Abstract
BACKGROUND
Conventional brain MRI lesion measures have unreliable associations with clinical progression in multiple sclerosis (MS). Gray matter imaging may improve clinical-MRI correlations.
METHODS
We tested if gray matter MRI measures and conventional measures of lesions/atrophy predicted clinical progression in a 4-year longitudinal study of 97 patients with MS. Baseline and follow-up brain MRI were analyzed for basal ganglia and thalamic normalized T2 signal intensity, whole brain T2-hyperintense lesion volume, and whole brain atrophy. Logistic regression tested the ability of baseline or on-study change in MRI to predict disability progression, as reported by area under the receiver operator characteristics curve (AUC).
RESULTS
Lower caudate T2-intensity at baseline (P = .04; AUC = .69) and on-study decreasing T2-intensity in the putamen (P = .03; AUC = .70) and thalamus (P = .01; AUC = .71) were the MRI variables associated with clinical progression when regression modeling was adjusted for length of follow-up interval, baseline EDSS, disease duration, age, and sex.
CONCLUSIONS
Gray matter T2-hypointensity, suggestive of excessive iron deposition is associated with worsening disability in patients with MS. Gray matter MRI assessment may be able to capture neurodegenerative aspects of the disease, with more clinical relevance than derived from conventional MRI measures.
Keywords: MRI, multiple sclerosis, neurologic disability, iron, T2 hypointensity, T2 hyperintense lesions, brain atrophy
Introduction
Magnetic resonance imaging (MRI) has become important in the diagnosis and monitoring of multiple sclerosis (MS) and has emerged as a key supportive therapeutic outcome measure in clinical trials. In addition, MRI metrics have a role in predicting the development of clinical disability.1–3 While conventional MRI surrogates show relatively weak-predictive strength for clinical change,4,5 gray matter changes assessed by MRI hold promise as clinically relevant new biomarkers.6
Gray matter involvement in MS manifest on MRI scans in several ways including lesions, atrophy, abnormal neuronal metabolites, reduced magnetization transfer ratio (MTR), increased diffusivity, and T2-hypointensity.6 T2-hypointensity can be seen in various gray matter areas including the red nucleus, thalamus, dentate nucleus, basal ganglia, and rolandic cortex7–10 and most likely represents pathological iron deposition as has also been suggested recently by magnetic field correlation imaging at 3T11 and phase imaging at 7T.12 Numerous studies have shown that T2-hypointensity is associated with physical disability,8,10 ambulatory impairment,8 cognitive impairment,9 brain atrophy,7 and the risk of developing brain atrophy.13 However, the relationship between T2-intensity-derived measurement of gray matter damage and longitudinal clinical changes is unknown.
In this study, we tested the hypothesis that T2-hypointensity of the deep gray matter in areas including the thalamus and basal ganglia is associated with subsequent clinical progression as measured by the expanded disability status scale (EDSS).14 We compared the strength of association between T2-intensity-derived gray matter damage and conventional global cerebral lesion/atrophy measures with regard to clinical impairment evolving over 4 years.
