Abstract
This article is a brief review of the basic science research conducted in the field of electrical stimulation for fracture healing. Direct electrical current, capacitive coupling, and inductive coupling have been studied as potential techniques to enhance fracture healing through the proliferation and differentiation of osteogenic cells. These techniques are particularly appealing as they offer a potential minimally invasive solution to the difficult clinical problem of delayed fracture healing and nonunion. Basic science studies have shown conclusively that electrical stimulation techniques lead to bone cell proliferation and have attempted to elucidate the intracellular processes by which this bone cell proliferation occurs. Further basic science and clinical research is required to enhance the effectiveness of this therapy for the treatment of fracture nonunions.
Keywords: Electrical stimulation, fracture healing, direct electrical current, capacitive coupling, inductive coupling
INTRODUCTION
The relationship between physical forces and bone biology has been recognized since the early 1800s.1,2 Mechanical forces (compression, distraction, and shear), electrical forces, magnetic forces, and ultrasonic waves have all been found to exert some level of effect on bone growth and healing.3–6 Electrical stimulation of bone has been touted as an effective and noninvasive method for enhancing bone healing, and treating fracture nonunion. Unfortunately, clinical evidence for the efficacy of electrical stimulation is limited. A recent meta-analysis by Mollon et al. could only identify four randomized controlled trials evaluating the clinical use of electrical stimulation to treat delayed union and nonunion of fractures.7 Despite the lack of clinical evidence, many in vitro and in vivo studies demonstrate the usefulness of electrical stimulation in bone healing at a cellular level. Furthermore, these studies provide us with an understanding of the underlying cellular mechanism by which electrical stimulation influences fracture healing.
BASIC SCIENCE OF ELECTRICAL STIMULATION OF BONE
The mechanical stress applied on bone results in the generation of electrical potentials.8,9 Electronegative potentials are generated with compression and electropositive potentials are generated with tension. Piezoelectric properties of the collagen matrix and electrokinetic effects (or streaming potentials) cause these electric potentials in response to the mechanical environment.10 It has been shown that bone is formed under electronegative potentials and resorbed under electropositive potentials.11 It is thought that this electrical stimulation is the path through which bone forms in response to applied load.
The observation regarding the electrical nature of bone osteogenesis has spurred the development and investigation of techniques for applying electrical fields to fracture sites in an effort to promote healing. Three techniques for the application of electrical stimulation in fracture healing have been described, which include direct electrical current, capacitive coupling, and inductive coupling [Table 1].
Table 1.
A summary of the techniques of electrical stimulation of bone
| Technique of electrical stimulation | Method of application | Advantages | Disadvantages |
|---|---|---|---|
| Direct electrical stimulation | One or multiple surgically implanted cathodes with one cutaneous electrode | Case series (Level IV) suggests clinical efficacy13 May enhance growth factor production | Invasive (requires surgical implantation of cathodes) |
| Capacitative coupling | Two cutaneous electrodes | Noninvasive Basic science studies show enhanced bone cell proliferation May enhance growth factor production | |
| Inductive coupling | Cutaneous electromagnetic coil | Noninvasive Basic science studies show enhanced bone cell proliferation May enhance growth factor production | Recent meta-analysis (Level I) failed to show clinical efficacy7 |
Electrical stimulation techniques have been applied to acute fractures, delayed unions, nonunions, and joint arthrodesis. Contraindications to electrical stimulation include segmental bone loss at the fracture site, synovial pseudoarthrosis, congential pseudoarthrosis, infected nonunions, and poor mechanical stability of the fracture site. In these clinical scenarios, surgical management to bone graft defects, eradicate infection, or stabilize the fracture with internal fixation is required before electrical stimulation can be considered. Electrical stimulation should be thought of as an adjunct to, not a replacement for, standard fracture care. We will examine each of the three methods of electrical stimulation in detail.
DIRECT ELECTRICAL CURRENT
Direct electrical current techniques are invasive and involve the implantation of one or multiple cathodes into the bone [Figure 1]. An anode is typically placed on the skin over the fracture site and a 5 to 100μA current is delivered.12 In 1981, Brighton et al. published a case series using direct electrical stimulation via four cathodes surgically implanted into a fracture nonunion site for 12 weeks (Level IV Evidence).13 They found that four 20-μA cathodes applied for 12 weeks produced solid bony union in 129 of 168 fracture nonunions (i.e., 76.8% union). The authors suggested that the presence of a synovial pseudoarthrosis, a large bone gap at the fracture site, or an osteomyelitis were contraindications to electrical stimulation therapy and therefore removed these patients from their clinical series. Direct electrical current has also been used to promote healing of spinal fusion, ankle fusions and charcot foot reconstructions.14–16
Figure 1.
