Abstract
The essential role of microtubules in mitosis makes them a major target of compounds useful for cancer therapy. In our search for potent antitumor agents, a novel series of 2-anilino-4-amino-5-aroylthiazoles was synthesized and evaluated for antiproliferative activity, inhibition of tubulin polymerization, and cell cycle effects. SAR was elucidated with various substitutions on the phenylamino and aroyl moiety at the 2- and 5-positions, respectively, of the 4-aminothiazole skeleton. Tumor cell exposure to several of these compounds led to the arrest of HeLa cells in the G2/M phase of the cell cycle and induction of apoptosis.
Introduction
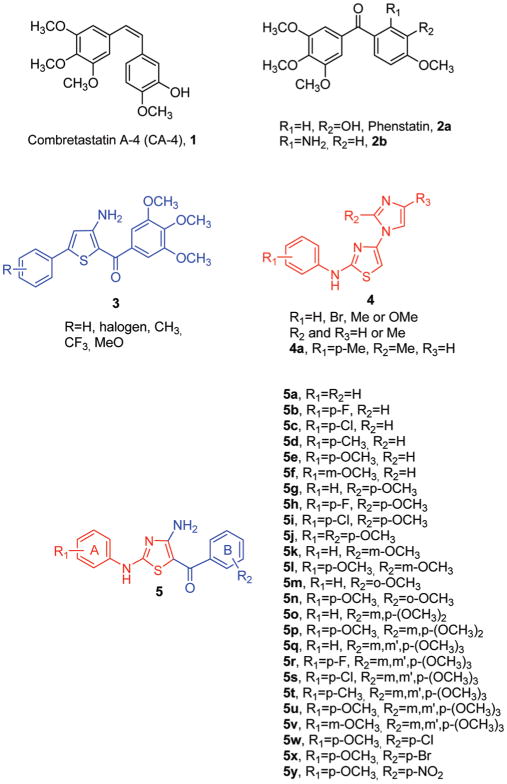
There has been in recent years an intense effort in the discovery and development of novel small molecules, many of which are natural products, able to inhibit tubulin polymerization and have potential for the treatment of cancer.1,2 One of the most important antimitotic agents is combretastatin A-4a (CA-4, 1; Chart 1). CA-4, isolated from the bark of the South African tree Combretumcaffrum,3 is one of the well-known natural tubulin-binding molecules affecting microtubule dynamics by binding to the colchicine site.4 Replacement of the double bond ofCA-4 with a carbonyl group furnished a synthetic benzophenone-typeCA-4 analogue named phenstatin (2a), which demonstrated interesting efficacy in a variety of tumor models.5 The 2-aminobenzophenone derivative 2b also strongly inhibited cancer cell growth and tubulin polymerization and caused mitotic arrest, as did 2a.6
Chart 1.
Inhibitors of Tubulin Polymerization
The classical bioisosteric equivalence between benzene and thiophene prompted us recently to synthesize a series of 2-(3′,4′,5′-trimethoxybenzoyl)-3-amino-5-phenyl thiophene derivatives with general formula 3, in which the thiophene system replaced the benzene moiety in the 2-amino phenstatin analogue 2a.7 The analysis of structures of 2-aminobenzophenone and 2-aroyl-3-aminothiophenes (compounds 2b and 3, respectively) showed that the ortho relationship between the aroyl group and the 2-amino moiety plays an essential role in activity.