Methods
Subjects
We retrospectively identified 97 patients with MS from a consecutive sample being prospectively enrolled and monitored as part of the comprehensive longitudinal investigation of MS at Brigham (CLIMB) study at the Brigham and Women’s Hospital (Partners MS Center). CLIMB is an ongoing prospective observational cohort study that began following patients in 2000.15 Inclusion into this study was based on the following criteria: (1) age 18–60 at baseline; (2) brain MRI at baseline and follow-up performed at the Brigham and Women’s Hospital on the 1.5T unit dedicated for MS care using the scanning protocol established for the CLIMB study; (3) baseline and follow-up neurologic examination with EDSS14 scoring performed by an MS specialist neurologist at the Partners MS Center; (4) the baseline and follow-up EDSS testing should have been performed in close approximation with baseline and follow-up MRI respectively; (5) the follow-up MRI and neurologic examination with EDSS scoring should have been performed 3 to 7 years after the baseline MRI scan; (6) established MS diagnosis at baseline of either relapsing-remitting (RR), secondary progressive (SP), or primary progressive (PP) by the International Panel criteria16 or patients with clinically isolated syndromes (CIS) converting to definitive MS. We identified 97 patients that were followed for (mean ± SD) 4.4 ± 1.1 years with the following baseline characteristics (Table 1): age 42.1 ± 9.1 years, disease duration 11.9 ± 8.5 years, EDSS score 3.1 ± 2.0, 66% (n = 64) relapsing-remitting, 6% (n = 6) primary progressive, 26% (n = 25) secondary progressive, and remaining 2% (n = 2) were CIS. All patients except 19 (20%) were treated with disease modifying therapy during the observation period. This study was approved by our institutional review board.
Table 1.
Demographic and Clinical Data at Baseline and Follow-Up
| Group | All | Stable (S) | Progressive (P) | S vs. P group, P value |
|---|---|---|---|---|
| Number of patients | 97 | 66 | 31 | |
| Sex ratio (M/F) | .35 | .27 | .55 | .14† |
| Number of males/females | 25/72 | 14/52 | 11/20 | |
| Age (years) (mean ± SD) | 42.2 ± 9.1 | 42.1 ± 9.1 | 42.3 ± 9.4 | |
| Median | 43.1 | 42.6 | 42.6 | .93†† |
| Range | 19–59 | 19–58 | 24–59 | |
| MS subtype (CIS/RRMS/SPMS/PPMS) | 2/64/25/6 | 2/43/16/5 | 0/21/9/1 | .62††† |
| Disease duration (years) (mean ± SD) | 11.9 ± 8.4 | 11.3 ± 7.9 | 13.0 ± 9.6 | |
| Median | 9.6 | 9.4 | 9.6 | .38†† |
| Range | 0.3–45.8 | 0.9–35 | 0.3–45.8 | |
| Length of period between 2 scans (years) (mean ± SD) | 4.5 ± 1.1 | 4.4 ± 1.2 | 4.6 ± 0.9 | |
| Median | 4.2 | 4.0 | 4.7 | .07†† |
| Range | 3.0–7.3 | 3.0–7.3 | 3.0–6.8 | |
| EDSS at baseline (mean ± SD) | 3.1 ± 2.0 | 2.9 ± 2.0 | 3.5 ± 2.0 | |
| Median | 2.5 | 2.3 | 2.5 | .97†† |
| Range | 0–6.5 | 0–6.5 | 0.0–6.0 | |
| EDSS at follow up (mean ± SD) | 3.5 ± 2.3 | 2.7 ± 1.9 | 5.2 ± 2.0 | |
| Median | 3.0 | 2.5 | 6.0 | < .0001†† |
| Range | 0–8.5 | 0–7.0 | 1.0–8.5 | |
| Patients on disease modifying therapy during observation period | 78 (80%) | 49 (74%) | 29 (94%) | .03† |
Key: M = male; F = female; MS = patients with multiple sclerosis; CIS = clinically isolated syndrome; RR = relapsing-remitting; SP = secondary progressive; PP = primary progressive. EDSS = Expanded Disability Status Scale; Values in table are mean ± SD, median or range;
Fisher’s exact test;
Wilcoxon test;
χ2 test.
Progression of Disability
Progression of neurologic disability at follow-up was defined by 1 point progression on EDSS if the baseline score was less than 6 or .5 point progression if the baseline score was 6 or higher. EDSS worsening at follow-up had to be sustained to be considered as progression; that is, persisting for at least 3 months. Patients were thus classified as stable or progressed on EDSS at the final follow-up (Table 1). At follow-up, 31 (32%) patients showed sustained progression in EDSS disability and thus were classified as progressive patients while 66 (68%) patients were classified as clinically stable.