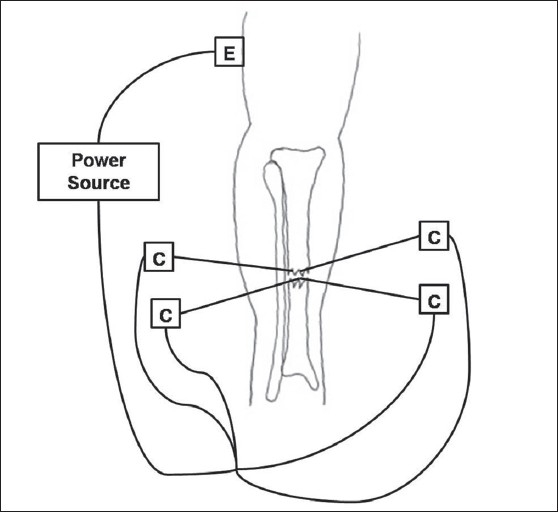
The technique of direct electrical stimulation of bone is illustrated for a tibia fracture. Four cathodes are implanted at the fracture site and a cutaneous electrode is placed at a distant site. An external power source is used to generate current. E = Electrode, C = Cathode
An electrochemical reaction occurring at the cathode is thought to, in part, result in the osteogenic effects of direct electrical stimulation. A faradic reaction at the cathode has been shown to lower oxygen concentration, increase pH, and produce hydrogen peroxide.17 Such a decrease in oxygen concentration has been found to enhance osteoblastic activity, whereas basic environments have been shown to both increase osteoblastic activity and decrease osteoclastic activity.18 The direct electrical current also results in increased proteoglycan and collagen synthesis. In addition, hydrogen peroxide may stimulate macrophages to release vascular endothelial growth factor (VEGF), an angiogenic factor that is critical for osteogenesis.19,20
CAPACITIVE COUPLING
Capacitive coupling is a noninvasive technique that involves placing two electrodes on the skin overlying the fracture such that the fracture site lies between the electrodes [Figure 2]. An alternating current is then used to create an electrical field within the fracture site. Potentials of 1–10 V at frequencies of 20–200 kHz are applied to the electrodes, which result in the development of electric fields of 1–100 mV/cm at the fracture site.21
Figure 2.
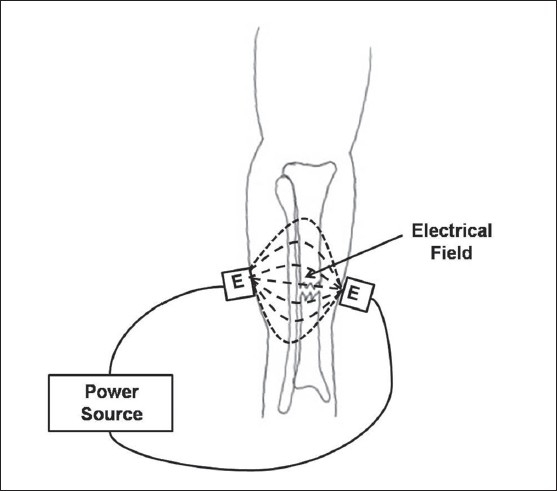
The technique of capacitative coupling is illustrated for a tibia fracture. Two coupled electrodes are placed on the skin overlying the fracture site and an external power source is used to generate current. An electrical field is produced between the electrodes and through the fracture site. E = Electrode
Brighton et al. found that the electrical field strength played a major role in determining the proliferation of bone cells when exposed to a capacitive coupling electric field.22 Stimulation of proliferation of rat calvarial bone cells was measured by 3H thymidine incorporation into DNA and alkaline phosphatase production. They found that an electrical field strength of 0.1–10 mV/cm induced proliferation of rat calvarial bone cells, and electrical field strengths less than 0.1 mV/cm did not induce proliferation.