Recently, investigators at Altana Pharma reported a series of [4-(imidazol-1-yl)thiazol-2-yl]phenylamine analogues with general structure 4, active at submicromolar concentrations as antiproliferative agents against human colon adenocarcinoma (RKOp27) cells and that act as inhibitors of microtubule polymerization by interfering with the colchicine site of tubulin.8
As a part of our search for novel antimitotic agents, these findings prompted us to synthesize a new series of 2-arylamino- 4-amino-5-aroylthiazole derivatives with general structure 5, obtained by incorporating the 3-amino and 2-aroyl moieties of compounds with general structure 3, into the 4- and 5-positions, respectively, of the 2-arylaminothiazole nucleus of general formula 4.9
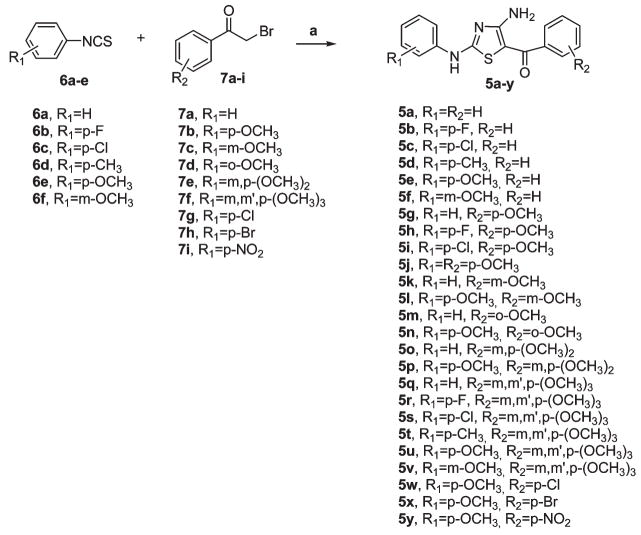
While compounds 5a–f were characterized by the unsubstituted 5-benzoylthiazole structure, we also prepared several derivatives in which the methoxy group was placed at the para (5g–j), meta (5k–l), or ortho (5m–n) position on the B-phenyl ring of the 5-benzoyl moiety. To analyze the effect of additional methoxy groups, we synthesized the 3′,4′-dimethoxy benzoyl (5o–p) and 3′,4′,5′-trimethoxybenzoyl (5q–v) analogues. Finally, to validate whether EWG’s at the para-position of the B-ring can replace the methoxy group of compound 5j with retention of activity, we prepared the chloro (5w), bromo (5x), and nitro (5y) analogues. Once one, two, or three methoxy substituents were placed on the 5-benzoyl moiety, we explored SAR by examining various substitutions with electron-withdrawing (F, Cl, Br, and NO2) and electron-releasing (Me and MeO) groups on the A-phenyl ring of the 2-anilino moiety.
Chemistry
The 2-anilino-4-amino-5-aroyl thiazoles 5a–y were synthesized in acceptable yield (35–55%) by a one-pot, three-component reaction in MeOH of substituted phenyl isothiocyanates 6a–f with α-bromoketones 7a–l and cyanamide in a 1:1:1 ratio and in the presence of MeONa (in situ generated by methanol and sodium) as base (Scheme 1).10
Scheme 1a.
a Reagents: (a) NH2CN, Na, MeOH, rt.
Results and Discussion
The novel series of thiazole derivatives 5a–y were evaluated for their antiproliferative activity against a panel of five tumor cell lines and compared with the reference compounds CA-4 (1) and 4a, as shown in Table 1. Of all the tested compounds, derivative 5q possessed the highest overall cytostatic potency, with IC50 values of 18, 23, 6, 9, and 14 nM against the L1210, FM3A, Molt4, CEM, and HeLa cell lines, respectively. It had comparable or slightly greater antiproliferative activity than CA-4 against three cell lines and was less potent in two lines. Comparing derivatives 5t and 4a, which shared a common 2-(p-methylanilino) thiazole moiety, 4a was from 40- to 150-fold less active than 5t.
Table 1.