MR Imaging
All patients underwent baseline and follow-up brain MRI on the same scanner using the same scanning protocol. MRI was obtained on a Signa 1.5-T unit (GE Signa, General Electric, Mil-waukee, WI) using a quadrature head coil. Axial brain imaging included dual-echo T2-weighted (TR = 3,000 ms; TE = 80/30 ms) images with 256 × 256 × 54 voxels and a nominal voxel size of .9375 × .9375 × 3 mm, without inter-slice gaps.
MRI Analysis
T2-Hyperintense lesion volume and whole brain atrophy
Using automated template-driven segmentation (TDS+) from the dual-echo images, T2-hyperintense lesion volume (T2LV) and normalized whole brain volume [brain parenchymal fraction (BPF)] were determined,17 the latter of which was an estimate of whole brain atrophy.18
Gray matter T2-intensity
Baseline and follow-up brain MRI-derived basal ganglia (caudate, putamen, and globus pallidus) and thalamic normalized-T2 signal intensity was measured as described previously.8,9,19
Reliability of MRI measurements
As previously described,17 the TDS+ segmentation achieved an intraclass correlation of .994, with an interscan coefficient of variation (COV) of 4.98% and a volume bias of .01 ± .68 mL. Our technique for the measurement of T2-intensity has previously shown intraobserver and interobserver COV of 1.7% and 4.5%. Test-retest (scan-rescan) COV ranged from 1.3% to 1.7% for T2-intensity.19
Statistical Analysis
Group differences in clinically progressive versus stable patients in their demographic and clinical measures were evaluated using the Fisher’s exact test, Wilcoxon test, or χ 2-square test. To test the ability of baseline and on study change in various MRI measures to predict progression of disability, logistic regression modeling was used. On-study change in an MRI measure was defined to be (follow-up MRI data-baseline MRI data)/baseline MRI data. Because of the variability in the follow-up interval, baseline EDSS score, disease duration, and age among subjects, all of these variables in addition to sex were controlled for in the primary regression model. In addition, we explored the effect of various other combinations of covariates in regression modeling. Predictive performance of the logistic regression models was reported by area under the receiver operator characteristics curve (AUC). A P-value less than .05 was considered statistically significant. Because this study was exploratory, no correction for multiple comparisons was completed.
Results
Table 1 shows demographic and clinical data in the overall cohort of 97 patients and compares stable and progressive groups that were retrospectively identified for this longitudinal study. We found no significant differences in terms of age, sex, MS subtype, and EDSS at baseline between progressive and stable patients; although, a trend towards longer length of follow-up (P = .07) was seen in the progressive patients. During the observation period a significant difference (P = .03) in terms of number of patients on disease-modifying treatment between stable [n = 49 (74%)] and progressive [n = 29 (94%)] groups was also seen. Although treatment was associated with clinical progression, we caution against over interpretation of this finding. This study was not designed to evaluate treatment effect and the choice of therapy was nonrandomized, based solely on patient/physician choice, potentially introducing a selection bias in comparing treated versus untreated patients.
To test the strength of either baseline or on-study change in MRI measures for their ability to predict progression of physical disability, T2LV, BPF and gray matter T2-intensity at baseline, and their longitudinal changes were related to final clinical status (stable vs. progressive). In regression modeling controlling for all of the major covariates, we found a significant relationship between MRI data and longitudinal clinical progression status (Table 2; Fig 1, 2) with regard to caudate T2-intensity at baseline (P = .04; AUC = .69), putamen T2-intensity% change (P = .01; AUC = .70) and thalamus T2-intensity% change (P = .01; AUC = .71). Regarding the direction of these associations, for caudate T2-intensity, a lower T2-intensity at baseline was associated with a higher risk of progression of disability (Fig 1); for putamen and thalamus T2-intensity% change, decreases over time were associated with an increased risk for progression of disability (Fig 2). Post-hoc exploratory regression modeling was performed using a fewer number of covariates (baseline EDSS score, disease duration, MRI interval, sex, and/or age) in various combinations. Results were similar to the primary regression modeling in that gray matter T2-hypointensity variables were always significantly associated with clinical progression and T2LV was not. However, in a few of the exploratory models, a lower baseline BPF was also associated with a higher risk of clinical progression. For example, in a model adjusting for MRI interval only (ie, time interval between the baseline and follow-up MRI scans) BPF at baseline (P = 0.046; AUC = .64) and putamen T2-intensity% change (P = .04; AUC = .64) were associated with clinical progression. When controlling for baseline EDSS score, disease duration, and MRI interval (but not age and sex), putamen T2-intensity% change (P = .02; AUC = .68) and thalamus T2-intensity% change (P = .01; AUC = .69) were associated with clinical progression, but T2LV and BPF were not.