Korenstein et al. found that there was a dose-dependent response to capacitive coupled fields whereby greater electrical field strength leads to greater proliferative response in osteoblast cells.23 An increase in the time the bone cells are exposed to the electrical field (or “duty cycle” of the capacitive coupling) has also been shown to increase bone cell proliferation.24
The chemical pathway by which capacitive coupling acts on the bone cell to cause proliferation and osteogenesis is a matter of current study.25 Bone cell proliferation resulting from capacitive coupling is accompanied by an increase in intracellular calcium concentration. It has been shown that the proliferative response of bone cells to a capacitive coupling is mediated by calcium translocation via voltage-gated calcium channels. Lorich et al. found that verapamil (a voltage-gated calcium channel blocker) halted the bone cell proliferation seen with capacitive coupling, whereas neomycin (a blocker of the inositol phosphate pathway) had no effect on this proliferation.26 A further study revealed that bromophenacyl bromide (an inhibiter of phospholipase A), indomethacin (an inhibiter of prostaglandin synthesis), and W-7 (a calmodulin antagonist) has a similar effect on bone cell proliferation.27 These studies suggest that, for capacitive coupling, signal transduction results from calcium ion translocation through voltage-gated calcium channels that leads to increases in prostaglandin, cytosolic calcium, and activated calmodulin [Figure 3].
Figure 3.
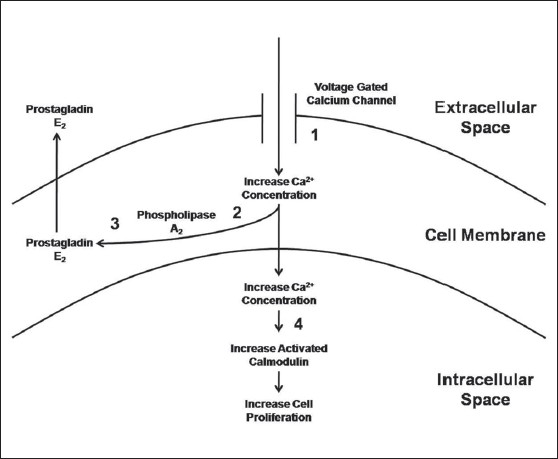
Schematic drawing depicting the signal transduction pathway followed by capacitive coupling electrical stimulation. Numbers represent the inhibitor that blocks the associated pathway: 1 = verapamil, 2 = bromophenacyl bromide, 3 = indomethacin, and 4 = W-7. (Adapted from Brighton C, Wang W, Seldes R, Zhang G, Pollack S: Signal transduction in electrically stimulated bone cells. J Bone Joint Surg. 2001; 83A:1514-1523.)
Wang et al. found that capacitive coupling up-regulates the mRNA expression for bone morphogenic proteins (BMPs)-2, -3, -4, -5, -6, -7, and -8, as well as gremlin and noggin.24 This increase in the production of growth factors that are important for the proliferation and differentiation of osteoblastic cells may represent an alternative mechanism by which capacitive coupling influences osteogenesis.
INDUCTIVE COUPLING
The third type of electrical stimulation used to enhance fracture healing is inductive coupling. Inductive coupling relies on the use of a pulsed electromagnetic field (PEMF) device that is placed on the skin over the fracture site [Figure 4]. The PEMF consists of a wire coil through which a current is passed and a magnetic field is generated. The magnetic field, in turn, induces an electrical field within the fracture site. The size of the electrical field that is induced within the fracture site is dependent on the magnitude of the magnetic field and the physical characteristics of the tissues surrounding and within the fracture site. The variability of the current flowing through the PEMF results in an induced magnetic field that is time variable, and thus the magnitude of an electrical field within the bone varies with time. Induced magnetic fields varying from 0.1 to 20G have been used to produce electrical fields varying from 1 to 100 mV/cm within bone.28 This time-varying electrical field is thought to simulate the normal response of bone cells physiologically to applied mechanical stress.29
Figure 4.