In Vitro Inhibitory Effects of Compounds 4a, 5a–y and CA-4 (1)
| IC50a (nM) |
|||||
|---|---|---|---|---|---|
| compd | L1210 | FM3A | Molt4 | CEM | HeLa |
| 5a | 8100 ± 200 | 8200 ± 100 | 7300 ± 1100 | 3900 ± 800 | 6100 ± 1600 |
| 5b | >10000 | >10000 | >10000 | >10000 | >10000 |
| 5c | >10000 | >10000 | >10000 | >10000 | >10000 |
| 5d | 2800 ± 400 | 1500 ± 900 | 210 ± 130 | 370 ± 120 | 900 ± 260 |
| 5e | 850 ± 170 | 730 ± 390 | 230 ± 140 | 300 ± 10 | 230 ± 50 |
| 5f | 8200 ± 600 | 5000 ± 800 | 1500 ± 200 | 2900 ± 300 | 3200 ± 1100 |
| 5g | >10000 | >10000 | 6900 ± 1900 | 9100 ± 5000 | >10000 |
| 5h | >10000 | >10000 | >10000 | >10000 | >10000 |
| 5i | >10000 | >10000 | >10000 | >10000 | >10000 |
| 5j | 470 ± 58 | 630 ± 110 | 320 ± 70 | 1200 ± 110 | 330 ± 10 |
| 5k | 7600 ± 2200 | 6200 ± 1900 | 1300 ± 100 | 2300 ± 200 | 1700 ± 0.0 |
| 5l | 35 ± 14 | 50 ± 26 | 4.0 ± 2.3 | 9.9 ± 1.8 | 37 ± 12 |
| 5m | 1100 ± 600 | 1100 ± 200 | 1300 ± 0.0 | 730 ± 490 | 1200 ± 100 |
| 5n | 31 ± 11 | 52 ± 15 | 18 ± 2 | 17 ± 3 | 17 ± 0.0 |
| 5o | >10000 | >10000 | 5500 ± 3700 | 6900 ± 1600 | 6000 ± 900 |
| 5p | 38 ± 28 | 71 ± 26 | 37 ± 31 | 10 ± 2 | 52 ± 12 |
| 5q | 18 ± 0.0 | 23 ± 10 | 6 ± 1.1 | 9.4 ± 3.6 | 14 ± 1 |
| 5r | 900 ± 480 | 58 ± 100 | 280 ± 170 | 400 ± 20 | 440 ± 40 |
| 5s | 420 ± 170 | 420 ± 10 | 110 ± 10 | 190 ± 70 | 180 ± 40 |
| 5t | 31 ± 6.0 | 15 ± 3.0 | 21 ± 1.1 | 41 ± 3.5 | 86 ± 10 |
| 5u | 1500 ± 1000 | 2200 ± 900 | 480 ± 150 | 1100 ± 0.0 | 560 ± 40 |
| 5v | 2100 ± 300 | 2000 ± 300 | 530 ± 0.00 | 1300 ± 0.0 | 1000 ± 200 |
| 5w | 84 ± 30 | 59 ± 37 | 13 ± 7 | 19 ± 6.9 | 83 ± 6 |
| 5x | 110 ± 0.00 | 100 ± 0.00 | 16 ± 6 | 39 ± 11 | 79 ± 4 |
| 5y | 280 ± 110 | 310 ± 160 | 29 ± 11 | 31 ± 12 | 350 ± 60 |
| 4a | 1800 ± 71 | 1200 ± 0 | 510 ± 11 | 1500 ± 71 | 530 ± 64 |
| CA-4 | 2.8 ± 1.1 | 42 ± 6.0 | 16 ± 1.4 | 12 ± 2.5 | 1.9 ± 1.6 |
IC50 = compound concentration required to inhibit tumor cell proliferation by 50%.
In comparing the effect of ERG’s or EWG’s at the para-position of the A-phenyl ring, compounds with electron-donating methyl or methoxy groups were generally more cytostatic than those with the electron-withdrawing fluoro or chloro moieties. In six of the eight potent analogues (i.e., 5l, 5n, 5p–q, and 5w–y), there was a para-methoxy substitution on the A-phenyl ring, and the only inactive compound with this substituent was 5u, while 5e and 5j had weak activity. The presence of the para-methoxy group in the A ring allowed a wide range of substituents in the B ring (meta- and ortho-methoxy groups in 5l and 5n, respectively; para-chloro, -bromo, and -nitro groups in 5w, 5x, and 5y, respectively; and even a meta-, para-dimethoxy substitution in 5p), but neither a bare B ring as in 5e, a 4′-methoxy group as in 5j, nor the 3′,4′, 5′-trimethoxy substitution pattern of 5u were tolerated. However, the 3′,4′,5′-trimethoxy substitution pattern was tolerated with either no A ring substituent (the highly active 5q) or the smaller A ring methyl substituent of 5t.
The results also showed that, with the notable exception of 5q, all compounds with no substituent in either ring A or ring B had minimal or reduced antiproliferative activity (5a–g, 5k, 5m, and 5o). Again, with the exception of 5q, the data showed that the ring B para-position is the least favorable for a methoxy group. However, para B ring EWG’s are also associated with high antiproliferative activity when a para-methoxy-substituted A ring is also present (5w–y).
In the series of 3′,4′,5′-trimethoxybenzoyl thiazole derivatives 5q–v, the unsubstituted 2-phenylamino derivative 5q was the most active inhibitor of cell growth. Introduction of a fluorine (5r) or chlorine (5s) into the ortho-position of A-phenyl ring caused on average a 27-fold reduction of potency relative to 5q, while the para-methyl compound 5t was almost as active as 5q. The replacement of the para-methyl with a para-methoxy (5u), as noted above, was highly detrimental to activity, resulting, on average, in an 83-fold reduction of potency. The compound with a meta-methoxy group (5v) was similarly inactive.