Table 2.
Predictive Value of MRI Variables for the Change in Clinical Status (Stable vs. Progressive) when Controlling for Baseline EDSS, Disease Duration, Age, Sex, and Time Interval between the MRI Scans
| Variable | Logistic regression P-value | AUC |
|---|---|---|
| BPF at baseline | .06 | .67 |
| BPF% change | .19 | .63 |
| T2LV at baseline | .63 | .65 |
| T2LV % change | .50 | .66 |
| Caudate T2-intensity at baseline | .04* | .69 |
| Caudate T2-intensity% change | .07 | .68 |
| Putamen T2-intensity at baseline | .14 | .64 |
| Putamen T2-intensity% change | .01* | .70 |
| Globus pallidus T2-intensity at baseline | .90 | .63 |
| Globus pallidus T2-intensity% change | .12 | .67 |
| Thalamus T2-intensity at baseline | .12 | .69 |
| Thalamus T2-intensity% change | .01* | .71 |
BPF = brain parenchymal fraction; T2LV = T2-lesion volume; AUC = area under the receiver operating characteristic curve; % change = (follow-up MRI data-baseline MRI data/baseline MRI data);
= P < .05. Lower baseline caudate T2-intensity and on-study decreasing T2-intensity in the putamen and thalamus were the only MRI variables significantly associated with clinical progression.
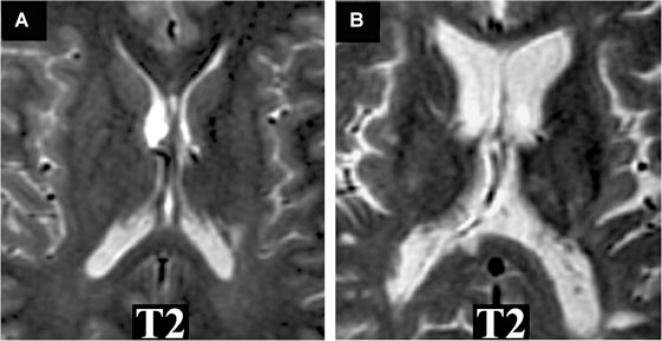
Fig 1.
MRI T2-hypointensity in gray matter and progression of disability in MS. T2-weighted axial brain images from 2 patients at baseline. Note: T2-hypointensity in the basal ganglia is present in the clinically progressive (B) versus stable patient (A). (A) A 44-year-old man with relapsing-remitting MS. The baseline MRI scan is shown (normalized T2-intensity in caudate = .535). The patient’s disability did not worsen clinically over the 4 years of follow-up: baseline expanded disability status scale (EDSS) score 2.5; follow-up EDSS score 0. (B) A 40-year-old man with relapsing-remitting MS. The baseline MRI scan is shown (normalized T2-intensity in caudate = .453). The patient experienced sustained clinical progression over the 4 years of follow-up: baseline EDSS score 6; follow-up EDSS score 7.
