSM-IV criteria for PMDD: selecting symptomatic and asymptomatic cycles for research. J Psychiatr Res. 2003;37:75–83. doi: 10.1016/s0022-3956(02)00053-5. [DOI] [PubMed] [Google Scholar]
- Spearow JL, Doemeny P, Sera R, Leffler R, Barkley M. Genetic variation in susceptibility to endocrine disruption by estrogen in mice. Science. 1999;285:1259–1261. doi: 10.1126/science.285.5431.1259. [DOI] [PubMed] [Google Scholar]
- Spitzer RL, Endicott J. Schedule for affective disorders and schizophrenia - lifetime version. New York, NY: Biometrics Research Department, New York State Psychiatric Institute; 1979. [Google Scholar]
- Spitzer RL, Williams JB, Gibbon M, First MB. Structured clinical interview for DSM-III-R, patient edition. New York, NY: Biometrics Research Department, New York State Psychiatric Institute; 1990. [Google Scholar]
- Stevens A, Ray DW, Zeggini E, John S, Richards HL, Griffiths CE, et al. Glucocorticoid sensitivity is determined by a specific glucocorticoid receptor haplotype. J Clin Endocrinol Metab. 2004;89:892–897. doi: 10.1210/jc.2003-031235. [DOI] [PubMed] [Google Scholar]
- Svare B. Genotype modulates the aggression-promoting quality of progesterone in pregnant mice. Horm Behav. 1998;22:90–99. doi: 10.1016/0018-506x(88)90033-5. [DOI] [PubMed] [Google Scholar]
- Tanaka Y, Sasaki M, Shiina H, Tokizane T, Deguchi M, Hirata H, et al. Catechol-O-methyltransferase gene polymorphisms in benign prostatic hyperplasia and sporadic prostate cancer. Cancer Epidemiol Biomarkers Prev. 2006;15:238–244. doi: 10.1158/1055-9965.EPI-05-0550. [DOI] [PubMed] [Google Scholar]
- Tunbridge E, Harrison P, Weinberger D. Catechol-o-methyltransferase, cognition and psychosis: Val158 Met and beyond. Biol Psychiatry. 2005 doi: 10.1016/j.biopsych.2005.10.024. in press. [DOI] [PubMed] [Google Scholar]
- Walf AA, Rhodes ME, Frye CA. Antidepressant effects of ERβ-selective estrogen receptor modulators in the forced swim test. Pharmacol Biochem Behav. 2004;78:523–529. doi: 10.1016/j.pbb.2004.03.023. [DOI] [PubMed] [Google Scholar]
- Walter P, Green S, Greene G, Krust A, Bornert J-M, Jeltsch J-M, et al. Cloning of the human estrogen receptor cDNA. Proc Natl Acad Sci U S A. 1985;82:7889–7893. doi: 10.1073/pnas.82.23.7889. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang-Gohrke S, Chang-Claude J, Becher H, Kieback DG, Runnebaum IB. Progesterone receptor gene polymorphism is associated with decreased risk for breast cancer by age 50. Cancer Res. 2000;60:2348–2350. [PubMed] [Google Scholar]
- Weinberger DR, Goldman D. Psychiatric genetics in an era of relative enlightenment. In: Watson JD, Witkowski JA, Inglis JR, editors. Rereading heredity in relation to eugenics: contemporary reflections the promise of human genetics. Cold Spring Harbor, NY: Cold Spring Harbor Laboratory Press; 2006. [Google Scholar]
- Wilson CA, Turner CW, Keye WR., Jr Firstborn adolescent daughters and mothers with and without premenstrual syndrome: a comparison. J Adolesc Health. 1991;12:130–137. doi: 10.1016/0197-0070(91)90455-u. [DOI] [PubMed] [Google Scholar]
- Wissink S, van der Burg B, Katzenellenbogen BS, van der Saag PT. Synergistic activation of the serotonin-1A receptor by nuclear factor-kappaB and esgtrogen. Mol Endocrinol. 2001;15:543–552. doi: 10.1210/mend.15.4.0629. [DOI] [PubMed] [Google Scholar]
- Wust S, van Rossum EF, Federenko IS, Koper JW, Kumsta R, Hellhammer DH. Common polymorphisms in the glucocorticoid receptor gene are associated with adrenocortical responses to psychosocial stress. J Clin Endocrinol Metab. 2004;89:565–573. doi: 10.1210/jc.2003-031148. [DOI] [PubMed] [Google Scholar]
- Xie T, Ho SL, Ramsden D. Characterization and implications of estrogenic down-regulation of human catechol-O-methyltransferase gene transcription. Mol Pharmacol. 1999;56:31–38. doi: 10.1124/mol.56.1.31. [DOI] [PubMed] [Google Scholar]
- Zeegers MP, Kiemeney LA, Nieder AM, Ostrer H. How strong is the association between CAG and GGN repeat length polymorphisms in the androgen receptor gene and prostate cancer risk? Cancer Epidemiol Biomarkers Prev. 2004;13:1765–1771. [PubMed] [Google Scholar]
- Zhang JX, Labaree DC, Mor G, Hochberg RB. Estrogen to antiestrogen with a single methylene group resulting in an unusual steroidal selective estrogen receptor modulator. J Clin Endocrinol Metab. 2004;89:3527–3535. doi: 10.1210/jc.2003-032005. [DOI] [PubMed] [Google Scholar]
- Zhao JH, Lissarrague S, Essioux L, Sham PC. GENECOUNTING: haplotype analysis with missing genotypes. Bioinformatics. 2002;18:1694–1695. doi: 10.1093/bioinformatics/18.12.1694. [DOI] [PubMed] [Google Scholar]