As noted above, ERG’s are not indispensable on the B-ring, as shown by the activity of derivatives 5w–y. In fact, in the para-position EWG’s resulted in compounds (5w–y) with substantially more cytostatic activity than was observed with the para-methoxy group of compound 5j.
To investigate whether the antiproliferative activities of this novel series of 4-aminothiazole derivatives involved an interaction with tubulin, a selected series of compounds (5a, 5f, 5l, 5n, 5p–q, 5t, and 5w) were evaluated for their in vitro inhibition of tubulin polymerization and for their inhibitory effects on the binding of [3H]colchicine to tubulin (Table 2). For comparison, CA-4 and 4a were examined in contemporaneous experiments. In the assembly assay, compound 5t was found to be the most active (IC50, 0.72 μM) and it was 2- and 5-times more potent than CA-4 and 4a (IC50’s of 1.4 and 4.0 μM, respectively). Derivatives 5l, 5n, and 5w had activity comparable to that of CA-4, while 5p and 5q were about half as potent as CA-4. Unexpectedly, derivatives 5a and 5f were 5- and 10-fold less active than 5t as inhibitors of tubulin assembly although they were 2 orders of magnitude less potent in their effects on cell growth. The reduced antiproliferative activities of 5a and 5f may result from poor permeability into cells, poor solubility in the tissue culture medium, or any other mechanism limiting the accessibility of these molecules to cellular tubulin. Alternatively, these molecules may be exerting their effect by a different, as yet uncovered, mechanism of action.
Table 2.
Inhibition of Tubulin Polymerization and Colchicine Binding by Compounds 4a, 5l, 5n, 5p–q, 5t, 5w, and CA-4
| compd | tubulin assemblya IC50 ± SD (μM) | colchicine bindingb % ± SD |
|---|---|---|
| 5a | 8.0 ± 0.7 | 35 ± 2 |
| 5f | 3.6 ± 0.4 | 43 ± 2 |
| 5l | 1.4 ± 0.2 | 84 ± 2 |
| 5n | 1.4 ± 0.2 | 77 ± 4 |
| 5p | 2.8 ± 0.3 | 63 ± 0.2 |
| 5q | 2.5 ± 0.3 | 75 ± 2 |
| 5t | 0.72 ± 0.01 | 87 ± 1 |
| 5w | 1.1 ± 0.1 | 89 ± 2 |
| 4a | 4.0 ± 0.4 | 58 ± 3 |
| CA-4 (1) | 1.4 ± 0.1 | 87 ± 3 |
Inhibition of tubulin polymerization. Tubulin was at 10 μM.
Inhibition of [3H]colchicine binding. Tubulin, colchicine, and tested compound were at 1, 5, and 1 μM, respectively.
For these eight compounds, 4a and CA-4, the order of activity as inhibitors of tubulin assembly was 5t > 5w > CA-4 = 5l = 5n > 5q > 5p > 5f > 4a ≫ 5a. The potent activity of derivatives 5l, 5n, and 5w showed that the 3′,4′, 5′-trimethoxybenzoyl group was not necessary for inhibiting tubulin assembly.
In the colchicine binding studies, derivatives 5l, 5t, and 5w were as potent as CA-4, which in these experiments inhibited colchicine binding by 87%. In general, inhibition of [3H]colchicine binding to tubulin correlated more closely with antiproliferative activity than inhibition of tubulin assembly.
The effects of compounds 5l, 5n, 5p–q, 5t, and 5w on the cell cycle were examined by flow cytometry after staining the cells with propidium iodide. HeLa cells were exposed for 24 h to different concentrations of the compounds. All tested compounds (see Figure 1 in the Supporting Information) caused an evident and rapid increase of cells in the G2-M phase, and this was already significant for all compounds at a concentration of 30 nM. Aconcomitant decrease of cells in the G1 and S phases was also observed. All the compounds also caused the appearance of a hypodiploid peak (sub-G1), indicative of apoptosis (see Figure 2 in the Supporting Information). Compared with the sub-G1 area (8.9%) in control cells, all compounds (except 5l) showed a significant increase in apoptotic cells in a concentration-dependent manner (see Figure 3 in the Supporting Information).