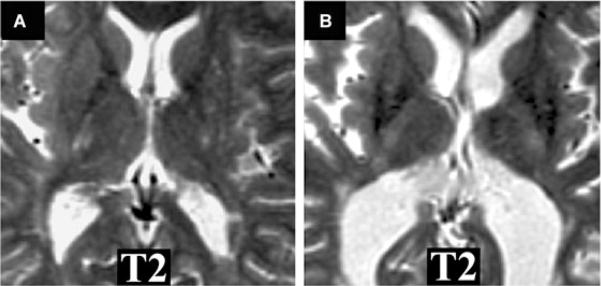
Fig 2.
MRI T2-hypointensity in gray matter and progression of disability in MS. T2-weighted axial brain images from 2 patients at follow-up. Note: T2-hypointensity in the basal ganglia is present in the clinically progressive (B) versus stable patient (A). (A) A 46-year-old woman with relapsing-remitting MS. The follow-up MRI scan is shown (normalized-T2 intensity in putamen = .513; thalamus = .482). The patient’s disability did not worsen clinically over the 3.2 years of follow-up: baseline expanded disability status scale (EDSS) score 2; follow-up EDSS score 1.5. (B) A 49-year-old woman with relapsing-remitting MS. The follow-up MRI scan is shown (normalized T2-intensity in putamen = .424; thalamus = .436). The patient experienced sustained clinical progression over the 3.5 years of follow-up: baseline EDSS score 1; follow-up EDSS score 3.5.
Discussion
In this study, we investigated the ability of MRI measures of deep gray matter damage, conventional lesions, and whole brain atrophy to predict 4-year longitudinal clinical change in a longitudinal study in a diverse group of patients with MS. The key findings in this study are that only the MRI variables of deep gray matter damage, as shown by T2-hypointensity were significantly associated with the risk of developing sustained progression of disability. While whole brain atrophy at baseline was significantly related to clinical progression in some models, it had a weaker and less reliable relationship overall than markers of deep gray matter damage. T2-hyperintense lesions at baseline or their on-study change did not show any association with clinical change. These data suggest that the destructive and neurodegenerative aspects of the disease, including the involvement of deep gray matter, are more closely related to disability than overt multifocal inflammatory/demyelination changes in white matter.
The most novel observation in this study is that both baseline T2-hypointensity and worsening T2-hypointensity in the deep gray matter are associated with clinical progression. Caudate T2-hypointensity at baseline and progressive T2-hypointensity in the putamen and thalamus were related to clinical deterioration during the 4-year observation period in the regression modeling that accounted for all covariates. Various studies have demonstrated a link between T2-hypointensity in the gray matter and clinical impairment in MS.20 The most likely cause of T2-hypointensity is excessive iron deposition, as suggested by MRI-histologic correlation,21 parallels with other neurodegen-erative diseases,20 and emerging studies with advanced 3T11 or 7 T MRI methods.12
A growing body of evidence suggests aberrant iron metabolism and excessive deposition of iron in the brain is associated with the disease.20,22–27 There are many potential causes of iron deposition in MS including blood-brain barrier dysfunction, decreased iron clearance due to axonal dysfunction, or dysregulation of iron transport proteins due to inflammation.20 Studies from experimental autoimmune encephalomyelitis, an animal model for MS, show abnormal brain iron metabolism, excessive iron deposition, and the beneficial effect of iron deficiency or iron chelation therapy.24–27 One hypothesis maintains that excessive iron deposition in the CNS is neurotoxic due to the production of free radicals, leading to lipid peroxidation.28 However, it is still unknown whether iron deposition is purely an epiphenomenon of tissue degeneration or if it contributes directly to neurotoxicity in the gray matter of patients with MS. Thus, it is not clear if the results of this study indicate that progressive T2-hypointensity reflects processes that are a marker or mediator of neurodegeneration and subsequent loss of brain function.