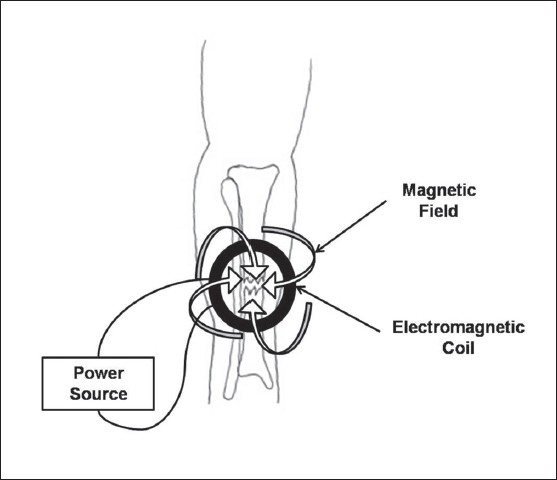
The technique of inductive coupling is illustrated for a tibia fracture. An inductively coupled electromagnetic coil is placed on the skin overlying the fracture site. An external power source produces a circular current within the coil which produces a magnetic field perpendicular to the direction of the current. This magnetic field induces an electrical field within the fracture site
Interestingly, inductive coupling results in an increased bone cell proliferation with increased cytosolic calcium concentration similar to that seen with capacitive coupling. However, the chemical pathway is different. The increase in bone cell proliferation with inductive coupling was blocked by TMB-8 (blocks release of intracellular calcium) and W-7 (blocks activation of calmodulin), but not with the inhibitors found to block bone cell proliferation encountered with capacitive coupling (verapamil, bromophenacyl bromide, or indomethacin).24 For bone cell proliferation due to inductive coupling, signal transduction must be mediated by release of intracellular calcium leading to increases in cytosolic calcium and activated calmodulin [Figure 5].
Figure 5.
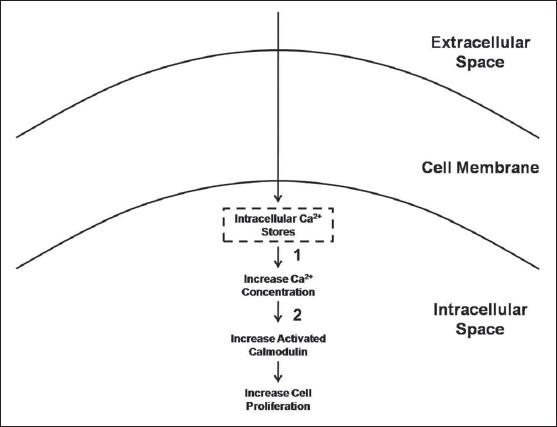
Schematic drawing depicting the signal transduction pathway followed by inductive coupling electrical stimulation. Numbers represent the inhibitor that blocks the associated pathway: 1 = TMB-8 and 2 = W-7. (Adapted from Brighton C, Wang W, Seldes R, Zhang G, Pollack S: Signal transduction in electrically stimulated bone cells. J Bone Joint Surg 2001;83A:1514-1523)
Similar to capacitive coupling, studies have shown increases in growth factors with the use of inductive coupling. BMP-2 and BMP-4 mRNA expression were found to be significantly increased in chick osteoblasts after undergoing inductive coupling.30 Another study cultured nonunion cells from fracture nonunion patients and found a significant increase in TGF-β (Transforming Growth Factor Beta) production in cells stimulated with inductive coupling versus control cells.31 This may represent an alternative method whereby inductive coupling may influence proliferation and differentiation of osteoblastic cells.
A meta-analysis by Mollon et al. examined four randomized controlled trials evaluating the clinical use of inductive coupling electrical stimulation to treat delayed union and nonunion of fractures.7 Their meta-analysis revealed high heterogeneity (I2 = 60.4%) that could not be explained by the bone investigated (tibia versus other bone) or the lesion treated (delayed union versus nonunion). They suggested that this heterogeneity may be due to the varied treatment devices and treatment times used in the different studies. The authors concluded that current evidence from randomized clinical trials is insufficient to suggest a clinical benefit for the use of this therapy for fresh fractures, osteotomies, delayed unions, and nonunions (level I evidence).
SUMMARY
There is extensive basic science research published on the effects of electrical stimulation for fracture healing. These studies have examined the effects of direct electrical stimulation, capacitive coupling and inductive coupling on bone cells in vitro. Through this research, we are now beginning to understand the mechanism of action of these modalities at the cellular level. Further research is required to better elucidate the chemical pathways within the bone cell that respond to electrical stimulation and result in proliferation and differentiation into osteoblastic cells. This may allow us to improve the effectiveness of electrical stimulation for enhancement of fracture healing and treatment of fracture nonunion in humans.