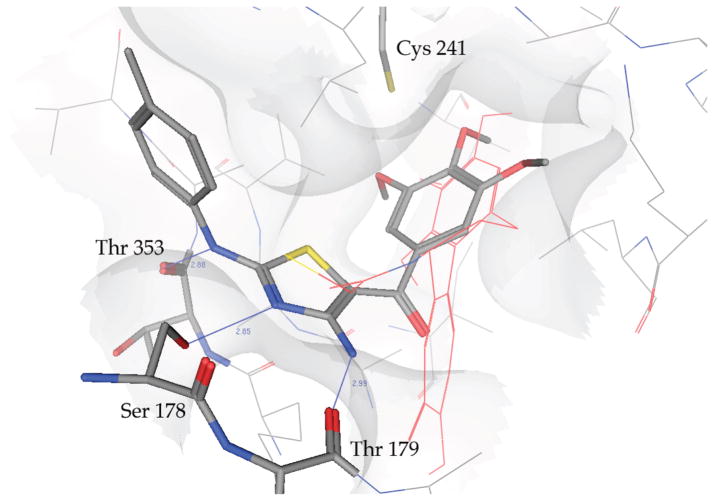
We also performed a series of molecular docking simulations in the colchicine site of tubulin with the most active compound 5t.11 The proposed binding of this compound is shown in Figure 1, and it is possible to see how the trimethoxyphenyl moiety is placed in proximity of Cys241 (residue numbering based on the crystal structure used). Furthermore, binding is stabilized by the presence of three hydrogen bonds between the thiazole core and Thr179, Ser178, and Thr353. These residues (Cys241, Thr179, and Ser178) were also found to be involved in the binding of other tubulin binding agents.12 Finally, the aromatic ring in position 2 of the thiazole is on the edge of the binding pocket.
Figure 1.
Proposed binding mode of 5t in the colchicine site. DAMA-colchicine in red.
In conclusion, we have discovered a new class of structurally simple synthetic inhibitors of tubulin polymerization based on a 2-anilino-4-amino-5-aroylthiazole molecular skeleton. With the exception of 3′,4′,5′-trimethoxybenzoyl derivatives 5u–v, we found that ERG’s on the A-phenyl ring enhanced antiproliferative activity, while EWG’s reduced antiproliferative activity. By comparing the effects of ERG’s and EWG’s on the B-phenyl ring, compounds (i.e., 5j, 5w, 5x, and 5y) with substituents with opposite electronic effects showed similar potency. We also showed by flow cytometry that selected compounds had cellular effects typical for microtubule- interacting agents, causing accumulation of apoptotic cells and cells in the G2/M phase of the cell cycle. The preparation of these compounds was carried out via an efficient procedure, and they constitute an interesting class of potent antitubulin agents which will be further evaluated as potential anticancer agents.
Experimental Section
General Procedure for the Synthesis of Compounds 5a–y
Sodium (188 mg, 8.2 mmol) was carefully dissolved in MeOH (10 mL) at ambient temperature. The resultant solution was added dropwise, over 10 min, to a mixture of cyanamide (345 mg, 8.2 mmol) and arylisothiocyanate (8.2 mmol, 1 equiv) dissolved in MeOH (5 mL), and cooled with an ice-bath. The appropriate phenacyl bromide (8.2 mmol, 1 equiv) was added in small portions, and the resulting mixture was stirred overnight at ambient temperature. The mixture was diluted with water (10 mL) and extracted with dichloromethane (3 × 15 mL). The organic phase was washed with brine (10 mL), dried over Na2SO4, and evaporated. The residue was purified by silica gel column chromatography or crystallized from ethyl ether or petroleum ether.
Supplementary Material
Acknowledgments
We wish to thank Alberto Casolari for technical assistance.
Footnotes
Abbreviations: CA-4, combretastatin A-4; EWG, electron-withdrawing group; ERG, electron-releasing group; SAR, structure–activity relationships; MeOH, methanol; MeONa, sodium methoxide.
Supporting Information Available: Detailed biological protocols, molecular modeling procedure, and spectroscopic data for compounds 5a–y. This material is available free of charge via the Internet at http://pubs.acs.org.