In this study, the global extent of conventional T2-hyper-intense lesions was not related to clinical progression. This is in agreement with prior studies demonstrating that these measures show relatively weak correlations with clinical status and have unreliable strength for predicting clinical progression.4 This is most likely because T2-hyperintense lesions lack the specificity required to define the underlying MS pathology and fail to capture clinically-relevant diffuse occult disease.29
Brain atrophy is an increasingly recognized manifestation of MS and has been shown to correlate better with physical disability when compared to conventional MRI lesion measures in cross-sectional and longitudinal studies.30 In agreement with previous studies, we demonstrated that patients with greater brain atrophy at baseline were at risk for developing clinical progression3,30–32 However, in our study, progression of whole brain atrophy during the 4-year observation period was not related to clinical progression at follow-up when adjusting for all covariates. These observations differ from reports showing a relationship between on-study progressive atrophy and evolving clinical impairment.3,32 These divergent results might reflect methodologic differences among studies, including MS clinical characteristics, covariates employed, and atrophy analysis techniques.
It should be noted that a significant correlation with worsening of disability was seen with baseline caudate T2-hypoin-tensity and progressive putamen and thalamus T2-hypoin-tensity only. The implication of the observed regional differences is unclear; however, likely they suggest a lack of statistical power due to small sample size. Several other limitations were evident in our study. Like most previous studies we have used normalized intensity from spin-echo T2-weighted images to examine iron deposition in the gray matter of patients with MS. However, T2 or T2* relaxometric techniques may be more sensitive and reliable for estimating iron levels.20,29 For T2 intensity measurement we have only performed ROI analysis on small regions, which might contribute to a high variability of intensity measurements. Analysis of global gray matter may reduce the error and increase the reproducibility of this method. Finally, we have performed our analysis on 1.5 T images, while high field systems (3T and greater) are especially sensitive to iron and likely would better detect iron related susceptibility changes missed by spin-echo T2 imaging.10–12,20 We are currently collecting data to examine such higher field relaxometry based approaches to the question at hand, which we will present in future publications.
In conclusion, the assessment of gray matter may be able to capture destructive and neurodegenerative aspects of the disease, with more clinical relevance than is derived from conventional MRI lesion and whole brain atrophy measurement in patients with MS. Further studies are warranted to confirm and extend our hypothesis regarding the role of gray matter iron accumulation and neurodegeneration playing a major role in predicting disease progression.
Acknowledgments
This work was supported by research grants to Dr. Bakshi from the National Institutes of Health (NIH-NINDS K23 NS42379-01 and R01 NS055083-01) and National Multiple Sclerosis Society (RG3705A1; RG3798A2). We thank Ms. Sophie Tamm for assistance with manuscript preparation.
References