Footnotes
Source of Support: Nil
Conflict of Interest: None.
REFERENCES
- 1.Boyer A. A treatise on surgical diseases: and the operations suited to them. In: Stevens AH, editor. translator. Translation of: Traites des maladies chirurgicales. New York: T and J Swords; pp. 1815–6. [Google Scholar]
- 2.Wolff J. Das Gesetz der Transformation der Knochen A. Hirschwald: Berlin, 1892. In: Manquet P, Furlong R, editors. The Law of Bone Remodelling. Berlin: Springer; 1986. [Google Scholar]
- 3.Fukada E, Yasuda I. On the piezoelectric effect of bone. J Physiol Soc Jpn. 1957;12:1158–62. [Google Scholar]
- 4.Duarte LR. The stimulation of bone growth by ultrasound. Arch Orthop Trauma Surg. 1983;101:153–9. doi: 10.1007/BF00436764. [DOI] [PubMed] [Google Scholar]
- 5.Goodship AE, Kennwright J. The influence of induced micromovement upon the healing of experimental tibial fractures. J Bone Joint Surg Br. 1985;67:650–5. doi: 10.1302/0301-620X.67B4.4030869. [DOI] [PubMed] [Google Scholar]
- 6.Valchanou VD, Michailov P. High energy shock waves in the treatment of delayed and non union of fractures. Int Orthop. 1991;15:181–4. doi: 10.1007/BF00192289. [DOI] [PubMed] [Google Scholar]
- 7.Mollon B, da Silva V, Busse JW, Einhorn TA, Bhandari M. Electrical stimulation for long-bone fracture-healing: a meta-analysis of randomized controlled trials. J Bone Joint Surg Am. 2008;90:2322–30. doi: 10.2106/JBJS.H.00111. [DOI] [PubMed] [Google Scholar]
- 8.Becker RO, Bassett CAL, Bachman CH. In: Bioelectric factors controlling bone structure. Frost H, editor. Boston, Little, Brown: Bone Biodynamics; 1964. pp. 209–32. [Google Scholar]
- 9.Yasuda I. Fundamental aspects of fracture treatment. J Kyoto Med SOC. 1953;4:392. [PubMed] [Google Scholar]
- 10.Otter M, Goheen S, Williams WS. Streaming potentials in chemically modified bone. J Orthop Res. 1988;6:346–59. doi: 10.1002/jor.1100060306. [DOI] [PubMed] [Google Scholar]
- 11.Rubinacci A, Black J, Brighton CT, Friedenberg ZB. Changes in bioelectric potentials on bone associated with direct current stimulation of osteogenesis. J Orthop Res. 1988;6:335–45. doi: 10.1002/jor.1100060305. [DOI] [PubMed] [Google Scholar]
- 12.Black J. Electrical Stimulation: Its Role in Growth, Repair, and Remodeling of the Musculoskeletal System. New York: Praeger; 1987. [Google Scholar]
- 13.Brighton CT, Black J, Friedenberg Z, Esterhai JL, Day LJ, Connolly JF. A multicenter study of the treatment of non-union with constant direct current. J Bone Joint Surg. 1981;63:2–13. [PubMed] [Google Scholar]
- 14.Gan JC, Glazer PA. Electrical stimulation therapies for spinal fusions: current concepts. Eur Spine J. 2006;15:1301–11. doi: 10.1007/s00586-006-0087-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Saxena A, DiDomenico LA, Widtfeldt A, Adams T, Kim W. Implantable electrical bone stimulation for arthrodeses of the foot and ankle in high-risk patients: a multicenter study. J Foot Ankle Surg. 2005;44:450–4. doi: 10.1053/j.jfas.2005.07.018. [DOI] [PubMed] [Google Scholar]
- 16.Petrisor B, Lau JT. Electrical bone stimulation: an overview and its use in high risk and Charcot foot and ankle reconstructions. Foot Ankle Clin. 2005;10:609–20. doi: 10.1016/j.fcl.2005.06.003. [DOI] [PubMed] [Google Scholar]
- 17.Bodamyali T, Kanczler JM, Simon B, Blake DR, Stevens CR. Effect of faradic products on direct current stimulated calvarial organ culture calcium levels. Biochem Biophys Res Commun. 1999;264:657–61. doi: 10.1006/bbrc.1999.1355. [DOI] [PubMed] [Google Scholar]