References
- 1.Amos LA. Microtubule structure and its stabilisation. Org Biomol Chem. 2004;2:2153–2160. doi: 10.1039/b403634d. [DOI] [PubMed] [Google Scholar]
- 2.Mahindroo N, Liou JP, Chang JY, Hsieh HP. Antitubulin agents for the treatment of cancer. A medicinal chemistry update. Exp Opin Ther Pat. 2006;16:647–691. [Google Scholar]
- 3.Pettit GR, Singh SB, Hamel E, Lin CM, Alberts DS, Garcia-Kendall D. Isolation and structure of the strong cell growth and tubulin inhibitor combretastatin A-4. Experientia. 1989;45:209–211. doi: 10.1007/BF01954881. [DOI] [PubMed] [Google Scholar]
- 4.Lin CM, Ho HH, Pettit GR, Hamel E. Antimitotic natural products combretastatin A-4 and combretastatin A-2: studies on the mechanism of their inhibition of the binding of colchicine to tubulin. Biochemistry. 1989;28:6984–6991. doi: 10.1021/bi00443a031. [DOI] [PubMed] [Google Scholar]
- 5.Pettit GR, Toki B, Herald DL, Verdier-Pinard P, Boyd MR, Hamel E, Pettit RK. Antineoplastic agents. 379. Synthesis of phenstatin phosphate. J Med Chem. 1998;41:1688–1695. doi: 10.1021/jm970644q. [DOI] [PubMed] [Google Scholar]
- 6.Liou JP, Chang CW, Song JW, Yang YN, Yeh CF, Tseng HY, Lo YK, Chang YL, Chang CM, Hsieh HP. Synthesis and structure–activity relationship of 2-aminobenzophenone derivatives as antimitotic agents. J Med Chem. 2002;45:2556–2572. doi: 10.1021/jm010365+. [DOI] [PubMed] [Google Scholar]
- 7.Romagnoli R, Baraldi PG, Remusat V, Carrion MD, Lopez Cara C, Preti D, Fruttarolo F, Pavani MG, Tabrizi MA, Tolomeo M, Grimaudo S, Balzarini J, Jordan MA, Hamel E. Synthesis and biological evaluation of 2-(3′,4′,5′-trimethoxybenzoyl)-3-amino 5-aryl thiophenes as a new class of tubulin inhibitors. J Med Chem. 2006;49:6425–6428. doi: 10.1021/jm060804a. [DOI] [PubMed] [Google Scholar]
- 8.Mahboobi S, Sellemer A, Hocher H, Eichhorn E, Bar T, Schmidt M, Maier T, Stadlweiser JF, Beckers TL. [4-(Imidazol-1-yl)thiazol-2-yl]phenylamines. A novel class of highly potent colchicine site binding tubulin inhibitors: synthesis and cytotoxic activity on selected human cancer cell lines. J Med Chem. 2006;49:5769–5776. doi: 10.1021/jm060545p. [DOI] [PubMed] [Google Scholar]
- 9.Only one compound of this series (5e) was reported in literature and showed an IC50 average value of 0.35 μM against different cancer cell lines. Sengupta S, Smitha SL, Thomas NE, Santhoshkumar TR, Devi KC, Sreejaleksmi KG, Rajasekharan KN. 4-Amino-5-benzoyl-2-(4-methoxyphenylamino) thiazole (DAT1): a cytotoxic agent towards cancer cells and a probe for tubulin–microtubule system. Br J Pharmacol. 2005;145:1076–1083. doi: 10.1038/sj.bjp.0706276.
- 10.Gewald K, Blauschmidt P, Mayer P. 4-Amino-thiazole. J Prakt Chem. 1967;35:97–104. [Google Scholar]
- 11.Ravelli RBG, Gigant B, Curmi PA, Jourdain I, Lachkar S, Sobel A, Knossow M. Insight into tubulin regulation from a complex with colchicine and a stathmin-like domain. Nature. 2004;428:198–202. doi: 10.1038/nature02393. [DOI] [PubMed] [Google Scholar]
- 12.De Martino G, Edler MC, La Regina G, Coluccia A, Barbera MC, Barrow D, Nicholson RI, Chiosis G, Brancale A, Hamel E, Artico M, Silvestri R. Arylthioindoles, potent inhibitors of tubulin polymerization. 2. Structure–activity relationships and molecular modeling studies. J Med Chem. 2006;49:947–954. doi: 10.1021/jm050809s. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.