- 1.Bakshi R, Thompson AJ, Rocca MA, et al. MRI in multiple sclerosis: current status and future prospects. Lancet Neurol. 2008;7:615–625. doi: 10.1016/S1474-4422(08)70137-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Brex PA, Ciccarelli O, O’Riordan JI, et al. A longitudinal study of abnormalities on MRI and disability from multiple sclerosis. N Engl J Med. 2002;346:158–164. doi: 10.1056/NEJMoa011341. [DOI] [PubMed] [Google Scholar]
- 3.Fisher E, Rudick RA, Simon JH, et al. Eight-year follow-up study of brain atrophy in patients with MS. Neurology. 2002;59:1412–1420. doi: 10.1212/01.wnl.0000036271.49066.06. [DOI] [PubMed] [Google Scholar]
- 4.Zivadinov R, Leist TP. Clinical–MRI correlations in multiple sclerosis. J Neuroimaging. 2005;15:10S–21S. doi: 10.1177/1051228405283291. [DOI] [PubMed] [Google Scholar]
- 5.Neema M, Stankiewicz J, Arora A, et al. MRI in multiple sclerosis: what’s inside the toolbox? Neurotherapeutics. 2007;4:602–617. doi: 10.1016/j.nurt.2007.08.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Pirko I, Lucchinetti CF, Sriram S, et al. Gray matter involvement in multiple sclerosis. Neurology. 2007;68:634–642. doi: 10.1212/01.wnl.0000250267.85698.7a. [DOI] [PubMed] [Google Scholar]
- 7.Bakshi R, Dmochowski J, Shaikh ZA, et al. Gray matter T2 hypointensity is related to plaques and atrophy in the brains of multiple sclerosis patients. J Neurol Sci. 2001;185:19–26. doi: 10.1016/s0022-510x(01)00477-4. [DOI] [PubMed] [Google Scholar]
- 8.Tjoa CW, Benedict RH, Weinstock-Guttman B, et al. MRI T2 hypointensity of the dentate nucleus is related to ambulatory impairment in multiple sclerosis. J Neurol Sci. 2005;234:17–24. doi: 10.1016/j.jns.2005.02.009. [DOI] [PubMed] [Google Scholar]
- 9.Brass SD, Benedict RH, Weinstock-Guttman B, et al. Cognitive impairment is associated with subcortical magnetic resonance imaging grey matter T2 hypointensity in multiple sclerosis. Mult Scler. 2006;12:437–444. doi: 10.1191/135248506ms1301oa. [DOI] [PubMed] [Google Scholar]
- 10.Zhang Y, Zabad RK, Wei X, et al. Deep grey matter “blackT2” on 3 tesla magnetic resonance imaging correlates with disability in multiple sclerosis. Mult Scler. 2007;13:880–883. doi: 10.1177/1352458507076411. [DOI] [PubMed] [Google Scholar]
- 11.Ge Y, Jensen JH, Lu H, et al. Quantitative assessment of iron accumulation in the deep gray matter of multiple sclerosis by magnetic field correlation imaging. AJNR Am J Neuroradiol. 2007;28:1639–1644. doi: 10.3174/ajnr.A0646. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Hammond KE, Metcalf M, Okuda DT, et al. In vivo high resolution MR imaging at 7T of multiple sclerosis with sensitivity to iron. Neurology. 2008;70:A8. doi: 10.1002/ana.21582. [DOI] [PubMed] [Google Scholar]
- 13.Bermel RA, Puli SR, Rudick RA, et al. Prediction of longitudinal brain atrophy in multiple sclerosis by gray matter magnetic resonance imaging T2 hypointensity. Arch Neurol. 2005;62:1371–1376. doi: 10.1001/archneur.62.9.1371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Kurtzke JF. Rating neurologic impairment in multiple sclerosis: an expanded disability status scale (EDSS) Neurology. 1983;33:1444–1452. doi: 10.1212/wnl.33.11.1444. [DOI] [PubMed] [Google Scholar]
- 15.Gauthier SA, Glanz BI, Mandel M, et al. A model for the comprehensive investigation of a chronic autoimmune disease: the multiple sclerosis CLIMB study. Autoimmun Rev. 2006;5:532–536. doi: 10.1016/j.autrev.2006.02.012. [DOI] [PubMed] [Google Scholar]
- 16.Polman CH, Reingold SC, Edan G, et al. Diagnostic criteria for multiple sclerosis: 2005 revisions to the “McDonald Criteria”. Ann Neurol. 2005;58:840–846. doi: 10.1002/ana.20703. [DOI] [PubMed] [Google Scholar]