- 18.Bushinsky DA. Metabolic alkalosis decreases bone calcium efflux by suppressing osteoclasts and stimulating osteoblasts. Am J Physiol. 1996;271:F216–22. doi: 10.1152/ajprenal.1996.271.1.F216. [DOI] [PubMed] [Google Scholar]
- 19.Cho M, Hunt TK, Hussain MZ. Hydrogen peroxide stimulates macrophage vascular endothelial growth factor release. Am J Physiol Heart Circ Physiol. 2001;280:H2357–63. doi: 10.1152/ajpheart.2001.280.5.H2357. [DOI] [PubMed] [Google Scholar]
- 20.Keramaris NC, Calori GM, Nikolaou VS, Schemitsch EH, Giannoudis PV. Fracture vascularity and bone healing: a systematic review of the role of VEGF. Injury. 2008;39:S45–57. doi: 10.1016/S0020-1383(08)70015-9. [DOI] [PubMed] [Google Scholar]
- 21.Aaron R, Ciombor D, Simon B. Treatment of nonunions with electric and electromagnetic fields. Clin Orthop Relat Res. 2004;419:21–9. doi: 10.1097/00003086-200402000-00005. [DOI] [PubMed] [Google Scholar]
- 22.Brighton CT, Okereke E, Pollack SR, Clark CC. In vitro bone-cell response to a capacitively coupled electrical field. The role of field strength, pulse pattern, and duty cycle. Clin Orthop Relat Res. 1992;285:255–62. [PubMed] [Google Scholar]
- 23.Korenstein R, Somjen D, Fischler H, Binderman I. Capacitative pulsed electric stimulation of bone cells. Induction of cyclic-AMP changes and DNA synthesi. Biochim Biophys Acta. 1984;803:302–7. doi: 10.1016/0167-4889(84)90121-6. [DOI] [PubMed] [Google Scholar]
- 24.Wang Z, Clark CC, Brighton CT. Up-regulation of bone morphogenetic proteins in cultured murine bone cells with use of specific electric fields. J Bone Joint Surg Am. 2006;88:1053–65. doi: 10.2106/JBJS.E.00443. [DOI] [PubMed] [Google Scholar]
- 25.Aaron RK, Boyan BD, Ciombor DM, Schwartz Z, Simon BJ. Stimulation of growth factor synthesis by electric and electromagnetic fields. Clin Orthop Relat Res. 2004;419:30–7. doi: 10.1097/00003086-200402000-00006. [DOI] [PubMed] [Google Scholar]
- 26.Lorich DG, Brighton CT, Gupta R, Corsetti JR, Levine SE, Gelb ID, et al. Biochemical pathway mediating the response of bone cells to capacitive coupling. Clin Orthop Relat Res. 1998;350:246–56. [PubMed] [Google Scholar]
- 27.Brighton CT, Wang W, Seldes R, Zhang G, Pollack SR. Signal transduction in electrically stimulated bone cells. J Bone Joint Surg Am. 2001;83:1514–23. doi: 10.2106/00004623-200110000-00009. [DOI] [PubMed] [Google Scholar]
- 28.Aaron RK, Steinberg M. Electrical stimulation of osteonecrosis of the femoral head. Semin Arthroplasty. 1991;2:214–21. [PubMed] [Google Scholar]
- 29.Nelson FR, Brighton CT, Ryaby J, Simon BJ, Nielson JH, Lorich DG, et al. Use of physical forces in bone healing. J Am Acad Orthop Surg. 2003;11:344–54. doi: 10.5435/00124635-200309000-00007. [DOI] [PubMed] [Google Scholar]
- 30.Nagai M, Ota M. Pulsating electromagnetic field stimulates mRNA expression of bone morphogenetic protein-2 and -4. J Dent Res. 1994;73:1601–5. doi: 10.1177/00220345940730100401. [DOI] [PubMed] [Google Scholar]
- 31.Guerkov HH, Lohmann CH, Liu Y, Dean DD, Simon BJ, Heckman JD, Schwartz Z, Boyan BD. Pulsed electromagnetic fields increase growth factor release by nonunion cells. Clin Orthop Relat Res. 2001;384:265–79. doi: 10.1097/00003086-200103000-00031. [DOI] [PubMed] [Google Scholar]