- 17.Wei X, Warfield S, Zou KH, et al. Quantitative analysis of MRI signal abnormalities of brain white matter with high reproducibility and accuracy. J Magn Reson Imaging. 2002;15:203–209. doi: 10.1002/jmri.10053. [DOI] [PubMed] [Google Scholar]
- 18.Pelletier D, Garrison K, Henry R. Measurement of whole-brain atrophy in multiple sclerosis. J Neuroimaging. 2004;14:11S–19S. doi: 10.1177/1051228404266264. [DOI] [PubMed] [Google Scholar]
- 19.Bakshi R, Benedict RH, Bermel RA, et al. T2 hypointensity in the deep gray matter of patients with multiple sclerosis: a quantitative magnetic resonance imaging study. Arch Neurol. 2002;59:62–68. doi: 10.1001/archneur.59.1.62. [DOI] [PubMed] [Google Scholar]
- 20.Stankiewicz J, Panter SS, Neema M, et al. Iron in chronic brain disorders: imaging and neurotherapeutic implications. Neurotherapeutics. 2007;4:371–386. doi: 10.1016/j.nurt.2007.05.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Smith MA, Castellani R, Napoli S, et al. Gray matter iron deposition in patients with multiple sclerosis: a histochemical study. Neurology. 2007;68:A118. [Google Scholar]
- 22.Craelius W, Migdal WM, Lussenhop CP, et al. Iron deposits surrounding multiple sclerosis plaques. Arch Pathol Lab Med. 1982;106:397–399. [PubMed] [Google Scholar]
- 23.Pedchenko TV, LeVine S. Desferrioxamine suppresses experimental allergic encephalomyelitis induced by MBP in SJL mice. J Neuroimmunol. 1998;84:188–197. doi: 10.1016/s0165-5728(97)00256-7. [DOI] [PubMed] [Google Scholar]
- 24.Forge JK, Pedchenko TV, LeVine SM. Iron deposits in the central nervous system of SJL mice with experimental allergic encephalomyelitis. Life Sci. 1998;63:2271–2284. doi: 10.1016/s0024-3205(98)00512-8. [DOI] [PubMed] [Google Scholar]
- 25.Levine SM, Chakrabarty A. The role of iron in the pathogenesis of experimental allergic encephalomyelitis and multiple sclerosis. Ann NY Acad Sci. 2004;1012:252–266. doi: 10.1196/annals.1306.021. [DOI] [PubMed] [Google Scholar]
- 26.Grant SM, Wiesinger JA, Beard JL, et al. Iron-deficient mice fail to develop autoimmune encephalomyelitis. J Nutr. 2003;3:2635–2638. doi: 10.1093/jn/133.8.2635. [DOI] [PubMed] [Google Scholar]
- 27.Mitchell KM, Dotson AL, Cool KM, et al. Deferiprone, an orally deliverable iron chelator, ameliorates experimental autoimmune encephalomyelitis. Mult Scler. 2007;13:1118–1126. doi: 10.1177/1352458507078916. [DOI] [PubMed] [Google Scholar]
- 28.Schipper HM. Heme oxygemase-1: Transducer of pathological brain iron sequestration under oxidative stress. Ann NY Acad Sci. 2004;1012:84–93. doi: 10.1196/annals.1306.007. [DOI] [PubMed] [Google Scholar]
- 29.Neema M, Stankiewicz J, Arora A, et al. T1- and T2-based MRI measures of diffuse gray matter and white matter damage in patients with multiple sclerosis. J Neuroimaging. 2007;17:16S–21S. doi: 10.1111/j.1552-6569.2007.00131.x. [DOI] [PubMed] [Google Scholar]
- 30.Bermel RA, Bakshi R. The measurement and clinical relevance of brain atrophy in multiple sclerosis. Lancet Neurol. 2006;5:158–170. doi: 10.1016/S1474-4422(06)70349-0. [DOI] [PubMed] [Google Scholar]
- 31.Gauthier SA, Mandel M, Guttmann CR, et al. Predicting short-term disability in multiple sclerosis. Neurology. 2007;68:2059–2065. doi: 10.1212/01.wnl.0000264890.97479.b1. [DOI] [PubMed] [Google Scholar]
- 32.Losseff NA, Wang L, Lai HM, et al. Progressive cerebral atrophy in multiple sclerosis. A serial MRI study Brain. 1996;119:2009–2019. doi: 10.1093/brain/119.6.2009. [DOI] [PubMed] [Google Scholar]